Abstract
Mammalian target of rapamycin (mTOR) is a key regulator of translational capacity. The mTOR inhibitor rapamycin can prevent forms of protein synthesis-dependent synaptic plasticity such as long-term facilitation in Aplysia and late-phase long-term potentiation (L-LTP) in the hippocampal CA1 region of rodents. In the latter model, two issues remain to be addressed: defining the L-LTP phase sensitive to rapamycin and identifying the site of rapamycin-sensitive protein synthesis. Here, we show that L-LTP is sensitive to application of rapamycin only during the induction paradigm, whereas rapamycin application after the establishment of L-LTP was ineffective. Second, we observed that Thr-389-phosphorylated p70 S6 kinase (p70S6K), the main active phosphoform of the mTOR effector p70S6K, was induced in an N-methyl-d-aspartateand phosphatidylinositol 3-kinase-dependent manner throughout the dendrites but not in the cell bodies of CA1 neurons in hippocampal slices after L-LTP induction. A similar dendrite-wide activation of p70S6K was induced in primary hippocampal neurons by depolarization with KCL or glutamate. In primary hippocampal neurons, the sites of dendritic activation of p70S6K appeared as discrete compartments along dendritic shafts like the hotspots for fast dendritic translation. Conversely, only a subset of dendritic spines also displayed activated p70S6K. Taken together, the present data suggest that the N-methyl-d-aspartate-, phosphatidylinositol 3-kinase-dependent dendritic activation of the mTOR-p70S6K pathway is necessary for the induction phase of protein synthesis-dependent synaptic plasticity. Newly synthesized proteins in dendritic shafts could be targeted selectively to activity-tagged synapses. Thus, coordinated activation of dendrite-wide translation and synaptic-specific activation is likely to be necessary for long-term synaptic plasticity.
Forms of long-term synaptic plasticity that require protein synthesis are believed to be cellular counterparts of long-term memory storage, whereas forms of synaptic plasticity that do not require protein synthesis are believed to be counterparts of short-term memory (1). In particular, dendritic protein synthesis is believed to play a crucial role in long-term synaptic plasticity and memory (1-4). Mammalian target of rapamycin (mTOR) regulates the translation initiation complex in a rapamycin-sensitive manner. It does so primarily through its downstream targets, the kinase p70 S6 kinase (p70S6K) and the elongation factor binding protein 4E-BP1. p70S6K is a major regulator of translation under the control of multiple signal transduction pathways including phosphatidylinositol 3-kinase (PI3K) (5). It increases translational capacity by promoting the expression of several members of the translational machinery whose mRNAs display oligopyrimidine tracts at their 5′ ends (6). 4E-BP1 is an inhibitor of the cap binding protein eukaryotic initiation factor 4E. 4E-BP1 phosphorylation by mTOR leads to increased translation of capped mRNAs (7). The major determinant of p70S6K activation is the phosphorylation state of Thr-389, which is regulated in a complex manner by mTOR and PI3K (5, 7-9).
Late-phase long-term potentiation (L-LTP) is a protein synthesis-dependent long-lasting form of synaptic plasticity induced by repeated tetanic stimulation (1). It was recently shown that hippocampal slices continuously incubated in rapamycin resulted in reduced stability of L-LTP, suggesting a role for the mTOR-p70S6K pathway in L-LTP (2). The present study was aimed at ascertaining which phase of L-LTP is sensitive to rapamycin and the site of activation of the rapamycin-sensitive pathway in L-LTP. The rapamycin sensitivity of L-LTP was found to be restricted to the induction phase. The rapamycin concentration used did not affect basal synaptic transmission, its effect was reversible, and it did not inhibit long-term potentiation (LTP) induced by a single tetanus. L-LTP induction was associated with the N-methyl-d-aspartate (NMDA)-, PI3K-, and mTOR-dependent activation of p70S6K in CA1 dendrites, but not in cell bodies. Consistent with the latter observation, in primary hippocampal neurons depolarization with KCL or glutamate induced dendritic activation of p70S6K in compartments reminiscent of hotspots of fast dendritic translation and in a subset of dendritic spines. These findings suggest that the NMDA-, PI3K-, and mTOR-dependent activation of dendritic p70S6K is necessary for the induction phase of protein synthesis-dependent synaptic plasticity. Recruitment of the mTOR-p70S6K pathway seemed to be primarily dendrite-wide and, to a lesser extent, involved dendritic spines.
Materials and Methods
Hippocampal Slices and Electrophysiological Techniques. Hippocampal slices were prepared as described (10, 11). Briefly, Wistar rats (28-35 days of age) were decapitated under halothane anesthesia. Brains were rapidly transferred into ice-cold artificial cerebrospinal fluid (ACSF) (130 mM NaCl/3.5 mM KCl/24 mM NaHCO3/1.25 mM NaH2PO4/2.2 mM CaCl2/10 mM glucose/2 mM MgSO4, oxygenated with a mixture of 95% O2/5%CO2, pH 7.4), and transverse hippocampal slices (400 μm) were obtained. Hippocampal slices were then incubated in oxygenated ACSF for at least 1 h at room temperature. For recording, slices were transferred to a submerged recording chamber, perfused with oxygenated ACSF and maintained at 31 ± 1°C and incubated for at least 40 min before recording. Bipolar stimulating electrodes were placed in the stratum radiatum to activate the Schaffer collateral/commissural fibers. Recordings of field excitatory postsynaptic potentials (fEPSPs) were made in the middle of the stratum radiatum with an Axoclamp 2B (Axon Instruments, Foster City, CA) by using microelectrodes filled with ACSF (resistance 3-5 MΩ). Stimulus intensities were ≈50% of those that induced population spikes. The initial slope of the rising phase of the fEPSP measured near its onset for an interval of 1.2 ms. A two-pathway paradigm was used with two stimulating electrodes placed in the stratum radiatum on opposite sides of the recording electrode, as described (11). The independence of the two pathways was demonstrated by the absence of paired-pulse facilitation of fEPSP and lack of potentiation in the untetanized pathway after LTP induction (11).
Neuronal Cell Culture. Primary cultures of hippocampal neurons were prepared from E18 Sprague-Dawley rat embryos according to Banker and Cowan (12). Hippocampi incubated in Hanks' buffered salt solution, without Ca2+ and Mg2+, with 10 mM Hepes (pH 7.5), 1 mM sodium pyruvate, and 0.25% trypsin (GIBCO/BRL) for 10 min at 37°C. Trypsin was then removed, DNase I (Roche Molecular Biochemicals) was added at a final concentration of 12.5 μg/ml, and the hippocampi were dissociated by trituration. Cells were plated on poly-d-lysine- and laminin-treated glass coverslips (Sigma) at a density of 25,000 cells/cm2 and maintained in Neurobasal medium supplemented with the B27 trophic additive (GIBCO/BRL).
Immunocytochemistry. Hippocampal slices and primary neurons were fixed with 4% paraformaldehyde in PBS and stained as described (13, 14). Hippocampal slices were cryostat sectioned at a thickness of 16 μm, thaw-mounted, and incubated with primary antibodies overnight at 4°C in 5% goat serum and 0.3% Triton X-100 in PBS. Primary neurons were incubated with primary antibodies overnight at 4°C in 10% goat serum, 2% BSA, and 0.3% Triton X-100 in PBS. The rabbit polyclonal and the mouse monoclonal to Thr-389-phosphorylated (Thr-389-P) p70S6K used were obtained from Cell Signaling Technology (Beverly, MA). FITC- or Texas red-conjugated anti-mouse or anti-rabbit secondary antibodies were obtained from Jackson ImmunoResearch. Rhodamine-conjugated phalloidin (Molecular Probes) was used to stain F-actin filaments. Hippocampal slices were analyzed by laser-scanning confocal microscopy with a Bio-Rad MRC-1024 LSCM as described (15). Primary neurons were viewed with a Zeiss Axiovert 100M microscope by using a ×63 lens (Zeiss) with a SensicamQE (Cooke, Auburn Hills, MI). For cell counting, 50 immunolabeled cells per coverslip were assessed for activation of phospho-Thr-389 p70S6K after depolarization. Each condition was repeated on four coverslips, and counts were based on three independent experiments. Five to seven images per condition were quantified in the hippocampal slice experiments. For signal quantification, digitized pictures were analyzed with NIH IMAGE software package for quantification of staining intensity. Data were analyzed by ANOVA with STATVIEW software (Abacus Concepts, Berkeley, CA).
Results
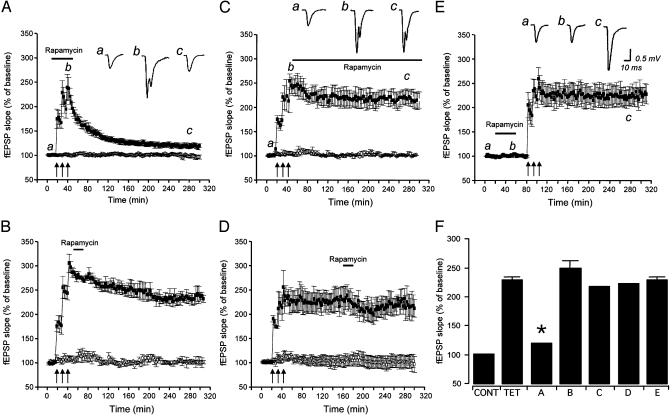
We investigated the time dependence of the sensitivity to rapamycin of L-LTP of fEPSP at Schaffer collateral/ commissural fiber-CA1 synapses in rat hippocampal slices. LLTP was induced by three 1-s tetani at 100 Hz delivered at 10-min intervals (2, 16). A transient application (40 min) of rapamycin (1 μM) during the L-LTP induction paradigm resulted in a decay of the level of potentiation (Fig. 1A), similarly to what was observed by others in continuous rapamycin incubation throughout the recording session (2). In fact, the fEPSP slopes after application of rapamycin during tetanization were 118.8 ± 0.6% of baseline 240-260 min after completion of the tetanization paradigm, and not significantly different from baseline fEPSP slopes (P > 0.05) (Fig. 1). However, fEPSP slopes were 228.3 ± 4.9% of baseline in untreated slices at the same time after tetanization, and significantly different from both baseline fEPSP slopes and fEPSP slopes in slices treated with rapamycin during tetanization (P < 0.01) (Fig. 1 A and F). No changes were seen in the untetanized pathway, suggesting that rapamycin application did not affect basal synaptic responses (Fig. 1 A-D). However, transient applications of rapamycin either early (5 min) or late (2 h) after L-LTP induction, or continuous perfusion in rapamycin starting 5 min after tetanization, were ineffective in reducing the level of potentiation (Fig. 1 B-D). In fact, at 240-260 min after tetanization, the levels of potentiation were 247.8 ± 12.6%, 221.4 ± 2.9%, and 217.2 ± 1.5% of baseline, respectively (Fig. 1F). Because the effect of a rapamycin application during L-LTP induction could theoretically outlast the application time, we validated that rapamycin is effectively washed out after termination of its application. To this aim, in a set of slices, rapamycin was applied for 40 min, and then the L-LTP-inducing tetanization paradigm was delivered after washing out the inhibitor. Slices treated in this manner demonstrated a level of potentiation consistent with an L-LTP and significantly different from untreated slices (Fig. 1E). In fact, at 240-260 min after tetanization, fEPSP slopes were 228.3 ± 4.9% of baseline (Fig. 1 E and F). This observation supports the notion that the action of rapamycin is reversible and that the rapamycin sensitivity of L-LTP is restricted to the induction phase.
Fig. 1.
The mTOR inhibitor rapamycin prevents L-LTP only when applied during induction. fEPSP at Schaffer collateral/commissural fiber-CA1 synapses were simultaneously monitored in two independent pathways (▪, tetanized pathway; ○, untetanized pathway). (A) When rapamycin (1 μM) was transiently (40 min) applied during L-LTP induction, a dramatic decay of fEPSPs was seen (n = 6). A transient rapamycin application 5 min after the delivery of the L-LTP tetanization paradigm (B) or 2 h after completion of the L-LTP induction paradigm (C) did not affect fEPSP slopes (n = 6), nor did a continuous incubation with rapamycin starting 5 min after tetanization (D). (E) L-LTP could be induced after a 40-min rapamycin application and washout, suggesting that the action of rapamycin was reversible. These observations suggest that rapamycin sensitivity of L-LTP is restricted to the induction phase. (F) fEPSP slopes as a percentage of baseline fEPSP in the control untetanized pathway (CONT), untreated tetanized slices (TET), and the conditions displayed in A-E. Measurements of fEPSP slopes taken 240-260 min after completion of the tetanization paradigm. Insets are representative traces of extracellular fEPSPs recorded at the times marked by lowercase letters (*, different from TET, B, C, D, and E; P < 0.01, NS from CONT).
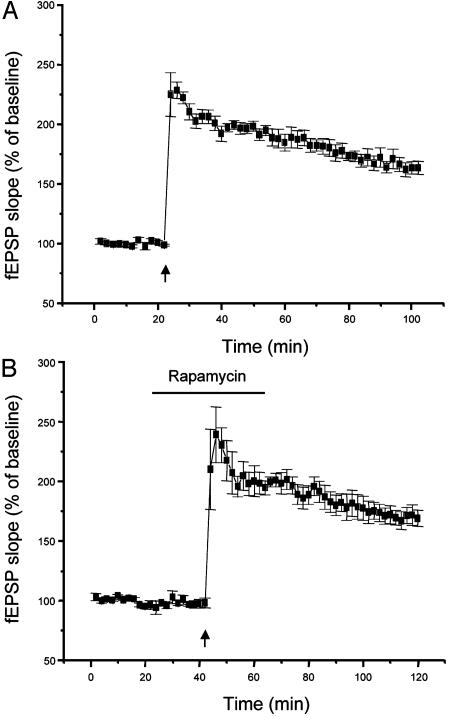
We also tested the rapamycin dependence of the conversion of short-term potentiation (STP) of fEPSP into LTP by group I metabotropic glutamate agonists, which requires translation but not de novo transcription (17). In this paradigm, a weak tetanic stimulation (50 Hz, 0.5 s) when delivered alone induced an STP decaying to control levels within 1 h (Fig. 2A) (17, 18). However, administration of a group I metabotropic glutamate receptor agonist such as trans-(±)-1-amino-1,3-cyclopentanedicarboxylic acid (50 μM for 8 min) during delivery of the weak tetanization promoted the conversion of such an STP into an LTP (17, 18). When rapamycin was transiently applied during the induction paradigm in the presence of trans-(±)-1-amino-1,3-cyclopentanedicarboxylic acid, the conversion of STP into LTP was abolished and potentiation decayed to control levels within 1 h (Fig. 2). This result supports the notion that multiple forms of protein synthesis-dependent LTP are sensitive to rapamycin during their induction phase. As expected, application of rapamycin during the induction of a protein synthesis-independent LTP did not alter the level of potentiation obtained (Fig. 3).
Fig. 2.
Rapamycin prevents the conversion of STP into LTP by a group I metabotropic glutamate receptor agonist. (A) Delivery of a tetanic stimulation (50 Hz, for a duration of 0.5 s) subthreshold for the induction of LTP (left arrow) resulted in an STP of fEPSP slopes that rapidly decayed to basal levels. However, an LTP was seen when the same subthreshold tetanic stimulation (right arrow) was delivered to the slices during a brief bath application of the group I metabotropic glutamate receptor agonist trans-(±)-1-amino-1,3-cyclopentanedicarboxylic acid (50 μM for 8 min), as shown by others (18). (B) Such a conversion of STP into LTP could be prevented by a 20-min application of rapamycin (1 μM) during the tetanization paradigm. In this case, fEPSP slopes decayed to baseline within 1 h.
Fig. 3.
The induction of a protein synthesis-independent LTP is not sensitive to rapamycin. (A) An LTP of fEPSP was obtained at Schaffer collateral/ commissural fiber-CA1 synapses with a tetanization paradigm consisting of two trains of 0.5-s duration, each at 100 Hz with an interval of 10 s, which only produces a protein synthesis-independent LTP (11, 23). (B) Application of rapamycin (1 μM) during the delivery of such a tetanization paradigm did not modify the level of potentiation of fEPSP slopes.
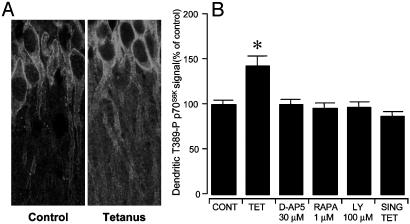
We then investigated the site of activation of the mTOR effector p70S6K in the CA1 region of hippocampal slices after tetanization as a marker for the activation of the mTOR-p70S6K pathway. Delivery of the L-LTP tetanization paradigm resulted in the induction of Thr-389-P p70S6K, the main active phospho form of p70S6K, in CA1 dendrites (Fig. 4). In CA1 dendrites, immunoreactivity for Thr-389-P p70S6K appeared distributed throughout the dendritic shafts and had a somewhat punctate appearance (Fig. 4A). Treatments with rapamycin, D-AP5 (an NMDA inhibitor), or LY294002 (a PI3K inhibitor) prevented dendritic activation of p70S6K (Fig. 4B). A single train of tetanization of 100 Hz for 1 s did not induce Thr-389-P p70S6K accumulation in the dendrites (Fig. 4B). Thr-389-P p70S6K immunoreactivity in CA1 somata was 98.2 ± 6.9% of untetanized controls, and it was not significantly different from controls (P = 0.89).
Fig. 4.
NMDA- and PI3K-dependent dendritic accumulation of an active phosphoform of p70S6K (Thr-389-P p70S6K) in tetanized hippocampal slices. (A) Induction of L-LTP at Schaffer collateral/commissural fiber-CA1 synapses in hippocampal slices resulted in the dendritic activation of p70S6K in CA1 neurons (Right). (Left) Control CA1 neurons are shown. (B) The induction of Thr-389-P p70S6K in CA1 dendrites could be prevented by treatment with the NMDA antagonist D-AP5, the mTOR inhibitor rapamycin (RAPA), or the PI3K inhibitor LY294002 during delivery of the tetanization paradigm. Slices that received a single train of tetanization of 100 Hz for 1 s (SING TET) did not show dendritic activation of p70S6K. Thr-389-P p70S6K immunoreactivity in CA1 somata after L-LTP induction was not significantly different (P > 0.05) from untetanized control slices (not shown) (*, P < 0.01, different from control and all other conditions).
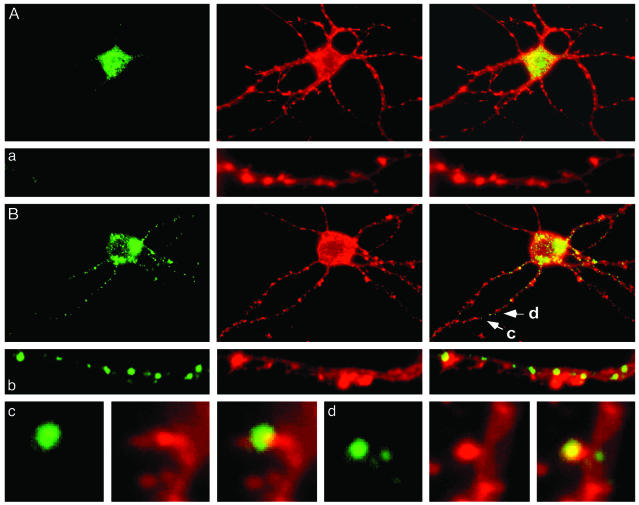
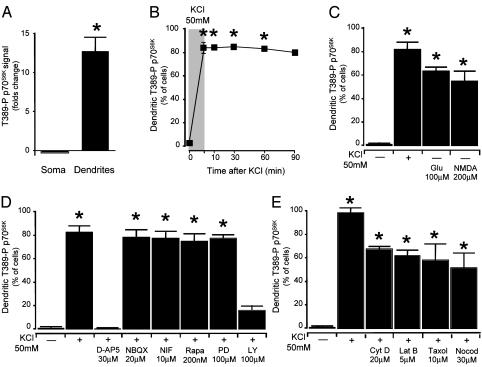
Dendritic induction of Thr-389-P p70S6K was also seen in primary hippocampal neurons. In untreated primary hippocampal neurons (17-21 DIV), immunoreactivity for Thr-389-P p70S6K was confined to the cell bodies (Figs. 5A and 6A). However, it was dramatically activated throughout the dendrites after depolarization with KCl (50 mM; Figs. 5B and 6A). In the dendrites, Thr-389-P p70S6K was arranged in highly distinct punctae along the length of dendritic shafts and, to a lesser extent, in dendritic spines (Fig. 5B). Activation of dendritic p70S6K was quite protracted (Fig. 6B). Accumulation of Thr-389-P p70S6K in the dendrites of primary hippocampal neurons after depolarization was blocked by pretreatment with the NMDA receptor antagonist D-AP5 (30 μM) or with the PI3K inhibitor LY294002 (100 μM), whereas the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate type glutamate receptor NBQX (20 μM) or the voltage-dependent calcium channels blocker nifedipine (10 μM) had no effect (Fig. 6D). Consistently, dendritic accumulation of Thr-389-P p70S6K could also be induced by application of glutamate (100 μM) or NMDA (200 μM) (Fig. 6C). Dendritic activation of p70S6K was not prevented by the mTor inhibitor rapamycin (up to 200 nM), possibly reflecting, at this developmental stage, low activity in the dendrites of PP2A, a phosphatase implicated in the dephosphorylation of Thr-389 (ref. 9; Fig. 6D). The actin polymerization inhibitors cytochalasin D (20 μM) and Latrunculin B (5 μM) and the inhibitors of microtubule polymerization taxol (10 μM) and nocodazole (30 μM) did not abolish dendritic activation of p70S6K (Fig. 6E). These observations suggest that local activation of p70S6K, rather than redistribution to the dendrites of Thr-389-P p70S6K from the somata, was the likely predominant mechanism of dendritic accumulation of Thr-389-P p70S6K.
Fig. 5.
Dendrite-wide induction of Thr-389-P p70S6K in primary hippocampal neurons. (A) Thr-389-P p70S6K was found predominantly in the soma of primary hippocampal neurons (21 days in vitro) (labeling for Thr-389-P p70S6K with a phosphorylation state-specific rabbit polyclonal antibody is shown in green (Right), counterstaining for actin with phycoerythrin-conjugated phalloidin in red in the middle, and the overlay in yellow (Left). Similar results were obtained with a mouse monoclonal antibody specific for Thr-389-P p70S6K. A representative dendrite is shown in a. (B) Thr-389-P p70S6K appeared in distinct punctae along dendrites after depolarization with KCl. A representative dendrite is shown in b. In dendrites (c and d), Thr-389-P p70S6K was also present in a subset of dendritic spines as revealed by double labeling for actin filaments (c and d as shown in B). The morphology of Thr-389-P p70S6K-immunoreactive puncta in dendritic shafts was reminiscent of the morphology of hotspots of dendritic translation recently demonstrated by Aakalu et al. and Job and Eberwine (3, 4) in similarly cultured primary hippocampal neurons.
Fig. 6.
Dendritic activation of Thr-389-P p70S6K is NMDA- and PI3K-dependent. (A) Quantification of immunoreactivity for Thr-389-P p70S6K in primary hippocampal neurons after depolarization with KCl (50 mM), as shown in Fig. 5. The intensity of Thr-389-P p70S6K immunoreactivity was greatly increased in dendrites after depolarization with KCl, whereas it was not significantly reduced in the somata. (B) Thr-389-P p70S6K was persistently activated in the dendrites after withdrawal of KCl. (C) Dendritic accumulation of Thr-389-P p70S6K could also be induced by application of glutamate or NMDA. (D) Accumulation of Thr-389-P p70S6K in dendrites was sensitive to the NMDA antagonist D-AP5 and the PI3K inhibitor LY294002 (NIF, nifedipine; Rapa, rapamycin; PD, PD98059; LY, LY294002). (E) Dendritic activation of Thr-389-P p70S6K was not prevented either by the actin polymerization inhibitors cytochalasin D and Latrunculin B or by the inhibitors of microtubule polymerization taxol and nocodazole (*, P < 0.01 from control).
Discussion
It was recently demonstrated that the persistence of L-LTP is severely reduced if hippocampal slices are pretreated and continuously perfused with the mTOR inhibitor rapamycin (2). In that study, the phase of L-LTP sensitive to rapamycin was not determined. Here, we tested the time dependence of the rapamycin sensitivity of two forms of LTP that require protein synthesis for their induction, but that differ in their requirement for de novo transcription (1, 17): an L-LTP induced by three tetani delivered at 10-min intervals (16) and an LTP induced by conversion of an STP into LTP by application of a group I metabotropic glutamate receptor agonist (17, 18). Both of these protein synthesis-dependent forms of LTP were sensitive to application of the mTOR inhibitor rapamycin during delivery of the induction paradigm. However, application of rapamycin after L-LTP induction had no effect. Interestingly, even a continuous application of rapamycin starting 5 min after completion of the L-LTP induction paradigm did not result in the decay of potentiation. Because the effect of rapamycin was readily reversible, these data suggest that the induction phase of protein synthesis-dependent LTP is sensitive to rapamycin. However, a requirement of protein synthesis for the maintenance of protein synthesis-dependent forms of LTP cannot be ruled out. In fact, there is considerable evidence that certain mRNAs, such as the activity-regulated cytoskeleton-associated protein, are delivered to distal synapses and translated over considerable time after induction of protein synthesis-dependent synaptic plasticity (19). However, the present data seem to point to a crucial requirement for the mTOR-p70S6K pathway during the induction phase of protein synthesis-dependent forms of LTPs. The requirement for the mTOR-p70S6K pathway was restricted to the time of delivery of tetanization, although dendritic activation of the pathway was quite protracted.
Previous studies also demonstrated the presence of several components of the translational machinery and of the mTOR pathway in dendrites of neurons in the hippocampus and other brain structures. In fact, ribosomes have been observed in dendritic shafts as well as in the necks of dendritic spines and in the vicinity of postsynaptic sites (20). mTOR, p70S6K, eukaryotic initiation factor 4E, 4E-BP1, and 4E-BP2 were also seen in CA1 dendrites and in primary hippocampal neurons by immunocytochemistry (2, 21). However, the contribution of the mTOR pathway in the soma and dendritic subcellular compartments to protein synthesis-dependent LTP remained to be determined. We investigated the site of activation of the mTOR pathway in L-LTP of Schaffer collateral/commissural fiber-CA1 synapses. It was observed that, immunoreactivity for Thr-389-P p70S6K, the main active phosphoform of the mTOR effector p70S6K, was very low in the dendrites of CA1 neurons in hippocampal slices. After delivery of the L-LTP-inducing paradigm, Thr-389-P p70S6K was induced in the dendrites but not in the cell bodies of hippocampal neurons in the CA1. Treatments that prevented the induction of L-LTP also blocked accumulation of the active p70S6K phosphoform in dendrites of hippocampal neurons. A single tetanization paradigm that induces protein synthesis-independent potentiation, but not L-LTP, did not result in dendritic activation of p70S6K. Therefore, it can be concluded that dendritic activation of p70S6K is necessary for the induction of L-LTP at Schaffer collateral/commissural fiber-CA1 synapses. In primary hippocampal neurons in culture, dendritic activation of p70S6K could be seen after depolarization or application of either glutamate or NMDA. In primary neurons, the main dendritic sites of p70S6K activation appeared as discrete puncta along dendritic shafts, whereas only a small subset of dendritic spines of primary hippocampal neurons displayed immunoreactivity for Thr-389-P p70S6K after depolarization. The Thr-389-P p70S6K immunoreactive puncta along dendritic shafts were highly reminiscent of the morphology of hotspots of dendritic translation described by Aakalu et al. (3) and Job and Eberwine in similarly cultured primary hippocampal neurons (4).
The accumulation of a p70S6K phosphoform with protranslational activity in the dendritic compartment could constitute a pivotal step in the formation of long-term memory by increasing the translational capacity of postsynaptic elements. The immunocytochemical data presented here suggest that the activation of the dendritic translational apparatus takes place primarily in the dendritic shaft and to a lesser extent in dendritic spines. Such a morphologic presentation suggests the existence of a combination of a primarily dendrite-wide, and to a lesser extent a synaptic-specific, induction of translation as a possible mechanism for synaptic modification. A dendrite-wide translational activation could result in synaptic-specific changes if coupled with the selective targeting of dendritically synthesized proteins by activity-dependent targeting mechanisms. This view is consistent with the synaptic tag theory, which suggests that L-LTP is associated with the tagging of activated synapses, allowing them to capture plasticity-related proteins synthesized at undetermined extrasynaptic sites such as the soma or the dendrites (22). Such a proposed mechanism is not inconsistent with the role of synaptic protein synthesis in plasticity (19). In fact, it is possible that the p70S6K-dependent induction of elements of the translational machinery (6) in dendritic shafts, and the subsequent synaptic targeting of these proteins as well as ribosomes, could be needed to enable increased translation of synaptic proteins in activated spines. Rapamycin has also been shown to block protein synthesis in synaptosomes (24). Because activated p70S6K is infrequently seen in spines, rapamycin ability to inhibit synaptic translation could be mediated by other targets of mTOR such as 4E-BP1, which controls translation of capped mRNAs (7).
Overall, the present data suggest that that NMDA-, PI3K-, and mTOR-dependent activation of dendritic p70S6K is necessary for the induction phase of protein synthesis-dependent synaptic plasticity. Whereas activation of the mTOR-p70S6K pathway was quite protracted, its requirement for enduring potentiation was restricted to the time of delivery of tetanization. The morphologic evidence presented here suggests that the activation of the presynaptic translational machinery is primarily dendrite-wide. Ultimately, newly synthesized proteins in dendritic shafts could be targeted selectively to activity-tagged synapses.
Acknowledgments
This work was supported by the National Institute of Mental Health.
Abbreviations: L-LTP, late-phase long-term potentiation; mTOR, mammalian target of rapamycin; NMDA, N-methyl-d-aspartate; PI3K, phosphatidylinositol 3-kinase; p70S6K, p70 S6 kinase; fEPSP, field excitatory postsynaptic potential; STP, short-term potentiation; Thr-389-P, Thr-389-phosphorylated.
References
- 1.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 2.Tang, S. J., Reis, G., Kang, H., Gingras, A. C., Sonenberg, N. & Schuman, E. M. (2002) Proc. Natl. Acad. Sci. USA 99, 467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aakalu, G., Smith, W. B., Nguyen, N., Jiang, C. & Schuman, E. M. (2001) Neuron 30, 489-502. [DOI] [PubMed] [Google Scholar]
- 4.Job, C. & Eberwine, J. (2001) Proc. Natl. Acad. Sci. USA 98, 13037-13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pullen, N. & Thomas, G. (1997) FEBS Lett. 410, 78-82. [DOI] [PubMed] [Google Scholar]
- 6.Jefferies, H. B., Fumagalli, S., Dennis, P. B., Reinhard, C., Pearson, R. B. & Thomas, G. (1997) EMBO J. 16, 3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Manteuffel, S. R., Dennis, P. B., Pullen, N., Gingras, A. C., Sonenberg, N. & Thomas, G. (1997) Mol. Cell. Biol. 17, 5426-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balendran, A., Currie, R., Armstrong, C. G., Avruch, J. & Alessi, D. R. (1999) J. Biol. Chem. 274, 37400-37406. [DOI] [PubMed] [Google Scholar]
- 9.Peterson, R. T., Desai, B. N., Hardwick, J. S. & Schreiber, S. L. (1999) Proc. Natl. Acad. Sci. USA 96, 4438-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanna, P. P., Berton, F., Cammalleri, M., Tallent, M. K., Siggins, G. R., Bloom, F. E. & Francesconi, W. (2000) Proc. Natl. Acad. Sci. USA 97, 8653-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna, P. P., Cammalleri, M., Berton, F., Simpson, C., Lutjens, R., Bloom, F. E. & Francesconi, W. (2002) J. Neurosci. 22, 3359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banker, G. A. & Cowan, W. M. (1977) Brain Res. 126, 397-342. [DOI] [PubMed] [Google Scholar]
- 13.Lutjens, R., Igarashi, M., Pellier, V., Blasey, H., Di Paolo, G., Ruchti, E., Pfulg, C., Staple, J. K., Catsicas, S. & Grenningloh, G. (2000) Eur. J. Neurosci. 12, 2224-2234. [DOI] [PubMed] [Google Scholar]
- 14.Sanna, P. P., Bloom, F. E. & Wilson, M. C. (1991) Brain Res. Dev. Brain Res. 59, 104-108. [DOI] [PubMed] [Google Scholar]
- 15.Sanna, P. P., Keyser, K. T., Deerink, T. J., Ellisman, M. H., Karten, H. J. & Bloom, F. E. (1992) Neuroscience 47, 745-751. [DOI] [PubMed] [Google Scholar]
- 16.Frey, U., Huang, Y. Y. & Kandel, E. R. (1993) Science 260, 1661-1664. [DOI] [PubMed] [Google Scholar]
- 17.Raymond, C. R., Thompson, V. L., Tate, W. P. & Abraham, W. C. (2000) J. Neurosci. 20, 969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otani, S., Ben-Ari, Y. & Roisin-Lallemand, M. P. (1993) Brain Res. 613, 1-9. [DOI] [PubMed] [Google Scholar]
- 19.Steward, O. & Worley, P. (2002) Neurobiol. Learn. Mem. 78, 508-527. [DOI] [PubMed] [Google Scholar]
- 20.Steward, O. & Reeves, T. M. (1988) J. Neurosci. 8, 176-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond, C. R., Redman, S. J. & Crouch, M. F. (2002) Neuroscience 109, 531-536. [DOI] [PubMed] [Google Scholar]
- 22.Frey, U. & Morris, R. G. (1997) Nature 385, 533-536. [DOI] [PubMed] [Google Scholar]
- 23.Yu, X. M., Askalan, R., Keil, G. J., II, & Salter, M. W. (1997) Science 275, 674-678. [DOI] [PubMed] [Google Scholar]
- 24.Dong, E., Caruncho, H., Liu, W. S., Smalheiser, N. R., Grayson, D. R., Costa, E. & Guidotti, A. (2003) Proc. Natl. Acad. Sci. USA 100, 5479-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]