Abstract
Adiponectin (Acrp30) is a physiologically active polypeptide hormone secreted by adipose tissue that shows insulin-sensitizing, antiinflammatory, and antiatherogenic properties. In humans, Acrp30 levels are inversely related to the degree of adiposity. In the current study, we tested the long-term weight-reducing and insulin-enhancing effects of Acrp30 cDNA delivered peripherally by a viral vector. To this end, we have generated a series of recombinant adeno-associated virus vectors of serotypes 1 and 5 encoding mouse Acrp30 cDNAs. The long-term expression of recombinant adeno-associated virus-Acrp30 vectors was tested after intramuscular or intraportal injection in female Sprague–Dawley rats with diet-induced obesity. We show that a single peripheral injection of 1012 physical particles of Acrp30-encoding vectors resulted in sustained (up to 280 days) significant reduction in body weight, concomitant with the reduction in daily food intake. Acrp30 treatment resulted in higher peripheral insulin sensitivity measured by the i.p. glucose tolerance test in fasted animals. Ectopic expression of the Acrp30 transgene resulted in modulation of hepatic gluconeogenesis and lipogenesis, as demonstrated by the reduction of the expression of two key genes: PEPCK (phosphoenolpyruvate carboxykinase) and SREBP-1c (sterol regulatory element-binding protein 1c) in the liver. These data show successful peripheral therapy in a clinically relevant model for human obesity and insulin resistance.
Obesity, recognized by the World Health Organization as a health problem of pandemic proportions, is a complex metabolic disorder of multifactorial etiology, which often leads to insulin resistance, dyslipidemia, hypertension, impaired fibrinolysis, and numerous other pathologic conditions, including cancers (1). In Western countries, 2–8% of health care costs are attributable to obesity (2), and this statistic is aggravated by a poor rate of successful treatment. To date, no effective pharmacological treatment has been established because current antiobesity drugs invoke a plethora of deleterious side effects (3, 4).
Among potential therapeutic molecules, leptin failed to fulfill expectations as the drug of choice for diet-induced obesity (DIO) because the vast majority of overweight patients are leptin resistant (5). When administered centrally, leptin mediated a more robust anorexigenic effect initially (6) but then appeared to promote the development of central leptin resistance in rat brain exposed to the extended constitutive expression of the leptin transgene (7, 8).
Unlike leptin, however, the expression of another adipose-secreted hormone, adiponectin (Acrp30) (9), is reduced in obese and diabetic mice (10), and plasma levels of Acrp30 are lower in obese compared with lean humans (11). Similarly, Acrp30 replenishment decreases body adiposity and ameliorates insulin resistance in various models of genetic obesity and DIO (12). The mechanism by which Acrp30 ameliorates insulin resistance and improves glucose metabolism in diabetes remains obscure, but evidence suggests that Acrp30 increases fatty acid oxidation in both muscle and liver and decreases hepatic glucose production (13–16). In addition to implications regarding treatment for obesity and diabetes, recent reports suggest antiatherogenic, antiinflammatory (17), and even hematopoiesis-related (18) effects of Acrp30. Experimental data on the modulation of Acrp30 levels in short-term animal experiments suggest that a primary function of this hormone is to augment insulin sensitivity. However, we believed that a longitudinal study would provide an answer to the question whether decreases in circulating levels of Acrp30 contribute directly to the development of obesity and diabetes. To investigate the primary physiological role of Acrp30 in a long-term experimental setting, we used a gene therapy approach for sustained peripheral expression of Acrp30 mediated by recombinant adeno-associated viral (rAAV) vectors. We show that a single intraportal injection of rAAV-Acrp30 counteracts the development of DIO and ameliorates insulin resistance for at least 41 weeks.
Methods
Animals and Diet. Sprague–Dawley rats (Harlan Breeders, Indianapolis) were cared for in accordance with the principles in the National Research Council's Guide for the Care and Use of Laboratory Animals (ref. 19; available at http://oacu.od.nih.gov/regs/guide/guidex.htm). Rats were housed individually at 23–24°C with a 12:12 h light/dark cycle. Animals were fed ad libitum one of the following two types of diet as indicated: standard laboratory chow (12% of kcal as fat; Lab Diet, St. Louis), hereafter referred to as normal diet (ND), and high-calorie diet (60% of kcal as fat; Research Diets, New Brunswick, NJ), hereafter referred to as high-fat diet (HF).
Vector Construction. cDNA encoding murine Acrp30 was obtained by RT-PCR-mediated cloning using total RNA isolated from white adipose tissue. On sequence verification, a single point mutation (A382/G) was identified that changed Met-113/Val. The cDNA was used “as is,” without reverting the residue back to the reported sequence (9), because sequence alignment of murine Acrp30 and human apM1 (20) revealed the presence of a Val residue in the respective position of the human ortholog, and the mutation was deemed functionally irrelevant. rAAV vector carrying murine Acrp30 cDNA was assembled by using pTR-UF backbone (21). The TR-flanked transgene expression cassette contained the following genetic elements: cytomegalovirus enhancer/chicken β-actin promoter (22), Acrp30 cDNA, woodchuck hepatitis virus posttranscriptional regulatory element (23), and bovine growth hormone polyadenylation site.
Packaging, Titering, and Administration of rAAV Vectors. The transfer plasmid pTR-Acrp30, containing the Acrp30-expression cassette, was packaged in AAV1 or AAV5 serotype capsids. Serotypes 1 and 5 were produced by the process known as “pseudotyping” by using the respective helper plasmids pXYZ1 or pXYZ5 (24), containing AAV1 and AAV5 capsid genes, respectively. Vectors were packaged, purified, concentrated, and titered as described (24). Purified rAAV vectors were >99% pure, as measured by polyacrylamide gel electrophoresis and silver-staining (data not shown). The average ratio of physical to infectious particles was 50:1.
A single dose of vector of 1012 DNase I-resistant particles per 500 μl of lactated Ringer's solution was administered into animals by either portal-vein injection (PVI) or injection into the quadriceps muscle.
Histology. Rats were killed by pentobarbital overdose, and serum was collected. The entire liver was harvested and weighed. A small portion was placed into RNAlater solution (Ambion, Austin, TX). Sections of liver, pancreas, adipose tissue, and muscle were harvested for routine histopathological evaluation. Tissue samples were fixed in 10% neutral buffered formalin overnight and embedded in paraffin. Paraffin sections (5 μm thick) were stained with hematoxylin and eosin and examined for inflammatory or degenerative changes by a pathologist who was unaware of the animal treatment group.
Plasma Acrp30, Leptin, and Glucose. Plasma Acrp30 was measured by using mouse and rat Acrp30 RIA kits (Linco Research Immunoassay, St. Charles, MO) or ELISA kits (B-Bridge International, Sunnyvale, CA). Alternatively, mouse Acrp30 was assayed by using Western blot analysis (see below). Plasma leptin was measured by using the LINCOplex rat endocrine panel kit (Linco Research Immunoassay).
i.p. Glucose Tolerance Test (IP GTT). IP GTT was performed at week 27 after vector injection. After overnight 17-h fast, unanesthetized rats were injected i.p. with a dose of 1.5 g of 50% glucose solution per kg of body weight (BW). Blood samples were obtained from the tail vein before glucose challenge and at 15, 30, 60, 90, and 120 min after glucose challenge. Blood glucose concentration was measured with an Elite XL (Bayer, Elkhart, IN) portable glucose meter.
RNA Isolation. Total RNA from the liver was isolated by using TRIzol reagent (GIBCO/BRL). RNA integrity was verified by denaturing agarose gel (1%) electrophoresis with ethidium bromide staining.
Relative Quantitative (RQ) RT-PCR Analysis. Equal amounts (2.5 μg) of RNA from 4–6 animals for each group were pooled to conduct RQ RT-PCR assay. MMLV (Moloney murine leukemia virus) reverse transcriptase (GIBCO/BRL) and random hexamer primers (Promega) were used to synthesize cDNA. cDNA was amplified by PCR using Taq DNA polymerase (Stratagene) and the following gene-specific primers: phosphoenolpyruvate carboxykinase (PEPCK), 5′-AGCCTCGACAGCCTGCCCCAGG-3′ (forward) and 5′-CCAGTTGTTGACCAAAGGCTTTT-3′ (reverse); and sterol regulatory element-binding protein 1c (SREBP-1c), 5′-TGGATTGCACATTTGAAGAC-3′ (forward) and 5′-AGGCCACAGTGCTCATTCTA-3′ (reverse). The 18S rRNA primer–Competimer mix (universal 18S internal standards kit; Ambion) was used as an internal standard in a multiplex PCR. For both PEPCK and SREBP1c primers, the optimal ratio of 18S primers to Competimers was found to be 1:9. To quantify PCR products, each PCR was spiked with 10 μCi (1 Ci = 37 GBq) of [α-32P]dCTP. The profile for PCRs was in accordance with the manufacturer's recommendations. The products were resolved on a 1.2% agarose gel and transferred overnight onto DE81 chromatography paper (Whatman). Blots were processed by using a Storm 860 PhosphorImager (Molecular Dynamics), and corrected relative values for the gene were obtained as a ratio of the 18S signal. RQ RT-PCR analyses were repeated two more times, and the intraassay coefficient of variation was <5%.
Western Blot Analysis. Transgene-derived murine Acrp30 in rat plasma was assayed after removal of albumin from samples by Affi-Gel Blue (Bio-Rad) microcolumn spin chromatography according to the manufacturer's recommendations. Twenty microliters of purified plasma samples was separated by using SDS/10% (unless indicated otherwise) polyacrylamide gel electrophoreses under reducing or nonreducing conditions as described (25), transferred onto poly(vinylidene difluoride) membrane (Bio-Rad), and probed with rabbit polyclonal anti-Acrp30 antibodies for mouse Acrp30 (PA1–054; Affinity BioReagents, Golden, CO) or rat Acrp30 (PA1–056; Affinity BioReagents), generated by using the synthetic peptide E17GITATEGPGAL28, which corresponds to the region of the highest diversity between the rat and murine hormones. After extensive washing and hybridization with respective secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology, Beverly, MA), immunoreactive bands were visualized by using an enhanced chemiluminescence kit (Amersham Biosciences). Murine plasma was used as either a positive or negative control, depending on the context of the experiment.
Statistical Analysis. Statistical analysis was done by unpaired Student's t test and one-way ANOVA, followed by Fisher's test. All data are expressed as mean ± SE; P values <0.05 were considered statistically significant.
Results
Effect of rAAV-Acrp30 on BW and Food Intake (FI). To conduct comprehensive testing of the metabolic effects of Acrp30 supplement therapy, we packaged the Acrp30 transgene cassette into rAAV1 and rAAV5 serotype capsids. It is well established that different AAV serotypes display different target-tissue tropisms. For example, AAV5 is reportedly a more efficacious vector for the transduction of liver (26) and brain (27), whereas AAV1 is a superior vector for muscle transduction (28). Because target tissue for Acrp30 is not entirely defined, our rationale was to use AAV1 and AAV5 serotypes to achieve the highest transduction rate in skeletal muscle and liver, organs involved in energy metabolism.
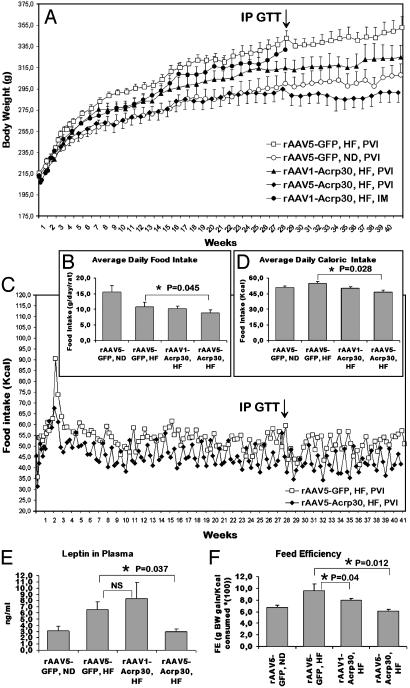
The effectiveness of Acrp30 gene therapy was tested in Sprague–Dawley rats, which are known to develop DIO when fed the HF. BW and FI were the primary readouts of the transgene action. Control groups, injected into the portal vein with rAAV-GFP vector, were fed either the ND or HF. As expected, control DIO rats gained weight faster than rats in the ND-fed group (Fig. 1A).
Fig. 1.
Effect of peripherally administered rAAV1-Acrp30 or rAAV5-Acrp30 in DIO female rats. All injections, unless indicated otherwise, were done intraportally. (A) Change in BW. (B) Average daily FI shown for all groups. Two groups display significant difference, as indicated. (C) Change in ingested kcal/day. For clarity, only two groups of rats are shown. (D) Average daily caloric intake shown for all groups. (E) Plasma leptin levels at the time that the animals were killed. NS, not statistically significant. (F) Plot of feed efficiency (FE), as explained in Results. □, DIO control rats injected with rAAV-GFP vector (n = 10) and fed the HF; ○, control rats injected with rAAV-GFP vector (n = 6) and fed the ND; •, rats injected intramuscularly with rAAV1-Acrp30 vector (n = 6) and fed the HF; ▴, rats injected with rAAV1-Acrp30 vector (n = 6) and fed the HF; ♦, rats injected with rAAV5-Acrp30 vector (n = 6) and fed the HF. The increase in ingested food and calories documented at the beginning of the experiment (C) is attributed to the switch to the HF, which is more palatable. Arrows in A and C indicate the time when IP GTT was conducted.
The group given PVI with rAAV1-Acrp30 vector showed a significant response during the experimental period. At 41 weeks, the average weight of this group was 8% less than the rAAV-GFP/HF control group. By contrast, BW gain in rAAV5-Acrp30-injected animals was dramatically lower than in the HF-fed control group (292 ± 11 g vs. 353 ± 16 g; P < 0.01) at 41 weeks. At 21 weeks, BW of rAAV5-Acrp30/HF rats reached a plateau that was seen in none of the other groups. Postmortem examination of carcasses revealed considerably lower visceral fat accumulation in animals treated with rAAV5-Acrp30 (data not shown). The lower adiposity was consistent with the concentration of leptin in plasma at the time that the animals were killed (Fig. 1E).
The HF-fed group injected intramuscularly with rAAV1-Acrp30 displayed a slower trend of BW gain, significant at 6–12 and 17–18 weeks. Because of the lack of the pronounced response, the intramuscular-injection group was terminated at 28 weeks after injection.
Consistent with BW data, the DIO control group consumed on average 54.9 kcal/day vs. 50.9 kcal/day for the ND-fed control group (Fig. 1 B–D). The rAAV1-Acrp30 and rAAV5-Acrp30 groups consumed 50.1 kcal/day and 46.7 kcal/day, respectively. Therefore, only the latter group demonstrated a significant decrease in FI (15% less compared with the PVI/rAAV-GFP/HF control).
FE is the ratio of weight gained to calories consumed. It is used as a digestive and metabolic indicator, reflecting metabolic efficiency that is not confounded by caloric intake. FE indicates the ease with which consumed energy is converted into BW. Fig. 1F illustrates that, compared with ND, HF increases the overall FE, whereas exogenous Acrp30 apparently reduces FE in HF-fed animals and more so if expressed in rAAV5 serotype vector background. FE seems to describe the differences in BW among the experimental groups more accurately. For the AAV1-Acrp30-injected group, despite the lower gross caloric intake, the FE parameter was slightly higher compared with the ND cohort, reflecting greater weight gain in this group of animals. Interestingly, the AAV5-Acrp30/PVI/HF group demonstrated the lowest FE, which is consistent with the BW data.
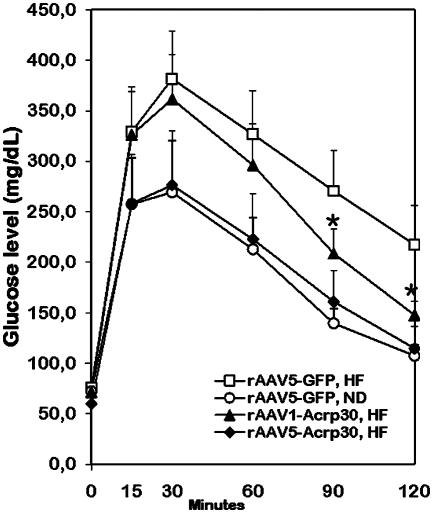
IP GTT. IP GTT was applied to measure the ability of HF-fed rats to withstand glucose challenge. Impaired GT is an indication of early stages of diabetes onset. Fasting glucose levels were identical in all groups (Fig. 2). However, an increase in blood glucose levels in response to glucose load was considerably higher in the rAAV-GFP/HF control group compared with the rAAV-GFP/ND cohort. The rAAV1-Acrp30 group revealed partial but statistically significant “recovery” in a descending quarter of the curve, whereas GT in the rAAV5-Acrp30 group was almost identical to GT observed in lean counterparts. These differences in GT are consistent with the BW data for the respective groups of animals shown in Fig. 1 A.
Fig. 2.
Effect of peripherally administered rAAV1-Acrp30 or rAAV5-Acrp30 on glucose tolerance (GT) in DIO female rats. □, DIO control rats injected with rAAV-GFP (n = 10) and fed the HF; ○, control rats injected with rAAV-GFP (n = 6) and fed the ND; ▴, rats injected with rAAV1-Acrp30 vector (n = 6) and fed the HF; ♦, rats injected with rAAV5-Acrp30 vector (n = 6) and fed the HF. IP GTT was conducted at week 28 after treatment (arrows in Fig. 1 A and C). *, P < 0.05, when compared with DIO control rats.
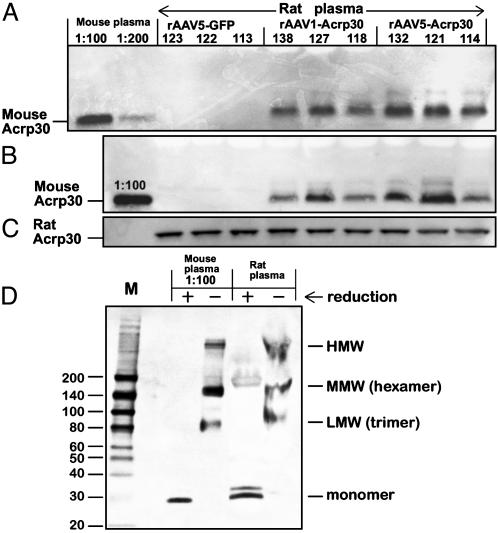
Transgene-Encoded Acrp30 in Plasma. Expression of a PVI-administered rAAV transgene driven by cytomegalovirus enhancer/chicken β-actin promoter reportedly delivers high concentrations of a secreted transgene product in plasma. For example, rAAV-mediated α-1 antitrypsin expression resulted in transgene product plasma concentrations of 0.8–1 mg/ml (29). Using a similar approach, we expected plasma concentrations of the exogenous murine Acrp30 to surpass concentrations of endogenous rat Acrp30. To our surprise, in the face of an apparent physiological effect, we were unable to detect any measurable increase in plasma Acrp30 by using mouse/rat cross-reactive RIA or ELISA on weeks 6, 12, 27, or 34, or postmortem. By employing murine-specific anti-Acrp30 antibodies, transgene-derived mouse Acrp30 was detected in undiluted plasma samples (Fig. 3). At 6 weeks after injection, the concentration of murine Acrp30 was ≈40–65 ng/ml in the AAV1-Acrp30/PVI/HF group and 50–90 ng/ml in the AAV5-Acrp30/PVI/HF group. The assessment was performed by using a standard calibration curve of serially diluted purified murine Acrp30 (data not shown). As expected, plasma from rats in the rAAV-GFP/HF control group was negative for murine Acrp30 (Fig. 3 A and B). The expression of the rAAV-mediated transgene was persistent: the plasma samples from same animals at 41 weeks after injection contained similar amount of murine Acrp30 (Fig. 3B). Same plasma samples diluted 100-fold were positive for endogenous rat Acrp30, as determined by anti-rat-specific Acrp30 antibodies (Fig. 3C, note absence of a signal in lane 1 containing murine plasma sample). Notably, vector-encoded murine Acrp30 was capable of forming multimer complexes similar to isoforms isolated from murine plasma (Fig. 3D), as confirmed by electrophoresis using nonreducing conditions and probed with murine-specific antibodies (25).
Fig. 3.
Western blot analysis of proteins in plasma from rats injected with rAAV1-Acrp30 or rAAV5-Acrp30. (A) Plasma from rats at day 40 after treatment. (B) Plasma from rats killed at day 297 after treatment. (C) Same as B, except diluted 1:100 and hybridized to rat-specific anti-Acrp30 antibodies. Plasma from normal mouse was used as a positive control sample (1:100 or 1:200 dilution). Numbers above each lane refer to individual experimental animals. (D) SDS/4–15% PAGE electrophoresis with subsequent immunoblotting. Before loading, prepurified plasma from one of the rAAV5-Acrp30 rats was treated under either reducing (+) or nonreducing (–) conditions. Diluted plasma from mouse, used as a positive control, was treated in the same way. High concentration of proteins in undiluted rat samples under nonreducing conditions resulted in slightly aberrant mobility of the multimer complexes; HMW, high molecular weight; MMW, middle molecular weight; LMW, low molecular weight (24).
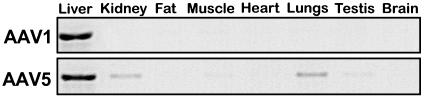
Biodistribution of rAAV Serotypes 1 and 5 on PVI. To determine the target organ and tissue transduced by the vector serotypes used for Acrp30 gene delivery, more Sprague–Dawley rats were injected intraportally with 2 × 1012 DNase I-resistant particles of rAAV1-GFP or rAAV5-GFP. PCR analysis of DNA isolated from different organs at 10 days after injection revealed that for both AAV serotypes tested, liver was the organ where most of the vector was detected (Fig. 4). For rAAV1-GFP, viral DNA was detected also in skeletal muscle. In contrast, rAAV5-GFP was detected in most of the tissues tested except heart and brain (Fig. 4 Lower), which indicates the higher efficiency and promiscuity of this serotype. The distribution of the transgene in the liver in both groups was evaluated also in each lobe separately. It is noteworthy that the distribution was similar throughout all lobes: right and left lateral, caudate, and both medial lobes (data not shown).
Fig. 4.
PCR analysis of viral DNA biodistribution in tissues of rats injected PVI with rAAV1-GFP (Upper) or rAAV5-GFP (Lower) vectors. PCR fragments of GFP cDNA (0.6 kb) were visualized by ethidium bromide staining. A negative image of the gel is shown.
Histological Examination of the Liver. Liver sections from 21 rats representing the HF and ND controls and the AAV1-Acrp30/HF and AAV5-Acrp30/HF groups were reviewed. Several rats in the control group had mild focal-to-multifocal mononuclear inflammation in the portal or parenchymal regions that was considered within the normal range of findings under the husbandry conditions. PVI of either rAAV-GFP or rAAV-Acrp30 did not result in changes in hepatic morphology. The degree of steatosis (fat accumulation) was similar also between the control and Acrp30 groups (data not shown).
Effect of Intraportally Administered rAAV-Acrp30 on Glucose and Lipid Metabolism-Related Molecules in the Liver. To elucidate the mechanisms by which rAAV-mediated ectopic expression of the transgene in the liver might protect against the development of obesity and impaired GT, we analyzed the expression of key genes regulating glucose and lipid metabolism.
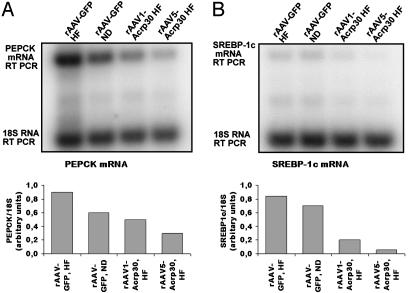
PEPCK catalyzes the conversion of oxaloacetate to phosphoenolpyruvate, which is the rate-limiting step of gluconeogenesis. The gene for PEPCK is expressed mainly in the liver, kidney cortex, and fat. High-level expression of the PEPCK gene in the liver during obesity and diabetes is a major factor in the elevated gluconeogenesis characteristic of this disease (30). Using RQ RT-PCR, we corroborated the finding (31) that in Sprague–Dawley rats HF significantly increases PEPCK levels (Fig. 5A). At the same time, expression of Acrp30 significantly reduced expression levels of PEPCK in the liver of both rAAV1-Acrp30-injected rats and rAAV5-Acrp30-injected rats (Fig. 5A). In both groups, PEPCK expression levels were lower than in control animals fed the ND.
Fig. 5.
RQ RT-PCR assay of the expression levels of mRNAs in the liver of female rats injected PVI with rAAV1-Acrp30 or rAAV5-Acrp30. (A) Expression of PEPCK mRNA. (B) Expression of SREBP-1c. A and B Upper are images of the respective filters with 32P-labeled RT PCR products, as indicated on the left. A and B Lower are graphic representations of the same data.
SREBP-1c is a major transcriptional factor regulating fatty acid synthesis in the liver (32). SREBP-1c levels are increased in the fatty livers of insulin-resistant obese mice (33). The elevated levels of SREBP-1c increase lipogenic gene expression, enhance fatty acid synthesis, and accelerate triglyceride accumulation (34). Similar to PEPCK, the levels of SREBP-1c in livers of rats fed the HF were elevated (Fig. 5B), whereas Acrp30 expression resulted in dramatic reduction of the expression of hepatic SREBP-1c (7-fold in the rAAV5-Acrp30 group vs. the rAAV-GFP/ND group). Therefore, the ectopic expression of Acrp30 down-regulates the expression of key genes controlling hepatic gluconeogenesis and de novo lipogenesis.
Discussion
Unlike leptin, levels of Acrp30 are reduced considerably during hyperlipidemia (10, 11), whereas short-term supplemental Acrp30 therapy in some cases has reduced adipose tissue mass and improved insulin sensitivity (13–15). In the current report, we have achieved similar long-term beneficial effects after a single injection of rAAV-Acrp30.
The primary target tissue and the precise mechanism of Acrp30 action are not yet fully understood. The Acrp30 activity is most likely regulated at several levels, including gene expression, posttranslational modifications, oligomeric complex formation, and proteolytic cleavage into a smaller and perhaps more active fragment (35). Therefore, to deliver the highest possible ectopic expression of Acrp30 in either liver or skeletal muscle and to provide a physiologically relevant ligand capable of exerting the whole-body metabolic effect, we used AAV vectors packaged into capsids of two separate serotypes known to posses distinct tissue tropisms (26, 28). In this article, we show varied weight-reducing effects of Acrp30 transgene delivered by a particular serotype. For example, PVI-administered rAAV1-Acrp30 vector produced modest anorexigenic effects (Fig. 1). However, rAAV5-Acrp30 provided a considerably stronger response, which completely negated HF-induced BW gain seen in the controls. Between two vector serotypes tested, AAV1 is generally considered a better vector for skeletal muscle transduction, even though intramuscular injection-administered rAAV1-Acrp30 produced only weak and transient BW-reducing effect (Fig. 1). This marginal response is apparently associated with the differential mode of Acrp30 action in muscle vs. liver. It appears that, in the skeletal muscle, the globular domain of Acrp30 (gAcrp30), a distinctive form of the hormone (13), stimulates glucose uptake and fatty-acid oxidation more potently than the full-length Acrp30 (36, 37). On the other hand, only a full-length form of Acrp30 is active in the liver (36). Recently, Yamauchi et al. (38) have isolated two distinct Acrp30 receptors: AdipoR1, abundantly expressed in skeletal muscle, and Adi-poR2, predominantly expressed in the liver. AdipoR1 is a high-affinity receptor for globular Acrp30 and a low-affinity receptor for the full-length ligand, whereas AdipoR2 is an intermediate-affinity receptor for both forms of Acrp30. Our data on the transient effect of the intramuscularly delivered rAAV1-Acrp30 indicate that ectopically expressed Acrp30 transgene does not produce a globular form capable of interacting with AdipoR1. It appears, however, that the liver is a more appropriate target organ for a full-length exogenous Acrp30 because of its putative interaction with AdipoR2.
The most unexpected finding in the current study was the relatively low plasma concentrations of murine Acrp30, ranging from 40 to 90 ng/ml. This range was about two orders of magnitude lower than physiological levels of the endogenous rat Acrp30 (Fig. 3), and it was not expected to produce any measurable response (15). However, it generated a systemic metabolic effect, sufficient to counteract the diet-induced adiposity gain. A local effect apparently has been achieved at the hepatic level on autocrine and/or paracrine interaction of exogenous Acrp30 with AdipoR2. Despite the ectopic origin, exogenous Acrp30 was nevertheless capable of forming higher-order structures (Fig. 3D) that were important for the proper secretion and physiological functions (25).
The biodistribution of tested vector serotypes (Fig. 4) indirectly corroborated the hepatic localization of the transgene. The majority of intraportally administered vectors of both serotypes tested displayed a pronounced hepatic tropism. Notably, AAV5-based vectors were the most efficient at transducing the majority of the assayed tissues, including testis, which may be a potential concern for future clinical application.
At the time of testing for GT (29 weeks after injection), the DIO group weighed on average 14% more than the ND group. This degree of obesity, corresponding to the human body-mass index parameter of ≈30, does not constitute a major disorder, such as metabolic syndrome X, with attendant diabetes, hypertension, dyslipidemia, and cardiovascular disease. It is a condition often characterized by prediabetic impaired GT, which may progress to fully developed type 2 diabetes if left untreated. At testing, both DIO and normal groups of animals had identical fasting glucose levels (Fig. 2). However, the DIO group demonstrated a weakened response to glucose challenge, a clear symptom of impaired GT. Remarkably, both Acrp30-treated groups of rats showed an improved GT, with an rAAV5-Acrp GT curve indistinguishable from the one in the ND control group.
Our data indicate that one of the therapeutic modalities of the ectopically expressed Acrp30 is mediated through its anorexigenic potential. Both rAAV1-Acrp30- and rAAV5-Acrp30-treated groups (Fig. 1) displayed reduction in FI, which is significant in the latter cohort. This observation contradicts findings of Fruebis et al. (13), who demonstrated that chronic treatment of DIO mice with gAcrp30 resulted in reduction of BW independently of FI. At this time, we cannot explain the discrepancy between the two studies other than by mentioning the assumption that gAcrp30 and a full-length hormone have different physiological functions (39) and target organs (38).
Recently, Acrp30 has been shown (36, 37) to activate AMP-activated protein kinase (AMPK), a “metabolic master switch” controlling pathways of hepatic ketogenesis, cholesterol synthesis, and triglyceride synthesis (40). On activation, AMPK phosphorylates numerous target proteins, resulting in an increased or decreased flux through the metabolic pathways in which the target proteins play a regulatory role. Alternatively, a form of AMPK expressed in the cell nucleus can also influence metabolism by regulating gene expression (41–43). In the current report, we provide evidence that rAAV-Acrp30 modulates the expression of two key hepatic genes subject to AMPK regulation, PEPCK and SREBP-1c.
The product of PEPCK gene catalyzes a rate-limiting step in gluconeogenesis, and the abnormal regulation of this gene has been associated with the development of type 2 diabetes and obesity (30, 44, 45). Fig. 5A shows a marked reduction in expression rates of the PEPCK gene in animals treated with rAAV-Acrp30, more pronounced in the case of AAV5 vector. This result is consistent with the observation of Combs et al. (16), who demonstrated that the hepatic expression of PEPCK and glucose-6-phosphatase were reduced by >50% after Acrp30 infusion.
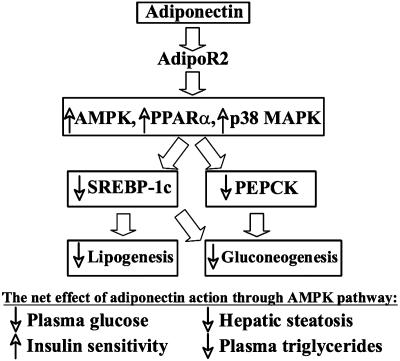
Another downstream gene modulated by Acrp30/AMPK cascade is SREBP-1c, an insulin-stimulated transcription factor that is implicated in the pathogenesis of insulin resistance, dyslipidemia, and type 2 diabetes (34, 46). In the liver, three SREBPs, including isoform 1-c, regulate the expression of >30 genes involved in lipid metabolism (32) and gluconeogenesis (47). Similar to PEPCK, expression of SREBP-1c is markedly down-regulated by rAAV-Acrp30 (Fig. 5B). Our results, therefore, suggest that on activating the AdipoR2 receptor, Acrp30 regulates two distinctive hepatic pathways, lipogenesis and gluconeogenesis, dichotomizing downstream at the point of the AMPK master switch (Fig. 6). Further studies are required to uncover missing links in these signaling cascades initiated by Acrp30.
Fig. 6.
Metabolic pathways regulated by Acrp30 in the liver. The up-regulation and down-regulation of genes and metabolic pathways are indicated by the respective up and down arrows. Block arrows indicate interactions documented in this report or elsewhere (37).
In summary, ectopic expression of adipokine Acrp30 transgene in the liver of the DIO rat model provides a long-term beneficial weight-reducing and insulin-enhancing effects. These effects appear to be mediated through diverse metabolic pathways that include regulation of FI, lipogenesis, and gluconeogenesis. Pending subsequent comprehensive studies of the mechanisms and safety of the described gene therapy approach, our findings present an alternative therapeutic modality in the treatment of obesity and type 2 diabetes.
Acknowledgments
We thank Dr. Yuri Sautin for technical advice on the project. This study was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK62302 (to S.Z.), and by the Medical Research Service of the Department of Veterans Affairs.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Acrp30, adiponectin; AMPK, AMP-activated protein kinase; BW, body weight; DIO, diet-induced obesity; FE, feed efficiency; FI, food intake; GT, glucose tolerance; HF, high-fat diet; IP GTT, i.p. GT test; ND, normal diet; PEPCK, phosphoenolpyruvate carboxykinase; PVI, portal-vein injection; rAAV, recombinant adeno-associated viral; RQ, relative quantitative.
References
- 1.Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. (2003) N. Engl. J. Med. 348, 1625–1638. [DOI] [PubMed] [Google Scholar]
- 2.Kolotkin, R. L., Meter, K. & Williams, G. R. (2001) Obes. Rev. 2, 219–229. [DOI] [PubMed] [Google Scholar]
- 3.Vastag, B. (2003) J. Am. Med. Assoc. 289, 1763–1764. [Google Scholar]
- 4.Michelakis, E. (2002) Vascul. Pharmacol. 38, 51–59. [DOI] [PubMed] [Google Scholar]
- 5.Jequier, E. (2002) Ann. N.Y. Acad. Sci. 967, 379–388. [DOI] [PubMed] [Google Scholar]
- 6.Halaas, J. L., Boozer, C., Blair-West, J., Fidahusein, N., Denton, D. A. & Friedman, J. M. (1997) Proc. Natl. Acad. Sci. USA 94, 8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpace, P. J., Matheny, M., Zhang, Y., Shek, E. W., Prima, V., Zolotukhin, S. & Tumer, N. (2002) Endocrinology 143, 3026–3035. [DOI] [PubMed] [Google Scholar]
- 8.Scarpace, P. J., Matheny, M., Zhang, Y., Tumer, N., Frase, C. D., Shek, E. W., Hong, B., Prima, V. & Zolotukhin, S. (2002) Neuropharmacology 42, 548–561. [DOI] [PubMed] [Google Scholar]
- 9.Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. (1995) J. Biol. Chem. 270, 26746–26749. [DOI] [PubMed] [Google Scholar]
- 10.Hu, E., Liang, P. & Spiegelman, B. M. (1996) J. Biol. Chem. 271, 10697–10703. [DOI] [PubMed] [Google Scholar]
- 11.Arita, Y., Kihara, S., Ouchi, N., Takahashi, M., Maeda, K., Miyagawa, J., Hotta, K., Shimomura, I., Nakamura, T., Miyaoka, K., et al. (1999) Biochem. Biophys. Res. Commun. 257, 79–83. [DOI] [PubMed] [Google Scholar]
- 12.Berg, A. H., Combs, T. P. & Scherer, P. E. (2002) Trends Endocrinol. Metab. 13, 84–89. [DOI] [PubMed] [Google Scholar]
- 13.Fruebis, J., Tsao, T. S., Javorschi, S., Ebbets-Reed, D., Erickson, M. R., Yen, F. T., Bihain, B. E. & Lodish, H. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941–946. [DOI] [PubMed] [Google Scholar]
- 15.Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. (2001) Nat. Med. 7, 947–953. [DOI] [PubMed] [Google Scholar]
- 16.Combs, T. P., Berg, A. H., Obici, S., Scherer, P. E. & Rossetti, L. (2001) J. Clin. Invest. 108, 1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto, Y., Kihara, S., Ouchi, N., Nishida, M., Arita, Y., Kumada, M., Ohashi, K., Sakai, N., Shimomura, I., Kobayashi, H., et al. (2002) Circulation 106, 2767–2770. [DOI] [PubMed] [Google Scholar]
- 18.Yokota, T., Meka, C. S., Medina, K. L., Igarashi, H., Comp, P. C., Takahashi, M., Nishida, M., Oritani, K., Miyagawa, J., Funahashi, T., et al. (2002) J. Clin. Invest. 109, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
- 20.Maeda, K., Okubo, K., Shimomura, I., Funahashi, T., Matsuzawa, Y. & Matsubara, K. (1996) Biochem. Biophys. Res. Commun. 221, 286–289. [DOI] [PubMed] [Google Scholar]
- 21.Zolotukhin, S., Potter, M., Hauswirth, W. W., Guy, J. & Muzyczka, N. (1996) J. Virol. 70, 4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niwa, H., Yamamura, K. & Miyazaki, J. (1991) Gene 108, 193–199. [DOI] [PubMed] [Google Scholar]
- 23.Donello, J. E., Loeb, J. E. & Hope, T. J. (1998) J. Virol. 72, 5085–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolotukhin, S., Potter, M., Zolotukhin, I., Sakai, Y., Loiler, S., Fraites, T. J., Jr., Chiodo, V. A., Phillipsberg, T., Muzyczka, N., Hauswirth, W. W., et al. (2002) Methods 28, 158–167. [DOI] [PubMed] [Google Scholar]
- 25.Waki, H., Yamauchi, T., Kamon, J., Ito, Y., Uchida, S., Kita, S., Hara, K., Hada, Y., Vasseur, F., Froguel, P., et al. (2003) J. Biol. Chem. 278, 40352–40363. [DOI] [PubMed] [Google Scholar]
- 26.Gao, G. P., Alvira, M. R., Wang, L., Calcedo, R., Johnston, J. & Wilson, J. M. (2002) Proc. Natl. Acad. Sci. USA 99, 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson, B. L., Stein, C. S., Heth, J. A., Martins, I., Kotin, R. M., Derksen, T. A., Zabner, J., Ghodsi, A. & Chiorini, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 3428–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao, H., Liu, Y., Rabinowitz, J., Li, C., Samulski, R. J. & Walsh, C. E. (2000) Mol. Ther. 2, 619–623. [DOI] [PubMed] [Google Scholar]
- 29.Song, S., Embury, J., Laipis, P. J., Berns, K. I., Crawford, J. M. & Flotte, T. R. (2001) Gene Ther. 8, 1299–1306. [DOI] [PubMed] [Google Scholar]
- 30.Friedman, J. E., Yun, J. S., Patel, Y. M., McGrane, M. M. & Hanson, R. W. (1993) J. Biol. Chem. 268, 12952–12957. [PubMed] [Google Scholar]
- 31.Bizeau, M. E., Short, C., Thresher, J. S., Commerford, S. R., Willis, W. T. & Pagliassotti, M. J. (2001) Am. J. Physiol. 281, R427–R433. [DOI] [PubMed] [Google Scholar]
- 32.Horton, J. D., Goldstein, J. L. & Brown, M. S. (2002) J. Clin. Invest. 109, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimomura, I., Bashmakov, Y. & Horton, J. D. (1999) J. Biol. Chem. 274, 30028–30032. [DOI] [PubMed] [Google Scholar]
- 34.Shimomura, I., Matsuda, M., Hammer, R. E., Bashmakov, Y., Brown, M. S. & Goldstein, J. L. (2000) Mol. Cell 6, 77–86. [PubMed] [Google Scholar]
- 35.Pajvani, U. B., Du, X., Combs, T. P., Berg, A. H., Rajala, M. W., Schulthess, T., Engel, J., Brownlee, M. & Scherer, P. E. (2003) J. Biol. Chem. 278, 9073–9085. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi, T., Kamon, J., Minokoshi, Y., Ito, Y., Waki, H., Uchida, S., Yamashita, S., Noda, M., Kita, S., Ueki, K., et al. (2002) Nat. Med. 8, 1288–1295. [DOI] [PubMed] [Google Scholar]
- 37.Tomas, E., Tsao, T. S., Saha, A. K., Murrey, H. E., Zhang, Cc. C., Itani, S. I., Lodish, H. F. & Ruderman, N. B. (2002) Proc. Natl. Acad. Sci. USA 99, 16309–16313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamauchi, T., Kamon, J., Ito, Y., Tsuchida, A., Yokomizo, T., Kita, S., Sugiyama, T., Miyagishi, M., Hara, K., Tsunoda, M., et al. (2003) Nature 423, 762–729. [DOI] [PubMed] [Google Scholar]
- 39.Tsao, T. S., Lodish, H. F. & Fruebis, J. (2002) Eur. J. Pharmacol. 440, 213–221. [DOI] [PubMed] [Google Scholar]
- 40.Winder, W. W. & Hardie, D. G. (1999) Am. J. Physiol. 277, E1–E10. [DOI] [PubMed] [Google Scholar]
- 41.Foretz, M., Carling, D., Guichard, C., Ferre, P. & Foufelle, F. (1998) J. Biol. Chem. 273, 14767–14771. [DOI] [PubMed] [Google Scholar]
- 42.Da Silva Xavier, G., Leclerc, I., Varadi, A., Tsuboi, T., Moule, S. K. & Rutter, G. A. (2003) Biochem. J. 371, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minokoshi, Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339–343. [DOI] [PubMed] [Google Scholar]
- 44.Valera, A., Pujol, A., Pelegrin, M. & Bosch, F. (1994) Proc. Natl. Acad. Sci. USA 91, 9151–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosella, G., Zajac, J. D., Baker, L., Kaczmarczyk, S. J., Andrikopoulos, S., Adams, T. E. & Proietto, J. (1995) Mol. Endocrinol. 9, 1396–1404. [DOI] [PubMed] [Google Scholar]
- 46.Kakuma, T., Lee, Y., Higa, M., Wang, Z., Pan, W., Shimomura, I. & Unger, R. H. (2000) Proc. Natl. Acad. Sci. USA 97, 8536–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakravarty, K., Leahy, P., Becard, D., Hakimi, P., Foretz, M., Ferre, P., Foufelle, F. & Hanson, R. W. (2001) J. Biol. Chem. 276, 34816–34823. [DOI] [PubMed] [Google Scholar]