Abstract
Herpes simplex virus type 1 (HSV-1) can induce a robust immune response initially thru the activation of pattern recognition receptors and subsequent type I interferon production that then shapes, along with other innate immune components, the adaptive immune response to the insult. While this response is necessary to quell virus replication, drive the pathogen into a “latent” state, and likely hinder viral reactivation, collateral damage can ensue with demonstrable cell death and foci of tissue pathology in the central nervous system (CNS) as a result of the release of inflammatory mediators including reactive oxygen species. Although rare, HSV-1 is the leading cause of frank sporadic encephalitis that, if left untreated, can result in death. A greater understanding of the contribution of resident glial cells and infiltrating leukocytes within the CNS in response to HSV-1 invasion is necessary to identify candidate molecules as targets for therapeutic intervention to reduce unwarranted inflammation coinciding with maintenance of the anti-viral state.
Keywords: Interferons, CNS, HSV-1, Encephalitis
1. Introduction to Herpes Simplex Virus-1
HSV-1 is a double-stranded DNA virus with a genome size of 152 kb encoding for at least 84 different polypeptides (McGeoch et al., 1988). During an in vitro acute infection, the lytic nature of the virus is driven by a sequential cascade of genes (referred to as lytic genes) expressed collectively over the course of the first 8-12 hours following entry into the host cell and includes the immediate early or α genes, early or β genes, and late or γ genes (Honess and Roizman, 1974). It is now appreciated that many of these genes encode proteins that serve dual functions: assist in the replication of virus and counter the innate or adaptive immune response to the pathogen. Ultimately, the success rate of the virus within the human host is dependent upon its ability to establish a latent infection and then reactivate at opportune times and shed into bodily secretions that are passed vertically or horizontally to a naive patient.
2. Herpes Spread Into the CNS
HSV-1 gains entry into cells by binding to heparan sulfate proteoglycan and invading the host cell (neurons) during primary infection (Shieh et al., 1992; WuDunn and Spear, 1989). It has been shown that this first step of adsorption and infection are severely impaired when enzymatic digestion of cell surface heparan sulfate is performed, yet unaffected with digestion of dermatan sulfate or chondroitin sulfate (WuDunn and Spear, 1989). When comparing mutant cells lacking heparan sulfate to wild types cells, binding is also impaired, (Shieh et al., 1992; Shieh and Spear, 1994) suggesting the likelihood of heparan sulfate being an initial receptor of HSV-1. Experimental evidence shows HSV-1 glycoprotein (g) C and gB are vital components for viral attachment to the host cell (Reske et al., 2007). Without heparan sulfate, the ability of the virus to attach and infect the host cell is severely impaired.
While the virus can infect cells with the loss of either glycoprotein, a loss of both gC and gB renders the virion noninfectious. It has also been shown gB binds to specific cell surface receptors and directly fuses with the cell membrane or enters through fusion within an endosome in a low pH-dependent or a pH-independent environment (Nicola et al., 2003; Nicola et al., 2005). Thus, gB is involved in attachment and fusion to many host cell types in some fashion and coordinates infection in a multitude of cell types including neurons. Following attachment and fusion, the virus then uses gD interaction with herpesvirus entry mediator, nectin-1, or 3-O-sulfated heparan sulfate for viral entry (Reske et al., 2007). However, the exact process and all viral and cellular components involved in attachment and fusion are still unclear. In the case of neurons, once the virus has entered in an in vivo model of infection, it will then travel via axonal retrograde transport to infect the cell nucleus of sensory ganglia such as the trigeminal ganglion within the first 24 hours following infection (Shimeld et al., 2001). The rapidity of the virus traveling to the neuronal body of the sensory ganglia all but assures escape from the adaptive immune response such that the host’s innate immune response is the primary arm of the immune system left to block HSV-1 replication and spread to the CNS. A significant portion of innate resistance lies with the type I interferon (IFN) response. Consequently, it should come as no surprise HSV-1 has a number of gene-encoded proteins that specifically target the type I IFN pathway including infected cell protein (ICP) 34.5, ICP0, and ICP27.
During primary infection HSV-1 replicates and thwarts viral-encoded protein translational arrest using ICP34.5 and ICP27 to inhibit activated protein kinase R and Jak/STAT signaling respectively in response to a vigorous immune response (Leib et al., 2000; Mossman and Smiley, 2002; Johnson et al., 2008). The virus circumvents host cell defense using ICP0 and virion host shutoff (vhs) antagonism of Stat I and thus IFN production (Yokota et al, 2001; Chee and Roizman, 2003; Halford et al., 2006; Pasieka et al., 2008; Harle et al., 2002). ICP0 accomplishes this process by inhibiting activation and nuclear accumulation of interferon regulatory factor (IRF)-3 and IRF-7 mediated by the ICPO RING finger domain (Lin et al., 2004; Melroe et al., 2004). In addition, ICP0 disperses nuclear domain-10 nuclear bodies that are normally associated with transcription regulation, growth suppression, and apoptosis (Maul et al., 1993; Everett and Zafiropoulos, 2004). Contrary to previous studies (Steiner et al., 1990; Sears et al., 1991; Ecob-Prince et al., 1993), a recent in vivo investigation has reported that virus lacking an early transcribed viral particle-16 (VP16), which mediates transcription of immediate early genes, does not exit latency (Thompson et al., 2009). In addition, it has also been reported ICP0 deficient HSV-1 maintains similar viral protein levels as the parental wild type counterparts in cell culture (Thompson et al., 2009) and are still able to reactivate (Preston, 2007). Taken together, these studies suggest VP16 could play a role in mediating transcription of early viral particles but also rely on ICP0-mediated interference of host anti-viral defenses to fully reactivate. Whether these coordinated events allow the virus to exit latency and reactivate still remains unproven. The impact of ICP0 expression in the CNS is not fully understood although ICP0 mutants do not replicate to the same extent as parental virus unless the type I IFN pathway is compromised (Halford et al., 2006). Conversely, over expression of type I IFNs in the CNS is found to enhance resistance to HSV-1 infection (Carr et al., 1998). Therefore, it would appear to be a race between type I IFN activation of antiviral pathways and HSV-encoded proteins to counter the effects of the IFN pathway and the adaptive immune response that ultimately dictates whether the virus will prevail and establish a latent infection.
After the acute infection, the virus establishes latency in A5+ and KH10+ sensory ganglion cells (Bertke et al., 2009) and is thought to remain dormant until reactivation. Experimentally though, HSV-1 causes localized incomplete or low level lytic infection (Feldman et al., 2002) causing a persistent immune response during latency (Cantin et al., 1995; Shimeld et al., 1995; Halford et al., 1996; Liu et al., 1996). Daily exposure to the antiviral drug acyclovir significantly reduces the localized immune response within the ganglion during latency (Halford et al., 1997). Thus, the virus continuously undergoes incomplete or low level reactivation until such circumstances allow it to fully reactivate and perpetuate spread to naive recipients.
3. Host Cell Response to HSV-1 Infection: The Toll-like receptor (TLR)
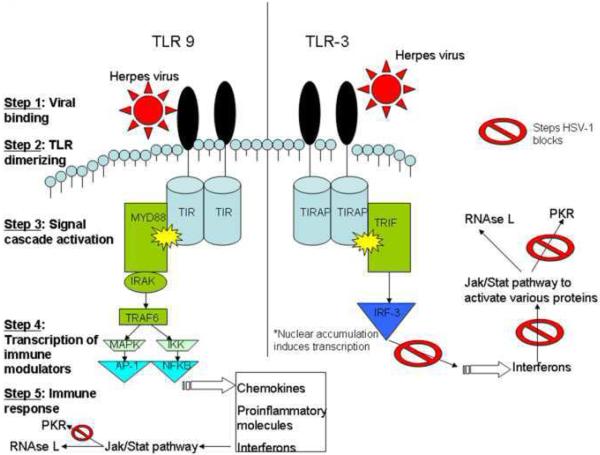
The lytic cycle of HSV-1 evokes an intricate immunological response in the CNS. One of the earliest steps in the immune process is the binding of viral invariant structures known as pathogen-associated molecular patterns (PAMPs) to toll-like receptors (TLRs), a type of pattern recognition receptor (PRR), to initiate the innate immune response and inflammation (Medzhitov and Janeway, 2002). TLRs are single membrane spanning receptors with extracellular leucine-rich repeats and an intracellular signaling Toll/IL-1 receptor domain [TIR] (Takeda et al., 2003) found on cell membranes and cell compartments that recognize specific molecular patterns. Once they bind foreign molecules like HSV-1 proteins or viral nucleic acid, they activate the innate immune response by dimerizing and inducing the production of chemokines, proinflammatory cytokines, and up-regulation of cell surface receptors through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), or a p38 mitogen-activated protein kinase (MAPK) and c-Jun NH2-terminal kinase (Jnk) activation of AP-1 (Akira et al., 2001; Karin, 1995). These molecules drive inflammation and prime the host adaptive immune response through further signaling pathways (Fig. 1). One critical anti-viral group of molecules elicited by 5 of the 10 human TLRs and 4 of the 12 mouse TLRs are the type I IFNs (Zhang et al., 2007a).
Figure 1.
Mechanisms of HSV-1-mediated antagonism of TLR-dependent, anti-viral pathways. TLR-3 signals in a MYD88-independent manner to induce interferon production, while TLR-9 activates inflammatory cytokines and interferons through a MYD88-dependent pathway in response to HSV-1 infection. HSV-1 blocks ND10 accumulation in the nucleus, PKR activity, and Jak/STAT signaling with a cumulative effect that renders the infected cell highly susceptible to viral replication.
Several TLRs have been implicated as important mediators of viral containment and/or destructive inflammation in response to HSV-1 infection of the CNS. Specifically, one of the most important TLRs to mediate host cell response to HSV-1 is TLR-2, which is found on the cell surface of microglia and astrocytes in the CNS (Kielian, 2006). TLR-2 signals through a myeloid differentiation factor 88 (MyD88) or toll-interleukin 1 receptor domain containing adaptor protein (TIRAP)-dependent cascade to activate downstream DNA binding proteins like NF-κB to increase transcription of various interleukins (IL) and tissue necrosis factor (TNF) (Yamamoto et al., 2002; Akira and Takeda, 2004; Akira et al., 2006; O’Neill and Bowie 2007). It has been reported TLR-2 deficient mice have a blunted cytokine and chemokine (monocyte chemoattractant protein1) response to lethal HSV-1 infection, yet a reduction in mortality, inflammatory brain lesions, partial or total paralysis, and/or seizures (Kurt-Jones et al., 2004). Another group has reported TLR-2 acts in concert with TLR-9 to control HSV-1 and HSV-2 infection through NK cell recruitment in the brain to decrease viral loads (Sato et al., 2006; Sorensen et al., 2008). How such observations can be reconciled is currently unknown. Identifying the role of various TLRs and downstream pathways activated in the CNS in response to HSV-1 continues to be an actively studied area.
Another aspect of the immune response regulated by TLR-2 is the induction of the IL-15 gene in response to HSV-1 (Ahmad et al., 2008). IL-15 with IL-21 elicits proliferation of naive and memory CD8+ T cells (Rodrigues et al., 2009) that likely monitor and control virus replication and spread. One detrimental outcome from CD8+ T cell activation is the targeted destruction of virally-infected cells which could have significant consequences as a contributor to neuropathology dependent upon the viral load within the CNS (Lellouch-Tubiana et al., 2000; Anglen et al., 2003).
TLR-3 is found in cell compartments of microglia, astrocytes, oligodendrocytes, and neurons (Bsibsi et al., 2002; Olson and Miller, 2004; Carpentier et al., 2005; Farina et al., 2005; Prehaud et al., 2005; Scumpia et al., 2005). It has been shown to prime the immune system in response to double-stranded HSV-1, non-transcribed RNA intermediates during infection through a MYD88-independent pathway resulting in the activation of NF-κB and IRF-3 (Alexopoulou et al., 2001; Doyle et al., 2002; Akira and Takeda, 2004; Takeda and Akira, 2004; Boehme and Compton, 2004; Akira et al., 2006; O’Neill and Bowie 2007; Onoguchi et al., 2007). While NF-κB is activated by other TLRs in response to HSV-1, IRF-3 is mobilized solely by TLR-3 or -4 and induces a specific set of genes necessary for viral defense (Doyle et al., 2002). In individuals lacking TLR-3 and thus IRF-3 activation, an impaired IFN-dependent containment of the virus has been reported (Casrouge et al., 2006). The susceptibility is likely due to a decreased ability to signal through TIR-domain-containing adaptor-inducing IFN-β (TRIF), a binding protein that interacts with TLR-3 (Sato et al., 2000; Yamamato et al., 2003; Town et al., 2006; Zhang et al., 2007b). Thus, cells are unable to activate antiviral effector molecules including RNAse L and double-stranded RNA-dependent protein kinase (PKR) to inhibit viral spread and can succumb to encephalitis like that reported in West Nile Virus meningoencephalitis (Samuel et al, 2006). TLR-3 activation in neuronal cells is associated with increased resistance to HSV-1 infection and an increase in the production and heightened response to IFNs (Prehaud et al., 2005; Delhaye et al., 2006; Boivin et al., 2008; Zhou et al., 2009). Such observations reinforce the central role of type I IFNs as necessary components to contain virus within the CNS (Delhaye et al., 2006).
TLR-9, a PRR that recognizes unmethylated CpG DNA, (Akira and Hemmi, 2003; Takeda et al., 2003; Hemmi et al., 2003) is found in endosomes/vacuolar compartments of microglia and astrocytes (Dalpke et al., 2002; Bsibsi, et al., 2002; Bowman et al., 2003; Olson et al., 2004; Jack et al., 2005). It mediates an early and rapid production of type I IFNs through an IRAK-4 and MYD88-dependent pathway in response to HSV-1 (Krug et al., 2004; Kawai et al., 2004; Yang et al., 2005; Rasmussen et al., 2007). Recently, TLR-9 has been found to operate through the PI(3)K-mTOR-p70S6K pathway in plasmacytoid dendritic cells to induce type I IFNs (Cao et al., 2008). TLR-9 has also been implicated in influencing hematopoiesis towards production of plasmacytoid and IFN producing killer dendritic cells at the expense of lymphoid precursors (especially B lymphocytes) (Welner et al., 2008). This becomes relevant in viral infections that activate TLR-9 since plasmacytoid dendritic cells can secrete large amounts of IFNs once mature and thus become a vital link between host innate and adaptive immune systems (Biron, 2001; Dai et al., 2004; Kadowaki et al., 2000).
4. The Interferon Response to HSV-1 in the CNS
Binding of viral particles and activation of PRRs, such as mannose receptors, TLRs, and cytosolic receptors induce type I IFN production through MYD88-dependent and -independent pathways (Malmgaard, 2004; Malmgaard et al., 2004). Type I IFNs are secreted by infected cells and work in an autocrine or paracrine manner to induce resistance to viral spread. They play a vital role in the host cell response signaling through the Jak-Stat pathway (Darnell et al., 1994; Aaronson and Horvath, 2002; Malmgaard, 2004) and induce expression of RNAse L, PKR, and Mx protein GTPases (Zhou et al., 1993; Samuel, 2001). RNAse L acts to degrade all intracellular mRNA (cellular and foreign), while PKR causes translation cessation. These two processes drastically reduce viral replication in the cell and are used by the cell to inhibit viral proliferation. In addition to these anti-viral properties, PKR and RNAse L are mediators of cell death through apoptosis (Zhou et al., 1997; Barber, 2005).
IFNs also augment maturation of dendritic cells and activate NK cells, dendritic cells, B and T lymphocytes (Mossman and Ashkar, 2005) and maintain and facilitate clonal expansion of activated T cells (von Hoegen, 1995; Marrack et al., 1999; Kolumam et al., 2005). IFN-α enhances TLR responsiveness by up-regulating TLR-3, -4, and -7 (Siren et al., 2005; Tissari et al., 2005) priming these cells to produce massive quantities of IFNs and cytokines (Garcia-Sastre and Biron, 2006). If such production arises in organized lymphoid tissue, the paracrine signaling acts to promote clonal expansion of cytotoxic T cell precursors (Curtsinger et al., 2005).
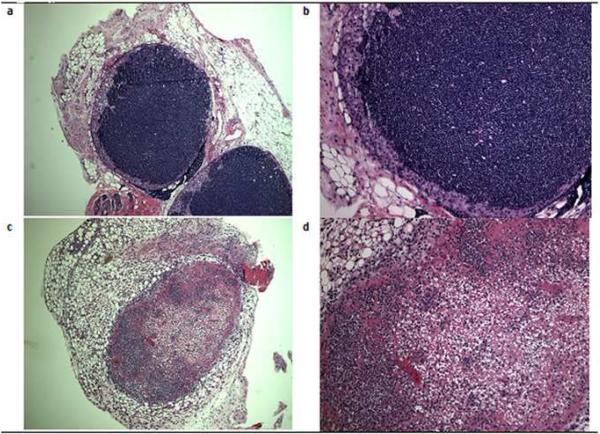
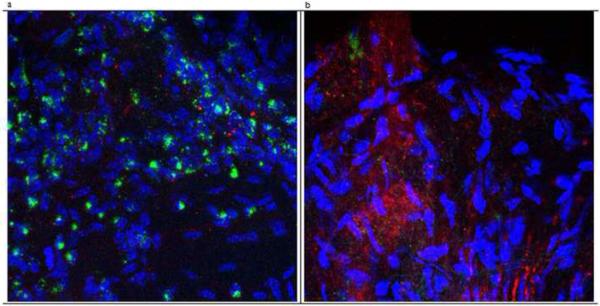
Experimentally in the absence of the type I IFN receptor (IFNAR), mice succumb to systemic HSV-1 dissemination (Luker et al., 2003) with two- to three-fold increased levels of circulating monocytes and neutrophils (Muller et al., 1994). The leukocytosis is likely due in part to TLR-9 driven myeloid cell differentiation (Welner et al., 2008). In the absence of the principal subunit of the type I IFN receptor IFNAR-A1 (CD118-/-), the increased sensitivity to HSV-1 infection is reflected by a loss of draining mandibular lymph node integrity. There is a massive loss of T and NK cells as well as macrophages in the lymph nodes (Conrady et al., 2009) evident by the pronounced loss of primary and secondary follicles by day 5 post infection (Fig. 2). As a result, the generation of HSV-specific cytotoxic T cells is dramatically reduced which likely contributes to poor viral surveillance in the peripheral and central nervous systems resulting in rapid death (Fig. 3) (Conrady et al., 2009).
Figure 2.
The absence of a functional type I IFN receptor results in a massive loss of cells populating the draining (mandibular) lymph node following ocular HSV-1 infection. Wild type and IFNRA1 deficient mice (CD118-/-) were infected with 1,000 plaque forming units of HSV-1 (McKrae strain) in the cornea. Five days post infection, the mice were exsanguinated and the mandibular lymph nodes were removed, sectioned, and H&E stained. The results are representative of three experiments, two mice/group/experiment. (a) Wild type mouse lymph node showing normal lymph node integrity at 40x magnification and (b) 100x magnification. (c) Lymph node from a CD118-/- mouse with widespread cell death resulting in a loss of cells at 40x magnification, and (d) 100x magnification.
Figure 3.
Type I IFN receptor deficient mice show increased expression of HSV-1 antigen and reduced CD3+ T cell infiltration in the trigeminal ganglia following ocular infection with HSV-1. Wild type and IFNRA1 deficient mice (CD118-/-) were infected with 1,000 plaque forming units of HSV-1 (McKrae strain) in the cornea. Five days post infection, the mice were exsanguinated and the trigeminal ganglia were removed and processed for whole mount staining using polyclonal phycoerythrin-conjugated anti-HSV-1 (red) and fluorescein isothiocyanate-conjugated anti-CD3 (green) antibodies. (a) Trigeminal ganglion of a wild type mouse at 400x magnification showing T cell infiltration with little appreciable HSV-1 antigen expression. (b) Trigeminal ganglion from a CD118-/- mouse at magnification of 400x with a constellation of HSV-1 antigen expression but few T cells residing in the tissue. Nuclei were stained with DAPI (blue). This figure is representative of three trigeminal ganglia per group.
Although T cell numbers are reduced within the nervous system of the CD118-/- mice, macrophage numbers are elevated (Conrady et al., 2009). The effect of increased macrophage trafficking to the CNS has not been firmly established. However, one group has found depletion of neutrophils or macrophages during HSV-1 infection increases mouse survival (Lundberg et al., 2008). The authors conclude the inflammatory response and increased mortality is due to a failure to control inflammation rather than HSV-1 destruction of the CNS. While the study seems to implicate macrophage and neutrophil-dependent CNS devastation in response to HSV-1 infection, further studies are required to eliminate the contribution of additional leukocyte populations in the neuropathology.
In addition to the risk of disseminated disease, the lack of IFNs or a pathway intermediate in the production of IFNs can have drastic consequences as well. For example, an autosomal recessive deficiency in UNC-93B (an endosomal protein important in TLR-3, TLR-7, and TLR-9 signaling) found in some children is found to increase susceptibility to herpes encephalitis due to a deficiency in IFN-α/β production through TLR-3 signaling, yet these children have a typical immune response to other viral encounters (cytomegalovirus, varicella zoster virus, Epstein-Barr virus, parvovirus B19, respiratory syncitial virus, parainfluenza-1, influenza A and B, human herpes virus-6, and immunized with live measles/mumps/rubella and poliovirus vaccines) (Casrouge et al., 2006). Thus, UNC-93B-dependent production of IFNs is necessary for host defenses against CNS HSV infection, yet seems to be redundant for effective immune responses to other pathogens.
Clearly, the inability to generate a robust adaptive immune response due to aberrant innate immune activation will ultimately lead to a loss in viral containment and spread from the peripheral nervous system to various areas of the brain. The toxic metabolites of large numbers of activated microglia including nitrous oxide could cause widespread damage (Marques et al., 2008) and thus severe CNS pathology. Therefore, the question arises: is it the loss of viral containment within the periphery resulting in a large viral burden in the CNS which then elicits resident cell (astrocyte and microglia) activation and production of toxic metabolites that ultimately harms the host, the recruitment of activated leukocytes (e.g., macrophages, CD4+ and CD8+ T cells) that release cytolytic molecules resulting in massive cell death of infected neurons, astrocytes, and oligodendrocytes, or the combination of these events that contributes to significant neuropathology including encephalitis? Ironically, one report suggests microglia, unlike astrocytes, harbor HSV-1 without overt cytopathic effect and therefore, may act as a reservoir for persistent low level release of virus and cytokines that over a long period of time may have detrimental consequences to the host (Baker et al., 1999).
5. Clinical Course: the human significance
HSV-1 is a highly successful human pathogen. This is shown by seroprevalance studies demonstrating widespread transmission in childhood such that 39% of individuals 14-19 years of age are already infected, and prevalence rates increase to 65.3% by middle adulthood (Xu et al., 2006). The vast majority of transmission in the general population is through saliva and less commonly through genital transmission, and infection is usually asymptomatic or manifest as a simple “cold sore” on the labia with little further sequelae. Nevertheless, a small fraction of patients HSV-1 infection develop more serious diseases of the CNS, the most notable of which is encephalitis. The yearly incidence of HSV-1 encephalitis has been estimated at 2-4 cases /1,000,000 individuals (Hjalmarrson et al., 2007; Skoldenberg et al., 1984; Whitley, 2006). It is also the most common cause of sporadic viral encephalitis and was responsible for 75% of hospitalizations for viral encephalitis in the United States prior to the introduction of West Nile Virus to North America (Khetsuriani et al., 2002).
HSV-1 encephalitis occurs in all age groups and has been described as having a bi-modal distribution although the majority of cases are in adults over the age of 50 (Hjalmarrson et al., 2007; Whitley, 1990.). Earlier reports suggested that it was not more common in immunosuppressed patients; however, more recent studies identified children specific mutations in UNC-93B and in TLR3 that impair host defenses against HSV (Zhang et al., 2008). By comparison, individuals with IFNγ-R-deficiency, STAT 1 deficiency, or pharmacological immunosuppression such as by anti-TNF-α monoclonal antibody are also at risk for HSV encephalitis as well as a variety of other viral and bacterial infections (Dupuis et al., 2003; Dorman et al., 1999; Bradford et. al., 2009.).
More than 80% of patients with HSV-1 encephalitis present with the triad of fever, headache, and altered level of consciousness and 97% of patients with biopsy proven HSV infection have CSF pleocytosis (Whitley et al., 1982). Other less common presentations are also possible such as a symmetrical brainstem inflammatory course with lesions associated with vasogenic edema (Miura et al., 2009). In addition, the presentation in neonates and young children differs from that of adults by being more frequently diffuse and or multi-focal (Kleinschmidt-DeMasters and Gilden, 2001; Batnitzky et al., 1986). These different presentations may reflect different mechanisms of CNS invasion between adults on the one hand and neonates / young children on the other. For example, recent data show that 34% of 32 children aged 9-44 months with primary HSV-1 gingivostomatitis were viremic by polymerase chain reaction (PCR) (Harel et al., 2004.). Although viremia can also be demonstrated in adults, it is possible that immaturity of the blood-brain barrier and the immune system in young children allow the virus to seed the CNS in a fashion not found in adults which lends itself to multi-focal or atypical disease in the setting of reactivation. Signs of temporal lobe involvement, e.g. personality changes, are suggestive of HSV encephalitis history, but clinical features and routine CSF parameters (protein concentration, leukocyte count, erythrocyte count) alone are not sufficient to distinguish CNS infection caused by HSV from encephalitis caused by other pathogens or to exclude it from further consideration (Whitley et al., 1982; Whitley et al., 1989). Definitive diagnosis requires demonstration of virus in the cerebral spinal fluid (CSF) or brain tissue by culture or histology, but it is most commonly accomplished by PCR methodology of CSF (Cinque et al., 1996.) Use of PCR containing multiple species-specific primer pairs has the advantage of distinguishing HSV-1 from other neurotropic herpesviruses such as HSV-2 and varicella zoster virus (VZV), as well as identifying mild or atypical cases of HSV-1 infection (Ihekwaba et al., 2008; Fodor et al., 1998). Gadolinium-enhanced magnetic resonance imaging is the optimal choice for neuroimaging and electroencephalography can reveal a characteristic temporal focus of periodic lateralized epileptiform discharges that suggests a diagnosis of HSV encephalitis (Steiner et al., 2005). On a histological level most cases in adults show a characteristic necrotizing, hemorrhagic encephalitis that is usually in a frontal-temporal distribution (Kleinschmidt-DeMasters and Gilden, 2001).
Before effective antiviral agents were available, HSV encephalitis had a mortality rate widely quoted at 70% and permanent neurological deficits afflicted many, if not most, of the survivors (Whitley et al., 1977). The superiority of acyclovir over vidarabine was established by 2 landmark clinical trials over 20 years ago and acyclovir remains the drug of choice for treatment for most patients today (Sköldenberg et al., 1984; Whitley et al., 1986). The main exception to this is individuals infected with acyclovir resistant virus. This is quite uncommon (< 1%) in the general population, but can be problematic in immunosuppressed patients reaching levels as high as 30% in some bone marrow transplant patients (Kimberlin, 2007). In such cases treatment options include cidofovir and foscarnet, drugs that are more toxic than acyclovir yet retain activity against acyclovir-resistant virus (Losada et al., 2002; Superti et. al., 2008). Nonetheless, even with treatment these studies and others report mortality rates of 14%-28% and severe neurological disabilities in an additional 9%-33% with a high prevalence of seizures (Raschilas et al., 2002; McGrath et al., 1997; Hjalmarrson et al., 2007.). In contrast only 38% to 65% of affected individuals have a good outcome, i.e. return to pre-infection level of functioning.
This unsatisfactory prognosis begs the question of the extent to which modulators of the immune response, e.g. glucocorticoids, could improve outcomes in HSV encephalitis as they have been shown to do in some forms of bacterial meningitis. However, unlike the situation with bacterial meningitis, there are no randomized, placebo-controlled trials demonstrating the effectiveness of corticosteroids in humans with HSV encephalitis on which to base strong recommendations (Steiner et al., 2005, Tunkel et al., 2008, Fitch and van de Beek, 2008). Nevertheless, experimental rodent models of HSV encephalitis and anecdotal experience in humans may be instructive. For example, studies using dexamethasone alone beginning 3 days after intranasal infection of mice showed reduced viral replication and prolonged survival compared with no treatment (Sergerie et al., 2007). In a different study, mice treated with acyclovir plus methylprednisolone resulted in a significant reduction of the severity of long-term MRI abnormalities and no increase in viral replication compared with acyclovir treatment alone (Meyding-Lamade et al., 2003). In addition, there are case reports in adult and pediatric clinical medicine also suggest that high doses of corticosteroids are not harmful, and can be helpful in selected patients such as those with severe brain edema (Nakano et al., 2003; Musallam et al., 2007). Furthemore, a retrospective analysis of 45 patients treated for HSV encephalitis, some of whom received dexamethasone, found that receiving this drug in addition to acyclovir was an independent predictor of a good outcome (Kamei et al., 2005). These data and others provided a rationale for a multicenter, randomized, placebo-controlled trial known as the GACHE trial (German trial of Acyclovir and Corticosteroids in Herpes-simplex-virus-Encephalitis) designed to answer the question of whether dexamethasone should be used along with acyclovir as adjunctive therapy to diminish collateral damage that ensues as a result of infection and the immune response to it (Martinez-Torres et al., 2008). Until systematic clinical studies like the GACHE trial show the efficacy of steroid therapy as well as the optimal protocol for their dosage and administration, the use of adjunctive corticosteroids remains an option for adjunctive therapy in patients with HSV encephalitis and severe brain edema or vasculitis left to the judgment of the attending physician (Fitch and van de Beek, 2008).
6. Potential long-term consequences of HSV-1
HSV-1 infection of the CNS can have lifelong effects such as permanent temporal lobe damage. Recent reports have surfaced that link Alzheimer’s disease and HSV-1 (Dobson and Itzhaki et al., 1999; Itzhaki and Wozniak, 2008) consistent with an earlier report (Middleton et al., 1980). HSV-1 has been shown to phosphorylate microtubule-associated tau, the main component of neurofibrillary tangles found in Alzheimer’s disease, at several sites including serine 202, threonine 212, serine 214, serine 396, and serine 404 (Wozniak et al., 2009). The virus has also been shown to lead to dramatic increases in the intracellular levels of β-amyloid proteins, the major protein found in senile plaques of Alzheimer’s disease in neuronal and glial cell cultures (Wozniak et al., 2007). These neurofibrillary tangles and senile plaques in the CNS are pathopnuemonic for a diagnosis of Alzheimer’s disease in the elderly with dementia. If indeed HSV-1 is proven to be one of several neurotropic pathogens that contribute to CNS disease including Alzheimer’s, alternative treatment options including vaccines (Lin et al., 2001) may limit the long-term consequences of CNS destruction.
7. Conclusion
While many studies are beginning to implicate the immune response to HSV-1 and its various cell populations (e.g. microglia, CD8+ T cells) in causing widespread CNS pathology (Fischer and Riechman, 2001; Anglen et al., 2003; Bien and Bauer, 2005; Skoldenberg et al., 2006; Sobel et al., 1986; Marques et al., 2008), the exact cause of the extensive destruction of the CNS is still unclear. The issue arises that only a few studies have incorporated human subjects or even animal models to study the response to HSV-1 making it difficult to apply to clinical medicine. The complex interaction between the immune response and the virus cannot be distilled down in vitro using cell lines and targeted mutations of select HSV-1 genes as this would exclude the host adaptive immune response, a major component involved in the maintenance of viral latency (Knickelbein et al., 2009) and tissue damage associated with much of the morbidity in the human host. Thus, emphasis should be placed on in vivo models including cases of human genetic anomalies (Casrouge et al., 2006) in order to attain a better understanding of components that contribute to CNS pathology in response to HSV-1.
8. Acknowledgements
The authors would like to thank Linh Sramek and Todd Wuest for technical assistance. This work was supported by NIH R01 AI053108 to DJJC. Additional support includes P20 RR017703 and NEI Core grant, EY12190.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Ahmad R, El Bassam S, Cordeiro P, Menezes J. Requirement of TLR2-mediated signaling for the induction of IL-15 gene expression in human monocytic cells by HSV-1. Blood. 2008;112:2360–2368. doi: 10.1182/blood-2008-02-137711. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signaling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Anglen CS, Truckenmiller ME, Schell TD, Bonneau RH. The dual role of CD8+ lymphocytes in the development of stress-induced herpes simplex encephalitis. J. Neuroimmunol. 2003;140:13–27. doi: 10.1016/s0165-5728(03)00159-0. [DOI] [PubMed] [Google Scholar]
- Baker M, Noisakran S, Gebhardt BM, Kriesel JD, Carr DJJ. The relationship between interleukin-6 and herpes simplex virus type 1: implications for behavior and immunopathology. Brain Behav. Immunity. 1999;13:201–211. doi: 10.1006/brbi.1999.0572. [DOI] [PubMed] [Google Scholar]
- Barber GN. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–570. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- Batnitzky S, Robinson RG, Wetzel LH, Lai CW, Halleran WJ, 3rd, McMillan JH. Imaging studies in neonatal herpes simplex encephalitis. Acta. Radiol. Suppl. 1986;369:197–199. [PubMed] [Google Scholar]
- Bertke AS, Patel A, Imai Y, Apakupakul K, Margolis TP, Krause PR. LAT exon 1 controls HSV viral species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J. Virol. 2009 doi: 10.1128/JVI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien CG, Bauer J. T-cells in human encephalitis. Neuromolecular Med. 2005;7:243–253. doi: 10.1385/NMM:7:3:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA. Interferons alpha and beta as immune regulators-a new look. Immunity. 2001:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J. Virol. 2004;78:7867–7873. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin N, Sergerie Y, Rivest S, Boivin G. Effect of pretreatment with Toll-like receptor agonists in a mouse model of herpes simplex virus type 1 encephalitis. J. Infect. Dis. 2008;198:664–672. doi: 10.1086/590671. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Bradford RD, Pettit AC, Wright PW, Mulligan MJ, Moreland LW, McLain DA, Gnann JW, Bloch KC. Herpes simplex encephalitis during treatment with tumor necrosis factor-alpha inhibitors. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveri D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by simplex virus type 1. J. Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carr DJJ, Veress LA, Noisakran S, Campbell IL. Astrocyte-targeted expression of IFN-alpha-1 protects mice from acute ocular herpes simplex virus type 1 infection. J. Immunol. 1998;161:4859–4865. [PubMed] [Google Scholar]
- Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- Chee AV, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 2003;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinque P, Cleator GM, Weber T, Monteyne P, Sindic CJ, van Loon AM. The role of laboratory investigation in the diagnosis and management of patients with suspected herpes simplex encephalitis: a consensus report. The EU Concerted Action on Virus Meningitis and Encephalitis. J. Neurol. Neurosurg. Psyschiatry. 1996;61:339–345. doi: 10.1136/jnnp.61.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrady CD, Thapa M, Wuest T, Carr DJJ. Loss of mandibular lymph node integrity is associated with an increase in sensitivity to HSV-1 infection in CD118-deficient mice. J. Immuno. 2009;182:3678–3687. doi: 10.4049/jimmunol.0803878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J. Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J. Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CB, Itzhaki RF. Herpes simplex virus type 1 and Alzheimer’s disease. Neurobiol. Aging. 1999;20:457–465. doi: 10.1016/s0197-4580(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Dorman SE, Uzel G, Roesler J, Bradley JS, Bastian J, Billman G, King S, Filie A, Schermerhorn J, Holland SM. Viral infections in interferon-gamma receptor deficieny. J. Pediatr. 1999;135:640–643. doi: 10.1016/S0022-3476(99)70064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Vaidya SA, O’Connell R, Dadgostar H, Dempsey PW, Wu TT, Rao G, Sun R, Haberland ME, Modlin RL, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat. Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- Ecob-Prince MS, Rixon FJ, Preston CM, Hassan K, Kennedy PG. Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency- associated transcripts. J. Gen. Virol. 1993;74:995–1002. doi: 10.1099/0022-1317-74-6-995. [DOI] [PubMed] [Google Scholar]
- Everett RD, Zafiropoulos A. Visualization by live-cell microscopy of disruption of ND10 during herpes simplex virus type 1 infection. J. Virol. 2004;78:11411–11415. doi: 10.1128/JVI.78.20.11411-11415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer GH, Reichman G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Fitch MT, van de Beek D. Drug Insight: steroids in CNS infectious diseases—new indications for an old therapy. Nat. Clin. Pract. Neurol. 2008;4:97–104. doi: 10.1038/ncpneuro0713. [DOI] [PubMed] [Google Scholar]
- Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, Tyler KL. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology. 1998;51:554–559. doi: 10.1212/wnl.51.2.554. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in détente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Persistent cytokine expression in the trigeminal ganglion latently infected with herpes simplex virus type I. J. Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- Halford WP, Weisend C, Grace J, Soboleski M, Carr DJJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 2006;3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel L, Smetana Z, Prais D, Book M, Alkin M, Supaev E, Mendelson E, Amir J. Presence of viremia in patients with primary herpetic gingivostomatitis. Clin. Infect. Dis. 2004;29:636–640. doi: 10.1086/422643. [DOI] [PubMed] [Google Scholar]
- Harle P, Sainz B, Carr DJJ, Halford WP. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology. 2002;293:295–304. doi: 10.1006/viro.2001.1280. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Kaisho T, Takeda K, Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-depedent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J. Immunol. 2003;170:3059–3064. doi: 10.4049/jimmunol.170.6.3059. [DOI] [PubMed] [Google Scholar]
- Hjalmarrson A, Blomqvist P, Skoldenberg B. Herpes simplex encephalitis in Sweden, 1990-2001: incidence, morbidity, and mortality. Clin. Infect. Dis. 2007;45:875–880. doi: 10.1086/521262. [DOI] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpes virus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihekwaba UK, Kudesia G, McKendrick MW. Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus. Clin. Infect. Dis. 2008;47:783–789. doi: 10.1086/591129. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer’s disease: the enemy within. J. Alzheimer’s Dis. 2008;13:393–405. doi: 10.3233/jad-2008-13405. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Song B, Knipe DM. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology. 2008;374:487–494. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei S, Sekizawa T, Shiota H, Mizutani T, Itoyama Y, Takasu T, Morishima T, Hirayanagi K. Evaluation of combination therapy using acyclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J. Neurol. Neurosurg. Psychiatry. 2005;76:1544–1549. doi: 10.1136/jnnp.2004.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;28:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uemastu S, Takeuchi O, Akira S. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin. Infect. Dis. 2002;35:175–182. doi: 10.1086/341301. [DOI] [PubMed] [Google Scholar]
- Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neuroscience Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin DW. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis, and treatment. Herpes. 2007;14:11–16. [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Gilden DH. Polymerase chain reaction as a diagnostic adjunct in herpesvirus infections of the nervous system. Brain Pathol. 2001;11:440–451. doi: 10.1111/j.1750-3639.2001.tb00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2009;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Hepres simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Machalek MA, Williams BR, Silverman RH, Virgin HW. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA. 2000;97:5684–5686. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lellouch-Tubiana A, Fohlen M, Robain O, Rozenberg F. Immunocytochemical characterization of long-term persistent immune activation in human brain after herpes simplex encephalitis. Neuropath. Applied Neurobio. 2000;26:285–294. doi: 10.1046/j.1365-2990.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 2004;78:1675–1684. doi: 10.1128/JVI.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WR, Jennings R, Smith TL, Wozniak MA, Itzhaki RF. Vaccination prevents latent HSV1 infection of mouse brain. Neurobiol. Aging. 2001;22:699–703. doi: 10.1016/s0197-4580(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada I, Canizares A, Hellin T, Marti-Belda P, Guerrero A. In vitro susceptibility study of herpes simplex virus to acyclovir and foscarnet. Are routine susceptibility studies necessary? Enferm. Infecc. Microbiol. Clin. 2002;1:25–27. doi: 10.1016/s0213-005x(02)72727-4. [DOI] [PubMed] [Google Scholar]
- Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J. Virol. 2003;77:11082–11093. doi: 10.1128/JVI.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Ramakrishna C, Brown J, Tyszka JM, Hamamura M, Hinton DR, Kovats S, Nalcioglu O, Weinberg K, Openshaw H, Cantin EM. The immune response to herpes simplex virus type 1 infection in susceptible mice is a major cause of central nervous system pathology resulting in fatal encephalitis. J. Virol. 2008;82:7078–7088. doi: 10.1128/JVI.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgaard L. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 2004;24:439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 2004;173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Urban SL, Lokensgard JR. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J. Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres F, Menon S, Pritsch M, Victor N, Jenetzky E, Jensen K, Schielke E, Schmutzhard E, de Gans J, Chung CH, Luntz S, Hacke W, Meyding-Lamade U. Protocol for German trial of acyclovir and corticosteroids in herpes simplex virus encephalitis (GACHE): a multicenter, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial. BMC Neurol. 2008;8:40. doi: 10.1186/1471-2377-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul GG, Guldner HH, Spivack JG. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0) J. Gen. Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGrath N, Anderson NE, Croxson MC, Powell KF. Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J. Neurol. Neurosurg. Psychiatry. 1997;63:321–326. doi: 10.1136/jnnp.63.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Melroe GT, DeLuca NA, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 2004;78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyding-Lamade UK, Oberlinner C, Rau PR, Seyfer S, Heiland S, Sellner J, Wildemann BT, Lamade WR. Experimental herpes simplex virus encephalitis: a combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J. Neurovirol. 2003;9:118–125. doi: 10.1080/13550280390173373. [DOI] [PubMed] [Google Scholar]
- Middleton PJ, Petric M, Kozak M, Rewcastle NB, McLachlan DR. Herpes-simplex viral genome and senile and presenile dementias of Alzheimer and Pick. Lancet. 1980;1:1038. doi: 10.1016/s0140-6736(80)91490-7. [DOI] [PubMed] [Google Scholar]
- Miura S, Kurita T, Noda K, Ayabe M, Aizawa H, Taniwaki T. Symmetrical brainstem encephalitis caused by herpes simplex virus. J. Clin. Neurosci. 2009;16:589–590. doi: 10.1016/j.jocn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Mossman KL, Smiley JR. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 2002;76:1995–1998. doi: 10.1128/JVI.76.4.1995-1998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman KL, Ashkar AA. Herpesviruse and the innate immune response. Viral Immunol. 2005;18:267–281. doi: 10.1089/vim.2005.18.267. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Musallam B, Matoth I, Wolf DG, Engelhard D, Averbuch D. Steroids for deteriorating herpes simplex virus encephalitis. Pediatr. Neurol. 2007;37:229–232. doi: 10.1016/j.pediatrneurol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Nakano A, Yamasaki R, Miyazaki S, Horiuchi N, Kunishige M, Mitsui T. Beneficial effect of steroid pulse therapy on acute viral encephalitis. Eur. Neurol. 2003;50:225–229. doi: 10.1159/000073864. [DOI] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 2003;77:113–122. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 2005;79:7609–7619. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- Pasieka TJ, Lu B, Crosby SD, Wylie KM, Morrison LA, Alexander DE, Menachery VD, Leib DA. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 2008;82:5527–5535. doi: 10.1128/JVI.02047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehaud C, Megret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong produces of beta interferon. J. Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CM. Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein ICP0. J. Virol. 2007;81:11781–11789. doi: 10.1128/JVI.01234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin. Infect. Dis. 2002;35:254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007;81:13315–13342. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske A, Pollara G, Krummenacher C, Chain BM, Katz DR. Understanding HSV-1 entry glycoproteins. Rev. Med. Virol. 2007;17:205–215. doi: 10.1002/rmv.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L, Nadakumar S, Bonorino C, Rouse BT, KKumaraguru U. IL-21 and IL-15 cytokine DNA augments HSV specific effector and memory CD8+ T cell response. Mol. Immunol. 2009;46:1494–1504. doi: 10.1016/j.molimm.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BR, Silverman RH, Gale M, Jr., Diamond MS. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Tniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. USA. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scumpia PO, Kelly KM, Reeves WH, Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52:153–162. doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- Sears AE, Hukkanen V, Labow MA, Levine AJ, Roizman B. Expression of the herpes simplex virus 1 alpha transinducing factor (VP16) does not induce reactivation of latent virus or prevent the establishment of latency in mice. J. Virol. 1991;65:2929–2935. doi: 10.1128/jvi.65.6.2929-2935.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie Y, Boivin G, Gosselin D, Rivest S. Delayed but not early glucocorticoid treatment protects the host during experimental herpes simplex virus encephalitis in mice. J. Infect. Dis. 2007;196:817–825. doi: 10.1086/511987. [DOI] [PubMed] [Google Scholar]
- Shieh MT, WuDunn D, Montgomery RI, Esko JD, Spear PG. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh MT, Spear PG. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J. Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C, Whiteland JL, Nicholls SM, Grinfeld E, Easty DL, Gao H, Hill TJ. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J. Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- Shimeld C, Efstathiou S, Hill T. Tracking the spread of lacZ-tagged herpes simplex virus type 1 between the eye and the nervous system of the mouse: comparison of primary and recurrent infection. J. Virol. 2001;75:5252–5262. doi: 10.1128/JVI.75.11.5252-5262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J. Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- Skoldenberg B, Forsgren M, Alestig K, Bergstrom T, Burman L, Dahlqvist E, Forkman A, Fryden A, Lovgren K, Norlin K. Acyclovir versus vidarabine in herpes simplex encephalitis. Randomised mulitcentre study in consecutive Swedish patients. Lancet. 1984;2:707–711. doi: 10.1016/s0140-6736(84)92623-0. [DOI] [PubMed] [Google Scholar]
- Skoldenberg B, Aurelius E, Hjalmarsson A, Sabri F, Forsgren M, Andersson B, Linde A, Strannegard O, Studahl M, Hagberg L, Rosengren L. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J. Neurol. 2006;253:163–179. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Collins AB, Colvin RB, Bhan AK. The in situ cellular immune response in acute herpes simplex encephalitis. Am. J. Path. 1986;125:332–338. [PMC free article] [PubMed] [Google Scholar]
- Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- Steiner I, Spivack JG, Deshmane SL, Ace CI, Preston CM. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, Kennedy PG. Viral encephalitis: a review of diagnostic methods and guidelines for management. Eur. J. Neurol. 2005;12:331–343. doi: 10.1111/j.1468-1331.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- Superti F, Ammendolia MG, Marchetti M. New advances in anti-HSV chemotherapy. Curr. Med. Chem. 2008;15:900–911. doi: 10.2174/092986708783955419. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin. Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS. Pathog. 2009;5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissari J, Siren J, Meri S, Julkunen I, Matikainen S. IFN-alpha enhances TLR-3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J. Immunol. 2005;174:4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA. Microglia recognize double-stranded RNA via TLR3. J. Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- Von Hoegen P. Synergistic role of type I interferons in the induction of protective cytotoxic T lymphocytes. Immunol. Letters. 1995;47:157–162. doi: 10.1016/0165-2478(95)00065-4. [DOI] [PubMed] [Google Scholar]
- Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch’ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N. Engl. J. Med. 1977;297:289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Soong SJ, Linneman C, Jr., Liu C, Pazin G, Alford CA. Herpes simplex encephalitis. Clinical assessment. JAMA. 1982;247:317–320. [PubMed] [Google Scholar]
- Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, Hanley D, Nahmias AJ, Soong SJ. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 1986;314:144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Cobbs CG, Alford CA, Soong SJ, Hirsch MS, Connor JD, Corey L, Hanley DF, Levin M, Powell DA, NIAD Collaborative Antiviral Study Group Diseases that mimic herpes simplex encephalitis. Diagnosis, presentation, and outcome. JAMA. 1989;262:234–239. [PubMed] [Google Scholar]
- Whitley RJ. Viral Encephalitis. N. Engl. J. Med. 1990;323:242–250. doi: 10.1056/NEJM199007263230406. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci. Lett. 2007;429:95–100. doi: 10.1016/j.neulet.2007.09.077. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Frost AL, Itzhaki RF. Alzheimer’s disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J. Alzheimers Dis. 2009;16:341–350. doi: 10.3233/JAD-2009-0963. [DOI] [PubMed] [Google Scholar]
- WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding heparan sulfate. J. Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signaling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Maródi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7, -8, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Kubota T, Suzutani T, Yoshida I, Miura S, Jimbow K, Fuji N. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and Janus kinases during an early infection stage. J. Virol. 2001;286:119–124. doi: 10.1006/viro.2001.0941. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol. Rev. 2007a;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007b;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, Picard C, Abel L, Jouanguy E, Casanova JL. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-α/β, IFN-γ, and IFN-λ in host defense. Immunol. Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- Zhou A, Paranjape J, Brown TL, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman RH. Interferon action and apoptosis are defective in mice devoid of 2′, 5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ye L, Wan Q, Zhou L, Wang X, Li J, Hu S, Zhou D, Ho W. Activation of Toll-like receptors inhibits herpes simplex virus-1 infection of human neuronal cells. J. Neurosci. Res. 2009;87:2916–2925. doi: 10.1002/jnr.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]