Abstract
Spectrin and protein 4.1 cross-link F-actin protofilaments into a network called the membrane skeleton. Actin and 4.1 bind to one end of β-spectrin. The adjacent end of α-spectrin, called the EF-domain, is calmodulin-like, with calcium-dependent and calcium-independent EF-hands. It has no known function. However, the sph1J/sph1J mouse has very fragile red cells and lacks the last 13 amino acids in the EF-domain, suggesting the domain is critical for skeletal integrity. Using pulldown binding assays, we find the α-spectrin EF-domain either alone or incorporated into a mini-spectrin binds native and recombinant protein 4.2 at a previously identified region of 4.2 (G3 peptide). Native 4.2 binds with an affinity comparable with other membrane skeletal interactions (Kd = 0.30 μm). EF-domains bearing the sph1J mutation are inactive. Binding of protein 4.2 to band 3 (Kd = 0.45 μm) does not interfere with the spectrin-4.2 interaction. Spectrin-4.2 binding is amplified by micromolar concentrations of Ca2+ (but not Mg2+) by three to five times. Calmodulin also binds to the EF-domain (Kd = 17 μm), and Ca2+-calmodulin blocks Ca2+-dependent binding of protein 4.2 but not Ca2+-independent binding. The data suggest that protein 4.2 is located near protein 4.1 at the spectrin-actin junctions. Because proteins 4.1 and 4.2 also bind to band 3, the erythrocyte anion channel, we suggest that one or both of these proteins cause a portion of band 3 to localize near the spectrin-actin junctions and provide another point of attachment between the membrane skeleton and the lipid bilayer.
Keywords: Calcium, Calmodulin, Erythrocyte, Membrane Structure, Metabolic Diseases, Band 3, Membrane Skeleton, Protein 4.2, Spectrin, Spherocytosis
Introduction
The red blood cell membrane skeleton is composed principally of short F-actin filaments cross-linked by α2β2-spectrin heterotetramers with the assistance of protein 4.12 to form a roughly hexagonal array (1). Actin and protein 4.1 bind to the actin binding domain at the amino terminus of the spectrin β-chain (2). The adjacent, carboxyl-terminal end of α-spectrin contains a calmodulin-like domain (3) (amino acids 2262–2418 in human α-spectrin) that is called the EF-domain and is thought to be inert and vestigial. However, the sph1J/sph1J mouse (4), which has severe hereditary spherocytosis and unstable red cell membranes, makes a mutant α-spectrin that lacks the last 13 amino acids of the EF-domain. The mutant protein is overexpressed by severalfold but is poorly incorporated into the red blood cell membrane skeleton (4), showing that the domain has some important but undiscovered function.
The complex formed by spectrin, actin, and protein 4.1 is part of a larger structure called the “junctional complex,” which also contains adducin, dematin, tropomyosin, tropomodulin, and p55 (5) and, according to recent work, is attached to integral membrane protein complexes containing various combinations of band 3, glucose transporter-1, the Kell and Duffy glycoproteins, the Rh protein, the XK protein, and glycophorin C (6–8).
The α- and β-spectrin chains are mostly composed of ∼106 amino acid, triple-helical subunits joined in tandem. There are 21 numbered repeats in α-spectrin (though “repeat” 10 is actually an SH3 domain inserted in repeat 9) and 16 repeats in β-spectrin. The two chains are aligned side-by-side in an anti-parallel arrangement and variably coiled about each other. The heterodimers interact with each other at their head ends through incomplete spectrin repeats (α0 and β17) to form heterotetramers. The tail ends of the heterodimers are capped with specialized domains; that is, an actin binding domain (ABD)3 in the case of β-spectrin and the EF-domain in α-spectrin. The ABD contains two calponin homology domains. The distal calponin homology 1 domain, which is closest to the amino terminus, binds both F-actin and protein 4.1. The proximal calponin homology 2 domain has similar capabilities, but it appears to be impotent unless activated by phosphatidyl 4,5-bisphosphate (2).
As noted, the EF-domain is structurally similar to calmodulin (3, 9) and has four EF-hands. The amino-terminal EF-hands are functional and bind Ca2+ with dissociation constants in the low millimolar range (Kd ∼ 0.5 mm) (3). The carboxyl-terminal (distal) pair does not bind Ca2+ (3). Like calmodulin, Ca2+ binding causes the proximal EF-hands in brain spectrin to change from a “closed” to an “open” state (9).
Using various in vitro binding assays, we find that the EF-domain binds protein 4.2, a 72,000-Da protein, in a Ca2+- and calmodulin-dependent manner and also binds calmodulin in the presence of Ca2+. Protein 4.2 is an erythrocyte membrane protein and an enzymatically inactive member of the transglutaminase gene family. It attaches to the membrane via the cytoplasmic domain of band 3 (the erythrocyte anion exchange channel) and also binds to ankyrin (10), spectrin (11), and CD47, a protein that is associated with the Rh complex (12–14) and helps protect normal red cells from phagocytosis (15). The location of the protein 4.2 binding site on spectrin was not previously identified but is now shown to be the EF-domain. The present experiments prove that the EF-domain is functionally active and is regulated by micromolar concentrations of calcium and calmodulin. All of protein 4.2 seems to be bound to band 3, which implies that a portion of band 3 resides near the spectrin-actin molecular junctions at the tail end of spectrin, far from the well known spectrin-ankyrin-band 3 complexes at the opposite end of spectrin.
EXPERIMENTAL PROCEDURES
Reagents
Bovine brain calmodulin was purchased from Calbiochem. Amido Black 10B was obtained from Bio-Rad. Calmodulin-Sepharose and glutathione-Sepharose 4B were purchased from GE Healthcare. PBS-1 composition was 150 mm NaCl, 5 mm sodium phosphate and 0.5 mm EGTA. PBS-2 composition was 150 mm NaCl, 5 mm sodium phosphate, pH 7.4, 0.5 mm EGTA, and 0.5 mm DTT. HEPES-saline composition was 150 mm NaCl, 10 mm HEPES, 0.5 mm EGTA, and 0.5 mm DTT.
2 m Tris Extract of Erythrocyte Membranes
Freshly drawn, de-identified whole human blood (100 ml) was washed 4 times in 10 volumes of ice-cold PBS-1. The washed and packed red cells in PBS-1 were made 0.4 mm in DFP to inactivate serine proteases and allowed to sit on ice for 20 min before lysis. The red cells were then lysed in 10–15 volumes of 5 mm sodium phosphate, 0.5 mm EGTA, pH 8.0, at 0 °C, and the membranes were collected by centrifugation (12,500 rpm, GSA rotor, Sorvall RC5B centrifuge, 25 min, 4 °C) and washed in the same buffer until white. Spectrin, actin, and residual hemoglobin were extracted by diluting the packed membranes in 15 volumes of 0.5 mm EGTA, pH 8.5 buffer, at 37 °C and incubating for 30 min at 37 °C. The spectrin/actin-depleted membranes were centrifuged in a Sorvall SS-34 rotor at 18,000 rpm for 25 min and washed once in the same buffer at 4 °C. The pellets were pooled and added to an equal volume of 4 m Tris, 5 mm sodium phosphate buffer, pH 7.2, 0.1% Tween 20, 0.5 mm EGTA, 0.5 mm DTT, 0.4 mm DFP, and 0.5 μg/μl each of pepstatin and leupeptin. This was stirred gently for 30 min on ice and then centrifuged in a Beckman 55.2Ti rotor (60 min, 45,000 rpm). The supernatant was dialyzed twice against 2 liters of 5 mm sodium phosphate, 0.5 mm EGTA, and 0.5 mm DTT. All experiments with the 2 m Tris extract were performed as described later under “Spectrin-Protein 4.2 and Spectrin-Calmodulin Binding Experiments,” except that a final volume of 400 μl was used in the binding incubation, and the results were quantified by gel densitometry.
Purification of Pig Protein 4.2
Pig protein 4.2 was prepared according to the protocol described by Friedrichs et al. (16) with the exception that the buffer for the anion exchange column was 5 mm sodium phosphate, pH 7.4, 0.5 mm EGTA and 0.5 mm DTT. The purified protein was verified by mass spectrometry and was >95% pure on SDS-PAGE.
Purification of the Cytoplasmic Domain of Band 3 (CDB3)
CDB3 was prepared by the method of Bennett and Stenbuck (17) with modifications. Spectrin- and actin-depleted human red blood cell membranes from 250 ml of blood were prepared as described above, resuspended in 10 volumes of 0.1 mm EGTA, pH 8.0, titrated to pH 11 with NaOH, mixed gently for 20 min at room temperature, and centrifuged, and the supernatant, containing all the peripheral membrane proteins, was discarded. The extracted membranes were washed once with 5 mm sodium phosphate, 0.5 mm EGTA, pH 8.0. The membranes were resuspended in one volume of the same buffer and digested with 1 μg/ml α-chymotrypsin (0 °C, 15 min). The digestion was terminated with 0.4 mm DFP. The supernatant was collected by centrifugation (180,000 × g, 45 min), loaded on a Q-Sepharose column in 5 mm sodium phosphate, 0.5 mm EGTA, 0.5 mm DTT, and eluted with a 0–600 mm KCl gradient. The fractions were evaluated with SDS-PAGE, and those containing CDB3 were pooled and dialyzed against binding buffer (150 mm NaCl, 10 mm Hepes, 0.5 mm EGTA, 0.5 mm DTT, pH 7.3).
Preparation of an Extract Enriched in Human Protein 4.2
Spectrin- and actin-depleted red cell membranes were resuspended in one volume of 0.1 mm EGTA, pH 8.0, titrated to pH 10.7 with NaOH, incubated (30 min, room temperature), and centrifuged (180,000 × g, 45 min). The supernatant was dialyzed against 150 mm NaCl, 0.5 mm DTT, 0.5 mm EGTA, 10 mm Hepes, pH 7.3. The extract contained mostly ankyrin and protein 4.2 (50–65%) by SDS-PAGE.
Expression and Purification of Recombinant Spectrin and Protein 4.2 Proteins
All constructs are listed in Table 1. Spectrin GST fusion proteins and the G3 spectrin-binding peptide from protein 4.2 (18) were generated by PCR amplification of cDNA clones from the human spectrin A-I (SPTA1) sequence (GenBankTM accession no. M61877) (19) using the corrected carboxyl-terminal sequence of Galluzzi et al. (20) (GenBankTM accession no. AF060556), from the human β-spectrin-1 sequence (21) (SPTB, GenBankTM accession no. J05500), or from human protein 4.2 (22) (HUMEMP42, GenBankTM accession no. M29399). The amplicons were subcloned in Amersham Biosciences vector pGEX-6p except for β-spectrin peptides, which were cloned into pGEX-2T. All constructs were sequenced to check fidelity and were expressed in Escherichia coli BL21.
TABLE 1.
Recombinant proteins
| Construct | Molecular mass | Molecular mass without GST | Nucleotides | Amino acids | Vector | Cleavage enzyme |
|---|---|---|---|---|---|---|
| Da | Da | |||||
| GST-αEF | 45,182 | 18,197 | 6970–7440 | 2262–2418 | pGEX-6p | PreScissiona |
| GST-αEFΔ13 | 43,653 | 16,668 | 6970–7402 | 2262–2405 | pGEX-6p | PreScission |
| GST-α18–21EF | 97,615 | 70,630 | 5603–7440 | 1806–2418 | pGEX-6p | PreScission |
| GST-α18–21EFΔ13 | 96,090 | 69,105 | 5603–7402 | 1806–2405 | pGEX-6p | PreScission |
| GST-βABD1–4 | 113,380 | 86,396 | 96–2324 | 1–743 | pGEX-2T | Thrombin |
| GST-4.2G3 | 36,208 | 9,223 | 1690–1935 | 410–492 | pGEX-6p | PreScission |
a PreScission protease (Amersham Biosciences).
To express GST fusion proteins, a 37 °C overnight culture was diluted 1:20 and grown at 30 °C for 2.5 h or to an A600 of 0.5 to 0.7. Isopropyl-β-d-thiogalactopyranoside was added to 1.0 mm, and the cultures were grown for an additional 2–3 h at 30 °C. After centrifugation, the pellets were stored at −80 °C. The proteins were isolated according to Harper et al. (23) with modifications. The pellets were resuspended in a small volume (10 ml/500 ml of culture) of 130 mm NaCl, 10 mm sodium phosphate, pH 7.4, 5 mm EDTA, 5 μg/ml leupeptin, 5 μg/ml pepstatin, and 5 μm DFP and sonicated on ice three times for 30 s each time. The lysed bacteria were centrifuged at 4 °C for 30 min at 180,000 × g, and the fusion proteins in the bacterial supernatant were bound to GSH-Sepharose beads using a volume in microliters equal to half that of the original culture in milliliters. For example, 250 μl of GSH beads 1:1 in PBS-1, pH 8.0, were added to the protein from a 250-ml bacterial culture. The suspension was tumbled at 4 °C for 60 min and then washed 4 times in phosphate-buffered saline at 4 °C.
After purification on GSH beads, some GST proteins were cleaved from the GST moiety with PreScission protease (Amersham Biosciences) while attached to the beads according to the manufacturer's directions. The cleaved proteins were dialyzed at 4 °C against either PBS-2, pH 7.3, or HEPES-saline, pH 7.3, depending on the experimental plan. The GST-α18–21EF or GST-α18–21EFΔ13 peptides were handled in the same manner when they were cleaved before incorporation into minispectrin heterodimers. Uncleaved GST proteins were eluted from the glutathione beads with 10 mm glutathione for 4 h at 4 °C and were then dialyzed twice against two liters of the appropriate buffer, described above.
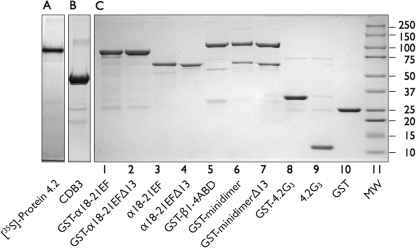
Examples of the recombinant proteins used in these studies are shown in Fig. 1B and described in Table 1. The recombinant proteins were 90–95% pure. Mass spectrometry showed that the minor, lower molecular weight bands evident in some samples were proteolytic degradation products of the recombinant protein.
FIGURE 1.
Proteins used in this study separated on 9% Laemmli-Tricine SDS gels (7). A, shown is an autoradiograph of the in vitro transcription/translation product of full-length (77 kDa) protein 4.2, labeled with [35S]methionine. B, shown is the cytoplasmic domain of band 3 cleaved and purified from red cell membranes stripped of peripheral proteins (17). C, shown are recombinant proteins used in this study. 8 μg of each protein were analyzed. In some cases (lanes 3, 4, and 9) the GST moiety had been cleaved from the recombinant fusion proteins with PreScission protease (Amersham Biosciences) before electrophoresis.
Preparation of a Minispectrin Heterodimer
Minispectrin heterodimers were prepared according to the protocol of Begg et al. (24) with the following changes. The minispectrins were formed by combining equimolar amounts of GST-βABD1–4 (ABD at the amino terminus of β-spectrin followed by spectrin repeats 1–4 of β-spectrin) with GST-cleaved α18–21EF or cleaved α18–21EFΔ13. After incubation on ice for 1 h, they were loaded on a 16/60 Sephacryl S-200 pre-poured column (Amersham Biosciences) in HEPES-saline. The column was equilibrated in 150 mm NaCl, 10 mm HEPES, pH 7.3, 0.5 mm EGTA, 0.5 mm DTT, and eluted with the same buffer at 10 ml/h. Two-ml fractions were collected. An aliquot of each fraction was analyzed by SDS-PAGE, and the fractions that had a 1:1 molar ratio of α- and β-spectrin were pooled (the first two or three fractions on the leading side of the elution profile). The minidimer preparation was used directly in binding assays without further dialysis.
Protein Determination
Protein concentrations were determined using the Bio-Rad dye binding reagent. Concentrations of recombinant proteins were calculated from molar extinction coefficients estimated from the amino acid analysis.
In Vitro Transcription and Translation
35S-Labeled full-length protein 4.2 was synthesized using the Promega TNT T7/SP6 Coupled Reticulocyte Lysate System. The reaction volume was scaled up 8 times to 400 μl using 8 μg of protein 4.2 cDNA, cloned in the riboprobe, pGem-7z (Promega), and allowed to proceed according to the instructions. The reaction was terminated by adding 16 μl of a solution made of 10 μl of DFP and 90 μl of 1 m Tris HCl, pH 8.0, incubating at 0 °C for 15 min, and then adding 80 μl of 100 mm sodium phosphate buffer, pH 11.5, plus 320 μl of water and incubating at room temperature for 10 min. The purpose of the alkaline treatment was to release protein 4.2 from any internal membranes. It was required to obtain satisfactory yields of labeled protein 4.2. The reaction was then centrifuged in a Beckman ultracentrifuge with the 42.2Ti rotor at 40,000 RPM for 30 min, and the supernatant was neutralized with 36 μl of 1 m sodium phosphate buffer, pH 7.4.
The apparent specific activity of the synthesized 35S-labeled protein 4.2 varied considerably in different experiments. The reasons were not investigated but were believed to include variation in the activity of different lots of the reticulocyte lysate kit used, variation in the amount of 35S-labeled protein 4.2 recovered on extraction of the reticulocyte membranes at pH 11.5, and variation in extraction of cold rabbit protein 4.2 from the membranes. This precluded calculation of binding affinities or capacities but did not affect comparisons of 35S-labeled protein 4.2 binding under different conditions within an experiment.
125I Labeling of Proteins
The protein 4.2 G3 peptide, CDB3, and calmodulin were labeled with 125I-Bolton-Hunter reagent (PerkinElmer Life Sciences). The 250-μCi vial of Bolton-Hunter reagent supplied in benzene was dried under a stream of nitrogen and resuspended with 250 μl of 1 m sodium borate, pH 8.5. This gave a solution of 1 μCi/μl. The desired number of μCi was added to the protein in PBS-1 buffer. The reaction was incubated 1 h at 0 °C before dialysis against 2 liters of HEPES-saline, pH 8.0, and then against 2 liters of binding buffer. Labeled proteins were centrifuged for 20 min at 40,000 RPM before binding experiments to remove aggregates.
Mass Spectrometry
Mass spectrometry was performed by Stephen P. Gygi at the Taplin Biological Mass Spectrometry Facility, Harvard Medical School, Boston, MA.
Electrophoresis
SDS gel electrophoresis was performed with 9% Laemmli acrylamide gels using a Tricine tank buffer (25). The upper buffer was a Tris/Tricine/SDS buffer (Bio-Rad catalog no. 161-0744), and the lower buffer was 0.2 m Tris, pH 8.9. We refer to these gels as 9% Laemmli Tricine SDS-PAGE. The gels were transferred to polyvinylidene difluoride membranes and stained with Amido Black.
Spectrin-Protein 4.2 and Spectrin-Calmodulin Binding Experiments
10–20 μg of GST-α-spectrin proteins were combined with increasing quantities of isotopically labeled protein 4.2, protein 4.2 G3 peptide, or calmodulin in a final volume of 200 or 300 μl. In competition experiments, all components were added at the same time. Measured (μg) quantities of 125I-Bolton-Hunter-labeled protein 4.2-G3 peptide or calmodulin were added, except for the in vitro transcribed and translated 35S-labeled protein 4.2, which was added by volume only. The binding buffer contained 150 mm NaCl, 5 mm phosphate buffer, pH 7.4, 0.5 mm EGTA, 0.5 mm DTT, 1 mg/ml gelatin, 0.05% Tween 20, 0.5 μg/ml leupeptin, 0.5 μg/ml pepstatin, and 0.5 μg/ml Pefabloc. When calcium or magnesium was included in the binding experiment, the 5 mm phosphate in the binding buffer was exchanged for 10 mm HEPES, pH 7.3 (HEPES-saline binding buffer). The calcium or magnesium was added directly to the binding reaction mixes, and the free calcium or magnesium concentration was calculated after accounting for the addition of EGTA. Likewise, any proteins that were used in calcium binding experiments were first dialyzed in HEPES-saline, pH 7.3, without calcium.
These components were mixed in a 1.5-ml tube and tumbled at room temperature for 16 h. Forty μl of glutathione beads, diluted 1:1 in PBS-1, were then added, and the reaction was allowed to continue for an additional hour at room temperature with tumbling. The reaction was then added to an Ultrafree-MC centrifugal filter device (Millipore, catalog no. UFC30GVNB) placed in a 10 × 75-mm polystyrene tube and centrifuged in a tabletop refrigerated Sorvall Legend RT centrifuge with the swinging rotor for 25 min at 3100 rpm at 23 °C. The beads were vortexed one time with 100 μl of fresh binding buffer and centrifuged again into the same polystyrene tube. The total effluent was considered the “free” component for binding. The filter containing the washed bound beads was transferred to a new polystyrene tube, and the bound proteins were eluted with 100 μl of 50 °C SDS-PAGE sample buffer and centrifuged as before. The protein in the sample buffer was the “bound” component of the binding reaction. Each binding point was done in duplicate, and binding of the labeled protein to GSH beads alone was subtracted as background at every point. The samples were counted in a β counter for 125I and in a β counter for [35S]methionine. The binding isotherms and binding constants were computed by nonlinear regression using the Prism 4 program by GraphPad.
Band 3-Protein 4.2 Binding Experiments
The interaction of band 3 and protein 4.2 was measured with the immunoprecipitation assay of Korsgren and Cohen (26) using 125I-labeled band 3 and an alkaline extract of human red blood cells membranes containing 50–65% protein 4.2.
Calcium Binding Experiments
Binding of 45Ca to the α-spectrin EF-domain and to protein 4.2 was measured by equilibrium dialysis with the 96-well equilibrium dialyzer from Harvard Apparatus. The manufacturer's protocol was followed with the following additions. HEPES-saline and 200,000 cpm of 45Ca2+ were added to both sides of the dialysis chambers with the protein of interest on one side. The final calcium concentration for both sides was adjusted with cold CaCl2. The apparatus was allowed to tumble at room temperature for 24 h. Then two 50-μl samples from each chamber were counted in a scintillation spectrometer.
Protein Structure Analysis
The KALIGN tool was used to align protein sequences. The Jpred 3 algorithm was used to predict secondary structures.
RESULTS
Binding of Native Protein 4.2 to the α-Spectrin EF-domain
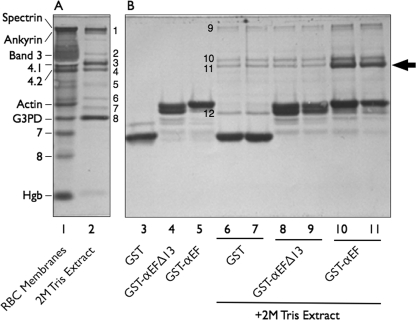
We first “fished” for interactions between the EF-domain and a solution of peripheral membrane proteins extracted by 2 m Tris at physiologic pH from erythrocyte membranes pretreated with a low ionic strength buffer to remove spectrin, actin, and residual hemoglobin. The Tris extraction was chosen because it is relatively gentle but removes all the remaining peripheral proteins. As expected, the extract contained ankyrin, proteins 4.1 and 4.2, p55, dematin, aldolase A, and glyceraldehyde-3-phosphate dehydrogenase (Fig. 2, lane 2). No spectrin was detected. Compared with GST alone (Fig. 2, lanes 6 and 7), a fusion protein of GST and the EF-domain (GST-αEF) bound a 72-kDa band that was identified as protein 4.2 by mass spectrometry (Fig. 2, lanes 10 and 11, arrow). A small amount of protein 4.1 was also pulled down in the experiment shown in Fig. 2, but the amount varied in different experiments, suggesting that protein 4.1 bound indirectly to the spectrin “bait” through protein 4.2. This fits with our preliminary evidence that the two proteins interact (data not shown). Remarkably, when the carboxyl-terminal 13 amino acids were deleted from the EF-domain (GST-αEFΔ13), as they are in the sph1J mouse mutation (4), the domain did not bind detectable amounts of protein 4.2 compared with the control (Fig. 2, lanes 8 and 9).
FIGURE 2.
Affinity purification of proteins interacting with the EF-domain of α-spectrin. A, Amido Black-stained 9% Laemmli-Tricine SDS gel of red cell membrane proteins (20 μg) and the protein composition of a 2 m Tris extract (5 μg) of spectrin- and actin-depleted membranes are shown. Protein bands (B) present in the extract were determined by mass spectrometry: B1, ankyrin; B2, unidentified; B3, protein 4.1; B4, protein 4.2; B5, protein p55; B6, dematin; B7, aldolase A; B8, glyceraldehyde-3-phosphate dehydrogenase (G3PD). RBC, red blood cells. Hgb, hemoglobin globin chains. B, shown is analysis of recombinant GST alone and GST-EF-domain fusion proteins retrieved by GSH beads before (lanes 3 to 5) and following (lanes 6 to 11) incubation with a 2 m Tris membrane protein extract that had been dialyzed into binding buffer. The gel is a separate section of the gel shown in A. Eight μg of each sample were applied to the gel with the exception of lanes 6, 8, and 10, where 16 μg were used. Protein bands B9-B12 were determined by mass spectrometry: B9, ankyrin; B10, protein 4.1; B11, protein 4.2; B12, glyceraldehyde-3-phosphate dehydrogenase. Note that the intact EF-domain (GST-αEF) retrieves protein 4.2 (arrow) from the extract, but the same domain is inactive if the last 13 amino acids are deleted (GST-αEFΔ13).
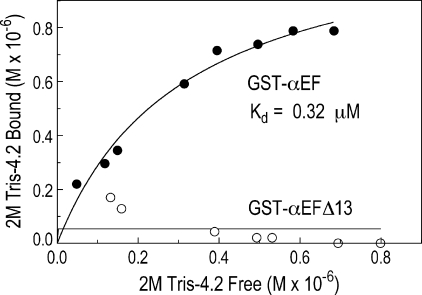
This experiment was repeated with increasing volumes of the 2 m Tris extract (Fig. 3). Full-length, native protein 4.2 bound to GST-αEF with a Kd of 0.32 μm in the experiment shown (average of 0.30 μm from 2 experiments), which is comparable with other interactions between erythrocyte membrane proteins (27). GST-αEFΔ13 did not bind at all, even at high doses (Fig. 3), and was essentially identical to GST alone (data not shown). The observed binding was similar to the low affinity, high capacity protein 4.2 binding site on spectrin observed by Golan et al. (11), which had a Kd of 0.28 μm.
FIGURE 3.
Protein 4.2 binds to the α-spectrin EF-domain but not to the carboxyl-terminal truncated domain present in the sph1J mouse. GST-αEF or GST-αEGΔ13 (0.48 μm each) were mixed with increasing volumes of a 2 m Tris extract of spectrin- and actin-depleted membranes and incubated at room temperature for 16 h, and the bound protein 4.2 was assayed by SDS gel electrophoresis and densitometry.
Similar binding curves were obtained in two additional experiments where an alkaline extract of stripped red cell membrane was substituted for the 2 m Tris extract and GST-α18–21EF was used instead of GST-αEF. GST-α18–21EF contains the four spectrin repeats that precede the EF domain. The extract contained 50–65% protein 4.2 and yielded an average Kd of 0.62 μm (data not shown). Binding of GST-α18–21EF to purified pig protein 4.2 was also tested and yielded a Kd of 0.23 μm (data not shown). Maximal binding in the experiments with human protein 4.2 averaged 1.1 molecules of protein 4.2 per molecule of spectrin peptide, but the range was wide (0.65–2.6 mol of 4.2/mol of spectrin peptide), which may indicate a tendency for protein 4.2 to aggregate in solution.
Compared with GST alone, no proteins bound to GST-αEF or GST-αEFΔ13 when a 2% Triton X-100 extract of the membranes remaining after the 2 m Tris extraction were used (data not shown). The Triton X-100 extract was enriched in band 3, ankyrin, and multiple proteins in the so-called band 4.9 region.
Binding of 35S-Labeled, in Vitro Transcribed and Translated Protein 4.2 to the α-Spectrin EF-domain
Because we found it very difficult to prepare full-length recombinant protein 4.2 in bacterial expression systems, as have others (28), we prepared the protein labeled with [35S]methionine by in vitro transcription and translation in a rabbit reticulocyte lysate system.
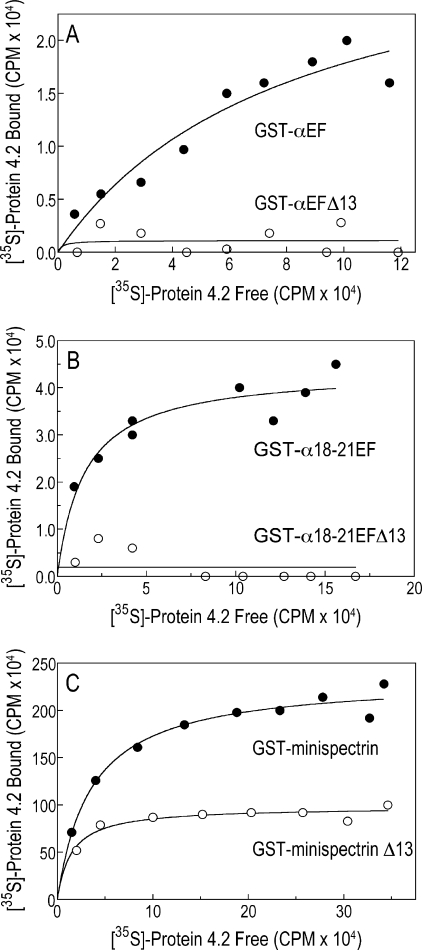
As shown in Fig. 4, synthetically prepared protein 4.2 bound to GST-αEF (Fig. 4A) and to GST-α18–21EF (Fig. 4B), but neither construct bound 4.2 if the terminal 13 amino acids were missing. The data in Fig. 4 suggest that the post-translational modifications of native protein 4.2, myristoylation (29) and palmitoylation (28), are not required for spectrin binding and confirm the importance of the conserved carboxyl-terminal segment of the EF-domain. We used GST-18–21αEF in most subsequent experiments because it is more resistant to proteolysis and is easier to prepare.
FIGURE 4.
In vitro synthesized 35S-labeled protein 4.2 binds to normal but not mutant α-spectrin EF-hands. Increasing volumes of 35S-labeled protein 4.2, prepared by in vitro transcription and translation, were incubated with various GST-tagged spectrin peptides, and bound 35S-labeled protein 4.2 was measured after retrieval of the GST peptides on GSH-Sepharose beads. A, GST-αEF and GST-αEFΔ13 are shown. B, GST-α18–21EF and GST-α18–21EFΔ13 are shown. C, GST-minispectrin with or without the αEFΔ13 mutation is shown.
To test whether the EF-domain retains the ability to bind 4.2 in native spectrin, we constructed a minispectrin by cleaving the GST moiety from GST-α18–21EF and GST-α18–21EFΔ13 and combining the resulting peptides with the actin binding domain and the first four repeats from β-spectrin (GST-β1–4ABD). The spectrin repeats in each peptide contain the sites that bind α- and β-spectrins together. They react spontaneously to form a minispectrin heterodimer, and the minispectrin, like the isolated EF-domain, binds protein 4.2 (Fig. 4C). This shows that the actin binding domain of β-spectrin does not enhance or interfere with the α-spectrin-protein 4.2 interaction when the two are side by side in the spectrin molecule. However, unlike isolated αEFΔ13, which is inert, the minispectrin containing the mutant α-chain EF-domain has some residual binding activity (Fig. 4C). This could be due to binding of protein 4.2 to GST-β1–4ABD. We have preliminary evidence that this occurs, but we have not yet carefully investigated the possibility.
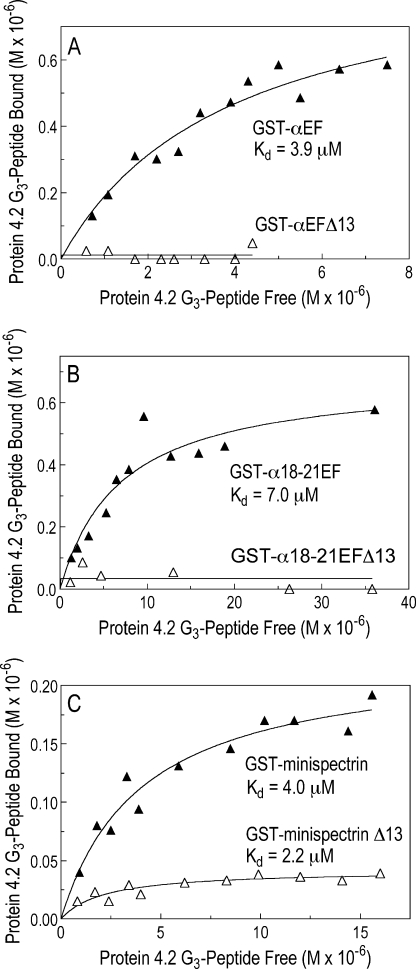
The Protein 4.2-G3 Spectrin-binding Peptide Binds to the EF-hand Domain
Mandal et al. (18) have shown that the “G3 peptide” of protein 4.2, which corresponds to amino acids 410–492 in the center of the protein sequence, contains a spectrin binding site, but the corresponding site for protein 4.2 on spectrin is unknown. 125I-Labeled recombinant 4.2-G3 bound GST-αEF and GST-α18–21EF with similar average Kd values of 4.1 μm (n = 2) and 6.6 ± 3.9 μm (n = 13), respectively (Fig. 5, A and B). There was no measurable binding of the 4.2-G3 peptide to either of the two recombinant mutant EF-hand peptides. This and the preceding data suggest the spectrin EF-hands contain a major 4.2 binding site and that site involves the conserved carboxyl-terminal 13 amino acids. However, the binding affinity of GST-αEF for 4.2-G3 was about 14-fold less than its affinity for full-length, native protein 4.2 (Fig. 3) and about 6 times less than the published affinity of the 4.2-G3 peptide for full-length, native spectrin (18). This suggests that other sites on protein 4.2 and spectrin or unknown post-translational modifications of the G3 or EF-domain peptides may enhance the avidity of the interaction when the full-length, native proteins are used.
FIGURE 5.
The 125I-labeled G3 peptide from protein 4.2 binds to the α-spectrin EF-domain and a minispectrin heterodimer. Increasing concentrations of the 125I-labeled G3 peptide from protein 4.2 were incubated with various GST-tagged spectrin peptides, and bound 125I-labeled G3 peptide was measured after retrieval of the GST peptides on GSH-Sepharose beads. A, GST-αEF and GST-αEFΔ13 (0.60 μm each) are shown. B, GST-α18–21EF and GST-α18–21EFΔ13 (0.20 μm each) are shown. C, GST-minispectrin with or without the αEFΔ13 mutation (0.34 μm each) is shown.
The 4.2-G3 peptide bound to the minispectrin heterodimer (Fig. 5C) with an affinity (Kd = 5.1 ± 2.8 μm, n = 4) similar to the isolated EF-domain. The minispectrin containing the mutant EF-domain bound less strongly (3.9 ± 1.5 μm, n = 4) and had half the binding capacity of the normal minispectrin (0.21 ± 0.15 versus 0.40 ± 0.22 μm, respectively).
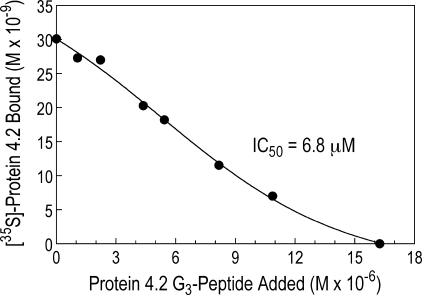
The Protein 4.2-G3 Peptide Fully Competes with Full-length Protein 4.2 for Binding to the α-Spectrin EF-domain
The addition of a 100-fold molar excess of unlabeled protein 4.2-G3 to a complex of 35S-labeled protein 4.2 bound to GST-α18–21EF completely displaced the full-length, 35S-labeled protein 4.2 in three of four experiments (Fig. 6) and did so with an inhibition coefficient (IC50 = 6.8 μm) comparable the binding affinity of 4.2-G3 for GST-α18–21EF (Kd = 7.0 μm obtained in separate experiments (Fig. 5B)). This shows that the G3 peptide contains all of the binding activity for the EF-domain of the full-length protein.
FIGURE 6.
The protein 4.2 G3 peptide fully competes with 35S-labeled full-length protein 4.2 for binding to the α-spectrin EF-domain. GST-α18–21EF (0.76 μm) was incubated with 35S-labeled protein 4.2 prepared by transcription and translation and increasing amounts (0 to 16.3 μm) of the protein 4.2 G3 peptide. The ability of the unlabeled G3 peptide to displace full-length 4.2 was measured after retrieval of the GST peptide on GSH-Sepharose beads.
Carboxyl-terminal 13 Amino Acids
We synthesized a peptide containing the last 13 amino acids of human α-spectrin and a control peptide with the same amino acids in a scrambled order and tested the ability of the two peptides to displace 35S-labeled protein 4.2 from GST-α18–21EF. Neither peptide was effective, even at a 165× molar excess (data not shown). We conclude that either the isolated peptide does not contain the binding site or does not fold into an active configuration by itself.
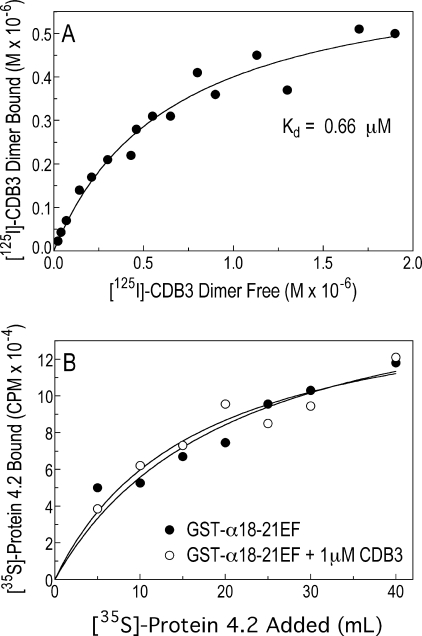
Binding of Band 3 to Protein 4.2 Does Not Interfere with the Spectrin-4.2 Interaction
Because protein 4.2 binds to band 3 in vitro (10, 26) and in vivo (30), it is important to determine whether band 3 interferes with the spectrin-4.2 interaction. Using 125I-labeled CDB3 and partially purified human 4.2 in an immunoprecipitation assay (10), we found that the two proteins bound with a Kd = 0.45 ± 0.15 μm (n = 4) (Fig. 7A), which is within the range (0.2–0.8 μm) previously reported (10). Importantly, the addition of 1 μm CDB3 did not alter the binding of 35S-labeled protein 4.2 to the EF domain fragment GST-18–21EF (Fig. 7B), showing that spectrin and protein 4.2 can bind in the presence of band 3.
FIGURE 7.
The cytoplasmic domain of band 3 binds to protein 4.2 but does not interfere with the spectrin-4.2 interaction. A, 125I-labeled CDB3 (0–2.4 μm) was incubated with 1 μm partially purified human protein 4.2, and the complex was immunoprecipitated with an anti-4.2 antibody and counted. B, shown is binding of 35S-labeled protein 4.2 to the GST-α18–21EF fragment of spectrin in the absence or presence of 1 μm CDB3. The data shown are the average of two experiments.
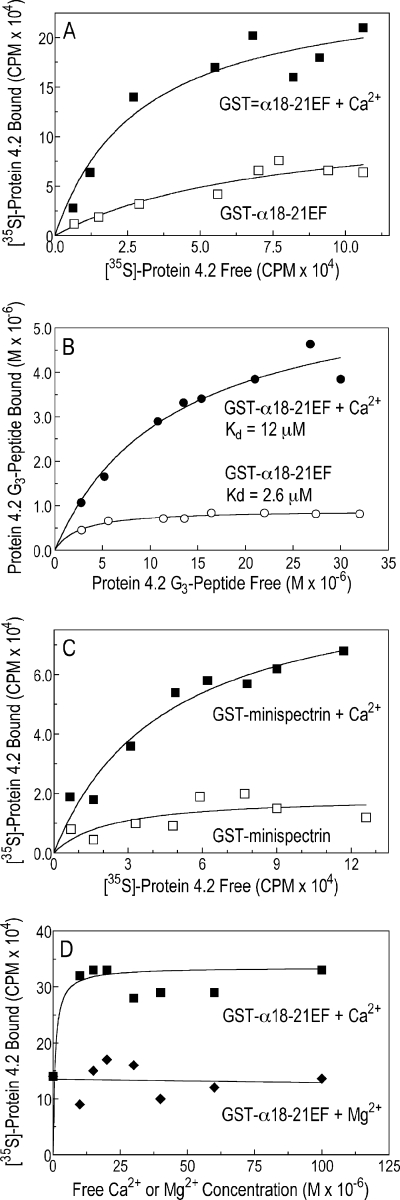
Binding of Protein 4.2 to the α-Spectrin EF-domain Is Amplified by Ca2+ but Not by Mg2+
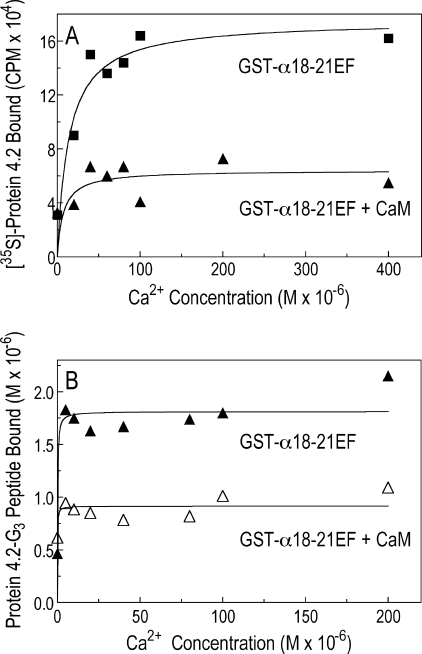
We tested whether the binding of protein 4.2 to spectrin was calcium-sensitive. Our initial studies used a high concentration of free Ca2+ (1.5 mm) because it is known that the EF-hands of erythrocyte spectrin bind Ca2+ ions in the nonphysiologic range (Kd ∼0.5 mm) (3, 31). At these concentrations, three-to-five times more full-length, 35S-labeled protein 4.2 (Fig. 8A) or 125I-labeled protein 4.2 G3 peptide (Fig. 8B) bound to the recombinant EF-domain (GST-α18–21EF) or to the minispectrin heterodimer (Fig. 8C). Surprisingly, when the Ca2+ stimulation was titrated, Ca2+ acted at a far lower concentration than expected. The effect was already maximal at 10 μm (Fig. 8D). We did not attempt to determine the lower limit of the Ca2+ effect, but 10 μm lies near the top of the physiological range for Ca2+ concentration in the red cell. Estimates using different techniques range from 0.1 to 15 μm (32–36). Moreover, the Ca2+ effect was specific. Mg2+ ions did not stimulate spectrin-4.2 binding in the same concentration range (Fig. 8D).
FIGURE 8.
Binding of protein 4.2 to the α-spectrin EF-domain is amplified by Ca2+ but not by Mg2+. A, shown is binding of 35S-labeled protein 4.2 to GST-α18–21EF in the absence or presence of free Ca2+ (1.5 mm). B, shown is binding of the 125I-labeled G3 peptide of protein 4.2 to GST-α18–21EF (0.75 μm) in the absence or presence of free Ca2+ (1.5 mm). C, shown is binding of 35S-labeled protein 4.2 to GST-minispectrin (0.43 μm) in the absence or presence of free Ca2+ (1.5 mm). D, shown is the specificity of Ca2+ compared with Mg2+ in the binding of 35S-labeled protein 4.2 to GST-α18–21EF (0.75 μm). All experiments used the HEPES binding buffer, which contains 0.5 mm EGTA (see “Experimental Procedures”) with or without added CaCl2 or MgCl2. Free Ca2+ and Mg2+ concentrations were calculated using the EGTA Calculator.
Using an equilibrium dialysis method, we found that 45Ca2+ binds weakly to GST-cleaved α18–21EF (Kd ∼0.3 mm, binding capacity was ∼5 mol of Ca2+/mol of α18–21EF; data not shown). The affinity is about the same as Travé et al. (3) reported (∼0.5 mm). They localized the Ca2+ site to EF12. This fits with our finding that Ca2+ binding is not significantly altered in the αEFΔ13 mutant, which disrupts EF34 (data not shown). We find that 45Ca2+ also binds to the G3 peptide of protein 4.2, although with an even lower affinity (Kd ∼1.0 mm). Maximal binding was ∼1.3 mol of Ca2+/mol of G3 (data not shown). This suggests that Ca2+ may work by binding to both molecules and bridging between them. The combination of two simultaneous binding interactions would greatly increase the apparent affinity of Ca2+ binding, perhaps explaining why it works at micromolar concentrations.
Calmodulin Inhibits the Ca2+-stimulated Binding of Protein 4.2 and Its G3 Peptide to Recombinant α-Spectrin
Calmodulin (CaM) reduced the maximal binding of full-length 35S-labeled protein 4.2 (Fig. 9A) or its spectrin-binding G3 peptide (Fig. 9B) to the EF-domain in the presence of Ca2+ by about 2–3-fold, essentially abolishing the Ca2+ stimulatory effect. CaM had no effect in the absence of Ca2+ (compare binding with or without CaM in the absence of added Ca2+ in Fig. 9B), and CaM did not change the sensitivity of the binding reaction to Ca2+. Again, it was already maximal at near physiological concentrations of Ca2+, in this case 5 μm (Fig. 9B). The fact that the 5 μm CaM present in the reaction was equally inhibitory from a 1:1 ratio of Ca2+:CaM to a 40:1 ratio (Fig. 9B) indicates that CaM did not inhibit the reaction simply by binding up free Ca2+ and suggests that CaM must interact with one or more of the protein constituents.
FIGURE 9.
Ca2+ and calmodulin regulate binding of protein 4.2 to the α-spectrin EF-domain. A, 35S-labeled protein 4.2 binding to GST-α18–21EF (0.4 μm) as a function of Ca2+ concentration in the presence or absence of calmodulin (5 μm) is shown. B, binding of the 125I-labeled G3 peptide of protein 4.2 (13.5 μm) to GST-α18–21EF (0.76 μm) as a function of Ca2+ concentration in the presence or absence of calmodulin (5 μm) is shown.
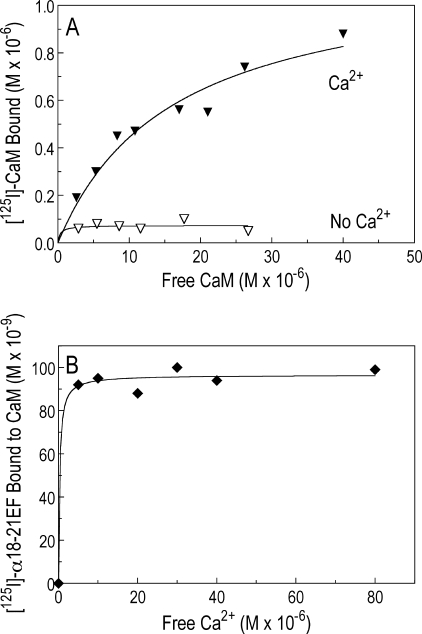
Recombinant α-Spectrin Binds Calmodulin
125I-Labeled CaM bound to GST-α18–21EF in the presence of excess Ca2+ (1.5 mm) but did not bind or only bound minimally in its absence (Fig. 10A). In a single experiment removal of the carboxyl-terminal 13 amino acids, which disrupts EF34, had no effect on CaM binding (data not shown). This implies that CaM binds to EF12. The dissociation constant (Kd = 16.4 ± 2.4 μm and binding capacity of 0.9 ± 0.3 mol of CaM/mol of GST-α18–21EF) is reasonably close to the Kd of 6.7 μm that Burns and Gratzer (37) obtained in analyzing the binding of 125I-labeled CaM to membrane cytoskeletons and full-length spectrin tetramers, which indicates that the binding site they discovered resides in the EF-domain. The interaction of 125I-labeled α18–21EF with CaM-Sepharose was also measured in the presence of varying amounts of Ca2+ and was already maximal at a Ca2+ concentration of 5 μm (Fig. 10B). This is roughly compatible with the affinity of Ca2+ for CaM, which has been estimated to have a Kd of 10.7 μm for each of the four binding sites (38). Taken together the data suggest that a Ca2+-CaM complex binds to the EF-domain and displaces protein 4.2.
FIGURE 10.
The α-spectrin EF-domain binds calmodulin. A, 125I-labeled CaM binding to GST-α18–21EF (0.76 μm) ± Ca2+ (1.5 mm) is shown. The incubation volume was 200 μl. B, 125I-labeled α18–21EF (4.2 μm) binding to CaM-Sepharose (10 μl, diluted 1:1 with HEPES binding buffer) with increasing free Ca2+. 125I-Labeled α18–21EF binding to Sepharose without CaM with increasing calcium was used as the background binding and was subtracted at each point.
DISCUSSION
The EF-domain at the carboxyl-terminal end of erythrocyte α-spectrin (SPTA1) is structurally similar to calmodulin (9) and has four EF-hands, each containing a helix-loop-helix motif. The amino-terminal pair of EF-hands (EF12) is functional, binds Ca2+ with affinities in the low millimolar range (Kd ∼ 0.5 mm) (3, 31), and like calmodulin, change from a closed to an open configuration when Ca2+ is bound (9). The carboxyl-terminal EF-hands belong to the Ca2+-insensitive class of EF-hands, typified by the carboxyl-terminal EF-hands (EF34) in muscle α-actinin, an actin binding relative of spectrins (Ref. 39; see also the Pfam website, Wellcome Trust Sanger Institute). Ca2+-insensitive EF-hands are in a semi-open state, and a pair combine to form a single peptide binding site (Ref. 39; see the Pfam website).
Spectrin EF-hands Must Have a Function
Because the EF-hands in erythrocyte α-spectrin do not bind Ca2+ in a physiological range and no binding functions have been assigned to them, they are generally thought to be inert or vestigial. However, this conflicts with the high degree of sequence conservation throughout the EF-domain (Fig. 11) and with the discovery that sph1J/sph1J (4) and sph4J/sph4J mice have nearly lethal spherocytic hemolytic anemias associated with defects in the EF-domain.4 The sph1J mutation deletes the last 13 amino acids of the α-spectrin chain (4), which includes the distal α-helix of EF4 (Fig. 11). Although sph1J α-spectrin is synthesized at 3–5× the normal rate in the affected animals, it fails to assemble into the membrane skeleton (4), indicating that it lacks some important binding function. Less is known about the sph4J mutation, which replaces a conserved Cys with a Tyr in the middle of the proximal α-helix of EF4 (Fig. 11). It must also lack an important function as red cells in the affected mice have extreme hemolysis but near normal amounts of spectrin.4 In addition, An and co-workers (42) have recently discovered that the Plasmodium falciparum merozoite protein PfEMP3 binds to the first two EF-hands (EF12) in the EF-domain and destabilizes the membrane.
FIGURE 11.
Sequence and structure of the α-spectrin EF-domain. Amino acids that are 70% identical among the α-spectrin family members listed are shaded. The Ca2+-sensitive EF-hands (EF1 and EF2) are on top, the Ca2+-insensitive EF-domain (EF34) is on the bottom. The predicted α-helices in human α-spectrin 1 are marked (h), as are the actual α-helices in chicken α-spectrin-2 (9).
Protein 4.2 Binds to the Spectrin EF-domain
We sought to identify proteins that interact with the EF-domain using GST pulldown binding assays. We began with extracts of native red cell membrane proteins rather than purified or recombinant proteins in case multiple proteins or post-translational modifications were needed for binding. The results showed that the EF-domain binds protein 4.2 and that deletion of the last 13 amino acids of α-spectrin-1, as in the sph1J mouse, abolishes the interaction with protein 4.2. The binding affinity (Kd = 3.0 × 10−7 m) is in the same range as other interactions of membrane skeletal proteins, and it is similar to the binding affinity between 4.2 and spectrin observed by Golan et al. (11) (Kd = 2.8 × 10−7 m) using full-length, native protein 4.2 and by Mandal et al. (18) (Kd = 7.0 × 10−7 m) using a recombinant piece of 4.2. Because the concentrations of spectrin and protein 4.2 are close to 4 × 10−6 m in the red cell, at a minimum, 76% of the protein 4.2 should be bound to spectrin if the Kd is 3 × 10−7 m, assuming the proteins have access to each other. However, because both spectrin and 4.2 are confined to the perimembrane space, the effective concentrations are much higher, and we expect the binding reaction to be nearly complete.
Spectrin Binding Site in Protein 4.2
The present studies show that the spectrin EF-hands bind to the G3 peptide of protein 4.2 (amino acids 410–492) (18). However, Mandal et al. (18), who identified the spectrin binding properties of the G3 peptide, traced the function to a smaller 23-amino acid fragment, amino acids 470–492, called the J3 peptide. This peptide (470GSLQEKEVLERVEKEKMEREKDN492) is highly charged, is predicted to be α-helical, and is a nonhomologous sequence within the transglutaminase family, where protein 4.2 resides. When an homology model of protein 4.2 is created using the known structures of transglutaminase homologs aligned with the sequence of protein 4.2, residues 470–492 lie in an exposed position on the surface of the molecule (43, 44). EF-hands bind α-helical peptides, which lie in the cleft between the planes formed by the EF-hands like a hot dog in a bun (39). We have not determined whether EF12 or EF34 is the binding site for the 4.2 J3 peptide. The Ca2+ sensitivity of binding suggests the former; the lack of binding by the sph1J EF-hands suggests the latter.
Ca2+ Augments Binding of Protein 4.2 to Spectrin
One of the surprises in this study is that physiologic or near physiologic concentrations of Ca2+ ions augment the binding of the spectrin EF-hands to protein 4.2 (Fig. 8). The effect is primarily an increase in the number of 4.2 molecules that bind rather than an increase in affinity. The data do not distinguish whether Ca2+ ions increase the number of 4.2 binding sites on spectrin or cause 4.2 molecules to oligomerize. We find that the spectrin EF-hands and protein 4.2 both bind Ca2+ as isolated proteins, but the Kd values are in the 300–1000 μm range, whereas the effect of Ca2+ on 4.2-spectrin binding is already maximal at 10 μm (Fig. 8D). This could be explained if Ca2+ binds simultaneously to both proteins, but we have no evidence to support that supposition. EF12 are responsible for Ca2+ binding in α-spectrin (3, 31). Homology modeling of protein 4.2 shows that glutamate resides Glu-469 and Glu-474, which are equivalent to the Ca2+-coordinating glutamates in the Ca2+-binding site of transglutaminases, lie right at the beginning of the spectrin binding J3 peptide discussed in the previous section (43, 44) and are also in the right position to affect 4.2-spectrin interactions. These glutamates are conserved in protein 4.2 sequences from many different mammalian species with the exception of mouse, which has an Ala at the equivalent of position 469, and short-tailed opossum, which has a Lys at the position equivalent to 474. It would be interesting to know if protein 4.2 binding to mouse α-spectrin lacks Ca2+ sensitivity.
Calmodulin Also Regulates the Binding of Protein 4.2 to Spectrin
Finally, we found that CaM also binds to the EF-domain in the presence of micromolar amounts of Ca2+ and blocks the binding of protein 4.2 (Figs. 9 and 10). It is well known that CaM binds to the 11th spectrin repeat in the middle of α-spectrin-2 (α-fodrin) (45, 46), where it enhances cleavage by Ca2+-dependent protease I (47) and inhibits the interaction of fodrin and actin (48). There is no previous evidence that CaM interacts with the EF-domain in α-spectrin-1 (erythrocyte spectrin). Indeed, it is widely believed that CaM does not interact with α-spectrin-1 at all, although careful but largely ignored studies performed more than 20 years ago prove the opposite. Burns and Gratzer (37) first showed that 125I-labeled CaM binds to erythrocyte membrane skeletons and to spectrin in a Ca2+, but not Mg2+-dependent, way. Backman and co-workers (49) also showed that CaM and erythrocyte spectrin interact in the presence of Ca2+ and that CaM interferes with the binding of actin to spectrin (50). They traced the binding site to the α-spectrin chain (49). 1–2 μm Ca2+ was needed to achieve full binding, which agrees with our observation that binding is complete at 5 μm Ca2+, the lowest concentration we tested (Figs. 9B and 10B). The observed dissociation constants, 6.7 μm (37), 13 μm (49), and 17 μm (this study), are in reasonable agreement. Burns and Gratzer (37) estimated that roughly 5% of spectrin molecules would have a CaM bound at a Ca2+ concentration of 1 μm but that the portion would increase to 30% if the Ca2+concentration rose to 10 μm. Because estimates of the physiologic range of Ca2+ in the red cell range from 0.1 to 15 μm (32–36), this suggests that under normal conditions CaM does not inhibit interactions between spectrin and protein 4.2 but is poised to do so if Ca2+ concentrations spike.
It is important to note that CaM also binds to the amino-terminal domain of erythrocyte protein 4.1 at both Ca2+-sensitive and Ca2+-insensitive sites (51) and reduces the affinity of the 4.1-CD44 interaction (52) as well as 4.1 interactions with band 3, glycophorin C, and protein p55. Also, CaM inhibits the binding of adducin to actin and the ability of adducin to enhance spectrin-actin binding (53, 54). Because protein 4.2 that is bound to α-spectrin lies right next to 4.1 bound to β-spectrin and both are very close to the adducin-actin and adducin-band 3 complexes, it is likely that complex interactions among all these proteins and perhaps others are regulated by Ca2+ and CaM in ways that we do not understand.
Consequences for Distribution of Band 3
Protein 4.2 binds to band 3 in vitro (10, 26) and in vivo as red cells that lack band 3 also lack protein 4.2 (30). Estimates of the number of band 3 molecules range from 0.9 to 1.6 × 106 monomers/red cell, with a median of 1.2 × 106 (55–63). The 120,000 ankyrin molecules in the red cell (64) account for 480,000 of the band 3 monomers (40% of the total), as ankyrin binds to band 3 tetramers (65, 66) (Fig. 12). The remaining band 3 is dimeric (65) and is generally assumed to be freely diffusing in the lipid bilayer. But if protein 4.2 binds to the spectrin EF-domain in vivo, then it is likely that some band 3 molecules are located in the actin junctional complex where proteins 4.1 and 4.2 reside (Fig. 12). Protein 4.1 binds band 3 in vitro, but it competes with ankyrin (67, 68), so it is likely to bind a separate population of band 3 in vivo (69). Red cells lacking protein 4.1 are deficient in glycophorin C, XK, Duffy, Rh, and possibly band 3 (7) (Fig. 12), which supports the idea that band 3 resides in the actin junctional complex. A recent study showing that the tail domains of α- and β-adducin bind band 3 (8) provides more direct evidence.
FIGURE 12.
Hypothesis regarding the topography of band 3. The ∼120,000 ankyrin molecules (64) bind ∼480,000 of the estimated 1,200,000 band 3 molecules in the human red blood cell as band 3 tetramers. These are located in the ankyrin macromolecular complex, with members of the Rh/RhAG complex, at the head end of spectrin. We assume that a small amount of protein 4.2, at least enough to bind the 17,000 molecules of CD47 (13), is also located in this complex. We show here that protein 4.2 binds to the EF domain of α-spectrin, near the binding site of protein 4.1 on β-spectrin. There are ∼240,000 spectrin dimers (64), ∼240,000 molecules of proteins 4.1, and ∼270,000–300,000 molecules of 4.2 in the red cell. Depending on stoichiometry, protein 4.2 and/or protein 4.1 (7) should bind at least an additional 240,000–480,000 copies of band 3 within the actin junctional complex, presumably as dimers. These linkages along with interactions of the ∼30,000 adducin oligomers with band 3 (8) and connections of proteins 4.1-p55 and glycophorin C (for review, see Ref. 27) create a second attachment site for the membrane skeleton. This leaves 120,000–240,000 band 3 dimers free to diffuse in the lipid bilayer. The membrane skeleton proteins are drawn roughly to size, and protein contacts are indicated where known. The relative positions of many of the proteins within the ankyrin complex and the actin junctional complex are not known, and some of the integral membrane proteins are present in much smaller numbers than band 3 (e.g. CD47), so the ankyrin complex and actin junctional complex must vary in composition. Finally, to show its various interactions, the actin protofilament is drawn perpendicular to the membrane plane, jutting out into the cytoplasm, whereas it actually lies parallel to the membrane. GPA, glycophorin A; GPB, glycophorin B; Ank, ankyrin 1 or ankyrin R.
We show here that protein 4.2 binds to the EF-domain on α-spectrin, which is very close to the protein 4.1 (2) and adducin (70) binding sites on β-spectrin. There are 240,000 spectrin dimers per red cell (64). The number of molecules of proteins 4.1 and 4.2 are not very accurately determined but are roughly the same as spectrin, with perhaps 15–25% more copies of 4.2 based on gel densitometry (27); for example, see lane 1 in Fig. 2. If we assume that proteins 4.1 and 4.2 each form a 1:1 complex with spectrin, then depending on the stoichiometry of their interactions with band 3, they could immobilize another 240,000–480,000 band 3 molecules (20–40%) near the tail end of spectrin (Fig. 12). It could be more if proteins 4.1 and 4.2 each bind separate band 3 dimers or if the roughly 30,000 molecules of adducin bind additional band 3s (8). If not, then there will be ∼240,000–480,000 untethered band 3 molecules (20–40%; 120,000–240,000 dimers) floating in the lipid bilayer. This is within the range of 25% freely diffusing band 3 measured in normal red cells using fluorescence-imaged microdeformation (71) and 46% measured using single particle tracking methods (72).
Is Protein 4.2 Also Part of the Ankyrin Macromolecular Complex?
Most models of the red cell membrane show protein 4.2 located adjacent to band 3 and ankyrin at the head end of spectrin, near the spectrin self-association site. This seems incompatible with protein 4.2 binding to the EF domain, which is located at the other end of the 100 nm spectrin dimer (Fig. 12).
There is reasonable evidence that protein 4.2 binds to ankyrin (10, 73) in vitro, but it is less clear whether ankyrin and protein 4.2 actually bind to each other on the membrane. Rybicki et al. (74) reported that mice lacking red cell ankyrin are also missing 73% of their protein 4.2 and that reconstitution of ankyrin improves 4.2 binding, suggesting that ankyrin is needed for optimal 4.2 binding. However, mice lacking ankyrin are also about 75% spectrin-deficient (75), and in humans with a heterozygous deletion of the ankyrin locus, the levels of protein 4.2 parallel the levels of spectrin, not ankyrin (76). Furthermore, patients and mice that lack protein 4.2 have normal ankyrin levels (77–79), and ankyrin does not extract more easily than normal from 4.2-deficient human red cell membranes (77).
Also, protein 4.2 does not play any direct role in ankyrin binding (26, 80). Reconstitution of stripped membranes with ankyrin or with protein 4.2 does not interfere or enhance the binding of the other protein (10). In fact, the ankyrin-4.2 complex does not bind to band 3 as well as either protein alone (10). This suggests that ankyrin and 4.2 may bind to separate band 3 molecules, which favors the idea that protein 4.2 may be localized to the EF-domain.
Golan et al. (11) find that red cells lacking protein 4.2 show no change in the immobile fraction of band 3, which they attribute to the band 3-ankyrin interaction. But there is a significant shift in the slowly rotating to rapidly rotating fraction of band 3. The authors suggest this is due to loss of low affinity sites between 4.2 and band 3 and suggest that protein 4.2 may interact with some membrane skeletal component other than ankyrin, possibly spectrin (11).
If the slowly rotating fraction of band 3 is the one tethered to protein 4.2 and spectrin (and to 4.1 and adducin), then it is probably not linked as tightly to the skeleton as band 3 attached through ankyrin, and it may be solubilized when membrane skeletons are prepared with Triton X-100. This may explain why band 3 was not observed near spectrin-actin junctions when skeletons were analyzed by immunoelectron microscopy (81).
Protein 4.2 also binds to CD47 in human (but not mouse (82)) red cells, and this interaction clearly occurs in vivo as human red cells that lack protein 4.2 are markedly deficient in CD47 (71, 82, 83). CD47 is part of the Rh/RhAG complex (13, 14), and RhAG binds to ankyrin (41), which strongly suggests that some protein 4.2 is located in the ankyrin macromolecular complex in human red cells; but perhaps not much. There are only about 17,000 molecules of CD47 per red cell (13), which is far less than the number of protein 4.2 molecules, and as noted above, there appear to be more copies of protein 4.2 than copies of protein 4.1 or spectrin in the red cell; that is, roughly 30,000–60,000 copies more (27). Therefore, even if the majority of protein 4.2 is bound to the EF domain of spectrin, there would still be enough protein 4.2 to accommodate all the CD47 molecules. This needs to be tested experimentally, but based on current knowledge, we suspect that protein 4.2 resides in both the ankyrin and actin junctional complexes in human red cells (Fig. 12).
Alternatively, the two complexes may actually be adjacent in the membrane, and protein 4.2 may somehow be shared. It is important to remember that spectrin is usually shown stretched out to its full contour length of 100 nm in models, but simple calculations show that spectrin dimers can only have a length of about 33–37 nm in vivo if the hexameric spectrin skeleton with its 30,000 molecular junctions is only a single layer thick and has an area equal to the area of the red cell, 140 μm2. In fact, this is what is observed in vivo (40). It is not known how spectrin molecules are folded to achieve this length, but some kinds of folding would bring the ankyrin and actin junctional complexes together in the membrane.
The EF-domain Must Do More than Bind Protein 4.2
Although it is interesting that protein 4.2 and Ca2+-CaM bind to the α-spectrin EF-domain, complete elimination of protein 4.2 in vivo only produces a very mild spherocytic hemolytic anemia in mice (79), whereas mice that are homozygous for sph1J (4) and sph4J α-spectrins have near lethal hemolysis.4 The obvious implication is that the EF-domain has other interactions that remain to be defined.
Acknowledgments
We thank Dr. Bernard G. Forget for the generous gift of the human α-spectrin cDNA, Steven Gygi of the Taplin Biological Mass Spectrometry Facility at Harvard Medical School for expert mass spectrometry analysis, and Carl M. Cohen for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL081608 (to S. E. L.) and RO1 HL088468 (to L. L. P.).
In this paper, protein 4.1 refers to the protein 4.1R paralogue, and α- and β-spectrins refer to α-spectrin-1 and β-spectrin-1, respectively.
Robledo, R. F., Lambert, A. J., Birkenmeier, C. S., Cirlan, M. V., Cirlan, A. F. M., Lux, S. E., and Peters, L. L. (2010) Blood, in press.
- ABD
- actin binding domain
- 4.2 G3
- the spectrin-binding G3 tryptic peptide of protein 4.2
- CaM
- calmodulin
- CDB3
- cytoplasmic domain of band 3
- DFP
- diisopropyl fluorophosphate
- DTT
- dithiothreitol
- αEF
- EF-hand domain at the carboxyl terminus of α-spectrin
- α18–21EF
- EF-hand domain preceded by spectrin repeats 18–21 of α-spectrin
- αEFΔ13
- αEF lacking the last 13 amino acids
- GST
- glutathione S-transferase
- PBS
- phosphate-buffered saline
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.Liu S. C., Derick L. H., Palek J. (1987) J. Cell Biol. 104, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An X., Debnath G., Guo X., Liu S., Lux S. E., Baines A., Gratzer W., Mohandas N. (2005) Biochemistry 44, 10681–10688 [DOI] [PubMed] [Google Scholar]
- 3.Travé G., Pastore A., Hyvönen M., Saraste M. (1995) Eur. J. Biochem. 227, 35–42 [DOI] [PubMed] [Google Scholar]
- 4.Wandersee N. J., Birkenmeier C. S., Bodine D. M., Mohandas N., Barker J. E. (2003) Blood 101, 325–330 [DOI] [PubMed] [Google Scholar]
- 5.Gilligan D. M., Bennett V. (1993) Semin. Hematol. 30, 74–83 [PubMed] [Google Scholar]
- 6.Khan A. A., Hanada T., Mohseni M., Jeong J. J., Zeng L., Gaetani M., Li D., Reed B. C., Speicher D. W., Chishti A. H. (2008) J. Biol. Chem. 283, 14600–14609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salomao M., Zhang X., Yang Y., Lee S., Hartwig J. H., Chasis J. A., Mohandas N., An X. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8026–8031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anong W. A., Franco T., Chu H., Weis T. L., Devlin E. E., Bodine D. M., An X., Mohandas N., Low P. S. (2009) Blood 114, 1904–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travé G., Lacombe P. J., Pfuhl M., Saraste M., Pastore A. (1995) EMBO J. 14, 4922–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsgren C., Cohen C. M. (1988) J. Biol. Chem. 263, 10212–10218 [PubMed] [Google Scholar]
- 11.Golan D. E., Corbett J. D., Korsgren C., Thatte H. S., Hayette S., Yawata Y., Cohen C. M. (1996) Biophys. J. 70, 1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindberg F. P., Lublin D. M., Telen M. J., Veile R. A., Miller Y. E., Donis-Keller H., Brown E. J. (1994) J. Biol. Chem. 269, 1567–1570 [PubMed] [Google Scholar]
- 13.Dahl K. N., Westhoff C. M., Discher D. E. (2003) Blood 101, 1194–1199 [DOI] [PubMed] [Google Scholar]
- 14.Bruce L. J., Beckmann R., Ribeiro M. L., Peters L. L., Chasis J. A., Delaunay J., Mohandas N., Anstee D. J., Tanner M. J. (2003) Blood 101, 4180–4188 [DOI] [PubMed] [Google Scholar]
- 15.Oldenborg P. A., Zheleznyak A., Fang Y. F., Lagenaur C. F., Gresham H. D., Lindberg F. P. (2000) Science 288, 2051–2054 [DOI] [PubMed] [Google Scholar]
- 16.Friedrichs B., Koob R., Kraemer D., Drenckhahn D. (1989) Eur. J. Cell Biol. 48, 121–127 [PubMed] [Google Scholar]
- 17.Bennett V., Stenbuck P. J. (1980) J. Biol. Chem. 255, 6424–6432 [PubMed] [Google Scholar]
- 18.Mandal D., Moitra P. K., Basu J. (2002) Biochem. J. 364, 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahr K. E., Laurila P., Kotula L., Scarpa A. L., Coupal E., Leto T. L., Linnenbach A. J., Winkelmann J. C., Speicher D. W., Marchesi V. T., Curtis P. J., Forget B. G. (1990) J. Biol. Chem. 265, 4434–4443 [PubMed] [Google Scholar]
- 20.Galluzzi L., Paiardini M., Magnani M., Nicolas G., Lecomte M. C., Harper S., Speicher D. W. (1999) Blood 93, 2421–2422 [PubMed] [Google Scholar]
- 21.Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. (1990) J. Biol. Chem. 265, 11827–11832 [PubMed] [Google Scholar]
- 22.Korsgren C., Lawler J., Lambert S., Speicher D., Cohen C. M. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper S. L., Begg G. E., Speicher D. W. (2001) Biochemistry 40, 9935–9943 [DOI] [PubMed] [Google Scholar]
- 24.Begg G. E., Harper S. L., Morris M. B., Speicher D. W. (2000) J. Biol. Chem. 275, 3279–3287 [DOI] [PubMed] [Google Scholar]
- 25.Schägger H., von Jagow G. (1987) Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 26.Korsgren C., Cohen C. M. (1986) J. Biol. Chem. 261, 5536–5543 [PubMed] [Google Scholar]
- 27.Grace R. F., Lux S. E. (2009) in Nathan and Oski's Hematology of Infancy and Childhood (Orkin S. H., Ginsburg D., Nathan D. G., Look A. T., Fisher D. E., Lux S. E. (eds) Vol. 1, 7th ed., pp 659–837, Elsevier, Philadelphia [Google Scholar]
- 28.Bhattacharyya R., Das A. K., Moitra P. K., Pal B., Mandal I., Basu J. (1999) Biochem. J. 340, 505–512 [PMC free article] [PubMed] [Google Scholar]
- 29.Risinger M. A., Dotimas E. M., Cohen C. M. (1992) J. Biol. Chem. 267, 5680–5685 [PubMed] [Google Scholar]
- 30.Peters L. L., Shivdasani R. A., Liu S. C., Hanspal M., John K. M., Gonzalez J. M., Brugnara C., Gwynn B., Mohandas N., Alper S. L., Orkin S. H., Lux S. E. (1996) Cell 86, 917–927 [DOI] [PubMed] [Google Scholar]
- 31.Lundberg S., Lehto V. P., Backman L. (1992) Biochemistry 31, 5665–5671 [DOI] [PubMed] [Google Scholar]
- 32.Ferreira H. G., Lew V. L. (1976) Nature 259, 47–49 [DOI] [PubMed] [Google Scholar]
- 33.Wiley J. S., Shaller C. C. (1977) J. Clin. Invest. 59, 1113–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons T. J. B. (1982) J. Membr. Biol. 66, 235–247 [DOI] [PubMed] [Google Scholar]
- 35.O'Rear E. A., Udden M. M., McIntire L. V., Lynch E. C. (1981) Am. J. Hematol. 11, 283–292 [DOI] [PubMed] [Google Scholar]
- 36.Calderón-Salinas J. V., Quintanar-Escorcia M. A., González-Martínez M. T., Hernández-Luna C. E. (1999) Hum. Exp. Toxicol. 18, 327–332 [DOI] [PubMed] [Google Scholar]
- 37.Burns N. R., Gratzer W. B. (1985) Biochemistry 24, 3070–3074 [DOI] [PubMed] [Google Scholar]
- 38.Burger D., Cox J. A., Comte M., Stein E. A. (1984) Biochemistry 23, 1966–1971 [Google Scholar]
- 39.Atkinson R. A., Joseph C., Kelly G., Muskett F. W., Frenkiel T. A., Nietlispach D., Pastore A. (2001) Nat. Struct. Biol. 8, 853–857 [DOI] [PubMed] [Google Scholar]
- 40.Ursitti J. A., Pumplin D. W., Wade J. B., Bloch R. J. (1991) Cell Motil. Cytoskeleton 19, 227–243 [DOI] [PubMed] [Google Scholar]
- 41.Nicolas V., Le Van Kim C., Gane P., Birkenmeier C., Cartron J. P., Colin Y., Mouro-Chanteloup I. (2003) J. Biol. Chem. 278, 25526–25533 [DOI] [PubMed] [Google Scholar]
- 42.Pei X., Guo X., Coppel R., Mohandas N., An X. (2007) J. Biol. Chem. 282, 26754–26758 [DOI] [PubMed] [Google Scholar]
- 43.Toye A. M., Ghosh S., Young M. T., Jones G. K., Sessions R. B., Ramaugé M., Leclerc P., Basu J., Delaunay J., Tanner M. J. (2005) Blood 105, 4088–4095 [DOI] [PubMed] [Google Scholar]
- 44.Satchwell T. J., Shoemark D. K., Sessions R. B., Toye A. M. (2009) Blood Cells Mol. Dis. 42, 201–210 [DOI] [PubMed] [Google Scholar]
- 45.Harris A. S., Croall D. E., Morrow J. S. (1988) J. Biol. Chem. 263, 15754–15761 [PubMed] [Google Scholar]
- 46.Simonovic M., Zhang Z., Cianci C. D., Steitz T. A., Morrow J. S. (2006) J. Biol. Chem. 281, 34333–34340 [DOI] [PubMed] [Google Scholar]
- 47.Harris A. S., Croall D. E., Morrow J. S. (1989) J. Biol. Chem. 264, 17401–17408 [PubMed] [Google Scholar]
- 48.Harris A. S., Morrow J. S. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3009–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berglund A., Backman L., Shanbhag V. P. (1986) FEBS Lett. 201, 306–310 [DOI] [PubMed] [Google Scholar]
- 50.Strömqvist M., Berglund A., Shanbhag V. P., Backman L. (1988) Biochemistry 27, 1104–1110 [DOI] [PubMed] [Google Scholar]
- 51.Han B. G., Nunomura W., Takakuwa Y., Mohandas N., Jap B. K. (2000) Nat. Struct. Biol. 7, 871–875 [DOI] [PubMed] [Google Scholar]
- 52.Nunomura W., Takakuwa Y., Tokimitsu R., Krauss S. W., Kawashima M., Mohandas N. (1997) J. Biol. Chem. 272, 30322–30328 [DOI] [PubMed] [Google Scholar]
- 53.Mische S. M., Mooseker M. S., Morrow J. S. (1987) J. Cell Biol. 105, 2837–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner K., Bennett V. (1987) Nature 328, 359–362 [DOI] [PubMed] [Google Scholar]
- 55.Halestrap A. P. (1976) Biochem. J. 156, 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ship S., Shami Y., Breuer W., Rothstein A. (1977) J. Membr. Biol. 33, 311–323 [DOI] [PubMed] [Google Scholar]
- 57.Funder J., Tosteson D. C., Wieth J. O. (1978) J. Gen. Physiol. 71, 721–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cousin J. L., Motais R. (1979) J. Membr. Biol. 46, 125–153 [DOI] [PubMed] [Google Scholar]
- 59.Wieth J. O. (1979) J. Physiol. 294, 521–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macara I. G., Cantley L. C. (1981) Biochemistry 20, 5095–5105 [DOI] [PubMed] [Google Scholar]
- 61.Cherry R. J., Nigg E. A. (1981) Prog. Clin. Biol. Res. 51, 59–77 [PubMed] [Google Scholar]
- 62.Sato Y., Chiba T., Suzuki Y. (1986) Biochim. Biophys. Acta 856, 11–18 [DOI] [PubMed] [Google Scholar]
- 63.Taylor A. M., Gröbner G., Williamson P. T., Watts A. (1999) Biochemistry 38, 11172–11179 [DOI] [PubMed] [Google Scholar]
- 64.Savvides P., Shalev O., John K. M., Lux S. E. (1993) Blood 82, 2953–2960 [PubMed] [Google Scholar]
- 65.Yi S. J., Liu S. C., Derick L. H., Murray J., Barker J. E., Cho M. R., Palek J., Golan D. E. (1997) Biochemistry 36, 9596–9604 [DOI] [PubMed] [Google Scholar]
- 66.Van Dort H. M., Moriyama R., Low P. S. (1998) J. Biol. Chem. 273, 14819–14826 [DOI] [PubMed] [Google Scholar]
- 67.Lombardo C. R., Willardson B. M., Low P. S. (1992) J. Biol. Chem. 267, 9540–9546 [PubMed] [Google Scholar]
- 68.An X. L., Takakuwa Y., Nunomura W., Manno S., Mohandas N. (1996) J. Biol. Chem. 271, 33187–33191 [DOI] [PubMed] [Google Scholar]
- 69.Workman R. F., Low P. S. (1998) J. Biol. Chem. 273, 6171–6176 [DOI] [PubMed] [Google Scholar]
- 70.Li X., Bennett V. (1996) J. Biol. Chem. 271, 15695–15702 [DOI] [PubMed] [Google Scholar]
- 71.Dahl K. N., Parthasarathy R., Westhoff C. M., Layton D. M., Discher D. E. (2004) Blood 103, 1131–1136 [DOI] [PubMed] [Google Scholar]
- 72.Kodippili G. C., Spector J., Sullivan C., Kuypers F. A., Labotka R., Gallagher P. G., Ritchie K., Low P. S. (2009) Blood 113, 6237–6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su Y., Ding Y., Jiang M., Jiang W., Hu X., Zhang Z. (2006) Mol. Cell. Biochem. 289, 159–166 [DOI] [PubMed] [Google Scholar]
- 74.Rybicki A. C., Musto S., Schwartz R. S. (1995) Blood 86, 3583–3589 [PubMed] [Google Scholar]
- 75.Lux S. E. (1979) Semin. Hematol. 16, 21–51 [PubMed] [Google Scholar]
- 76.Lux S. E., Tse W. T., Menninger J. C., John K. M., Harris P., Shalev O., Chilcote R. R., Marchesi S. L., Watkins P. C., Bennett V., McIntosh S., Collins F. S., Francke U., Ward D. C., Forget B. G. (1990) Nature 345, 736–739 [DOI] [PubMed] [Google Scholar]
- 77.Ideguchi H., Nishimura J., Nawata H., Hamasaki N. (1990) Br. J. Haematol. 74, 347–353 [DOI] [PubMed] [Google Scholar]
- 78.Ghanem A., Pothier B., Marechal J., Ducluzeau M. T., Morle L., Alloisio N., Feo C., Ben Abdeladhim A., Fattoum S., Delaunay J. (1990) Br. J. Haematol. 75, 414–420 [DOI] [PubMed] [Google Scholar]
- 79.Peters L. L., Jindel H. K., Gwynn B., Korsgren C., John K. M., Lux S. E., Mohandas N., Cohen C. M., Cho M. R., Golan D. E., Brugnara C. (1999) J. Clin. Invest. 103, 1527–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. (1980) J. Biol. Chem. 255, 11965–11972 [PubMed] [Google Scholar]
- 81.Derick L. H., Liu S. C., Chishti A. H., Palek J. (1992) Eur. J. Cell Biol. 57, 317–320 [PubMed] [Google Scholar]
- 82.Mouro-Chanteloup I., Delaunay J., Gane P., Nicolas V., Johansen M., Brown E. J., Peters L. L., Van Kim C. L., Cartron J. P., Colin Y. (2003) Blood 101, 338–344 [DOI] [PubMed] [Google Scholar]
- 83.Bruce L. J., Ghosh S., King M. J., Layton D. M., Mawby W. J., Stewart G. W., Oldenborg P. A., Delaunay J., Tanner M. J. (2002) Blood 100, 1878–1885 [DOI] [PubMed] [Google Scholar]