Abstract
Tandem of P domains in a weak inwardly rectifying K+ channel 1 (TWIK1) is a K+ channel that produces unusually low levels of current. Replacement of lysine 274 by a glutamic acid (K274E) is associated with stronger currents. This mutation would prevent conjugation of a small ubiquitin modifier peptide to Lys-274, a mechanism proposed to be responsible for channel silencing. However, we found no biochemical evidence of TWIK1 sumoylation, and we showed that the conservative change K274R did not increase current, suggesting that K274E modifies TWIK1 gating through a charge effect. Now we rule out an eventual effect of K274E on TWIK1 trafficking, and we provide convincing evidence that TWIK1 silencing results from its rapid retrieval from the cell surface. TWIK1 is internalized via a dynamin-dependent mechanism and addressed to the recycling endosomal compartment. Mutation of a diisoleucine repeat located in its cytoplasmic C terminus (I293A,I294A) stabilizes TWIK1 at the plasma membrane, resulting in robust currents. The effects of I293A,I294A on channel trafficking and of K274E on channel activity are cumulative, promoting even more currents. Activation of serotoninergic receptor 5-HT1R or adrenoreceptor α2A-AR stimulates TWIK1 but has no effect on TWIK1I293A,I294A, suggesting that Gi protein activation is a physiological signal for increasing the number of active channels at the plasma membrane.
Keywords: Cell/Endocytosis, Cell/Trafficking, Channels/Potassium, Membrane/Channels, Membrane/Recycling, Receptors/Recycling, Signal Transduction/G Proteins
Introduction
TWIK12 (KCNK1, K2P1) has been cloned from human kidney (1). It has four membrane-spanning segments (M1–M4) and two pore-forming loops (P1 and P2) and forms covalent homodimers (2). Following the characterization of TWIK1, related two-P domain K+ (K2P) channels were isolated from Drosophila, Caenorhabditis elegans, and mammals (3–7). Fifteen related genes are present in the human genome. K2P channels produce background K+ currents modulated by a large variety of physical (temperature, membrane stretch) and chemical (pH, polyunsaturated fatty acids, phospholipids, hormones, and neurotransmitters) stimuli. Gene inactivation has revealed the implication of different K2P channels in a variety of physiological and pathophysiological functions, including cell volume regulation (8), bicarbonate transport and proximal renal tubular acidosis (9), cerebellar excitability and altered motor performance (10), adrenal gland development and primary aldosteronism (11), polyunsaturated fatty acid-mediated neuroprotection (12), sensitivity to volatile anesthetics (12), perception of pain (13), and mood control (14).
Among K2P channels, TWIK1 displays several unique features. Like its closest homolog TWIK2 (KCNK6, K2P6) (15), it produces currents with a rapidly inactivating component. Because of this inactivation, their steady-state current-voltage relationships are much more similar to that of the weak inwardly rectifying ROMK1 current (1) than those of TASK K2P currents that follow the Goldman-Hodgkin-Katz equation (16). Another unique feature of TWIK1 is its low level of functional expression. In Xenopus oocytes, only modest currents are induced despite the high amount of injected cRNA. In transfected mammalian cells, TWIK1 does not produce measurable currents. How can this failure of TWIK1 to produce currents be explained? A first hypothesis is that TWIK1 channels are expressed at the cell surface but silenced. A silencing mechanism recently proposed is the conjugation of a small ubiquitin modifier (SUMO) peptide to lysine 274. In Xenopus oocytes, substitution of lysine 274 by a glutamic acid residue that cannot be used for sumoylation gives rise to robust current expression (17). This work has first gained considerable interest not only because it identified a novel mechanism of ion channel regulation, but also for its general implication in cell biology (18). However, when we analyzed the problem ourselves, we failed to observe any biochemical evidence supporting TWIK1 sumoylation in oocytes, in mammalian cells, or even in vitro. Furthermore, we did not observe any current increase by changing lysine 274 to arginine, a substitution that should have also prevented sumoylation and silencing of the channel. We concluded that the increase of current associated with K274E, and which is absent in K274R, could probably be attributed to a charge effect modifying the TWIK1 gating and that SUMO conjugation at lysine 274 does not underlie TWIK1 silencing (19).
Here, we further explore an alternative hypothesis for TWIK1 silencing: TWIK1 is a functional channel but is not preferentially expressed at the plasma membrane. We have shown previously that TWIK1 is present mainly in the subapical recycling endosomal compartment in native proximal tubule cells in the kidney and in cultured polarized cells (20). In a variety of nonpolarized cells, TWIK1 was detected almost exclusively in the corresponding pericentriolar recycling compartment (20). In this article, we provide new data on the mechanisms that control this surface expression/retrieval of TWIK1. We show that TWIK1 contains a diisoleucine-based motif required for its rapid endocytosis. We also provide evidence for a Gi-dependent stabilization of TWIK1 at the cell surface.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Rabbit anti-TWIK1 polyclonal antibodies have already been described (2). Goat anti-TWIK1 polyclonal antibody (clone V-20) was from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-HA monoclonal antibody (clone HA.7) and chemicals, including clonidine and 5-hydroxytryptamine (5-HT), were purchased from Sigma-Aldrich.
Molecular Biology
The HA tag was added in-frame at the 5′ end of the human TWIK1 sequence by PCR. The amplification product was inserted into the EcoRI/BamHI sites of pCI-iresCD8 (21). Deletions were performed by PCR using oligonucleotides introducing a stop codon followed by a BamHI restriction site. Point mutations and chimeras were obtained by PCR using standard procedures.
Cell Culture and Electrophysiology
Protocols for COS and Madin-Darby canine kidney (MDCK) cell cultures, transient transfection, and development of stable cell lines were described earlier (20). Xenopus oocyte preparation and injection, and oocyte and cell electrophysiological recordings were performed as described previously (19).
Electron Microscopy and Immunochemistry
Cells were fixed with 4% formaldehyde in 0.1 m phosphate buffer, rinsed in the same buffer, and embedded in gelatin (22) before partial dehydration with ethanol and final embedding in LR White resin (23). Immunocytochemistry was performed as described previously (22), by using affinity-purified polyclonal antibodies directed against TWIK1 diluted 1:200. Quantification of colloidal gold density along the boundary of cells was carried out as described (24). F-actin was labeled with phalloidin coupled to Alexa Fluor 647 (Invitrogen). Immunocytochemistry on MDCK cells was performed as described previously (19).
Biochemistry
For cell surface quantification experiments, cells were plated in 12-well dishes and transfected with pCI-CD8 empty or containing sequences encoding either wild type or I293,294A mutant of TASK3-HA/TWIK1 chimera. Forty-eight h after transfection, cells were incubated in complete growth medium containing anti-HA antibody (1:200 dilution). After 2 h, cells were washed, and channel·antibody complexes were detected using secondary goat anti-mouse antibodies coupled with horseradish peroxidase and ECL substrate (Thermo). Luminescence was quantified by using a Luminoskan Ascent from Thermo.
RESULTS
Mutation K274E Has No Effect on TWIK1 Trafficking
We have shown previously that in transfected mammalian cells TWIK1 produced currents only when fused to the HcRed protein (20). We used this strategy to produce functional TWIK1K274E channels and to show the stimulatory effect of the K274E substitution (19). However, we did not check the effect of this mutation on TWIK1 trafficking. Intracellular distributions of TWIK1 and TWIK1K274E were evaluated in stably transfected MDCK cells by fluorescence and electron microscopy (Fig. 1). MDCK cells are epithelial cells of nephric tubule origin that form confluent monolayers of polarized cells on porous membranes. As reported previously, in nonpolarized cells TWIK1 was detected in the same intracellular compartment as Vamp8, a marker of the pericentriolar and vesiculotubular compartment corresponding to recycling endosomes (Fig. 1A). This endosomal compartment is located distal to early endosomes and is not related to the late endosomal/lysosomal degradation pathway. No significant surface staining of TWIK1 could be observed. In kidney proximal tubule cells, TWIK1 is localized to the subapical recycling compartment, a compartment corresponding to the pericentriolar recycling compartment in nonpolarized cells. Electron microscopy from polarized MDCK cells shows that TWIK1 is present mainly underneath the apical brush border with only a few immunogold particles found at a position corresponding to the plasma membrane (Fig. 1B). Mutation K274E has no effect on TWIK1 distribution in nonpolarized or polarized MDCK cells. TWIK1K274E was detected in the same Vamp8-positive compartment as TWIK1 (Fig. 1A). In polarized cells, quantification by electron microscopy confirms that the density of TWIK1K274E detected at the plasma membrane is not significantly different from that of TWIK1 (Fig. 1B). A comparison of the channels present at the surface was obtained by counting the number of gold particles along the ciliated apical plasma membrane. For cells expressing TWIK1, the value is 36 particles along 76.7 μm of membrane, i.e. 2.13 particles/μm, and it is 38 along 81.4 μm (2.14 particles/μm) for TWIK1K274E. This result demonstrates that mutation K274E has no effect on TWIK1 trafficking and gives more support to the hypothesis that K274E modifies channel activity by modifying TWIK1 gating.
FIGURE 1.
K274E does not affect TWIK1 distribution in transfected MDCK cells. A, nonpolarized cells co-expressing GFP-Vamp8 and TWIK1 or TWIK1K274E. Overlapping red and green labeling is in yellow in the merge panels. Nuclei are in blue. Insets represent magnification of the perinuclear staining. B, immunolocalization of TWIK1 by electron microscopy in polarized cells. Open and filled arrowheads show immunogold labeling, respectively, inside the cell and at the plasma membrane. Scale bar, 200 nm.
Identification of an Unconventional Dileucine-based Motif Involved in TWIK1 Silencing
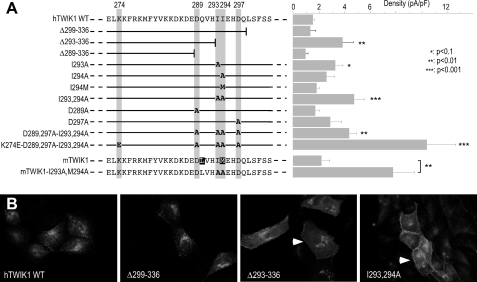
To identify a signal sequence involved in TWIK1 silencing, we designed a screening method based on a combination of mutagenesis, electrophysiology, and fluorescence microscopy. TWIK1 mutants were transiently expressed in COS-7, a fibroblast cell line well suited for electrophysiology. Whole cell currents were measured by voltage clamp at 0 mV (Fig. 2A, right panel). In parallel, TWIK1 mutants were transiently expressed and immunolabeled in MDCK cells that are more suitable than COS-7 for subcellular localization of membrane proteins (Fig. 2B). We progressively deleted the cytoplasmic C terminus of TWIK1 (Fig. 2A, left panel). Deletion of the last 37 residues (Δ299–336) had no effect on intracellular retention (Fig. 2B). Recorded currents were not significantly different from those currents expressed by control COS-7 cells (Fig. 2A). Removing 6 additional residues (Δ293–336) yielded a truncated mutant producing more current and immunostaining compatible with expression at the plasma membrane (Fig. 2, A and B). A more truncated channel (Δ289–336) lost this current activity and was detected in endoplasmic reticulum, suggesting protein misfolding (data not shown). These results show that the sequence extending from residues 293 to 299 is important for functional expression of TWIK1 at the plasma membrane. Interestingly, this segment contains two adjacent isoleucines, Ile-293 and Ile-294. Such a doublet is reminiscent of the repeat present in so-called dileucine signals of internalization. These motifs facilitate endocytosis by forming a link between membrane proteins and the vesicular budding machinery through interaction with adaptor proteins and recruitment of the clathrin coat protein (25). In TWIK1, substitution of these isoleucines by alanines is associated with channel relocalization to the plasma membrane and production of currents similar to those produced by mutant Δ293–336. Substitution of a single isoleucine has milder effect (Fig. 2A). In mouse TWIK1, one of the two isoleucines is not conserved. Ile-294 is replaced by a methionine. However, the silencing signal is conserved: mouse TWIK1I293A,M294A produces more current than mouse TWIK1 (Fig. 2A). Conversely, substitution of isoleucine 294 by methionine in human TWIK1 did not alleviate silencing. These results demonstrate that these residues at position 294 are functionally equivalent and that the motif involved in TWIK1 silencing is functionally conserved in mouse and human.
FIGURE 2.
Diisoleucine motif controls functional expression and distribution of TWIK1. A left, sequence of a portion of the C-terminal domain of TWIK1 that contains the diisoleucine doublet, and schematic representation of the mutants. Mutated residues are in bold. Right, current recorded at +40 mV in transfected COS-7 cells (n = 3–5). B, immunolocalization in MDCK-transfected cells.
Dileucine-based motifs involved in endocytosis usually contain acidic residues at positions −4 or +3 of the leucine repeat. By affecting the spatial location of the dileucine repeat, these negatively charged residues are important for trafficking. We mutated aspartates Asp-289 and Asp-297 alone or in combination with I293,294A. Mutation D297A is associated with a current increase, but this change is not significant (Fig. 2A).
As reported previously (19) and in agreement with the fact that mutation K274E has no effect on TWIK1 trafficking (Fig. 1), TWIK1K274E does not produce currents in COS-7 cells. However, K274E is associated with a current increase when introduced into TWIK1I293A,I294A (or D289A,D297A-I293A,I294A as shown in Fig. 2A). This indicates that these mutations that increase respectively channel activity and channel density in the plasma membrane have additive effects.
TWIK1I293A,I294A Is Expressed Mainly at the Plasma Membrane
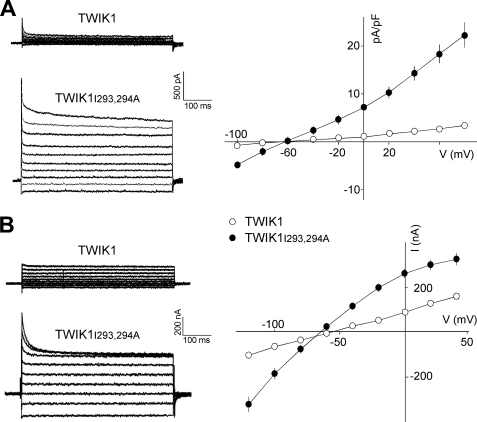
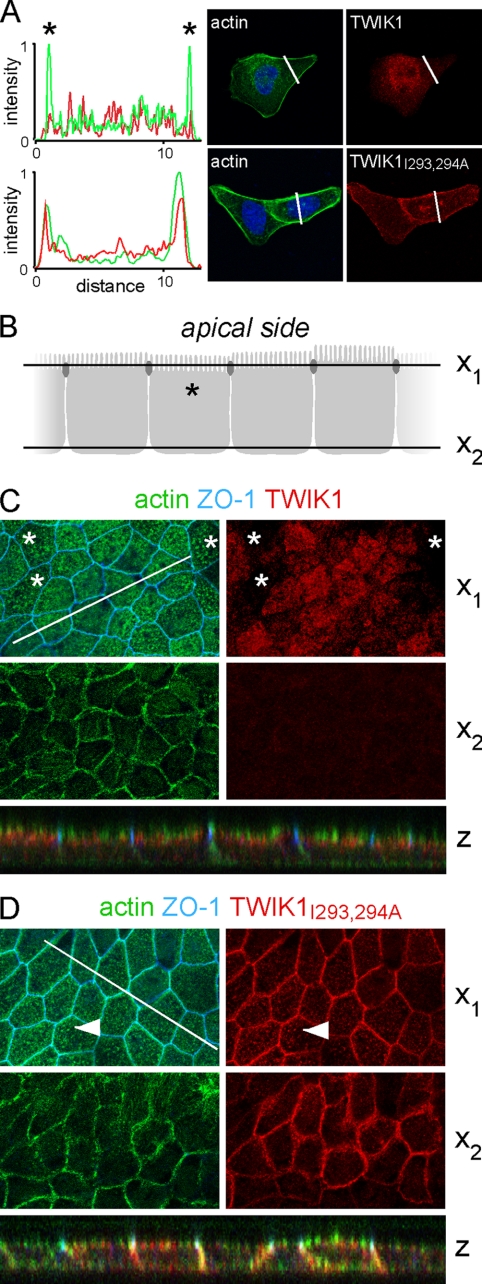
The importance of the diisoleucine repeat was verified and further characterized in stably transfected MDCK cells. Fig. 3A shows currents produced by TWIK1 and TWIK1I293A,I294A. They display a fast inactivation component as observed in Xenopus oocytes (Fig. 3B) and thoroughly characterized for the closely related channel TWIK2 (15). The kinetics of TWIK1 inactivation are extremely fast and overlap the membrane capacitive discharge associated with the voltage pulse. This fast inactivating peak current is clearly not a stimulation artifact and constitutes a hallmark of the TWIK currents. As observed in COS-7 (Fig. 2A) and MDCK (Fig. 3A) cells, TWIK1I293A,I294A produces larger currents than TWIK1 in oocytes (Fig. 3B). Next we compared subcellular distributions of TWIK1 and TWIK1I293A,I294A in nonpolarized and polarized cells. TWIK1I293A,I294A is found at the cell surface. Its immunolabeling overlaps the submembraneous F-actin stained by fluorescein isothiocyanate-phalloidin (Fig. 4A). In polarized cells, we used confocal microscopy to discriminate apical and basolateral membranes (Fig. 4B). TWIK1 was detected exclusively below the actin-rich microvilli of the brush border (Fig. 4C). TWIK1I293A,I294A does not show this subapical localization. It has the same localization as actin in the basolateral membrane and in apical microvilli (Fig. 4D). These results confirm that the isoleucine repeat is responsible for the absence of TWIK1 at the cell surface. They also confirm that TWIK1 is a functional channel that produces measurable currents when it reaches the plasma membrane.
FIGURE 3.
Electrophysiological characterization of TWIK1 and TWIK1I293A,I294A in MDCK cells (A) and Xenopus oocytes (B). A left, current traces recorded in MDCK cells during voltage pulses ranging from −100 mV to +80 mV in 20-mV steps from a holding potential of −80 mV. Right, current density was determined at all test potentials from the steady-state currents and the whole cell capacitance recorded as in the left panel. Each value is the mean ± S.E., n cells for TWIK1 (n = 10), TWIK1I293A,I294A (n = 9). B left, current traces recorded in Xenopus oocytes during voltage pulses ranging from −120 mV to +40 mV in 20-mV steps from a holding potential of −80 mV. Right, current-voltage relationships deduced from steady-state currents recorded as in the left panel. Each value is the mean ± S.E., n cells for TWIK1 (n = 29), TWIK1I293A,I294A (n = 31).
FIGURE 4.
TWIK1 and TWIK1I293A,I294A distributions in stably transfected MDCK cells. A, immunolocalization in nonpolarized cells. Cell nuclei are in blue. Actin is labeled with fluorescein isothiocyanate-phalloidin. Left, line scan through cells expressing TWIK1 or TWIK1I293A,I294A. Scan sections are shown as a white line on the images. Stars indicate signals at the peripheral plasma membrane. B–D, immunolocalization in polarized MDCK cells by confocal microscopy. B, schematic representation of the polarized cells monolayer, with the x1 and x2 axes corresponding to cross-sections shown in C and D. ZO-1 is expressed in tight junctions represented here as dark ovals. C and D, cross-sections in monolayer of polarized cells expressing TWIK1 (C) or TWIK1I293A,I294A (D). Z is the reconstituted xz image corresponding to a z section along the white line. Asterisks mark cells in which the actin-rich microvilli constituting the brush-border staining are labeled as dot. The position of the x1 axis in these cells is indicated in B. Filled arrowheads show the colabeling of actin and TWIK1I293A,I294A in these microvilli (not seen with TWIK1 in cells labeled with an asterisk).
The Diisoleucine-based Motif Can Be Transferred to Another K2P Channel
TASK3 is a K2P channel expressed at the plasma membrane. A chimera TASK3CtTWIK1, in which the cytoplasmic C terminus of TASK3 is replaced by the cytoplasmic C terminus of TWIK1, has the same intracellular distribution as that of TWIK1 (supplemental Fig. 1A). It colocalizes with vamp8-GFP (not shown) demonstrating that TWIK1 sorting to recycling endosomes is conferred by its cytoplasmic C terminus. When the diisoleucine motif is mutated, the corresponding channel TASK3CtTWIK1I293A,I294A now reappears at the cell surface (supplemental Fig. 1A). The proportions of TASK3, TASK3CtTWIK1, and TASK3CtTWIK1I293A,I294A present at the plasma membrane were quantified by taking advantage of a HA tag introduced in the extracellular M2P3 loop. Transfected cells were incubated in the presence of anti-HA antibody after fixation and permeabilization (total labeling) or on living cells kept at 4 °C (cell surface labeling). HA tag·HA antibody complexes were detected using a secondary antibody coupled to horseradish peroxidase and quantified by luminescence (supplemental Fig. 1B). The results confirm that TASK3 and TASK3CtTWIK1I293A,I294A are present in similar proportions at the cell surface (respectively, 41.5 ± 18.6 and 66.1 ± 12.2%, n = 3), whereas TASK3CtTWIK1 is mostly intracellular (9.8 ± 12.8%, n = 3). Together, these results show that the cytoplasmic C terminus of TWIK1 constitutes a transferable cassette that contains all of the motifs required for targeting to recycling endosomal compartment. These results also show that the diisoleucine repeat prevents the stable expression of the protein at the plasma membrane.
TWIK1 Is Rapidly Internalized through a Dynamin-dependent Mechanism
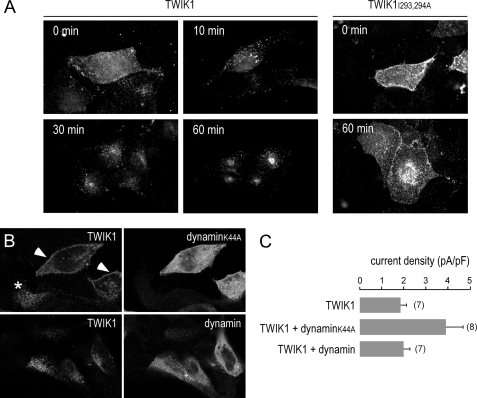
We next examined alternative hypotheses about TWIK1 trafficking following biosynthesis: Either TWIK1 is directly sorted and addressed to the recycling endosomes, or it reaches the plasma membrane before being endocytosed and routed to the recycling compartment. In the latter case, the diisoleucine motif would constitute a strong signal for endocytosis. We investigated the TWIK1 dynamics at the cell surface. Transiently transfected MDCK cells expressing TWIK1 were incubated at 4 °C with antibodies directed against its extracellular M1P1 loop (2). After washing, cells were incubated for various periods of time at 37 °C before fixation and labeling of the TWIK1·antibody complexes (Fig. 5A). A diffuse staining at the cell surface was observed when cells were fixed directly after the 4 °C incubation with the antibody (Fig. 5A, upper left panel). A 10-min incubation at 37 °C induced a redistribution of the labeling into bright vesicles. Redistribution is already observed after 5 min (data not shown). Progressively, the staining evolved to give a punctate and perinuclear distribution at 60 min. Under the same conditions, TWIK1I293A,I294A gave a surface staining that was not modified by incubation at 37 °C even after 60 min (Fig. 5A). Together, these results show that TWIK1 reaches the cell surface before being rapidly internalized and addressed to the recycling endosomal compartment. They also show that the diisoleucine-based motif controls TWIK1 endocytosis from the cell surface. Previously, we have shown that overexpression of TWIK1 was able to inhibit clathrin-dependent internalization of the transferrin receptor suggesting a link between clathrin-dependent endocytosis and TWIK1 (20). We co-expressed TWIK1 with a dominant negative mutant of dynamin I (dynaminK44A) that is known to block clathrin-mediated endocytosis (26, 27). Fig. 5B shows that expression of dynaminK44A, but not dynamin, induces a partial redistribution of TWIK1 to the cell surface. This relocation is correlated with a higher density of current (Fig. 5C). The effect of dynaminK44A is always more drastic in MDCK cells transiently expressing TWIK1 compared with MDCK cells stably expressing TWIK1 (data not shown). This suggests that dynaminK44A affects neosynthesized channels present at the plasma membrane and promotes their accumulation in this location.
FIGURE 5.
Dynamic TWIK1 retrieval from the cell surface. A, transfected MDCK cells were incubated at 4 °C in the presence of an antibody directed against the M1P1 external loop of TWIK1. After washes, cells were incubated at 37 °C for the indicated period of time, then fixed, permeabilized, and TWIK3·antibody complexes were labeled with a fluorescent secondary antibody. The same experiment was performed on MDCK cells expressing TWIK1 (left panels) and TWIK1I293A,I294A (right panels). B, MDCK cells were co-transfected with TWIK1 and dynamin-GFP or dynaminK44-GFP. Twenty-four h after transfection, cells were fixed and permeabilized. Total TWIK1 was detected by immunocytochemistry. A redistribution of TWIK1 at the cell surface is clearly visible in cells co-expressing TWIK1 and dynaminK44 (filled arrowheads) but not in cells expressing TWIK1 alone (asterisk) or cells co-expressing TWIK1 and dynamin. C, current densities measured at +40 mV in conditions corresponding to B. Each value is the mean ± S.E. (error bars), and the number of tested cell is indicated.
Activation of Gi-coupled Receptors Increases TWIK1 Currents
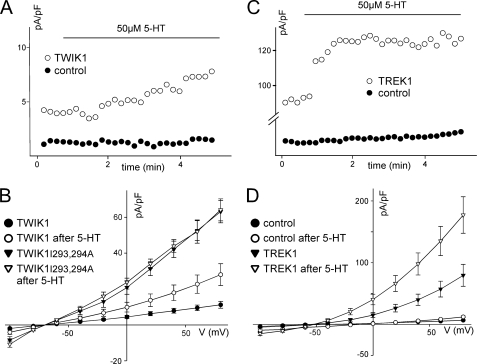
The data presented above indicate that TWIK1 silencing is related to its intracellular sequestration in recycling endosomes. In a recent article, Deng et al. (28) reported the inhibitory action of serotonin in neurons of the entorhinal cortex and linked this inhibition to the activation of a TWIK1 current. They also showed that in HEK cells, TWIK1 was stimulated by activation of the serotonin receptor 5-HT1R. We observed a similar effect. Upon application of serotonin, a current appears in TWIK1-expressing HEK cells (17.9 ± 4.3 pA/pF at +40 mV after 10 min of 5-HT application (50 μm) versus 7.9 ± 1.3 pA/pF before application, n = 15) that is not present in control cells (3.5 ± 0.6 pA/pF after 5-HT application versus 2.8 ± 0.5 pA/pF before application, n = 15, Fig. 6, A and B). This current displays a rapidly inactivating component (data not shown), as observed in MDCK cells and Xenopus oocytes (Fig. 3). As expected, TWIK1I293A,I294A produces much more current than TWIK1, but there is no significant effect of serotonin application (44 ± 3.6 pA/pF at +40 mV after 10 min of 5-HT application versus 42.1 ± 4.6 pA/pF before application, n = 5).
FIGURE 6.
Activation of the serotonin receptor 5-HT1R stimulates TWIK1 and TREK1 channels in HEK cells. Cells were transfected with a plasmid for simultaneous expression of 5-HT1R and GFP (pCi5HT1R-Ires-GFP) and with a plasmid for the simultaneous expression of Discosoma red fluorescent protein and the indicated K+ channel thanks to an Internal Ribosome Entry Site (Ires) (pTWIK1-Ires-DsRed, pTWIK1I293A,I294A-Ires-DsRed, and pTREK1-Ires-DsRed). Cells co-expressing 5-HT1R and the indicated channel were visualized by green and red fluorescences. Control cells express only 5-HT1R (showing green fluorescence but not red fluorescence). Forty-eight h after transfection, HEK cells co-expressing 5-HT1R and TWIK1 or TWIK1I293A,I294A (A and B), or TREK1 (C and D) were perfused with 5-HT as indicated, and currents were measured at +40 mV (A and C). The corresponding current-voltage relationships deduced from steady-state currents recorded before and after a 10-min application of 5-HT are shown in B and D.
Binding of 5-HT to 5-HT1R activates Gi protein that inhibits adenylate cyclase, resulting in a decrease in cAMP levels and cAMP-dependent protein kinase activity. TWIK-related K+ channels (TREK1) are inhibited by cAMP-dependent protein kinase-dependent phosphorylation of a serine residue located in their cytoplasmic C terminus (21, 29). Activation of Gi-coupled receptors results in an increase of TREK1 currents related to a dephosphorylation of the same residue (30). As expected, activation of 5-HT1R results in an increase of TREK1 currents in HEK cell (92.8 ± 8 pA/pF at +40 mV after 10 min of 5-HT application versus 50.3 ± 9.8 pA/pF before application, n = 8, Fig. 6, C and D). This effect is rapid. After less than 1 min (Fig. 6C), the effect is maximal. For TWIK1, the effect is much slower (Fig. 6A), and >7 min is necessary to reach a plateau (data not shown), suggesting that unlike TREK1, TWIK1 is not regulated by dephosphorylation of channels already present in the plasma membrane. The slow kinetics of activation of TWIK1 and the absence of effect on TWIK1I293,294A strongly suggest that 5-HT1R activation acts on the trafficking of TWIK1 and results in more channels at the plasma membrane. We have observed the same effect in MDCK cells by activating endogenous Gi-coupled adrenergic receptor α2A-AR (31): application of α2-AR agonist clonidine promotes current expression in TWIK1-expressing cells: 20.75 ± 1.4 pA/pF at +40 mV after 10 min of clonidine application (10 μm) versus 4.3 ± 0.4 pA/pF before application that is not present in control cells (3.7 ± 2 pA/pF after 10 min versus 1.9 ± 0.4 pA/pF before application).
DISCUSSION
Low or absent functional expression of TWIK1 in heterologous expression systems has led to contradictory reports. Here, we provide key insights on this unusual behavior. TWIK1 is a functional channel but is constitutively and rapidly internalized from the cell surface before being stored in recycling endosomes. At any time, a modest pool of neosynthesized channels is present at the cell surface en route to the recycling endosomes. Given the high level of expression in Xenopus oocytes, the fraction of channels present in the plasma membrane could be sufficient to generate a small but detectable current. In mammalian cells, the expression level may be lower, or constitutive endocytosis more effective, resulting in no measurable currents. Here, we also show that stimulation of Gi-coupled receptors 5-HT1R and α2A-AR leads to an increase of TWIK1 current. This effect does not seem to be related to a regulation of channels already present at the cell surface because TWIK1I293A,I294A is not stimulated.
We have previously established that TWIK1 is expressed in the pericentriolar endosomal recycling compartment of nonpolarized cells and in the corresponding subapical recycling compartment of polarized epithelial cells. We have also shown that TWIK1 interacts with a complex of proteins comprising the nucleotide exchange factor EFA6 and the small G protein ARF6 (20), a protein actively involved in the recycling of membrane proteins and plasma membrane (32). These results suggested that the absence of TWIK1 at the plasma membrane was a major factor in the lack of TWIK1 current upon heterologous expression. As an additional support for this hypothesis, we have shown that a fusion protein comprising HcRed fluorescent protein fused to the N terminus of TWIK1 is able to reach the plasma membrane producing macroscopic currents in MDCK and COS-7 cells. We have proposed that the steric hindrance and/or the masking of retrieval signals resulting from the fused peptide was able to relieve intracellular retention of TWIK1 or to slow its retrieval from the plasma membrane. Now, we have identified a retrieval motif consisting of a diisoleucine repeat located in the cytoplasmic C terminus of TWIK1. When this motif is mutated, the resulting TWIK1I293A,I294A channel redistributes at the cell surface and produces macroscopic currents (Figs. 2 and 4). In the mouse TWIK1 protein, isoleucine 24 is replaced by a methionine. However, methionine 294 plays the same role as isoleucine, both residues being interchangeable without affecting surface retrieval (Fig. 2A). Dileucine-based motifs are classical signals for endocytosis (25). They can accommodate an isoleucine instead of the second leucine. Their consensus sequence is D/E(XXXL)L/I. They form a link between membrane proteins and the vesicular budding machinery through interaction with adaptor proteins and recruitment of the clathrin coat protein (33, 34). Sometimes both leucine residues are replaced by isoleucines. For example, K+ channel Kir2.3 contains a diisoleucine motif that is required for internalization (35). However, the two isoleucines cannot be replaced by leucines without losing Kir2.3 endocytosis. In TWIK1, both isoleucines can be replaced by leucines without affecting endocytosis (data not shown), indicating that diisoleucine-based motifs in TWIK1 and Kir2.3 are both unconventional but different.
In polarized MDCK cells, replacement of isoleucines by alanines abolishes the subapical distribution of TWIK1: the channel redistributes in apical and basolateral membranes (Fig. 4D). These data indicate that after synthesis, TWIK1 reaches the plasma membrane in a nonpolarized manner, before being internalized and specifically addressed to the subapical recycling compartment. What could be the signal responsible for this localization? Our study shows that this signal is located between residues 269 and 299 and can be transferred to TASK3 by replacing its C terminus by the TWIK1 C terminus (supplemental Fig. 1). So far, the detailed study of this sorting signal has been impeded by the fact that many mutations in this region lead to retention of the mutants in the endoplasmic reticulum (data not shown). Also, given that mutation of the diisoleucine motif blocks TWIK1 in the plasma membrane, we could not establish whether this unconventional dileucine motif necessary for endocytosis has also a role in the subsequent sorting of TWIK1 and its final localization in the recycling endosomal compartment.
Many channels and receptors transit through recycling endosomes where they accumulate to various extents. For instance, pacemaker channel HCN4 (36) and aquaporin AQ2 (37) are detected mainly in this compartment. The cystic fibrosis transmembrane conductance regulator CFTR also recycles at the cell surface from recycling endosomes (38, 39). Such a recycling has also been reported for AMPA receptors (40), KCNQI/KCNQ2 (41) and TRPV5, V6 channels (42). All of these membrane proteins that internalize but are not addressed to late endosomes and lysosomes for degradation recycle to the plasma membrane in a controlled manner (43, 44). For example, HCN4 density at the cell surface is increased following stimulation by insulin in a mechanism involving phospholipase D (36). KCNQ1/KCNE1 exocytosis is enhanced by the serum- and glucocorticoid-inducible kinase 1 and requires ion and activation of phosphoinositide 3-phosphate 5-kinase and generation of phosphatidylinositol 3,5-biphosphate (41). AQ2 increases at the cell surface following vasopressin binding to its Gs-coupled receptor cAMP-dependent protein kinase activation (45). Here, we show that stimulation of a Gi-coupled serotonin receptor could provide a physiological signal for a TWIK1 increase at the cell surface. The effect is much slower than that for TREK1, which is regulated by dephosphorylation, and is not observed for TWIK1I293A,I294A, which is already present at the cell surface. Currently, we do not know whether the increase of TWIK1 is due to a forward signal that allows its exit from a particular recycling subcompartment or to inhibition of its endocytosis leading to accumulation at the cell surface. The slow kinetics suggest that the latter hypothesis is the more likely. A similar behavior has already been described: Vasopressin causes AQ2 to accumulate in “endocytosis-resistant” membrane domains (46). TWIK1 and Gi-coupled receptors to hormones and neuromodulators are both expressed in many tissues. Recruitment of TWIK1 K+ channels to the cell surface may constitute a general mechanism of cell excitability modulation by hormones binding to Gi-coupled receptors. Changes in membrane potential resulting from this regulation may also play a role in modifying transport in the renal proximal tubule where TWIK1 is well expressed. In rat proximal tubule membrane brush border, ionic permeability is under regulation of hormones that activate or deactivate G proteins that govern adenylate cyclase activity (Gi/Gs). For example, activation of Gi by angiotensin II significantly decreases Cl− permeability relative to that of K+ (47). In future studies, it would be interesting to evaluate the contribution of cell surface TWIK1 readdressing in these variations of membrane ionic permeability. It is also interesting to note that by binding to the cytoplasmic C terminus of TWIK1, the complex made of EFA6·ARF6GDP may mask the diisoleucine-based motif and modulate TWIK1 endocytosis. Whether the two pathways involving EFA6 and Gi and leading to an increase of TWIK1 at the cell surface act independently or in a coordinated manner remains to be established.
Supplementary Material
Acknowledgment
We thank Maud Larroque for technical assistance.
This work was supported by the Fondation pour la Recherche Médicale (Equipe labellisée to F. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- TWIK1
- tandem of P domains in a weak inwardly rectifying K+ channel 1
- K2P channel
- two-P domain K+ channel
- HA
- hemagglutinin
- 5-HT
- 5-hydroxytryptamine
- 5-HT1R
- 5-HT1 receptor
- MDCK
- Madin-Darby canine kidney
- pF
- picofarad
- GFP
- green fluorescent protein
- TREK1
- TWIK-related K+ channel 1.
REFERENCES
- 1.Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. (1996) EMBO J. 15, 1004–1011 [PMC free article] [PubMed] [Google Scholar]
- 2.Lesage F., Reyes R., Fink M., Duprat F., Guillemare E., Lazdunski M. (1996) EMBO J. 15, 6400–6407 [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein S. A., Bayliss D. A., Kim D., Lesage F., Plant L. D., Rajan S. (2005) Pharmacol. Rev. 57, 527–540 [DOI] [PubMed] [Google Scholar]
- 4.Kim D. (2005) Curr. Pharm. Des. 11, 2717–2736 [DOI] [PubMed] [Google Scholar]
- 5.Lesage F., Lazdunski M. (2000) Am. J. Physiol. Renal Physiol. 279, F793–F801 [DOI] [PubMed] [Google Scholar]
- 6.Patel A. J., Honoré E. (2001) Trends Neurosci. 24, 339–346 [DOI] [PubMed] [Google Scholar]
- 7.Talley E. M., Sirois J. E., Lei Q., Bayliss D. A. (2003) Neuroscientist 9, 46–56 [DOI] [PubMed] [Google Scholar]
- 8.Barriere H., Belfodil R., Rubera I., Tauc M., Lesage F., Poujeol C., Guy N., Barhanin J., Poujeol P. (2003) J. Gen. Physiol. 122, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warth R., Barrière H., Meneton P., Bloch M., Thomas J., Tauc M., Heitzmann D., Romeo E., Verrey F., Mengual R., Guy N., Bendahhou S., Lesage F., Poujeol P., Barhanin J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8215–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aller M. I., Veale E. L., Linden A. M., Sandu C., Schwaninger M., Evans L. J., Korpi E. R., Mathie A., Wisden W., Brickley S. G. (2005) J. Neurosci. 25, 11455–11467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heitzmann D., Derand R., Jungbauer S., Bandulik S., Sterner C., Schweda F., El Wakil A., Lalli E., Guy N., Mengual R., Reichold M., Tegtmeier I., Bendahhou S., Gomez-Sanchez C. E., Aller M. I., Wisden W., Weber A., Lesage F., Warth R., Barhanin J. (2008) EMBO J. 27, 179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heurteaux C., Guy N., Laigle C., Blondeau N., Duprat F., Mazzuca M., Lang-Lazdunski L., Widmann C., Zanzouri M., Romey G., Lazdunski M. (2004) EMBO J. 23, 2684–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alloui A., Zimmermann K., Mamet J., Duprat F., Noël J., Chemin J., Guy N., Blondeau N., Voilley N., Rubat-Coudert C., Borsotto M., Romey G., Heurteaux C., Reeh P., Eschalier A., Lazdunski M. (2006) EMBO J. 25, 2368–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heurteaux C., Lucas G., Guy N., El Yacoubi M., Thümmler S., Peng X. D., Noble F., Blondeau N., Widmann C., Borsotto M., Gobbi G., Vaugeois J. M., Debonnel G., Lazdunski M. (2006) Nat. Neurosci. 9, 1134–1141 [DOI] [PubMed] [Google Scholar]
- 15.Patel A. J., Maingret F., Magnone V., Fosset M., Lazdunski M., Honoré E. (2000) J. Biol. Chem. 275, 28722–28730 [DOI] [PubMed] [Google Scholar]
- 16.Duprat F., Lesage F., Fink M., Reyes R., Heurteaux C., Lazdunski M. (1997) EMBO J. 16, 5464–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajan S., Plant L. D., Rabin M. L., Butler M. H., Goldstein S. A. (2005) Cell 121, 37–47 [DOI] [PubMed] [Google Scholar]
- 18.Wilson V. G., Rosas-Acosta G. (2005) Sci. STKE 2005, pe32. [DOI] [PubMed] [Google Scholar]
- 19.Feliciangeli S., Bendahhou S., Sandoz G., Gounon P., Reichold M., Warth R., Lazdunski M., Barhanin J., Lesage F. (2007) Cell 130, 563–569 [DOI] [PubMed] [Google Scholar]
- 20.Decressac S., Franco M., Bendahhou S., Warth R., Knauer S., Barhanin J., Lazdunski M., Lesage F. (2004) EMBO Rep. 5, 1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink M., Duprat F., Lesage F., Reyes R., Romey G., Heurteaux C., Lazdunski M. (1996) EMBO J. 15, 6854–6862 [PMC free article] [PubMed] [Google Scholar]
- 22.Gounon P. (2002) in Methods in Microbiology, Molecular Cellular Microbiology (Sansonetti P., Zychlinsky A. eds) vol. 31, 551–557, Academic Press, San Diego [Google Scholar]
- 23.Newman G. R., Hobot J. A. (2001) in Resin Microscopy and One-Section Immunocytochemistry, 2nd Ed., Springer-Verlag, Berlin [Google Scholar]
- 24.Griffiths G. (1993) in Fine Structure Immunochemistry, Springer-Verlag, Berlin [Google Scholar]
- 25.Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 26.Damke H., Baba T., Warnock D. E., Schmid S. L. (1994) J. Cell Biol. 127, 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herskovits J. S., Burgess C. C., Obar R. A., Vallee R. B. (1993) J. Cell Biol. 122, 565–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng P. Y., Poudel S. K., Rojanathammanee L., Porter J. E., Lei S. (2007) Mol. Pharmacol. 72, 208–218 [DOI] [PubMed] [Google Scholar]
- 29.Patel A. J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. (1998) EMBO J. 17, 4283–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cain S. M., Meadows H. J., Dunlop J., Bushell T. J. (2008) Mol. Cell. Neurosci. 37, 32–39 [DOI] [PubMed] [Google Scholar]
- 31.Okusa M. D., Lynch K. R., Rosin D. L., Huang L., Wei Y. Y. (1994) Am. J. Physiol. Renal Physiol. 267, F347–F353 [DOI] [PubMed] [Google Scholar]
- 32.Altschuler Y., Liu S., Katz L., Tang K., Hardy S., Brodsky F., Apodaca G., Mostov K. (1999) J. Cell Biol. 147, 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonifacino J. S., Lippincott-Schwartz J. (2003) Nat. Rev. Mol. Cell. Biol. 4, 409–414 [DOI] [PubMed] [Google Scholar]
- 34.Edeling M. A., Smith C., Owen D. (2006) Nat. Rev. Mol. Cell. Biol. 7, 32–44 [DOI] [PubMed] [Google Scholar]
- 35.Mason A. K., Jacobs B. E., Welling P. A. (2008) J. Biol. Chem. 283, 5973–5984 [DOI] [PubMed] [Google Scholar]
- 36.Hardel N., Harmel N., Zolles G., Fakler B., Klocker N. (2008) Cardiovasc. Res. 79, 52–60 [DOI] [PubMed] [Google Scholar]
- 37.Sun T. X., Van Hoek A., Huang Y., Bouley R., McLaughlin M., Brown D. (2002) Am. J. Physiol. Renal Physiol. 282, F998–F1011 [DOI] [PubMed] [Google Scholar]
- 38.Gentzsch M., Chang X. B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E., Riordan J. R. (2004) Mol. Biol. Cell 15, 2684–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picciano J. A., Ameen N., Grant B. D., Bradbury N. A. (2003) Am. J. Physiol. Cell Physiol. 285, C1009–C1018 [DOI] [PubMed] [Google Scholar]
- 40.Park M., Penick E. C., Edwards J. G., Kauer J. A., Ehlers M. D. (2004) Science 305, 1972–1975 [DOI] [PubMed] [Google Scholar]
- 41.Seebohm G., Strutz-Seebohm N., Birkin R., Dell G., Bucci C., Spinosa M. R., Baltaev R., Mack A. F., Korniychuk G., Choudhury A., Marks D., Pagano R. E., Attali B., Pfeufer A., Kass R. S., Sanguinetti M. C., Tavare J. M., Lang F. (2007) Circ. Res. 100, 686–692 [DOI] [PubMed] [Google Scholar]
- 42.van de Graaf S. F., Hoenderop J. G., van der Kemp A. W., Gisler S. M., Bindels R. J. (2006) Pflügers Arch. Eur. J. Physiol. 452, 407–417 [DOI] [PubMed] [Google Scholar]
- 43.Hoekstra D., Tyteca D., van IJzendoorn S. C. (2004) J. Cell Sci. 117, 2183–2192 [DOI] [PubMed] [Google Scholar]
- 44.Mellman I., Nelson W. J. (2008) Nat. Rev. Mol. Cell. Biol. 9, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nedvetsky P. I., Tamma G., Beulshausen S., Valenti G., Rosenthal W., Klussmann E. (2009) Handb. Exp. Pharmacol. 190, 133–157 [DOI] [PubMed] [Google Scholar]
- 46.Bouley R., Hawthorn G., Russo L. M., Lin H. Y., Ausiello D. A., Brown D. (2006) Biol. Cell 98, 215–232 [DOI] [PubMed] [Google Scholar]
- 47.Lipkowitz M. S., London R. D., Beck J. C., Abramson R. G. (1992) Am. J. Physiol. Renal Physiol. 263, F144–F151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.