Abstract
As the mechanisms for learning and memory are elucidated, modulation of learning and memory becomes a central issue. We studied the modulation of learning and memory by investigating the circadian regulation of short- and long-term sensitization of the siphon withdrawal reflex in Aplysia. We found that Aplysia exhibited diurnal and circadian rhythms of long-term sensitization (LTS) with significantly greater LTS occurring when animals were trained and tested during the day relative to those trained and tested at night. In contrast to the modulation of LTS, short-term sensitization was not regulated by the circadian clock. Time of training rather than time of testing determined the circadian rhythm of LTS. Animals trained during the subjective day demonstrated LTS when tested during either the day or the night. Conversely, when animals were trained during the night, LTS was not observed when animals were tested either at night or during the day. Thus, the circadian rhythm of LTS is a rhythm in learning rather than a rhythm in recall. The threshold required to elicit siphon withdrawal and the duration of siphon withdrawal were not regulated by the circadian clock. These results indicate that the circadian oscillator exerts strong modulatory influences on one form of long-term memory in Aplysia.
How organisms learn has been of interest for centuries as people seek to understand and improve learning and memory abilities. One aspect of understanding learning and memory is determining how these processes are normally modulated. What conditions modulate learning and memory, and how does regulation occur? General health, motivation, age, stress, sleep patterns, and time of day may modulate learning and memory. The circadian clock regulates many behavioral and physiological processes, making it likely that circadian modulation of learning and memory also occurs (1).
Circadian modulation of learning and memory has been investigated with varied results. Some studies have indicated time of day influences memory in humans (2–4), whereas other studies have found little impact of time of day on memory (5). Researchers studying hippocampus-dependent learning found that rats trained at midday showed decreased retention compared with animals trained earlier or later (6). However, other researchers found no time-of-day effects on multiple learning tasks (7). Furthermore, two laboratories studying contextual fear conditioning in mice reported different effects of the circadian clock on learning and memory (8, 9). The issue of circadian modulation of learning and memory remains to be determined.
Indirect evidence for circadian modulation of learning and memory comes from in vitro studies of long-term potentiation. Researchers have documented diurnal and circadian differences in long-term potentiation in the hippocampus (10, 11). Additional studies have also found that excitability of CA3 neurons is regulated diurnally (12). Thus, some mechanisms exist for learning and memory to be rhythmic. Experiments demonstrating that consolidation of learning or recall of events can be disrupted by phase-shifting the circadian clock also suggest that interactions occur between learning and memory and the circadian clock (13–16). However, disruption of learning in this case could be due to stress or other factors accompanying phase shifting.
Aplysia have proven to be advantageous for studies of learning and memory at the behavioral, cellular, and molecular levels. Sensitization of the siphon-withdrawal reflex is a nonassociative form of learning by which a noxious stimulus increases the duration of the reflex. Many of the signaling processes involved in short-term sensitization (STS) and long-term sensitization (LTS) are well understood (17–20), making Aplysia a useful model for studying the circadian modulation of learning and memory. We investigated circadian modulation of both STS and LTS by using the siphon-withdrawal reflex. We found that the circadian clock regulated LTS but not STS. Moreover, the influence of the circadian clock on learning seemed to depend on the time of training rather than the time of testing. Our results suggest that the circadian clock might play an important role in the capacity to form long-term memory in vivo.
Methods
Aplysia californica (100–150 g) were housed in artificial seawater at 15°C and entrained to light–dark (LD) 12:12 h cycles for at least 10 d before any behavioral manipulation. Entrainment of the ocular circadian clock to LD cycles occurs within a few days (21). To examine the effects of LD cycles (Figs. 3, 4, 5, 6), animals were entrained and then transferred to constant darkness (DD) at least 1 d before the start of behavioral analysis. The circadian activity and ocular rhythms (in vitro and in vivo) display normal free-running rhythms during the 2nd day of DD, with the phase of these rhythms similar to the phase of the prior LD cycles because the periods of the free-running rhythms in Aplysia are close to 24 h (1, 22). Thus, circadian time (CT) approximates the previous zeitgeber time (ZT) for several days in DD. All experiments in darkness were performed under dim red light.
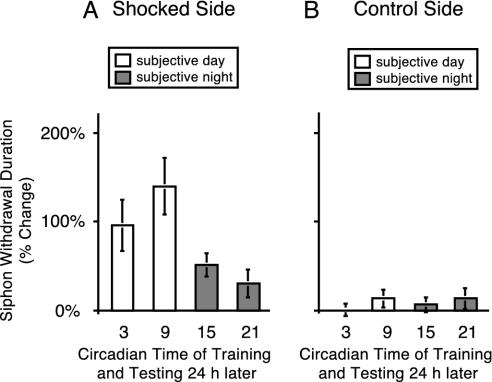
Fig. 3.
Rhythm in LTS expressed in DD. (A) Animals were entrained by LD cycles and then trained for LTS during the second day of DD. Animals were trained at CT 3, 9, 15, and 21 (n = 12 for CT 9 and CT 21, n = 6 for CT 3 and CT 15) and then tested 24 h after training. LTS expressed a significant rhythm under constant conditions (ANOVA, P < 0.02). LTS was not significant on the trained side of the animal at CT 21 (t test, P > 0.1). Tukey–Kramer post hoc analyses determined that LTS at CT 9 was significantly different from LTS at CT 15 or CT 21 (P < 0.05). (B) Training produced unilateral LTS. No rhythm was observed when the unstimulated control side was tested (ANOVA, P > 0.8).
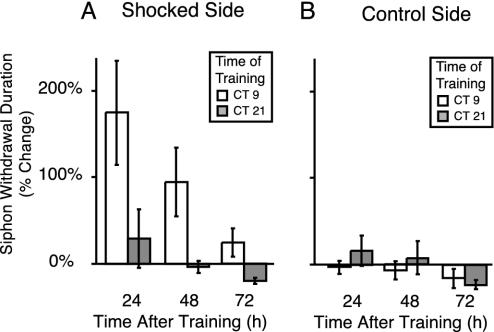
Fig. 4.
The circadian rhythm of LTS persisted for several days in constant conditions. (A) A subset of the animals shown in Fig. 3 was used to determine whether the rhythm of LTS persisted under constant conditions. Posttraining measurements were taken at 48 and 72 h from animals maintained in DD that had been trained either in the subjective day (CT 9; n = 6) or subjective night (CT 21; n = 6). A significant difference in LTS between animals trained at CT 9 and CT 21 was detectable at 24, 48, and 72 h after training (Kruskal–Wallis, P < 0.01; Nemenyi post hoc analyses, P < 0.05). Thus, the difference in LTS between animals trained and tested in the day versus the night persisted in constant conditions for at least3din animals that had been in constant conditions for 4 d. Significant LTS was not observed on the trained side of the animal compared with the control side of the animal for animals trained at CT 21 and tested 24 h later (t test, P > 0.7). (B) No circadian differences were observed on the untrained side of the animal (ANOVA, P > 0.7).
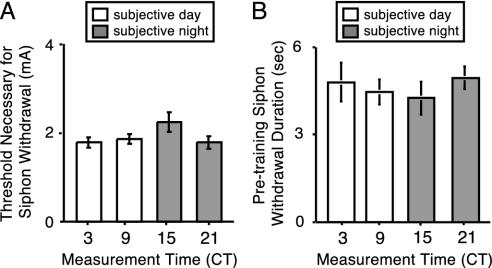
Fig. 5.
Baseline behavior of siphon withdrawal was not rhythmic in constant conditions. Pretraining baseline measurements of the threshold current required to elicit siphon withdrawal and the duration of the behavior are shown for animals examined before training at different times on the second day of DD. (A) The threshold current was not significantly different throughout the circadian cycle (ANOVA, P > 0.18). (B) The baseline pretraining siphon withdrawal duration did not significantly differ with time of day (ANOVA, P > 0.8). The number of animals is the same as in Fig. 3.
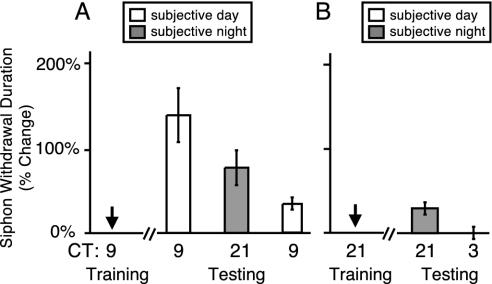
Fig. 6.
The circadian rhythm of LTS was due to time of training (learning), not the time of testing (recall). (A) To determine whether the time of training established the rhythm in LTS, animals were trained (LTS) on the second day of DD at CT 9. Animals were tested at either CT 21 (36 h after training; n = 5) or CT 9 (48 h after training; n = 5). Data for animals trained at CT 9 and tested 24 h later have been replotted from Fig. 3. Animals trained at CT 9 and tested 36 h later at CT 21 demonstrated robust LTS (t test, P < 0.01) that was significantly different from animals trained at CT 21 and tested at CT 21 (24 h after training; in B, one-tailed t test, P < 0.05). The decrease in memory over time in this experiment was similar to that observed in the earlier experiments (Figs. 1 and 4). (B) To determine whether the time of testing established the circadian rhythm in LTS, animals were trained (LTS) on the second day of DD at CT 21 and tested at CT 21 (24 h after training; n = 3), or tested 30 h after training at CT 3 (a time when learning has been observed; n = 3). A small, but not significant, amount of LTS was seen as before (Fig. 2) for animals trained and tested at CT 21 (compared with control side of the animals; t test, P > 0.14). Animals trained at CT 21 and tested at CT 3 showed no significant LTS (t test, P > 0.9).
Training and testing for sensitization of the siphon-withdrawal reflex were done as described (23, 24). The change in the duration of siphon withdrawal after training relative to its value before training was used to measure the memory for sensitization training. Portions of both parapodia were removed 7 d before training to visualize siphon withdrawal. Animals were fed seaweed 5 d before training and then isolated from food (25). To elicit the siphon-withdrawal behavior, electrodes were implanted on each side of the tail 3 d before training. The threshold current required to elicit siphon withdrawal was determined for each side of the animal. Siphon withdrawal was elicited by current from an AC stimulator (1504 Isolated Variable AC Line Supply, Global Specialties, Cheshire, CT) through implanted electrodes. Pretraining and posttraining siphon-withdrawal durations were elicited by using a 20-msec shock at 2× threshold current (usually ≈4 mA). Five pretraining measurements (interstimulus interval, 10 min) of siphon withdrawal duration were made on each side 30 min after threshold determination. Siphon-withdrawal duration was measured from the beginning of siphon contraction to the point at which the siphon began to relax. The stimulus used to elicit pretraining siphon withdrawal did not cause habituation. Pretraining measurements and subsequent posttraining measurements were made by a single observer who was blind to experimental manipulations. The order of pretraining and posttraining tests between sides of the animal was determined independently with respect to determination as to the side of the animal that received sensitization training.
After measuring the pretraining duration of siphon withdrawal, sensitization training involved electrical stimulation by an electrode placed on the skin on one side of the body. LTS training consisted of four 10-sec blocks of 10 shocks (500 msec, 60 mA delivered at 1 Hz) with 30-min intervals between blocks of shocks. STS training consisted of a single 10-sec block of ten 500 msec, 60-mA shocks delivered at 1 Hz. Posttraining measurements of siphon withdrawal duration were made 24 h after the end of LTS training and 10 or 20 min after the end of STS training. All measurements of LTS and STS were expressed as percent changes from pretraining measurements.
The sensitizing stimulus elicited inking responses during training from all animals. No differences were observed in the immediate response of the animal to sensitization training with respect to time of day. Sensitization training was conducted by multiple individuals throughout the course of this study. Sensitization training of the animals was never done by the observer responsible for measuring siphon-withdrawal durations. In experiments conducted in DD (Figs. 3, 4, 5, 6), the trainer was blind as to the CT of the animal and the hypothesis being tested.
All comparisons for parametric data were made by using Student's t test, ANOVA, or Bonferroni t test; P values of <0.05 were considered significant. Post hoc analyses were done when appropriate with the Tukey–Kramer test. Nonparametric data were analyzed by the Kruskal–Wallis ANOVA test followed by the Nemenyi test for post hoc analyses.
Results
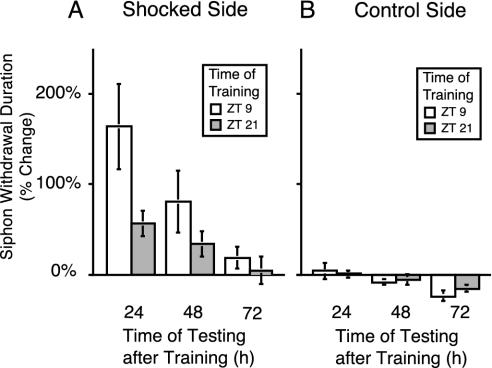
LTS Expressed a Diurnal Rhythm. To investigate diurnal modulation of memory formation, Aplysia entrained to LD cycles received LTS training during the day at ZT 9 (ZT 0 is lights on) or during the night at ZT 21. LTS training consisted of four trains of shocks to the animal's side for a period of 1.5 h (see Methods). Posttraining measurements of the duration of siphon withdrawal were made 24, 48, and 72 h after training. Siphon withdrawal was elicited by a current pulse (2× threshold) through implanted electrodes on either side of the tail. Learning is expressed as the ratio of pretraining and posttraining siphon-withdrawal durations. Animals trained at ZT 9 or ZT 21 and tested 24 h later displayed long-term memory, as seen by the increase in the duration of siphon withdrawal. Animals trained at ZT 9 exhibited significantly greater LTS (≈3-fold difference) compared with animals trained at ZT 21 (Fig. 1A). Behavioral training produced unilateral LTS, because no sensitization was observed on the unstimulated side of the animal (Fig. 1B). Furthermore, no diurnal differences in siphon-withdrawal duration were observed when the untrained side of the animal was tested. Diurnal differences in LTS persisted for several days. Animals trained at ZT 9 demonstrated significant increases in siphon-withdrawal duration for 3 d after training, whereas animals trained at ZT 21 exhibited no memory on the third day after training (Fig. 1 A).
Fig. 1.
Diurnal rhythm in LTS. (A) Animals were entrained to the LD cycle and then trained for LTS at ZT 9 (day; n = 11) or ZT 21 (night; n = 9). Means and standard errors of changes in posttraining siphon-withdrawal duration compared with pretraining baseline levels are plotted. Animals trained during the day showed significantly increased LTS compared with animals trained at night 24 h after training (Bonferroni t test, P < 0.05). (B) No LTS was produced on the unstimulated control side of the animal. No diurnal differences were observed in the responses between animals trained at ZT 9 and ZT 21 when the control side was tested (ANOVA, P > 0.6).
It is possible that the difference in LTS observed at ZT 9 and ZT 21 is due to faster memory decay in animals trained and tested at night, although initially animals trained during the night or the day had similar amounts of learning. To determine whether this was the case, the data of Fig. 1 were used to estimate the rate of memory decrease. For animals trained during the day, the rate of decrease in LTS obtained by using a linear fit of the data were -2.45% change in siphon-withdrawal duration per h, and the rate of decrease of LTS for animals trained at night was significantly less, -0.75 ± 0.02% change in siphon withdrawal duration per h. Thus, less memory at night does not appear to be due to faster rates of memory loss at night, but it rather suggests different amounts of initial learning. Memory decay during the 24- to 72-h period after training can be calculated another way with the data normalized to the amount of LTS observed at 24 h. If the data are analyzed this way, no significant difference occurs in the rate of memory decay between animals trained and tested during the day (-1.54 ± 0.18) and the night (-1.34 ± 0.04). Thus, both analysis methods of memory decay lead to the same conclusion, that less LTS observed for animals trained and tested during the night cannot be due to a greater rate of memory decay at night.
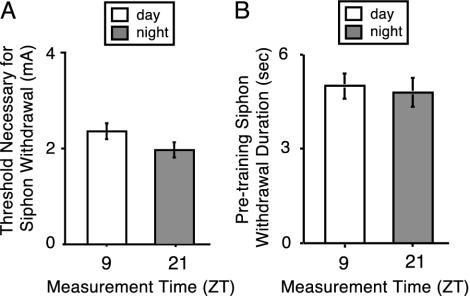
No Diurnal Regulation of the Baseline Behavior of Siphon Withdrawal. It is possible that the diurnal difference in LTS (Fig. 1) could be due to light- or dark-induced differences in either induction or expression of baseline siphon-withdrawal behavior. If light (or dark) regulates the mechanisms that trigger siphon withdrawal, then the threshold required to elicit siphon withdrawal should express a rhythm similar to that shown in Fig. 1. No significant difference was found in the threshold required to elicit the siphon-withdrawal response between animals tested at ZT 9 and ZT 21 (Fig. 2A). On the other hand, if light regulates the expression of the behavior, then the pretraining baseline siphon-withdrawal duration should express a rhythm. No significant differences were observed in the duration of siphon withdrawal between ZT 9 and ZT 21 (Fig. 2B). Together, these results suggest that the diurnal differences observed in LTS were due to modulation of learning and memory events rather than to diurnal regulation of the behavioral expression of siphon withdrawal.
Fig. 2.
Baseline behavior of siphon withdrawal was not diurnally regulated. Pretraining baseline measurements of the threshold necessary to produce the siphon withdrawal and siphon-withdrawal duration are shown for animals examined at ZT 9 and ZT 21. (A) The threshold current required to elicit siphon withdrawal was not significantly different in animals measured in the daytime compared with dark time (t test, P > 0.18). (B) The duration of the baseline siphon-withdrawal response before LTS training measured at 2× threshold current did not significantly differ with time of day (t test, P > 0.75). The data of this figure were the pretraining baseline measurements taken for the experiments shown in Fig. 1.
LTS Expressed a Circadian Rhythm. To investigate whether the diurnal modulation of LTS was a direct effect of the LD cycle, animals were trained and tested in DD. Animals were subjected to LTS training during the 2nd day of DD and tested 24 h after training. LTS varied significantly depending on when LTS training was conducted during the circadian cycle (Fig. 3A). As expected from the diurnal rhythm of LTS, sensitization was highest in animals trained during the subjective day compared with those trained during the subjective night. These results suggest that the rhythm of LTS was caused by the circadian clock. No circadian differences in duration of siphon withdrawal were detected on the control side of the animals after 2 d in DD.
If the circadian clock was responsible for the observed rhythm in LTS, then the rhythm should persist under constant conditions. To determine whether the differences in LTS persisted for more than 2 d in DD, behavioral measurements were taken at 48 h and 72 h after LTS training. LTS was significantly higher for animals trained in the subjective day than in the subjective night for at least 3 d after training (Fig. 4). Thus, the rhythmic modulation of LTS persisted in constant conditions for at least 3 d in animals that had been maintained in DD for 4 d. These results further support the conclusion that the rhythm in LTS is a circadian rhythm. Memory decay for animals trained in the subjective day in DD (-1.61 ± 0.04% change in LTS per h) was similar to the memory decay seen for animals trained during the day under LD cycles. The rate of memory decay could not be calculated for animals trained and tested at CT 21 because significant LTS was not observed 24 h after training at CT 21.
As a control for any possible time of day cues other than the entraining LD cycle that could set the phase of the rhythm in DD, one group of animals in the LTS experiments conducted in constant conditions was entrained to a 7:00 a.m. to 7:00 p.m. lights-on cycle, one group of animals was entrained to a reverse LD cycle (7:00 p.m. to 7:00 a.m. lights on), and two groups of animals were entrained to a third LD cycle (11:00 a.m. to 11:00 p.m. lights on). In each group, LTS was higher during the subjective day than the subjective night (data not shown). Thus, the phase of the previous LD-entraining cycle determined the phase of the LTS rhythm. The results of these experiments taken all together demonstrate that the circadian clock modulates LTS.
Baseline Behavior of Siphon Withdrawal Did Not Express a Circadian Rhythm. To determine whether the circadian modulation of LTS was due to regulation of the expression of siphon-withdrawal behavior, baseline properties of siphon-withdrawal behavior were analyzed in DD as they were under LD conditions (Fig. 2). As in the experiments conducted in LD, no significant difference was found in the threshold current required to elicit the siphon-withdrawal response between animals trained in the subjective day and the subjective night (Fig. 5A). Additionally, no circadian differences were observed between the duration of siphon withdrawal in the subjective day and night (Fig. 5B). The lack of rhythms in siphon-withdrawal behavior itself suggests that the circadian rhythm in LTS was due to regulation of a learning event and not the result of circadian changes in the expression of the memory.
Circadian Rhythm of Learning Depended on Time of Training, Not Time of Testing. The circadian rhythm seen in LTS could be due to an underlying rhythm of learning in the animal, a rhythm of recall seen during behavioral testing or a combination of both. To determine the contribution of training time, animals were trained at CT 9, a time when memory has been shown to occur days later (Fig. 4), and then tested either at CT 21, a time when learning was not observed (Fig. 3; 36 h after training) or at CT 9 (48 h after training), a time when learning was observed. Animals trained at CT 9, a time when significant learning occurs, and tested 30 h later at CT 21 showed robust LTS (Fig. 6A) that was significantly different from animals trained at CT 21 and tested 24 h later at CT 21 (Figs. 3 and 6B). Thus, memory can be recalled during the subjective night when animals were trained during the subjective day. This result demonstrates that the time of training plays an important role in determining the amount of memory formed. The smaller response of animals trained at CT 9 and tested at CT 21, compared with animals trained and tested 24 h later at CT 9, was due largely to the decay of memory that normally occurs.
In a second set of experiments examining the relative contributions of training and testing times, animals were trained at CT 21, a time when significant learning was not observed 24 h later (see Fig. 3), and tested at CT 3, a time when increases in LTS were previously observed (Fig. 3). When animals were trained at CT 21 and tested at CT 3, no significant learning was observed (Fig. 6B). As previously observed, a small, although not significant, amount of LTS occurred (see Fig. 3) when animals were trained and tested at CT 21 (Fig. 6B). The lack of significant LTS at CT 3 was not due to the 30-h posttraining testing time because the memory persists for ≈72 h (Fig. 4). Thus, testing animals at a time when recall has been shown to occur (CT 3) did not reveal learning of animals trained at CT 21. The complementary results shown in Fig. 6 demonstrate that the circadian modulation of learning and the formation of memory depended on the time of training of the animal. Although the time of testing does not appear to be a major factor, it is still possible that a rhythm in recall might play a minor role.
Additionally, these results support the conclusion that the circadian clock does not regulate the baseline behavior of siphon withdrawal. If the behavior itself expressed a circadian rhythm, then one would have expected animals trained in the subjective day to express LTS when tested during the day and not express LTS when animals were tested at night. However, when animals were trained during the subjective day (CT 9), the animals exhibited robust LTS when tested either in the subjective day (CT 9) or the subjective night (CT 21; Fig. 6A).
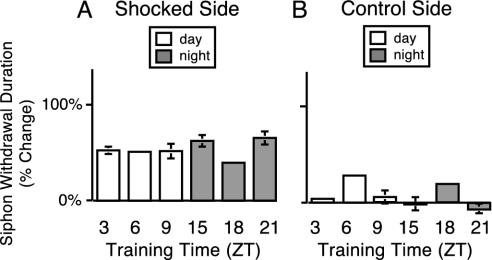
STS Did Not Express a Diurnal Rhythm. Major differences exist in the mechanisms involved in STS and LTS, such as the requirement for transcription and translation in LTS but not STS (20). However, some early induction steps, such as the release of serotonin (5-HT) and engagement of the cAMP-signaling pathway, are involved in the formation of both LTS and STS (20, 26, 27). To investigate whether the circadian clock modulates STS, animals were subjected to STS training at different times throughout the LD cycle. Behavioral measurements were made before and 20 min after the end of a single training session (10-sec block of ten 500-msec, 60-mA shocks delivered at 1 Hz). Although animals exhibited STS at all times tested, a diurnal rhythm in STS was not expressed (Fig. 7A). Thus, all types of learning in Aplysia are not modulated by the circadian clock. Additionally, no diurnal differences in STS were seen on the control side of the animal (Fig. 7B). To determine whether a rhythm might exist at earlier time points after training, animals were tested 10 min (instead of 20 min as above) after the end of a single short-term training session at ZT 9 and ZT 21. No diurnal rhythm in STS was apparent in these experiments either (n = 5 at ZT 9, n = 6 at ZT 21; P > 0.85; data not shown). The lack of a rhythm in STS is consistent with earlier conclusions (Figs. 2 and 4) that the withdrawal reflex itself does not express a rhythm.
Fig. 7.
STS was not rhythmic. (A) Animals received STS at different times in the LD cycle. Mean changes in siphon-withdrawal duration 20 min after training compared with pretraining baseline levels are plotted (n = 6–7atZT 3, 9, 15, and 21 and n = 2 at ZT 6 and 18). STS was not diurnally regulated (ANOVA, P > 0.4). (B) No diurnal differences were observed in responses of the control side of the animal (ANOVA, P > 0.09).
Discussion
The present research extends our understanding of learning and memory by demonstrating circadian modulation of a well studied, nonassociative form of long-term memory. Some studies in mammals have suggested circadian modulation of learning (6, 8, 9). LTS demonstrated both diurnal and circadian rhythms in Aplysia (Figs. 1 and 3). Differences in LTS persisted for 3 days after training between animals trained in the day and animals trained at night (Figs. 1 and 4). Animals trained during the subjective day showed significantly higher retention than animals trained during the subjective night.
The rhythm in LTS did not appear to be due to a rhythm of expression or a rhythm of the siphon-withdrawal behavior itself, because STS, siphon-withdrawal duration, and the threshold required to elicit siphon withdrawal were not rhythmic (Figs. 2, 5, and 7). Also, the finding that animals displayed sensitization behavior both during the day and the night when animals were trained during the day further demonstrates that the expression of the behavior is not responsible for the LTS rhythm (Fig. 6A). Furthermore, the rhythm in LTS did not appear to be due to a rhythm in extinction or memory decay (Figs. 1. and 4).
The rhythm in LTS was established mainly by training and not recall, because time of training rather than time of testing determined the memory (Fig. 6). Animals trained during the subjective day demonstrated significant learning at all times tested. Conversely, animals trained during the subjective night displayed no LTS when tested during either the subjective day or night. The results from the current study differ from research in mice in which a rhythm of recall was observed (9). The differences in these results might be due to species differences or the particular types of behavior analyzed. Thus, rhythms in learning of other behaviors in Aplysia might reveal rhythms in recall.
Short-term memory was not regulated by the circadian clock. The lack of circadian regulation of short-term memory in Aplysia is consistent with previous research in mammals demonstrating that short-term memory is not susceptible to disruption by phase shifts (28). Thus, short-term learning is neither normally modulated by the circadian clock nor subject to interruption by changes in the circadian clock. These results provide further delineation between the mechanisms for long-term and shortterm learning and memory.
During learning and the formation of memory, information may be stored in a time-stamped manner such that peak recall may occur in 24-h intervals after training or the time of day may become a contextual component of learning (29–32). The rhythm of LTS is a case of modulation by the circadian clock and not a contextual time-stamping event, because animals trained in the subjective day exhibited LTS when tested either in the subjective day or night.
How does the circadian clock modulate learning and memory? This question of circadian regulation raises issues at both the cellular and subcellular levels. In Aplysia, rhythms in feeding, locomotor activity, serotonin levels in the hemolymph, and ApC/EBP gene expression have been observed (22, 25, 33–35). However, the only documented circadian oscillator is located in the eye (36). Although the possibility of extraocular circadian oscillators has been suggested, additional oscillators have not been localized (37–39). Circadian oscillators in the eye could modulate learning and memory by hormonal or neural mechanisms. The eye is neurosecretory and could regulate behavior through factors secreted in the hemolymph (40). Levels of 5-HT in the hemolymph express a diurnal rhythm, but 5-HT levels are constant in DD (34), whereas the rhythm in LTS persists in DD. Thus, the diurnal rhythm of 5-HT could not account for the circadian rhythm of LTS, but other humoral factors remain a possibility. Additionally, the eye could generate the rhythm in LTS through neural projections from the circadian pacemaker cells to the cerebral ganglia and the pleural-pedal ganglia, which are anatomically the site of long-term facilitation (18, 24, 41).
Modulation of LTS by the circadian clock could occur at any point in the pathway by which LTS is induced. For example, the amount of 5-HT released by LTS training or the site of 5-HT release after LTS training may be regulated by the circadian clock. Although tail shocks have been shown to produce 5-HT release in the vicinity of both sensory neuron cell bodies and synaptic regions (27), no study has analyzed differential 5-HT release during the day compared with the night. Activation of 5-HT receptors in different locations or activation of different types of 5-HT receptors could be also regulated by the circadian clock (42).
Other potential mechanisms for circadian regulation of learning and memory center on second messenger signaling pathways or the regulation of transcription factors involved in long-term learning and memory. During LTS, 5-HT induction of the cAMP-signaling pathway activates protein kinase A and mitogen-activated protein kinase, resulting in gene expression mediated by the cAMP-responsive-element-binding protein transcription factor (20, 43). Any step in this pathway could be regulated in Aplysia by the circadian clock. The circadian clock has been shown to regulate the activity of mitogen-activated protein kinase (44–46), cAMP-responsive-element-binding protein activation, and cAMP-responsive-element-mediated gene expression (47–49) in other organisms. Downstream, immediate early genes could be regulated by the circadian clock, such as the transcription factor ApC/EBP, which was recently shown to be rhythmically expressed in the eye of Aplysia (35).
What is the adaptive significance of circadian regulation of learning? In this regard, two aspects of our results are striking. Aplysia demonstrated long-term learning primarily during the day, whereas short-term learning occurred both during the day and the night. What is the significance of LTS predominantly occurring during the day and not at night? Two general possibilities arise, one mechanistic and one ecological. First, Aplysia learn primarily during the day because learning during the night may interfere in some way with consolidation of memory initiated in the day. Sleep deprivation can lead to some impairment in learning, particularly in regard to sleep deprivation after new learning paradigms (50, 51). The restriction in learning at night is limited to some types of learning and memory, because Aplysia do exhibit short-term memory at night (Fig. 7). It will be important in future studies to determine whether other types of long-term memory in Aplysia are modulated by the circadian clock and, if so, whether these occur primarily during the day or night.
Second, Aplysia learn during the day because much more important information (i.e., predators, feeding activity, etc.) occurs during the day than during the night. Because A. californica is diurnal, these animals may be geared toward higher levels of learning and memory during the day. Using this line of reasoning, one might suppose that nocturnal animals would demonstrate increased learning and memory during the night when these animals are active. Indeed, one research group examining contextual fear conditioning in mice found that the mice learned significantly better during the early night than during the early day (8). It will be interesting to study learning and memory in nocturnal species of Aplysia.
Future research examining diurnal and nocturnal animals and different behaviors will help define the role of circadian modulation of learning and memory. Moreover, mechanistic studies of circadian modulation of learning and memory may help us understand learning and memory itself and how the circadian clock is able to regulate outputs.
Acknowledgments
We thank Ms. Marta Nunez-Regueiro for assistance with this research and Drs. Greg Cahill and Terry Walters for helpful discussions and comments. This work was supported by National Institute of Mental Health Grant MH 41979 and National Institute of Neurological Disorders and Stroke Grant NS 28462.
Abbreviations: 5-HT, serotonin; DD, constant darkness; CT, circadian time; LD, light–dark; LTS, long-term sensitization; STS, short-term sensitization; ZT, zeitgeber time.
References
- 1.Takahashi, J. S., Turek, F. W. & Moore, R. Y. (2001) Circadian Clocks (Plenum, New York).
- 2.Maury, P. & Queinnec, Y. (1992) Br. J. Psychol. 83, 249-260. [DOI] [PubMed] [Google Scholar]
- 3.Nesca, M. & Koulack, D. (1994) Can. J. Exp. Psychol. 48, 359-379. [DOI] [PubMed] [Google Scholar]
- 4.Koulack, D. (1997) Percept. Mot. Skills 85, 99-104. [DOI] [PubMed] [Google Scholar]
- 5.Leirer, V. O., Tanke, E. D. & Morrow, D. G. (1994) Exp. Aging Res. 20, 127-134. [DOI] [PubMed] [Google Scholar]
- 6.Rudy, J. W. & Pugh, C. R. (1998) J. Exp. Psychol. Anim. Behav. Process 24, 316-324. [DOI] [PubMed] [Google Scholar]
- 7.McDonald, R. J., Hong, N. S., Ray, C. & Martin, R. R. (2002) Learn. Motiv. 33, 230-252. [Google Scholar]
- 8.Valentinuzzi, V. S., Kolker, D. E., Vitaterna, M. H., Ferrari, E., Takahashi, J. S. & Turek, F. W. (2001) Anim. Learn. Behav. 29, 133-142. [Google Scholar]
- 9.Chaudhury, D. & Colwell, C. S. (2002) Behav. Brain Res. 133, 95-108. [DOI] [PubMed] [Google Scholar]
- 10.Harris, K. M. & Teyler, T. J. (1983) Brain Res. 261, 69-73. [DOI] [PubMed] [Google Scholar]
- 11.Raghavan, A. V., Horowitz, J. M. & Fuller, C. A. (1999) Brain Res. 833, 311-314. [DOI] [PubMed] [Google Scholar]
- 12.Kole, M. H., Koolhaas, J. M., Luiten, P. G. & Fuchs, E. (2001) Neurosci. Lett. 307, 53-56. [DOI] [PubMed] [Google Scholar]
- 13.Tapp, W. N. & Holloway, F. A. (1981) Science 211, 1056-1058. [DOI] [PubMed] [Google Scholar]
- 14.Fekete, M., van Ree, J. M., Niesink, R. J. & de Wied, D. (1985) Physiol. Behav. 34, 883-887. [DOI] [PubMed] [Google Scholar]
- 15.Cho, K., Ennaceur, A., Cole, J. C. & Suh, C. K. (2000) J. Neurosci. 20, RC66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devan, B. D., Goad, E. H., Petri, H. L., Antoniadis, E. A., Hong, N. S., Ko, C. H., Leblanc, L., Lebovic, S. S., Lo, Q., Ralph, M. R. & McDonald, R. J. (2001) Neurobiol. Learn. Mem. 75, 51-62. [DOI] [PubMed] [Google Scholar]
- 17.Byrne, J. H., Baxter, D. A., Buonomano, D. V., Cleary, L. J., Eskin, A., Goldsmith, J. R., McClendon, E., Nazif, F. A., Noel, F. & Scholz, K. P. (1991) Ann. N.Y. Acad. Sci. 627, 124-149. [DOI] [PubMed] [Google Scholar]
- 18.Bailey, C. H., Bartsch, D. & Kandel, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 13445-134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carew, T. J. (1996) Neuron 16, 5-8. [DOI] [PubMed] [Google Scholar]
- 20.Kandel, E. R. (2001) Science 294, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 21.Eskin, A. (1979) Fed. Proc. 38, 2573-2579. [PubMed] [Google Scholar]
- 22.Lickey, M. E., Block, G. D., Hudson, D. J. & Smith, J. T. (1976) Photophysiology 23, 253-273. [DOI] [PubMed] [Google Scholar]
- 23.Scholz, K. P. & Byrne, J. H. (1987) Science 235, 685-687. [DOI] [PubMed] [Google Scholar]
- 24.Cleary, L. J., Lee, W. L. & Byrne, J. H. (1998) J. Neurosci. 18, 5988-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kupfermann, I. (1974) Behav. Biol. 10, 1-26. [DOI] [PubMed] [Google Scholar]
- 26.Byrne, J. (1987) Physiol. Rev. 67, 329-439. [DOI] [PubMed] [Google Scholar]
- 27.Marinesco, S. & Carew, T. J. (2002) J. Neurosci. 22, 2299-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reijmers, L. G., Leus, I. E., Burbach, J. P., Spruijt, B. M. & van Ree, J. M. (2001) Physiol. Behav. 72, 305-309. [DOI] [PubMed] [Google Scholar]
- 29.Holloway, F. A. & Wansley, R. A. (1973) Behav. Biol. 9, 1-14. [DOI] [PubMed] [Google Scholar]
- 30.Pereyra, P., De La Iglesia, H. O. & Maldonado, H. (1996) Physiol. Behav. 59, 19-25. [DOI] [PubMed] [Google Scholar]
- 31.Daan, S. (2000) J. Biol. Rhythms 15, 296-299. [DOI] [PubMed] [Google Scholar]
- 32.Ralph, M. R., Ko, C. H., Antoniadis, E. A., Seco, P., Irani, F., Presta, C. & McDonald, R. J. (2002) Behav. Brain Res. 136, 179-184. [DOI] [PubMed] [Google Scholar]
- 33.Strumwasser, F. (1973) Physiologist 16, 9-42. [PubMed] [Google Scholar]
- 34.Levenson, J., Byrne, J. H. & Eskin, A. (1999) J. Neurosci. 19, 8094-8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hattar, S., Lyons, L. C. & Eskin, A. (2002) J. Neurochem. 83, 1401-1411. [DOI] [PubMed] [Google Scholar]
- 36.Jacklet, J. W. (1969) Science 164, 563-564. [Google Scholar]
- 37.Lickey, M. E., Wozniak, J. A., Block, G. D., Hudson, D. J. & Augter, G. K. (1977) J. Comp. Physiol. 118, 121-143. [Google Scholar]
- 38.Page, T. L. & Hudson, D. J. (1979) Fed. Proc. 38, 2580-2582. [PubMed] [Google Scholar]
- 39.Jordan, W. P., Lickey, M. E. & Hiaasen, S. O. (1985) J. Comp. Physiol. A 156, 293-303. [Google Scholar]
- 40.Harf, L., Arch, S. & Eskin, A. (1976) Brain Res. 111, 295-299. [DOI] [PubMed] [Google Scholar]
- 41.Olson, L. M. & Jacklet, J. W. (1985) J. Neurosci. 5, 3214-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, Z. Y. & Schacher, S. (1998) J. Neurosci. 18, 3991-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michael, D., Martin, K. C., Seger, R., Ning, M. M., Baston, R. & Kandel, E. R. (1998) Proc. Natl. Acad. Sci. USA 95, 1864-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obrietan, K., Impey, S. & Storm, D. R. (1998) Nat. Neurosci. 1, 693-700. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi, Y., Sanada, K. & Fukada, Y. (2001) FEBS Lett. 491, 71-75. [DOI] [PubMed] [Google Scholar]
- 46.Sanada, K., Okano, T. & Fukada, Y. (2002) J. Biol. Chem. 277, 267-271. [DOI] [PubMed] [Google Scholar]
- 47.Obrietan, K., Impey, S., Smith, D., Athos, J. & Storm, D. R. (1999) J. Biol. Chem. 274, 17748-17756. [DOI] [PubMed] [Google Scholar]
- 48.Lonze, B. E. & Ginty, D. D. (2002) Neuron 35, 605-623. [DOI] [PubMed] [Google Scholar]
- 49.Travnickova-Bendova, Z., Cermakian, N., Reppert, S. M. & Sassone-Corsi, P. (2002) Proc. Natl. Acad. Sci. USA 99, 7728-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maquet, P. (2001) Science 294, 1048-1052. [DOI] [PubMed] [Google Scholar]
- 51.Siegel, J. M. (2001) Science 294, 1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]