Abstract
Fragile X syndrome (FXS) is a common inherited cause of mental retardation resulting from the absence of the fragile X mental retardation protein (FMRP). FMRP is thought to regulate the translation of target mRNAs, including its own transcript. Here we show that the levels of FMRP are rapidly up-regulated in primary cortical neurons in response to the type-I metabotropic glutamate receptor (mGluR) agonist S-3,5-dihydrophenylglycine. These changes require new protein synthesis but not transcription and are specific to mGluR activation. We also demonstrate that the mRNA for PSD-95, a scaffolding protein involved in synaptic plasticity, contains a highly conserved canonical binding site for FMRP within its 3′ UTR. Furthermore, PSD-95 is rapidly translated in response to S-3,5-dihydrophenylglycine. Finally, we show that these mGluR-dependent changes in PSD-95 expression are lost in neurons derived from FMRP knockout mice, a model of FXS. Taken together, these studies suggest that FMRP is required for mGluR-dependent translation of PSD-95 and provide insights into the pathophysiology of FXS.
Fragile X syndrome is the most common inherited cause of mild to moderate mental retardation (1, 2). The syndrome typically results from transcriptional silencing of the fragile X mental retardation gene, FMR1, and loss of the fragile X mental retardation protein (FMRP) (for a review, see ref. 3). Through RGG and KH domains, FMRP binds up to 4% of total brain mRNA, including its own transcript (4-7). FMRP is highly expressed in the cytoplasm and dendrites of neurons (8), often in large messenger ribonucleoprotein particles, implicating FMRP in translational regulation (9-12).
FMRP is translated in response to activation of metabotropic glutamate receptors (mGluRs) (13, 14) and may be required for mGluR-dependent translation (15). Moreover, FMRP is required for normal type-I mGluR-dependent long-term depression, a process that requires protein synthesis (16). Thus, we hypothesized that FMRP-bound mRNAs would be translated in cultured neurons in response to application of a type-I mGluR agonist.
Here we show that application of the type-I mGluR agonist S-3,5-dihydrophenylglycine (DHPG) onto cortical cultures leads to the rapid and robust translation of both FMRP and the synaptic scaffolding protein PSD-95, whose mRNA contains a canonical FMRP binding site. Furthermore, the mGluR-dependent translation of PSD-95 is lost in cultures derived from FMR1 knockout (KO) mice. Taken together, these findings support a role for FMRP in activity-dependent translation and may provide insights into mGluR-dependent synaptic plasticity.
Methods
Animals and Cortical Cultures. C57/B6 FMR1-/- mice [generously provided by W. T. Greenough (University of Illinois at Urbana-Champaign, Urbana) (15, 17)] were obtained by backcrossing FVB FMR1-/- lines for more than six generations. High-density (2,500/mm2; ref. 18) and low-density (160/mm2; ref. 19) primary cortical cultures were prepared from E17 WT or FMR1 KO C57/B6 mice (see Figs. 4 and 5) or Sprague-Dawley rats (Harlan-Sprague-Dawley) (see Figs. 1, 2, 3) and used at 14-15 days in vitro. Western blots were performed and analyzed as described (14) with anti-FMRP (mAb 2160, Chemicon), anti-PSD-95 (7E3-1B8, Affinity BioReagents, Neshanic Station, NJ), anti-mGluR 1 and 5 (Upstate Biotechnology, Lake Placid, NY), and anti-β-actin (Sigma). For FMRP, only the top band was quantitated and normalized to β-actin.
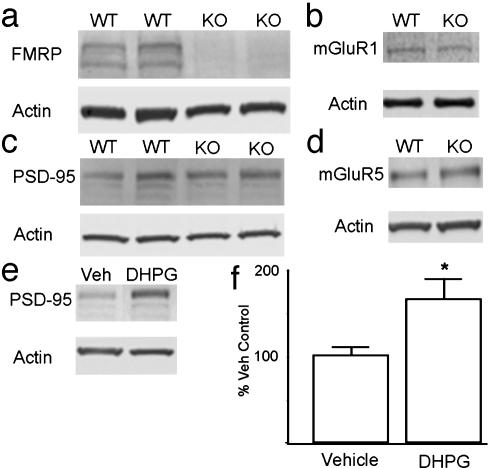
Fig. 4.
Characterization of FMRP WT and KO cultures. (a) Expression of FMRP (Upper) or actin (Lower) by immunoblot of primary cortical neuronal cultures derived from C57/B6 WT and FMR1-/- (KO) mice. (b) Representative immunoblot of mGluR 1 expression in WT or KO primary cortical neuronal cultures. (c) Expression of PSD-95 (Upper) in WT or KO neuronal cultures. (d) Representative immunoblot of mGluR 5 expression in WT or KO cultures. (e) Representative immunoblot of PSD-95 in WT cultures after treatment with vehicle (Veh) or 20 min of DHPG. (f) Summary of multiple experiments evaluating the effects of DHPG on PSD-95 expression in WT cultures.
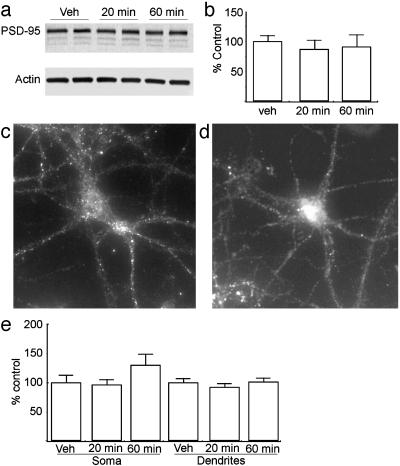
Fig. 5.
DHPG-dependent expression of PSD-95 is absent in FMR1 KO neurons. (a) Representative immunoblot of PSD-95 (Upper) and β-actin (Lower) in FMR1-/- neurons treated with vehicle (Veh) or 100 μM DHPG for 20 or 60 min. (b) Summary of three independent experiments quantifying PSD-95 expression by immunoblot after treatment with DHPG. (c) Representative image of an untreated neuron at 14 days in vitro derived from an FMR1-/- mouse stained for PSD-95. (d) Image of an FMR1-/- neuron treated with DHPG for 20 min and stained for PSD-95. (e) Quantification of immunofluorescence data for PSD-95 expression after DHPG treatment of FMR1-/- neurons. Histograms are as in Fig. 1.
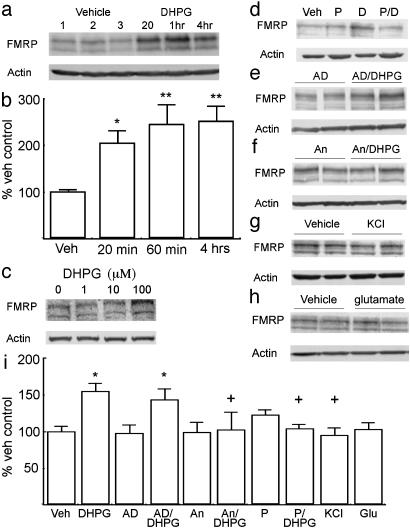
Fig. 1.
FMRP expression is up-regulated in cortical cultures in response to activation of type-I mGluRs. (a) A representative immunoblot of FMRP (Upper) and β-actin (Lower) expression in cultured neurons treated with vehicle or 100 μM DHPG for the times shown. (b) Summary of multiple experiments (more than five in all cases) evaluating the effect of DHPG on FMRP expression. (c) Representative immunoblot showing the effects of different concentrations of DHPG on FMRP expression at 20 min. Lanes from left to right are 0, 1, 10, and 100 μM.(d) Effect of mGluR antagonist on DHPG-mediated FMRP expression. Immunoblot of FMRP after vehicle alone (Veh), the type-I mGluR antagonist PHCCC (P), 20 min of DHPG alone (D), or PHCCC and DHPG together (P/D) is shown. (e and f) Representative immunoblots showing FMRP expression after treatment with actinomycin D (AD) (e) or anisomycin (An) (f). After a 10-min pretreatment, cells were treated with either vehicle (AD) or DHPG (AD/DHPG) for 20 min. (g) FMRP after 20 min of 50 mM KCl. (h) FMRP after 20 min of 50 μM glutamate. (i) Summary of experiments shown in d-h on the expression of FMRP in cultured neurons. Throughout figure, y axes are percentage vehicle controls, and error bars represent SEM. **, P < 0.0001 versus control; *, P < 0.05 versus control; +, P < 0.05 versus 20-min DHPG treatment alone.
Fig. 2.
PSD-95 mRNA contains a conserved FMRP binding site. The published RNA selection consensus sequence and FMRP binding site in the FMRP mRNA (6, 22) are aligned with the putative G-quartet in the 3′ UTR of the human, rat, and mouse PSD-95 mRNA. N, any ribonucleotide; D, any ribonucleotide except C. W represents A or U. Nucleotides listed in bold are conserved among rats, humans, and mice.
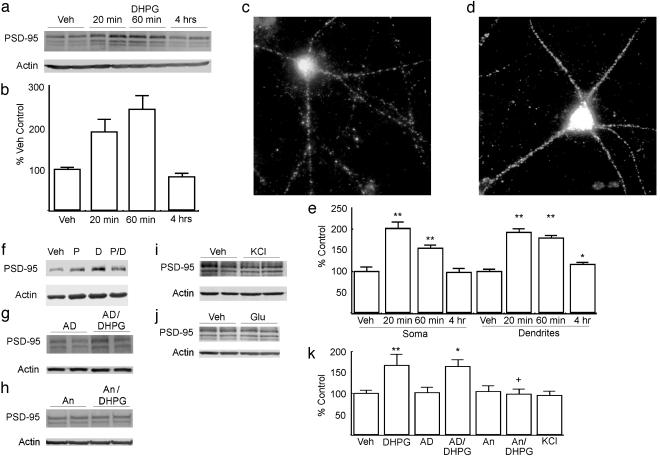
Fig. 3.
PSD-95 is rapidly translated in response to type-I mGluR activation in cortical cultures. (a) Immunoblot of PSD-95 (Upper) and β-actin (Lower) from neurons treated with vehicle (Veh) or 100 μM DHPG for the times shown. (b) Summary of immunoblot data of DHPG treatment on PSD-95 expression in cultured neurons. (c) Representative image of an untreated primary cortical neuron at 14 days in vitro stained for PSD-95. (d) Representative image of a neuron treated with DHPG for 1 h stained for PSD-95. (e) Summary of three independent experiments (>20 neurons per group) quantifying the effect of DHPG on PSD-95 immunofluorescence. Data represent the average intensity of the PSD-95 signal as a percentage of vehicle-treated controls in either the soma or dendrites at 20 min, 1 h, or 4 h after treatment with 100 μM DHPG. (f) Immunoblot of FMRP after vehicle alone (Veh), the type-I mGluR antagonist PHCCC (P), 20 min of DHPG alone (D), or PHCCC and DHPG together (P/D). (g and h) Representative immunoblots showing PSD-95 expression after treatment with actinomycin D (AD) (g) or anisomycin (An) (h). After a 10-min pretreatment, cells were treated with either vehicle (An) or DHPG (An/DHPG) for 20 min. (i) PSD-95 after 20 min of 50 mM KCl. (j) PSD-95 after 20 min of 50 μM glutamate. (k) Summary of multiple experiments evaluating the expression of PSD-95 in cultured neurons. **, P < 0.0001 vs. controls; *, P < 0.05 vs. controls.
Immunofluorescence and Immunoblotting. For immunofluorescence, low-density cultures fixed in 4% paraformaldehyde and dehydrated with methanol were incubated with primary antibodies in 0.5% BSA, 0.2% Triton X-100, and 5% normal goat serum in PBS overnight at 4°C followed by Cy5-linked secondary antibodies (Jackson ImmunoResearch). Images of more than five stained cells per cover slip were captured with a cooled charge-coupled device camera on a Nikon inverted microscope with a ×60/1.4-NA oil immersion objective. Background subtraction, shading correction, and data analysis were performed in a blinded fashion by using METAMORPH (19). Data are expressed as the average pixel intensity of traced dendrites or soma as a percentage of vehicle-treated controls.
Results
To test our hypothesis that FMRP would be translated in cultured neurons in response to type-I mGluR activation, we applied 100 μM DHPG to high-density primary cortical neurons. Twenty minutes after DHPG, FMRP levels were >2-fold above untreated cultures for at least 4 h (Fig. 1 a and b). DHPG at 100 μM was optimal (Fig. 1c), and this concentration was used thereafter. To assure that this increase in FMRP was, in fact, mediated by activation of type-I mGluRs, we pretreated cultures with either vehicle or the potent type-I mGluR-specific antagonist N-phenyl-7-(hydroxyimino)cyclopropachromen-1α-carboxamide (PHCCC) (20) and then stimulated with DHPG. As with previous experiments, FMRP expression was significantly elevated after treatment with DHPG for 20 min, which was completely blocked by PHCCC (Fig. 1 d and i). Thus, DHPG induction of FMRP production appears to depend on type-I mGluR activation.
FMRP is rapidly translated in synaptoneurosomes in response to DHPG (13); therefore, we examined whether the increase in FMRP was regulated at the level of transcription or translation. Actinomycin D (5 μg/ml) had no effect on the DHPG-dependent increase in FMRP levels (Fig. 1 e and i). In contrast, pretreatment with the protein synthesis inhibitor anisomycin (40 μM) completely abrogated DHPG-dependent FMRP expression (Fig. 1 f and i).
In synaptoneurosomes, increases in mRNA loading onto polyribosomes and subsequent protein synthesis can be activated by depolarization with KCl as well as mGluR agonists (21). To test the specificity of the changes in FMRP levels seen in cultured cortical neurons, we applied either 50 mM KCl or 50 μM glutamate and measured FMRP expression. No changes in FMRP levels were seen after 20 min of either glutamate or KCl treatment, suggesting that these changes are specific to activation of mGluRs (Fig. 1 g-i).
Two separate groups have recently identified an mRNA sequence motif that is specifically bound by FMRP (6, 22). The so-called purine or G-quartet sequence is present in semaphorin, munc13, and Rab6-interacting protein mRNAs among others (24). Using sequence analysis, we noted a highly conserved G-quartet in the 3′ UTR of PSD-95 mRNA (Fig. 2). PSD-95 is a multi-PDZ domain containing protein that participates in the synaptic scaffolding at the postsynaptic density. PSD-95 is a critical component in synaptic plasticity, as demonstrated by learning deficits and abnormal long-term potentiation and long-term depression in PSD KO mice (23, 24). Furthermore, PSD-95 has been implicated in synapse maturation, with overexpression of PSD-95 in cultured hippocampal neurons leading to increases in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor clustering, dendritic spine size and number, and associated changes in miniature excitatory postsynaptic potential amplitude and efficacy (24).
Based on this evidence, we hypothesized that DHPG would affect PSD-95 expression in primary cortical neurons. PSD-95 expression was maximally elevated 20 min after the onset of DHPG stimulation (Fig. 3 a and b). However, unlike FMRP, PSD-95 increases were transient, returning to baseline by 4 h after the onset of treatment (Fig. 3 a and b). As with FMRP, these DHPG-dependent effects were blocked by pretreatment with the type-I mGluR antagonist PHCCC (Fig. 3f). To localize these changes, we used immunofluorescence in low-density cortical cultures to examine PSD-95 expression. As described, PSD-95 staining within cultured neurons was diffuse within the cell soma with discrete puncta seen throughout the dendritic arbor (Fig. 3c). At 20 min and 1 h from the onset of DHPG treatment, PSD-95 staining within these neurons was elevated 2-fold compared with controls, and these changes were seen both in the cell soma and within the dendrites (Fig. 3 c-e). By 4 h, these changes returned to basal levels within the soma, with a small residual increase seen in dendritic staining (Fig. 3e). When neurons were pretreated with actinomycin D, there was no significant effect on the DHPG-mediated increases in PSD-95 (Fig. 3 g and k). In contrast, these increases were blocked by pretreatment with anisomycin (Fig. 3 h and k). Furthermore, as with FMRP, PSD-95 expression was not affected by treatment with KCl or glutamate (Fig. 3 i-k).
Thus, application of DHPG rapidly increases the translation of two different putative FMRP targets in cortical neurons. To test the hypothesis that PSD-95 increases were mediated through an FMRP-dependent mechanism, we prepared primary cortical neurons from C57/B6 FMR1-/- mice. Basal levels of PSD-95 within these cortical cultures were not significantly altered from nontransgenic controls (Fig. 4b). As in cultures from rats, application of DHPG to cultures derived from C57/B6 WT mice for 20 min significantly elevated expression of PSD-95 (Fig. 4e). In contrast, treatment of cortical cultures derived from KO mice with DHPG had no effect on PSD-95 expression as measured by Western blot (Fig. 5 a and b). Furthermore, in low-density cultures DHPG had no effect on PSD-95 immunofluorescence within the cell soma or dendrites of FMR1-/- neurons (Fig. 5 c-e). The absence of a response to DHPG was not a result of lost or reduced expression of mGluRs, because there were no differences in either mGluR1 or mGluR5 by Western blot analysis of WT and KO cultures or brain homogenates (Fig. 4 c and d).
Discussion
We have shown that both FMRP and the putative FMRP mRNA target PSD-95 are rapidly translated in cultured cortical neurons in response to activation of type-I mGluRs. These changes are specific to the activation of type-I mGluRs, because neither depolarization with KCl nor application of glutamate replicates these activity-dependent increases in FMRP and PSD-95. Furthermore, mGluR-dependent expression of PSD-95 is absent in primary cortical neurons derived from FMR1 KO animals, suggesting that FMRP is required for this activity-dependent translation event. Because FMRP is associated with both mRNAs and ribosomes in neurons and can inhibit translation in vitro (8, 11, 12), our observations suggest that FMRP plays a direct role in translational regulation in vivo as well.
The kinetics of both PSD-95 and FMRP up-regulation are surprisingly fast, especially for PSD-95, which has previously been shown by 35S pulse-chase experiments to be relatively stable under basal conditions (25). The most likely explanation for this difference is that PSD-95 produced in response to DHPG is unstable compared with the majority of preexisting PSD-95 before stimulation. That is, there may be two pools of PSD-95: one that is stable and slowly turned over, likely in association with the postsynaptic density or other macromolecular complexes, and a second pool of unassociated PSD-95 that is more rapidly degraded (25). There are some data to support this hypothesis. In young (2-to 4-day-old) cultured neurons, PSD-95 is produced in the cell soma and then condensed into perinuclear homomultimer complexes before transport into synaptic sites in dendrites (26-28). The majority of dendritic PSD-95 under basal conditions in cultures at 2-3 weeks is incorporated into postsynaptic densities in spines (29, 30), and the turnover of PSD-95 within these spines is relatively slow, with a half-life of >24 h. In contrast, PSD-95 that is either diffuse within the shaft or associated with clusters in the shaft is much less stable, with virtually complete turnover over a 24-h period and dissociation of specific clusters occurring in <1 h (29, 31). Thus, the localization of PSD-95 within cells influences its stability. We observed increases in both diffuse and clustered PSD-95 staining within dendrites after DHPG treatment, although we have not quantified whether these new clusters are within spines or are associated with synapses.
The mechanism for rapid turnover of newly synthesized PSD-95 is unclear. One possibility is calpain-mediated degradation of PSD-95, which is operative in early postnatal development (32). As activation of type-I mGluRs increases calcium levels through release from intracellular stores, calpain activation and degradation of PSD-95 may be increased after DHPG. It is worth noting that, although most of the PSD-95 produced in response to DHPG is gone by 4 h after the stimulus onset, there is a significant increase in residual dendritic PSD-95 protein in the cultures. Thus, some of the protein produced may be incorporated into a more stable PSD-95 pool as a component of either new or already existing synaptic PSD clusters.
We show that neither depolarization with KCl nor activation of all ionotropic and mGluRs with the endogenous ligand glutamate leads to changes in expression of FMRP or PSD-95 in cultured neurons, in agreement with some previous studies (25). At first, these results seem somewhat surprising, given that past studies in synaptoneurosomes have shown that KCl or glutamate can activate translation of dendritic mRNAs (21) and that glutamate is activating type-I mGluRs in addition to other glutamatergic receptors. Our findings suggest that the context of mGluR activation is important. This idea is supported by recent studies that show a difference in outcome between activation of synaptic and nonsynaptic glutamate receptors (33). In these studies, bath application of glutamate onto cells triggers signaling cascades different from activation of synaptic N-methyl-D-aspartate (NMDA) receptors, triggering activation of apoptotic pathways and decreases in cAMP response element-binding protein (CREB) activity, rather than CREB phosphorylation and brain-derived neurotrophic factor expression (33). Bath application of glutamate and KCl depolarization on our cortical neurons would be expected to activate both synaptic and nonsynaptic NMDA receptors, leading to the proapoptotic effects described above. In synaptoneurosomes, the cell bodies are removed; therefore, activation by glutamate or KCl can occur in the absence of any soma-derived nonsynaptic ”override.” In support of this, pretreatment of synaptoneurosomes with NMDA inhibits mGluR-dependent changes in polyribosome profiles in a calcium-dependent manner (34). Thus, by activating type-I mGluRs alone, we may be producing a more physiologically appropriate signal for the cells that drives local protein translation.
Our findings represent the identification of specific proteins that are translated in response to activation of type-I mGluRs in intact cells, although a number of studies suggest that there are likely multiple proteins translated in response to such a stimulus (13, 21, 36). Weiler and colleagues (13, 21) were able to detect a general increase in [35S]methionine incorporation after stimulation of mGluRs in synaptoneurosomes, a cortically derived subcellular preparation of dendritic endings, and they were able to show that one of the mRNAs translated in this manner encoded FMRP. More recently, Job and Eberwine (36) demonstrated that GFP mRNA transfected into cultured neurons is translated in both the dendrites and cell soma after addition of DHPG.
Recent experiments using cDNA microarray techniques to evaluate FMRP complexes immunoprecipitated from mouse brains have identified a number of possible mRNA targets for FMRP, and a subset of these mRNAs also contains the G-quartet sequence identified as the preferred FMRP binding site (22, 37). To date, the best evidence that one of these mRNAs is actually regulated by FMRP in vivo comes from work in Drosophila, where the fly homolog of FMRP, Drosophila fragile X-related protein (dFXR), has recently been identified (38, 39). dFXR binds to the futsch mRNA in vitro, and expression of futsch is inversely related to expression of dFXR, suggesting that dFXR acts to inhibit futsch translation under normal conditions (39). The mRNA for the mammalian homolog of futsch, the microtubule-associated protein MAP1B, is found in FMRP complexes in mouse brain and contains a G-quartet (22, 37). Because both FMRP and PSD-95 mRNAs contain G-quartet sequences and PSD-95 translation appears to require FMRP, we anticipate that other structurally related mRNAs will be similarly regulated.
The function of mGluR-dependent production of FMRP and PSD-95 remains to be determined, but activation of type-I mGluRs by DHPG in slices and cultures induces numerous downstream effects that require protein synthesis. Application of DHPG onto either hippocampal or cerebellar slices induces a long-term depression that requires dendritic protein synthesis but not transcription (35, 40, 41). Furthermore, DHPG application has also been reported to enhance both hippocampal long-term potentiation and the epileptogenic effects of picrotoxin in a protein synthesis-dependent fashion (42, 43). The requirements for protein synthesis in these various paradigms are very brief (10-15 min), suggesting that a ”pulse” of translation is rapidly activated and then turned off (42, 44). Our data suggest that both FMRP and PSD-95 are translated during such a short time frame in response to DHPG, supporting the possibility that these proteins may be involved in these types of mGluR-dependent synaptic plasticity.
Recent work by Huber et al. (16) demonstrates that type-I mGluR-dependent long-term depression is actually enhanced in fragile X KO mice, suggesting that FMRP may act in a negative feedback manner to inhibit mGluR-activated translation. Yet our results in FMR1 KO cultures show that there is a loss rather than an enhancement of mGluR-dependent translation of PSD-95, indicating that FMRP may also play a role in activity-dependent translational initiation of some bound mRNAs. This hypothesis is supported by recent observations in synaptoneurosomes that demonstrate a decrease in DHPG-dependent mRNA loading onto ribosomes and new protein translation in preparations from FMR1 KO mice (15).
In summary, we demonstrate the rapid mGluR-dependent translation of two proteins, FMRP and PSD-95, and provide evidence that these effects are mediated in part through an FMRP-dependent mechanism. Because both proteins are involved in dendritic spine maturation and morphology (24, 45), our data suggest some of fragile X syndrome neuropathology may be due to aberrant PSD-95 expression and mGluR-dependent synaptic plasticity. In addition, they provide a framework for future experiments targeted at defining the specific functions of FMRP and its downstream mRNA targets.
Acknowledgments
We thank Dandan Sun and colleagues, Meggan Czapiga, Erik Dent, Kate Kalil, and Bill Greenough. P.K.T. was supported by the Medical Scientist and Neuroscience Training Programs of the University of Wisconsin. This work was funded by grants from the FRAXA Research Foundation (to K.J.M.) and the National Institutes of Health (to K.J.M. and J.S.M.).
Abbreviations: FMRP, fragile X mental retardation protein; mGluR, metabotropic glutamate receptor; DHPG, S-3,5-dihydrophenylglycine; PHCCC, N-phenyl-7-(hydroxyimino)cyclopropachromen-1α-carboxamide; KO, knockout.
References
- 1.Turner, G., Webb, T., Wake, S. & Robinson, H. (1996) Am. J. Med. Genet. 64, 196-197. [DOI] [PubMed] [Google Scholar]
- 2.de Vries, B. B., van den Ouweland, A. M., Mohkamsing, S., Duivenvoorden, H. J., Mol, E., Gelsema, K., van Rijn, M., Halley, D. J., Sandkuijl, L. A., Oostra, B. A., et al. (1997) Am. J. Hum. Genet. 61, 660-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin, P. & Warren, S. T. (2000) Hum. Mol. Genet. 9, 901-908. [DOI] [PubMed] [Google Scholar]
- 4.Ceman, S., Brown, V. & Warren, S. T. (1999) Mol. Cell. Biol. 19, 7925-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley, C. T., Sutcliffe, J. S., Kunst, C. B., Leiner, H. A., Eichler, E. E., Nelson, D. L. & Warren, S. T. (1993) Nat. Genet. 4, 244-251. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffer, C., Bardoni, B., Mandel, J. L., Ehresmann, B., Ehresmann, C. & Moine, H. (2001) EMBO J. 20, 4803-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, V., Small, K., Lakkis, L., Feng, Y., Gunter, C., Wilkinson, K. D. & Warren, S. T. (1998) J. Biol. Chem. 273, 15521-15527. [DOI] [PubMed] [Google Scholar]
- 8.Feng, Y., Gutekunst, C. A., Eberhart, D. E., Yi, H., Warren, S. T. & Hersch, S. M. (1997) J. Neurosci. 17, 1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, Y., Absher, D., Eberhart, D. E., Brown, V., Malter, H. E. & Warren, S. T. (1997) Mol. Cell 1, 109-118. [DOI] [PubMed] [Google Scholar]
- 10.Corbin, F., Bouillon, M., Fortin, A., Morin, S., Rousseau, F. & Khandjian, E. W. (1997) Hum. Mol. Genet. 6, 1465-1472. [DOI] [PubMed] [Google Scholar]
- 11.Laggerbauer, B., Ostareck, D., Keidel, E. M., Ostareck-Lederer, A. & Fischer, U. (2001) Hum. Mol. Genet. 10, 329-338. [DOI] [PubMed] [Google Scholar]
- 12.Li, Z., Zhang, Y., Ku, L., Wilkinson, K. D., Warren, S. T. & Feng, Y. (2001) Nucleic Acids Res. 29, 2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiler, I. J., Irwin, S. A., Klintsova, A. Y., Spencer, C. M., Brazelton, A. D., Miyashiro, K., Comery, T. A., Patel, B., Eberwine, J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5395-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd, P. K., Malter, J. S. & Mack, K. J. (2003) Brain Res. Mol. Brain Res. 110, 267-278. [DOI] [PubMed] [Google Scholar]
- 15.Greenough, W. T., Klintsova, A. Y., Irwin, S. A., Galvez, R., Bates, K. E. & Weiler, I. J. (2001) Proc. Natl. Acad. Sci. USA 98, 7101-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. (2002) Proc. Natl. Acad. Sci. USA 99, 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comery, T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schomberg, S. L., Su, G., Haworth, R. A. & Sun, D. (2001) J. Neurophysiol. 85, 2563-2575. [DOI] [PubMed] [Google Scholar]
- 19.Dent, E. W., Callaway, J. L., Szebenyi, G., Baas, P. W. & Kalil, K. (1999) J. Neurosci. 19, 8894-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annoura, H., Fukunaga, A., Uesugi, M., Tatsuoka, T. & Horikawa, Y. (1996) Bioorg. Med. Chem. Lett. 6, 763-766. [Google Scholar]
- 21.Weiler, I. J. & Greenough, W. T. (1993) Proc. Natl. Acad. Sci. USA 90, 7168-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darnell, J. C., Jensen, K. B., Jin, P., Brown, V., Warren, S. T. & Darnell, R. B. (2001) Cell 107, 489-499. [DOI] [PubMed] [Google Scholar]
- 23.Migaud, M., Charlesworth, P., Dempster, M., Webster, L. C., Watabe, A. M., Makhinson, M., He, Y., Ramsay, M. F., Morris, R. G., Morrison, J. H., et al. (1998) Nature 396, 433-439. [DOI] [PubMed] [Google Scholar]
- 24.El-Husseini, A. E., Schnell, E., Chetkovich, D. M., Nicoll, R. A. & Bredt, D. S. (2000) Science 290, 1364-1368. [PubMed] [Google Scholar]
- 25.El-Husseini, A. E., Schnell, E., Dakoji, S., Sweeney, N., Zhou, Q., Prange, O., Gauthier-Campbell, C., Aguilera-Moreno, A., Nicoll, R. A. & Bredt, D. S. (2002) Cell 108, 849-863. [DOI] [PubMed] [Google Scholar]
- 26.Craven, S. E. & Bredt, D. S. (2000) J. Biol. Chem. 275, 20045-51. [DOI] [PubMed] [Google Scholar]
- 27.El-Husseini, A. E., Craven, S. E., Chetkovich, D. M., Firestein, B. L., Schnell, E., Aoki, C. & Bredt, D. S. (2000) J. Cell Biol. 148, 159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craven, S. E., El-Husseini, A. E. & Bredt, D. S. (1999) Neuron 22, 497-509. [DOI] [PubMed] [Google Scholar]
- 29.Okabe, S., Kim, H. D., Miwa, A., Kuriu, T. & Okado, H. (1999) Nat. Neurosci. 2, 804-811. [DOI] [PubMed] [Google Scholar]
- 30.Rao, A., Kim, E., Sheng, M. & Craig, A. M. (1998) J. Neurosci. 18, 1217-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okabe, S., Miwa, A. & Okado, H. (2001) J. Neurosci. 21, 6105-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, X., Rong, Y. & Baudry, M. (2000) Neurosci. Lett. 286, 149-153. [DOI] [PubMed] [Google Scholar]
- 33.Hardingham, G. E., Fukunaga, Y. & Bading, H. (2002) Nat. Neurosci. 5, 405-414. [DOI] [PubMed] [Google Scholar]
- 34.Weiler, I. J., Childers, W. S. & Greenough, W. T. (1996) J. Neurochem. 66, 197-202. [DOI] [PubMed] [Google Scholar]
- 35.Huber, K. M., Kayser, M. S. & Bear, M. F. (2000) Science 288, 1254-1257. [DOI] [PubMed] [Google Scholar]
- 36.Job, C. & Eberwine, J. (2001) Proc. Natl. Acad. Sci. USA 98, 13037-13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown, V., Jin, P., Ceman, S., Darnell, J. C., O'Donnell, W. T., Tenenbaum, S. A., Jin, X., Feng, Y., Wilkinson, K. D., Keene, J. D., et al. (2001) Cell 107, 477-487. [DOI] [PubMed] [Google Scholar]
- 38.Wan, L., Dockendorff, T. C., Jongens, T. A. & Dreyfuss, G. (2000) Mol. Cell. Biol. 20, 8536-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M. & Broadie, K. (2001) Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]
- 40.Snyder, E. M., Philpot, B. D., Huber, K. M., Dong, X., Fallon, J. R. & Bear, M. F. (2001) Nat. Neurosci. 4, 1079-1085. [DOI] [PubMed] [Google Scholar]
- 41.Xiao, M. Y., Zhou, Q. & Nicoll, R. A. (2001) Neuropharmacology 41, 664-671. [DOI] [PubMed] [Google Scholar]
- 42.Raymond, C. R., Thompson, V. L., Tate, W. P. & Abraham, W. C. (2000) J. Neurosci. 20, 969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merlin, L. R., Bergold, P. J. & Wong, R. K. (1998) J. Neurophysiol. 80, 989-993. [DOI] [PubMed] [Google Scholar]
- 44.Karachot, L., Shirai, Y., Vigot, R., Yamamori, T. & Ito, M. (2001) J. Neurophysiol. 86, 280-289. [DOI] [PubMed] [Google Scholar]
- 45.Irwin, S. A., Patel, B., Idupulapati, M., Harris, J. B., Crisostomo, R. A., Larsen, B. P., Kooy, F., Willems, P. J., Cras, P., Kozlowski, P. B., et al. (2001) Am. J. Med. Genet. 98, 161-167. [DOI] [PubMed] [Google Scholar]