Abstract
MEK/ERK activities are increased in many primary lung cancers, and MEK inhibitors have been tested clinically for treatment of non-small cell lung cancers. The molecular mechanisms of resistance to MEK inhibitors have not been clearly demonstrated, however, and no molecular biomarker that can predict lung cancer response to MEK inhibitors is available. By determining the dose-responses of 35 human lung cancer cell lines to MEK-specific inhibitor AZD6244, we identified subsets of lung cancer cell lines that are either sensitive or resistant to this agent. Subsequent molecular characterization showed that treatment with AZD6244 suppressed ERK phosphorylation in both sensitive and resistant cells, suggesting that resistance is not mediated by the activities of MEK/ERK themselves. Interestingly, we found that levels of phosphorylated AKT were dramatically higher in the resistant cancer cells than in the sensitive cells. Stable transfection of dominant-negative AKT into resistant cells by retroviral infection restored their susceptibility to AZD6244. These results indicate that phosphorylated AKT may be a biomarker of response to AZD6244 and that modulation of AKT activity may be a useful approach to overcome resistance to MEK inhibitors.
Keywords: AZD6244, MEK inhibitor, resistance, AKT, lung cancer
Introduction
The recent advent of pathway-targeted chemotherapeutic agents, such as gefitinib1 and erlotinib,2 has led to improved therapy for a small subgroup of patients with advanced lung cancer. However, research findings have demonstrated that tumors with similar clinical characteristics (location, pathology, size, stage) may respond differently to the same drug. The discovery that mutations in the tyrosine kinase domain of epidermal growth factor receptor are associated with response of non-small cell lung cancer (NSCLC) to gefitinib3–5 or erlotinib3 made it possible to identify patients who may benefit from treatment with one of these agents.
The mitogen-activated protein kinase kinase (MEK) is one of the critical molecules in the Ras/Raf/MEK/ERK cascade, which mediates signal transduction from Ras/Raf to extracellular signal-regulated kinases (ERK) through phosphorylation of both tyrosine (Tyr185) and threonine (Thr183) residues of ERK proteins. Humans and other mammals have two MEK proteins, MEK1 and MEK2, which are encoded by their corresponding genes and are expressed ubiquitously.6 The constitutively active form of MEK is sufficient for cellular transformation, as reflected in a number of highly tumorigenic cell lines.7,8 Moreover, activation of MEK/ERK has been implicated in development of a broad range of human tumors, including lung cancers.9,10 Enhanced activation of MEK/ERK has been detected in 30–60% of primary lung cancers,9,11–14 is associated with hyperactivation rather than overexpression of ERK, and is linked to poor survival.12
The important role of Ras/Raf/MEK/ERK pathway in tumorigenesis has led to clinical trials of MEK inhibitors for treatment of various solid tumors, including lung cancers.15,16 Treatment has resulted in stabilization of disease in a small subset of patients with lung cancer, melanoma, pancreatic cancer or colon cancer. The clinical studies showed, however, that inhibition of phosphorylated ERK in tumor tissues from patients receiving MEK inhibitor therapy did not correlated with clinical benefit,17 indicating that both the presence of activated ERK before treatment and suppression of ERK activation after treatment are not sufficient to guide patient selection or to predict the response to treatment with MEK inhibitors. Therefore, molecular biomarkers that can be used for predicting response to MEK inhibitors are needed to improve the design of future clinical trials of those agents and evaluation of the results, and to optimize outcomes of targeted lung cancer therapy with MEK inhibitors. The identification of molecular mediators of resistance may also facilitate the design of drugs to overcome resistance.
In our search for molecular biomarkers that can be used to predict response to MEK inhibitors, we evaluated the dose-responses of human lung cancer cell lines to AZD6244, a highly selective MEK inhibitor that is used in clinical trials for treatment of solid tumors, including lung cancers.18–20 Subsets of AZD6244-sensitive and -resistant human lung cancer cell lines were identified and evaluated for their molecular differences.
Results
Effect of AZD6244 on viability of lung cancer cells in vitro
We first tested the antiproliferative effect of AZD6244 on 35 lung cancer cell lines by SRB assay and determined their median inhibitory concentrations. The response to AZD6244 varied greatly (up to more than 1,000 fold) among the cell lines (data not shown). On the basis of their response to AZD6244, we selected the four most sensitive cell lines (Calu-6, H2347, H3122 and H2009) and the four most resistant cell lines (H196, H522, HCC2450 and Calu-3) for further studies. The dose-responses of those eight cell lines are shown in Figure 1A. We also performed clonogenic assays to verify the responses of those eight cell lines to AZD6244 (Fig. 1B). As in the cell viability assay, treatment with AZD6244 resulted in a dramatic dose-dependent reduction of colony formation in cell lines Calu-6, H2347, H3122 and H2009. Colony formation was suppressed by more than 50% in those sensitive cells at of 5 µM or less which is in the range of concentrations achieved in the serum of patient receiving oral AZD6244. In contrast, colony formation by cell lines H196, H522, HCC2450 and Calu-3 was either not affected or only mildly suppressed even at doses of 50 µM or higher (Fig. 1B). The median inhibitory concentrations determined by either cell viability assay or clonogenic assay were comparable in those eight cell lines, and varied from less than 0.5 µM to more than 100 µM (Table 1). The mutation status of Braf, EGFR and KRAS for each cell lines is shown in Table 1. Among four sensitive cell lines, two (Calu-6 and H2009) have KRAS mutations. Most of cell lines used in this study have wild-type Braf and EGFR genes. The Braf mutation status in H196 and H3122 cell lines were unreported. We performed a PCR-based sequence analysis for exon 11 and 15 of Braf gene, exon 18–21 of EGFR gene, and exon 1–2 of KRAS gene, which contains hot-spot mutations are reported to be associated with sensitivity of chemotherapy.21 The results showed that both cell lines are wild-type for all genes.
Figure 1.
Dose-response to AZD6244 in various NSCLC cell lines. (A) Cell viability assay. Cells were treated with medium containing different concentrations of AZD6244 for 96 h. Cell viability was determined by SRB, and relative cell viability was plotted as described in Materials and Methods. Values represent mean ± SE of three independent triplicate assays. (B) Clonogenic assay. Cells were treated with medium containing various concentrations of AZD6244 for 96 h. The medium was then removed, and fresh drug-free medium was added to allow clonogenic growth. The values represent the mean ± SE of three independent experiments performed in triplicate.
Table 1.
IC50 and mutation status of eight lung cancer cell lines
| IC50 (µM) | Genes | ||||
|---|---|---|---|---|---|
| Cell lines |
SRB | Clongenic | Braf | EGFR | KRAS |
| Calu6 | 0.32 | 0.28 | WT | WT | Mutated |
| H2347 | 0.31 | 0.59 | WT | WT | WT |
| H3122 | 2.52 | 1.38 | WT | WT | WT |
| H2009 | 0.99 | 0.53 | WT | WT | Mutated |
| H522 | 140.77 | 151.6 | WT | WT | WT |
| H2450 | 149.98 | 137.35 | WT | WT | WT |
| H196 | 162.12 | 182.5 | WT | WT | WT |
| Calu3 | 183.12 | 193.48 | WT | WT | WT |
Induction of apoptosis by AZD6244 in sensitive lung cancer cells lines
To investigate whether AZD6244-mediated reduction of viability of sensitive cells was caused by suppression of cell growth or induction of apoptosis, we analyzed apoptosis and cell cycle profiles after treatment with AZD6244. Sensitive or resistant cells were treated with 10 µM of AZD6244 for 72 h, and cells were harvested for cell cycle analysis. The results show that treatment with AZD6244 led to a dramatic increase in apoptotic (sub-G1) cells in a time-dependent manner in the sensitive Calu-6, H2347, H3122 and H2009 cells but not in the resistant HCC2450 cells (Fig. 2A). The apoptosis induced by AZD6244 in sensitive lung cancer cells was confirmed by western blot analysis. Treatment with AZD6244 resulted in a dramatic, time-dependent increase of caspase-3 cleavage in the sensitive Calu-6 cells but not in the resistant HCC2450 cells (Fig. 2B). Moreover, we also detected that AZD6244 could induce apoptosis in sensitive cell line Calu-6 in dose response (Fig. 2C). Together, those results demonstrate that treatment with AZD6244 induced apoptosis in sensitive lung cancer cells.
Figure 2.
Apoptosis induction by AZD6244 in cultured lung cancer cells. (A) Representative flow cytometric histograms of cells stained with propidium iodide. The numbers represent percentages of sub-G1 phase cells. (B) Western blot analysis. Calu-6 and HCC2450 cells were exposed to 10 µM AZD6244 for 0, 4, 24 or 72 h. Whole-cell extracts were analyzed by western blot with antibody to caspase-3. The figure represents one of three western blots with similar results. (C) Calu-6 Cells were treated with increasing doses of AZD6244 for 72 h. Apoptosis was analyzed with flow cytometry.
Phosphorylated AKT is elevated in AZD6244-resistant cell lines
To investigate the mechanism of intrinsic resistance of lung cancer cells to MEK inhibitor AZD6244, we harvested resistant and sensitive cells during log-phase growth and tested their endogenous expression of molecules in the Ras/Raf/MEK/ERK pathway and the phosphatidylinositide-3 kinase (PI3K)/AKT pathway, both of which mediate signal transduction from growth factor receptors. Western blot analysis showed no obvious difference in expression of B-Raf and p-ERK among the sensitive and resistant cells. Interestingly, all four resistant cell lines expressed high levels of p-AKT (Ser473), which was barely detectable in the sensitive cells (Fig. 3). Furthermore, patterns of p-AKT (Thr308), p-p38 and p-mTOR expression were similar, although to a less dramatic degree (Fig. 3). This observation was verified by AKT activity assay. AKT kinase activity was higher in resistant cell lines than in sensitive cell lines (Fig. 3).
Figure 3.
Expression of various molecules in AZD6244-sensitive and -resistant cell lines. Western blot analysis of eight lung cancer cell lines. The name of the cell line is at the top of the appropriate lane, while the names for each molecule or its phosphorylation sites are marked on the right. α-Tubulin was used as a loading control. Akt activity assay was performed as described in Materials and Methods, using GSK-3α/β as substrate for phosphorylation. Experiments were repeated three times with similar results.
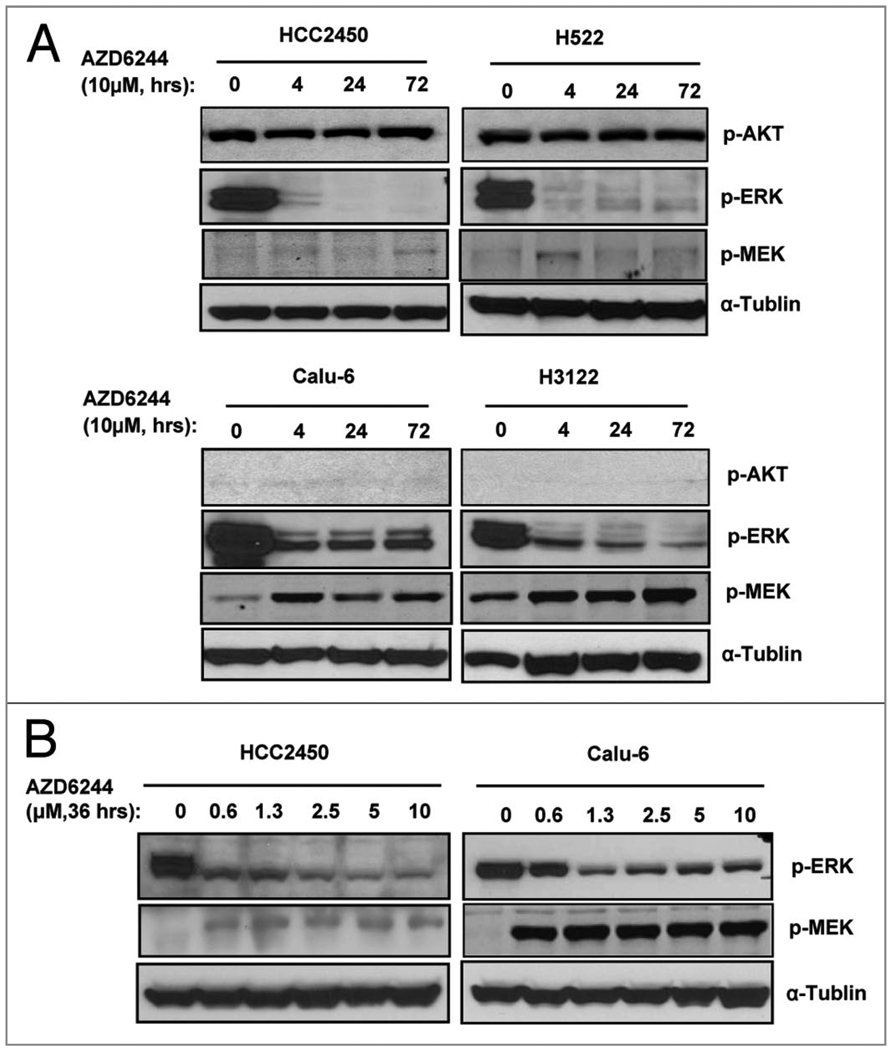
We then tested whether treatment with AZD6244 would alter levels of p-AKT, p-ERK and p-MEK. Because ERK is phosphorylated by MEK, inhibiting MEK by AZD6244 is expected to suppress p-ERK. As expected, treatment with 10 µM of AZD6244 resulted in suppression of p-ERK at all time points tested (4, 24 and 72 h) in the sensitive Calu-6 and H3122 cells. Also as expected, treatment with AZD6244 had no obvious impact on levels of p-AKT in either sensitive or resistant cells. Interestingly, the level of p-ERK was suppressed in resistant cell lines HCC2450 and H522 to the same degree as in the sensitive cells (Fig. 4A). A dose-response analysis showed that both sensitive Calu-6 cells and resistant HCC2450 cells responded similarly in term of suppression phosphorylated ERK (Fig. 4B). This indicates that MEK was inhibited by AZD6244 in both sensitive and resistant cells, regardless of the different responses in their cell growth profile or apoptosis induction. We also investigate p-MEK expression after treatment with AZD6244. An obvious upregulation of p-MEK was detected in sensitive cell lines Calu-6 and H3122 after AZD6244 treatment. This upregulation was much weaker in resistant cell lines H522 and HCC2450. This result indicated that p-MEK upregulation might not contribute to resistance to AZD6244.
Figure 4.
Effect of AZD6244 on phosphorylation of ERK and AKT. (A) AZD6244-sensitive Calu-6 and H3122, and -resistance HCC2450 and H522 cells were treated with 10 µM AZD6244 for 0, 4, 24 or 72 h as indicated. Cells were collected for western blot analysis for levels of p-AKT, p-ERK and p-MEK. (B) Calu-6 and HCC2450 cells were treated with AZD6244 at doses range of 0.6 to 10 µM for 36 h as indicated. Cells were collected for western blot analysis for levels of p-ERK and p-MEK. α-Tubulin was used as a loading control. The figure represents one of three western blots with similar results.
AZD6244-resistant phenotype reversed by dominant-negative AKT
To further investigate the role of AKT in resistance to AZD6244, we infected resistant HCC2450 and H522 cells with a retroviral vector expressing HA-tagged dominant-negative AKT (dnAKT). Cells infected with an empty vector were used as a control. After brief selection with Geneticin, expression of dnAKT was verified in dnAKT-transfected cells by anti-HA tag antibody (Fig. 5A). We then treated parental, vector-transfected and dnAKT-transfected cells with various doses of AZD6244 and determined cell viability at 96 h after the treatment. The results showed that transfection with dnAKT sensitized both HCC2450 and H522 cells to AZD6244 (Fig. 5B). IC50 values for AZD6244 in parental or vector-transfected HCC2450 cells were 189.6 µM and 167.2 µM, respectively, whereas the IC50 for dnAKT-transfected cells was 1.9 µM. Similarly, transfection of dnAKT reduced IC50 from 169.3 µM to 1.8 µM in H522 cells. Cell cycle analysis on those cells revealed that transfection with dnAKT led to a dramatic increase in apoptosis induction by AZD6244. In both HCC2450 and H522 cells, treatment with 10 µM of AZD6244 for 3 days resulted in only background levels of apoptotic cells (3.5%–6.5%) in parental and vector transfected cells. In contrast, the apoptotic cells increased to 22%–29% in the dnAKT transfected cells (Fig. 5C), similar to those observed in sensitive cell lines. This result demonstrates that inhibition of AKT can restore susceptibility to AZD6244 in resistant cells.
Figure 5.
Effect of dominant-negative AKT (dnAKT). (A) Lung cancer cell line HCC2450 and H522 was infected with an empty retroviral vector or a dnAKT-expressing vector. After a brief selection, dnAKT expression was detected by anti-HA-tag antibody. α-Tubulin was used as a loading control. (B) Dose-response of AZD6244 in vector-transfected and dnAKT-transfected HCC2450 cells. Cells were exposed to increasing concentrations of AZD6244 for 96 h. Cell viability was determined by SRB as described in Figure 1. (C) Apoptosis induction by AZD6244. Cells were treated with 10 µM AZD6244 for 72 h. Apoptosis was analyzed as described in Figure 2. The numbers represent percentages of apoptotic sub-G1 phase cells.
Discussion
AZD6244 is a synthetic small-molecule tyrosine kinase inhibitor that is selective for MEK1/2. It has been investigated in clinical trials for treatment of melanoma, advanced NSCLC, or colorectal cancer. Although cancer cell cytotoxicity of AZD6244 has been observed,18–20 intrinsic and acquired resistance to this compound occurs, and molecular biomarkers that are able to predict response to this agent may be useful clinically. It has been reported previously that Braf mutations, especially Braf (V600E) mutation, correlated with sensitivity to MEK inhibitors in various cancer cells.21 Activating mutations in Raf proteins, direct upstream activators of MEK, are expected to lead to elevations in MEK/ERK activity. It is thus conceivable that suppression of MEK can inhibit the pro-survival functions derived from activation of Raf proteins. Clinical studies indicated, however, that both presence of activated ERK and suppression of ERK activation after the treatment are not sufficient to predict the benefit of treatment with MEK inhibitors.17 We found that phosphorylation of ERK was suppressed by AZD6244 in both sensitive and resistant cells, suggesting that resistance to MEK inhibitors is not caused by MEK/ERK activities themselves. In fact, Solit et al. observed a similar degree of p-ERK suppression in both sensitive and resistant cells treated with MEK inhibitor CI-1040, even though the downstream molecule cyclin D1 was suppressed in sensitive cells but not in resistant cells.21 In this study, we did not observe association between AZD6244 sensitivity and Braf mutations, as all cell lines analyzed here were Braf wild type.
Recent studies showed that a lack feedback inhibition between ERK and Raf, resulting in activated MEK upregulation in Braf mutant cells are associated with resistant to AZD6244.22,23 Our study revealed that upregulation of p-MEK after treatment with AZD6244 was more dramatic in sensitive cell lines than in resistant cell lines, suggesting that feedback upregulation of MEK may not play roles in the resistance to AZD6244.
In this study, we found that AZD6244-resistant cells express higher levels of p-AKT than sensitive cells. Both the Ras/Raf/MEK/ERK pathway and the PI3K/AKT pathway mediate signals from various growth factor receptors. Interestingly, these two pathways regulate several common downstream molecules that are critical in cell survival and cell cycle progression. For example, both pathways regulate expression of cyclin D1,24,25 and both phosphorylate or regulate expression of forkhead transcriptional factors,26–28 Bad29–31 and caspase-9,32,33 all of which play critical roles in apoptosis. Thus, it is possible that, in cells with low levels of AKT, suppression of MEK is sufficient to alter the expression or function of those downstream molecules and to induce apoptosis. In cells with elevated AKT activity, however, suppression of MEK is insufficient to induce those effects because the expression or functions of the downstream molecules can be maintained by the PI3K/AKT pathway. This hypothesis is supported by our data showing that suppression of AKT with expression of a dominant-negative AKT can dramatically restore the susceptibility of resistant cells to AZD6244.
Amplification, overexpression or mutational activation in the p110 or p85 subunits of P13K is observed frequently in solid tumors. Deletion of PTEN has been observed in a widely varying group of cancers, and results in increased PI3K activity and overdependence on this pathway.34,35 Amplification and overexpression of p-AKT are common in solid cancers. Although mutations in AKT genes are rare in human cancers, AKT can be aberrantly activated via numerous mechanisms that affect elements upstream of AKT. These include mutation or amplification of PI3K, loss of PTEN function, and mutational activation of receptor kinases and oncogenes. Previous in vitro and in vivo studies implicated AKT as a participant in progression in the early stages of human lung tumorigenesis. AKT is activated by tobacco-specific carcinogens in lung epithelial cells and is constitutively active in premalignant and malignant bronchial cell lines but not in their normal counterparts.36,37 Clinical studies showed that AKT is an indicator of poor prognosis and may increase the risk of lung cancer relapse with distant metastasis.38,39
Constitutively active AKT has been shown to be related to resistance to chemotherapeutic and molecular targeted drugs, including paclitaxel, tumor necrosis factor-related apoptosis-inducing ligand and cisplatin.40–44 Strategies for overcoming chemotherapy resistance caused by the constitutively activated PI3K/AKT pathway have been investigated by using various inhibitors. For example, administration of LY294002, which inhibits the catalytic activity of p110, has been shown in mouse tumor models to induce antitumour activity and to enhance the cytotoxicity of therapeutic antimicrotubule agents.45 Derivatives of rapamycin, such as CCI-779, have been shown to inhibit the growth of several lines of PTEN-negative tumor cells in vitro and to increase the cytotoxic activity of traditional therapies.46 Together, those findings and the results reported here strongly support a role for constitutively active AKT in resistance to anticancer chemotherapies. In conclusion, our results suggest that active AKT in cancer cells may serve not only as a biomarker for predicting response to treatment but also as a molecular target for overcoming resistance to chemotherapeutic agents, including MEK inhibitor AZD6244.
Materials and Methods
Materials
AZD6244 was provided by AstraZeneca Pharmaceuticals. It was dissolved in dimethyl sulfoxide (DMSO) at 25 mM and stored at −80°C. Antibodies against caspase-3, p-AKT (Ser473), p-AKT (Thr308), p-MEK, MEK, p-Erk, pTEN, p-p38 and p-mTOR, and the AKT kinase assay kit, were purchased from Cell Signaling Technology, Inc., Raf-B antibody was purchased from Santa Cruz Biotechnology, Inc., and hemagglutinin (HA)-tag and α-tubulin antibodies, protease inhibitor cocktail, and sulforhodamine B (SRB) from Sigma Chemical Corporation. Protein assay materials were purchased from Bio-Rad, and Geneticin from Life Technologies, Inc.
Cell culture
All lung cancer cell lines were maintained at 37°C in high-glucose Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS) containing 100 µg/ml ampicillin and 0.1 mg/ml streptomycin, in a humidified atmosphere containing 5% CO2 and 95% air.
Cell viability assay
Cell viability was assessed by using the Sulforhodamine B (SRB) assay; each assay was carried out in quadruplicate. Lung cancer cells were seeded at about 3,000 per well in 96-well plates and incubated for 24 h in 10% FBS-supplemented DMEM. The cells were then treated with AZD6244 at the indicated concentrations. Cells treated with DMSO were used as controls. Cells were fixed 96 h after treatment by adding 50 µL of 10% trichloroacetic acid at 4°C for 1 h. They were then stained with 70 µL of 0.4% SRB for 60 min and washed with 1% acetic acid; 200 µL of Tris base (10 mmol/L; pH, 10.5) was added. Absorbance readings at 570 nM were determined by using a microplate analyzer. The relative survival rate was calculated by the following equation: relative cell viablity (%) = ODT/ODC × 100% (ODT is the absorbance of treatment groups and ODC the absorbance of control groups). Median inhibitory concentrations (IC50 values) were determined by using CurveExpert 1.3 software according to dose-response curves. Experiments were repeated at least three times.
Colony-forming assay
Cells were seeded into 60-mm dishes (2,000 cells/dish in triplicate) and allowed to attach overnight to initiate log-phase growth. The cells were then cultured in 10% FBS-supplemented DMEM containing various concentrations of AZD6244 (0, 1, 5, 10, 50 or 100 µM) for 96 h, and then in fresh drug-free medium for an additional 5–7 days to allow clonogenic growth. At the end of this period, the plates were washed with cold phosphate-buffered saline solution (PBS) and the contents stained with 4% crystal violet in 50% methanol. Colonies of >50 normal-appearing cells were then counted via microscopy. Experiments were repeated at least three times.
Western blot analysis
Whole-cell lysates were prepared by washing the cells with PBS and subjecting them to lysis with Laemmli sample buffer supplemented with protease inhibitor cocktail. After the lysates were sonicated for 15 sec, the protein concentrations were quantified using the Bio-Rad protein assay kit. Equivalent proteins were loaded, separated by 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes at 80 V for 2 h. The membranes were blocked for 1 h with 5% nonfat dried milk in Tris buffer containing 0.1% Tween (TBST) and probed with diluted primary antibody at 4°C overnight. The membranes were then washed three times in TBST buffer and probed with horseradish peroxidase-linked goat anti-mouse or goat anti-rabbit IgG, and the immunoreactive bands were visualized with the enhanced chemiluminescent detection system (Ampharmacia Biotech). Experiments were repeated at least three times.
Plasmid transfection
The cDNA encoding a dominant-negative form of AKT1 (dnAKT) (mutations: K179A, T308A and Y473A) was cloned into pLNCX vector (Strategene, La Jolla, CA) to generate plasmid pLNCX-dnAKT. The empty pLNCX vector was used as control. Plasmids were isolated and purified by using a plasmid maxi kit from Qiagen. The day before transfection, 1 × 105 HEK-293 phoenix cells were plated in 35-mm plates. The cells were transfected with pLNCX-dnAKT or pLNCX by using the FuGENE HD transfection reagent (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Cell culture medium was collected 48 h after transfection and filtered through a 0.45-µm filter. The medium was stored at −80°C or used fresh. Target HCC2450 or H522 cells were seeded at 1 × 105 cells/plate in 35-mm plates and allowed to attach overnight. The following day, 3 ml of medium containing retrovirus was added to each dish. Cells were selected for growth in 1,000 µg/ml G418. Surviving cells were pooled together after approximately 3 weeks, and subsequent clones were isolated by limiting dilution cloning.
Cell cycle and apoptosis assay
Cells were harvested by trypsinization. They were washed twice in cold PBS, and then were fixed with ice-cold 70% methanol and incubated at 4°C overnight. Cells were then washed with PBS and incubated with 25 µg/ml propidium iodide containing 30 µg/ml RNase for 30 min at room temperature. Cells were analyzed on an EPICS Profile II flow cytometer (Coulter Corp., Hialeah, FL) with the Multicycle Phoenix Flow Systems program (Phoenix Flow Systems, San Diego, CA). Experiments were repeated at least three times.
Akt kinase activity assay
Cell were washed twice with PBS, subjected to lysis in cell lysis buffer, and sonicated for 15 sec. The extracts were centrifuged to remove cellular debris, and the protein concentrations of the supernatants were determined by using the Bio-Rad protein assay reagent. Two hundred µl cell lysate sample was incubated with 20 µl immobilized anti-Akt antibody at 4°C overnight with gentle rocking. The resulting immune-precipitates were washed three times with lysis buffer and twice with Akt kinase buffer. Kinase assays were performed for 30 min at 30°C under continuous agitation in kinase buffer containing 200 µM ATP and 1 µg of GSK-3 fusion protein. Reaction products were resolved by 10% SDS-PAGE followed by western blotting with an anti-phospho-GSK-3α/β antibody (Cell Signaling Technology, Inc., Danvers, MA) according to the manufacturer’s instructions for the nonradioactive Akt kinase assay. Experiments were repeated at least three times.
Statistical analysis
Data were expressed as the mean ± SE and calculated as the mean values with 95% confidence intervals. Statistical comparison between experimental groups was performed by two-way ANOVA test using Microsoft Excel software. Values of p < 0.05 were considered statistically significant.
Acknowledgements
We thank Katy Hale for editorial review. This work was supported by a Sponsored Research Grant from AstraZenica Pharmaceutics, the National Cancer Institute Specialized Program of Research Excellence (SPORE) Grant CA70907 (J. Minna and J. Roth), R01 Grant CA092487 (B. Fang), and Cancer Center Support Grant CA16672.
Abbreviations
- NSCLC
non-small cell lung cancer
- DMSO
dimethyl sulfoxide
- SRB
sulforhodamine B
- IC50
median inhibitory concentration
- dnAKT
dominant-negative AKT
References
- 1.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 2.Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J of Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 7.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 8.Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 10.Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mountzios G, Planchard D, Besse B, Validire P, Girard P, Devisme C, et al. Mitogen-activated protein kinase activation in lung adenocarcinoma: a comparative study between ever smokers and never smokers. Clin Cancer Res. 2008;14:4096–4102. doi: 10.1158/1078-0432.CCR-07-4150. [DOI] [PubMed] [Google Scholar]
- 12.Vicent S, López-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchón C, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer. 2004;90:1047–1052. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41:123–130. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 14.Blackhall FH, Pintilie M, Michael M, Leighl N, Feld R, Tsao MS, et al. Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003;9:2241–2247. [PubMed] [Google Scholar]
- 15.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 16.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Boerner SA, Winkler JD, Lorusso PM. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta. 2006;1773:1248–1255. doi: 10.1016/j.bbamcr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Haass NK, Sproesser K, Nguyen TK, Contractor R, Medina CA, Nathanson KL, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–239. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 19.Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;8:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 20.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 21.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, et al. V600EBRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA. 2009;106:4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friday BB, Yu C, Dy GK, Smith PD, Wang L, Thibodeau SN, et al. BRAF disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008;68:6145–6153. doi: 10.1158/0008-5472.CAN-08-1430. [DOI] [PubMed] [Google Scholar]
- 24.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci USA. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 27.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 28.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 31.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 32.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 33.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 34.Kokubo Y, Gemma A, Noro R, Seike M, Kataoka K, Matsuda K, et al. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA) Br J Cancer. 2005;92:1711–1719. doi: 10.1038/sj.bjc.6602559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi Y, Zhang J, Murga C, Yu H, Koller E, Monia BP, et al. PTEN, but not SHIP and SHIP2, suppresses the PI3K/Akt pathway and induces growth inhibition and apoptosis of myeloma cells. Oncogene. 2002;21:5289–5300. doi: 10.1038/sj.onc.1205650. [DOI] [PubMed] [Google Scholar]
- 36.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West KA, Linnoila IR, Belinsky SA, Harris CC, Dennis PA. Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3'-kinase/Akt pathway in vitro and in vivo. Cancer Res. 2004;64:446–451. doi: 10.1158/0008-5472.can-03-3241. [DOI] [PubMed] [Google Scholar]
- 38.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Tsurutani J, Fukuoka J, Tsurutani H, Shih JH, Hewitt SM, Travis WD, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 40.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for antiestrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- 42.Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NFkappaB activation and cFLIP(L) upregulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 43.Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J Biol Chem. 2003;278:23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 44.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 45.Hu L, Hofmann J, Lu Y, Mills GB, Jaffe RB. Inhibition of phosphatidylinositol 3'-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 46.Jin W, Wu L, Liang K, Liu B, Lu Y, Fan Z. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer. 2003;89:185–191. doi: 10.1038/sj.bjc.6601048. [DOI] [PMC free article] [PubMed] [Google Scholar]