Abstract
Fungal taxonomists routinely encounter problems when dealing with asexual fungal species due to poly- and paraphyletic generic phylogenies, and unclear species boundaries. These problems are aptly illustrated in the genus Phoma. This phytopathologically significant fungal genus is currently subdivided into nine sections which are mainly based on a single or just a few morphological characters. However, this subdivision is ambiguous as several of the section-specific characters can occur within a single species. In addition, many teleomorph genera have been linked to Phoma, three of which are recognised here. In this study it is attempted to delineate generic boundaries, and to come to a generic circumscription which is more correct from an evolutionary point of view by means of multilocus sequence typing. Therefore, multiple analyses were conducted utilising sequences obtained from 28S nrDNA (Large Subunit - LSU), 18S nrDNA (Small Subunit - SSU), the Internal Transcribed Spacer regions 1 & 2 and 5.8S nrDNA (ITS), and part of the β-tubulin (TUB) gene region. A total of 324 strains were included in the analyses of which most belonged to Phoma taxa, whilst 54 to related pleosporalean fungi. In total, 206 taxa were investigated, of which 159 are known to have affinities to Phoma. The phylogenetic analysis revealed that the current Boeremaean subdivision is incorrect from an evolutionary point of view, revealing the genus to be highly polyphyletic. Phoma species are retrieved in six distinct clades within the Pleosporales, and appear to reside in different families. The majority of the species, however, including the generic type, clustered in a recently established family, Didymellaceae. In the second part of this study, the phylogenetic variation of the species and varieties in this clade was further assessed. Next to the genus Didymella, which is considered to be the sole teleomorph of Phoma s. str., we also retrieved taxa belonging to the teleomorph genera Leptosphaerulina and Macroventuria in this clade. Based on the sequence data obtained, the Didymellaceae segregate into at least 18 distinct clusters, of which many can be associated with several specific taxonomic characters. Four of these clusters were defined well enough by means of phylogeny and morphology, so that the associated taxa could be transferred to separate genera. Aditionally, this study addresses the taxonomic description of eight species and two varieties that are novel to science, and the recombination of 61 additional taxa.
Keywords: Boeremia, coelomycetes, Didymella, Didymellaceae, DNA phylogeny, Epicoccum, Leptosphaerulina, Macroventuria, Peyronellaea, Phoma, Pleosporales, taxonomy, Stagonosporopsis
INTRODUCTION
Coelomycetous fungi (Grove 1935) are geographically widespread and are found in numerous ecological niches. Sutton (1980) mentions exponents of this anamorph group inhabiting soil, organic debris, and water, as well as species that parasitise other fungi, lichens, insects and vertebrates. A substantial percentage of the coelomycetes is associated with plant material, either as opportunists or as primary pathogens (Sutton 1980).
Difficulties in morphological identification have resulted in a poor understanding of the generic and species boundaries in the coelomycetes (Sutton 1977, 1980, Nag Raj 1981, Van der Aa et al. 1990, Torres et al. 2005a, b, De Gruyter et al. 2009). In an attempt to improve the classification of the coelomycetes, Sutton (1980) proposed to divide the order into six suborders, which unfortunately proved to be highly artificial from an evolutionary perspective (De Gruyter et al. 2009).
The current common procedure for isolate identification, which chiefly relies on similarity of DNA sequences to those found in public DNA libraries (Hyde & Soytong 2007), combined with the high level of incorrectly identified sequences in these databases (Bridge et al. 2003, 2004, Nilsson et al. 2006) placed the likelihood of achieving correct identifications of coelomycetous fungi under intense scrutiny. As pointed out by De Gruyter et al. (2009), for appropriate morphological identifications within the coelomycete genera in vitro studies are essential, for example in the cases in which quarantine pathogens are involved (Aveskamp et al. 2008). For the current generic delimitation of this class, the use of conidiogenesis characters as taxonomic criteria is of major importance (Hughes 1953; Boerema 1965, Boerema & Bollen 1975, Sutton 1964, 1977, 1980, Singh et al. 1997).
Phoma
The genus Phoma Sacc. emend. Boerema & G.J. Bollen (Pleosporales) is a good example of a coelomycetous genus made fascinating by its great ecological diversity, but taxing investigators with profound difficulties in making identifications. The majority of the taxa within this mitosporic genus have been found in association with land plants, causing mainly leaf and stem spots (Aveskamp et al. 2008, Zhang et al. 2009). Approximately 50 % of the Phoma taxa that were redescribed by Boerema et al. (2004) are recognised as relevant phytopathogenic fungi, including a series of pathogens with quarantine status (Boerema et al. 2004, Aveskamp et al. 2008). Although most taxa are continuously present in the environment as saprobic soil organisms, many species switch to a pathogenic lifestyle when a suitable host is encountered (Aveskamp et al. 2008). The genus further comprises several species and varieties that are recognised as endophytic, fungicolous and lichenicolous fungi (e.g. Hawksworth 1981, Xianshu et al. 1994, Sullivan & White 2000, Hawksworth & Cole 2004, Diederich et al. 2007, Schoch et al. 2009a). In addition, approximately 10 species are known as pathogens of humans (e.g. De Hoog et al. 2000, Balis et al. 2006) and other vertebrates, such as cattle (Costa et al. 1993) and fish (Ross et al. 1975, Hatai et al. 1986, Voronin 1989, Faisal et al. 2007). Next to such an active role in vertebrate pathology, Phoma spp. may indirectly affect animal health by the production of toxic secondary metabolites (Bennett 1983, Pedras & Biesenthal 2000, Rai et al. 2009), as is known for Ph. sorghina in straw roofs in South Africa (Rabie et al. 1975) and may be the case in Ph. pomorum in cattle feed (Sørensen et al. 2009). An almost completely unexplored habitat of Phoma spp. is the marine environment (Kohlmeyer & Volkmann-Kohlmeyer 1991), in which Phoma species are regularly found that are completely new to science (e.g. Osterhage et al. 2000, Yarden et al. 2007).
The genus Phoma has always been considered to be one of the largest fungal genera, with more than 3 000 infrageneric taxa described (Monte et al. 1991). The number of species described in Phoma rose to this level due to the common practice of host associated nomenclature, in combination with the paucity in micromorphological characters and a high variability in cultural characteristics. These factors have resulted in the fact that the systematics of the genus never has been fully understood (Aveskamp et al. 2008). Based on various morphological features depicted by earlier workers, probably less than one-tenth of the 3 200 species listed in MycoBank (www.mycobank.org, Crous et al. 2004, Robert et al. 2005) can currently still be recognised as a separate Phoma taxon. Many of those names were thus already reduced to synonymy after an extensive study of the genus (Boerema et al. 2004), and after a thoroughly revised generic concept of the morphologically similar genera Ascochyta (Boerema & Bollen 1975) and Phyllosticta (Van der Aa 1973, Van der Aa & Vanev 2002). Many other species could be recombined into other coelomycete genera, such as Asteromella, Microsphaeropsis, Phomopsis, Pleurophoma, Pyrenochaeta and Stagonospora (Sutton 1964, 1980, Boerema & Bollen 1975). In addition, Coniothyrium and Paraconiothyrium have regularly been mistaken for Phoma (Verkley et al. 2004, Damm et al. 2008, Woudenberg et al. 2009). In their studies, Boerema et al. (2004) recognised a total of 215 Phoma taxa and eight teleomorph species with an unnamed Phoma anamorph, although this is probably just the tip of the iceberg as, thus far, only 40 % of the herbarium species mentioned in literature could be recovered and studied properly. Additionally, novel species are described regularly in this genus (e.g. Hawksworth & Cole 2004, Torres et al. 2005a, Li et al. 2006, Diederich et al. 2007, Aveskamp et al. 2009a, Davidson et al. 2009).
A subdivision of the asexual genus Phoma that is currently widely applied divides the genus into nine sections, including the sections Phoma, Heterospora, Macrospora, Paraphoma, Peyronellaea, Phyllostictoides, Pilosa, Plenodomus and Sclerophomella (Boerema 1997). These sections are primarily based on just a few morphological or physiological characters and have not been confirmed as biologically realistic by molecular biological studies. The number of taxa per section may vary, ranging from almost 70 species in section Phoma to only two in section Pilosa. In Table 1, a list is provided with the main characters of every section (Boerema 1997). This subdivision into sections has led to an identification system that is considered to be extremely helpful in morphological identification (Boerema et al. 2004). However, as was hypothesised by Boerema et al. (2004), the classification has proved to be artificial. Molecular evidence has shown that the sections are linked to phylogenetically distinct teleomorph genera (Reddy et al. 1998, Torres et al. 2005b, De Gruyter et al. 2009). Even these teleomorph genera are not always monophyletic (Morales et al. 1995, Câmara et al. 2002, Kodsueb et al. 2006, Inderbitzin et al. 2009). In addition, characters that are thought to be specific for a certain section appeared to be polyphyletic, as is illustrated for dictyochlamydospores and setose pycnidia, the main characters for the sections Peyronellaea (Aveskamp et al. 2009a) and Paraphoma (Grondona et al. 1997, De Gruyter et al. 2010) respectively. Furthermore, Phoma section Phoma, a group of species which is characterised by the absence of chlamydospores, septate conidia, and pycnidial ornamentation or wall thickening, is considered to be a repository for degenerated and insufficiently understood species that could not be placed elsewhere.
Table 1.
Overview of the characters of the various Phoma sections in the Boeremaean classification system. Adapted from Boerema et al. (2004).
| Section | Teleomorph | Synanamorph | Sectional character |
|---|---|---|---|
| Heterospora | — | Stagonosporopsis | Production of distinctly large conidia in addition to the regular conidia |
| Macrospora | Mycosphaerella | — | Conidia large, measuring 8-19 × 3-7 μm |
| Paraphoma | — | — | Setose pycnidia |
| Peyronellaea | — | Epicoccum* | Multicellular chlamydospores |
| Phoma | Didymella | Phialophora* | — |
| Phyllostictoides | Didymella | — | Small septate conidia in addition to the regular conidia |
| Pilosa | Pleospora | — | Pycnidia covered by pilose outgrows |
| Plenodomus | Leptosphaeria | Sclerotium* | Pycnidia scleroplectenchymatous |
| Phialophora* | |||
| Sclerophomella | Didymella | — | Pycnidia thick-walled |
Synanamorph only recorded in a single species.
The genus Phoma is typified by Phoma herbarum (Boerema 1964). This species has thus far not been linked to any teleomorph, but several other species that are currently accommodated in Phoma do have a sexual state. The species in the section Pilosa are linked to the teleomorph genus Pleospora, while many species in the section Plenodomus have a sexual state in Leptosphaeria. As mentioned above, Leptosphaeria is para- or possibly polyphyletic (Morales et al. 1995, Câmara et al. 2002). A teleomorph in the poorly studied genus Didymella is associated with approximately 40 Phoma species placed in sections Phoma, Phyllostictoides and Sclerophomella (Boerema et al. 2004). Moreover, Phoma has been linked in literature to several other teleomorph genera, such as Mycosphaerella (Corlett 1991, De Gruyter 2002, Crous et al. 2009a, b), Belizeana (Kohlmeyer & Volkmann-Kohlmeyer 1987), Atradidymella (Davey & Currah 2009) and Fenestella, Cucurbitaria, Preussia, and Westerdykella (Von Arx 1981, Zhang et al. 2009). None of these hypothesised teleomorph-anamorph linkages is supported by molecular evidence. All must be investigated by study of type material. However, these associations are unlikely as the mentioned teleomorph genera are not linked to the Pleosporales. The species and teleomorph relations are also not recognised by Boerema et al. (2004), except for two Phoma species of the section Macrospora, Ph. rabiei and Ph. zeae-maydis which were linked to “Mycosphaerella” teleomorphs as M. rabiei (Kaiser 1997, De Gruyter 2002) and M. zeae-maydis (Mukunya & Boothroid 1973) respectively. Both species also have names in Didymella. The use of those names is recommended, since Mycosphaerella has been shown to be phylogenetically widely separated from all known Phoma species (De Gruyter et al. 2009, Crous et al. 2009a, b).
Characteristic strains of the genus concerned have been used in a Multilocus Sequence Typing (MLST) study of the Dothideomycetes, which indicated that Phoma is phylogenetically embedded in the Pleosporales (Schoch et al. 2006, 2009b, Zhang et al. 2009). A similar, but smaller scale study aiming to delineate the species in the unofficial suborder Phialopycnidiineae (Sutton 1980), revealed that Phoma is highly polyphyletic, as reference species of the various sections were recovered in distinct clades of the reconstructed phylogeny (De Gruyter et al. 2009). Type species of the sections Heterospora, Plenodomus, Paraphoma and Pilosa appeared to be ancestral to a cluster comprising types of the other sections, as well as to members of the anamorph genera Ascochyta, Microsphaeropsis, Chaetasbolisia, Coniothyrium and Paraconiothyrium. This group has been elevated to family level and is now recognised as the Didymellaceae (De Gruyter et al. 2009). A Blast-search in public sequence libraries revealed a high genetic similarity between species ascribed to the Didymellaceae and two other teleomorph genera, Macroventuria and Leptosphaerulina, although these genera are morphologically clearly distinct from Didymella (Van der Aa 1971, Von Arx 1981, Zhang et al. 2009). The genetic similarity between those two genera has been observed before by Kodsueb et al. (2006), but the phylogenetic relationship with the genus Didymella was not noted in their study. Members of these two genera have therefore also been included in this study.
To solve the problems in quarantine species identification of isolates taken from samples obtained during phytosanitary border controls, a comprehensive taxonomic system is required (Aveskamp et al. 2008). As DNA-based techniques do become more and more important in identification and detection of plant pathogens (Bridge 2002), such a taxonomic system should be in line with sequence data. One of the major initiatives in this field is the development of DNA Barcodes (Hebert et al. 2003, Summerbell et al. 2005), which has been promising in the rapid detection of potentially serious plant pathogens (Armstrong & Ball 2005).
Three genes have in recent years been proposed as standard loci for use in DNA barcoding in fungi. These comprise the internal transcribed spacers (ITS) of the rDNA operon ITS region (Druzhinina et al. 2005), actin (ACT, Aveskamp et al. 2009b), and cytochrome c oxidase subunit I (COI, Seifert et al. 2007). The last locus was successfully applied in DNA Barcoding of Penicillium (Seifert et al. 2007, Chen et al. 2009). However, COI analysis applied to a subset of Ph. exigua related strains, did not reveal taxon-specific conserved SNPs (Aveskamp et al. 2009b), whilst in an attempt to barcode Aspergillus, COI was found to have limited value (Geiser et al. 2007). Although ACT has proven helpful in resolving the phylogeny of Phoma exigua below species level (Aveskamp et al. 2009b), it could not be applied in the present study, as interspecific variation proved to be too high to align the obtained sequences properly. The use of ITS as fungal barcode locus is most popular (Seifert 2009) and has been applied in several taxonomic groups, such as Trichoderma and Hypocrea (Druzhinina et al. 2005), and Trichophyton (Summerbell et al. 2007) and in ecological groups such as wood-inhabiting fungi (Naumann et al. 2007). The power of this locus for barcoding lies in the multiple copies that are present within each cell; this phenomenon results in lower detection thresholds than can be obtained with single-copy loci. Despite the general practicality of using ITS in barcoding, the locus is relatively conservative and may oversimplify species delimitations or blur generic boundaries in some groups (Nilsson et al. 2008). In the present study, a combination of four loci is therefore applied. These include two loci that are renowned for their capacity to resolve phylogenies above family level, namely parts of the LSU (Large Subunit – 28S) and SSU (Small Subunit – 18S) nrDNA. Additinally two loci were applied that mainly provide resolution at species level – or even below. In addition to the abovementioned ITS regions, also part of the β-tubulin gene was utilised, which was successfully applied in a preliminary study on Phoma species of the section Peyronellaea (Aveskamp et al. 2009a).
For the present study, four objectives were defined. The main objective of this study was to reach consensus on the circumscription of the genus Phoma. A modified definition of the genus is not only helpful in taxonomy, but will also be of interest to plant quarantine officers (Aveskamp et al. 2008). Teleomorph associations of Phoma are still uncertain, and here we attempt to shed light on the sexual state of Phoma s. str. Species representing all Phoma sections were included and DNA sequences were compared with those of other species in the Pleosporales.
Secondly, we aimed to integrate morphological and cultural features with DNA sequence data to resolve the generic limits of taxa currently placed in the Didymellaceae. The number of genera in this family is still unclear. Although De Gruyter et al. (2009) found a series of genera that, according to their reconstructed phylogeny, clustered in this family, many were not clearly defined or were morphologically distant from each other, although all anamorph taxa found are accommodated in the coelomycetes (Sutton 1980). Examples of these taxa were included in this study, although the number of Ascochyta, Coniothyrium and Microsphaeropsis species is too high to take all infrageneric taxa of these adjacent genera into account.
Further, we aimed to validate the Phoma sections, which are widely applied in Phoma species recognition. Are the sections representing evolutionary units, and what is the taxonomical value of the characters used to define the sections? To judge the value of the Boeremaean taxonomic system, representative species of all sections were studied, including the sectional type species. The main focus was, however, to resolve the sections associated with Didymellaceae. A single generic name, based on priority but regardless of whether it is an “anamorph” or “teleomorph” genus, is used for all unambiguous monophyletic phylogenetic lineages (Crous et al. 2006, 2009a, b). Finally, we aimed to assess the molecular variation within species that have historically been placed in Phoma. Genes were tested for their potential reliability as standard barcoding genes for Phoma species.
For this study, a sequence data set was generated and morphological data assembled for the more than 300 well-vouchered strains available in the culture collections of CBS (CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands) and PD (Plantenziektenkundige Dienst, Dutch Plant Protection Service, Wageningen, the Netherlands). In addition, five species recognised in a recent study in the section Peyronellaea (Aveskamp et al. 2009a) have also been included, as well as several strains that could not be associated with any of the species that were accepted in Phoma by Boerema et al. (2004), and that were maintained as unnamed Phoma species in the culture collections mentioned above. These strains were recognised as taxonomic novelties and are described at species or variety level in the present paper. Furthermore, several species were relocated to more appropriate genera based on the results obtained.
MATERIALS AND METHODS
Strain selection
A total of 324 strains, belonging to 206 species were selected for the present study. The majority of these species (159) belonged to the genus Phoma or its associated teleomorphs, the remainder to genera that are regularly confused with this genus and that belong to the Pleosporales according to the studies published by De Gruyter et al. (2009). Besides the anamorphous species that were included, representatives of the teleomorph genera Didymella, Leptosphaeria, Leptosphaerulina, Macroventuria and Pleospora were also included. The recently described genus Atradidymella (Davey & Currah 2009) was not available for study and therefore excluded.
Strains were obtained from CBS and PD culture collections in lyophilised form or from the liquid nitrogen collection. Freeze-dried strains were revived overnight in 2 mL malt/peptone (50 % / 50 %) liquid medium. Subsequently, the cultures were transferred and maintained on oatmeal agar (OA, Crous et al. 2009c). The strains that were stored at -196 °C were directly plated on the same agar medium.
DNA extraction, amplification and sequence analysis
Genomic DNA extraction was performed using the Ultraclean Microbial DNA isolation kit (Mo Bio Laboratories, Carlsbad, CA, U.S.A.), according to the instructions of the manufacturer. All DNA extracts were diluted 10 × in milliQ water and stored at 4 °C before their use as PCR templates.
For nucleotide sequence comparisons fragments of four loci were analysed: LSU, SSU, ITS, and TUB. Amplification of LSU and SSU was conducted utilising the primer combination LR0R (Rehner & Samuels 1994) and LR7 (Vilgalys & Hester 1990) for LSU sequencing and the primer pair NS1 and NS4 (White et al. 1990) for SSU. The PCRs were performed in a 2720 Thermal Cycler (Applied Biosystems, Foster City, California) in a total volume of 12.5 μL. The PCR mixture contained 0.5 μL 10 × diluted genomic DNA, 0.2 μM of each primer, 0.5 Unit Taq polymerase E (Genaxxon Bioscience, Germany), 0.04 mM (SSU) or 0.06 mM (LSU) of each of the dNTP, 2 mM MgCl2 and 1 × PCR buffer E incomplete (Genaxxon Bioscience). Conditions for amplification for both regions were an initial denaturation step of 5 min at 94 °C, followed by 35 cycles of denaturation, annealing and elongation and a final elongation step of 7 min at 72 °C. For the SSU amplification, the 35 cycles consisted of 30 s at 94 °C, 50 s at 48 °C and 90 s at 72 °C; for the LSU 45 s at 94 °C, 45 s at 48 °C and 2 min at 72 °C. The loci ITS and TUB were amplified as described by Aveskamp et al. (2009a), using the primer pairs V9G (De Hoog & Gerrits van den Ende 1998) and ITS4 (White et al. 1990) for ITS sequencing and the BT2Fw and BT4Rd primer pair (Aveskamp et al. 2009a) for sequencing of the TUB locus. PCR products were analysed by electrophoresis in a 1.0 % (w/v) agarose gel containing 0.1 ug/mL ethidium bromide in 1 × TAE buffer (0.4 M Tris, 0.05 M glacial acetetic acid 0.01 M ethylenediamine tetraacetic acid [EDTA], pH 7.85). The amplicons were visualised under UV light. Hyperladder I (Bioline, Luckenwalde, Germany) was applied as size standard.
The obtained amplicons were sequenced in both directions using the same primer combinations, except for LSU, where an additional primer, LR5 (White et al. 1990) was further required to assure complete coverage of the locus. Sequencing reactions were prepared with the BigDye terminator chemistry v. 3.1 (Applied Biosystems) according to the manufacturer's recommendations. Sequence products were purified with Sephadex G-50 Fine (Amersham Biosciences, Roosendaal, the Netherlands) and subsequently separated and analysed on an ABI Prism 3730 DNA Sequencer (Applied Biosystems). Consensus sequences were computed from the forward and reverse sequences using the BioNumerics v. 4.61 software package (Applied Maths, St-Martens-Latem, Belgium). The consensus sequences are deposited in GenBank (For GenBank accession numbers see Tables 2, 3).
Table 2.
Isolates of Phoma and related genera used for DNA analyses. The GenBank accession numbers in bold have been obtained from other studies.
| Strain no.1 | Holomorph2 |
GenBank no. |
Original substrate | Locality | |
|---|---|---|---|---|---|
| SSU | LSU | ||||
| CBS 129.79 | Ampelomyces quisqualis | EU754029 | EU754128 | Mildew on Cucumis sativus | Canada |
| CBS 543.70 | Aposphaeria populina | EU754031 | EU754130 | Populus canadensis | Netherlands |
| CBS 246.79; PD 77/655 | Ascochyta caulina T | EU754032 | EU754131 | Atriplex hastata | Germany |
| CBS 544.74 | Ascochyta hordei var. hordei | EU754035 | EU754134 | Triticum aevestum | South Africa |
| CBS 117477 | Ascochyta sp. | GU238202 | GU237926 | Salicornia australis | New Zealand |
| CBS 265.94 | Asteromella tiliae | EU754040 | EU754139 | Tilia platyphilos | Austria |
| CBS 431.74; PD 74/2447 | Boeremia exigua var. exigua B | EU754084 | EU754183 | Solanum tuberosum | Netherlands |
| CBS 341.67; CECT 20055; IMI 331912 | Boeremia foveata B | GU238203 | GU237947 | Solanum tuberosum | U.K. |
| CBS 148.94 | Chaetasbolisia erysiphoides | EU754041 | EU754140 | Unknown | Unknown |
| CBS 216.75; PD 71/1030 | Chaetosphaeronema hispidulum | EU754045 | EU754144 | Anthyllis vulneraria | Germany |
| CBS 589.79 | Coniothyrium concentricum | EU754053 | EU754152 | Yucca sp. | Netherlands |
| CBS 797.95 | Coniothyrium fuckelii | GU238204 | GU237960 | Rubus sp. | Denmark |
| CBS 400.71 | Coniothyrium palmarum | EU754054 | EU754153 | Chamaerops humilis | Italy |
| CBS 122787; PD 03486691 | Coniothyrium sp. | EU754052 | EU754151 | Unknown | Germany |
| CBS 183.55 | Didymella exigua T | EU754056 | EU754155 | Rumex arifolius | France |
| CBS 524.77 | Didymella fabae | EU754034 | EU754133 | Phaseolus vulgaris | Belgium |
| CBS 581.83A | Didymella rabiei | GU238205 | GU237970 | Cicer arietinum | Syria |
| CBS 173.73; ATCC 24428; IMI 164070 | Epicoccum nigrum T | GU238206 | GU237975 | Dactylis glomerata | U.S.A. |
| CBS 298.36 | Leptosphaeria biglobosa | GU238207 | GU237980 | Brassica napus var. napobrassica | Unknown |
| CBS 127.23; MUCL 9930 | Leptosphaeria maculans | EU754090 | EU754189 | Brassica sp. | Netherlands |
| CBS 939.69 | Leptosphaerulina australis | EU754068 | EU754167 | Soil | Netherlands |
| CBS 525.71 | Macroventuria anomochaeta T | GU238208 | GU237984 | Decayed canvas | South Africa |
| CBS 442.83 | Microsphaeropsis olivacea | EU754072 | EU754171 | Taxus baccata | Netherlands |
| CBS 331.37 | Neottiosporina paspali | EU754073 | EU754172 | Paspalum notatum | U.S.A. |
| CBS 122786; PD 99/1064-1 | Paraconiothyrium minitans | EU754075 | EU754174 | Unknown | Unknown |
| CBS 626.68; IMI 108771 | Peyronellaea gardeniae T | GQ387534 | GQ387595 | Gardenia jasminoides | India |
| CBS 528.66; PD 63/590 | Peyronellaea glomerata B | EU754085 | EU754184 | Chrysanthemum sp. | Netherlands |
| CBS 531.66 | Peyronellaea pinodella B | GU238209 | GU238017 | Trifolium pratense | U.S.A. |
| CBS 235.55 | Peyronellaea pinodes | GU238210 | GU238021 | Unknown | Netherlands |
| CBS 588.69 | Peyronellaea zeae-maydis T | EU754093 | EU754192 | Zea mays | U.S.A. |
| CBS 110110 | Phaeosphaeria oryzae | GQ387530 | GQ387591 | Oryza sativa | South Korea |
| CBS 297.74 | Phialophorophoma litoralis | EU754078 | EU754177 | Sea water | Montenegro |
| CBS 285.72 | Phoma apiicola B | GU238211 | GU238040 | Apium graveolens var. rapaceum | Germany |
| CBS 337.65; ATCC 16195; IMI 113693 | Phoma capitulum B | GU238212 | GU238054 | Soil | India |
| CBS 522.66 | Phoma chrysanthemicola T | GQ387521 | GQ387582 | Chrysanthemum morifolium | U.K. |
| CBS 100311 | Phoma complanata | EU754082 | EU754181 | Heracleum sphondylium | Netherlands |
| CBS 345.78; PD 76/1015 | Phoma dimorphospora | GU238213 | GU238069 | Chenopodium quinoa | Peru |
| CBS 527.66 | Phoma eupyrena B | GU238214 | GU238072 | Soil | Germany |
| CBS 161.78 | Phoma fallens B | GU238215 | GU238074 | Olea europaea | New Zealand |
| CBS 170.70; ATCC 22707; CECT 20011; IMI 163514; PD 70/Alk | Phoma fimeti T | GQ387523 | GQ387584 | Apium graveolens | Netherlands |
| CBS 178.93; PD 82/1062 | Phoma flavescens T | GU238216 | GU238075 | Soil | Netherlands |
| CBS 314.80 | Phoma flavigena T | GU238217 | GU238076 | Water | Romania |
| CBS 633.92; ATCC 36786; VKM MF-325 | Phoma fungicola | EU754028 | EU754127 | Microsphaera alphitoides on Quercus sp. | Ukraine |
| CBS 284.70 | Phoma glaucispora B | GU238218 | GU238078 | Nerium oleander | Italy |
| CBS 175.93; PD 92/370 | Phoma haematocycla T | GU238219 | GU238080 | Phormium tenax | New Zealand |
| CBS 615.75; PD 73/665, IMI 199779 | Phoma herbarum B | EU754087 | EU754186 | Rosa multiflora | Netherlands |
| CBS 448.68 | Phoma heteromorphospora B | EU754088 | EU754187 | Chenopodium album | Netherlands |
| CBS 467.76 | Phoma incompta B | GU238220 | GU238087 | Olea europaea | Greece |
| CBS 253.92; PD 70/998 | Phoma lini B | GU238221 | GU238093 | Water | U.S.A. |
| CBS 529.66; PD 66/521 | Phoma macrostoma var. macrostoma B | GU238222 | GU238098 | Malus sylvestris | Netherlands |
| CBS 316.90 | Phoma medicaginis var. medicaginis | GU238223 | GU238103 | Medicago sativa | Czech Republic |
| CBS 509.91; PD 77/920 | Phoma minutispora | GU238224 | GU238108 | Saline soil | India |
| CBS 501.91; PD 83/888 | Phoma multipora B | GU238225 | GU238109 | Unknown | Egypt |
| CBS 376.91; CBS 328.78, PD 77/1177 | Phoma opuntiae B | GU238226 | GU238123 | Opuntia ficus-indica. | Peru |
| CBS 560.81; PD 92/1569; PDDCC 6614 | Phoma paspali T | GU238227 | GU238124 | Paspalum dilatatum | New Zealand |
| CBS 445.81; PDDCC 7049 | Phoma pratorum T | GU238228 | GU238136 | Lolium perenne | New Zealand |
| CBS 111.79; PD 76/437; IMI 386094 | Phoma radicina B | EU754092 | EU754191 | Malus sylvestris | Netherlands |
| CBS 138.96; PD 82/653 | Phoma samarorum B | GQ387517 | GQ387578 | Phlox paniculata | Netherlands |
| CBS 343.85; IMI 386097 | Phoma terricola T | GQ387563 | GQ387624 | Globodera pallida | Netherlands |
| CBS 630.68; PD 68/141 | Phoma valerianae B | GU238229 | GU238150 | Valeriana phu | Netherlands |
| CBS 539.63 | Phoma vasinfecta T | GU238230 | GU238151 | Chrysanthemum sp. | Greece |
| CBS 306.68 | Phoma violicola B | GU238231 | GU238156 | Viola tricolor | Unknown |
| CBS 523.66; PD 66/270 | Pleospora betae B | EU754080 | EU754179 | Beta vulgaris | Netherlands |
| CBS 191.86; IMI 276975 | Pleospora herbarum T | GU238232 | GU238160 | Medicago sativa | India |
| CBS 257.68; IMI 331911 | Pleurophoma cava | EU754100 | EU754199 | Soil | Germany |
| CBS 398.61; IMI 070678 | Pseudorobillarda phragmitis T | EU754104 | EU754203 | Phragmites australis | U.K. |
| CBS 122789; PD 03486800 | Pyrenochaeta acicola | EU754105 | EU754204 | Hordeum vulgare | Unknown |
| CBS 306.65 | Pyrenochaeta lycopersici T | EU754106 | EU754205 | Lycopersicon esculentum | Germany |
| CBS 407.76 | Pyrenochaeta nobilis T | EU754107 | EU754206 | Laurus nobilis | Italy |
| CBS 252.60; ATCC 13735 | Pyrenochaeta romeroi T | EU754108 | EU754209 | Man | Venezuela |
| CBS 524.50 | Sporormiella minima | DQ678003 | DQ678056 | Goat dung | Panama |
| CBS 343.86 | Stagonospora neglecta var. colorata | EU754119 | EU754218 | Phragmites australis | France |
| CBS 101.80; PD 75/909; IMI 386090 | Stagonosporopsis andigena B | GU238233 | GU238169 | Solanum sp. | Peru |
| CBS 133.96; PD 79/127 | Stagonosporopsis cucurbitacearum | GU238234 | GU238181 | Cucurbita sp. | New Zealand |
| CBS 631.68; PD 68/147 | Stagonosporopsis dennisii B | GU238235 | GU238182 | Solidago floribunda | Netherlands |
| CBS 164.31 | Stenocarpella macrospora | EU754121 | EU754220 | Zea mays | Unknown |
ATCC: American Type Culture Collection, Virginia, U.S.A.; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CECT: Colección Española de Cultivos Tipo, Valencia University, Spain; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; MUCL: Mycotheque de l'Universite catholique de Louvain, Louvain-la-Neuve, Belgium; PD: Plant Protection Service, Wageningen, the Netherlands; PDDCC: Plant Diseases Division Culture Collection, Auckland, New Zealand; VKM: All-Russian Collection of Microorganisms, Pushchino, Russia.
T: Ex-type strain; B: Reference strain according to Boerema et al. (2004).
Table 3.
Strains from the Didymellaceae used for DNA analyses. The GenBank accession numbers in bold have been obtained from other studies.
| Strain no.1 | Holomorph2 |
GenBank no. |
Original substrate | Locality | ||
|---|---|---|---|---|---|---|
| LSU | ITS | TUB | ||||
| CBS 544.74 | Ascochyta hordei var. hordei | EU754134 | GU237887 | GU237488 | Triticum aevestum | South Africa |
| CBS 109.79; PD 77/747 | Boeremia crinicola B | GU237927 | GU237737 | GU237489 | Crinum powellii | Netherlands |
| CBS 118.93; PD 70/195 | Boeremia crinicola | GU237928 | GU237758 | GU237490 | Crinum sp. | Netherlands |
| CBS 101194; PD 79/687; IMI 373349 | Boeremia diversispora | GU237929 | GU237716 | GU237491 | Phaseolus vulgaris | Netherlands |
| CBS 102.80; PD 79/61; CECT 20049; IMI 331907 | Boeremia diversispora B | GU237930 | GU237725 | GU237492 | Phaseolus vulgaris | Kenya |
| CBS 119730 | Boeremia exigua var. coffeae | GU237942 | GU237759 | GU237504 | Coffea arabica | Brazil |
| CBS 109183; IMI 300060; PD 2000/10506 | Boeremia exigua var. coffeae B | GU237943 | GU237748 | GU237505 | Coffea arabica | Cameroon |
| CBS 431.74; PD 74/2447 | Boeremia exigua var. exigua B | EU754183 | FJ427001 | FJ427112 | Solanum tuberosum | Netherlands |
| CBS 101150; PD 79/118 | Boeremia exigua var. exigua | GU237933 | GU237715 | GU237495 | Cichorium intybus | Netherlands |
| CBS 101197; PD 95/721 | Boeremia exigua var. forsythiae | GU237931 | GU237718 | GU237493 | Forsythia sp. | Netherlands |
| CBS 101213; PD 92/959 | Boeremia exigua var. forsythiae B | GU237932 | GU237723 | GU237494 | Forsythia sp. | Netherlands |
| CBS 101196; PD 79/176 | Boeremia exigua var. heteromorpha | GU237934 | GU237717 | GU237496 | Nerium oleander | France |
| CBS 443.94 | Boeremia exigua var. heteromorpha B | GU237935 | GU237866 | GU237497 | Nerium oleander | Italy |
| CBS 569.79; PD 72/741 | Boeremia exigua var. lilacis B | GU237936 | GU237892 | GU237498 | Syringa vulgaris | Netherlands |
| CBS 114.28 | Boeremia exigua var. linicola | GU237937 | GU237752 | GU237499 | Linum usitatissimum | Netherlands |
| CBS 116.76; ATCC 32332; CECT 20022; CECT 20023; IMI 197074 | Boeremia exigua var. linicola B | GU237938 | GU237754 | GU237500 | Linum usitatissimum | Netherlands |
| CBS 100167; PD 93/217 | Boeremia exigua var. populi T | GU237939 | GU237707 | GU237501 | Populus (x) euramericana | Netherlands |
| CBS 101202; PD 82/942 | Boeremia exigua var. populi | GU237940 | GU237719 | GU237502 | Salix sp. | Netherlands |
| CBS 101207; PD 94/614 | Boeremia exigua var. pseudolilacis T | GU237941 | GU237721 | GU237503 | Syringa vulgaris | Netherlands |
| CBS 100354; PD 84/448 | Boeremia exigua var. viburni B | GU237944 | GU237711 | GU237506 | Viburnum opulus | Netherlands |
| CBS 101211; PD 93/838 | Boeremia exigua var. viburni | GU237945 | GU237722 | GU237507 | Viburnum sp. | Netherlands |
| CBS 109176; CECT 2828; PD 94/1394 | Boeremia foveata B | GU237946 | GU237742 | GU237508 | Solanum tuberosum | Bulgaria |
| CBS 341.67; CECT 20055; IMI 331912 | Boeremia foveata B | GU237947 | GU237834 | GU237509 | Solanum tuberosum | U.K. |
| CBS 366.91; PD 70/811 | Boeremia hedericola | GU237948 | GU237841 | GU237510 | Hedera helix | Netherlands |
| CBS 367.91; PD 87/229 | Boeremia hedericola B | GU237949 | GU237842 | GU237511 | Hedera helix | Netherlands |
| CBS 378.67; PD 76/276 | Boeremia lycopersici B | GU237950 | GU237848 | GU237512 | Lycopersicon esculentum | Netherlands |
| CBS 109172; PD 84/143 | Boeremia lycopersici | GU237951 | GU237739 | GU237513 | Lycopersicon esculentum | Netherlands |
| CBS 100353; PD 87/718 | Boeremia noackiana B | GU237952 | GU237710 | GU237514 | Phaseolus vulgaris | Guatemala |
| CBS 101203; PD 79/1114 | Boeremia noackiana | GU237953 | GU237720 | GU237515 | Phaseolus vulgaris | Colombia |
| CBS 109170; PD 75/796 | Boeremia sambuci-nigrae | GU237954 | GU237738 | GU237516 | Sambucus nigra | Netherlands |
| CBS 629.68; CECT 20048; IMI 331913; PD 67/753 | Boeremia sambuci-nigrae T | GU237955 | GU237897 | GU237517 | Sambucus nigra | Netherlands |
| CBS 126.93; PD 73/642 | Boeremia strasseri | GU237956 | GU237773 | GU237518 | Mentha sp. | Netherlands |
| CBS 261.92; ATCC 244146; PD 92/318 | Boeremia strasseri | GU237957 | GU237812 | GU237519 | Mentha piperita | U.S.A. |
| CBS 109175; PD 79/524 | Boeremia telephii B | GU237958 | GU237741 | GU237520 | Sedum spectabile | Netherlands |
| CBS 760.73; PD 71/1616 | Boeremia telephii B | GU237959 | GU237905 | GU237521 | Sedum spectabile | Netherlands |
| CBS 148.94 | Chaetasbolisia erysiphoides | EU754140 | GU237785 | GU237522 | Unknown | Unknown |
| CBS 187.83; PD 82/128 | Didymella adianticola B | GU238035 | GU237796 | GU237576 | Polystichum adiantiforme | U.S.A. |
| CBS 258.92; PD 89/1887 | Didymella adianticola | GU238036 | GU237811 | GU237577 | Polystichum adiantiforme | Costa Rica |
| CBS 102634; PD 75/248 | Didymella applanata | GU237997 | GU237726 | GU237555 | Rubus idaeus | Netherlands |
| CBS 205.63 | Didymella applanata T | GU237998 | GU237798 | GU237556 | Rubus idaeus | Netherlands |
| CBS 234.37 | Didymella cannabis | GU237961 | GU237804 | GU237523 | Cannabis sativa | Unknown |
| CBS 102635; PD 77/1131 | Didymella catariae | GU237962 | GU237727 | GU237524 | Nepeta catenaria | Netherlands |
| CBS 183.55 | Didymella exigua T | EU754155 | GU237794 | GU237525 | Rumex arifolius | France |
| CBS 524.77 | Didymella fabae | GU237963 | GU237880 | GU237526 | Phaseolus vulgrais | Belgium |
| CBS 649.71 | Didymella fabae | GU237964 | GU237902 | GU237527 | Vicia faba | Netherlands |
| PD 83/492 | Didymella fabae | GU237965 | GU237917 | GU237528 | Phaseolus vulgaris | Netherlands |
| PD 84/512 | Didymella macropodii | GU237966 | GU237919 | GU237529 | Crucifer | Unknown |
| CBS 100190; PD 82/736 | Didymella macropodii | GU237967 | GU237708 | GU237530 | Brassica napus | Germany |
| CBS 126.54 | Didymella pisi | GU237968 | GU237772 | GU237531 | Pisum sativum | Netherlands |
| CBS 122785; PD 78/517 | Didymella pisi | GU237969 | GU237763 | GU237532 | Pisum sativum | Netherlands |
| CBS 534.65 | Didymella rabiei | GU237970 | GU237886 | GU237533 | Cicer arietinum | India |
| CBS 581.83a | Didymella rabiei | GU237971 | GU237894 | GU237534 | Cicer arietinum | Syria |
| CBS 121.75; ATCC 32164; IHEM 3403; IMI 194767; PD 73/584 | Didymella urticicola T | GU237972 | GU237761 | GU237535 | Urtica dioica | Netherlands |
| PD 73/570 | Didymella urticicola | GU237973 | GU237914 | GU237536 | Urtica dioica | Netherlands |
| CBS 454.64 | Didymella vitalbina | FJ515646 | FJ515605 | FJ515623 | Clematis vitalba | France |
| CBS 138.25 | Diplodina coloradensis | EU754158 | GU237784 | GU237537 | Senecio sp. | Unknown |
| CBS 172.34 | “Dothiorella ulmi” | EU754160 | GU237789 | GU237538 | Ulmus sp. | U.S.A. |
| CBS 125.82; IMI 1331914; CECT 20044 | Epicoccum nigrum | GU237974 | FJ426995 | FJ427106 | Human | Netherlands |
| CBS 173.73; ATCC 24428; IMI 164070 | Epicoccum nigrum T | GU237975 | FJ426996 | FJ427107 | Dactylis glomerata | U.S.A. |
| CBS 246.60; ATCC 22237; ATCC 16652; IMI 081601 | Epicoccum pimprinum T | GU237976 | FJ427049 | FJ427159 | Soil | India |
| PD 77/1028 | Epicoccum pimprinum | GU237977 | FJ427050 | FJ427160 | Unknown | Unknown |
| CBS 179.80; PD 76/1018 | Epicoccum sorghi | GU237978 | FJ427067 | FJ427173 | Sorghum vulgare | Puerto Rico |
| CBS 627.68; PD 66/926 | Epicoccum sorghi | GU237979 | FJ427072 | FJ427178 | Citrus sp. | France |
| CBS 213.55 | Leptosphaerulina americana | GU237981 | GU237799 | GU237539 | Trifolium pretense | U.S.A. |
| CBS 275.59; ATCC 13446 | Leptosphaerulina arachidicola | GU237983 | GU237820 | GU237543 | Arachis hypochea | Taiwan |
| CBS 317.83 | Leptosphaerulina australis | EU754166 | GU237829 | GU237540 | Eugenia aromatica | Indonesia |
| CBS 939.69 | Leptosphaerulina australis | EU754167 | GU237911 | GU237541 | Soil | Netherlands |
| CBS 235.58 | Leptosphaerulina trifolii | GU237982 | GU237806 | GU237542 | Trifolium sp. | Netherlands |
| CBS 525.71 | Macroventuria anomochaeta T | GU237984 | GU237881 | GU237544 | decayed canvas | South Africa |
| CBS 502.72 | Macroventuria anomochaeta | GU237985 | GU237873 | GU237545 | Medicago sativa | South Africa |
| CBS 526.71 | Macroventuria wentii | GU237986 | GU237881 | GU237546 | Unidentified plant material | U.S.A. |
| CBS 432.71 | Microsphaeropsis olivacea | GU237987 | GU237863 | GU237548 | Sorothamus sp. | Netherlands |
| CBS 233.77 | Microsphaeropsis olivacea | GU237988 | GU237803 | GU237549 | Pinus laricio | France |
| CBS 442.83 | Microsphaeropsis olivacea | EU754171 | GU237865 | GU237547 | Taxus baccata | Netherlands |
| CBS 132.96; PD 93/853 | Peyronellaea alectorolophi T | GU237989 | GU237778 | GU237550 | Rhinanthus major | Netherlands |
| CBS 185.85; PD 80/1191 | Peyronellaea americana B | GU237990 | FJ426972 | FJ427088 | Zea mays | U.S.A. |
| CBS 568.97; PD 94/1544; ATCC 44494 | Peyronellaea americana | GU237991 | FJ426974 | FJ427090 | Glycine max | U.S.A. |
| PD 82/1059 | Peyronellaea americana | GU237992 | FJ426980 | FJ427096 | Nematode cyst | Unknown |
| CBS 360.84 | Peyronellaea anserina B | GU237993 | GU237839 | GU237551 | Potatoflour | Netherlands |
| CBS 363.91; PD 79/712 | Peyronellaea anserina | GU237994 | GU237840 | GU237552 | Pisum sativum | Netherlands |
| CBS 315.90; PD 80/1190 | Peyronellaea arachidicola | GU237995 | GU237827 | GU237553 | Arachis hypogaea | Zimbabwe |
| CBS 333.75; ATCC 28333; IMI 386092; PREM 44889 | Peyronellaea arachidicola T | GU237996 | GU237833 | GU237554 | Arachis hypogaea | South Africa |
| CBS 269.93; PD 78/1087 | Peyronellaea aurea B | GU237999 | GU237818 | GU237557 | Medicago polymorpha | New Zealand |
| CBS 444.81; PDDCC 6546 | Peyronellaea australis T | GU238000 | GU237867 | GU237558 | Actinidia chinensis | New Zealand |
| PD 77/919 | Peyronellaea australis | GU238001 | GU237915 | GU237559 | Actinidea chinensis | Unknown |
| CBS 109.92; PD 73/1405 | Peyronellaea calorpreferens T | GU238002 | FJ426983 | FJ427098 | Undefined food material | Netherlands |
| CBS 630.97; ATCC 96683; IMI 361196; PD 96/2022 | Peyronellaea calorpreferens | GU238004 | GU237925 | GU237560 | Heterodera glycines | U.S.A. |
| CBS 875.97; PD 93/1503 | Peyronellaea calorpreferens | GU238003 | GU237908 | GU237561 | Indoor environment | U.S.A. |
| CBS 123380; PD 84/1013 | Peyronellaea coffeae-arabicae T | GU238005 | FJ426993 | FJ427104 | Coffea arabica | Ethiopia |
| CBS 123398; PD 84/1014 | Peyronellaea coffeae-arabicae | GU238006 | FJ426994 | FJ427105 | Coffea arabica | Ethiopia |
| PD 92/1460 | Peyronellaea curtisii | GU238012 | FJ427041 | FJ427151 | Sprekelia | Netherlands |
| CBS 251.92; PD 86/1145 | Peyronellaea curtisii B | GU238013 | FJ427038 | FJ427148 | Nerine sp. | Netherlands |
| CBS 377.91; PD 79/210 | Peyronellaea eucalyptica B | GU238007 | GU237846 | GU237562 | Eucalyptus sp. | Australia |
| CBS 508.91; PD 73/1413 | Peyronellaea eucalyptica | GU238008 | GU237878 | GU237563 | Water | Croatia |
| CBS 302.79; PD 79/1156 | Peyronellaea gardeniae | GQ387596 | FJ427002 | FJ427113 | Air | Netherlands Antilles |
| CBS 626.68; IMI 108771 | Peyronellaea gardeniae T | GQ387595 | FJ427003 | FJ427114 | Gardenia jasminoides | India |
| CBS 464.97; MUCL 9882 | Peyronellaea glomerata | GU238009 | FJ427012 | FJ427123 | Indoor environment | Netherlands |
| CBS 528.66; PD 63/590 | Peyronellaea glomerata B | EU754184 | FJ427013 | FJ427124 | Chrysanthemum sp. | Netherlands |
| CBS 103.25 | Peyronellaea lethalis | GU238010 | GU237729 | GU237564 | Unknown | Unknown |
| CBS 463.69 | Peyronellaea musae B | GU238011 | FJ427026 | FJ427136 | Mangifera indica | India |
| CBS 377.93; PD 80/976 | Peyronellaea obtusa B | GU238014 | GU237847 | GU237565 | Daucus carota | Netherlands |
| CBS 391.93; PD 80/87 | Peyronellaea obtusa B | GU238015 | GU237858 | GU237566 | Spinacia oleracea | Netherlands |
| CBS 318.90; PD 81/729 | Peyronellaea pinodella | GU238016 | FJ427051 | FJ427161 | Pisum sativum | Netherlands |
| CBS 531.66 | Peyronellaea pinodella B | GU238017 | FJ427052 | FJ427162 | Trifolium pratense | U.S.A. |
| CBS 100580; PD 98/1135 | Peyronellaea pinodella | GU238018 | GU237713 | GU237567 | Glycine max | Hungary |
| CBS 567.97; PD 97/2160 | Peyronellaea pinodella | GU238019 | GU237891 | GU237568 | Glycine max | Hungary |
| CBS 159.78b | Peyronellaea pinodes | GU238020 | GU237786 | GU237569 | Pisum sativum | Iraq |
| CBS 285.49 | Peyronellaea pinodes | GU238022 | GU237823 | GU237571 | Primula auricula | Switzerland |
| CBS 235.55 | Peyronellaea pinodes | GU238021 | GU237805 | GU237570 | Unknown | Netherlands |
| CBS 525.77 | Peyronellaea pinodes | GU238023 | GU237883 | GU237572 | Pisum sativum | Belgium |
| CBS 525.77a | Peyronellaea pinodes | GU238024 | GU237882 | GU237573 | Pisum sativum | Belgium |
| CBS 539.66; ATCC 16791; IMI 122266; PD 64/914 | Peyronellaea pomorum var. pomorum B | GU238028 | FJ427056 | FJ427166 | Polygonum tataricum | Netherlands |
| CBS 285.76; ATCC 26241; IMI 176742; VKM F-1843 | Peyronellaea pomorum var. circinata T | GU238025 | FJ427053 | FJ427163 | Heracleum dissectum | Russia |
| CBS 286.76; ATCC 26242; IMI 176743; VKM F-1844 | Peyronellaea pomorum var. circinata | GU238026 | FJ427054 | FJ427164 | Allium nutans | Russia |
| CBS 388.80; PREM 45736 | Peyronellaea pomorum var. cyanea T | GU238027 | FJ427055 | FJ427165 | Triticum sp. | South Africa |
| CBS 381.96; PD 71/706 | Peyronellaea protuberans B | GU238029 | GU237853 | GU237574 | Lycium halifolium | Netherlands |
| CBS 281.83 | Peyronellaea sancta T | GU238030 | FJ427063 | FJ427170 | Ailanthus altissima | South Africa |
| LEV 15292 | Peyronellaea sancta | GU238031 | FJ427065 | FJ427172 | Gleditsia triacantha | Unknown |
| CBS 110.92; PD 76/1010 | Peyronellaea subglomerata B | GU238032 | FJ427080 | FJ427186 | Triticum sp. | U.S.A. |
| PD 78/1090 | Peyronellaea subglomerata | GU238033 | FJ427081 | FJ427187 | Zea mays | Unknown |
| CBS 588.69 | Peyronellaea zeae-maydis T | EU754186 | FJ427086 | FJ427190 | Zea mays | U.S.A. |
| CBS 179.97 | Phoma acetosellae | GU238034 | GU237793 | GU237575 | Rumex hydrolapathum | Netherlands |
| CBS 379.93; PD 82/945 | Phoma aliena | GU238037 | GU237851 | GU237578 | Berberis sp. | Netherlands |
| CBS 877.97; PD 94/1401 | Phoma aliena | GU238038 | GU237910 | GU237579 | Buxus sempervirens | Netherlands |
| CBS 381.91; PD 79/1110 | Phoma anigozanthi B | GU238039 | GU237852 | GU237580 | Anigozanthus maugleisii | Netherlands |
| CBS 107.96; PD 73/598 | Phoma aquilegiicola B | GU238041 | GU237735 | GU237582 | Aconitum pyramidale | Netherlands |
| CBS 108.96; PD 79/611 | Phoma aquilegiicola B | GU238042 | GU237736 | GU237583 | Aquilegia sp. | Netherlands |
| CBS 125.93; PD 77/1029 | Phoma arachidis-hypogaeae B | GU238043 | GU237771 | GU237584 | Arachis hypogaea | India |
| CBS 383.67; PD 65/223 | Phoma aubrietiae B | GU238044 | GU237854 | GU237585 | Aubrietia hybrida cv. Superbissima | Netherlands |
| CBS 627.97; PD 70/714 | Phoma aubrietiae B | GU238045 | GU237895 | GU237586 | Aubrietia sp. | Netherlands |
| CBS 714.85; PD 74/265 | Phoma bellidis B | GU238046 | GU237904 | GU237587 | Bellis perennis | Netherlands |
| PD 94/886 | Phoma bellidis | GU238047 | GU237923 | GU237581 | Bellis sp. | Netherlands |
| CBS 109942; PD 84/402 | Phoma boeremae T | GU238048 | FJ426982 | FJ427097 | Medicago littoralis cv. Harbinger | Australia |
| CBS 120105 | Phoma brasiliensis T | GU238049 | GU237760 | GU237588 | Amaranthus sp. | Brazil |
| CBS 357.84 | Phoma bulgarica T | GU238050 | GU237837 | GU237589 | Trachystemon orientale | Bulgaria |
| CBS 124515; PD 82/1058 | Phoma bulgarica | GU238051 | GU237768 | GU237590 | Trachystemon orientale | Bulgaria |
| CBS 448.83 | Phoma calidophila T | GU238052 | FJ427059 | FJ427168 | Soil | Egypt |
| PD 84/109 | Phoma calidophila | GU238053 | FJ427060 | FJ427169 | Cucumis sativus | Europe |
| CBS 128.93; PD 79/140 | Phoma chenopodiicola B | GU238055 | GU237775 | GU237591 | Chenopodium quinoa cv. Sajana | Peru |
| CBS 129.93; PD 89/803 | Phoma chenopodiicola | GU238056 | GU237776 | GU237592 | Chenopodium quinoa cv. Sajana | Peru |
| CBS 102.66 | Phoma clematidina | FJ515630 | FJ426988 | FJ427099 | Clematis sp. | U.K. |
| CBS 108.79; PD 78/522 | Phoma clematidina T | FJ515632 | FJ426989 | FJ427100 | Clematis sp. | Netherlands |
| CBS 507.63; MUCL 9574; PD 07/03486747 | Phoma clematidis-rectae T | FJ515647 | FJ515606 | FJ515624 | Clematis sp. | Netherlands |
| PD 95/1958 | Phoma clematidis-rectae | FJ515648 | FJ515607 | FJ515625 | Clematis sp. | Netherlands |
| CBS 100409 | Phoma commelinicicola B | GU238057 | GU237712 | GU237593 | Tradescantia sp. | New Zealand |
| CBS 100311 | Phoma complanata | EU754181 | GU237709 | GU237594 | Heracleum sphondylium | Netherlands |
| CBS 268.92; PD 75/3 | Phoma complanata | EU754180 | GU237815 | GU237595 | Angelica sylvestris | Netherlands |
| CBS 506.91; IMI 215229; PD 91/876 | Phoma costarricensis B | GU238058 | GU237876 | GU237596 | Coffea sp. | Nicaragua |
| CBS 497.91; PD 79/209 | Phoma costarricensis | GU238059 | GU237870 | GU237597 | Coffea arabica | Unknown |
| CBS 193.82 | Phoma crystallifera T | GU238060 | GU237797 | GU237598 | Chamaespartium sagittale | Austria |
| CBS 124513; PD 73/1414 | Phoma dactylidis T | GU238061 | GU237766 | GU237599 | Dactylis glomerata | U.S.A. |
| CBS 133.93; PD 88/961; IMI 173142 | Phoma destructiva var. destructiva | GU238064 | GU237779 | GU237602 | Solanum lycopersicum | Guadeloupe |
| CBS 378.73; CECT 2877 | Phoma destructiva var. destructiva B | GU238063 | GU237849 | GU237601 | Lycopersicon esculentum | Tonga |
| CBS 162.78; PD 77/725 | Phoma destructiva var. diversispora | GU238062 | GU237788 | GU237600 | Lycopersicon esculentum | Netherlands |
| CBS 507.91; PD 74/148 | Phoma dictamnicola B | GU238065 | GU237877 | GU237603 | Dictamnus albus | Netherlands |
| CBS 109179; PD 90/835-1 | Phoma digitalis | GU238066 | GU237744 | GU237604 | Digitalis sp. | Netherlands |
| CBS 229.79; LEV 7660 | Phoma digitalis B | GU238067 | GU237802 | GU237605 | Digitalis purpurea | New Zealand |
| CBS 346.82 | Phoma dimorpha T | GU238068 | GU237835 | GU237606 | Opuntiae sp. | Spain |
| CBS 186.83; PD 82/47 | Phoma draconis B | GU238070 | GU237795 | GU237607 | Dracaena sp. | Rwanda |
| CBS 123.93; PD 77/1148 | Phoma eupatorii B | GU238071 | GU237764 | GU237608 | Eupatorium cannabinum | Netherlands |
| CBS 374.91; PD 78/391 | Phoma eupyrena B | GU238072 | FJ426999 | FJ427110 | Solanum tuberosum | Netherlands |
| CBS 527.66; ATCC 22238 | Phoma eupyrena B | GU238073 | FJ427000 | FJ427111 | Soil | Germany |
| CBS 633.92; ATCC 36786; VKM MF-325 | Phoma fungicola | EU754127 | GU237900 | GU237609 | Microsphaera alphitoides on Quercus sp. | Ukraine |
| CBS 112.96 | Phoma glaucii | GU238077 | GU237750 | GU237610 | Dicentra sp. | Netherlands |
| CBS 114.96; PD 94/888 | Phoma glaucii B | FJ515649 | FJ515609 | FJ515627 | Chelidonium majus | Netherlands |
| CBS 377.67 | Phoma gossypiicola B | GU238079 | GU237845 | GU237611 | Gossypium hirsutum | U.S.A. |
| CBS 104.80; PD 74/1017 | Phoma henningsii B | GU238081 | GU237731 | GU237612 | Acacia mearnesii | Kenya |
| CBS 502.91; PD 86/276 | Phoma herbarum | GU238082 | GU237874 | GU237613 | Nerium sp. | Netherlands |
| CBS 615.75; PD 73/665; IMI 199779 | Phoma herbarum B | EU880896 | FJ427022 | FJ427133 | Rosa multiflora | Netherlands |
| CBS 629.97; PD 76/1017 | Phoma herbicola B | GU238083 | GU237898 | GU237614 | Water | U.S.A. |
| CBS 105.80; PD 75/908 | Phoma huancayensis T | GU238084 | GU237732 | GU237615 | Solanum sp. | Peru |
| CBS 390.93; PD 77/1173 | Phoma huancayensis | GU238085 | GU237857 | GU237616 | Chenopodium quinoa | Peru |
| CBS 220.85 | Phoma humicola B | GU238086 | GU237800 | GU237617 | Franseria sp. | U.S.A. |
| CBS 123394 | Phoma infossa | GU238088 | FJ427024 | FJ427134 | Fraxinus pennsylvanica | Argentina |
| CBS 123395 | Phoma infossa T | GU238089 | FJ427025 | FJ427135 | Fraxinus pennsylvanica | Argentina |
| CBS 252.92; PD 80/1144 | Phoma insulana B | GU238090 | GU237810 | GU237618 | Olea europaea | Greece |
| CBS 124.93; PD 87/269 | Phoma labilis B | GU238091 | GU237765 | GU237619 | Solanum lycopersicum | Netherlands |
| CBS 479.93; PD 70/93 | Phoma labilis | GU238092 | GU237868 | GU237620 | Rosa sp. | Israel |
| CBS 347.82 | Phoma longicolla | GU238094 | GU237836 | GU237621 | Opuntiae sp. | Spain |
| CBS 124514; PD 80/1189; VPRI 1239 | Phoma longicolla T | GU238095 | GU237767 | GU237622 | Opuntiae sp. | Spain |
| CBS 223.69 | Phoma macrostoma var. incolorata B | GU238096 | GU237801 | GU237623 | Acer pseudoplatanus | Switzerland |
| CBS 109173; PD 83/908 | Phoma macrostoma var. incolorata B | GU238097 | GU237740 | GU237624 | Malus sylvestris | Netherlands |
| CBS 529.66; PD 66/521 | Phoma macrostoma var. macrostoma B | GU238098 | GU237885 | GU237625 | Malus sylvestris | Netherlands |
| CBS 482.95 | Phoma macrostoma var. macrostoma | GU238099 | GU237869 | GU237626 | Larix decidua | Germany |
| CBS 259.92; IMI 286996; PD 91/272 | Phoma matteuciicola B | GU238100 | GU237812 | GU237627 | Matteuccia struthiopteris | Canada |
| CBS 112.53 | Phoma medicaginis var. macrospora B | GU238101 | GU237749 | GU237628 | Medicago sativa | U.S.A. |
| CBS 404.65; IMI 116999 | Phoma medicaginis var. macrospora B | GU238102 | GU237859 | GU237629 | Medicago sativa | Canada |
| CBS 316.90 | Phoma medicaginis var. medicaginis | GU238103 | GU237828 | GU237630 | Medicago sativa | Czech Republic |
| CBS 105.95 | Phoma microchlamydospora T | GU238104 | FJ427028 | FJ427138 | Eucalyptus sp. | U.K. |
| CBS 491.90 | Phoma microchlamydospora | GU238105 | FJ427029 | FJ427139 | Unidentified vegetable | U.K. |
| CBS 315.83 | Phoma minor | GU238106 | GU237826 | GU237631 | Syzygium aromaticum | Indonesia |
| CBS 325.82 | Phoma minor T | GU238107 | GU237831 | GU237632 | Syzygium aromaticum | Indonesia |
| CBS 110.79; PD 65/8875; MUCL 8247 | Phoma multirostrata | GU238110 | FJ427030 | FJ427140 | Cucumis sativus | Netherlands |
| CBS 274.60; IMI 081598 | Phoma multirostrata T | GU238111 | FJ427031 | FJ427141 | Soil | India |
| CBS 368.65; PD 92/1757; HACC 154 | Phoma multirostrata | GU238112 | FJ427033 | FJ427143 | Soil | India |
| PD 83/48 | Phoma multirostrata | GU238113 | FJ427037 | FJ427147 | Cucumis sativus | Unknown |
| CBS 117.93; PD 83/90 | Phoma nebulosa | GU238114 | GU237757 | GU237633 | Mercurialis perennis | Netherlands |
| CBS 503.75; ATCC 32163; DSM 63391; IMI 194766; PD 75/4 | Phoma nebulosa B | GU238115 | GU237875 | GU237634 | Urtica dioica | Austria |
| CBS 358.71 | Phoma negriana B | GU238116 | GU237838 | GU237635 | Vitis vinifera | Germany |
| PD 79/74 | Phoma negriana | GU238117 | GU237916 | GU237636 | Vitis vinifera | Netherlands |
| CBS 116.96; PD 95/7930 | Phoma nigripycnidia B | GU238118 | GU237756 | GU237637 | Vicia cracca | Russia |
| CBS 114.93; PD 74/228 | Phoma novae- verbascicola | GU238119 | GU237753 | GU237638 | Verbascum sp. | Netherlands |
| CBS 127.93; PD 92/347 | Phoma novae-verbascicola B | GU238120 | GU237774 | GU237639 | Verbascum densiflorum | Netherlands |
| CBS 654.77 | Phoma omnivirens | GU238122 | FJ427043 | FJ427153 | Unknown | India |
| CBS 991.95 | Phoma omnivirens | GU238121 | FJ427044 | FJ427154 | Soil | Papua New Guinea |
| CBS 560.81; PD 92/1569; PDDCC 6614 | Phoma paspali T | GU238124 | FJ427048 | FJ427158 | Paspalum dilatatum | New Zealand |
| CBS 561.81; PDDCC 6615 | Phoma paspali | GU238125 | GU237889 | GU237640 | Lolium perenne | New Zealand |
| CBS 124516; PD 84/453 | Phoma pedeiae | GU238126 | GU237769 | GU237641 | Orchidaceae | Netherlands |
| CBS 124517; PD 92/612A | Phoma pedeiae T | GU238127 | GU237770 | GU237642 | Schefflera elegantissima | Netherlands |
| CBS 267.92; PD 76/1014 | Phoma pereupyrena T | GU238128 | GU237814 | GU237643 | Coffea arabica | India |
| CBS 268.93; CBS 108.93; PD 88/720 | Phoma piperis B | GU238129 | GU237816 | GU237644 | Peperomia pereskifolia | Netherlands |
| PD 90/2011 | Phoma piperis | GU238130 | GU237921 | GU237645 | Peperomia sp. | Netherlands |
| CBS 284.93; PD 75/907 | Phoma plurivora | GU238131 | GU237822 | GU237646 | Medicago sativa | Australia |
| CBS 558.81; PDDCC 6873 | Phoma plurivora T | GU238132 | GU237888 | GU237647 | Setaria sp. | New Zealand |
| CBS 109181; PD 83/757 | Phoma polemonii B | GU238133 | GU237746 | GU237648 | Polemonium caeruleum | Netherlands |
| CBS 116.93; PD 71/884 | Phoma poolensis B | GU238134 | GU237755 | GU237649 | Antirrhinum majus | Netherlands |
| CBS 113.20; PD 92/774 | Phoma poolensis | GU238135 | GU237751 | GU237650 | Unknown | Unknown |
| CBS 372.91; PD 75/690 | Phoma putaminum B | GU238137 | GU237843 | GU237651 | Ulmus sp. | Netherlands |
| CBS 130.69; CECT 20054; IMI 331916 | Phoma putaminum B | GU238138 | GU237777 | GU237652 | Malus sylvestris | Denmark |
| CBS 109177; LEV 15165; PD 2000/9941 | Phoma rhei B | GU238139 | GU237743 | GU237653 | Rheum rhaponticum | New Zealand |
| CBS 298.89 | Phoma saxea | GU238140 | GU237824 | GU237654 | Limestone | Germany |
| CBS 419.92 | Phoma saxea T | GU238141 | GU237860 | GU237655 | Corroded mediterranean marble | Germany |
| CBS 122.93; PD 77/1049 | Phoma selaginellicola B | GU238142 | GU237762 | GU237656 | Selaginella sp. | Netherlands |
| CBS 160.78; LEV 11451 | Phoma senecionis B | GU238143 | GU237787 | GU237657 | Senecio jacobaea | New Zealand |
| CBS 249.92; PD 78/1088 | Phoma subherbarum | GU238144 | GU237808 | GU237658 | Solanum sp. | Peru |
| CBS 250.92; DAOM 171914; PD 92/371 | Phoma subherbarum B | GU238145 | GU237809 | GU237659 | Solanum sp. | Peru |
| CBS 305.79A; DAOM 170848 | Phoma subherbarum | GU238146 | GU237825 | GU237660 | Zea mays | Peru |
| CBS 135.93; PD 83/87 | Phoma sylvatica B | GU238147 | GU237781 | GU237661 | Melampyrum pratense | Netherlands |
| CBS 874.97; PD 93/764 | Phoma sylvatica B | GU238148 | GU237907 | GU237662 | Melampyrum pratense | Netherlands |
| CBS 436.75 | Phoma tropica T | GU238149 | GU237864 | GU237663 | Saintpaulia ionantha | Germany |
| CBS 876.97; PD 82/1008 | Phoma versabilis B | GU238152 | GU237909 | GU237664 | Silene sp. | Netherlands |
| PD 2000/1379 | Phoma versabilis | GU238153 | GU237913 | GU237665 | Stellaria media | Netherlands |
| CBS 500.91; PD 83/322 | Phoma viburnicola B | GU238154 | GU237871 | GU237666 | Ilex aquifolium | Netherlands |
| CBS 523.73; PD 69/800 | Phoma viburnicola B | GU238155 | GU237879 | GU237667 | Viburnum cassioides | Netherlands |
| CBS 383.68 | Phoma xanthina B | GU238157 | GU237855 | GU237668 | Delphinium sp. | Netherlands |
| PD 84/407 | Phoma xanthina | GU238158 | GU237918 | GU237669 | Delphinium sp. | Netherlands |
| CBS 131.93; PD 69/140 | Phoma zantedeschiae | GU238159 | FJ427084 | FJ427188 | Calla sp. | Netherlands |
| CBS 105.96; PD 74/230 | Stagonosporopsis actaeae B | GU238165 | GU237733 | GU237670 | Cimicifuga simplex | Netherlands |
| CBS 106.96; PD 94/1318 | Stagonosporopsis actaeae T | GU238166 | GU237734 | GU237671 | Actaea spicata | Netherlands |
| CBS 176.93; PD 86/547 | Stagonosporopsis ajacis | GU238167 | GU237790 | GU237672 | Delphinium sp. | Netherlands |
| CBS 177.93; PD 90/115 | Stagonosporopsis ajacis T | GU238168 | GU237791 | GU237673 | Delphinium sp. | Kenya |
| CBS 101.80; PD 75/909; IMI 386090 | Stagonosporopsis andigena B | GU238169 | GU237714 | GU237674 | Solanum sp. | Peru |
| CBS 269.80; PD 75/914 | Stagonosporopsis andigena | GU238170 | GU237817 | GU237675 | Solanum sp. | Peru |
| CBS 102636; PD 73/1409 | Stagonosporopsis artemisiicola B | GU238171 | GU237728 | GU237676 | Artemisia dracunculus | France |
| CBS 178.25; MUCL 9915 | Stagonosporopsis astragali B | GU238172 | GU237792 | GU237677 | Astragalus sp. | Unknown |
| CBS 248.90 | Stagonosporopsis caricae | GU238175 | GU237807 | GU237680 | Carica papaya | Chile |
| PD 06/03082531 | Stagonosporopsis caricae | GU238176 | GU237912 | GU237681 | Carica papaya | Brazil |
| CBS 282.76 | Stagonosporopsis caricae | GU238177 | GU237821 | GU237682 | Brassica sp. | Indonesia |
| CBS 713.85; ATCC 76027; PD 83/826 | Stagonosporopsis crystalliniformis T | GU238178 | GU237903 | GU237683 | Lycopersicon esculentum | Colombia |
| CBS 771.85; IMI 386091; PD 85/772 | Stagonosporopsis crystalliniformis | GU238179 | GU237906 | GU237684 | Solanum tuberosum | Colombia |
| CBS 109171; PD 91/310; PDDCC 272 | Stagonosporopsis cucurbitacearum | GU238180 | GU237922 | GU237685 | Cucurbita sp. | Netherlands |
| CBS 133.96; PD 79/127 | Stagonosporopsis cucurbitacearum | GU238181 | GU237780 | GU237686 | Cucurbita sp. | New Zealand |
| CBS 631.68; PD 68/147 | Stagonosporopsis dennisii B | GU238182 | GU237899 | GU237687 | Solidago floribunda | Netherlands |
| CBS 135.96; IMI 19337; PD 95/4756 | Stagonosporopsis dennisii | GU238183 | GU237782 | GU237688 | Solidago canadensis | Canada |
| CBS 320.90; PD 86/932 | Stagonosporopsis dorenboschii B | GU238184 | GU237830 | GU237689 | Physostegia virginiana | Netherlands |
| CBS 426.90; IMI 386093; PD 86/551 | Stagonosporopsis dorenboschii T | GU238185 | GU237862 | GU237690 | Physostegia virginiana | Netherlands |
| CBS 109182; PD 74/231 | Stagonosporopsis heliopsidis B | GU238186 | GU237747 | GU237691 | Heliopsis patula | Netherlands |
| PD 95/6189; DAOM 221138 | Stagonosporopsis heliopsidis | GU238187 | GU237924 | GU237692 | Ambrosia artemisiifolia | Canada |
| CBS 104.42 | Stagonosporopsis hortensis B | GU238198 | GU237730 | GU237703 | Unknown | Netherlands |
| CBS 572.85; PD 79/269 | Stagonosporopsis hortensis B | GU238199 | GU237893 | GU237704 | Phaseolus vulgaris | Netherlands |
| CBS 425.90; PD 81/520 | Stagonosporopsis ligulicola var. inoxydabilis T | GU238188 | GU237861 | GU237693 | Chrysanthemum parthenii | Netherlands |
| PD 85/259 | Stagonosporopsis ligulicola var. inoxydabilis | GU238189 | GU237920 | GU237694 | Matricaria sp. | Netherlands |
| CBS 500.63; MUCL 8090 | Stagonosporopsis ligulicola var. ligulicola B | GU238190 | GU237872 | GU237695 | Chrysanthemum indicum | Germany |
| CBS 137.96; PD 84/75 | Stagonosporopsis ligulicola var. ligulicola B | GU238191 | GU237783 | GU237696 | Chrysanthemum indicum | Netherlands |
| CBS 562.81; PDDCC 6884 | Stagonosporopsis loticola T | GU238192 | GU237890 | GU237697 | Lotus pedunculatus | New Zealand |
| CBS 628.97; PD 79/72; PDDCC 3870 | Stagonosporopsis loticola | GU238193 | GU237896 | GU237698 | Lotus tenuis | New Zealand |
| CBS 101494; PD 98/5247 | Stagonosporopsis lupini B | GU238194 | GU237724 | GU237699 | Lupinus albus | U.K. |
| CBS 375.84; PD 80/1250 | Stagonosporopsis lupini | GU238195 | GU237844 | GU237700 | Lupinus mutabilis | Peru |
| CBS 634.92; IMI 193307 | Stagonosporopsis oculo-hominis T | GU238196 | GU237901 | GU237701 | Human | U.S.A. |
| CBS 109180; PD 79/175 | Stagonosporopsis rudbeckiae B | GU238197 | GU237745 | GU237702 | Rudbeckia bicolor | Netherlands |
| CBS 379.91; PD 77/675 | Stagonosporopsis trachelii B | GU238173 | GU237850 | GU237678 | Campanula isophylla | Netherlands |
| CBS 384.68 | Stagonosporopsis trachelii B | GU238174 | GU237856 | GU237679 | Campanula isophylla | Sweden |
| CBS 273.92; PD 76/1019 | Stagonosporopsis valerianellae | GU238200 | GU237819 | GU237705 | Valerianella locusta | Netherlands |
| CBS 329.67; PD 66/302 | Stagonosporopsis valerianellae B | GU238201 | GU237832 | GU237706 | Valerianella locusta var. oleracea | Netherlands |
ATCC: American Type Culture Collection, Virginia, U.S.A.; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CECT: Colección Española de Cultivos Tipo, Valencia University, Spain; DAOM: Canadian Collection of Fungal Cultures, Ottawa, Canada; DSM: Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany; HACC: Research Laboratory, Hindustan Antibiotics Ltd., Pimpri Poona, India; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; LEV: Plant Health and Diagnostic Station, Auckland, New Zealand; MUCL: Mycotheque de l'Universite catholique de Louvain, Louvain-la-Neuve, Belgium; PD: Plant Protection Service, Wageningen, the Netherlands; PDDCC: Plant Diseases Division Culture Collection, Auckland, New Zealand; PREM: National Collection of Fungi: Culture Collection, Pretoria, South Africa; VKM: All-Russian Collection of Microorganisms, Pushchino, Russia; VPRI: Victorian Plant Disease Herbarium, Victoria, Australia.
T: Ex-type strain; B: Reference strain according to Boerema et al. (2004).
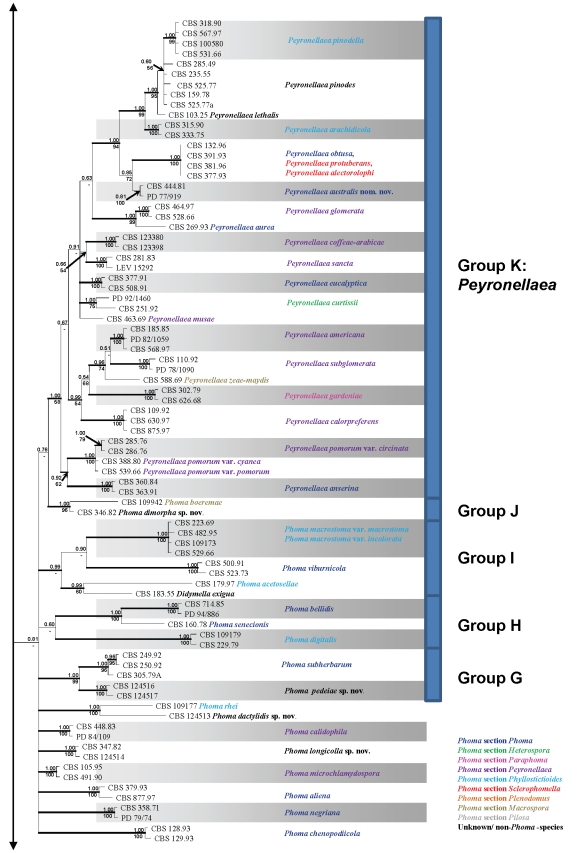
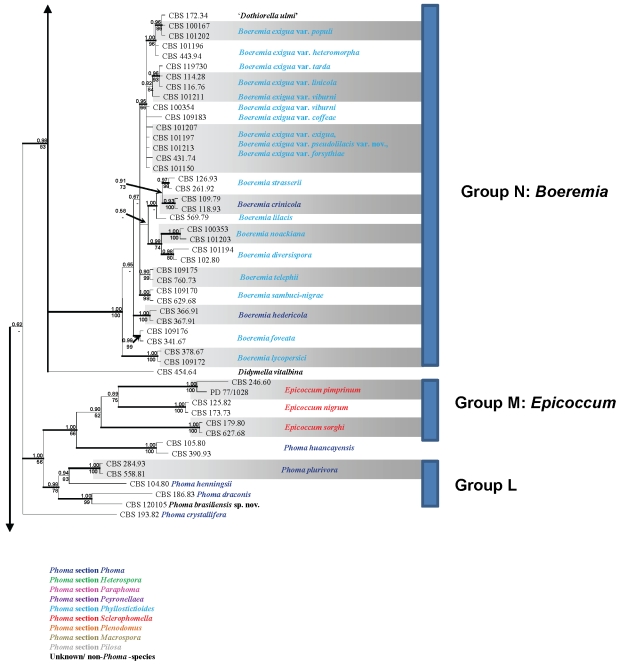
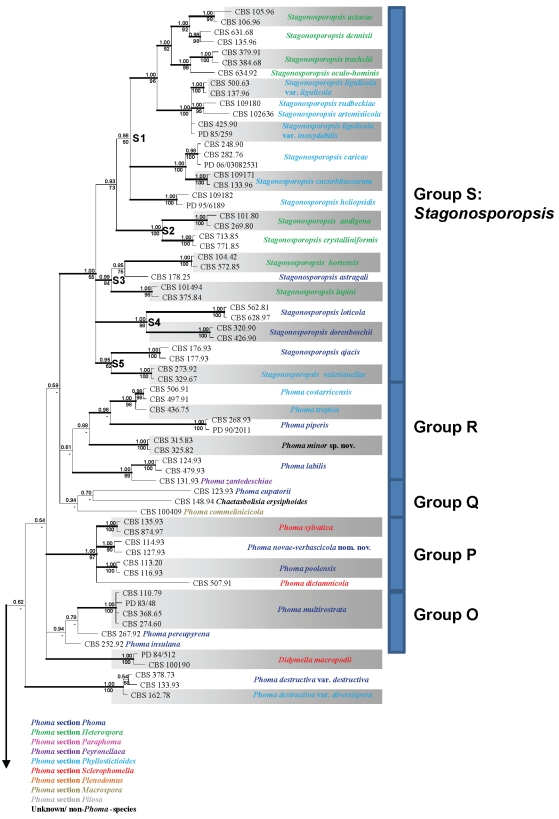
Obtained consensus sequences were assembled and aligned using the same BioNumerics software and adjusted manually where necessary. As SSU was highly conserved in deeper node phylogenies, revealing almost no phylogenetic informative nuclear polymorphisms, and as ITS and TUB proved to be unalignable due to a high level of polymorphism if all taxa studied would be taken into account, it was decided to conduct two separate analyses. The first analysis comprised SSU and LSU loci, and was applied to 76 taxa of which most species included belonged to genera that were often confused with Phoma (Sutton 1980, De Gruyter et al. 2009). A second set of analyses was conducted on 274 taxa, and focussed on the species that had proven to be related to the Didymellaceae from preliminary studies.
Each of the phylogenetic analyses consisted of two methods: Bayesian Interference (BI) and Maximum Likelihood (ML). For BI analysis, the nucleotide substitution models were determined for each locus separately with MrModeltest v. 2.2 (Nylander 2004). According to this software, the General Time Reversible substitution was determined to be the best model for SSU, TUB and LSU in both data sets, with inverse gamma rates and dirichlet base frequencies (GTR + I + G). For the ITS dataset, the software suggested the Symmetrical Model as the best model for substitution of nucleotides. Also in this locus, the inverse gamma rates and dirichlet base frequencies were used (SYM + I + G). The actual Bayesian calculations were performed in MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001). One tree was saved per 100 generations, and the run was automatically ended when the standard deviation of split frequencies was below 0.01. The temperature value of the Bayesian run was set at 0.2. To avoid suboptimal trees being taking into account for the consensus tree, a burn-in of 25 % of the saved trees was used. The resulting “50 % majority rule consensus” trees were visualised with TreeView v. 1.6.6 (Page 1996).
A second measure of branch support was obtained by conducting a ML analysis using RAxML software (Stamatakis et al. 2005) through the CIPRES Website (www.phylo.org). The same partitions were used as in the BI analyses, but because RAxML implements only the GTR substitution model, the symmetrical model for the ITS partition was waived. The robustness of trees in the ML analyses was evaluated by bootstrapping the datasets. The number of bootstrap replicates was automatically determined by the RAxML software (Stamatakis et al. 2008). The obtained trees in both analyses are lodged with TreeBASE (www.treebase.org).
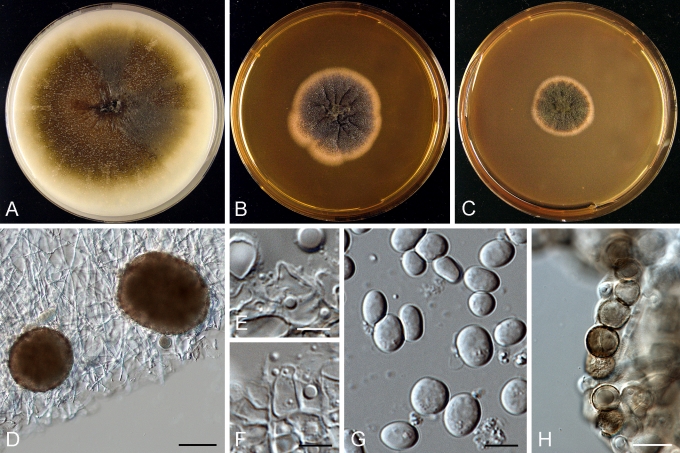
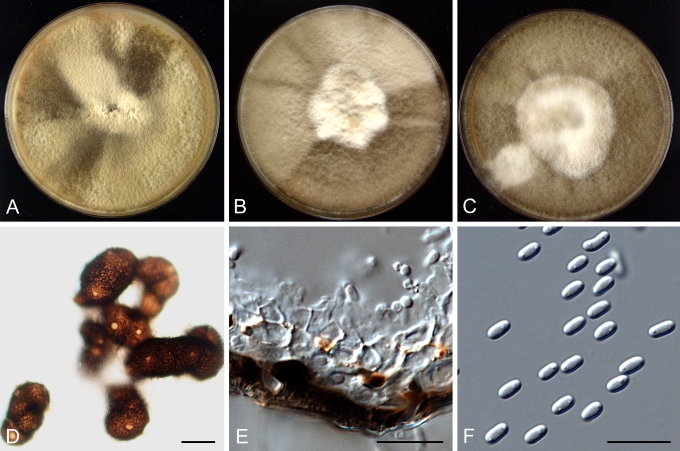
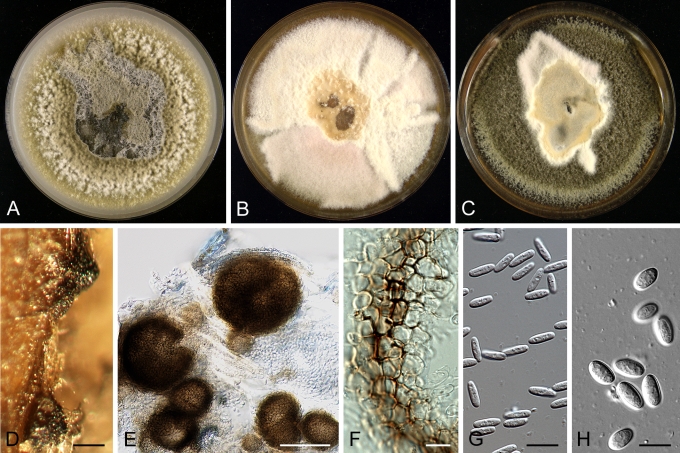
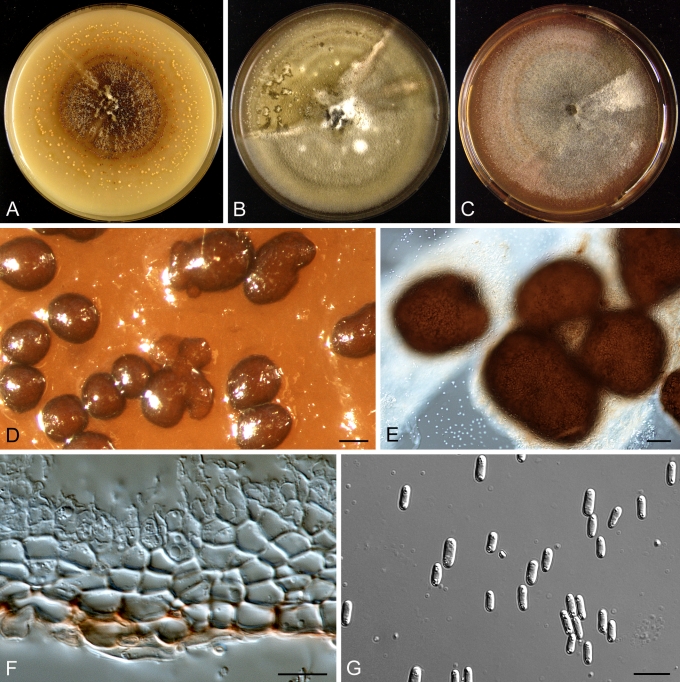
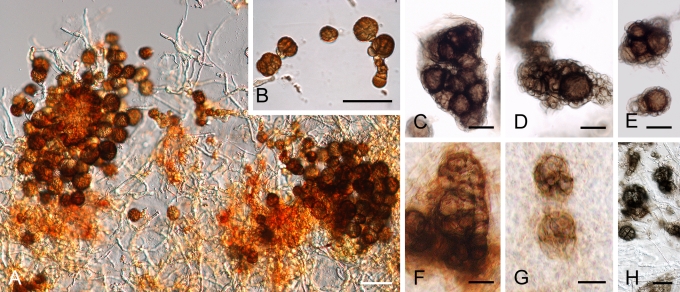
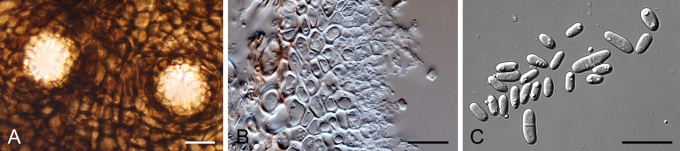
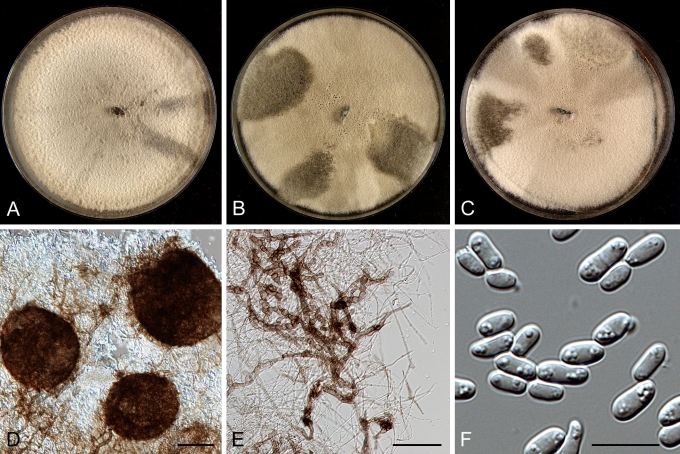
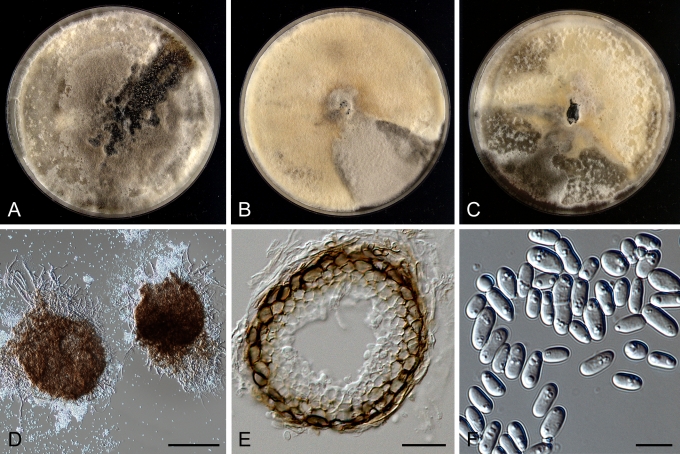
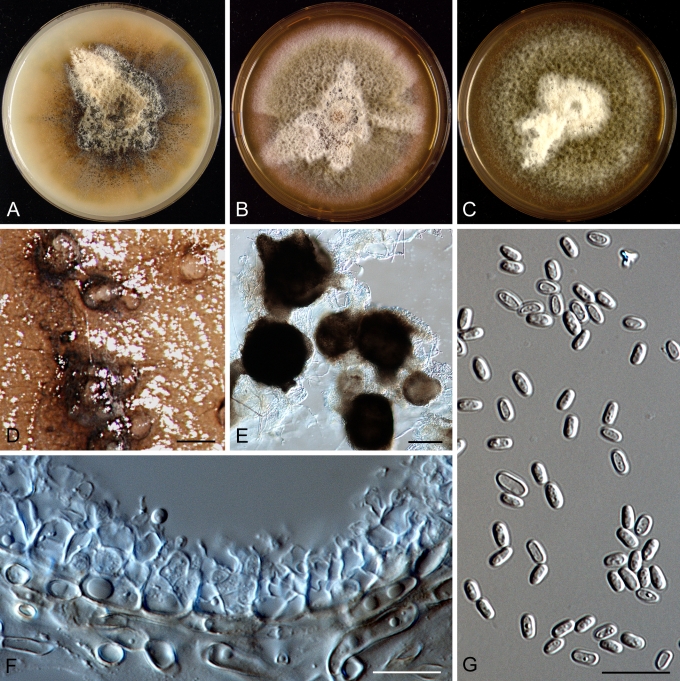
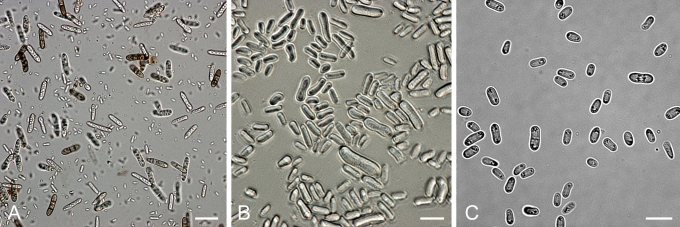
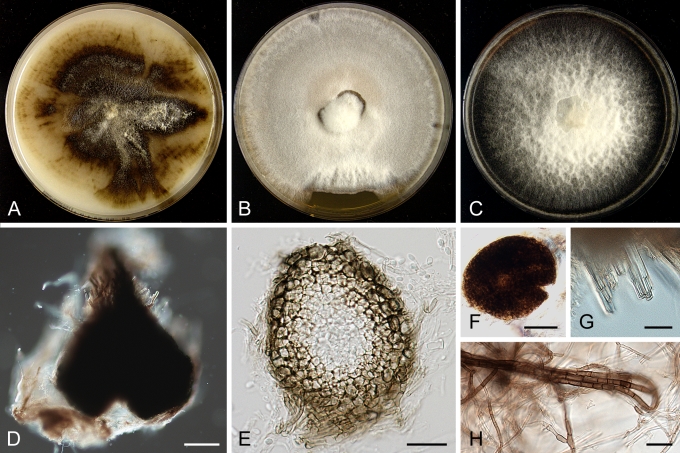
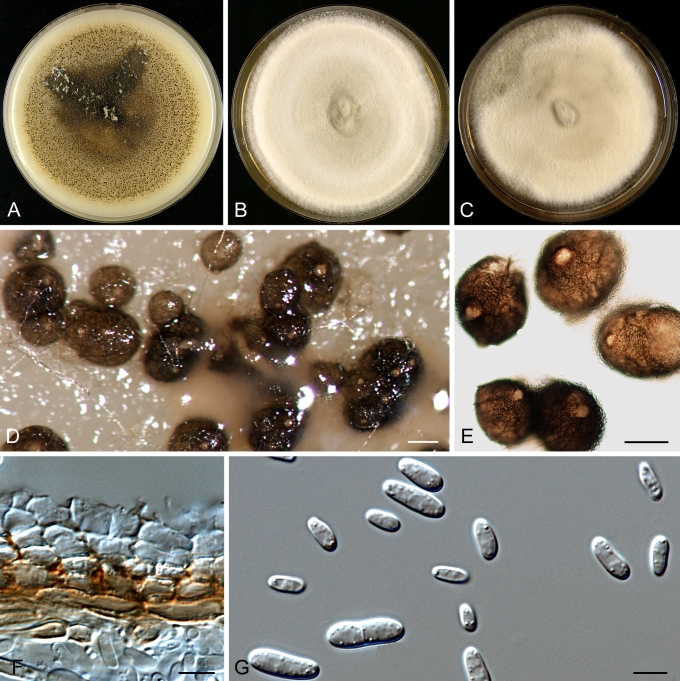
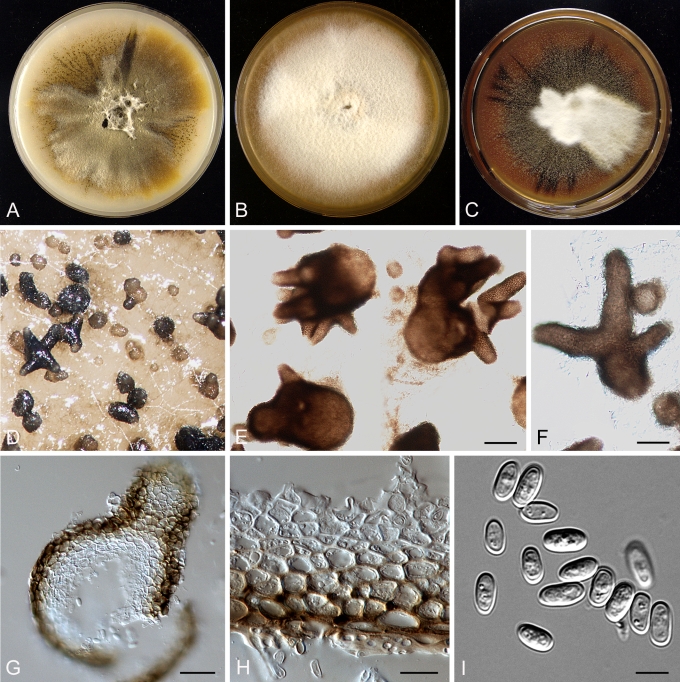
Morphology
Morphological studies of the strains were performed on OA, malt extract agar (MEA) and cherry decoction agar (CHA) (Crous et al. 2009c). The cultures were incubated according to the methodologies described by Boerema et al. (2004). Eight days after inoculation, the colony growth was measured. At the 15th day after incubation, the colony colours were rated using the colour charts of Rayner (1970). Micromorphological features were studied after maturation of the pycnidia. Therefore, fungal structures were mounted in tap water using a scalpel blade and examined under a stereo light microscope. Perennial structures that were formed in the agar medium, such as chlamydospores, were cut out from the medium, and mounted in lactic acid. Remaining agar was removed from these samples by gently heating the glass slides. The sizes of the various structures were determined by averaging the measurements of 30 samples of each structure, except for conidiogenous cells and pycnidial wall characters, of which the size ranges were estimated based on 5–10 samples. Fifth and 95th percentiles were determined for all measurements and are provided in parentheses. By application of a droplet of 1N NaOH, the production of metabolite E+ was determined (Dorenbosch 1970, Noordeloos et al. 1993). The structure of the pycnidial wall and shape of conidiogenous cells were studied using microtome sections of 6 μm thickness, prepared with a Leica CM3050 freezing microtome and mounted in lactic acid. Taxonomic recombinations and novel species and descriptions were deposited in MycoBank.
RESULTS
Systematics of the genus Phoma
DNA phylogenetical analysis
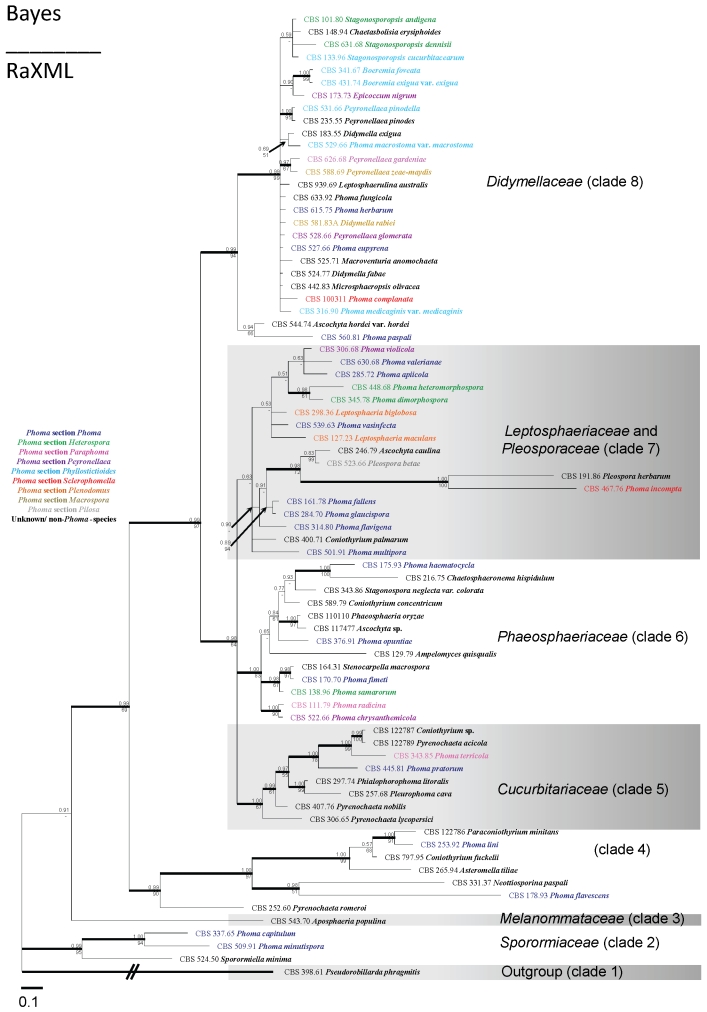
Due to alignment difficulties multiple datasets, consisting of different sets of loci, were utilised. For a generic overview, LSU and SSU were included in the first alignment, which consisted of 76 taxa. A list of species names and numbers, original substrates, geographical origins and GenBank accession numbers of the strains used in this study is provided in Table 2. The aligned sequence matrix had a total length of 2 210 characters including alignment gaps (LSU: 1 258 and SSU: 952 bp). Of those characters, 1 809 (LSU: 994 and SSU: 815) were constant and 401 were variable (LSU: 264 and SSU: 137). The Bayesian analysis run was aborted after 10 000 000 generations as a point of stationarity was reached in the average standard deviation of split frequencies, at a value of 0.0288. The applied “burn-in” percentage of 25 % was well after stationarity in the probability of the trees was reached. The tree topologies and support values of the ML analysis, differed only slightly from the trees obtained from the Bayesian analyses, supporting the probability of the tree. The tree is rooted to Pseudorobillarda phragmitis (CBS 398.61).
Based on the LSU-SSU phylogenetic study performed here for the various anamorph and teleomorph species in the Phoma complex, eight clades were revealed (Fig. 1), including one which only comprises the outgroup specimen. The various clades will be treated below, but for additional synonymy on the Phoma species we refer to Boerema et al. (2004). The findings in these clades are largely in congruence with the observations of De Gruyter et al. (2009).
Fig. 1.
(p. 15) Fifty percent majority rule consensus tree from a BI analysis of Large and Small subunit sequences of Phoma and related genera (n = 76). At the nodes the BI Posterior Probabilities are presented above the branch, and bootstrap percentages of the ML analysis are given below the branch. Branches that were less than 50 % supported in the ML analyses are indicated with a hyphen. The bar indicates the number of substitutions per site. The tree is rooted with Pseudorobillarda phragmitis (CBS 398.61).
Species that were ascribed to the Phoma section Phoma by Boerema et al. (2004) appear to be genetically highly heterogeneous, as these species are recovered in almost every clade. Species that were ascribed to Phoma section Heterospora appear to be linked to at least three distinct clades. Also polymorphism is observed for sections Paraphoma, Peyronellaea and Sclerophomella, as well as for Coniothyrium and Ascochyta. The type species of this latter genus, A. pisi, is not included in the present tree, but is genetically similar to the Didymellaceae.
Treatment of the clades
Clade 1, Outgroup:
Pseudorobillarda phragmitis was selected as outgroup on the basis of the studies conducted by De Gruyter et al. (2009). This species, although being recognised as a coelomycete, is not only phylogenetically, but also morphologically distinct from Phoma, although Sutton (1980) classified it in the Phialopycnidiineae.
Clade 2, Sporormiaceae:
In the basal lineages, Sporormiella minima (CBS 524.50) was recovered, representing the Sporormiaceae, which was recently recircumscribed (Barr 2000). In the same clade, two species were recovered that are described in Phoma section Phoma: Ph. capitulum and Ph. minutispora. Both species are distinguishable from other species in this Boeremaean section by the production of relatively small subglobose conidia (measuring ca. 2–5 × 1.5–3 μm) with a few, large guttules. Within the Sporormiaceae, teleomorphs species have been reported with phoma-like anamorphs, such as Westerdykella dispersa (Von Arx 1981). Two Sporormiaceae-associated genera, Sporormia and Preussia, have been mentioned as possible teleomorph for Ph. deserticola (Von Arx & Storm 1967), a species that was regarded as miscellaneous by Boerema et al. (2004). Also these anamorphs produce minute (sub-) globose conidia (Von Arx 1981, Boerema et al. 2004). Although the Sporormiaceae belongs to the Pleosporales (Barr 2000, 2002, Shearer et al. 2009, Suetrong et al. 2009), it forms a rather basal clade to most of the other Phoma species, and a taxonomic revision of Ph. capitulum and Ph. minutispora should therefore be considered.
Clade 3, Melanommataceae:
One species that belongs to the Melanommataceae was included in the phylogenetical reconstruction of the phomoid Pleosporales. This species, Aposphaeria populina (CBS 543.70), is recovered in the basal lineages of the reconstructed tree (Mugambi & Huhndorf 2009, Suetrong et al. 2009, Tanaka et al. 2009). The close association of this family with the Sporormiaceae and their phylogenetic placement in the basal lineages of the Pleosporales is in congruence with results obtained in earlier studies (Kruys et al. 2006, De Gruyter et al. 2009). Although some earlier workers regularly mistook several Phoma species for members of the genus Aposphaeria (e.g. Saccardo 1884), none of the Phoma species included in this study were clustering with the Melanommataceae.
Clade 4:
This clade comprises a range of species that almost all belong to different genera. Phoma lini and Ph. flavescens are the two Phoma representatives found in this clade, although they are not sister species. Based on morphological data, both species were accommodated in Phoma section Phoma (De Gruyter et al. 1993). Both species produce a yellow diffusible pigment in vitro, although a positive reaction to NaOH is only observed in Ph. lini. Both Ph. flavescens and Ph. lini are closely related to Paraconiothyrium minitans (≡ Coniothyrium minitans; Verkley et al. 2004). With this formal recombination into Paraconiothyrium, it was aimed to differentiate Par. minitans, which produces complex, thick-walled pycnidia from other Coniothyrium species that normally produce more phomoid pycnidia (Verkley et al. 2004). The close relationship between Par. minitans with C. fuckelii that is found here is in congruence with the observations of Damm et al. (2008), although the teleomorph name, Leptosphaeria coniothyrium, would suggest a association with the Leptosphaeriaceae (clade 8).
The likeliness of the findings of Pyrenochaeta romeroi (CBS 252.60), Asteromella tiliae (CBS 265.94) and Neottiosporina paspali (CBS 331.37) in this clade was already discussed by De Gruyter et al. (2009).
Clade 5, Cucurbitariaceae:
Clade 5 comprises mainly taxa with setose pycnidia, including several representative species of the genus Pyrenochaeta. In addition, a Coniothyrium sp., Phialophorophoma litoralis and Pleurophoma cava grouped in this clade, as well as two Phoma species, Ph. pratorum (section Phoma) and Ph. terricola, (section Paraphoma). Another representative of the section Paraphoma that is included in this study is Ph. radicina, which is however found in clade 6. The taxonomy of setose species that are currently classified in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma is revised (De Gruyter et al. 2010). Also in several non-Paraphoma species in the genus Phoma setose or semi-pilose pycnidia do occur incidentally (Boerema et al. 2004). However, thus far, no setae-forming Ph. pratorum strains have been recorded. The finding of this species in the present clade is thus highly remarkable.
The Coniothyrium strain in this clade (CBS 122787) was previously identified as C. cerealis, and is found to be closely related to Pyrenochaeta acicola (BPP = 0.99, RBS = 100 %). As was illustrated in a previous study of Muthumeenakshi et al. (2001) C. cerealis is quite distantly related to other Coniothyrium species. However, based on comparison with sequence data available in GenBank, it is unlikely that its previous identification was correct. This finding further illustrates the polyphyly of the genus Coniothyrium, which further has been retrieved in clades 4, 6 (Phaeosphaeriaceae), 7 (Leptosphaeriaceae and Pleosporaceae) and 8 (Didymellaceae). As mentioned before, some species of this genus have been associated with the teleomorph genus Leptosphaeria, and are thus expected to cluster with the Leptosphaeriaceae (clade 7). None of the species recovered in clade 5 has been associated with a teleomorph.
Clade 6, Phaeosphaeriaceae:
The species that are found in the well-supported clade 6 (BPP = 1.00; RBS = 83 %), belong to the morphologically heterogeneous group of the Phaeosphaeriaceae (Boehm et al. 2009, Zhang et al. 2009). Most findings in this clade have already been discussed in the previous paper of De Gruyter et al. (2009). In addition to that study, six Phoma species are retrieved in this clade. Phoma radicina, type of Phoma section Paraphoma, is found in close association with Ph. chrysanthemicola (BPP = 1.00; RBS = 90 %). The association between Ph. radicina and the Phaeosphaeriaceae is further discussed by De Gruyter et al. (2010). Its close association with Ph. chrysanthemicola has been observed before by Aveskamp et al. (2008a), but the link with the Phaeosphaeriaceae has not been established. Strains of Ph. chrysanthemicola exhibit some semi-setose pycnidia that are, however, often fully covered by mycelial hairs (Boerema 1993). This is a feature that is in common with Ph. radicina, which has, as type species of the section Paraphoma, clearly visible setae. In contrast, the main characteristic of Ph. chrysanthemicola, the presence of pseudosclerotioid masses, has never been observed in the latter species. However, also not all strains of Ph. chrysanthemicola exhibit this character (Dorenbosch 1970).
Phoma fimeti forms a subclade with Ph. samarorum and a strain that was previously identified as Stenocarpella macrospora (BPP = 0.98; RBS = 67 %), but that is probably misidentified (De Gruyter et al. 2009). Especially the finding of Ph. samarorum is noteworthy, as it is found rather distinct from two clusters of other species belonging to the section Heterospora, which are retrieved among the Leptosphaeriaceae and Didymellaceae (clades 7 and 8). In contrast to these other Heterospora species, the large conidia of Ph. samarorum that can be observed in planta are clearly distinct by the subulate top cells, and measures up to 17 × 3.5 μm (Boerema et al. 1997). The strain identified as Stenocarpella macrospora is now sterile and therefore not studied morphologically. This species is known to produce similar-shaped, septate conidia, which are however pigmented and considerably larger, 44–82 × 7.5–11.5 μm (Sutton 1980). The close association with Ph. fimeti is therefore remarkable as this species is known to produce only minute, aseptate conidia, measuring (2–)2.5–4(–5) × (1.5–)2–2.5(–3) μm (De Gruyter & Noordeloos 1992).
The remaining two Phoma species in this clade, Ph. haematocycla and Ph. opuntiae, also produce such minute conidia. Phoma haematocyla, a flax-associated species from New Zealand, is retrieved in a subclade that also accommodates Chaetasphaeronema hispidulum (BPP = 1.00; RBS = 100 %).
All Phoma species found here are morphologically rather distinct, hence their placement in four different Phoma sections (Boerema et al. 2004). None of the Phoma species accommodated in this clade is associated with a teleomorph. The main teleomorph associated with the Phaeosphaeriaceae is Phaeosphaeria, although also incidentally a Leptosphaeria species is associated with this family (Câmara et al. 2002). An anamorph genus that is often confused with Phoma is Microsphaeropsis (Boerema 1997), which is linked to Phaeosphaeria (Câmara et al. 2002). Both anamorph genera differ in conidial pigmentation, which is commonly only present in mature conidia of Microsphaeropsis. Younger conidia are, however, often colourless. It may be that the Phoma species in this clade actually belong to what is now known as Microsphaeropsis, but have lost the pigmentation character during evolution.
Clade 7, Leptosphaeriaceae and Pleosporaceae:
Clade 7 is a large clade comprising many Phoma species from various Boeremaean sections. Three reference species encountered here have been associated with the Leptosphaeriaceae before, these include Leptosphaeria maculans, L. biglobosa and Coniothyrium palmarum (Reddy et al. 1998, Verkley et al. 2004, De Gruyter et al. 2009), or with the Pleosporaceae, such as Pleospora herbarum, Ascochyta caulina and Ph. betae (Dong et al. 1998, Kodsueb et al. 2006, Inderbitzin et al. 2009, De Gruyter et al. 2009).
The two Leptosphaeria species in this study that were associated with a Phoma anamorph cluster together in the present clade: L. maculans (anam Ph. lingam) and L. biglobosa, which produces an unnamed, phomoid anamorph that is highly similar to Ph. lingam (Shoemaker & Brun 2001). Both species are serious pathogens of Brassicaceae (Fitt et al. 2006). Leptosphaeria biglobosa was found to be closely related to Ph. lingam in previous studies (Mendes-Perreira et al. 2003) and was for a long time recognised as a weakly pathogenic variety of the latter species (Johnson & Lewis 1990, Schäfer & Wöstemeyer 1992, Morales et al. 1993, Pongam et al. 1999, Williams & Fitt 1999, Purwantara et al. 2000, Shoemaker & Brun 2001, Voigt et al. 2001).
The phylogenic relation of Phoma species currently classified in sections Pleonodomus and Pilosa is currently investigated (De Gruyter et al. in prep.). However, the present results reveal that a number of species from other Phoma sections fits in the Leptosphaeriaceae and Pleosporaceae. These include Ph. apiicola, Ph. fallens, Ph. flavigena, Ph. glaucispora, Ph. multipora, Ph. valerianeae and Ph. vasinfecta. In contrast to the species that are accommodated in sections Pilosa and Plenodomus, pilose or scleroplectenchymatous pycnidia have never been recorded in these seven species; hence the placements in section Phoma.
Phoma multipora was ascribed to section Phoma. However, the original morphological description mentions the presence of elongated conidiophores (Pawar et al. 1967), which indicates that this species does not belong to the genus Phoma according to the present-day concept.
In addition, some representatives of other sections are found in clade 7, such as Ph. incompta (section Sclerophomella) and Ph. violicola, which is associated with the section Peyronellaea. Based on previous studies in the section Peyronellaea however, also Ph. chrysanthemicola and Ph. schachtii may be expected to cluster with the species in this clade (Aveskamp et al. 2009a). Remarkably, also two representatives of the section Heterospora are found in this clade. Phoma heteromorphospora is the assigned type species of this section (Boerema et al. 1997), whereas Ph. dimorphospora is morphologically closely allied, in congruence with the molecular results obtained here. Both species have a slow growth-rate and occur on Chenopodium spp., but can be distinguished by the absence of the conidial dimorphism in Ph. dimorphospora in vitro. Moreover, the latter species is commonly found in North and South America, whilst Ph. heteromorphospora occurs mainly in Europe (Boerema et al. 2004).
With the exception of Ph. samarorum (clade 6 – Phaeosphaeriaceae), the other species of the section Heterospora are found in clade 8, which represents the Didymellaceae. The major difference between the Heterospora species in the present clade in contrast to those in the Didymellaceae is the size of the septate conidia, which are up to 9 × larger in vivo than the regular conidia in Ph. heteromorphospora and Ph. dimorphospora, whereas, in the Didymellaceae clade, the septate conidia are only 1.5–4.5 × larger.
Also, Coniothyrium palmarum, which represents the type of its genus, clusters in this clade. Just as in Phoma, the species in Coniothyrium have only a limited number of morphological features that can aid in taxonomy. This has led to an unwanted situation in which species morphologically placed in this genus have been shown in phylogenetic examination to be dispersed among multiple families (Verkley et al. 2004). Although, based on type species, an anamorph-teleomorph link has been established between Coniothyrium and Leptosphaeria (Crous 1998), many heterogeneous species are Coniothyrium-like, and belong phylogenetically to different families or even classes (Cortinas et al. 2006). In this study we found “Coniothyrium” species accommodated in at least three different clades (Fig. 1). Coniothyrium clematidis-rectae is phylogenetically linked to the Didymellaceae (Fig. 2 – see below). Phoma and Coniothyrium are considered to be highly similar and are only distinguished on basis of the pigmentation of the conidia and the structure of the pycnidial wall (Boerema et al. 2004).
Fig. 2.
Fifty percent majority rule consensus tree from a BI analysis of LSU, ITS and TUB sequences of Didymellaceae (n = 274). At the nodes the BI Posterior Probabilities are presented above the branch, and bootstrap percentages of the analysis are given below the branch. Branches that were less than 50 % supported in the ML analyses are indicated with a hyphen. The bar indicates the number of substitutions per site. The tree is rooted with Ascochyta hordei var. hordei (CBS 544.74) and Phoma paspali (CBS 560.81 & CBS 561.81).>
This clade also accommodates Pleospora betae, a notorious leaf and seed pathogen of beet (Beta vulgaris, Bugbee & Cole 1981), and Pl. herbarum, which is the type species of the genus Pleospora. The genetic distance between the two species was already observed in a study utilising SSU nrDNA sequences (Dong et al. 1998). Also three Phoma species that are found in close association with these “true” Pleosporaceae and that are found basal to this clade, Ph. fallens, Ph. flavigena and Ph. glaucispora have glabrous pycnidia and, like Ph. betae, aseptate conidia, hence their link to Phoma section Phoma. Absence of an ostiole is only recorded in Ph. glaucispora (De Gruyter et al. 1998).
Pleospora is linked to the anamorph genus Stemphylium (Simmons 1969), Alternaria and Dendryphion (Von Arx 1981). The pluriform nature of the Pleospora anamorphs strongly contrasts with the relatively uniform morphology of the teleomorphic structures (Holm 1962, Kodsueb et al. 2006, Inderbitzin et al. 2009). The polyphyletic nature of Pleospora has been hypothesised by Holm (1962) and Berbee (1996), but only recently have molecular studies confirmed its taxonomic complexity (Dong et al. 1998, Kodsueb et al. 2006, Inderbitzin et al. 2009).
Clade 8, Didymellaceae:
The major cluster observed in the generic phylogeny is the top clade in Fig. 1, which represents the Didymellaceae clade. This clade is well supported (BPP = 0.99, RBS = 94 %), but with the loci used, a high level of basal polytomy is recorded within the clade. The ancestral species in this clade are the Graminae-pathogens, Ascochyta hordei and Ph. paspali. The latter species has been considered to be an indigenous pathogen of grasses in Australia and New Zealand (Johnston 1981, Boerema et al. 2004), but based on sequence comparisons this species is probably also present in Europe (Wirsel et al. 2001, C. Gueidan pers. comm.).
Clade 8 comprises most Phoma species, including CBS 615.75, the representative strain of Ph. herbarum (Boerema et al. 2004), which is type species of the genus (Boerema 1964). This clade also includes the type species of the Phoma sections Phoma, Peyronellaea, Phyllostictoides, Sclerophomella and Macrospora. Some phytopathologically and medically relevant species of the section Heterospora are also associated with this clade, although some species of this section are found in other clades, such as Ph. samarorum (clade 6) and Ph. dimorphospora, and the sectional type Ph. heteromorphospora (clade 7). Finally, a single species of the setose section Paraphoma, Ph. gardeniae, is found in the Didymellaceae. Based on the sequence data obtained in this study, it is estimated that approximately 70 % of the species recognised by Boerema et al. (2004) can be associated with the Didymellaceae.
Besides the many Phoma species, several other anamorph fungi are found within this clade, including Ampelomyces quercinus, Ascochyta fabae (teleom. Didymella fabae), Asc. hordei var. hordei, Asc. pinodes (teleom. Didymella pinodes), Chaetasbolisia erysiphoides, Didymella exigua, Epicoccum nigrum (synanamorph Ph. epicoccina) and Microsphaeropsis olivacea. Of these species, Asc. pisi, C. erysiphoides and M. olivacea are recognised as type species for their respective genera. De Gruyter et al. (2009) already discussed the probability of finding most of these non-Phoma taxa in the Didymellaceae clade.
It should be noted that not all Ascochyta species are found within this clade, indicating that this genus is also polyphyletic. Whereas A. hordei var. hordei is found to be one of the basal taxa of clade 8, the legume associated pathogens A. fabae, A. pinodes and the type species A. pisi are found in close association with several species of Phoma. This result is in congruence with the observations in the study of Peever et al. (2007). Also the recently described Didymella clematidis has an anamorph state in Ascochyta and is closely related to Phoma taxa in this major clade (Woudenberg et al. 2009). A representative strain Asc. caulina and a new Ascochyta species that is still due to be published (G.J.M. Verkley, pers. comm.), however, have been found to be only distantly related and are found in clades 7 and 6, respectively.
Where a sexual state is known for the Phoma and Ascochyta species in clade 8, it is Didymella. The type species of this teleomorph genus, D. exigua, is also found within this clade, although it is not associated with a Phoma anamorph state. The family Didymellaceae was introduced for this group by De Gruyter et al. (2009). However, type species of two other teleomorph genera have also been found within this clade. DNA sequences of Leptosphaerulina australensis resemble a high level of similarity with those of the various Phoma and Didymella strains, although none are identical. Also sequences of LSU and ITS sequence data obtained from GenBank of L. americana, L. argentinensis, L. chartarum, L. crassiasca and L. trifolii (GenBank accession no. AY278318, AY849949, EU272493, U79485, AY8315585 respectively) were highly similar or even identical to the Didymellaceae sequences obtained in the present study (data not shown). These observations are in congruence with the results obtained by Silva-Hanlin & Hanlin (1999), who found that D. bryoniae (anam. Ph. cucurbitacearum) was closely related with L. chartarum and L. crassiasca. Also Macroventuria anomochaeta, which represents the genus Macroventuria (Van der Aa 1971) groups in Didymellaceae. The close genetical resemblance of Macroventuria and Leptosphaerulina found in the present study is in congruence with the results of Kodsueb et al. (2006).
The loci employed here for phylogenetic analysis are sufficient to identify clades at the family level, but for proper resolution at generic level or lower, additional gene regions need to be sequenced. As the majority of Phoma species is embedded in the Didymellaceae clade, we will define further generic and species boundaries within this recently established family in the subsequent part of this paper.
Systematics of the Didymellaceae
DNA phylogenetic analysis
The alignment that was used to delineate the Didymellaceae consisted of 274 sequences belonging to 196 species. A list of the species names and numbers, original substrates, geographical origins and GenBank Accession numbers of the strains used in this study is provided in Table 3. The sequence matrix had a total length of 2 188 characters including the alignment gaps (LSU: 1 327; ITS: 508 and TUB: 353). Of those characters, 1 788 (LSU: 1 233; ITS: 374 and TUB: 181) were constant, whereas 400 characters (LSU: 94; ITS: 144 and TUB: 192) were variable.
The analysis run of the LSU-ITS-TUB sequence matrix in MrBayes was aborted after obtaining 20 000 trees, which was well after stationarity in the probability of the trees was reached, whereas the standard deviation of split frequencies was below 0.02. From the obtained tree population, the 25 % burn-in was discarded and the consensus tree and posterior probabilities were calculated. The topology and support values of the BI tree were in congruence with the optimal tree obtained in the ML analysis.
Systematics: treatment of clades
As most other anamorph genera, Phoma has largely been used as a convenient form genus, rather than a phylogenetic entity. With the number of Phoma species that are being analysed on DNA sequence level rapidly increasing, the question is raised whether form genera should be maintained or that more natural groupings, merging both phylogeny and morphological data, should be erected. Of course, as greater numbers of taxa are collected and analysed, the taxonomic boundaries of more clades will be resolved. However, for the present, only those genera that could be resolved based on available cultures are treated. The groups mentioned below refer to those indicated A–R in Fig. 2. The unresolved clades are left untreated, and are thus not discussed.
The taxa in this part of the study were selected based on genetic and/or morphological similarities with the species that were associated with the Didymellaceae in Fig. 1. Although numerous taxa from various genera have been associated with “Phoma”, the number of genera that could be included in the selection for the Didymellaceae was limited. Next to Phoma, the only species found were those accommodated on basis of previous morphological studies in either Ampelomyces, Ascochyta, Chaetasbolisia, Coniothyrium, Didymella, Diplodina, Dothiorella, Epicoccum, Leptosphaerulina, Macroventuria, or Microsphaeropsis. Of three of these generic representatives, viz. Chaetasbolisia, Diplodina and Dothiorella, we suspect that some cultures have been preserved under an incorrect name. The species representing Ampelomyces, A. quercinus, was correctly identified, but as suggested earlier, the taxonomic placement in this genus appears to be incorrect (Szentiványi et al. 2005).
Strains belonging to a single species proved to be genetically identical or at least highly similar, indicating that the initial identification of these strains had been carried out correctly.
Several well-supported clusters are recognised within this family that are treated here as novel groups of the Didymellaceae. In this section these separate groups are treated. However, although multiple genes were employed in this study to generate a phylogenetic reconstruction of the family, high levels of basal polytomy were observed as well (Fig. 2). Application of general nrDNA loci alone did not reduce this high level of polytomy, whilst interspecies variation in several well-supported clades was reduced drastically.
Group A – outgroup and basal lineages:
The tree presented in Fig. 2 is rooted to Ascochyta hordei and Ph. paspali, which proved to be ancestral to the Didymellaceae in Fig. 1. The latter species was described by Johnston (1981) as a species from grasses in New Zealand and Australia, but in recent years, isolates with similar genotypes were isolated from iron-rich volcanic soil from France (C. Gueidan, pers. comm.), and from common reed (Phragmites australis) in Germany (Wirsel et al. 2001). These isolates were, however, never studied morphologically.
Another species used as outgroup is Ascochyta hordei var. hordei (CBS 544.74), which was obtained from a South African Triticum aestivum, indicating that also within the Didymellaceae, species that are ascribed to Ascochyta do not form a monophyletic group. Also CBS 259.92, the isotype of Ph. matteuciicola, proved to be basal to most other Phoma species. Phoma matteuciicola is commonly known as a pathogen of many fern species (De Gruyter et al. 2002). Within the basal lineages, also a group comprising Ph. humicola and the novel species Ph. saxea is found, although this group is only supported by BI analysis (BPP = 0.92, RBS < 50 %). Although Phoma humicola is known as a saprobic soil fungus, it is sometimes mistaken for the notorious potato pathogen Ph. foveata (Group N), due to a similar biochemical reaction to NaOH and the formation of citrine green crystals on MEA (De Gruyter et al. 1998). However, conidia of Ph. humicola are always eguttulate in contrast to those of Ph. foveata. Phoma saxea has been found twice in Germany on rock material, and will be further described below.
Phoma humicola J.C. Gilman & E.V. Abbott, Iowa St. Coll. J. Sci. 1(3): 266. 1927.
Specimen examined: U.S.A., Nevada, Death Valley, from a dead leaf of Franseria sp., 1971, G.H. Boerema, CBS H-16390, culture CBS 220.85.
Phoma matteuciicola Aderkas, Gruyter, Noordel. & Strongman, Canad. J. Pl. Pathol. 14(3): 227. 1992.
Specimen examined: Canada, Nova Scotia, Five Mile River, from leaf base of Matteuccia struthiopteris, May 1981, P. von Aderkas, holotype DAOM 183092, culture ex-holotype CBS 259.92 = IMI 286996 = PD 91/272.
Notes: Gangrene in ostrich fern was originally attributed to Ph. exigua var. foveata (von Aderkas & Brewer 1983), which is here recombined as Boeremia foveata, but Von Aderkas et al. (1992) recognised a new species as causal agent of this disease. The phylogeny presented here supports these observations, as Ph. matteuciicola is found rather distinct from B. foveata.
Phoma paspali P.R. Johnst., New Zealand J. Bot. 19(2): 181. 1981.
Specimens examined: New Zealand, Auckland, Kaikohe, from a dead leaf of Paspalum dilatatum, Jan. 1979, P.K. Buchanan, isotype CBS H-7623, culture ex-isotype CBS 560.81 = PD 92/1569; Waikato District, Ruakura, from Lolium perenne, Jan. 1979, G.H. Boerema, CBS 561.81 = PDDCC 6615.
Phoma saxea Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515591. Fig. 3.
Fig. 3.
Phoma saxea (CBS 419.92). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia. E–F. Conidiogenous cells. G. Conidia. H. Chain of unicellular chlamydospores. Scale bars: D = 100 μm; E–G = 5 μm; H = 20 μm.
Conidia dimorpha, intra idem pycnidia formata. Conidia typus 1 (sub)globosa, glabra, hyalina, continua, (3–)3.5–5.5 μm diam., (0–)3–10(–15) guttulis praedita. Conidia typus 2 cylindrica vel ellipsoidea, glabra, hyalina, continua, (3.5–)4.5–7(–7.5) × 2.5–3.5(–4) μm, plerumque eguttulata, vel 1–3 guttulis praedita. Matrix conidiorum salmonea. Chlamydosporae continuae, globosae, viridulae, in catenas usque 35 positae, (8.5–)10–16.5(–17.5) × (6–)8–12.5(–14) μm.
Etymology: Refers to the substratum on which both isolates of this species were found, stone material.
Pycnidia solitary, (sub-)globose, glabrous or covered with hyphal outgrows, (90–)135–280(–310) × (90–)105–260(–275) μm. Ostioles single, papillate, with wide openings, ca. 40–80 μm diam. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 2–3 layers, 10–17 μm thick, outer cell layer brown pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, variable in appearance, flask-shaped, oblong or isodiametric ca. 5.5–7.5 × 3–4 μm. Conidia of two types, both originating from the same pycnidia. Conidia of type 1: (sub-)globose, thin-walled, smooth, hyaline, aseptate (3–)3.5–5.5 μm diam, with (0–)3–10(–15) guttules. Conidia of type 2: cylindrical to ellipsoidal, thin-walled, smooth, hyaline, aseptate, (3.5–)4.5–7(–7.5) × 2.5–3.5(–4) μm, mainly egutullate or with up to 3 minute guttules. Conidial matrix salmon. Chlamydospores ubiquitously present in the agar, unicellular, globose, in long chains of up to 35 elements, greenish pigmented, measuring (8.5–)10–16.5(–17.5) × (6–)8–12.5(–14) μm.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular. Immersed mycelium flat, olivaceous to greenish olivaceous, citrine-green or coral near the colony margin. Aerial mycelium absent, but sometimes some grey erect tufts are encountered near the colony centre; reverse concolourous. Colonies on MEA 20–25 mm diam after 7 d, margin regular. Immersed mycelium violet-slate, but saffron near the colony margin. Abundant pycnidia are present on the agar surface; reverse iron-grey, saffron near the colony margin. Colonies on CHA similar as on MEA, but somewhat slower growing, 10–15 mm diam. after 7 d, and some sparse white aerial mycelia hyphae are present in the colony. Application of NaOH results in a greenish yellow discolouration of the agar, best to be observed on OA medium.
Specimens examined: Germany, Oldenburg, from corroded Mediterranean marble, June 1992, J. Kuroczkin, holotype designated here CBS H-20240, culture ex-holotype CBS 419.92; Oldenburg, from limestone, 1987, J. Kuroczkin, CBS 298.89.
Notes: The pycnidial wall of Phoma saxea is extremely thin and almost hyaline when the conidia have exuded. Older pycnidia collapse and remain as a double-layered, disc-like structure on the agar.
Both strains of this species have been isolated from stone material, such as limestone (CBS 298.89) and corroded Mediterranean marble (CBS 419.92). Although the genus is known from all kinds of substrates, the number of rock-inhabiting Phoma isolates is relatively low. Selbmann et al. (2002) report on Ph. herbarum from Antarctic rock, and Boerema et al. (2004) list several species from rock-like materials, such as cement (Ph. herbarum), wall-plaster (Ph. heteroderae – here recombined into Ph. calorpreferens) and crockery (Ph. pomorum). In addition, multiple species are recorded from rock-inhabiting lichens (Nelson et al. 2009, Ruibal et al. 2009). These species, listed by Hawksworth & Cole (2004) are, however, unculturable and could therefore not be compared with Ph. saxea in vitro. However, the morphological descriptions suggest that the mentioned species and Ph. saxea are different taxonomic entities.
Group B:
Four of the six species clustering in Group B produce a Didymella teleomorph. Only Ph. polemonii and Ph. xanthina presently have no known sexual state. The species in this clade are collected from a wide variety of dicots, although all individual taxa appear to be host-specific (Boerema et al. 2004). Also the micromorphological features of these species are highly variable.
A single strain that was kept in the CBS collection as Diplodina coloradensis was found in this clade as well. However, this genus name has been accommodated in the Gnomoniaceae (Diaporthales), indicating that this strain has been preserved under an incorrect name and should be renamed. However, as this strain proved to be sterile, no proper redescription of the material could be provided.
Didymella applanata (Niessl) Sacc., Syll. Fung. 1: 546. 1882.
Basionym: Didymosphaeria applanata Niessl, Oesterr. Bot. Z. 25(4): 129. 1875.
Anamorph: Phoma argillacea (Bres.) Aa & Boerema, in De Gruyter, Boerema & Van der Aa, Persoonia 18(1): 17. 2002.
Basionym: Phyllosticta argillacea (Bres.), Hedwigia 1894: 206. 1894.
Specimens examined: The Netherlands, Baarn, from Rubus idaeus, Sep. 1963, A. van Dijkman, CBS H-11943, culture CBS 205.63; from Rubus idaeus, 1975, G.H. Boerema, CBS 102634 = PD 75/248.
Didymella cannabis (G. Winter) Arx, in Müller & Arx, Beitr. Kryptogamenfl. Schweiz 11(2): 365. 1962.
Basionym: Sphaerella cannabis G. Winter, Hedwigia 11(10): 145. 1872.
Anamorph. Phoma cannabis (L.A. Kirchn.) McPartl., Mycologia 86(6): 871. 1995.
Basionym: Depazea cannabis L.A. Kirchn., Lotos 6: 183. 1856.
Specimen examined: Unknown origin, from Cannabis sativa, Oct. 1937, K. Röder, CBS 234.37.
Notes: The studied culture (Röder 1937) is now sterile, and could therefore not be described here morphologically.
Didymella catariae (Cooke & Ellis) Sacc., Syll. Fung. 1: 557. 1882.
Basionym: Sphaeria catariae Cooke & Ellis, Grevillea 5: 96. 1876.
Anamorph: Phoma nepeticola (Melnik) Dorenb. & Gruyter, Persoonia 18(1): 18. 2002.
Basionym: Ascochyta nepeticola Melnik, Novoste Sist. Nizsh. Rast. 1968: 178. 1968.
Specimen examined: The Netherlands, from the stem of Nepeta catenaria, 1977, M.M.J Dorenbosch, CBS 102635 = PD 77/1131.
Didymella urticicola Aa & Boerema, in Boerema, Trans. Brit. Mycol. Soc. 67(2): 303. 1976.
Anamorph: Phoma urticicola Aa & Boerema, in Boerema, Trans. Brit. Mycol. Soc. 67(2): 303. 1976.
Specimens examined: The Netherlands, Wageningen, from a dead stem tip of Urtica dioica, Mar. 1973, G.H. Boerema, holotype CBS H-11971, culture ex-holotype CBS 121.75 = ATCC 32164 = IHEM 3403 = IMI 194767 = PD 73/584; from Urtica dioica, 1973, G.H. Boerema, PD 73/570.
Phoma polemonii Cooke, Grevillea 13(68): 94. 1885.
Specimen examined: The Netherlands, from Polemonium caeruleum, 1983, J. de Gruyter, CBS 109181 = PD 83/757.
Phoma xanthina Sacc., Michelia 1(4): 359. 1884.
Specimens examined: The Netherlands, Baarn, from leaves of Delphinium sp., May 1968, H.A. van der Aa, CBS H-8938, culture CBS 383.68; from Delphinium sp., 1984, G.H. Boerema, PD 84/407.
Group C:
The species in Group C cluster in two subgroups: One comprising the Clematis pathogens Ph. clematidina and Coniothyrium clematidis-rectae, the other subgroup comprising Ph. aquilegiicola and Ph. glaucii, two pathogens of Ranunculaceae and Papaveraceae, respectively. All three Phoma species in this group were morphologically linked to the section Heterospora (Boerema et al. 1997), but are distinct from the species in clade S by the absence of conidia that represent the Stagonosporopsis synanamorph in culture, although smaller septate conidia do occur. In these species the Stagonosporopsis synanamorph is only known from in vivo material (Boerema 1993, Boerema et al. 1997).
The several species that were associated with the Ph. clematidina morphotype have recently been distinguished in a study of Woudenberg et al. (2009). In the same study, the authors showed that C. clematidis-rectae is closely related and, based on sequence analysis, a member of the family Didymellaceae. The major character on which this species is regarded as distinct from Ph. clematidina is by the production of pale brown pigmented conidia. In addition, the conidiogenesis of Coniothyrium is annellidic with percurrent proliferation, in contrast to the conidiogenesis in Phoma, which is considered to be solely phialidic with periclinal thickening (Boerema & Bollen 1975, Sutton 1980). Evidence for the presence of annellides has, however, not been observed in C. clematidis-rectae, while conidial pigmentation is relatively pale in comparison to other Coniothyrium species. Pigmented conidia have also been observed in various Phoma species before (Dorenbosch 1970, Boerema et al. 2004, Aveskamp et al. 2009a). These features may indicate that this species is actually a Phoma with early conidial pigmentation. Therefore C. clematidis-rectae is recombined into Phoma below.
Phoma aquilegiicola M. Petrov, Acta Inst. Bot. Acad. Sci. USSR Pl. Crypt. [Trudy Bot. Inst. Akad. Nauk SSSR] Fasc. 1: 281. 1933.
Specimens examined: The Netherlands, from a stem of Aconitum pyramidale, 1973, G.H. Boerema, CBS 107.96 = PD 73/598; from a stem of Aquilegia sp., 1979, G.H. Boerema, CBS 108.96 = PD 79/611.
Phoma clematidina (Thüm.) Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen (Jaarboek 1978) 153: 17. 1979. emend. Woudenberg et al., Persoonia 22: 59. 2009.
Basionym: Ascochyta clematidina Thüm., Bull. Soc. Imp. Naturalistes Moscou 55: 98. 1880.
Specimens examined: Russia, Minussinsk, from leaves of Clematis glaucae, N. Martianoff, isotype LE 40082; The Netherlands, Spaubeek, from the stem of Clematis sp., July 1978, G.H. Boerema, epitype CBS H-16193, culture exepitype CBS 108.79 = PD 78/522; from Clematis sp., I. de Boer, Nov. 1949, CBS 201.49; Boskoop, from Clematis jackmanii, C. Dorsman, Oct. 1962, CBS 195.64; Wageningen, from Selaginella sp. M.M.J. Dorenbosch, 1966, CBS 520.66; U.K., England, from Clematis sp., Jan. 1966, F.T. Last, CBS 102.66.
Phoma clematidis-rectae (Petr.) Aveskamp, Woudenberg & Gruyter, comb. nov. MycoBank MB515592.
Basionym: Coniothyrium clematidis-rectae Petr., Fungi Polon. 576. 1921.
Pycnidia solitary or confluent, immersed or produced on the agar surface, globose, glabrous, (80–)85–130(–155) μm diam, in older cultures pycnidia may become larger and grow after maturation to 220–250 μm diam. Ostioles 1(–4), wide, non-papillate to papillate or, in older cultures, on a elongated neck. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 4–5 layers, (10–)11–19(–19.5) μm thick, outer 1–2 layers pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, ampulliform to doliiform, measuring 3–4.5(–5) × 2.5–4.5 μm. Conidia ellipsoidal to cylindrical, thin-walled, smooth, aseptate, (3–) 4–7(–8) × 2–3(–3.5) μm, with (2–)5–12 guttules, initially hyaline, but mature conidia become slightly brownish pigmented. Conidial matrix sepia.
Culture characteristics: Colonies on OA 42–52 mm diam after 7 d, margin regular. Immersed mycelium dark brick to sepia or iron-grey, but hyaline near the colony margin. Pycnidia in concentric rings give the colony an olivaceous tinge. Aerial mycelium absent; reverse concolourous. Colonies on MEA 27–52 mm diam after 7 d, margin regular. Aerial mycelium incidentally occurs in sectors in some strains, grey to olivaceous. Immersed mycelium rosy-buff to rosy-vinaceous with olivaceous and grey tinges; reverse olivaceous iron-grey to saffron. Application of NaOH did not have any effect.
Specimens examined: The Netherlands, Boskoop, from Clematis sp., 1963, G.H. Boerema, CBS H-20275, culture CBS 507.63 = PD 07/03486747 = MUCL 9574; from Clematis sp., 1995, J. de Gruyter, PD 95/1958.
Notes: In congruence with the studies of Woudenberg et al. (2009), this species was found to be closely related to Ph. clematidina and other Didymellaceae species. In contrast, it is only distantly related to the type species of Coniothyrium, C. palmarum. Therefore, a recombination into Phoma is proposed here. The present species is clearly distinct from Ph. clematidina by the production of pigmented conidia, although the level of pigmentation is low, which distinguishes Ph. clematidis-rectae from the species remaining in Coniothyrium that produce darker, olivaceous conidia.
Phoma glaucii Brunaud, “Ph. glauci”, Ann. Soc. Sci. Nat. La Rochelle 1892: 97. 1892.
Specimens examined: The Netherlands, near Lisse, from Dicentra sp., 1979, G.H. Boerema, CBS 112.96; Wageningen, from a leaf of Chelidonium majus, 1994, G.H. Boerema, CBS 114.96 = PD 94/888.
Groups D & E – Leptosphaerulina and Macroventuria:
The most remarkable findings in the Didymellaceae are the Leptosphaerulina and Macroventuria (clade E) teleomorph genera. The species belonging to these teleomorphs are found amidst the Didymellaceae, causing the genus Didymella to be paraphyletic. The species in both genera are closely related to each other, as was already pointed out by Kodsueb et al. (2006), who, however, missed the link with Didymella. A phomoid anamorph state has, thus far, not been recorded for any of the species in these teleomorph genera.
Leptosphaerulina is morphologically distinct from Macroventuria and Didymella, although all three genera are known for their hyaline ascospores (Van der Aa 1971, Von Arx 1981). Leptosphaerulina produces large, longitudinally and transversally septated ascospores, resembling those of Pleospora and Cucurbitaria, although the ascospores of these genera are pigmented. The major difference between Didymella and Macroventuria is the presence of setae on the pseudothecia of the latter genus, whereas Didymella ascomata are commonly glabrous. According to the original description (Van der Aa 1971), Macroventuria strains resemble Venturia by their setose pycnidia, but differ in their restricted number of the asci.
Leptosphaerulina americana (Ellis & Everh.) J.H. Graham & Luttr., Phytopathology 51: 686. 1961.
Basionym: Pleospora americana Ellis & Everh., in North American Pyrenomycetes: 336. 1892, nom. nov. pro Pleospora hyalospora Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia: 238. 1890, non Pleospora hyalospora Speg.
Specimen examined: U.S.A., Georgia, from Trifolium pratense, Apr. 1954, E.S. Luttrell, CBS 213.55.
Leptosphaerulina arachidicola W.Y. Yen, M.J. Chen & K.T. Huang, J. Agric. Forest. 10: 167. 1956.
Specimen examined: Taiwan, from a leaf of Arachis hypogaea, 1956, K.T. Huang, CBS 275.59 = ATCC 13446.
Note: CBS 275.59 is degenerated and forms only very tiny sclerotia in vitro.
Leptosphaerulina australis McAlpine, Fungus Diseases of stone-fruit trees in Australia: 103. 1902.
Specimens examined: Indonesia, Lampung, from Eugenia aromatica, Dec. 1982, H. Vermeulen, CBS 317.83. The Netherlands, Baarn, from soil, Sep. 1969, J.A. Stalpers, CBS 939.69.
Leptosphaerulina trifolii (Rostr.) Petr., Sydowia 13: 76. 1959.
Basionym: Sphaerulina trifolii Rostr., Bot. Tidsskr. 22: 265. 1899.
Specimen examined: The Netherlands, from Trifolium sp., 1958, CBS 235.58.
Macroventuria anamochaeta Aa, Persoonia 6(3): 362. 1971.
Specimens examined: South Africa, Karroo Desert, from decayed canvas, Aug. 1971, M.C. Papendorf, holotype CBS H-14192, ex-holotype culture CBS 525.71; Cape Province, from a trunk of Medicago sativa, June 1972, W.F.O. Marasas, CBS 502.72.
Macroventuria wentii Aa, Persoonia 6(3): 361. 1971.
Specimen examined: U.S.A., Nevada, Death Valley, from plant litter, Aug. 1971, F.W. Went, holotype CBS H-14195, ex-holotype culture CBS 526.71.
Group F:
As a sister group to Leptosphaerulina, several host-specific Phomaspecies are found that induce leaf spots on a variety of plant species, including Ph. infossa, Ph. anigozanthi, Ph. arachidis-hypogaeae and Ph. gossypiicola. The latter species causes leaf spots and stem canker on cotton plants (Gossypium spp.). However, other plant species may also become symptomatic when deliberately infected (Holliday & Punithalingam 1970). Phoma infossa has originally been reported from stems of ash trees (Fraxinus sp.) in New York State (Ellis & Everhart 1888), but has recently been associated with a severe foliar disease of green ash (F. pennsylvanica) in Argentina (Aveskamp et al. 2009a). All species produce aseptate conidia in culture, although Ph. gossypiicola is known to also produce 2- to multi-celled conidia in vivo, hence the Ascochyta gossypii synonym (De Gruyter 2002).
In contrast to these plant pathogens, a fungicolous species also occurs in the present clade. Species from the genera Phoma and Ampelomyces have been “frequently confused with each other” (Sullivan & White 2000), which explains why Ph. fungicola is found here. This species was previously known as Amp. quercinus and is recombined in the subsequent taxonomical section. The finding of this species in the Didymellaceae is in congruence with sequence results obtained by Sullivan & White (2000) and Szentiványi et al. (2005). Also Amp. humuli, another fast-growing species, proved to be phylogenetically similar to species that currently represent the Didymellaceae (Kiss & Nakasone 1998). Additionally, it has been suggested that the fast growing species Amp. artemisiae and Amp. uncinulae (Rudakov 1979, Kiss 1997) actually do, in fact, not represent Ampelomyces, but belong to the genus Phoma; these species were incorrectly identified based on their host-association (Kiss et al. 2004). The species in Ampelomyces are all recognised as parasites of fungi that cause powdery mildew (Kiss 1997). However, it is suggested that also the ubiquitous species Ph. glomerata has fungicolous capacities, and may be suited as mycoparasitic control agent of powdery mildew (Sullivan & White 2000).
Only one of the Phoma species embedded in this clade has been associated with a teleomorph. In the description of Ph. anigozanthi, the sexual state is recorded as Sphaerella millepunctata (apud Gruyter & Noordeloos 1992). Sphaerella is practically synonymised with Mycosphaerella (e.g. Aptroot 2006), but as described above, several of the Mycosphaeralla species have subsequently been recombined into Didymella. In the present study no evidence of teleomorph formation in vitro has been observed, which is in congruence with the results of Gruyter & Noordeloos (1992). As also type material of Ph. anigozanthi and S. multipunctata could not be obtained, this taxonomic link is still to be confirmed.
Phoma anigozanthi Tassi, Boll. Reale Orto Bot. Siena 3 (2 – 1899): 148. 1900.
Specimen examined: The Netherlands, from a leaf of Anigozanthus maugleisii, 1979, H. Cevat CBS H-5199, culture CBS 381.91 = PD 79/1110.
Phoma arachidis-hypogaeae (V.G. Rao) Aa & Boerema, Persoonia 15(3): 388. 1993.
Basionym: Phyllosticta arachidis-hypogaeae V.G. Rao, Sydowia 16 (1962): 275. 1963.
Specimen examined: India, Madras, from a leaf of Arachis hypogaea, 1977, CBS 125.93 = PD 77/1029.
Phoma fungicola Aveskamp, Gruyter & Verkley, nom. nov. pro Cicinobolus quercinus Syd. Ann. Mycol. 13: 42. 1915. MycoBank MB515593.
Basionym: Cicinobolus quercinus Syd., Ann. Mycol. 13: 42. 1915.
≡ Ampelomyces quercinus (Syd.) Rudakov, Mikol. Fitopatol. 13(2): 109. 1979. not Phoma quercina Sacc & Roum. Syll. fung. 3: 96. 1881, = Phomopsis quercina Sacc.) Höhn., not Phoma quercina (Peck) Sacc. Syll. fung. 3: 96. 1884.
Etymology: Epithet refers to the fungicolous lifestyle of this species.
Pycnidia always solitary, produced on the agar surface, globose, peroblate to suboblate, glabrous, measuring (50–)65–130(–150) × (65–)95–200(–220) μm with a single, conspicuous, non-papillate ostiole. Pycnidial wall pale brown, pseudoparenchymatous, composed of isodiametric cells, 3–5 layers, (6–)8.5–14.5(–16) μm thick, outer 1–2 layers slightly pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, doliiform to ampulliform, variable in size, ca. (3–)3.5–5 × 3–4(–5) μm. Conidia variable in shape and size, subglobose to oval or obtuse, thin-walled, smooth, aseptate, measuring (5–)5.5–7.5(–8.5) × 3–4.5(–5) μm, with 0–2(–3) minute guttules, initially hyaline, but brown at maturity. Conidial exudates not recorded.
Culture characteristics: Colonies on OA 55–68 mm diam after 7 d, margin regular. Aerial mycelium white, floccose to woolly. Immersed mycelium greenish olivaceous to olivaceous near the colony centre. Abundant black pycnidia are scattered over the medium; reverse concolourous. Colonies on MEA 65–75 mm diam after 7 d, margin regular. Aerial mycelium covering the whole colony, compact, white to pale grey, with olivaceous tinges near the colony centre; reverse olivaceous-black.
Specimen examined: Ukraine, Crimea, in the vicinity of Feodosiya, on Microsphaera alphitoides from Quercus sp., 1979, O.L. Rudakov, CBS H-20276, culture CBS 633.92 = ATCC 36786, VKM MF-325.
Notes: The epithet used for the description of this species in the genera Cicinobolus and Ampelomyces could not be transferred to the genus Phoma as Ph. quercina is already occupied. This name, however, refers to a Phyllosticta species (Van der Aa & Vanev 2002). Therefore, a new name is proposed here for the present species.
Kiss & Nakasone (1998) already found that several fast-growing Ampelomyces species were phylogenetically distinct from the type species, which is characterised by a rather slow growth rate, and suggested that A. quercinus belonged to Phoma. This finding was supported by results obtained in later studies (Sullivan & White 2000, Szentiványi et al. 2005).
Phoma gossypiicola Gruyter, Persoonia 18(1): 96. 2002.
Specimen examined: U.S.A., Texas, from a leaf of Gossypium sp., 1963, L.S. Bird CBS H-9006, culture CBS 377.67.
Phoma infossa Ellis & Everh., J. Mycol. 4(10): 102. 1888, emend. Aveskamp et al., Mycologia 101. 373. 2009.
Specimens examined: Argentina, Buenos Aires Province, La Plata, from leafs of Fraxinus pennsylvanica, 2008, M. Murace, neotype CBS H-20145, culture ex-neotype CBS 123395; Buenos Aires Province, La Plata, from leafs of Fraxinus pennsylvanica, 2008, M. Murace, CBS 123394.
Group G:
This group (BPP = 1.00, RBS = 99 %) consists of Ph. subherbarum and Ph. pedeiae sp. nov. Although the first species name suggests a close resemblance with the type species Ph. herbarum, it is phylogenetically distinct. Both Ph. herbarum and Ph. subherbarum are accommodated in section Phoma, but are distinct in colony characters: in contrast to Ph. herbarum, Ph. subherbarum does not react to the application of a droplet of NAOH (De Gruyter et al. 1993). The growth rate of Ph. subherbarum is also considerably faster, as a colony can cover the plate surface within 1 wk.
Boerema et al. (2004) hypothesised that Ph. subherbarum is from American origin. In contrast, both strains of Ph. pedeiae were found in the Netherlands. Both species in this clade appear to have a plurivorous nature. The novel species Ph. pedeiae is described below.
Phoma pedeiae Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515594. Fig. 4.
Fig. 4.
Phoma pedeiae (CBS 124517). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia. E. Section of the pycnidial wall. F. Conidia. Scale bars: D = 100 μm; E–F = 10 μm.
Conidia ellipsoidea vel cylindrica, glabra, hyalina, continua, 3–4.5 × 1.5–2.5 μm, 0–2(–3) guttulis praedita. Matrix conidiorum cremeo-alba.
Etymology: Named after the institute that has facilitated most of the research on the taxonomy of the genus Phoma and affiliated genera in the past decade, the PD (Plantenziektenkundige Dienst – Dutch Plant Protection Service). Both isolates of this species were collected and preserved by employees of this institute.
Pycnidia solitary or confluent, produced on the agar surface, globose to ellipsoidal, glabrous, (90–)100–230(–255) × (75–) 90–155(–165) μm with 1–2 conspicuous, non-papillate ostioles. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 3–5 layers, 11–17 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped, relatively small, ca. 3.5–4(–4.5) × 3–4 μm. Conidia ellipsoidal to cylindrical, thin-walled, smooth, hyaline, aseptate 3–4.5 × 1.5–2.5 μm, with 0–2(–3) guttules. Conidial matrix creme-white.
Culture characteristics: Colonies on OA, 65–75 mm diam after 7 d, margin regular. Immersed mycelium olivaceous. Aerial mycelium floccose, white or smoke-grey to greenish olivaceous. Abundant black pycnidia are scattered over the medium; reverse concolourous with some reddish tinges. Colonies on MEA 55–65 mm diam after 7 d, margin regular. Aerial mycelium covering the whole colony, floccose, smoke-grey to greenish olivaceous, white near the centre of the colony; reverse olivaceous-black or bay. Agar colour changes to bay due to diffusible pigments produced by the fungus. Colonies on CHA similar as on MEA, but somewhat faster growing, 70–80 mm diam after 7 d. Application of NaOH did not have any effect.
Specimens examined: The Netherlands, Aalsmeer region, on Schefflera elegantissima, 1992, J. de Gruyter, holotype designated here CBS H-20239, culture ex-holotype CBS 124517 = PD 92/612A; on Orchidaceae sp., 1984, J. de Gruyter, CBS 124516 = PD 84/453.
Notes: Phoma pedeiae has been found in association with several tropical ornamental pot plants in Dutch greenhouses. Only mild disease symptoms were recorded from this species, and therefore the fungus was not further studied. Phylogenetically, this species is found in close relation to Ph. subherbarum (BPP= 1.00; RBS = 99 %), which is probably a weak pathogen and saprobe of different plant substrates occurring on the American continent (De Gruyter et al. 1993).
Phoma subherbarum Gruyter, Noordel. & Boerema, Persoonia 15(3): 387. 1993.
Specimens examined: Canada, from Zea mays, holotype L 992.177.439, culture ex-holotype CBS 250.9292 = DAOM 171914 = PD 92/371; from Zea mays, May 1978, G.A. Neish, CBS 305.79A = DAOM 170848; Peru, from Solanum sp., CBS 249.92 = PD 78/1088.
Group H:
Phoma bellidis and Ph. senecionis are found in association with two plant genera from the Compositae family: Bellis spp. and Senecio spp. respectively (De Gruyter et al. 1993). The distantly related Ph. digitalis is a pathogen of Digitalis spp. (Scrophulariaceae), but shares the feature with Ph. bellidis that it is also recorded as a seed-pathogen (Boerema & Dorenbosch 1979). In contrast, Ph. senecionis is only known as a necrophyte.
Phoma bellidis Neerg., Friesia 4: 74. 1950.
Specimens examined: The Netherlands, from seed of Bellis perennis, 1985, G.H. Boerema, CBS H-5200, culture CBS 714.85 = PD 74/265; from Bellis sp., 1994, J. de Gruyter, PD 94/886.
Phoma digitalis Boerema apud Boerema & Dorenbosch, Verslagen Meded. Plziektenk. Dienst Wageningen 153: 19. 1979.
Specimen examined: The Netherlands, Ommen, from Digitalis sp., 1990, J. de Gruyter, CBS 109179 = PD 90/835-1.
Phoma senecionis P. Syd., Hedwigia, Beibl. 38: 136. 1899.
Specimen examined: New Zealand, Raetihi, from a stem of Senecio jacobaea, Feb. 1977, S. Ward, CBS 160.78 = LEV 11451.
Group I:
Group I comprises three Phoma taxa (Ph. acetosellae, Ph. macrostoma var. macrostoma and var. incolorata) that were placed in the section Phyllostictoides on the basis of the presence of septate conidia (Gruyter et al. 2002), but also accommodates Ph. viburnicola. The placement of this species in section Phoma can be debated, as a single septate conidium has been observed in strain CBS 500.91, one of the strains that was designated as reference strain (De Gruyter & Noordeloos 1992). Also D. exigua (CBS 183.55 - Neotype) is found in this clade, the type species of the genus Didymella (De Gruyter et al. 2009), which does however not produce an anamorph state. The four species do not exhibit a shared pathological feature or geographic origin. The variety incolorata differs from var. macrostoma in lacking a red to violet pigment in the hyphae and any reaction to NaOH.
Phoma acetosellae (A.L. Sm. & Ramsb.) Aa & Boerema, in De Gruyter, Boerema & Van der Aa, Persoonia 18(1): 16. 2002.
Basionym: Phyllosticta acetosellae A.L. Sm. & Ramsb., Trans. Brit. Mycol. Soc. 4: 173. 1912.
Specimens examined: France, Corrèze, Monteil sur Bois, from a leaf of Rumex acetosella, 1976, H.A. van der Aa, CBS H-16138, culture 631.76. The Netherlands, Baarn, from a stem of Rumex hydrolapathum, March 1996, H.A. van der Aa, CBS 179.97.
Phoma macrostoma var. incolorata (A.S. Horne) Boerema & Dorenb., Persoonia 6(1): 55. 1970.
Basionym: Polyopeus purpureus var. incolorata A.S. Horne, J. Bot. 58: 240. 1920.
Specimens examined: Switzerland, Vierwaldstättersee, near Brunnen, from a leaf of Acer pseudoplatanus, Oct. 1968, J. Gemmen, CBS H-20240, culture CBS 223.69. The Netherlands, from Malus sylvestris, 1983, J. de Gruyter, CBS 109173 = PD 83/908.
Phoma macrostoma var. macrostoma Mont., Annls Sci. Nat., Bot. III 11: 52. 1849.
Specimens examined: Germany, near München, from the bark of Larix decidua, 1995, G.J. Verkley, CBS 482.95. The Netherlands, Wageningen, from wood of Malus sylvestris, Sep. 1969, G.H. Boerema, CBS H-16431, culture CBS 529.66 = PD 66/521.
Phoma viburnicola Oudem., Contr. Flora Mycol. d. Pays-Bas 17: 247. 1901.
Specimens examined: The Netherlands, Wageningen, Aboretum, from Viburnum cassioides, 1969, G.H. Boerema, CBS H-16605, culture CBS 523.73 = PD 69/800; from Chamaecyparis lawsoniana, 1981, G.H. Boerema, CBS 371.91 = PD 81/413; Baarn, from a leaf of Ilex aquifolium, 1993, J. de Gruyter, CBS 500.91 = PD 83/222.
Group J:
This small group (BPP = 1.00, RBS = 96 %) comprises only two species. Because of the production of dictyochlamydospores, Phoma boeremae was suggested to belong to the section Peyronellaea (Group K, Aveskamp et al. 2009a), to which the present group is closely related. No such structures were, however, observed in its sister species, Ph. dimorpha sp. nov. This species is known from a single strain, which sporulates poorly and may be degenerated.
Phoma boeremae Gruyter, Persoonia 18 (1): 91. 2002.
Specimen examined: Australia, Victoria, Burnley Gardens, from seed of Medicago littoralis cv. Harbinger, Febr. 1982, M. Mebalds, neotype L 996.294.536, ex-neotype culture CBS 109942 = PD 84/402.
Phoma dimorpha Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515595. Fig. 5.
Fig. 5.
Phoma dimorpha (CBS 346.82). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia on stem of Urtica dioica. E. Pycnidia. F. Pycnidial wall. G–H. Conidia in vitro (G) and in vivo (H). Scale bars: D–E = 100 μm; F = 20 μm; G–H = 10 μm.
Conidia dimorpha, in vitro cylindrica, glabra, hyalina, continua, 8–9.5(–10.5) × (2–)2.5–3(–3.5) μm, (5–)6–8(–10) guttulis minutis apolaribus praedita, in vivo eguttulata, (8–)9–12(–12.5) × (4.5–)5–5.5(–6.5) μm.
Etymology: The epithet refers to the two different conidial types that are observed.
Pycnidia produced only scarcely in vitro, in clusters of ca. 4–10 elements, globose, glabrous, non-papillate, produced on the agar surface, relatively small, measuring (65–)85–170(–190) μm diam. Ostioles single, non-pappillate. Pycnidial wall pseudoparenchymatous, composed of isodiametric cells, 4–7 layers, 14–20 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped, ca. 5.5–7 × 4.5–6.5 μm. Conidia cylindrical, thin-walled, smooth, hyaline, aseptate 8–9.5(–10.5) × (2–)2.5–3(–3.5) μm, with (5–)6–8(–10) minute apolar guttules. In vivo eguttulate and somewhat broader, measuring (8–)9–12(–12.5) × (4.5–)5–5.5(–6.5) μm. Conidial exudates not observed.
Culture characteristics: Colonies on OA 45–50 mm diam after 7 d, margin regular. Immersed mycelium olivaceous-black, in some sectors covered by a low mat of floccose white to grey aerial mycelium, towards colony margin the aerial mycelium is gradually becoming more felted and white; reverse olivaceous buff to dark mouse-grey. Colonies on MEA 50–55 mm diam after 7 d, margin regular. Immersed mycelium hyaline, amber or iron-grey. Only sparsely small white tufts of whitish aerial mycelium are produced in older cultures; reverse concolourous. Colonies on CHA 55–60 mm diam after 7 d, margin regular. Immersed mycelium hyaline, honey to isabelline or dark mouse-grey. Aerial mycelium more proliferent near colony margin initially white, later developing to iron-grey with olivaceous-grey tinges; reverse black, but hyaline near colony centre. Application of NaOH did not have any effect. In older cultures white dendritic crystals are formed both in the aerial mycelium and in immersed in the agar.
Specimen examined: Spain, Canary Isles, Gran Canaria, from phyllocladium of Opuntiae sp., June 1982, H.A. Van der Aa, holotype designated here CBS H-20234, culture ex-holotype CBS 346.82.
Notes: Although sufficient pycnidial primordia are formed on OA, maturation of pycnidia is only incidentally observed in vitro. Therefore the characters of the pycnidia and the pycnidial wall described here are based on only three samples. Formation of mature pycnidia can be induced by addition of a sterilised stem piece of stinging nettle (Urtica dioica). The conidia that were described from in vivo material were obtained using this technique.
Several other Phoma species are known from Opuntiae, including Ph. opuntiae (Phoma sensu lato) and Ph. longicolla sp. nov. (see below). The conidia of Ph. opuntiae are, however, considerably smaller, measuring 2.5–3.5 × 1–1.5 μm (De Gruyter & Noordeloos 1992), whereas the main difference with Ph. longicolla are the pycnidia, which are uniostiolate and significantly larger in the latter species.
Group K – Peyronellaea:
This group (BPP = 1.00, RBS = 58 %) comprises many of the chlamydospore forming species, including the majority of the species that were accommodated in Phoma section Peyronellaea (Boerema et al. 1965a, 1968, 1971, 1973, 1977). Also Ph. glomerata, type species of this section is accommodated here (Boerema 1997). However, as section, Peyronellaea has a polyphyletic nature (Aveskamp et al. 2009a). Phoma chrysanthemicola, Ph. violicola and the recently established species Ph. schachtii (Aveskamp et al. 2009a) have been found to be basal to the Didymellaceae (Fig. 1), whilst several species producing botryoid chlamydospores, representing the genus Epicoccum as emended below, are clustered in group M. Also Ph. infossa and Ph. omnivirens, which have proven to produce dictyochlamydospores in culture (Aveskamp et al. 2009a), are not situated in this part of the phylogenetic tree. Peyronellaea calidophila and Ph. microchlamydospora reside in the basal lineages of this clade.
Also several Phoma species that were not included in Peyronellaea in the Boeremaean taxonomical system, but that do produce either uni- or multicellular chlamydospores, are included in this clade. In Ph. gardeniae, Ph. narcissi, and Ph. zeae-maydis multicellular chlamydospores have been observed, whereas Ph. pinodella, Ph. arachidicola, and Ph. heteroderae are species that form unicellular chlamydospores. Several species in this clade, however, have never been recorded to produce any unicellular or multicellular chlamydospores. These species are Ph. alectorolophi, Ph. obtusa and Ph. protuberans, which will be treated in a subsequent section of this paragraph, and Ph. anserina, Ph. aurea, Ph. nigricans and Ph. eucalyptica. However, two of these species, Ph. anserina and Ph. eucalyptica are well-known for the formation of swollen cells and anastomosis in culture (De Gruyter & Noordeloos 1992), which may be regarded as a precursor to chlamydospore formation. The ancestral location of Ph. anserina in this clade may also be an indication that chlamydospore production has not completely been developed yet in this group. The high posterior probabillity for this group justifies the recognition of a separate genus in the Didymellaceae. Therefore the genus name Peyronellaea Goid. is re-established, and the associated species are recombined into this genus below.
The plurivorous species Ph. calorpreferens and Ph. heteroderae share identical LSU, ITS and TUB genes. Also morphologically the representative strains of these species are highly similar. A synonymisation of these species is therefore proposed in this paper.
Another notable subgroup within this clade is a cluster formed by Didymella pinodes, D. lethalis, D. arachidicola and Ph. pinodella. Recently, Irinyi et al. (2009) synonymised Ph. sojicola with Ph. pinodella, based on morphological observations and sequence data of ITS, β-tubulin and translation elongation factor 1-α. This indicates that the notorious pathogen of green pea (Pisum sativum) is also capable of infecting soybean (Glycine max). These observations are supported by the results obtained in the present study. As reported in previous studies (Faris-Mokaiesh et al. 1996, Onfroy et al. 1999, Fatehi et al. 2003, Peever et al. 2007), Ph. pinodella appears to be very closely related to D. pinodes (anam. Ascochyta pinodes) and because these species share the same host range they are often confused. Both species can however easily be differentiated on basis of the amount of septate conidia formed in vitro, abundantly in D. pinodes, and in very small numbers in Ph. pinodella.
Because Ph. pinodella is morphologically so similar to Ph. medicaginis, it was once regarded as a variety of this species by Boerema et al. (1965b). The variety was elevated to species rank after careful observation (White & Morgan-Jones 1987), but the varietal name is however currently still in common use (e.g. Onfroy et al. 1999, Fatehi et al. 2003, Taylor & Ford 2007). The results obtained in this study however, illustrate a substantial phylogenetical distance to Ph. medicaginis, and warrant recognition at species level, in the in the re-instated genus Peyronellaea.
The close association of Ph. arachidicola with Ph. pinodella and D. pinodes is reflected by the morphology of these species, which all produce, next to septate and aseptate conidia, also globose to ellipsoidal unicellular chlamydospores, which may be formed in chains. These chlamydospores measure 5–20 μm diam, which is somewhat larger than the species in group N. The close relationship of these three species has been hypothesised before, and was based on chemical analysis of the crystals produced by these taxa (Noordeloos et al. 1993).
Didymella arachidicola is a specific pathogen of groundnut (Arachis hypogaea), another host plant of the family Fabaceae with which the other species in this subclade are also associated.
In the Ph. pinodella / D. pinodes subcluster (BPP = 1.00, RBS = 94 %), four teleomorph species are found with a coelomycete anamorph state. Next to D. pinodes, these are D. alectorolophi, D. arachidicola, and D. lethalis. A fifth teleomorph is the sexual state of Ph. pinodella (as Ph. medicaginis var. pinodella) that is reported and described by Bowen et al. (1997), but that has not been named thus far. From a phylogenetic point of view, this record is very plausible as all species in the subclade in which Ph. pinodella is embedded, do form a Didymella-like teleomorph (Fig. 2). However, as we did not include mating type tests in our studies, and as the species is probably heterothallic (Bowen et al. 1997), pseudothecia were not observed in the present study. A formal name for the teleomorph of Ph. pinodella could therefore not be proposed here either.
A fifth species in group K that has a known teleomorph is Ph. zeae-maydis. This species is however only distantly related to the four species mentioned above. Nevertheless, it can be concluded that the sole teleomorph genus that is associated with group K is Didymella-like. This would further support the suggestion (Peever et al. 2007) that the teleomorph name for A. pinodes that is often referred to by plant pathologists, Mycosphaerella pinodes, should be omitted.
Remarkably, three species are found in this clade that are identical based on sequence analyses, but that are morphologically rather distinct. Also sequence comparisons of parts of the actin and calmodulin genes did not reveal any differences between those four strains (Aveskamp & Woudenberg, unpubl. data). Phoma alectorolophi and Ph. protuberans are associated with Phoma section Sclerophomella (Boerema et al. 1997, De Gruyter et al. 2002), because of the thick-walled pycnidia formed in culture and in vivo. However, because of the production of relatively large secondary conidia, a link with sections Heterospora or Phyllostictoides can also be advocated. Colony characters, microscopic features and ecology indicate that the two species should actually be rather distinct. A third taxon found in this group is Ph. obtusa, a saprobic species that has a thin pycnidial wall and lacks septate conidia. Nevertheless, these three species are recovered in a clade in which solely chlamydospore-forming species reside, a character that never has been recorded in any of these taxa. The explanation of the contrast between the level of genetic and morphological similarity will be one of the main challenges in Phoma taxonomy.
Peyronellaea Goid. ex Togliani, Ann. Sperim. Agrar. II 6: 93. 1952, emend. Aveskamp, Gruyter & Verkley
Conidiomata pycnidial, globose to subglobose, measuring 50–380 μm diam, on agar surface or immersed, solitary or confluent, ostiolate or poroid. Pycnidial wall pseudoparenchymatous, counting 2–8 cell layers of which the outer 1–3 are brown or olivaceous pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, ampulliform or doliiform, ca. 3.5–7 × 3.5–6 μm. Conidia generally aseptate, ellipsoidal to subglobose, thin-walled, smooth, hyaline, but in older cultures conidia may become pigmented, generally measuring 4–15 × 2–4 μm, but larger or septated conidia may occur in at least one species. Unicellular chlamydospores often abundantly formed in and on the agar and in the aerial mycelium, globose, intercalary, brown or olivaceous pigmented, measuring 5–22 μm diam. Multicellular chlamydospores mainly alternarioid, terminal or intercalary, often in chains, brown or olivaceous pigmented, 10–50 × 7–25 μm. Pseudothecia only present in a few species, (sub-)globose, up to 200 μm diam, but in one species also flattened pseudothecia occur. Asci cylindrical to clavate, measuring 35–65 × 11–17 μm, always 8-spored, biseriate. Ascospores ellipsoid, measuring 12–24 × 4–8 μm, uniseptate, upper cell usually larger than the lower cells.
Type species: Peyronellaea glomerata (Corda) Goid. ex Togliani
Peyronellaea alectorolophi (Rehm.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515597.
Basionym: Didymella alectorolophi Rehm, apud Ade, Hedwigia 64: 294. 1923.
≡ Phoma alecotorolophi Boerema, Gruyter & Noordel., Persoonia 16(3): 366. 1997.
Specimen examined: The Netherlands, from seed of Rhinanthus major, 1993, L 992.167.515, culture CBS 132.96 = PD 93/853.
Peyronellaea americana (Morgan-Jones & J.F. White) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515596.
Basionym: Phoma americana Morgan-Jones & J.F. White, Mycotaxon 16(2): 406. 1983.
Specimens examined: Argentina, Buenos Aires Province, Olavarria, from leaves of Triticum aestivum cv. Buck Diamante, Aug. 2002, A. Perelló, CBS 112525. Denmark, Copenhagen, from seed of Phaseolus vulgaris, May 1965, S.B. Mathur, CBS 256.65. Nigeria, from Sorghum vulgare, 1979, PD 79/58. South Africa, from Zea mays, 1978, PD 78/1059. U.S.A., Arkansas, from pod lesions of Glycine max, 1981, H.J. Walters, CBS 568.9797 = ATCC 44494 = PD 94/1544; Georgia, from Zea mays, 1985, G.H. Boerema, CBS H-16144, culture CBS 185.85 = PD 80/1191; from Zea mays, 1980, PD 80/1143. Unknown origin, from a nematode cyst, 1982, G.H. Boerema, PD 82/1059.
Peyronellaea anserina (Marchal) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515598.
Basionym: Phoma anserina Marchal, Champignon Copr. 11: 1891.
Specimens examined: The Netherlands, from Pisum sativum, 1979, CBS 363.91 = PD 79/712; Ter Apel, from potato flour, 1983, CBS 360.84.
Peyronellaea arachidicola (Khokhr.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515599.
Basionym: Mycosphaerella arachidicola Khokhr., Bolezni i vrediteli maslichnykh kul'tur 1(2): 29. 1934.
≡ Didymella arachidicola (Khokhr.) Tomilin, Opredelitel' gribov roda Mycosphaerella Johans: 285. 1979.
Anamorph: Phoma arachidicola Marasas, Pauer & Boerema, Phytophylactica 6(3): 200. 1974.
Specimens examined: South Africa, Cape Province, Jan Kempdorp, Vaalharts Research Station, from a leaf of Arachis hypogaea, Mar. 1972, G.D. Pauer, isotype of Ph. arachidicola CBS H-7601, ex-isotype culture CBS 333.75 = ATCC 28333 = IMI 386092 = PREM 44889; Zimbabwe, from Arachis hypogaea, 1980, CBS 315.90 = PD 80/1190.
Peyronellaea aurea (Gruyter, Noordel. & Boerema) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515600.
Basionym: Phoma aurea Gruyter, Noordel. & Boerema, Persoonia 15(3): 394. 1993.
Specimen examined: New Zealand, Auckland, from a stem of Medicago polymorpha, 1978, holotype L 992.177.422, ex-holotype culture CBS 269.93 = PD 78/1087.
Peyronellaea australis Aveskamp, Gruyter & Verkley, nom. nov. pro Phoma nigricans P.R. Johnst. & Boerema, MycoBank MB515601.
≡ Phoma nigricans P.R. Johnst. & Boerema, New Zealand J. Bot. 19(4): 394. 1982.
Etymology: Epithet refers to the Southern Hemisphere, where this fungus is mainly found.
Specimens examined: New Zealand, from Actinidea chinensis, 1977, P.R. Johnston, PD 77/919; Auckland, Mt. Albert, from a leaf of Actinidia chinensis, Apr. 1979, P.R. Johnston, isotype CBS H-7619, ex-isotype culture CBS 444.81 = PDDCC 6546.
Note: A new name was sought for this species, as the epithet “nigricans” already was occupied in Peyronellaea, referring to a species which is now synonymised with Pey. pomorum var. circinata (see below).
Peyronellaea calorpreferens (Boerema, Gruyter & Noordel.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515602.
Basionym: Phoma pomorum var. calorpreferens Boerema, Gruyter & Noordel. apud Boerema, Persoonia 15: 207. 1993.
≡ Phoma calorpreferens (Boerema, Gruyter & Noordel.) Aveskamp, Gruyter & Verkley, Mycologia 101: 370. 2009.
= Phoma heteroderae Sen Y. Chen, D.W. Dicks. & Kimbr., Mycologia 88: 885.1996.
Conidiomata pycnidial, solitary or confluent, partially or completely immersed in the agar, (sub-)globose or irregular due to the presence of 1(–4) slightly papillate ostioles, measuring (70–)100–200(–250) μm diam. Pycnidial wall pseudoparenchymatous, composed of isodiametric cells, 2–5 layers thick, with many hyphal outgrows, some setae-like. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped, ca. 3–5.5 × 3–6.5 μm. Conidia broadly ellipsoidal to ovoid to cylindrical, thin-walled, smooth, hyaline, (3.5–)4–8.5(–12) × 2–3.5(–4.5) μm, aseptate, with (1–)2–5(–8) polar guttules. Conidial matrix pale pink. Chlamydospores highly variable in shape and size, mostly unicellular but also multicellular. Where unicellular, pale brown to brown, guttulate, intercalary, solitary or in chains, globose, 7.5–19(–26) μm, thick-walled and often with a distinct “envelope”. Where multicellular dictyosporous alternarioid or botryoid, brown to black, terminal or occasionally intercalary in chains of unicellular chlamydospores, measuring ca. (16–)21–55 × (7–)12–30(–33) μm.
Specimens examined: The Netherlands, from undefined food material, 1973, G.H. Boerema, holotype L 990.290.418, ex-holotype culture CBS 109.92 = PD 73/1405. U.S.A., Florida, Gainesville, from eggs of Heterodera glycines from greenhouse soil, CBS 630.97 = ATCC 96683 = IMI 361196 = PD 96/2022; from indoor environment, 1993, CBS 875.97 = PD 93/1503.
Notes: Peyronellaea calorpreferens is a taxon that was recently elevated from variety level to species rank, as Phoma calorpreferens (Aveskamp et al. 2009a). Due to its morphological and genetical similarity with Ph. heteroderae, it is concluded that both taxa are actually one and the same species. According to the International code of Botanical Nomenclature (McNeal et al. 2006) the epithet calorpreferens has priority, as its basionym Ph. pomorum var. calorpreferens was published earlier.
The type of Peyronellaea calorpreferens has been recovered from food materials, but Boerema (1993) hypothesises about the plurivorous nature of this taxon, and mainly records it as a worldwide occurring soil- and seedborne opportunist, whereas Chen et al. (1996) record this species (as Ph. heteroderae) from eggs of a cyst nematode, Heterodera glycines.
Peyronellaea coffeae-arabicae (Aveskamp, Verkley & Gruyter) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515603.
Basionym: Phoma coffeae-arabicae Aveskamp, Verkley & Gruyter, Mycologia 101(3): 371. 2009.
Specimens examined: Ethiopia, from Coffea arabica, 1984, M.M.J. Dorenbosch, holotype CBS H-20143, ex-holotype culture CBS 123380 = PD 84/1013; from Coffea arabica, 1984, M.M.J. Dorenbosch, CBS 123398 = PD 84/1014.
Peyronellaea curtisii (Berk.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515604.
Basionym: Hendersonia curtisii Berk., in Cooke, Nuovo Giorn. Bot. Ital. 10: 19. 1878.
≡ Stagonosporopsis curtisii (Berk.) Boerema, in Boerema & Dorenbosch, Verslagen Meded. Plziektenk. Dienst Wageningen 157: 20. 1981.
= Phyllosticta narcissi Aderh., Centralbl. Bakteriol., 2 Abth. 6: 632. 1900.
≡ Phoma narcissi (Aderh.) Boerema, Gruyter & Noordel., Persoonia 15(2): 215. 1993.
Specimens examined: The Netherlands, from Nerine sp., May 1992, J. de Gruyter, culture 251.92 = PD 86/1145; from Sprekelia sp., PD 92/1460. Unknown origin, from Ismene sp., 1971, PD 71/6. Unknown origin, from Hippeastrum sp., 1976, PD 76/61.
Peyronellaea eucalyptica (Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515605.
Basionym: Phoma eucalyptica Sacc., Syll. Fung. 3: 78. 1884.
Specimens examined: Australia, Western Australia, from a leaf of Eucalyptus sp., 1979, CBS 377.91 = PD 79/210. Croatia, Adriatic Sea, from seawater, 1973, CBS 508.91 = PD 73/1413. Indonesia, Sumatra, Sulavesi, from Eugenia aromatica, 1982, CBS 378.91 = PD 82/107.
Peyronellaea gardeniae (S. Chandra & Tandon) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515606.
Basionym: Pyrenochaeta gardeniae S. Chandra & Tandon, Mycopathol. Mycol. Appl. 29: 274. 1966.
≡ Phoma gardeniae (S. Chandra & Tandon) Boerema, in Boerema & Dorenbosch, Verslagen Meded. Plziektenk. Dienst Wageningen 156: 27. 1980.
Specimens examined: India, Allahabad, from the leaf of Gardenia jasminoides, 1966, S. Chandra and R.N. Tandon, isotype CBS H-7605, ex-isotype culture CBS 626.68 = IMI 108771. Netherlands Antilles, Curacao, from air sample, 1978, A. Kikstra, CBS 302.79 = PD 79/1156.
Peyronellaea glomerata (Corda) Goid. ex Togliani, Ann. Sperim. Agrar. III 6: 93. 1952.
Basionym: Coniothyrium glomeratum Corda, Icon. Fung. (Prague) 4: 39. 1840.
≡ Phoma glomerata (Corda) Wollenw. & Hochapfel, Z. Parasitenk. 3(5): 592. 1936.
Specimens examined: Germany, Berlin-Zehlendorf, Domäne Düppel, from a tuber of Solanum tuberosum, 1936, H.W. Wollenweber, CBS 293.36 = MUCL 9882; Monheim, from Hordeum sativum, 1984, M. Hossfeld, CBS 834.84; from indoor environment, 2003, C. Rudolph, CBS 112448. Romania, Bukarest, from a church wall-fresco, Nov. 1971, I. Ionita, CBS 133.96 = PD 79/127. Russia, Novosibirsk, Hortus Botanicus, from a leaf of Populus nigra, 1963, T.T. Kuznetsova, CBS 284.76 = ATCC 26238 = IMI 176748 = VKM F-1842; Novosibirsk, Hortus Botanicus, from a leaf of Rubus idaeus, 1963, T.T. Kuznetsova, CBS 287.76 = ATCC 26240 = IMI 176746 = VKM F-1847; Novosibirsk, Hortus Botanicus, from a leaf of Populus alba, 1963, T.T. Kuznetsova, CBS 288.76 = ATCC 26243 = VKM F-1845; Novosibirsk, Hortus Botanicus, from a leaf of Allium nutans, 1963, T.T. Kuznetsova, CBS 289. 76 = ATCC 26239 = IMI 176745 = VKM F-1846; Novosibirsk, Hortus Botanicus, from a leaf of Ribes nigrum, 1963, T.T. Kuznetsova, CBS 290.76 = ATCC 26244 = IMI 176747 = VKM F-1848; from Heracleum sp., 1973, PD 73/1415. The Netherlands, from a root of Lycopersicon esculentum, 1949, D. Verleur, CBS 304.49 = MUCL 9884; from Chrysanthemum sp., 1963, CBS 528.66 = PD 63/590; from indoor bathroom environment, 1997, M. Komen, CBS 464.97; from Medicago sativa, PD 77/47. U.K., from air, PD 74/1023. U.S.A., Virginia, from Juniperus sp., Jan. 2002, A.Y, Rossman, CBS 120109. Unknown origin, from Cucumis sativus, PD 81/767; from Capsicum sp., PD 83/782.
Peyronellaea lethalis (Ellis & Bartholomew) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515607.
Basionym: Ascochyta lethalis Ellis & Bartholomew, Fungi Columb. 1808. 1903.
= Mycosphaerella lethalis R. Stone, Ann. Mycol. 10: 587. 1912.
≡ Didymella lethalis (R. Stone) Sivan., Bitunicate Ascomycetes and their Anamorphs: 424. 1984.
Specimen examined: Unknown origin and substrate, 1925, A.W. Archer, CBS 103.25.
Peyronellaea musae P. Joly, Revue Mycol. 26: 97. 1961.
≡ Phoma jolyana Piroz. & Morgan-Jones, Trans. Brit. Mycol. Soc. 51: 200. 1968.
Specimens examined: India, from fruit of Mangifera indica, May 1969, CBS 463.69; from Malus sylvestris, PD 83/326.
Notes: Phoma jolyana was originally described in the genus Peyronellaea, as Pey. musae. The epithet “jolyana” was later proposed for this species, as the epithet musae was already occupied in Phoma (Pirozynski & Morgan-Jones 1968). Here, we reinstate this fungus under its original name.
Peyronellaea obtusa (Fuckel) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515608.
Basionym: Phoma obtusa Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 378. 1870.
Specimens examined: The Netherlands, from a root of Daucus carota, July 1993, J. de Gruyter, CBS 377.93 = PD 80/976; from Spinacia oleracea, July 1993, J. de Gruyter, CBS 391.93 = PD 80/87.
Peyronellaea pinodella (L.K. Jones) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515609.
Basionym: Ascochyta pinodella L.K. Jones, Bull. New York State Agric. Exp. Sta. 547: 10. 1927.
≡ Phoma medicaginis var. pinodella (L. K. Jones) Boerema apud Boerema, Dorenbosch & Leffring, Netherlands J. Pl. Pathol. 71: 88. 1965.
≡ Phoma pinodella (L.K. Jones) Morgan-Jones & K.B. Burch, Mycotaxon 29: 485. 1987.
Specimens examined: Hungary, from Glycine max, 1996, G. Kövics, CBS 567.97 = PD 97/2160; from seed of Glycine max, 1997, G. Kövics, CBS 100580 = PD 98/1135. The Netherlands, from Pisum sativum, 1981, CBS 318.90 = PD 81/729. U.S.A., Minnesota, from Trifolium pretense, 1966, CBS 531.66.
Notes: Phoma sojicola, which was erected in 1999 (Kövics et al. 1999), has recently been synonymised with the present species, based on morphological and genetical similarities (Irinyi et al. 2009). The present study supports these findings.
Peyronellaea pinodes (Berk. & A. Bloxam) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515610.
Basionym: Sphaeria pinodes Berk. & A. Bloxam, Ann. Mag. Nat. Hist., Ser. III 7: 454. 1861.
≡ Didymella pinodes (Berk. & A. Bloxam) Petr., Ann. Mycol. 22(1/2): 16. 1924.
≡ Mycosphaerella pinodes (Berk. & A. Bloxam) Vestergr., Ann. Mycol. 10(5): 581. 1912.
= Ascochyta pinodes L.K. Jones, Bull. New York State Agric. Exp. Sta. 547: 4. 1927.
Specimens examined: Belgium, Gembloux, from Pisum sativum, 1977, G. Sommereyns, CBS 525.77. Iraq, Basrah province, from Pisum sativum, 1977, CBS 159.78. Switzerland, Glarus Kanton, Filzbach, from a leaf of Primula auricula, June 1949, E. Müller, CBS 285.49. The Netherlands, from an unknown substrate, 1955, M.H. van Raalte, CBS 235.55.
Peyronellaea pomorum var. circinata (Kusnezowa) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515612.
Basionym: Peyronellaea circinata Kusnezowa, Novoste Sist. Nizsh. Rast. 8: 189. 1971.
≡ Phoma jolyana var. circinata (Kusnezowa) Boerema, Dorenb. & Kesteren, Kew Bull. 31: 535. 1977 [1976].
≡ Phoma pomorum var. circinata (Kusnezowa) Aveskamp, Gruyter & Verkley, Mycologia 101(3): 377. 2009.
= Peyronellaea nigricans Kusnezowa, Novoste Sist. Nizsh. Rast. 8: 191. 1971.
Specimens examined: Russia, Siberia, Novosibirsk, from Heracleum dissectum, 1963, T.T. Kusnezowa, isotype CBS H-3747, ex-isotype culture CBS 285.76 = ATCC 26241 = IMI 176742 = VKM F-1843; Siberia, Novosibirsk, from a leaf of Allium nutans, 1963, T.T. Kusnezowa, CBS 286.76 = ATCC 26242 = IMI 176743 = VKM F-1844.
Peyronellaea pomorum var. cyanea (Jooste & Papendorf) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515614.
Basionym: Phoma cyanea Jooste & Papendorf, Mycotaxon 12: 444. 1981.
≡ Phoma pomorum var. cyanea (Jooste & Papendorf) Aveskamp, Gruyter & Verkley, Mycologia 101(3): 377. 2009.
Specimen examined: South Africa, Heilbron, from straw of Triticum sp., 1972, W.J. Jooste, holotype PREM 45736, ex-holotype culture CBS 388.80.
Peyronellaea pomorum var. pomorum (Thüm.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515611.
Basionym: Phoma pomorum var. pomorum Thüm., Fungi Pomicoli: 105. 1879.
Specimen examined: The Netherlands, Wageningen, from Polygonum tataricum, 1964, M.M.J. Dorenbosch, CBS H-16540, culture CBS 539.66 = ATCC 16791 = IMI 122266 = PD 64/914.
Peyronellaea protuberans (Lév.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515613.
Basionym: Phoma protuberans Lév., Ann. Sci. Nat. Bot. III 5: 281. 1846.
Specimen examined: The Netherlands, from a leaf of Lycium halifolium, 1971, CBS 381.96 = PD 71/706.
Peyronellaea sancta (Aveskamp, Gruyter & Verkley) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515615.
Basionym: Phoma sancta Aveskamp, Gruyter & Verkley, Mycologia 101(3): 377. 2009.
Specimens examined: Argentina, from Opuntia ficus-indica, 1997, CBS 644.97. South Africa, from dead branches of Ailanthus altissima, Oct. 1982, C. Jansen CBS H-16332, ex-holotype culture CBS 281.83. Unknown origin, from Gleditsia triancantha culture LEV 15292.
Peyronellaea subglomerata (Boerema, Gruyter & Noordel.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515616.
Basionym: Phoma subglomerata Boerema, Gruyter & Noordel., Persoonia 15(2): 204. 1993.
Specimens examined: U.S.A., North Dakota, from Triticum sp., 1976, CBS 110.92 = PD 76/1010. Unknown origin, from Zea mays, 1978, PD 78/1090.
Peyronellaea zeae-maydis (Mukunya & Boothr.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515617.
Basionym: Mycosphaerella zeae-maydis Mukunya & Boothr., Phytopathology 63: 530. 1973.
≡ Didymella zeae-maydis (Mukunya & Boothr.) Arx, Beih. Nova Hedwigia 87: 288. 1987.
Anamorph: Phyllosticta maydis Arny & R.R. Nelson, Phytopathology 61: 1171. 1971.
≡ Phoma zeae-maydis Punith., Mycopathologia 112(1): 50. 1990.
Specimen examined: U.S.A., Wisconsin, Hancock, from Zea mays, June 1969, D.C. Arny, ex-holotype culture CBS 588.69.
Group L:
Phoma draconis, Ph. henningsii, Ph. plurivora and the novel species Ph. brasiliensis cluster basally to the Epicoccum species in group M. The species clustered here, however, all lack chlamydospores. These species do, like the chlamydospore-forming species mentioned above, solely produce unicellular conidia, and have glabrous, thin-walled, pseudoparenchymatous pycnidial walls composed of isodiametric cells.
Phoma brasiliensis Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515618. Fig. 6.
Fig. 6.
Phoma brasiliensis (CBS 120105a). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D–E. Pycnidia. F. Section of the pycnidial wall. G. Conidia. Scale bars: D = 200 μm; E = 100 μm; F–G = 10 μm.
Conidia cylindrica, glabra, hyalina, continua, 6–9(–10) × 2–3(–3.5) μm, (3–)4–6(–8) guttulis parvis praedita. Matrix conidiorum alba.
Etymology: Epithet refers to the country of origin, Brazil.
Conidiomata pycnidial, mainly solitary but also confluent, globose to irregularly shaped, glabrous, on the agar surface and immersed, (220–)250–370(–550) × (150–)190–290(–320) μm. Usually with a single inconspicuous non-papillate ostiole. Pycnidial wall pseudoparenchymatous, composed of 5–9 layers of oblong to isodiametric cells, 18–27 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, globose to flask-shaped, ca. 4–5 × 3.5–4 μm. Conidia variable in size, cylindrical, thin-walled, smooth, hyaline, aseptate 6–9(–10) × 2–3(–3.5) μm, with (3–)4–6(–8) small polar guttules. Conidial matrix white.
Culture characteristics: Colonies on OA 50–53 mm diam after 7 d, margin regular. Aerial mycelium sparse, tufted near the centre of the colony, white. Immersed mycelium hyaline. Abundant pycnidia produced semi-immersed in concentric rings. Pycnidia in the outer rings pale luteous, darkening towards the centre of the colony via buff, honey, hazel to brown-vinaceous; reverse concolourous. Colonies on MEA 59–63 mm diam after 7 d, margin regular. Immersed mycelium completely covered by a mycelial mat, which is densely floccose, greenish olivaceous to greenish grey, with elements of citrine, olivaceous black and white; reverse concolourous. Hyphae locally containing red amorphous chrystaline material. Colonies on CHA 62–67 mm diam after 7 d, margin regular. Aerial mycelium floccose, white. Abundant dark pycnidia are formed on the agar surface. Application of NaOH results in a luteous discolouration of the agar, later changing to reddish, best to be observed on OA medium.
Specimen examined: Brazil, from Amaranthus sp., Nov. 2007, E. Rosskopf, holotype designated here CBS H-20235, ex-holotype culture CBS 120105.
Notes: This species is thus far only known from a single isolate from a wild Amaranthus sp. in Brazil. According to Boerema et al. (2004), no other Phoma species have been recorded from the same host.
Phoma draconis (Berk. ex Cooke) Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 159 (Jaarboek 1982): 24. 1983.
Basionym: Phyllosticta draconis Berk. ex Cooke, Grevillea 19: 8. 1891.
Specimen examined: Rwanda, from a leaf of Dracaena sp., Jan. 1982, G.H. Boerema, CBS H-16207, culture CBS 186.83 = PD 82/47.
Phoma henningsii Sacc., Syll. Fung. 10: 139. 1892.
Specimen examined: Kenya, Maguga, from the bark of Acacia mearnsii, June 1992, T.W. Olembo, CBS H-16354, culture CBS 104.80 = PD 74/1017.
Phoma plurivora P.R. Johnst., New Zealand J. Bot. 19(2): 181. 1981.
Specimens examined: Australia, from Medicago sativa, 1975, CBS 248.93 = PD 95/907. New Zealand, Auckland, Mt Albert, from a leaf of Setaria sp., Feb. 1979, P.R. Johnston, CBS H-7624, ex-isotype culture CBS 558.81 = PDDCC 6873.
Group M – Epicoccum:
This group (BPP = 1.00, RBS = 66 %) comprises three species that are accommodated in the section Peyronellaea. The Peyronellaea species in this group, Ph. sorghina, Ph. pimprina and Epicoccum nigrum (chlamydospore-based synanamorph of Ph. epicoccina; Arenal et al. 2000, 2004) are characterised by the production of botryoid or epicoccoid chlamydospores, in contrast to the species in group K, which produce alternarioid dictyochlamydospores. The distinct morphology and phylogenetic position justify the recombination into a separate genus. As the oldest generic name in this clade is Epicoccum, new combinations for Ph. pimprina and Ph. sorghina are proposed below.
Epicoccum Link, Mag. Gesell. Naturf. Freunde Berlin 7: 32. 1815, emend. Aveskamp, Gruyter & Verkley. Fig. 7.
Fig. 7.
Globose chlamydospores of Epicoccum spp. A–B. E. nigrum (CBS 173.73). C–E. E. sorghi (CBS 246.60). F–H. E. pimprinum (CBS 179.80). Scale bars: A–B = 50 μm; C–H = 20 μm.
Conidiomata pycnidial, globose to subglobose, measuring 50–250 μm diam, on agar surface or immersed, mostly solitary but incidentally confluent. Ostioles papillate or on pronounced necks. Pycnidial wall pseudoparenchymatous, counting 2–8 cell layers of which the outer 1–3 are brown-olivaceous pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, ampulliform, ca. 3–7 × 3–7 μm. Conidia variable in shape, initially hyaline but in later stages a slight brownish pigmentation may be found, thin-walled, smooth, always aseptate 3–8.5(–10) × 1.5–4(–4.5) μm. Chlamydospores unicellular or multicellular, intercalary or terminal, smooth, verrucose or incidentally tuberculate, subhyaline to dark brown, where unicellular globose, measuring 5–15 μm diam, where multicellular globose or irregular shaped, smooth, verrucose or incidentally tuberculate, measuring 8–35 μm.
Type species: Epicoccum nigrum Link.
Epicoccum nigrum Link, Mag. Gesell. Naturf. Freunde Berlin 7: 32. 1815.
≡ Phoma epicoccina Punith., M.C. Tulloch & C.M. Leach, Trans. Brit. Mycol. Soc. 59(2): 341 (1972).
Specimens examined: Germany, Berlin, from soil, 1985, H.J. Halfmann, CBS 505.85. The Netherlands, Geleen, from human toe nail, Dec. 1981, CBS 125.82 = IMI 331914 = CECT 20044; Randwijk, from Malus sp., J. Köhl, 2003, CBS 115825. U.S.A., Oregon, from seeds of Dactylis glomerata, 1967, CBS 173.73 = ATCC 24428 = IMI 164070.
Epicoccum pimprinum (P.N. Mathur, S.K. Menon & Thirum.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515619.
Basionym: Phoma pimprina P.N. Mathur, S.K. Menon & Thirum., Sydowia 13: 146. 1959.
Specimens examined: India, Poona, Pimpri, from soil, Mar. 1959, S.K. Menon, ex-isotype culture CBS 246.60 = ATCC 22237 = ATCC 16652 = IMI 81601; from soil, 1977, PD 77/1028.
Epicoccum sorghi (Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515620.
Basionym: Phyllosticta sorghina Sacc., Michelia 1 (2): 140. 1878.
≡ Phoma sorghina (Sacc.) Boerema, Dorenb. & Kesteren, Persoonia 7(2): 139. 1972.
For a complete synonymy see Boerema et al. (1977).
Specimens examined: France, Antibes, from a twig of Citrus sp., 1966, CBS 627.68 = PD 66/926. Guinea-Bissau, Gacheu Région, from Oryza sativa, Oct. 1978, CBS 181.81. India, from a fruit of Coffea sp., July 1968, C.V. Subramanian, CBS 846.68; Jabalpur, from Panicum miliare, Jan. 1972, D. Sharma, CBS 293.72. Martinique, from a leaf of Lycopersicon esculentum, June 1989, B. Hostachy, CBS 301.89. Papua New Guinea, from Stellaria sp., A. Aptroot, Oct. 1995, CBS 886.95; Central Province, Varirata National Park near Port Moresby, from soil, A. Aptroot, Oct. 1995, CBS 986.95. Puerto Rico, Mayaguez, from Sorghum vulgare, Apr. 1976, R. Alconera, CBS 179.80= PD 76/1018. South Africa, Potchefstroom, from a leaf of Zea mays, Nov. 1978, W.J. Jooste, CBS 180.80 = PD 78/1100.
Notes: The strains that were previously accommodated in Ph. sorghina are morphologically and phylogenetically highly diverse (Aveskamp et al. 2009a, Pažoutová 2009), and probably represent multiple species. These species were, however, not treated in the present study.
Group N – Boeremia gen. nov.:
This group represents species that are morphologically similar to what is currently known as Ph. exigua. Group N is a well-defined clade (BPP = 1.00, RBS = 100 %) and comprises all taxa that were previously recognised as separate Ph. exigua varieties by Abeln et al. (2002). Phoma foveata and Ph. sambuci-nigrae are embedded here as well, two species that previously were known as varieties of Ph. exigua, but were elevated to species rank due to their phytopathological relevance (Ph. foveata, Boerema et al. 1987) or distinct physiological characters (Ph. sambuci-nigrae, Monte et al. 1991). As already noted by Aveskamp et al. (2009b) also Ph. telephii, Ph. strasseri and Ph. lycopersici are closely related. This study also reveals the close relationship with Ph. tarda, a pathogen of coffee. Phoma hedericola, a frequently occurring causal agent of leaf spots on poison ivy (Hedera helix) and Ph. crinicola, a pathogen of Amaryllidaceae are embedded in this clade. In contrast to the other species in this clade, which are linked to Phoma section Phyllostictoides, Ph. hedericola and Ph. crinicola are associated with Phoma section Phoma, due to the absence of septate conidia (De Gruyter & Noordeloos 1992, De Gruyter et al. 1993). The sequence data of CBS 172.34, a strain recorded as Dothiorella ulmi, appeared to be genetically identical to Ph. exigua, as was already noted by De Gruyter et al. (2009). Based on morphological studies of other strains, Dothiorella ulmi was suggested to be recombined into Plectophomella (Redfern & Sutton 1981), a genus that is linked to the Pezizomycotina. Morphological features of the present strain appeared to be similar to Ph. exigua, suggesting that this strain was probably preserved under an incorrect name, and actually belongs to Ph. exigua var. populi.
Of the species within this clade, a teleomorph is only named in Ph. lycopersici (Didymella lycopersici), although Stewart (1957) has reported the existence of pseudothecia of Ph. tarda in nature, a finding that also has been reported by Salgado et al. (2007). This contradicts with the fact that none of the varieties embedded in the Ph. exigua has been found in association with a teleomorph thus far.
For further delineation of this clade, a comparison of actin gene sequences is proposed (Aveskamp et al. 2009b), although not all species and varieties in this complex can be recognised using this gene only. Thus far the varieties of Ph. exigua could only be delineated using two fingerprint techniques: Amplified Fragment Length Polymorphism (AFLP, Abeln et al. 2002) and DAF (DNA Amplification Fingerprinting) using mini-hairpin primers (Aveskamp et al. 2009b). Based on this latter technique Aveskamp et al. (2009b) recognised two varieties within Ph. exigua that had not been described before. These two infraspecific taxa, var. gilvescens and var. pseudolilacis are treated and described below.
Based on the phylogenetic reconstruction obtained here, the taxa previously known as Ph. exigua var. noackiana and Ph. exigua var. diversispora cluster in a distinct clade from the other varieties in this complex, and are elevated to species level here. Also actin sequence data and DAF analysis (Aveskamp et al. 2009b), AFLP data (Abeln et al. 2002) reveal a basal topology of these species compared to Ph. exigua. Morphological data obtained by Van der Aa (2000) also suggest that these species are not completely fitting in the Ph. exigua concept.
The species and varieties in this clade differ from other Phoma taxa based on their ostiole morphology. In contrast to other species, which have a smoothly lined ostiole, the taxa present in this clade have distinct hyaline cells lining their ostiolar openings (Fig. 8A). In addition, these species, with the exception of Ph. hedericola, produce septate conidia in addition to the regular aseptate ones, although in general the septate conidia are produced in smaller numbers in culture than on the host. These conidia are mostly 1-septate, as only in Ph. exigua incidentally multiseptate conidia occur, and are often only slightly larger than the regular aseptate ones (Fig. 8C). Due to the morphological and genetic distinctiveness, we propose a new generic name for the taxa in this clade.
Fig. 8.
Boeremia gen. nov. A. Ostiole configuration of B. exigua var. exigua (CBS 431.74). B. Pycnidial wall and conidiogenous cells of B. telephii (CBS 760.73) C. Aseptate and septate conidia of B. lycopersici (CBS 378.67). Scale bars: A = 20 μm; B–C = 10 μm.
Boeremia Aveskamp, Gruyter & Verkley, gen. nov. MycoBank MB515621. Fig. 8.
Conidiomata pycnidialia, plerumque globosa vel subglobosa, glabra vel eminentiis sparsis hypharum vestita, superficialia vel in agaro immersa, solitaria vel confluentia, 75–370 μm diam. Ostiola papillata vel epapillata, tempore maturitatis interne cellulis hyalinis papillatis. Paries pycnidii pseudoparenchymatus, e 2–8 stratis cellularum compositus, extima 1–3 strata brunnea. Cellulae conidiogenae phialidicae, hyalinae, glabrae, ampulliformes vel dolliiformes, ca. 3–7.5 × 3–6.5 μm. Conidia hyalina, tenuitunicata, glabra, plerumque continua, 2.5–12 × 2–4 μm, et interdum uni- vel biseptata, usque 15 × 5 μm.
Conidiomata pycnidial conidiomata variable in shape and size, mostly globose to subglobose, glabrous or with few mycelial outgrowths, on agar surface or immersed, solitary or confluent, measuring 75–370 μm diam. Ostioles 1–2(–3), non-pappillate or pappillate, lined internally with a pappillate hyaline cells when mature. Pycnidial wall pseudoparenchymatous, counting 2–8 cell layers of which the outer 1–3 are brown pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, ampulliform to doliiform, ca. 3–7.5 × 3–6.5 μm. Conidia variable in shape, hyaline, thin-walled, smooth, mainly aseptate, 2.5–12 × 2–4 μm, but regularly 1(–2)-septate conidia may be found which measure up to 15 × 5 μm. Pseudothecia, only rarely recorded in one species in vivo, subglobose, up to 300 μm diam. Asci cylindrical or subclavate, measuring 50–95 × 6–10 μm, always 8-spored, biseriate. Ascospores ellipsoid, measuring 12–18 × 5–6 μm, uniseptate.
Type species: Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley
Etymology: Named after Gerhard H. Boerema, who made great contributions to our understanding of the taxonomy of phomoid fungi.
Boeremia crinicola (Siemasko) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515622.
Basionym: Phyllosticta crinicola Siemasko, Acta Soc. Bot. Poloniae 1: 22. 1923.
≡ Phoma crinicola (Siemasko) Boerema apud Boerema & Dorenbosch, Verslagen Meded. Plziektenk. Dienst Wageningen 153: 18.1979.
Specimens examined: The Netherlands, Haarlem, from a bulb of Crinum powellii, Mar. 1976, G.H. Boerema, CBS H-16198, CBS 109.79 = PD 77/747; Alkmaar, from a bulb of Crinum sp., 1970, G.H. Boerema, CBS 118.93 = PD 70/195.
Boeremia diversispora (Bubák) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515623.
Basionym: Phoma diversispora Bubák, Oest. Bot. Z. 55: 78. 1905
≡ Phoma exigua var. diversispora (Bubák) Boerema apud Boerema & van Kesteren, Gewasbescherming 11: 122. 1980
For a complete description see Boerema et al. (1981a, 2004), and Van der Aa et al. (2000).
Specimens examined: Brazil, leaf of Phaseolus, F. Noack, holotype B. Kenya, from a pod of Phaseolus vulgaris, 1979, G.H. Boerema, epitype designated here CBS H-16308, ex-epitype culture CBS 102.80 = CECT 20049 = IMI 331907 = PD 79/61. The Netherlands, near Tilburg, from Phaseolus vulgaris, 1979, J. de Gruyter, CBS 101194 = PD 79/687 = IMI 373349.
Notes: Phoma diversispora was originally described by Bubák as a pathogen of cowpea (Vigna unguiculata) causing Black Node Disease (Van der Aa et al. 2000), but was later classified as a variety of Ph. exigua by Boerema & Van Kesteren (1980) and Boerema et al. (1981a), on basis of its morphology. The present study, however, revealed the B. exigua varieties to be phylogenetically distinct from the present species, which justifies re-establishment of the taxon as separate species in the genus Boeremia. The present species is closely related to B. noackiana, formerly known as Ph. exigua var. noackiana (see below).
Boeremia exigua var. coffeae (Henn.) Aveskamp, Gruyter & Verkley, stat. et comb. nov. MB515632.
Basionym: Ascochyta coffeae Henn., Hedwigia 41: 307. 1902; not Phoma coffeae Delacr., Bull. Soc. Mycol. France 13: 122. 1897.
= Ascochyta tarda R.B. Stewart, Mycologia 49: 430. 1957.
≡ Phoma tarda (R.B. Stewart) H. Verm., Coffee Berry Dis. Kenya: 14. 1979.
For a complete description see De Gruyter et al. (2002).
Specimens examined: Brazil, Patrocínio, from leaf of Coffea arabica, L.H. Pfenning, CBS 119730. Cameroon, Bemenda, from Coffea arabica, CBS 109183 = PD 2000/10506 = IMI 300060.
Notes: Boeremia exigua var. coffeae was originally described from leaves of coffee plants (Coffea arabica, Stewart 1957) as Ascochyta coffeae and A. tarda. The observed late euseptation in this species proved to be a character common for Phoma species accommodated in section Phyllostictoides, leading to a recombination into Phoma, as Ph. tarda. Phylogenetic results obtained in the present study reveal genetic similarity between the present species and the B. exigua species complex. The cultures of B. exigua varieties are somewhat slower growing than those of the present species, which completely covers the agar surface (90 mm diam) within 7 d. The pycnidia of B. exigua var. tarda may grown to up to 255 μm (De Gruyter et al. 2002), but other micromorphological characters fit within the scope of B. exigua as described for Ph. exigua by Van der Aa et al. (2000) and De Gruyter et al. (2002). It is concluded, therefore, that Ph. tarda should be reduced to a variety of the B. exigua. Multiple Phoma species have been found in association with Coffea arabica, such as Ph. coffeae-arabicae, Ph. coffeicola, Ph. coffeiphila, Ph. costarricensis, Ph. excelsa, and Ph. pereupyrena (Saccas 1981, Aveskamp et al. 2009a). None of these species however matches the description that is applied to taxa in the B. exigua complex.
Boeremia exigua var. exigua (Desm.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515624.
Basionym: Phoma exigua Desm., Ann. Sci. Nat. Bot. III 11: 282. 1849.
Specimens examined: Germany, Artern, from Foeniculum vulgare, Apr. 1984, S. Petzoldt, CBS 391.84. The Netherlands, from a tuber of Solanum tuberosum, 1928, CBS 236.28; Emmeloord, from a tuber of Solanum tuberosum, 1974, G.H. Boerema, CBS 431.74 = PD 74/2447; Emmeloord, from Cichorium intybus, 1979, G.H. Boerema, CBS 101150 = PD 79/118; Ommen, from Digitalis sp., 1990, J. de Gruyter, CBS 101152 = PD 90/835-3.
Boeremia exigua var. forsythiae (Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515625.
Basionym: Phyllosticta forsythiae Sacc., Michelia 1(1): 93. 1997.
≡ Phoma exigua var. forsythiae (Sacc.) Aa, Boerema & Gruyter, Persoonia 17: 452. 2000.
Specimens examined: The Netherlands, from Forsythia sp., 1992, J. de Gruyter, CBS 101213 = PD 92/959; from Forsythia sp., 1995, J. de Gruyter, CBS 101197= PD 95/721.
Boeremia exigua var. gilvescens Aveskamp, Gruyter & Verkley, var. nov. MycoBank MB515626. Fig. 9.
Fig. 9.
Boeremia exigua var. gilvescens (CBS 101150). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia. E. Chains of wollen cells. F. Conidia. Scale bars: D = 100 μm; E = 100 μm; F = 10 μm.
Varietas Phomae exiguae similis, sed matrix conidiorum flavida vel luteola. In agaro et in mycelio aereo catenis cellularum inflatarum (11.5–)12.5–27.5(–31) × (5.5–)7.5–14.5(–18) μm.
Etymology: Varietal name refers to the yellow conidial matrix, which distinguishes this variety.
Culture characteristics: Colonies on OA 75–80 mm diam after 7 d, margin regular or irregular. Immersed mycelium sparsely visible due to coverage by the aerial mycelium, hyaline or black to greenish olivaceous, with many pycnidia; reverse mouse-grey to olivaceous. Colonies on MEA 70–75 mm diam after 7 d, margin regular or irregular. Immersed mycelium completely covered by a compact aerial mat, which is smoke-grey with some mouse-grey zones; reverse black. Colonies on CHA at least (75–)80 mm diam after 7 d, but often the agar surface is completely covered, margin regular or somewhat crenate. Immersed mycelium completely covered by a compact smoke-grey mat of aerial mycelium, or, in some zones floccose, olivaceous with white tufts; reverse shows a dendritic leaden-black zone around the colony centre, with black zones near the colony border. Application of NaOH did not have any effect.
Pycnidial and conidial shapes and sizes fit within the Ph. exigua species concept. Conidial matrix yellowish or pale luteous. Brown pigmented swollen cells occur in chains in the agar and in the aerial mycelium, measuring (11.5–)12.5–27.5(–31) × (5.5–)7.5–14.5(–18) μm.
Specimens examined: Philippines, from Solanum tuberosum,1990, L.J. Turkensteen, CBS 101156 = PD 90/731; The Netherlands; from a graft of Ulmus, 1961, H.M. Heybroek, CBS 373.61; Baarn, from leaves of Dactylis purpurea, 1970, H.A. van der Aa, holotype designated here CBS H-16281, culture ex-holotype CBS 761.70; Lisse, from Dahlia, 1982, J. de Gruyter, CBS 101151 = PD 82/1022.
Notes: This novel variety of B. exigua, distinguished from other B. exigua varieties on basis of DAF analysis (Aveskamp et al. 2009b), is closely related to B. exigua var. exigua, but different in the colour of its conidial matrix (yellowish) and absence of a positive reaction to NaOH. This variety may be identical to Ph. exigua var. inoxydabilis Boerema & Vegh, but as the type culture has been lost (Van der Aa et al. 2000) a proper comparison of the varieties cannot be made. Additionally, Ph. exigua var. inoxydabilis was originally only known from periwinkle (Vinca minor, Vegh et al. 1974), whereas the strains associated to the present taxon are isolated from a wide range of host plants.
Boeremia exigua var. heteromorpha (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515627.
Basionym: Phoma heteromorpha Schulzer & Sacc., Hedwigia 23: 107. 1884.
≡ Phoma exigua var. heteromorpha (Schulzer & Sacc.) Noordel. & Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 166: 109.1989.
Specimens examined: France, Antibes, from Nerium oleander, 1979, CBS 101196 = PD 79/176. Italy, Perugia, from Nerium oleander, 1994, A. Zazzerini, CBS 443.94.
Boeremia exigua var. lilacis (Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515628.
Basionym: Phoma herbarum f. lilacis Sacc., Michelia 2(1): 93. 1880.
Specimen examined: The Netherlands, Wageningen, from a twig of Syringa vulgaris, June 1976, G.H. Boerema, CBS H-163131, culture CBS 569.79 = PD 72/741.
Notes: Although in the present study this variety clusters outside the B. exigua cluster, it is phylogenetic affiliation is ambiguous. In previous studies in which fingerprint markers and actin sequences were applied to delineate this species complex (Abeln et al. 2002, Aveskamp et al. 2009b) the present taxon clusters within Ph. exigua, and is therefore recombined as B. exigua var. lilacis. Further analysis of this complex is, however, advocated.
Boeremia exigua var. linicola (Naumov & Vassiljevsky) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515629.
Basionym: Ascochyta linicola Naumov & Vassiljevsky, Mater. Micol. Fitopatol. 5: 3. 1926.
Specimens examined: The Netherlands, Zierikzee, from Linum usitatissimum, 1928, H.A. Diddens, CBS 114.28; Flevoland, from a stem of Linum usitatissimum, 1976, G.H. Boerema, CBS 116.76 = ATCC 32332 = CECT 20022 = CECT 20023 = IMI 197074 = PD 75/544.
Boeremia exigua var. populi (Gruyter & P. Scheer) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515630
Basionym: Phoma exigua var. populi Gruyter & P. Scheer, J. Phytopathol. 146(8–9): 413. 1998.
Specimens examined: The Netherlands, Deil, from a twig of Populus X euramericana cv. Robusta, Feb. 1993, A.J.P. Oort, holotype L 995.263.325, ex-holotype culture CBS 100167 = PD 93/217; Rotterdam, from Salix sp., 1982, J. de Gruyter, CBS 101202 = PD 82/942.
Boeremia exigua var. pseudolilacis Aveskamp, Gruyter & Verkley, var. nov. MycoBank MB515631. Fig. 10.
Fig. 10.
Boeremia exigua var. pseudolilacis (CBS 101207). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia. E. section of young pycnidium. F. Conidia. Scale bars: D = 100 μm; E = 20 μm; F = 5 μm.
Varietas haec in cultura habitu Phomae exiguae var. exiguae et var. gilvescentis similis, sed matrix conidiorum roseo-bubalina et citius crescens.
Etymology: Refers to the former placement in and close resemblance to Ph. exigua var. lilacis.
Colonies on OA 70–75 mm diam after 7 d, margin regular. Immersed mycelium black to greenish olivaceous, sparsely visible due to coverage by a mat of mouse-grey woolly to compact aerial mycelium; reverse mouse-grey to olivaceous. Colonies on MEA 70–75 mm diam. after 7 d, margin regular. Immersed mycelium completely covered by a compact aerial mat, which is smoke-grey with some mouse-grey to white zones; reverse black. Colonies on CHA slower growing, 70–80 mm diam after 7 d, margin regular, appearance similar as on MEA. Application of NaOH did not have any effect. Pycnidial and conidial shapes and sizes fit within the B. exigua species concept. Conidial matrix rosy-buff.
Specimen examined: The Netherlands, near Boskoop, from Syringa vulgaris, 1994, J. de Gruyter, holotype CBS H-20371, culture ex-holotype CBS 101207 = PD 94/614.
Notes: This novel variety of B. exigua, distinguished from other B. exigua varieties on basis of DAF analysis (Aveskamp et al. 2009b) and AFLP (Abeln et al. 2002), is closely related to B. exigua var. exigua and B. exigua var. gilvescens. Upon collection, the strain representing B. exigua var. pseudolilacis has probably erroneously been identified as var. lilacis due to its host association.
Boeremia exigua var. viburni (Roum. ex. Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515633.
Basionym: Ascochyta viburni Roum. ex Sacc., Syll. Fung. 3: 387. 1884.
≡ Phoma exigua var. viburni (Roum. ex. Sacc.) Boerema apud De Gruyter & P. Scheer, J. Phytopathol. 146: 414. 1998.
Specimens examined: The Netherlands, Boskoop, from Viburnum opulus, 1984, G.H. Boerema, CBS 100354 = PD 83/448; from Lonicera sp., 1993, J. de Gruyter, CBS 101211 = PD 93/838.
Boeremia foveata (Foister) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515653.
Basionym: Phoma foveata Foister, Trans. & Proc. Bot. Soc. Edinburgh 33: 66. 1940.
Specimens examined: Bulgaria, from a tuber of Solanum tuberosum, 1994, J. de Gruyter, CBS 109176 = CECT 2828 = PD 94/1394. U.K., from a tuber of Solanum tuberosum, Mar. 1937, C.E. Foister, ex-isotype culture CBS 200.37; Northern Ireland, Belfast, from a tuber of Solanum tuberosum, 1966, C. Logan, CBS 341.67 = CECT 20055 = IMI 331912.
Boeremia hedericola (Durieu & Mont.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515634.
Basionym: Phyllosticta hedericola Durieu & Mont., Flore d'Algérie Cryptog. 1: 611. 1849.
≡ Phoma hedericola (Durieu & Mont.) Boerema, Trans. Brit. Mycol. Soc. 67: 295. 1976.
Specimens examined: The Netherlands, Meppel, from a leaf of Hedera helix, 1970, CBS 366.91 = PD 70/811; from Hedera helix, 1987, J. de Gruyter, CBS 367.91 = PD 87/229.
Note: Strain CBS 367.91 is sterile.
Boeremia lycopersici (Cooke) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515635.
Basionym: Phoma lycopersici Cooke, Grevelia 13: 94. 1885. Teleomorph: Didymella lycopersici Kleb., Z. Pflanzenkrankh. 31: 9. 1921.
Specimens examined: The Netherlands, Heerde, from fruit of Lycopersicon esculentum, Aug. 1967, G.H. Boerema, CBS 378.67 = PD 76/276; from Lycopersicon esculentum, 1984, J. de Gruyter, CBS 109172 = PD 84/143.
Boeremia noackiana (Allesch.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515636.
Basionym: Phyllosticta noackiana Allesch., Bol. Inst. Agron. Campinas 9: 85. 1898.
≡ Phoma exigua var. noackiana (Allesch.) Aa, Boerema & Gruyter, Persoonia 17: 450. 2000.
For a complete description see Van der Aa et al. (2000).
Specimens examined: Colombia, from Phaseolus vulgaris, 1979, J. de Gruyter, CBS 101203 = PD 79/1114. Guatemala, from Phaseolus vulgaris, 1987, IPO Wageningen, CBS 100353 = PD 87/718.
Notes: Boeremia noackiana is genetically a sister species to B. diversispora, and was also noted by Boerema et al. (2004) as “the American cousin”. Just like B. diversispora, the present species is known from beans, although the main host appears to be Phaseolus vulgaris. The two species have many characters in common with B. exigua (Van der Aa et al. 2000, Boerema et al. 2004) and with each other, but are distinguished based on enzyme analysis (Obando-Rojas, 1989) and molecular fingerprinting methods such as AFLP (Abeln et al. 2002) and DAF (Aveskamp et al. 2009b). Additionally, B. noackiana is characterised by a relative fast growth rate on MEA: (6–)6.5–7.5 mm diam after 7 d, and is further distinguished from B. diversispora by its relatively uniform conidia. Due to the relatively large genetical distance to the B. exigua complex, this taxon is elevated to species level.
Boeremia sambuci-nigrae (Sacc.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515637.
Basionym: Phoma herbarum f. sambuci-nigrae Sacc., Syll. Fung. 3: 133. 1884.
≡ Phoma exigua var. sambuci-nigrae (Sacc.) Boerema & Höweler, Persoonia 5(1): 26. 1967.
≡ Phoma sambuci-nigrae (Sacc.) E. Monte, Bridge & B. Sutton, Mycopathologia 115: 102. 1991.
Specimens examined: The Netherlands, Wageningen, from a leaf of Sambucus nigra, 1967, lectotype CBS H-16314, ex-lectotype culture CBS 629.68 = CECT 20048 = IMI 331913 = PD 67/753; Baarn, Maarschalksbos, from a leaf of Sambucus nigra, Nov. 1967, H.A. van der Aa, CBS 104.68= CECT 20010; from Sambucus nigra, 1975, G.H. Boerema, CBS 109170 = PD 75/796.
Boeremia strasseri (Moesz) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515638.
Basionym: Phoma strasseri Moesz, Bot. Közlem. 22: 45. 1924. nom. nov. pro Phoma menthae Strasser, Verh. zool. Bot. Ges. Wien 60: 317. 1910 [non Phoma menthae Roum. (date of publication unknown)].
Specimens examined: The Netherlands, Arnhem, from a stem of Mentha sp., 1973, CBS 126.93 = PD 73/642. U.S.A., Oregon, from Mentha piperita, 1970, H.A. van der Aa, CBS 261.92 = ATCC 244146 = PD 92/318.
Note: As the older name Ph. menthae is illegitimate, the epithet “strasseri” prevails.
Boeremia telephii (Vestergr.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515639.
Basionym: Ascochyta telephii Vestergr., Öfvers. Förh. Kongl. Svenska Vetenska.-Akad. 54: 41. 1897.
≡ Phoma telephii (Vestergr.) Kesteren, Netherlands J. Pl. Pathol. 78: 117.1972.
Specimens examined: The Netherlands, Utrecht, from a stem of Sedum telephium, 1971, G.H. Boerema, CBS 760.73 = PD 71/1616; from Sedum spectabile, 1975, G.H. Boerema, CBS 109175 = PD 79/524.
Group O:
Three species are clustered in group O, which all were accommodated in the Boeremaean section Phoma. These species, Ph. multirostrata, Ph. pereupyrena and Ph. insulana are characterised by the production of small (5–15 μm diam), unicellular chlamydospores, comparable to those formed by some species in group K. The absence of septate conidia and a thin pycnidial wall are further characters of the species accommodated in group O.
The strains accommodated in Ph. multirostrata reveal a high variation in spore size. Boerema et al. (1986) introduced three varieties within this species, but based on morphological observations and DNA sequence analyses, these varieties were not recognised by later researchers and thus the varieties were merged again (Morgan-Jones 1988, Aveskamp et al. 2009a).
Phoma insulana (Mont.) Boerema & Malathr., in Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 158 (Jaarboek 1981): 28. 1982.
Basionym: Phyllosticta insulana Mont., Ann. Sci. Nat. Bot. IV 5: 343.1856.
Specimen examined: Greece, from the berries of Olea europaea, 1980, G.H. Boerema, CBS 252.92 = PD 80/1144.
Phoma multirostrata (P.N. Mathur, S.K. Menon & Thirum.) Dorenb. & Boerema, Mycopathol. Mycol. Appl. 50(3): 256. 1973, emend. Aveskamp et al. Mycologia 101: 375. 2009.
Basionym: Sphaeronaema multirostratum P.N. Mathur, S.K. Menon & Thirum., Sydowia 13: 146. 1959. (as “Sphaeronema”).
Specimens examined: India, Maharashtra, Poona, Talegaon, from poultry farm soil, Mar. 1959, M.J. Thirumalachar, isotype CBS H-7616, culture CBS 274.60 = IMI 081598; Maharashtra, Poona, Talegaon, from soil, Mar. 1959, M.J. Thirumalachar, CBS H-16499, culture CBS 368.65 = PD 92/1757. The Netherlands, Hoorn, greenhouse, from the stem of Cucumis sativus, Aug. 1967, G.H. Boerema, CBS H-16502, culture CBS 110.79 = PD 65/8875. Unknown origin, from Cucumis sativus, 1983, PD 83/48.
Phoma pereupyrena Gruyter, Noordel. & Boerema, Persoonia 15(3): 390. 1993.
Specimen examined: India, from a leaf of Coffea arabica, 1976, CBS 267.92 = PD 76/1014.
Group P:
This well-supported clade (BPP = 1.00, RBS = 97 %) comprises Ph. dictamnicola and Ph. sylvatica, which are both associated with the section Sclerophomella (Boerema et al. 1998). In addition, both varieties of Ph. poolensis are recovered here. As in the Sclerophomella species, an ostiole is commonly absent in Ph. poolensis var. poolensis, a character which supports the sequence data found in the present study. In contrast, the second variety of this species, Ph. poolensis var. verbascicola, always produces ostiolate pycnidia (De Gruyter et al. 1993). Both Ph. poolensis varieties can further be differentiated on the basis of the β-tubulin sequence, and are morphologically distinguishable in the colour of the conidial matrix. The conidia of the type variety are on average somewhat smaller, measuring ca. 3.5–5 × 1.5–2 μm, than those of var. verbasicola, which measure 3.5–5.5 × 1.5–2.5 μm. Both varieties are known from plant hosts belonging to the Scophulariaceae, but whereas var. poolensis is recorded as causal agent of leaf spots and basal stem rot in snapdragon (Antirrhinum majus), var. verbascicola is only known as saprobe of Verbascum spp., although inoculation trials indicated that it may also have a role as pathogen (Boerema et al. 2004). Given all these differences, it is considered to be justified to erect a separate species for Ph. poolensis var. verbascicola as Ph. novae-verbascicola.
Although none of the species in this group has been confirmed to have a teleomorph (Boerema et al. 1998), it has been suggested that Didymella winteriana is the teleomorph of Phoma sylvatica (Munk 1957). Given the topology of the tree, this association with a Didymella species is plausible, although a sexual structure was not observed in the present study, nor in the previous studies of Boerema & De Gruyter (1998).
Phoma dictamnicola Boerema, Gruyter & Noordel., Persoonia 15(1): 90. 1992.
Specimen examined: The Netherlands, Arnhem, from a stem of Dictamnus albus, 1974, J. de Gruyter, CBS 507.91 = PD 74/148.
Phoma novae-verbascicola Aveskamp, Gruyter & Verkley, nom. nov. pro Phyllosticta verbascicola Ellis & Kellerm. MycoBank MB515640.
Basionym: Phyllosticta verbascicola Ellis & Kellerm., Bull. Torrey Bot. Club 11: 115. 1884.
≡ Phoma poolensis var. verbascicola (Ellis & Kellerm.) Aa & Boerema, in De Gruyter, Noordeloos & Boerema, Persoonia 15(3): 385. 1993. Not Phoma verbascicola (Schwein.) Cooke, in Ravenel. 1878.
Etymology: The epithet refers to the host plant, Verbascum spp.
For a full description see De Gruyter et al. (1993).
Specimens examined: The Netherlands, Zeist, Abburg nursery, holotype L 9893.00.134; Haarlem, from dead stem material of Verbascum densiflorum, 1992, J. de Gruyter, CBS 127.93 = PD 92/347; from stem of Verbascum sp., 1974, G.H. Boerema, CBS 114.93 = PD 74/228.
Notes: This species is distinguishable from Ph. poolensis by to the presence of 1–2(–5) ostioles, the colourless to whitish matrix and the smaller conidia. On MEA, the aerial mycelium is more compact or woolly than that of Ph. poolensis.
The variety epithet could not be elevated to species level, as Phoma verbascicola is already occupied. This basionym, however, probably refers to immature pseudothecia of a Pleospora species (Boerema et al. 1996). Therefore, a new name is proposed here for the present species.
Phoma poolensis Taubenh., Dis. Greenhouse Crops 203. 1919.
Specimens examined: Denmark, from a stem of Antirrhinum majus, July 1938, P. Neergaard, CBS 253.38. The Netherlands, Wageningen, from a stem of Scrophularia nodosa, 1974, G.H. Boerema, CBS 115.93 = PD 74/206; Bennekom, from a stem of Antirrhinum majus, 1973, G.H. Boerema, CBS 116.93= PD 71/884. Unknown origin and substrate, 1920, E.M. Smiley, CBS 113.20 = PD 92/774.
Phoma sylvatica Sacc. Michelia 2(2): 337. 1881.
Specimens examined: The Netherlands, Wageningen, from Melampyrum pratense, 1983, J. de Gruyter, CBS 135.93 = PD 83/87; Wageningen, from a stem of Melampyrum pratense, 1993, J. de Gruyter, CBS 874.97 = PD 93/764.
Group Q:
The Phoma species embedded in this group, Ph. commelinicicola and Ph. eupatorii are morphologically distinct, hence their accommodation in the sections Phoma and Macrospora respectively. The accommodation of Chaetasbolisia erysiphoides in this clade, the type of its genus, is unexpected. Attempted morphological studies revealed that this strain was sterile, and therefore recombination of the species could not be supported by morphological data. The descriptions provided in literature (Sutton 1980, Patel et al. 1997, Reynolds 1999) suggest, however, that this genus could very well represent a group of setose Phoma species, although this cannot be resolved due to a lack of isolates. The presence of setae is not recorded in other species in group Q, and moreover, is within the Didymellaceae only known from Peyronellaea gardeniae (Group K), and from pycnidia in some older cultures from Epicoccum sorghi (Group M), Peyronellaea glomerata (Group K) and Phoma herbarum (Boerema et al. 2004). The topology and the clustering of these species cannot be further explained by the morphology or ecology, nor by their geographical distribution.
Phoma commelinicola (E. Young) Gruyter, Persoonia 18(1): 93. 2002.
Basionym: Phyllosticta commelinicola E. Young, Mycologia 7: 144. 1915.
Specimen examined: New Zealand, South Auckland, Alfriston, from Tradescantia sp., 1997, K. Ramsay, CBS 100409.
Phoma eupatorii Died., Ann. Mycol. 10(5): 447. 1912.
Specimen examined: The Netherlands, Arnhem, from Eupatorium cannabinum, 1977, G.H. Boerema, CBS 123.93 = PD 77/1148.
Group R:
This group comprises five species that previously were accommodated in the sections Phoma, Peyronellaea and Phyllostictoides. As the name of Ph. tropica already suggests, it concerns a thermotolerant species, which is mainly found in European greenhouses on a wide range of hosts, but which probably has a tropical origin (Schneider & Boerema 1975), as do most other species found in the present clade. The sole host of Ph. costarricensis is coffee bean (Coffea arabica), while Ph. piperis is associated with Indian Long Pepper (Piper longus), and the novel species Ph. minor has been isolated twice from clove (Syzygium aromaticum) in Indonesia. In addition, Ph. labilis is a warmth-preferring plurivorous species that has been isolated in European greenhouses and from nature in the Middle East, Turkey and Indonesia (Boerema et al. 2004). Phoma zantedeschiae is widespread throughout the Western Hemisphere, but always in association with arum or calla (Zantedeschia sp.), a genus that is indigenous in southern Africa (Boerema & Hamers 1990). Thus far, however, no data of temperature-growth studies are available for these species except for Ph. tropica. Several other thermotolerant species, such as Ph. calidophila, Ph. calorpreferens and Ph. multirostrata, are, however, not accommodated in this group. These three species are soil-borne, in contrast to Ph. tropica and Ph. costarricensis, which are associated with leaf-spots.
Phoma tropica and Ph. costarricensis are both closely related, and colony characters are highly similar. However, the strains available revealed a significant difference in conidial and pycnidial sizes, consistent with the data obtained in previous studies (Schneider & Boerema 1975, De Gruyter & Noordeloos 1992).
Phoma costarricencis Echandi, Rev. Biol. Trop. 5: 83. 1957.
Specimens examined: Nicaragua, from a twig of Coffea sp., 1991, CBS 506.91 = PD 91/876 = IMI 215229. Unknown origin, from Coffea arabica, 1979, CBS 497.91 = PD 79/209.
Notes: Strain CBS 497.91 was initially identified as Ph. tropica. The close phylogenetic association between this species and Ph. costarricensis concurs with their overlapping morphological characters (see Schneider & Boerema 1975, De Gruyter & Noordeloos 1992).
Phoma labilis Sacc., Michelia 2(7): 341. 1881.
Specimens examined: Israel, from a stem of Rosa sp., 1970, G.H. Boerema, CBS 479.93 = PD 70/93. The Netherlands, Barendrecht, from a stem of Lycopersicon esculentum, 1987, J. de Gruyter, CBS 124.93 = PD 87/269.
Phoma minor Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515641. Fig. 11.
Fig. 11.
Phoma minor (CBS 325.82). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D–E. Pycnidia. F. Section of the pycnidial wall. G. Conidia. Scale bars: D = 200 μm; E = 100 μm; F–G = 10 μm.
Conidia ellipsoidea, ovoidea vel leniter allantoidea, glabra, hyalina, continua, (3–)3.5–4.5(–5) × 1.8–2.5(–3) μm, (0–)1–3(–4) guttulis minutis praedita. Matrix conidiorum alba.
Etymology: Epithet derived from the small-sized conidia.
Conidiomata pycnidial, solitary, (sub-)globose to broadly ellipsoidal, glabrous or with some hyphal outgrows, on the agar surface and immersed, (125–)150–280(–330) × (125–)150–220(–245) μm. Ostioles (1–5), slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, outer cell layer pigmented, 2–4 layers, 8–15 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped or somewhat isodiametric, ca. 4–5.5(–6.2) × 3–4.5(–4.7) μm. Conidia ellipsoidal to ovoid or slightly allantoid, thin-walled, smooth, hyaline, aseptate (3–)3.5–4.5(–5) × 1.8–2.5(–3) μm, with (0–)1–3(–4) minute guttules. Conidial matrix white.
Culture characteristics: Colonies on OA (44–)45–50(–54) mm diam after 7 d, margin regular. Aerial mycelium flat, grey, but locally well-developed in densely floccose white tufts. Immersed mycelium olivaceous with rosy-buff tinges near the colony margin; reverse concolourous. Colonies on MEA 46-48 mm diam after 7 d, margin regular. Immersed mycelium hyaline, with abundant semi-immersed pycnidia, but almost completely covered by an aerial mycelial mat. Aerial mycelium pluriform, with a compact white mat and some felty glaucous grey or dull green zones, near colony margin white; reverse black to grey-olivaceous. Colonies on CHA 50–54 mm diam. after 7 d, margin regular. Aerial mycelium similar as on MEA, although the felty white and glaucous grey zones are less abundant; reverse slate blue to leaden-black. Application of NaOH results in a greenish yellow discolouration of the agar, best to be observed on OA medium.
Specimens examined: Indonesia, Sumatra, from Syzygium aromaticum, Apr. 1982, R. Kasim, holotype designated here CBS H-20236, ex-holotype culture CBS 325.82; Lampung, from Syzygium aromaticum, Dec. 1982, H. Vermeulen, CBS 315.83.
Notes: As for Ph. eucalyptica, this species has been recorded in association with clove trees (Syzygium aromaticum, Boerema et al. 2004). Both species, although genetically distinct, have many characters in common, notably the colony characters on OA, the high variation in ostiole number and a similar reaction to application of NaOH. Although Phoma minor produces relatively small conidia, the conidia of Ph. eucalyptica are even smaller, measuring only 2–4 × 1–2 μm (De Gruyter & Noordeloos 1992).
Phoma piperis (Tassi) Aa & Boerema, Persoonia 15(3): 398. 1993.
Basionym: Phyllosticta piperis Tassi, Boll. Reale Orto Bot. Siena 3(2): 28. 1900.
Specimens examined: The Netherlands, Tiel, from a leaf of Peperomia pereskiifolia, 1988, J. de Gruyter, CBS 268.93 = CBS 108.93 = PD 88/720; Tiel, from Peperomia sp., 1990, J. de Gruyter, PD 90/2011
Phoma tropica R. Schneid. & Boerema, Phytopathol. Z. 83 (4): 361. 1975.
Specimen examined: Germany, Horrheim, from Saintpaulia ionantha, 1973, R. Schneider, isotype CBS H-7629, ex-isotype culture CBS 436.75.
Phoma zantedeschiae Dippen., S. African J. Sci. 28: 284. 1931.
Specimen examined: The Netherlands, from a bulb of Zantedeschiae sp., 1969, G.H. Boerema, CBS 131.93 = PD 69/140.
Group S – Stagonosporopsis:
This large group (BPP = 1.00, RBS = 55 %) comprises mainly species with Stagonosporopsis synanamorphs. In the Boeremaean classification system, these species were embedded in Phoma section Heterospora (Boerema et al. 1997). As with the other sections, this group also appeared to be artificial. Based on LSU and SSU sequences, the type species of the section Heterospora, Ph. heteromorphospora, clusters outside the Didymellaceae (De Gruyter et al. 2009), as do Ph. samarorum and Ph. dimorphospora. Three species, Ph. clematidina, Ph. glaucii and Ph. aquilegiicola form a separate clade (Group C) within the Didymellaceae, and are treated above. Also Ph. nigripycnidia and Ph. narcissi are not accommodated here.
In contrast to the Heterospora species that are absent in this clade, several current Phoma taxa recovered here have been associated with the section Phyllostictoides, such as Ph. artemisiicola, Ph. caricae-papayae, Ph. cucurbitacearum, Ph. heliopsidis, Ph. rudbeckiae, and the quarantine-organisms Ph. ligulicola var. ligulicola and var. inoxydabilis (De Gruyter 2002). These are all included in subclade S1 (BPP = 0.93, RBS = 73 %). These species do produce a percentage of multicellular conidia in culture that are often considerably larger than the regular aseptate ones. However, Boerema et al. (1997) decided to exclude the Ph. ligulicola varieties and Ph. cucurbitacearum from section Heterospora, as the sizes of the Stagonosporopsis-like conidia do not always exceed that of the aseptate conidia in these species. A sister clade to subclade S1 is S2, which hosts the potato pathogens Ph. andigena and Ph. crystalliniformis – formerly known as Ph. andina var. crystalliniformis. Both species originate from the Andes region, and are regarded as serious quarantine pathogens in large parts of the world (Smith et al. 1992).
In addition, three other subclades can be recognised in this clade. One (S3) comprises the species Ph. schneiderae and Ph. subboltshauserii (both of the section Heterospora) as well as Ph. astragali. This species is known as a pathogen of Astragalus spp., and is characterised by a high percentage of “distorted” conidia, but thus far, no records have been made of a Stagonosporopsis-like synanamorph. Whereas records of Ph. astragali and Ph. schneiderae are mainly limited to the American continent, Ph. subboltshauseri appears to occur worldwide on Fabaceae. However, Boerema et al. (2004) suggested that the original host of this species may have been Phaseolus, which is native to the Americas.
A fourth and fifth (S4, S5) subclade in this group comprise species that are accommodated in section Phoma, and therefore lack any further features than a plain, globose pycnidium and aseptate, hyaline conidia. The species found here are Ph. dorenboschiae, Ph. loticola (both S4), Ph. ajacis and Ph. valerianellae (both S5).
In group S several taxa have been found with a teleomorph in Didymella, such as Ph. ligulicola var. ligulicola (teleomorph D. ligulicola var. ligulicola), Ph. ligulicola var. inoxydabilis (D. ligulicola var. inoxydabilis), and Ph. cucurbitacearum (D. bryoniae). Also the teleomorph of Ph. caricae-papayae has been recovered in this study, and found to be a Didymella, which is in line with the other teleomorph observations in this clade. The current teleomorph state of this species is accommodated in Mycosphaerella as M. caricae (Sivanesan 1990).
As the species in the present clade form a well-defined group within the Didymellaceae, the taxa are recombined into the genus Stagonosporopsis. This further implies that the names of the Stagonosporopsis synanamorphs of Ph. samarorum and Ph. narcissi (S. fraxini and S. curtisii respectively) should no longer be used.
Stagonosporopsis Died., Ann. Mycol. 10(2): 142. 1912. emend. Aveskamp, Gruyter & Verkley. Fig. 12.
Fig. 12.
Conidial dimorphism in three species of Stagonosporopsis. A. S. actaeae (CBS 106.96). B. S. lupini (CBS 101494). C. S. cucurbitacearum (CBS 109171). Scale bars: A = 20 μm; B–C = 10 μm.
Conidiomata pycnidial, globose to subglobose, measuring 70–300 μm diam, on agar surface or immersed, solitary or confluent, ostiolate or poroid. Pycnidial wall pseudoparenchymatous, counting 2–6 cell layers of which the outer 1–3 are brown/olivaceous pigmented. Conidiogenous cells phialidic, hyaline, simple, smooth, ampulliform or doliiform, ca. 4–7.5 × 3–6 μm. Conidia often in two types: majority aseptate, hyaline, ellipsoidal to subglobose, thin-walled, smooth, measuring (3–)3.5–10 × 1.5–3(–3.5) μm. Conidia of the second type can be produced both in vivo and in vitro in the same pycnidia as the smaller spores, unicellular or with up to 3 septa, measuring up to 30 × 8 μm. Pseudothecia, if present, occurring only in vivo, globose to subglobose, sometimes with a somewhat conical neck, measuring 90–230 μm diam. Asci cylindrical or subclavate, measuring 50–90 × 9–13 μm, always 8-spored, biseriate. Ascospores ellipsoid, fusiform or obovoid, measuring 12–18 × 4–7 μm, uniseptate, guttulate.
Stagonosporopsis actaeae (Allesch.) Died., Ann. Mycol. 10: 141. 1912.
Basionym: Actinonema actaeae Allesch., Ber. bayer. bot. Ges. 5: 7. 1897.
= Phoma actaeae Boerema, Gruyter & Noordeloos, Persoonia 16(3): 347. 1997.
Specimens examined: The Netherlands, Zeist, from a stem of Cimicifuga simplex, 1974, G.H. Boerema, CBS 105.96 = PD 74/230; Limburg, Schaersbergerbos, from a leaf of Actaea spicata, 1994, J. de Gruyter, L 992.167.501, culture CBS 106.96 = PD 94/1318.
Notes: In contrast to the earlier description of the Phoma anamorph of this species (Boerema et al. 1997), the larger conidia regularly produces up to 3-septate conidia (see Fig. 12A). In the study mentioned above and in the present one the same strains were examined morphologically.
Stagonosporopsis ajacis (Thüm.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515653.
Basionym: Phyllosticta ajacis Thüm., apud Bolle & von Thümen, Boll. Soc. Adriat. Sci. Nat. Trieste 6: 329. 1880.
= Phoma ajacis Aa & Boerema, apud De Gruyter, Noordeloos & Boerema, Persoonia 15(3): 383. 1993.
Specimens examined: Kenya, from Delphinium sp., 1990, Hopman, L 993.034.225, culture CBS 177.93 = PD 90/115. The Netherlands, Ter Aar, from Delphinium sp., 1986, CBS 176.93 = PD 86/547.
Stagonosporopsis andigena (Turkenst.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515655.
Basionym: Phoma andigena Turkenst., apud Boerema, Gruyter & Noordeloos, Persoonia 16(1): 131. 1995.
Specimens examined: Peru, Dep. Junin, Huancayo, near Vallis Mantaro, from a leaf of Solanum sp., 1975, L.J. Turkensteen, CBS 101.80 = PD 75/909 = IMI 386090; Dep. Junin, Huancayo, near Vallis Mantaro, from a leaf of Solanum sp., 1975, L.J. Turkensteen, CBS 269.80 = PD 75/914.
Stagonosporopsis artemisiicola (Hollós) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515656.
Basionym: Phoma artemisiicola Hollós, Mat. Természettud. Közlem. 35: 40. 1926. (as “artemisaecola”)
Specimen examined: France, from a stem base of Artemisia dracunculus, 1973, CBS 102636 = PD 73/1409.
Stagonosporopsis astragali (Cooke & Harkn.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515657.
Basionym: Phoma astragali Cooke & Harkn., Grevillea 13: 111. 1885.
Specimen examined: Unknown origin, from Astragalus sp., 1925, A.W. Archer, CBS 178.25 = MUCL 9915.
Stagonosporopsis caricae (Sydow & P. Sydow) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515658.
Basionym: Mycosphaerella caricae Sydow & P. Sydow, Ann.. Mycol. 11: 403. 1913.
≡ Phoma caricae-papayae (Tarr.) Punith., Trans Brit. Mycol. Soc. 75: 340. 1980.
≡ Ascochyta caricae-papayae Tarr., The fungi and plant diseases of Sudan: 53. 1955.
= Ascochyta caricae Pat., Bull. Soc. Mycol. France 7: 178. 1891.
≡ Phoma caricae Punith., CMI Descriptions of Pathogenic Fungi and Bacteria 634: 1. 1979.
For description of the teleomorph see Sivanesan (1990). Punithalingam (1979b) provides an extensive description, Ph. caricae is a synonym of the anamorph.
Specimens examined: Brazil, from Carica papaya, 2006, J. de Gruyter, PD 06/03082531. Chile, from fruit of Carica papaya, Feb. 1990, H.A. van der Aa, CBS 248.90. Indonesia, Java, Segunung, from Brassica sp., Feb. 1976, H. Vermeulen, CBS 282.76.
Notes: Phoma caricae-papayae has been associated with an undescribed teleomorph state in Mycosphaerella or Didymella (Boerema et al. 2004). Sivanesan (1990) synonymised Ph. caricae with M. caricae, apparently not noting that Ph. caricae already was synonymised with Ph. caricae-papayae by Punithalingam (1980). As Mycosphaerella is phylogenetically unrelated to Phoma (De Gruyter et al. 2009), this taxonomic association is unlikely, and the observed sexual state observed was probably Didymella-like.
This species has solely been associated with pawpaw (Carica papaya, Caricaceae), but a single strain, deposited at CBS as D. exigua and that was isolated from Brassica leaves from Java, Indonesia (CBS 282.76), was genetically identical to the reference strain of Ph. caricae-papayae. Herbarium material of this strain consisted of an inoculated lupine stem on cornmeal agar (CBS H-11960) and represented a conidial state similar to this of Ph. caricae-papayae. This indicated that probably the Didymella teleomorph had been observed, but that it was preserved under an incorrect name as it was only distantly related to the ex-type strain of Didymella exigua (CBS 183.55). This finding provides evidence that S. caricae is not restricted to pawpaw.
Stagonosporopsis crystalliniformis (Loer., R. Navarro, M. Lôbo & Turkenst.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515659.
Basionym: Phoma andina var. crystalliniformis Loer., R. Navarro, M. Lôbo & Turkenst., Fitopatología 21(2): 100. 1986.
≡ Phoma crystalliniformis (Loer., R. Navarro, M. Lôbo & Turkenst.) Noordel. & Gruyter, apud Noordeloos, de Gruyter, van Eijk & Roeijmans, Mycol. Res. 97: 1344. 1993.
Specimens examined: Colombia, Antioquia, Rionegro, from a stem base of Lycopersicon esculentum, 1983, R. Navarro, holotype CBS H-3926, ex-holotype culture CBS 713.85 = ATCC 76027 = PD 83/826; Guachacal, from a leaf of Solanum tuberosum, Nov. 1985, W.M. Loerakker, CBS 771.85 = IMI 386091 = PD 85/772.
Stagonosporopsis cucurbitacearum (Fr.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515660.
Basionym: Sphaeria cucurbitacearum Fr., Syst. Mycol. 2(2): 502. 1823.
≡ Phoma cucurbitacearum (Fr.) Sacc., Syll. Fung. 3: 148. 1884.
= Sphaeria bryoniae Fuck., Jahrb. Nassauischen Vereins Naturk. 23–24: 112. 1870.
≡ Didymella bryoniae (Fuckel) Rehm, Ber. Naturhist. Vereins Augsburg 26: 27. 1881.
Specimens examined: New Zealand, from Cucumis sp., 1979, CBS 133.96= PD 79/127. The Netherlands, Horst, from Cucumis sp., 1991, J. de Gruyter, CBS 109171 = PD 91/310.
Note: Strain CBS 133.96 could not be identified morphologically, as it proved to be sterile.
Stagonosporopsis dennisii Boerema, Gruyter & Noordel., Persoonia 16(3): 350. 1997.
= Phoma dennisii Boerema, Trans. Brit. Mycol. Soc. 67(2): 307. 1976.
Specimens examined: Canada, Ontario, from a stem of Solidago canadensis, 1995, G.P. White, CBS 135.96 = IMI 19337 = PD 94/4756. The Netherlands, Arnhem, from a stem of Solidago floribunda, 1968, CBS 631.68 = PD 68/147.
Stagonosporopsis dorenboschii (Noordel. & Gruyter) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515661.
Basionym: Phoma dorenboschii Noordel. & Gruyter, Persoonia 15(1): 83. 1992.
Specimens examined: The Netherlands, Rijnsburg, from Physostegia virginiana, 1986, D. Kruger, holotype L 988.202.121, isotype CBS H-7604, ex-holotype culture CBS 426. 90 = IMI 386093 = PD 86/551; from Physostegia virginiana, 1986, CBS 320.90 = PD 86/932.
Stagonosporopsis heliopsidis (H.C. Greene) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515662.
Basionym: Phyllosticta heliopsidis H.C. Greene, Trans. Wisconsin Acad. Sci. 50: 158. 1961.
≡ Phoma heliopsidis (H.C. Greene), Aa & Boerema apud De Gruyter Boerema & van der Aa, Persoonia 18(1): 40. 2002.
Specimens examined: Canada, Island of Montréal, from Ambrosia artemisiifolia, PD 95/6189 = DAOM 221138. The Netherlands, from Heliopsis patula, 1974, CBS 109182 = PD 74/231.
Stagonosporopsis hortensis (Sacc. & Malbr.) Petr., Ann. Mycol. 19(1/2): 21. 1921.
Basionym: Hendersonia hortensis Sacc. & Malbr., in Saccardo, Michelia 2(8): 629. 1882.
= Phoma subboltshauserii Boerema, Gruyter & Noordel., Persoonia 16(3): 360. 1997.
Specimens examined: The Netherlands, from an unknown substrate, Mar. 1942, N. Hubbeling, CBS 104.42; from Phaseolus vulgaris, 1979, G.H. Boerema, CBS 572.85 = PD 79/269.
Stagonosporopsis ligulicola var. inoxydabilis (Boerema) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515664.
Basionym: Didymella ligulicola var. inoxydabilis Boerema, Stud. Mycol. 32: 10. 1990.
Anamorph: Phoma ligulicola var. inoxydabilis Boerema, Stud. Mycol. 32: 10. 1990.
Specimens examined: The Netherlands, from Chrysanthemum parthenii, 1981, G.H. Boerema, holotype CBS H-7611, culture ex-holotype CBS 425.90 = PD 81/520; from Matricaria sp. 1985, J. de Gruyter, PD 85/259.
Stagonosporopsis ligulicola var. ligulicola (K.F. Baker, Dimock & L.H. Davis) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515663.
Basionym: Mycosphaerella ligulicola K.F. Baker, Dimock & L.H. Davis, Phytopathology 39: 799. 1949.
Anamorph: Phoma ligulicola var. ligulicola Boerema, Stud. Mycol. 32: 9. 1990.
Specimens examined: Germany, Berlin, from Chrysanthemum indicum, 1963, R. Schneider, CBS H-11952, culture CBS 500.63 = MUCL 8090. The Netherlands, near Lisse, from a leaf of Chrysanthemum indicum, 1984, CBS 137.96 = PD 84/75.
Stagonosporopsis loticola (Died.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515665.
Basionym: Phoma loticola Died., Kryptog.-Fl. Mark Brandenburg. 9, Pilze 7(1): 152. 1912.
Specimens examined: New Zealand, Auckland, Mt. Albert, from Lotus pedunculatus, 1981, P.R. Johnston, isotype CBS H-7612, ex-isotype culture CBS 562.81 = PDDCC 6884; Auckland, from the stem of Lotus tenuis, 1979, P.R. Johnston, CBS 628.97 = PD 79/72.
Stagonosporopsis lupini (Boerema & R. Schneid.) Boerema, Gruyter & P. Graaf, Persoonia 17(2): 283. 1999.
Basionym: Ascochyta lupini Boerema & R. Schneid., apud Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 162: 28. 1984.
≡ Phoma schneiderae (Boerema & R. Schneid.) Boerema, Gruyter & P. Graaf, Persoonia 17(2): 282. 1999.
Specimens examined: Peru, Puno, from Lupinus mutabilis, 1980, CBS H-9061, culture CBS 375.84= PD 80/1250. U.K., Cambridgeshire, Mepal, from Lupinus albus, Apr. 1998, P. van de Graaf, holotype L 998.099.105, ex-holotype culture CBS 101494 = PD 98/5247.
Stagonosporopsis oculo-hominis (Punith.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515666.
Basionym: Phoma oculi-hominis Punith., Trans Brit. Mycol. Soc. 67: 142. 1976.
≡ Phoma dennisii var. oculo-hominis (Punith.) Boerema, Gruyter & Noordel., Persoonia 16: 351. 1997.
Specimen examined: U.S.A., Tennessee, Nashville, from a man's corneal ulcer, 23 Apr. 1975, Y.M. Clayton, ex-holotype culture CBS 634.92 = IMI 193307.
For a complete description see Punithalingam (1976) and Boerema et al. (1997).
Notes: Stagonosporopsis oculo-hominis is a species that thus far has been reported only once in a clinical case in Tennessee, U.S.A., when it was isolated from a man's corneal ulcer (Punithalingam 1976). Due to morphological similarities it has been recombined into a variety of Ph. dennisii by Boerema et al. (1997), but the genetical data presented here suggest that this entity should be recognised at species level in Stagonosporopsis. It is distinguishable from S. dennisii by the absence of a diffusible pigment in the agar, and by the absence of a discolouration after application of NaOH to the culture. Further, the septate conidia are significantly smaller than those of S. dennisii: 9–16 × 4.5 μm versus 14.5–24 × 4–7 μm, respectively.
Stagonosporopsis rudbeckiae (Fairm.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515667.
Basionym: Phoma rudbeckiae Fairm., Proc. Rochester Acad. Sci. 1: 51. 1890.
Specimen examined: The Netherlands, from Rudbeckia bicolor, 1979, CBS 109180 = PD 79/175.
Stagonosporopsis trachelii (Allesch.) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515668.
Basionym: Phoma trachelii Allesch., Fungi Bavaria exs. 4: 360. 1897.
= Ascochyta bohemica Kabát & Bubák apud Bubák & Kabát, Hedwigia 44: 361. 1905.
≡ Stagonosporopsis bohemica (Kabát & Bubák) Boerema, Gruyter & Noordel., Persoonia 16(3): 361. 1997.
Specimens examined: Sweden, Svalöv, from Campanula isophylla, 1968, W. Södergren, CBS H-8972, ex-holotype culture 384.68. The Netherlands, from a leaf of Campanula isophylla, 1977, CBS 379.91 = PD 77/675.
Notes: Although this species has been described in Stagonosporopsis before (as S. bohemica, Boerema et al. 1997), this was based on a later homonym, and thus a recombination based on the oldest epithet is proposed here.
Stagonosporopsis valerianellae (Gindrat, Semecnik & Bolay) Aveskamp, Gruyter & Verkley, comb. nov. MycoBank MB515669.
Basionym: Phoma valerianellae Gindrat, Semecnik & Bolay, Revue Hort. Suisse Romande 40: 350. 1967.
Specimens examined: The Netherlands, Wageningen, from Valerianella locusta var. oleracea, 1966, G.H. Boerema, holotype L 965.300.24, isotype CBS H-7631, ex-isotype culture CBS 329.67 = PD 66/302; from Valerianella locusta, 1982, CBS 273.92 = PD 76/1019.
Residual species in the Didymellaceae:
The following Phoma species are embedded in the Didymellaceae, but could not be confidently assigned to one of the groups or new genera in this study due to lack of support for their respective clades. Several of the species listed here belong to this family based on LSU and/or ITS sequence data, but due to missing sequencing data on one of the loci used, these species could not be assigned. These species are provisionally retained under their current holomorph name until further analyses are conducted to place them in the new phylogenetic system.
Didymella macropodii Petr., Hedwigia 68: 219. 1928.
Anamorph: Phoma nigrificans (P. Karst.) Boerema, Loer. & Wittern, J. Phytopathol. 115(3): 270. 1986.
Basionym: Sphaeronaema nigrificans P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 16: 17. 1888. (as “Sphaeronema”).
Specimens examined: Germany, from Brassica napus, 1982, G.H. Boerema, CBS 100190 = PD 82/736. Poland, near Gryfice, from Thlaspi arvense, 1990, J. Marcinkowska, CBS 100191. The Netherlands, from an unidentified crucifer, 1984, G.H. Boerema, PD 84/512.
Didymella rabiei (Kovatsch.) Arx, in Müller & Arx, Beitr. Kryptogamenfl. Schweiz 11(2): 364. (1962).
Basionym: Mycosphaerella rabiei Kovatsch., The blight of chick pea: 70. 1936.
Anamorph: Phoma rabiei (Pass.) Khune ex Gruyter, Persoonia 18(1): 89. 2002.
Basionym: Zythia rabiei Pass., Comment. Soc. Crittog. Ital. 2(3): 437. 1867.
Specimens examined: India, from the seeds of Cicer arietinum, 1965, S. Sinha, CBS 534.65. Syria, from Cicer arietinum, 1981, W. Barz, CBS 581.83A.
Notes: The placement of this teleomorph in either the Didymella or Mycosphaerella has been debated regularly in the past (Müller and Arx 1962, Von Arx 1987, Trapero-Casas & Kaiser 1992, Wilson & Kaiser 1995, De Gruyter 2002, Barve et al. 2003). The most recent emendment was by De Gruyter (2002) who judged in favour of Mycosphaerella rabiei Kovatsch. ex Gruyter. However, as the genus Mycosphaerella is phylogenetically not linked with the Pleosporales (Schoch et al. 2006, 2009b, Crous et al. 2009c), the placement in Didymella appears to be more correct.
Didymella adianticola Aa & Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 159 (Jaarboek 1982): 25. 1983.
Anamorph: Phoma adianticola (E. Young) Boerema, Verslagen Meded. Plziektenk. Dienst Wageningen 159 (Jaarboek 1982): 25. 1983
Basionym: Phyllosticta adianticola E. Young, Mycologia 7: 144. 1915.
Specimens examined: Costa Rica, from a leaf of Polystichum adiantiforme, 1989, J. de Gruyter, CBS 258.92 = PD 89/1887. U.S.A., Florida, from a leaf of Polystichum adiantiforme, 1982, G.H. Boerema, CBS H-16142, culture CBS 187.83 = PD 82/128.
Phoma aliena (Fr.) Aa & Boerema, apud Gruyter, Noordeloos & Boerema, Persoonia 16(4): 486. 1998.
Basionym: Sphaeria aliena Fr., Syst. Mycol. 2(2): 502. 1823.
Specimens examined: The Netherlands, from a twig of Berberis sp., 1982, J. de Gruyter, CBS 379.93 = PD 82/945; near Boskoop, from a twig of Buxus sempervirens, 1994, J. de Gruyter, CBS 877.97 = PD 94/1401.
Phoma aubrietiae (Moesz) Boerema, Gewasbescherming 1(4): 66. 1970.
Basionym: Sclerophomella aubrietiae Moesz, Balkán-Kutat Tud. Eredm. 3: 144. 1926.
Specimens examined: The Netherlands, Bodegraven, from seed of Aubrietia hybrida cv. Superbissima, 1965, G.H. Boerema, CBS H-16154, culture CBS 383.67 = PD 65/223; from a stem of Aubrietia sp., 1970, G.H. Boerema, CBS 627.97 = PD 70/714.
Phoma bulgarica Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515671. Fig. 13.
Fig. 13.
Phoma bulgarica (CBS 357.84). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D. Pycnidia in vivo, isolated from manually infected sterilised stems of Urtica dioica. E. Pycnidial section. F. Pycnidium. G. Crystals. H. Hyphal strand. Scale bars: D, F = 100 μm; E, H = 50 μm; G = 10 μm.
Pycnidia solitaria, subglobosa, elongata vel obpyriformia, glabra, epapillata, brunnea, superficialia vel in agaro immersa, (140–)170–250(–295) μm. Pycnidia fertilia non vidi.
Etymology: Epithet refers to the country of origin, Bulgaria.
Conidiomata pycnidial solitary, subglobose to elongated or obpyriform, glabrous, non-papillate, brown, on the surface and immersed in the agar, measuring (140–)170–250(–295) μm. Pycnidia proved to be sterile. In older cultures pycnidial primordia are formed, which are surrounded by clusters of needle-shaped crystals.
Culture characteristics: Colonies on OA, 45–65 mm diam after 7 d, margin regular. Immersed mycelium hyaline, largely covered by mat of felty to compact whitish grey to lavender grey aerial mycelium; reverse iron-grey, but vinaceous-black where the aerial mycelium is present. Colonies on MEA 40–50 mm diam after 7 d, margin regular. Immersed mycelium mainly hyaline, incidentally black when clustering into thicker hyphal strands. Aerial mycelium sparse, flat, olivaceous green to white near the colonies margin; reverse greenish olivaceous to olivaceous black. Colonies on CHA 70–85 mm diam after 7 d, or even covering the total agar surface, margin regular. Immersed mycelium as on MEA. Aerial mycelium occurring around the colony centre, white, compact to floccose; reverse leaden black. Application of NaOH did not have any effect.
Specimens examined: Bulgaria, Silkossia, Strandga Mountain, from leafs of Trachystemon orientale, 20 June 1980, S. Vanev, holotype designated here CBS H-20242, ex-holotype culture CBS 357.84; from Trachystemon orientale, 1982, CBS 124515 = PD 82/1058.
Notes: Strain PD 82/1058 differed from CBS 357.84 (which is described above) by a significantly different colony pattern on MEA. This strain was characterised by a growth of ca. 20 mm diam. after 7 d, with a strongly lobate margin. White to buff aerial mycelium was present in a few irregular zones, and had a compact to floccose structure. Pycnidial primordial are only produced in culture on MEA after addition of an autoclaved piece of Urtica dioica (stinging nettle).
Phoma calidophila Aveskamp, Gruyter & Verkley, Mycologia 101: 368. 2009.
Specimens examined: Egypt, from desert soil, Feb. 1980, M.I.A. Abdel-Kader, neotype CBS H-20168, ex-neotype culture CBS 448.83. Unknown European origin, from Cucumis sativus, 1984, G.H. Boerema, PD 84/109.
Phoma chenopodiicola Gruyter, Noordel. & Boerema, Persoonia 15(3): 395. 1993.
Specimens examined: Peru, from a stem of Chenopodium quinoa cv. Sajana, 1979, CBS 128.93= PD 79/140; from a stem of Chenopodium quinoa cv. Sajana, 1979, CBS 129.93 = PD 89/803.
Phoma complanata (Tode) Desm., Michelia 2 (7): 337. 1881. Basionym: Sphaeria complanata Tode, Fungi Mecklenburg. Sel. (Lüneburg) 2: 22. 1791.
Specimens examined: The Netherlands, Tilburg, from a stem of Heracleum sphondylium, Nov. 1997, H.A. van der Aa, CBS H-16194, culture CBS 100311; from a stem of Angelica sylvestris, 1974, G.H. Boerema, CBS 268.92 = PD 75/3.
Phoma crystallifera Gruyter, Noordel. & Boerema, Persoonia 15(3): 393. 1993.
Specimen examined: Austria, Kärnten, Wallenberg near Völkermarkt, from Chamaespartium sagittale, Apr. 1982, H.A. van der Aa, holotype L 992.177-456, ex-holotype culture CBS 193.82.
Phoma dactylidis Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515671. Fig. 14.
Fig. 14.
Phoma dactylidis (CBS 124513). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D-E. Pycnidia. F. Section of the pycnidial wall. G. Conidia. Scale bars: D–E = 100 μm; F–G = 5 μm.
Conidia dimorpha, intra idem pycnidium formata. Conidia typus 1 ellipsoidea vel ovoidea, interdum leniter allantoidea, glabra, hyalina, continua, 4.5–9(–9) × (2–)2.5–3.5 μm, (2–)3–6(–8) guttulis praedita. Conidia typus 2 cylindrica vel ellipsoidea, glabra, hyalina, saepe uniseptata, (9–)9.5–13.5(–14.5) × (2.5–)3.5–4.5 μm, interdum septata et guttulis (2–)4–8(–15) in quoque cellula. Matrix conidiorum salmonea.
Etymology: Named after the associated plant host genus, Dactylis sp.
Conidiomata pycnidial, solitary or confluous, produced on the agar surface, (sub-)globose, with some hyphal outgrows, (115–)135–230(–250) × (75–)95–195(–105) μm. Ostioles 1–4(–5), papillate. Pycnidial wall pseudoparenchymatous, composed of isodiametric cells, 4–8 cell layers, outer 2–4 cell layers pigmented, 10–27 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped, ca. 4.5–6.5 × 3–5 μm. Conidia dimorphic, both originating from the same pycnidia. Conidia of type 1: ellipsoidal to ovoid, sometimes somewhat allantoid, thin-walled, smooth, hyaline, aseptate 4.5–9(–9) × (2–)2.5–3.5 μm, with (2–)3–6(–8) guttules. Regularly also large conidia occur: cylindrical to ellipsoidal, thin-walled, smooth, hyaline, often uniseptate (9–)9.5–13.5(–14.5) × (2.5–)3.5–4.5 μm, but sometimes septate and septate somewhat constricted at the septum, with (2–)4–8(–15) guttules per cell. Conidial matrix salmon.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular. Immersed mycelium hyaline, but some greenish black zones may occur, with tufts of with aerial mycelium. Abundant greenish black pycnidia are scattered over the medium, which are salmon coloured near the colony margin; reverse concolourous. Colonies on MEA 45–50 mm diam after 7 d, margin regular. Immersed mycelium completely covered by a felty greyish white aerial mycelium; reverse grey-olivaceous, becoming brown-olivaceous near the colony margin. Colonies on CHA similar as on MEA, but somewhat faster growing, 55–60 mm diam. after 7 d; reverse completely black. Application of NaOH results in a slight greenish discolouration of the agar, best to be observed on OA medium.
Specimen examined: U.S.A., Oregon, on Dactylis glomerata, 1973, holotype designated here CBS H-20237, ex-holotype culture CBS 124513 = PD 73/1414.
Notes: Phoma dactylidis has thus far only been isolated once from the leaves of Dactylis glomerata in Oregon, U.S.A.. Other Phoma pathogens of Dactylis include Ph. paspali and Ph. pratorum, which both occur in New Zealand, but are relatively distantly related to Ph. dactylidis. Additionally, two related taxa have been found on this host, viz. the novel variety Boeremia exigua var. gilvescens and Epicoccum nigrum (Punithalingam et al. 1972). The clustering of this species suggests ecological or morphological similarities with Ph. rhei (BPP = 1.00; RBS = 100 %).
Phoma destructiva var. destructiva Plowr., Gard. Chron. II 16: 621. 1881.
Specimens examined: Guadeloupe, from fruit of Lycopersicon esculentum, 1987, CBS 133.93 = PD 88/961 = IMI 173142. Tonga, Friendly Islands, from decaying fruit of Lycopersicon esculentum, 1967, G.F. Laundon, CBS H-16200, culture CBS 378.73 = CECT 2877.
Phoma destructiva var. diversispora Gruyter & Boerema, apud De Gruyter, Boerema & Van der Aa, Persoonia 18(1): 28. 2002.
Specimen examined: The Netherlands, Berkel en Rodenrijs, from a leaf of Lycopersicon esculentum, Oct. 1977, G.H. Boerema, holotype CBS H-16199, ex-holotype culture CBS 162.78 = PD 77/725.
Phoma eupyrena Sacc., Michelia 1(5): 525. 1879.
Specimens examined: Germany, Kiel-Kitzeberg, from wheat field soil, 1966, W. Gams, CBS 527.66 = ATCC 22238; The Netherlands, from the tuber of Solanum tuberosum, 1991, J. de Gruyter, CBS 374.91 = PD 78/391.
Phoma herbarum Westend., Bull. Acad. Roy. Sci. Belgique, Cl. Sci. 19(3): 118. 1852.
Specimens examined: Belgium, Herb. Crypt. Belge. Fasc. 20, No. 965, lectotype, on stems of Onobrychis viciifolia, 1854. Sweden, Sofieheim, from wood pulp, Apr. 1937, E. Rennerfelt, CBS 276.37 = MUCL 9920. The Netherlands, Emmeloord, from the stem of Rosa multiflora cv. Cathayensis, Apr. 1965, G.H. Boerema, CBS 615.75 = PD 73/665 = IMI 199779; Naaldwijk, from a stem base of Nerium sp., 1986, J. de Gruyter, CBS 502.91 = PD 82/276; Oirschot, from a twig of Thuja sp., 1987, J. de Gruyter, CBS 503. 91 = PD 87/499. U.K., from paint, Aug. 1936, K.S.G. Cartwright, CBS 109.36. U.S.A., Maryland, Washington area, from the fruit of Malus sylvestris, July 1963, M.A. Smith, CBS 567.63 = ATCC 15053 = MUCL 9889.
Phoma herbicola Wehm., Mycologia 38: 319. 1946.
Specimen examined: U.S.A., Montana, Missoula, head of Seeley Lake, from water, CBS H-16581, culture CBS 629.97 = PD 76/1017.
Phoma huancayensis Turkenst., Fitopatologia 13: 68. 1978.
Specimens examined: Peru, Dep. Junin, Huancayo, near Vallis Mantaro, from a stem of Solanum sp., Feb. 1974, L.J. Turkensteen, isotype CBS H-7609, ex-isotype culture CBS 105.80 = PD 75/908; from Chenopodium quinoa, 1977, CBS 390.93 = PD 77/1173.
Phoma longicolla Aveskamp, Gruyter & Verkley, sp. nov. MycoBank MB515672. Fig. 15.
Fig. 15.
Phoma longicolla (CBS 124514). A–C. Fourteen-day-old colonies on OA (A), MEA (B) and CHA (C). D–F. Pycnidia. G. Section of the pycnidial wall. H. Conidia. Scale bars: E–G = 100 μm; H = 50 μm; I–J = 10 μm.
Conidia late ellipsoidea vel ovoidea, glabra, hyalina, continua, 6–8.5(–10) × (3.5–)4–5(–5.5) μm, (2–)3–9(–12) guttulis polaris praedita. Matrix conidiorum cremeo-alba.
Etymology: Refers to the elongated necks of the ostioles.
Conidiomata pycnidial, initially solitary, globose, glabrous, slightly papillate and olivaceous buff, produced on the agar surface, measuring (45–)50–115(–130) μm diam. Later developing to black broadly globose to irregular conidiomata with many white hyphal outgrows and with a clear elongated neck around the ostioles, giving it a irregular shape, measuring (170–)200–270(–285) × (115–)125–205(–220) μm. Ostioles 1–3(–4), on a long elongated neck (up to 200 μm long). Often these pycnidia merge to an irregular mass of confluent conidiomata. Pycnidial wall pseudoparenchymatous, composed of isodiametric cells, 5–7 layers, 17–22 μm thick. Conidiogenous cells phialidic, hyaline, simple, smooth, flask-shaped, ca. 4–5 × 3–5 μm. Conidia broadly ellipsoidal to ovoid, thin-walled, smooth, hyaline, aseptate 6–8.5(–10) × (3.5–)4–5(–5.5) μm, with (2–)3–9(–12) polar guttules. Conidial matrix crème-white.
Culture characteristics: Colonies on OA 50–55 mm diam. after 7 d, margin regular. Immersed mycelium hyaline with abundant pycnidia, in some sectors covered by a low mat of felty to floccose mouse grey aerial mycelium, with tufts of white mycelium near the colonies margin. In the sectors with aerial mycelium, pycnidia are only sparsely present; reverse hyaline, but leaden black and olivaceous grey where the aerial mycelium is present. Colony on MEA 50–55 mm diam. after 7 d, margin regular. Immersed mycelium completely covered by a floccose crème mat of white aerial mycelium; reverse greenish olivaceous to olivaceous-black. Colony on CHA 55–60 mm diam. after 7 d, margin regular. Immersed mycelium brown vinaceous to black. Aerial mycelium is occurring in sectors, felty, pale grey to white; reverse black with incidentally a pale purplish grey zone. Application of NaOH did not have any effect.
Specimens examined: Spain, Canary Isles, from Opuntia sp., 1980, J. de Gruyter, holotype designated here CBS H-20238, ex-holotype culture CBS 124514 = PD 80/1189; Canary Isles, Gran Canaria, from Opuntia sp., June 1982, H.A. van der Aa, CBS 347.82.
Notes: This species was isolated twice from Opuntia on the Canary Isles. Around the time of the second isolation (CBS 347.82), also Ph. dimorpha sp. nov. was isolated from the same location and host substrate. This species is described above. A third species that is found in association with Opuntia is Ph. opuntiae, which is, however, rather distinct in morphology and phylogeny.
Phoma medicaginis var. macrospora Boerema, R. Pieters & Hamers, Netherlands J. Pl. Pathol. 99(Suppl. 1): 19. 1993.
Specimens examined: Canada, Saskatchewan, Saskatoon, from seed of Medicago sativa, 1965, H.W. Mead, CBS 404.65 = IMI 116999. U.S.A., Minnesota, from Medicago sativa, Sep. 1953, M.F. Kernkamp, holotype CBS H-16487, ex-holotype culture CBS 112.53.
Phoma medicaginis var. medicaginis Malbr. & Roum. apud Roumeguère, Fungi Selecti Galliaei Exs. 37: 3675. 1886.
Specimens examined: Czech Republic, from Medicago sativa, CBS 316.90 = CCM F-187. Italy, Perugia, from a leaf of Medicago sativa, 1963, M. Ribaldi, CBS H-16483, culture CBS 479.63. The Netherlands, from a leaf of Medicago sativa, 1966, M.M.J. Dorenbosch, CBS 533.66 = ATCC 16929 = PD 66/370. Turkey, Ankara, from Medicago sativa, 1942, S. Kuntay, CBS 107.42. U.S.A., Minnesota, from Medicago sativa, Sep. 1953, M.F. Kernkamp, CBS 110.53; Minnesota, from Medicago sativa, Sep. 1953, M.F. Kernkamp, CBS 111.53.
Phoma microchlamydospora Aveskamp & Verkley, Mycologia 101: 374. 2009.
Specimens examined: U.K., from an unknown vegetable plant, 1990, D. Hyall, CBS 491.90; from leaves of Eucalyptus sp., 1994, A.M. Ainsworth, holotype CBS H-20147, ex-holotype culture CBS 105.95.
Phoma nebulosa (Pers.) Berk., Outl. Brit. Fung. (London): 314. 1860.
Basionym: Sphaeria nebulosa Pers., Observ. Mycol. 2: 69. 1799.
Specimens examined: Austria, Kaprun, from a stem of Urtica dioica, Jan. 1975, G.H. Boerema, CBS H-16510, culture CBS 503.75= ATCC 32163 = DSM 63391 = IMI 194766 = PD 75/4. The Netherlands, from a stem of Mercurialis perennis, 1983, CBS 117.93 = PD 83/90.
Phoma negriana Thüm., Die Pilze des Weinstockes, Vienna: 185. 1878. Originally described as “Ph. negrianum”.
Specimens examined: Germany, Oberdollendorf am Rhein, from Vitis vinifera, July 1969, L. Kiewnik, CBS H-16511, culture CBS 358.71. The Netherlands, from Vitis vinifera, 1979, PD 79/74; from Vitis vinifera, 1979, PD 79/75; from Vitis vinifera, 1979, PD 79/76.
Phoma nigripycnidia Boerema, Gruyter & Noordel., Persoonia 16(3): 356. 1997.
Specimen examined: Russia, from a leaf of Vicia cracca, 1969, M. Ondrej, holotype L 992.163.150, ex-holotype culture CBS 116.96 = CCMF 243 = PD 95/7930.
Phoma omnivirens Aveskamp, Verkley & Gruyter, Mycologia 101: 375. 2009.
Specimens examined: Belgium, Gembloux, from Phaseolus vulgaris, 1968, L. Obando, holotype CBS H-20151, ex-holotype culture CBS 341.86. India, Japalbur, from an unknown substrate, 1977, D.P. Tiwari, CBS 654.77. Papua New Guinea, Varirata National Park, from soil, Aug. 1995, A. Aptroot, CBS 991.95. Varirata National Park. From soil, Aug. 1995, A. Aptroot, CBS 992.95. Tanzania, from Statice sp., 1990, J. de Gruyter, CBS 123397 = PD 90/1555. The Netherlands, from Chrysanthemum indicum, 1981, J. de Gruyter, CBS 123396 = PD 81/122.
Phoma putaminum Speg., Atti Soc. Crittog. Ital. 3: 66. 1881.
Specimens examined: The Netherlands, from a branch of Ulmus sp., 1975, G.H. Boerema, CBS 372.91 = PD 75/960. Denmark, from the rhizosphere of Malus sylvestris, Mar. 1968, E. Sønderhousen, CBS 130.69 = CECT 20054 = IMI 331916.
Phoma rhei (Ellis & Everh.) Aa & Boerema apud De Gruyter, Boerema & Van der Aa, Persoonia 18 (1): 42. 2002.
Basionym: Ascochyta rhei Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 1893: 160. 1893.
Specimen examined: New Zealand, from a leaf of Rheum rhaponticum, CBS 109177 = LEV 15165 = PD 2000/9941.
Phoma selaginellicola Gruyter, Noordel., Aa & Boerema, Persoonia 15(3): 399. 1993.
Specimen examined: The Netherlands, from a leaf of Selaginella sp., 1977, G.H. Boerema, CBS 122.93 = PD 77/1049.
Phoma versabilis Boerema, Loer. & Hamers, Persoonia 16(2): 154. 1996.
Specimens examined: Germany, Westfalen, Oberdresselendorf, from stems of Cardamine impatiens, Oct. 1925, A. Ludwig, holotype L 995.229.369. The Netherlands, Wageningen, from a stem of Silene sp., 1982, G.H. Boerema, CBS 876. 97 = PD 82/1008; from Stellaria media, 2000, J. de Gruyter, PD 2000/1379.
DISCUSSION
What is Phoma?
According to the generic concept which is applied today, species of Phoma are relatively simple coelomycetes that are characterised by the in vitro production of mainly unicellular, hyaline conidia from monophialidic, doliiform to flask-shaped conidiogenous cells in pycnidial conidiomata (Boerema & Bollen 1975).
Many species that currently are accommodated in sections Paraphoma, Pilosa and Plenodomus are phylogenetically basal to the Didymellaceae, in which most other Phoma taxa, including the type species are accommodated. These results support the work of Reddy et al. (1998), who advocated that the genus Plenodomus should be reinstalled as a separate genus. Torres et al. (2005b) subsequently made a novel description in this genus, Pl. morganjonesii. A paper by De Gruyter et al. is in preparation, in which all species of Phoma section Plenodomus recognised by Boerema et al. (1994, 1996) and Boerema & De Gruyter (1999), will be taxonomically revised.
However, in the present study, it has become clear that the phylogenetic boundaries between Phoma and several closely related genera that are defined on their conidial characters are ambiguous. Species that produce consistently two-celled hyaline conidia classified therefore traditionally in the genus Ascochyta appear to have evolved independently multiple times during evolution together with typical Phoma taxa, in several lineages of the pleosporalean tree (Fig. 1). Also other conidial characters, such as the pigmentation of spores, as formed by Phoma clematidis-rectae (formerly in Coniothyrium) and Microsphaeropsis olivacea, appear not to be reliable for the delimitation of the genus Phoma. Thus, based on the trees presented in this study, it can be concluded that Phoma, as defined by Saccardo (1880, 1884) and emended by Boerema & Bollen (1975) is highly polyphyletic.
The close relation of Phoma with Ascochyta has often been observed before, as strains of both genera are often highly similar in morphology (Wollenweber & Hochapfel 1937, Brewer & Boerema 1965, Boerema & Bollen 1975, Boerema 1997), physiology (Noordeloos et al. 1993, Faris-Mokaiesh et al. 1995), pathogenicity (Mendes-Perreira et al. 1999, Davidson et al. 2009) and nucleotide sequences (Faris-Mokaiesh et al. 1995, Fatehi et al. 2003, Schoch et al. 2006, Peever et al. 2007, Chilvers et al. 2009, De Gruyter et al. 2009). In the Saccardoan system, both genera were only distinguished by the presence or absence of conidial septa, and by the type of substrate: Ascochyta species were considered to be specific leaf-pathogens, whereas Phoma was solely associated with stem lesions (Boerema & Bollen 1975).
Brewer & Boerema (1965) contrasted the septation process of the conidia in Ascochyta pisi to this process in Phoma exigua. These authors suggested that in Phoma species euseptation occurs only after secession, whereas in Ascochyta the septation of the spores was considered to be an elemental part of conidiogenesis. Later, this was determined to be a genus-specific character (Boerema 1970). Additionally, Boerema and Bollen (1975) stated that both genera are distinct in conidiogenesis. According to these authors, the Ascochyta species produce conidia from either an accumulation of annelations, which give the conidigenous cell an annelidic appearance, or from a gradually thickening collar of periclinal annelations. In contrast, Phoma species produce true phialides with a collarette. This micromorphological difference of the conidiogenesis can only be observed using electron microscopy, as the appearance of a Phoma collarette is highly similar to the periclinal thickening of Ascochyta species. This observation is however not consistent with the conidial ontogeny of Ph. fumosa, which was observed to be annellidic by Sutton & Sandhu (1969).
The application of these characters for the purpose of generic delimitation was heavily questioned (Punithalingam 1979a), and nowadays these characters are hardly applied in the taxonomy of both genera, simply because the use of electron microscopy is expensive and sectioning of pycnidia is too time consuming. Due to this unclear classification system, and to the fact that not all species produce exclusively septate or aseptate conidia, species had synonyms in both genera (Boerema 1972, Boerema & Dorenbosch 1973, Van der Aa et al. 2000, Mel'nik 2000). Even nowadays the status of many species is unclear as Phoma and Ascochyta synonyms are often used simultaneously. Examples are Ph. rabiei and its synonym A. rabiei (Singh & Reddy 1993, Singh et al. 1997, Barve et al. 2003, Chongo et al. 2004, Pande et al. 2005, Hernandez-Bello et al. 2006, Peever et al. 2007), and Ph. gossypiicola and its synonym, A. gossypii (e.g. Shen et al. 2005). The concept of Ph. clematidina has appeared to comprise several taxa belonging in multiple genera, amongst which a Didymella with an unnamed Ascochyta anamorph (Woudenberg et al. 2009).
The results presented in this study further suggest a close relation between Microsphaeropsis and Phoma. Morphological studies of members of both genera (Jones 1976) reveal that conidiogenesis is similar, although the conidia of Microsphaeropsis differ from those of Phoma by the dark pigmentation and the presence of a double-layered cell wall. The pigmentation occurs only after conidial secession. Therefore, young pycnidia with colourless pycnidia may be easily confused with a Phoma species (Boerema et al. 2004).
In general, it can be concluded that Phoma should only be regarded as a general concept, as members sharing this morphology are found throughout the Pleosporales, although most members are found in the Didymellaceae. The type species of Phoma is only distantly related to the other members of this genus, but relatively close to Ascochyta pisi, the type species of the older name Ascochyta. However, based on the results observed in the present study, this genus is poorly elucidated. Therefore, we opt to retain the taxonomy of Phoma as is, with the exception of the groups that can be resolved further, such as Boeremia, Epicoccum, Peyronellaea and Stagonosporopsis.
Taxonomic revisions
The observations presented in the present paper suggest that LSU and SSU data, which contain approximately 270 informative sites in the alignment, are sufficient to distinguish various major groups in the Pleosporales. However, other, more variable loci should also be analysed to determine the phylogenetical basis for the species that are congeneric with the ex-type strain of Phoma. These species were found throughout the pleosporalean phylogeny that was reconstructed in the present paper. Molecular studies on the species that are currently accommodated in the section Plenodomus and Pilosa are in progress (De Gruyter et al. in prep.).
The type species of the genus Phoma, Ph. herbarum, resides in the Didymellaceae clade, a result that is in congruence with the observations of De Gruyter et al. (2009). However, based on the data generated in the present study, also the type species of Ascochyta (A. pisi), Chaetasbolisia (C. erysiphoides), and Microsphaeropis (M. olivaceae) are located in the same group (Fig. 2). Of those species, Phoma carries the oldest name, which was deposited by Fries in 1821, but as Phoma sensu Saccardo (1880) was conserved against Phoma Fries (McNeill et al. 2006), the genus Ascochyta, which was erected in 1830, would be the preferred name for the species in this genus. Nevertheless, because of the impact that recombination of Phoma in Ascochyta would have in phytopathology, we suggest to keep both generic names in use for the unresolved species in the Didymellaceae, disregarding the fact that both names are polyphyletic. Both genera can be regarded as polyphyletic concepts, until a proper study of the teleomorph genera related to the Didymellaceae has been conducted. Also the younger genera Chaetasbolisiaand Microsphaeropsis should be retained as separate taxonomic entities, until at least all taxa are restudied both morphologically and phylogenetically. However, the clades that are resolved, and that are characterised by shared morphological or physiological characters, or have a shared ecological role, are elevated to generic level here. Consequences of this approach are the reinstatement of the genus Peyronellaea Goid., expansion of the formerly monotypic genus Epicoccum Link, emendment of the concept of Stagonosporopsis Died. and the erection of the novel genus Boeremia.
Teleomorph relations
In Phoma several teleomorphs have been recognised, but for the majority of Phoma species the sexual structures have yet to be discovered, as the induction of these structures requires special conditions; or simply because the species has lost its ability to propagate sexually. Boerema et al. (2004) only recognised ca. 40 species that produce teleomorphs.
The finding of multiple teleomorphs with phenotypically indistinguishable associated anamorphs is not uncommon in mycology, yet unwanted, and should be resolved in due course as more data become available. For example, such a situation also applies to major genera such as Aspergillus (Pitt & Samson 2007), Botryosphaeria (Crous et al. 2006), Geotrichum (De Hoog & Smith 2004), Mycosphaerella (Crous et al. 2009a, b) and Penicillium (Pitt 1979).
Boerema et al. (2004) linked Phoma to four teleomorph genera: Didymella, Leptosphaeria, Mycosphaerella and Pleospora. In recent studies it was shown that the association of Phoma with Mycosphaerella was untenable, because the involved teleomorphs were apparently morphologically similar but in fact Didymella. The genus Mycosphaerella is phylogenetically distinct and not even associated with the Pleosporales (Schoch et al. 2006, 2009a, b, Crous et al. 2009a, b), whereas their associated Phoma anamorphs proved to be genetically similar to Didymella (De Gruyter et al. 2009). As a consequence, the pawpaw (Carica papaya) pathogen M. caricae has been recombined into D. caricae in the present study. Also the Didymellaceae clade is not yet completely resolved.
Next to Didymella, also Leptosphaerulina and Macroventuria are accommodated in the Didymellaceae. Macroventuria resembles Venturia (Van der Aa et al. 1971); the ascospore morphology being highly comparable to that of Didymella. In contrast, Leptosphaerulina is distinct in morphology, producing ascospores with longitudinal and transverse septa, more resembling the ascospores of Pleospora and Cucurbitaria (Von Arx 1981). Didymella is a poorly studied genus that is in need of a comprehensive revision, as it plays such a crucial role in the delimitation of phytopathologically important genera. When studied more intensively, this genus may very well be split up into multiple genera that have a proper morphological basis.
Sexual states have thus far only been reported for a limited number of Phoma species. It seems unlikely that the ability to produce sexual reproductive structures is lost in so many species, whilst other, closely related species, or even species that emerge from these “asexual” species, do have a teleomorph state. It may be assumed that the sexual state of these species is cryptic, and can only be induced under the right conditions. These teleomorph structures, that probably much resemble the sexual structures formed by the genus Didymella, are probably the missing links that are required for further taxonomical delineation of the species in the Didymellaceae.
Can the sections be maintained?
The present study was initiated chiefly to clarify the status of Phoma and to judge the validity of the sections introduced by Boerema (1997). Aveskamp et al. (2008) already illustrated the ambiguity of some sections, as multiple characters that are regarded to be section-specific may be present in a single species. For example, Ph. zeae-maydis was regarded as the type species of the section Macrospora, due to the presence of its relatively large aseptate spores (De Gruyter 2002). However, this species also produces multicellular chlamydospores, resembling the chlamydospores formed in species that are accommodated in the section Peyronellaea. The recombination of this species into Pey. zeae-maydis in the present study, which is based on DNA phylogeny, indicates that the spore size is not an informative character at above-species level.
Another example of the ambiguity of the Boeremaean section is Ph. destructiva. Infraspecific taxa of this species are accommodated in two sections: Ph. destructiva var. diversispora was accommodated in section Phyllostictoides, wheras the type variety was linked to section Phoma due to the absence of septate conidia. Boerema et al. (2004) acknowledged this ambiguity problem and were forced to key out several species in multiple sectional dichotomous keys. In the previous study of De Gruyter et al. (2009) this ambiguity could not be illustrated as only sectional representatives were included. Here it is illustrated that, although some sections can be partially maintained, most of the sections are not supported from an evolutionary perspective.
Section Heterospora
The majority of the species that were ascribed to Phoma section Heterospora is recovered in Group R, from which the species are all recombined into the genus Stagonosporopsis in the present paper. The type species of section Heterospora however, Ph. heteromorphospora, is recovered basal to the Didymellaceae together with Ph. dimorphospora. Also Ph. samarorum is not retrieved in the main Phoma clade, but is associated with the Phaeosphaeriaceae.
Also within the Didymellaceae, the Heterospora section appears to be polyphyletic as Ph. aquilegiicola, Ph. glaucii and Ph. clematidina are distantly related to most other Heterospora species and form a distinct clade together with another Clematidinapathogen, Ph. clematidis-rectae, a species that has been regularly confused with the Phoma clematidina complex (Woudenberg et al. 2009). The species in this clade can be distinguished from the main body of the Heterospora species as they lack the production of large Stagonospora-type conidia in culture, although smaller, septate conidia may occur.
Section Macrospora
The five large-spored species of the section Macrospora included in this study are found scattered throughout the Didymellaceae, indicating that spore size is not a good taxonomic criterion for delimiting taxa above species level. Phoma zeae-maydis is genetically similar to most Peyronellaea species. This association is supported by the finding of dictyochlamydospores in most species in this clade (Aveskamp et al. 2009a).
Section Paraphoma
Also Phoma section Paraphoma (Van der Aa et al. 1990) appears to be polyphyletic. The section comprises 12 taxa that produce pycnidial conidiomata with setae (De Gruyter & Boerema 2002). Members of this section are found in clades 5, 6, and 8 of Fig. 1. Phoma gardenia is the only setae-producing species known in the Didymellaceae. Because of its ability to produce dictyochlamydospores, and based on the DNA phylogeny presented in Fig. 2, it is recombined into the genus Peyronellaea here.
The type species for the former section Paraphoma is Ph. radicina, which is accommodated in the Phaeosphaeriaceae group (clade 6). Remarkably, no other species that were ascribed to the section Paraphoma are found in the same family. Instead, Ph. chrysanthemicola (formerly ascribed to the section Peyronellaea) is found in close association with Ph. radicina. Both species are recognised as soil fungi and have a wide distribution with records from Europe, North-America and Asia (Boerema et al. 2004). The close association between Ph. samarorum, Ph. chrysanthemicola and Ph. radicina has been recorded before in a phylogenetical reconstruction of the section Peyronellaea in a study of Aveskamp et al. 2009a. The resolution of the clade in that study was, however, higher as the complete ITS regions 1 and 2 were applied in genetic analyses (Aveskamp et al. 2009a). Further linkage of the morphological and ecological characters to the phylogeny will be one of the main challenges for taxonomists working on the species in this group.
A third Paraphoma species, Ph. terricola, is recovered in clade 5 of Fig. 1, which resembles the Cucurbitaceae. This family also hosts the setae-lacking species Ph. pratorum, which was classified in section Phoma. Several other coelomycete fungi are accommodated here as well, including Phialophorophoma litoralis, Pleurophoma cava, a sterile strain that once has been identified as Coniothyrium sp. and various Pyrenochaeta species. The close morphological relation between the genera Pyrenochaeta, Pleurophoma and Phoma section Paraphoma was already noted by Boerema et al. (1996) and Grondona et al. (1997). Like Phialophorophoma litoralis and Pleurophoma cava, Pyrenochaeta is characterised by the formation of elongated, filiform, multiseptate conidiophores, a character that is however not found in the various Phoma species embedded in this clade (De Gruyter et al. 2009). A further delineation of the species associated with the genera Pyrenochaeta and Pleurophoma and the Phoma section Paraphoma will be provided in a follow-up paper by De Gruyter et al. (2010).
Section Peyronellaea
The chlamydospore-producing species have been treated before by Aveskamp et al. (2009a), who revealed that also Phoma section Peyronellaea is artificial from an evolutionary point of view. Most species, including the type Ph. glomerata, cluster in group K of Fig. 2, along with many other (uni- and multicellular) chlamydospore producing species. To be in accordance with the phylogenetic results, this cluster is elevated to generic level, which is named after the section Peyronellaea. A second group of species belonging to this section is recovered in clade L, which groups species that produce botryoid or epicoccoid dictyochlamydospores, including Epicoccum nigrum. Two species, Ph. pimprina and Ph. sorghina are recombined into Epicoccum here. Species that produce pseudoscleroid chlamydospores, such as Ph. violicicola and Ph. chrysanthemicola were found to cluster outside the Didymellaceae.
Section Phoma
Species ascribed to Phoma section Phoma are retrieved in practically all clades of the trees produced in the present study. This supports the general idea that this section has been used as a “waste-bin” for phomoid taxa that could not be placed in other sections or genera due to the lack or presence of typical sectional characters.
The type species of this section, and also of the genus as a whole, is Ph. herbarum (Boerema 1964). The reference strains of this species are accommodated amongst the basal polytomous species of the Didymellaceae. This suggests that it has branched off from most other members of this family in an early phase of the development of the Didymellaceae and probably evolved further without recombining with other taxa.
Although the description of Ph. crinicola is highly similar to that of other species in the B. exigua clade presented in Fig. 2, it has never been recognised as such due to the absence of septate conidia. Nevertheless, the remaining characters do not contradict with the description given for Ph. exigua (Van der Aa et al. 2000). The pycnidia of Ph. crinicola usually carry a single ostiole, but pycnidia are regularly observed lacking an apparent ostiole. This may correspond with the ostiolar openings of many species found within the exigua clade, which are often lined or filled with papillate, hyaline cells.
Similar findings are Ph. aurea and Ph. nigricans in clade K, which is mainly filled with chlamydospore-forming species that were previously associated with the section Peyronellaea. Both species were originally described from New Zealand (Johnston & Boerema 1981, De Gruyter et al. 1993), but may be commonly present on the whole Australasian continent (De Gruyter et al. 1993, 1998). Two other species, belonging to section Phoma, but found in this clade are Ph. anserina and Ph. eucalyptica. Both species produce swollen cells in older cultures (De Gruyter & Noordeloos 1992), which may be an initial phase of chlamydospore formation.
Fifteen species are phylogenetically only distantly related to the Didymellaceae, and should therefore be excluded from the genus. These species include the current Ph. apiicola, Ph. capitulum, Ph. fallens, Ph. fimeti, Ph. flavescens, Ph. flavigena, Ph. glaucispora, Ph. haematocycla, Ph. lini, Ph. minutispora, Ph. multipora, Ph. opuntiae, Ph. pratorum, Ph. valerianae, and Ph. vasinfecta. The problem in recombining these species is, however, the absence of characters that could link these taxa to a specific genus. No teleomorphs are known in this group.
Section Phyllostictoides
All taxa belonging to Phoma section Phyllostictoides are retrieved in the Didymellaceae clade of Fig. 1 (Clade 8). This is remarkable as this large section has been regarded, just like section Phoma, to be a repository for all species that could not be accommodated elsewhere. Nevertheless, within the Didymellaceae this section falls apart as species occur in many distinct clades.
The major body of the Phyllostictoides species is retrieved in group N, in which all Ph. exigua-related species and varieties are found (Aveskamp et al. 2009b), as well as Ph. crinicola and Ph. hedericola, which were associated with Phoma section Phoma. A second group in which many Phyllostictoides taxa cluster is clade R. This clade comprises many species of the former section Heterospora, and several species that were excluded from this section and transferred to Phyllostictoides by Boerema et al. (1997), such as Ph. cucurbitacearum and Ph. ligulicola.
Section Pilosa
Only one of both members of the section Pilosa was included in the present study. The type of this section, Ph. betae, produces a teleomorph in Pleospora, a genus that is typified by Pl. herbarum. Both species are related and are found in the Pleosporaceae and Leptosphaeriaceae clade, although the genetic distance between these species is significant. This finding illustrates the difficulties that are experienced when delineating the Pleosporaceae (Dong et al. 1998).
Section Plenodomus
Thus far the only section created by Boerema that still may be monophyletic is the section Plenodomus, of which all the members are found in the Leptosphaeriaceae. However, some species associated with other sections, such as Ph. apiicola, Ph. valerianae, Ph. vasinfecta (section Phoma) and Ph. violicola (section Peyronellaea) are also linked to this clade and are found to be closely related to the Plenodomus species. The section Plenodomus is associated with a Leptosphaeria teleomorph, but for the aberrant Phoma states found in this clade, no teleomorphs are known. Boerema et al. (2004) mentioned five Leptosphaeria species that produce Phoma anamorphs, but that do not fit within the Plenodomus concept. These species, including L. sacchari, L. haematitis, L. libanotis, L. purpurea and L. weimeri were however not to our disposal, and were therefore not studied. Apparently the genus Leptosphaeria produces multiple anamorphs.
Most taxonomic studies on the Leptosphaeriaceae reveal a monophyletic group, although in these studies, only a limited number of species, belonging to either Leptosphaeria or Phoma section Plenodomus, have been included (Morales et al. 1995, Reddy et al. 1998, Torres et al. 2005b). Other studies indicate that this genus is paraphyletic (Dong et al. 1998, Câmara et al. 2002). Due to the inclusion of only two Leptosphaeria species in the present study, it cannot be unambiguously stated whether this section is mono- or paraphyletic.
Both species included, L. maculans and L. biglobosa, are assumed to represent a heterogeneous assemblage of cryptic taxa (Howlett et al. 2001, Mendes-Pereira et al. 2003, Barrins et al. 2004, Voigt et al. 2005). Although many recombinations have been made in the past, this has obscured a proper understanding of Phoma section Plenodomus and Leptosphaeria (Boerema et al. 1996). Due to the complexity of this group, we will attempt to resolve its phylogeny in a separate paper (De Gruyter et al. prep.).
Section Sclerophomella
The thickened, sclerotisised pycnidial wall, and the formation of poroid pycnidial openings instead of an ostiole, are the main characters of Phoma section Sclerophomella. These characters appear not to reflect the evolutionary history of the genus. Only in group O, a cluster of species is retrieved that is known for their ostiole absence, although not in all species the thickened pycnidial wall is observed. Most other species belonging to section Sclerophomella appear to be unrelated as they have emerged from non-Sclerophomella multiple times during evolution. Therefore these species are found scattered throughout the phylogeny of the Pleosporales. The type species of this section is Ph. complanata, which is found in the basal polytomy of the Didymellaceae.
Many of the morphological characters that were used by Boerema et al. (1997) to create an infrageneric subdivision of Phoma, appear not to be evolutionary informative when compared to sequence data. The main characters that were applied to distinguish sections, like the thickness of the pycnidial walls, chlamydospore structure and presence of Stagonosporopsis synanamorphs are only of limited value. Several characters, such as percentage of septated spores may be genetically driven, but are certainly also highly influenced by the growth media and culturing conditions (Rai 2000). This has led to much confusion surrounding the taxonomic placement of many species in either Ascochyta or Phoma, such as A. rabiei (e.g. Barve et al. 2003, Pande et al. 2005, Peever et al. 2007) vs. Ph. rabiei (e.g. Singh & Reddy 1993, Singh et al. 1997, De Gruyter 2002).
In short, the Boeremaean sectional subdivision is hardly of any evolutionary relevance, suggesting that future classification of taxonomic novelties into these sections should be avoided. Nevertheless, the morphological identification system that was developed based on this subdivision (Boerema et al. 2004) is still applicable, as this system can be still aid in morphological species recognition.
DNA Barcoding
A further aim of this study was the development of species-specific DNA barcodes for species of Phoma. The preferred DNA barcode region for Fungi is ITS (Druzhininia et al. 2005, Summerbell et al. 2005, Seifert 2008, 2009). Cytochrome Oxidase I (COI) was for a long time considered to be a good candidate gene for barcoding fungi (Seifert et al. 2007, Nguyen & Seifert 2008), although some recent studies indicate the variation between copies within a single strain (Geiser et al. 2007, Gilmore et al. 2009). Also Aveskamp et al. (2009b) found that the COI locus was not robust, and thus far, COI barcodes have only been applied in an oligonucleotide array identification system for Penicillium spp. (Chen et al. 2009). The value of ITS as primary barcode region is, however, not sufficient to delineate all taxa. Especially amongst the species clustered in clade N, which represents the species that are associated to the Ph. exigua species complex, ITS is not sufficient to distinguish the various species. This finding is in congruence with results obtained in previous studies, in which the ITS region has been applied in an attempt to distinguish the species within the Ph. exigua complex but without success (MacDonald et al. 2000, Abeln et al. 2002, Cullen et al. 2006). Nevertheless, the other taxa included in this study have been found on long-branched clades, which are mainly due to the variation in TUB and ITS sequences. Another locus that is considered to be helpful for developing DNA barcodes, and which can distinguish many more taxa in the Ph. exigua complex is the Actin gene (Aveskamp et al. 2009b), which is sequenced with a primer combination developed by Carbone & Kohn (1999). This locus has, however, not been included in the present study, as infraspecific genetic variation, even within the Didymellaceae, was too high to align the obtained sequences. Also Calmodulin and Translation Elongation Factor 1-α loci have been tested, but none of the primers combinations used (Carbone & Kohn 1999) could guarantee successful amplification of all strains.
Observations and results presented here represent only a preliminary step towards resolving questions related to the taxonomy of the genus Phoma. With the numerous species awaiting to be discovered, the taxonomic system of this complex will probably be changed again as more clades are added. Nevertheless, it is hoped that the present study on Phoma systematics, together with the “Phoma Identification Manual”, will provide a solid foundation on which the Didymellaceae in general, and the Phoma species in particular, can be further delineated.
Acknowledgments
We thank Mrs Karin Rosendahl-Peters (Plantenziektenkundige Dienst), Dr Amy Rossman (Systematic Botany and Mycology Laboratory), Prof dr dr hc mult Wolfgang E. Krumbein and Dr Gorbushina (University of Oldenburg) for providing cultures. Jeroen Korving (Hubrecht Laboratory, Utrecht) is thanked for his help in preparing the microtome sections. Dr Cecile Gueidan is kindly thanked for her comments on Phoma paspali. Many thanks also to Mrs Trix Merckx and Mrs Arien van Iperen who helped us with the deposit of strains and herbarium material. Mrs Marjan Vermaas is kindly thanked for her assistance in preparing the photoplates. This research is supported by the Dutch Ministry of Agriculture, Nature and Food Quality through an endowment of the FES programme “Versterking infrastructuur Plantgezondheid”.
Taxonomic novelties: New genus: Boeremia Aveskamp, Gruyter & Verkley. New species: Phoma brasiliensis Aveskamp, Gruyter & Verkley, Ph. bulgarica Aveskamp, Gruyter & Verkley, Ph. dactylidis Aveskamp, Gruyter & Verkley, Ph. dimorpha Aveskamp, Gruyter & Verkley, Ph. longicolla Aveskamp, Gruyter & Verkley, Ph. minor Aveskamp, Gruyter & Verkley, Ph. pedeiae Aveskamp, Gruyter & Verkley, Ph. saxea Aveskamp, Gruyter & Verkley. New varieties: Boeremia exigua var. gilvescens Aveskamp, Gruyter & Verkley, B. exigua var. pseudolilacis Aveskamp, Gruyter & Verkley. New combinations: Boeremia crinicola (Siemasko) Aveskamp, Gruyter & Verkley, B. diversispora (Bubák) Aveskamp, Gruyter & Verkley, B. exigua var. exigua (Desm.) Aveskamp, Gruyter & Verkley, B. exigua var. heteromorpha (Schulzer & Sacc.) Aveskamp, Gruyter & Verkley, B. exigua var. lilacis (Sacc.) Aveskamp, Gruyter & Verkley, B. exigua var. linicola (Naumov & Vassiljevsky) Aveskamp, Gruyter & Verkley, B. exigua var. populi (Gruyter & Scheer) Aveskamp, Gruyter & Verkley, B. exigua var. coffeae (Henn.) Aveskamp, Gruyter & Verkley, B. exigua var. viburni (Roum. ex. Sacc.) Aveskamp, Gruyter & Verkley, B. foveata (Foister) Aveskamp, Gruyter & Verkley, B. lycopersici (Cooke) Aveskamp, Gruyter & Verkley, B. noackiana (Allesch.) Aveskamp, Gruyter & Verkley, B. sambuci-nigrae (Sacc.) Aveskamp, Gruyter & Verkley, B. strasseri (Moesz) Aveskamp, Gruyter & Verkley, B. telephii (Vestergr.) Aveskamp, Gruyter & Verkley, Epicoccum pimprinum (P.N. Mathur, S.K. Menon & Thirum.) Aveskamp, Gruyter & Verkley, E. sorghi (Sacc.) Aveskamp, Gruyter & Verkley, Peyronellaea americana (Morgan-Jones & J.F. White) Aveskamp, Gruyter & Verkley, Pey. alectorolophi (Rehm.) Aveskamp, Gruyter & Verkley, Pey. anserina (Marchal) Aveskamp, Gruyter & Verkley, Pey. arachidicola (Khokhr.) Aveskamp, Gruyter & Verkley, Pey. aurea (Gruyter, Noordel. & Boerema) Aveskamp, Gruyter & Verkley, Pey. calorpreferens (Boerema, Gruyter & Noordel.) Aveskamp, Gruyter & Verkley, Pey. coffeae-arabicae (Aveskamp, Verkley & Gruyter) Aveskamp, Gruyter & Verkley, Pey. curtisii (Berk.) Aveskamp, Gruyter & Verkley, Pey. eucalyptica (Sacc.) Aveskamp, Gruyter & Verkley, Pey. gardeniae (S. Chandra & Tandon) Aveskamp, Gruyter & Verkley, Pey. lethalis (Ellis & Bartholomew) Aveskamp, Gruyter & Verkley, Pey. pomorum var. pomorum (Thüm.) Aveskamp, Gruyter & Verkley, Pey. pomorum var. circinata (Kusnezowa) Aveskamp, Gruyter & Verkley, Pey. pomorum var. cyanea (Jooste & Papendorf) Aveskamp, Gruyter & Verkley, Pey. obtusa (Fuckel) Aveskamp, Gruyter & Verkley, Pey. pinodella (L.K. Jones) Aveskamp, Gruyter & Verkley, Pey. pinodes (Berk. & A. Bloxam) Aveskamp, Gruyter & Verkley, Pey. protuberans (Lév.) Aveskamp, Gruyter & Verkley, Pey. sancta (Aveskamp, Gruyter & Verkley) Aveskamp, Gruyter & Verkley, Pey. subglomerata (Boerema, Gruyter & Noordel.) Aveskamp, Gruyter & Verkley, Pey. zeae-maydis (Arny & R.R. Nelson)Aveskamp, Gruyter & Verkley, Phoma clematidis-rectae (Petr.) Aveskamp, Woudenberg & Gruyter, Ph. noackiana (Allesch.) Aveskamp, Gruyter & Verkley, Stagonosporopsis ajacis (Thüm.) Aveskamp, Gruyter & Verkley, S. andigena (Turkenst.) Aveskamp, Gruyter & Verkley, S. artemisiicola (Hollós) Aveskamp, Gruyter & Verkley, S. astragali (Cooke & Harkn.) Aveskamp, Gruyter & Verkley, S. caricae (Sydow & P. Sydow) Aveskamp, Gruyter & Verkley, S. crystalliniformis (Loer., R. Navarro, M. Lôbo & Turkenst.) Aveskamp, Gruyter & Verkley, S. cucurbitacearum (Fr.) Aveskamp, Gruyter & Verkley, S. dorenboschii (Noordel. & Gruyter) Aveskamp, Gruyter & Verkley, S. heliopsidis (H.C. Greene) Aveskamp, Gruyter & Verkley, S. ligulicola var. ligulicola (K.F. Baker, Dimock & L.H. Davis) Aveskamp, Gruyter & Verkley, S. ligulicola var. inoxydabilis (Boerema) Aveskamp, Gruyter & Verkley, S. loticola (Died.) Aveskamp, Gruyter & Verkley, S. oculo-hominis (Punith.) Aveskamp, Gruyter & Verkley, S. rudbeckiae (Fairm.) Aveskamp, Gruyter & Verkley, S. trachelii (Allesch.) Aveskamp, Gruyter & Verkley, S. valerianellaea (Gindrat, Semecnik & Bolay) Aveskamp, Gruyter & Verkley. New names: Peyronellaea australis Aveskamp, Gruyter & Verkley, Phoma fungicola Aveskamp, Gruyter & Verkley, Ph. novae-verbascicola Aveskamp, Gruyter & Verkley.
References
- Aa HA van der (1971). Macroventuria, a new genus in the Venturiaceae. Persoonia 6: 359–363. [Google Scholar]
- Aa HA van der (1973). Studies in Phyllosticta I. Studies in Mycology 5: 1–110. [Google Scholar]
- Aa HA van der, Kesteren HA van (1979). Some pycnidial fungi occurring on Atriplex and Chenopodium. Persoonia 10: 267–276. [Google Scholar]
- Aa HA van der, Kesteren HA van (1980). Phoma heteromorphospora nom. nov. Persoonia 10: 267–276. [Google Scholar]
- Aa HA van der, Vanev S (2002). A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- Aa HA van der, Noordeloos ME, Gruyter J de (1990). Species concepts in some larger genera of the Coelomycetes. Studies in Mycology 32: 3–19. [Google Scholar]
- Aa HA van der, Boerema GH, Gruyter J de (2000). Contributions towards a monograph of Phoma (Coelomycetes) – VI-1. Section Phyllostictoides: Characteristics and nomenclature of its type species Phoma exigua. Persoonia 17: 435–456. [Google Scholar]
- Abeln ECA, Stax AM, Gruyter J de, Aa HA van der (2002). Genetic differentiation of Phoma exigua varieties by means of AFLP fingerprints. Mycological Research 106: 419–427. [Google Scholar]
- Aderkas P von, Brewer D (1983). Gangrene of the ostrich fern caused by Phoma exigua var. foveata. Canadian Journal of Plant Pathology 5: 164–167. [Google Scholar]
- Aderkas P von, Gruyter J de, Noordeloos ME, Strongman DB (1992). Phoma matteucciicola sp. nov., the causal agent of gangrene disease of ostrich fern. Canadian Journal of Plant Pathology 14: 227–228. [Google Scholar]
- Aptroot A (2006). Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series no. 6. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- Arenal F, Platas G, Monte E, Pelaéz F (2000). ITS sequencing support for Epicoccum nigrum and Phoma epicoccina being the same biological species. Mycological Research 104: 301–303. [Google Scholar]
- Arenal F, Platas G, Peláez F (2004). Taxonomic reconsideration of Epicoccum nigrum and Phoma epicoccina based on DNA sequences and morphological observations. Mycotaxon 89: 465–471. [Google Scholar]
- Arx JA von (1981). The genera of fungi sporulating in pure culture. 3rd edn. J. Cramer, Vaduz, Liechtenstein.
- Arx JA von (1987). Plant pathogenic fungi. J. Cramer, Berlin, Germany.
- Arx JA von, Storm PK (1967). Über einige aus dem Erdboden isolierte, zu Sporormia, Preussia, und Westerdykiella gehörende Ascomyceten. Persoonia 4: 407–415. [Google Scholar]
- Armstrong KF, Ball SL (2005). DNA Barcodes for biosecurity: invasive species identification. Philosophical Transactions of the Royal Society of London B 360: 1813–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp MM, Gruyter J de, Crous PW (2008). Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Diversity 31: 1–18. [Google Scholar]
- Aveskamp MM, Verkley GJM, Gruyter J de, Murace MA, Perelló A, Woudenberg JHC, Groenewald JZ, Crous PW (2009a). DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101: 363–382. [DOI] [PubMed] [Google Scholar]
- Aveskamp MM, Woudenberg JHC, Gruyter J de, Turco E, Groenewald JZ, Crous PW (2009b). Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): a case study in the Phoma exigua species complex. Molecular Plant Pathology 10: 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balis E, Velegraki A, Fragou A, Pefanis A, Kalabokas T, Mountokalakis T (2006). Lung mass caused by Phoma exigua. Scandinavian Journal of Infectious Diseases 38: 552–555. [DOI] [PubMed] [Google Scholar]
- Barr ME (2000). Notes on coprophilous bitunicate Ascomycetes. Mycotaxon 76: 105–112. [Google Scholar]
- Barr ME (2002). Teichosporaceae, another family in the Pleosporales. Mycotaxon 82: 373–389. [Google Scholar]
- Barrins JM, Ades PK, Salisbury PA, Howlett BJ (2004). Genetic diversity of Australian isolates of Leptosphaeria maculans, the fungus that causes blackleg of canola (Brassica napus). Australasian Plant Pathology 33: 529–536. [Google Scholar]
- Barve MP, Arie T, Salimath SS, Muehlbauer FJ, Peever TL (2003). Cloning and characterization of the mating type (MAT) locus from Ascochyta rabiei (teleomorph Didymella rabiei) and a MAT phylogeny of legume associated Ascochyta spp. Fungal Genetics and Biology 39: 151–167. [DOI] [PubMed] [Google Scholar]
- Bennett JW (1983). Secondary metabolism and differentiation in mycelia fungi. In: Secondary metabolism and differentiation in fungi (Bennett JW, Ciegler A, eds). Marcel Dekker Inc. New York, U.S.A.: 1-32
- Berbee ML (1996). Loculoascomycete origins and evolution of filamentous ascomycete morphology based on 18S rDNA gene sequence data. Molecular Biology and Evolution 13: 462–470. [DOI] [PubMed] [Google Scholar]
- Boehm EWA, Mugambi GK, Miller AN, Huhndorf SM, Marincowitz S, Spatafora JW, Schoch CL (2009). A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keys to world species. Studies in Mycology 64: 49–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerema GH (1964). Phoma herbarum Westend., the type-species of the form-genus Phoma Sacc. Persoonia 3: 9–16. [Google Scholar]
- Boerema GH (1965). Spore development in the form-genus Phoma. Persoonia 3: 413–417. [Google Scholar]
- Boerema GH (1970). Additional notes on Phoma herbarum. Persoonia 6: 15–48. [Google Scholar]
- Boerema GH (1972). Ascochyta phaseolorum synonymous with Phoma exigua. Netherlands Journal of Plant Pathology 78: 113–115. [Google Scholar]
- Boerema GH (1993). Contributions towards a monograph of Phoma (Coelomycetes) - II. Section Peyronellaea. Persoonia 15: 197–221. [Google Scholar]
- Boerema GH (1997). Contributions towards a monograph of Phoma (Coelomycetes) - V. Subdivision of the genus in sections. Mycotaxon 64: 321–333. [Google Scholar]
- Boerema GH (2003). Contributions towards a monograph of Phoma (Coelomycetes) - X. Section Pilosa (Taxa with a Pleospora teleomorph) and nomenclatural notes on some other taxa. Persoonia 18: 153–161. [Google Scholar]
- Boerema GH, Bollen GJ (1975). Conidiogenesis and conidial septation ads differentiating criteria between Phoma and Ascochyta. Persoonia 8: 111–144. [Google Scholar]
- Boerema GH, Dorenbosch MMJ (1973). The Phoma and Ascochyta species described by Wollenweber and Hochapfel in their study on fruit-rotting. Studies in Mycology 3: 1–50. [Google Scholar]
- Boerema GH, Dorenbosch MMJ (1979). Mycologisch-taxonomisch onderzoek. Verslagen en Mededelingen Plantenziektenkundige Dienst Wageningen 153: 17–21. [Google Scholar]
- Boerema GH, Hamers MEC (1990). Check-list for scientific names of common parasitic fungi. Series 3c: Fungi on bulbs: `additional crops'belonging to the Araceae, Begoniaceae, Compositae, Oxalidaceae and Ranunculaceae. Netherlands Journal of Plant Pathology 96: Suppl. 1.
- Boerema GH, Gruyter J de (1998). Contributions towards a monograph of Phoma (Coelomycetes) – VII. Section Sclerophomella: Taxa with thick-walled pseudoparenchymatous pycnidia. Persoonia 17: 81–95. [Google Scholar]
- Boerema GH, Gruyter J de (1999). Contributions towards a monograph of Phoma (Coelomycetes) – III-Supplement. Additional species of section Plenodomus. Persoonia 17: 273–280. [Google Scholar]
- Boerema GH, Kesteren HA van (1980). Vermeldingswaardige schimmelaantastingen in de periode 1975–1979. A. Aantastingen door schimmels die in Nederland niet bekend waren. Gewasbescherming 11: 115–127. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Kesteren HA van (1965a). Remarks on species of Phoma referred to Peyronellaea. Persoonia 4: 47–68. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Leffring L (1965b). A comparative study of the black stem fungi on lucerne and red clover and the footrot fungus on pea. Netherlands Journal of Plant Pathology 71: 79–89. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Kesteren HA van (1968). Remarks on species of Phoma referred to Peyronellaea II. Persoonia 5: 201–205. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Kesteren HA van (1971). Remarks on species of Phoma referred to Peyronellaea III. Persoonia 6: 171–177. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Kesteren HA van (1973). Remarks on species of Phoma referred to Peyronellaea IV. Persoonia 7: 131–139. [Google Scholar]
- Boerema GH, Dorenbosch MMJ, Kesteren HA van (1977). Remarks on species of Phoma referred to Peyronellaea V. Kew Bulletin 31: 533–544. [Google Scholar]
- Boerema GH, Cruger G, Gerlagh M, Nirenberg H (1981a). Phoma exigua var. diversispora and related fungi on Phaseolus beans. Zeitschrift für Pflanzenkrankheiten und Pflanzenschütz 88: 597–607. [Google Scholar]
- Boerema GH, Kesteren HA van, Loerakker WM (1981b). Notes on Phoma. Transactions of the British Mycological Society 77: 61–74. [Google Scholar]
- Boerema GH, Loerakker WM, Hamers MEC (1987). Check-list for scientific names of common parasitic fungi. Supplement 2a (additions and corrections): Fungi on field crops: beet and potato; caraway, flax and oilseed poppy. Netherlands Journal of Plant Pathology 93 (suppl. 1): 1–20. [Google Scholar]
- Boerema GH, Gruyter J de, Kesteren HA van (1994). Contributions towards a monograph of Phoma (Coelomycetes) - III. 1. Section Plenodomus: Taxa often with a Leptosphaeria teleomorph. Persoonia 15: 431–487. [Google Scholar]
- Boerema GH, Loerakker WM, Hamers MEC (1996). Contributions towards a monograph of Phoma (Coelomycetes) - III. 2. Misapplications of the type species name and the generic synonyms of the section Plenodomus. Persoonia 16: 141–190. [Google Scholar]
- Boerema GH, Gruyter J de, Noordeloos ME (1997). Contributions towards a monograph of Phoma (Coelomycetes) IV. - Section Heterospora: Taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia 16: 335–371. [Google Scholar]
- Boerema GH, Gruyter J de, Noordeloos ME, Hamers MEC (2004). Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI publishing, Wallingford, U.K.
- Bowen JK, Lewis BG, Matthews P (1997). Discovery of the teleomorph of Phoma medicaginis var. pinodella in culture. Mycological Research 101: 80–84. [Google Scholar]
- Brewer JG, Boerema GH (1965). Electron microscope observations on the development of pycnidiospores in Phoma and Ascochyta spp. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen C. 68: 86–97. [Google Scholar]
- Bridge PD (2002). The history and application of molecular mycology. Mycologist 16: 90–99. [Google Scholar]
- Bridge PD, Roberts PJ, Spooner BM, Panchal G (2003). On the unreliability of published DNA sequences. New Phytologist 160: 43–48. [DOI] [PubMed] [Google Scholar]
- Bridge PD, Spooner BM, Roberts PJ (2004). Reliability and use of published sequence data. New Phytologist 161: 15–17. [Google Scholar]
- Bugbee WM, Cole DF (1981). The effect of seed infected with Phoma betae on rot and sucrose yield of stored sugar beet. Phytopathology 71: 357–359. [Google Scholar]
- Câmara MPS, Palm ME, Berkum P van, O'Neill NR (2002). Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94: 630–640. [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Chen SY, Dickson DW, Kimbrough JW (1996). Phoma heteroderae sp. nov. isolated from the eggs of Heterodera glycines. Mycologia 88: 885–891. [Google Scholar]
- Chen W, Seifert KA, Lévesque CA (2009). A high density COX1 barcode oligonucleotide array for identification and detection of species of Penicillium subgenus Penicillium. Molecular Ecology Resources 9: 114–129. [DOI] [PubMed] [Google Scholar]
- Chilvers MI, Rogers JD, Dugan FM, Stewart JE, Chen W, Peever TL (2009). Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycological Research 113: 391–400. [DOI] [PubMed] [Google Scholar]
- Chongo G, Gossen BD, Buchwaldt L, Adhikari T, Rimmer SR (2004). Genetic diversity of Ascochyta rabiei in Canada. Plant Disease 88: 4–10. [DOI] [PubMed] [Google Scholar]
- Costa EO, Gandra CR, Pires MF, Couthino SD, Castilho W, Teixeira CM (1993). Survey of bovine mycotic mastitis in dairy herds in the State of São Paulo, Brazil. Mycopathologia 124: 13–17. [DOI] [PubMed] [Google Scholar]
- Corlett M (1991). An annotated list of the published names in Mycosphaerella and Sphaerella. Mycologia Memoir 18: 1–328. [Google Scholar]
- Cortinas M-N, Burgess T, Dell B, Xu D, Crous PW, Wingfield BD, Wingfield MJ (2006). First record of Colletogloeopsis zuluense comb. nov., causing a stem canker of Eucalyptus in China. Mycological Research 110: 229–236. [DOI] [PubMed] [Google Scholar]
- Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoirs 21: 1–170. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, et al. (2009a). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, et al. (2009b). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA (2009c). Fungal Biodiversity. CBS Laboratory Manual Series. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands.
- Cullen DW, Toth IK, Boonham N, Walsh K, Barker I, Lees AK (2007). Development and validation of conventional and quantitative polymerase chain reaction assays for the detection of storage rot potato pathogens, Phytophthora erythroseptica, Pythium ultimum, Phoma foveata. Journal of Phytopathology 155: 309–315. [Google Scholar]
- Damm U, Verkley GJM, Crous PW, Fourie PH, Haegi A, Riccioni L (2008). Novel Paraconiothyrium species on stone fruit trees and other woody hosts. Persoonia 20: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey ML, Currah RS (2009). Atradidymella muscivora gen. et sp. nov. (Pleosporales) and its anamorph Phoma muscivora sp. nov.: A new pleomorphic pathogen of boreal bryophytes. American Journal of Botany 96: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Davidson JA, Hartley D, Priest M, Krysinska-Kaczmarek M, Herdina McKay A, Scott ES (2009). A new species of Phoma causes ascochyta blight symptoms on field peas (Pisum sativum) in South Australia. Mycologia 101: 120–128. [DOI] [PubMed] [Google Scholar]
- Diederich P, Kocourkova J, Etayo J, Zhurbenko M (2007). The lichenicolous Phoma species (coelomycetes) on Cladonia. Lichenologist 39: 153–163. [Google Scholar]
- Dong J, Chen W, Crane JL (1998). Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other Loculoascomycetes based on nuclear ribosomal DNA sequences. Mycological Research 102: 151–156. [Google Scholar]
- Dorenbosch MMJ (1970). Key to nine ubiquitous soil-borne phoma-like fungi. Persoonia 6: 1–14. [Google Scholar]
- Druzhinina IS, Kopchinskiy AG, Komón M, Bisset J, Szakacs G, Kubicek CP (2005). An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genetics and Biology 42: 813–828. [DOI] [PubMed] [Google Scholar]
- Ellis JB, Everhart BM (1888). New species of fungi from various localities. Journal of Mycology 4: 97–118. [Google Scholar]
- Faisal M, Elsayed E, Fitzgerald SD, Silva V, Mendoza, L (2007). Outbreaks of phaeohyphomycosis in the chinook salmon (Oncorhynchus tshawyscha) caused by Phoma herbarum. Mycopathologia 163: 41–48. [DOI] [PubMed] [Google Scholar]
- Faris-Mokaiesh S, Boccara M, Denis J-B, Derrien A, Spire D (1996). Differentiation of the “Ascochyta complex” fungi of pea by biochemical and molecular markers. Current Genetics 29: 182–190. [DOI] [PubMed] [Google Scholar]
- Fatehi J, Bridge PD, E. Punithalingam (2003). Molecular relatedness within the “Ascochyta pinodes-complex”. Mycopathologia 156: 317–327. [DOI] [PubMed] [Google Scholar]
- Fitt BDL, Brun H, Barbetti MJ, Rimmer SR (2006). World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology 114: 3–15. [Google Scholar]
- Geiser DM, Klich MA, Frisvad JC, Peterson SW, Varga J, Samson RA (2007). The current status of species recognition and identification in Aspergillus. Studies in Mycology 59: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore SR, Gräfenhan T, Louis-Seize G, Seifert KA (2009) Multiple copies of cytochrome oxidase 1 in species of the fungal genus Fusarium. Molecular Ecology Resources 9 (Suppl. 1): 90–98. [DOI] [PubMed] [Google Scholar]
- Grondona I, Monte E, Garcia-Acha I, Sutton BC (1997). Pyrenochaeta dolichi: an example of a confusing species. Mycological Research 101: 1405–1408. [Google Scholar]
- Grove WB (1935). British stem- and leaf-fungi (Coelomycetes). A contribution to our knowledge of the Fungi Imperfecti belonging the de Sphaeropsidales and the Melanconiales. Vol 1: Sphaeropsidales. To the end of the Sphaerioideae which have colourless or nearly colourless spores. Cambridge University Press, London, U.K.
- Gruyter J de (2002). Contributions towards a monograph of Phoma (Coelomycetes) – IX. Section Macrospora. Persoonia 18: 85–102. [Google Scholar]
- Gruyter J de, Boerema GH (2002). Contributions towards a monograph of Phoma (Coelomycetes) - VIII. Section Paraphoma: Taxa with setose pycnidia. Persoonia 17: 541–561. [Google Scholar]
- Gruyter J de, Noordeloos ME (1992). Contributions towards a monograph of Phoma (Coelomycetes) - I. 1. Section Phoma: Taxa with very small conidia in vitro. Persoonia 15: 71–92. [Google Scholar]
- Gruyter J de, Noordeloos ME, Boerema GH (1993). Contributions towards a monograph of Phoma (Coelomycetes) - I. 2. Section Phoma: Additional taxa with very small conidia and taxa with conidia up to 7 μm long. Persoonia 15: 369–400. [Google Scholar]
- Gruyter J de, Noordeloos ME, Boerema GH (1998). Contributions towards a monograph of Phoma (Coelomycetes) - I. 3. Section Phoma: Taxa with conidia longer than 7μm. Persoonia 16: 471–490. [Google Scholar]
- Gruyter J de, Boerema GH, Aa HA van der (2002). Contributions towards a monograph of Phoma (Coelomycetes) - VI. 2. Section Phyllostictoides: outline of its taxa. Persoonia 18: 1–53. [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009). Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research 113: 508–519. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia: In press. [DOI] [PubMed]
- Hatai K, Fujimaki Y, Egusa S (1986). A visceral mycosis in ayu fry, Plecoglossus altivelis Temmink and Schlegel, caused by a species of Phoma. Journal of Fish Diseases 9: 111–116. [Google Scholar]
- Hawksworth DL (1981). The lichenicolous Coelomycetes. Bulletin of the British Museum (Natural History), Botany series 9: 1–98. [Google Scholar]
- Hawksworth DL, Cole MS (2004). Phoma fuliginosa sp. nov., from Caloplaca trachyphylla in Nebraska, with a key to the known lichenous species. Lichenologist 36: 7–13. [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society of London B 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Bello MA, Chilvers MI, Akamatsu H, Peever TL (2006). Host specificity of Ascochyta spp. infecting legumes of the Viciae and Cicerae tribes and pathogenicity of an interspecific hybrid. Phytopathology 96: 1148–1156. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000). Atlas of Clinical Fungi. 2nd edn. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands & Universitat Rovira I Virgili, Reus, Spain.
- Hoog GS de, Smith M.Th. (2004). Ribosomal gene phylogeny and species delimitation in Geotrichum and its teleomorphs. Studies in Mycology 50: 489–515. [Google Scholar]
- Holliday P, Punithalingam E (1970). Ascochyta gossypii. CMI Descriptions of pathogenic Fungi and Bacteria 271.
- Holm L (1962). Lewis E. Wehmeyer, a world monograph of the genus Pleospora and its segregates. Svensk Botanisk Tidskrift 56: 377–381. [Google Scholar]
- Howlett BJ, Idnurm A, Pedras MSC (2001). Leptosphaeria maculans, the causal agent of blackleg disease of brassicas. Fungal Genetics and Biology 33: 1–14. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hughes SJ (1953). Conidiophores, conidia and classification. Canadian Journal of Botany 31: 577–659. [Google Scholar]
- Hyde KD, Soytong K (2007). Understanding microfungal diversity – a critique. Cryptogamie Mycologie 28: 281–289. [Google Scholar]
- Inderbitzin P, Mehta YR, Berbee ML (2009). Pleospora species with Stemphylium anamorphs: a four locus phylogeny resolves new lineages yet does not distinguish among species in the Pleospora herbarum clade. Mycologia 101: 329–339. [DOI] [PubMed] [Google Scholar]
- Irinyi L, Kövics GJ, Sándor E (2009). Taxonomical re-evaluation of Phoma-like soybean pathogenic fungi. Mycological Research 113: 249–260. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Lewis BG (1990). DNA polymorphism in Leptosphaeria maculans. Physiological and Molecular Plant Pathology 37: 417–424. [Google Scholar]
- Johnston PR (1981). Phoma on New Zealand grasses and pasture legumes. New Zealand Journal of Botany 19: 173–186. [Google Scholar]
- Johnston PR, Boerema GH (1981). Phoma nigricans sp. nov. and Ph. pratorum sp. nov., two common saprophytes from New Zealand. New Zealand Journal of Botany 19: 393–396. [Google Scholar]
- Jones JP (1976). Ultrastructure of conidium ontogeny in Phoma pomorum, Microsphaeropsis olivaceum, and Coniothyrium fuckelii. Canadian Journal of Botany 54: 831–851. [Google Scholar]
- Kaiser WJ (1997). Inter- and intranational spread of Ascochyta pathogens of chickpea, faba beans and lentil. Canadian Journal of Plant Pathology 19: 215–224. [Google Scholar]
- Kiss L (1997). Genetic diversity in Ampelomyces isolates, hyperparasites of powdery mildew fungi, inferred from RFLP analysis of the rDNA ITS region. Mycological Research 101: 1073–1080. [Google Scholar]
- Kiss L, Nakasone KK (1998). Ribosomal DNA internal transcribed spacer sequences do not support the species status of Ampelomyces quisqualis, a hyperparasite of powdery mildew fungi. Current Genetics 33: 362–367. [DOI] [PubMed] [Google Scholar]
- Kiss L, Russell JC, Szentiványi O, Xu X, Jeffries P (2004). Biology and biocontrol potential of Ampelomyces mycoparasites, natural antagonists of powdery mildew fungi. Biocontrol Science and Technology 14: 635–651. [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R (2006). The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28SrDNA. Mycologia 98: 571–583. [DOI] [PubMed] [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1987). Marine fungi from Belize with descriptions of two new genera of Ascomycetes. Botanica Marina 30: 195–204. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1991). Illustrated key to the filamentous higher marine fungi. Botanica Marina 34: 1–61. [Google Scholar]
- Kövics GJ, Gruyter J de, Aa HA van der (1999). Phoma sojicola comb. nov. and other hyaline-spored coelomycetes pathogenic on soybean. Mycological Research 103: 1065–1070. [Google Scholar]
- Kruys Å, Eriksson OE, Wedin M (2006). Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycological Research 110: 527–536. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Q, Mei R-H, Lu G-Z. (2006). Phoma adonidicola sp. nov. on Adonis palaestina. Mycotaxon 98: 237–240. [Google Scholar]
- Macdonald JE, White GP, Coté MJ (2000). Differentiation of Phoma foveata from Ph. exigua using a RAPD generated PCR-RFLP marker. European Journal of Plant Pathology 106: 67–75. [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, Marhold K, Nicolson DH, Prado J, Silva PC, Skog JE, Wiersema JH, Turland NJ (2006). International Code of Botanical Nomenclature (Vienna Code) adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005. Gantner Verlag, Ruggell, Liechtenstein.
- Mel'nik VA (2000). Key to the fungi of the genus Ascochyta Lib. (Coelomycetes). In: Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft. Heft 379. (Mel'nik VA, Braun U, Hagedorn G, eds). Perley Buchverlag, Berlin Germany.
- Mendes-Pereira E, Faris-Mokaiesh S, Bertrandy J, Spire D (1999). Characterisation of mycelial glycoproteins of the `Ascochyta pea complex' (Ascochyta pisi Lib., Mycosphaerella pinodes (Berk. and Blox.) and Phoma medicaginis var. pinodella (Jones) Boerema. Agronomie 19: 21–29. [Google Scholar]
- Mendes-Pereira E, Balesdent MH, Brun H, Rouxel T (2003). Molecular phylogeny of the Leptosphaeria maculans - L. biglobosa species complex. Mycological Research 107: 1287–1304. [DOI] [PubMed] [Google Scholar]
- Monte E, Bridge PD, Sutton BC (1991). An integrated approach to Phoma systematics. Mycopathologia 115: 89–103. [DOI] [PubMed] [Google Scholar]
- Morales VM, Pelcher LE, Taylor JL (1993). Comparison of the 5.8s rDNA and internal transcribed spacer sequences of isolates of Leptosphaeria maculans from different pathogenicity groups. Current Genetics 23: 490–495. [DOI] [PubMed] [Google Scholar]
- Morales VM, Jasalavich CA, Pelcher LE, Petrie GA, Taylor JL (1995). Phylogenetic relationship among several Leptosphaeria species based on their ribosomal DNA sequences. Mycological Research 99: 593–603. [Google Scholar]
- Morgan-Jones G (1988). Studies in the genus Phoma. XV. Concerning Phoma multirostrata, a leaf spot-inducing and soil-borne species in warm climates. Mycotaxon 33: 339–351. [Google Scholar]
- Mugambi GK, Huhndorf SM (2009). Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Studies in Mycology 64: 103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukunya DM, Boothroyd CW (1973). Mycosphaerella zeae-maydis sp. n., the sexual stage of Phyllosticta maydis. Phytopathology 63: 529–532. [Google Scholar]
- Müller E, Arx JA von (1962). Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11(2): 1–922. [Google Scholar]
- Munk A (1957). Danish Pyrenomycetes: a preliminary flora. Dansk botanisk Arkiv 17.
- Muthumeenakshi S, Goldstein AL, Stewart A, Wipps JM (2001). Molecular studies on intraspecific diversity and phylogenetic position of Coniothyrium minitans. Mycological Research 105: 1065–1074. [Google Scholar]
- Nag Raj TR (1981). Coelomycete systematics. In: Biology of conidial fungi, vol 1. (Cole T, Kendrick WB, eds). Academic Press, New York, U.S.A.
- Naumann A, Navarro-González M, Sánchez-Hernández O, Hoegger PJ, Kües U (2007). Correct identification of wood-inhabiting fungi by ITS analysis. Current Trends in Biotechnology and Pharmacy 1: 41–61. [Google Scholar]
- Nelsen MP, Lücking R, Grube M, Mbatchou JS, Muggia L, et al. (2009). Unravelling the phylogenetic relationships of lichenised fungi in Dothideomyceta. Studies in Mycology 64: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HDT, Seifert KA (2008). Description and DNA barcoding of three new species of Leohumicola from South Africa and the United States. Persoonia 21: 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RH, Ryberg M, Kristiansson E, Abarenkov K, Larsson K-H, Köljalg U (2006). Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS One 1: e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson RH, Kristiansson E, Ryberg M, Hallenberg N, Larsson K-H (2008). Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evolutionary Bioinformatics Online 4: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeloos ME, Gruyter J de, Eijk GW van, Roeijmans HJ (1993). Production of dendritic crystals in pure cultures of Phoma and Ascochyta and its value as a taxonomic character relative to morphology, pathology and cultural characteristics. Mycological Research 97: 1343–1350. [Google Scholar]
- Nylander JAA (2004). MrModeltest v. 2.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden.
- Obando-Rojas L (1989). Contribution à l'etude de l'ascochytose du haricot Phaseolus vulgaris L.: identification de souches fongiques en cause et recherché de sources de résistance au sein du genre Phaseolus. Ph.D. dissertation. Faculté universitaire des sciences agronomiques de Gembloux, Gembloux Agricultural University, Belgium.
- Onfroy C, Tivoli B, Corbiere R, Bouznad Z (1999). Cultural, molecular and pathogenic variability of Mycosphaerella pinodes and Phoma medicaginis var. pinodella isolates from dried pea (Pisum sativum) in France. Plant Pathology 48: 218–229. [Google Scholar]
- Osterhage C, Schwibbe M, König GM, Wright AD (2000). Differences between marine and terrestrial Phoma species as determined by HPLC-DAD and HPLC-MS. Phytochemical analysis 11: 288–294. [Google Scholar]
- Page RDM (1996). TREEVIEW: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Pande S, Siddique KHM, Kishore GK, Bayaa B, Gaur PM, Gowda CLL, Bretag TW, Crouch JH (2005). Ascochyta blight of chickpea (Cicer arietinum L.): a review of biology pathogenicity, and disease management. Australian Journal of Agricultural Research 56: 317–332. [Google Scholar]
- Patel US, Pandey AK, Rajak RC. Chaetasbolisia indica sp. nov. from India. Mycological Research 101: 335–336.
- Pawar VH, Mathur PN, Thirumalachar MJ (1967). Species of Phoma isolated from marine soils in India. Transactions of the British Mycological Society 50: 259–265. [Google Scholar]
- Pažoutová S (2009). Genetic variation of Phoma sorghina isolates from Southern Africa and Texas. Folia Microbiologica 54: 217–229. [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Biesenthal CJ (2000). HPLC analyses of cultures of Phoma species: differentiation among groups and species through secondary metabolite profiles. Canadian Journal of Microbiology 46: 685–691. [DOI] [PubMed] [Google Scholar]
- Peever TL, Barve MP, Stone LJ, Kaiser WJ (2007). Evolutionary relationships among Ascochyta species infecting wild and cultivated hosts in legume tribes Cicereae and Vicieae. Mycologia 99: 59–77. [DOI] [PubMed] [Google Scholar]
- Pirozynski KA, Morgan-Jones G (1968). Notes on Microfungi. III. Transaction of the British Mycological Society 51: 185–206. [Google Scholar]
- Pitt JI (1979). The genus Penicillium and its telemorphic states Eupenicillium and Talaromyces. Academic Press, London, U.K.
- Pitt JI, Samson RA (2007). Nomenclatural considerations in naming species of Aspergillus and its teleomorphs. Studies in Mycology 59: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongam P, Osborn TC, Williams PH (1999). Assessment of genetic variation among Leptosphaeria maculans isolates using pathogenicity data and AFLP analysis. Plant Disease 83: 149–154. [DOI] [PubMed] [Google Scholar]
- Punithalingam E (1976). Phoma oculi-hominis sp. nov. from corneal ulcer. Transactions of the British Mycological Society 67: 142–143. [Google Scholar]
- Punithalingam E (1979a). Graminicolous Ascochyta species. Mycological Papers 142: 1–214. [Google Scholar]
- Punithalingam E (1979b). Phoma caricae. CMI Descriptions of Pathogenic Fungi and Bacteria. No. 634.
- Punithalingam E (1980). A combination in Phoma for Ascochyta caricae-papayae. Transactions of the British Mycological Society 75: 340–341. [Google Scholar]
- Punithalingam E, Tulloch M, Leach CM (1972). Phoma epicoccina sp. nov. on Dactylis glomerata. Transactions of the British Mycological Society 59: 341–345. [Google Scholar]
- Purwantara A, Barrins JM, Cozijnsen AJ, Ades PK, Howlett BJ (2000). Genetic diversity of isolates of the Leptosphaeria maculans species complex from Australia, Europe and North America using amplified fragment length polymorphism analysis. Mycological Research 104: 772–781. [Google Scholar]
- Rabie CJ, Rensburg SJ van, Watt JJ van der, Lubben A (1975). Onyalai - the possible involvement of a mycotoxin produced by Phoma sorghina in the aetiology. South African Medical Journal 57: 1647–1650. [PubMed] [Google Scholar]
- Rai MK (2000). The genus Phoma (Identity and Taxonomy). International Book Distributors, Dehra Dun, India.
- Rai M, Deshmukh P, Gade A, Ingle A, Kövics GJ, Irinyi L (2009). Phoma Saccardo: Distribution, secondary metabolite production and biotechnological applications. Critical Reviews in Microbiology 35: 182–196. [DOI] [PubMed] [Google Scholar]
- Rayner RW (1970). A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, U.K.
- Reddy PV, Patel R, White Jr JF (1998). Phylogenetic and developmental evidence supporting reclassification of cruciferous pathogens Phoma lingam and Phoma wasabiae in Plenodomus. Canadian Journal of Botany 76: 1916–1922. [Google Scholar]
- Redfern DB, Sutton BC (1981). Canker and dieback of Ulmus glabra caused by Plectophomella concentrica, and its relationship to Ph. ulmi. Transactions of the British Mycological Society 77: 381–390. [Google Scholar]
- Rehner SA, Samuels GJ (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Reynolds DR (1999). Capnodium citri: The sooty mold fungi comprising the taxon concept. Mycopathologia 148: 141–147. [DOI] [PubMed] [Google Scholar]
- Robert V, Stegehuis G, Stalpers JA (2005). The MycoBank engine and related databases. http://www.mycobank.org. Accessed October 16th, 2009.
- Röder K (1937). Phyllosticta cannabis (Kirchner?) Speg. Eine Nebenfruchtform von Mycosphaerella cannabis (Winter). Zeitschrift für Pflanzenkrankheiten (Pflanzen pathologie) und Pflanzenschutz 47: 526–531. [Google Scholar]
- Ross AJ, Yasutake T, Leek S (1975). Phoma herbarum, a fungal plant saprophyte, as a fish pathogen. Journal of the Fisheries Research Board of Canada 32: 1648–1652. [Google Scholar]
- Rudakov OL (1979). Fungi of the genus Ampelomyces Ces. ex Schlecht. Mikologiya i Fitopatologiya 13: 104–110. [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, et al. (2009). Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Studies in Mycology 64: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA (1880). Conspectus generum fungorum Italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium systemate sporologico dispositorum. Michelia 2: 1–38. [Google Scholar]
- Saccardo PA (1884). Sylloge Sphaeropsidearum et Melanconiearum omnium hucusque cognitorum. Sylloge Fungorum 3: 1–860. [Google Scholar]
- Saccas AM (1981). Etude de la flore cryptogamique des cafeiers en Afrique Centrale. Institut Français du Café et du Cacao, Paris, France.
- Salgado M, Lima CS, Almeida AR, Santos LL, Pfenning LH (2007). Primeiro relato da ocorrência de Didymella sp., fase sexuada de Phoma tarda, em Coffea arabica no Brasil. In: 5. Simpósio de Pesquisa dos Cafés do Brasil, 2007, Águas de Lindóia SPh. Anais. Brasília DF: Embrapa Café, 2007.
- Schäfer C, Wöstemeyer J (1992). Random primer dependent PCR differentiates aggressive from non-aggressive isolates of the oilseed rape pathogen Phoma lingam (Leptosphaeria maculans). Journal of Phytopathology 136: 124–136. [Google Scholar]
- Schneider R, Boerema GH (1975). Phoma tropica n. sp., ein a Gewächshauspflanzen häufig vorkommender, nicht pathogener Pilz. Phytopathologische Zeitschrift 83: 239–243. [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hableton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZS, Boehm EWA, BurgessTI, et al. (2009a). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Sung G-H, López-Giráldez F, Townsend JP, Miadlikowska J, et al. (2009b). The Ascomycota Tree of Life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Seifert KA (2008). Integrating DNA barcoding into the mycological sciences. Persoonia 21: 162–167. [Google Scholar]
- Seifert KA (2009). Progress towards DNA barcoding of fungi. Molecular Ecology Resources 9 (Suppl. 1): 83–89. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Samson RA, DeWaard JR, Houbraken J, Lévesque CA, Moncalvo J-M, Louis-Seize G, Hebert PDN (2007). Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proceedings of the National Academy of Sciences of the United States of America 104: 3901–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbmann L, Onofri S, Fenice M, Federici F, Petruccioli M (2002). Production and structural characterization of the exopolysaccharide of the Antarctic fungus Phoma herbarum CCFEE 5080. Research in Microbiology 153: 585–592. [DOI] [PubMed] [Google Scholar]
- Shen G, Wang YC, Zhang WL, Zheng XB (2005). Development of a PCR assay for the molecular detection of Phytophthora boehmeriae in infected cotton. Journal of Phytopathology 153: 291–296. [Google Scholar]
- Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, et al. (2009). The molecular phylogeny of freshwater Dothideomycetes. Studies in Mycology 64: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA, Brun H (2001). The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Canadian Journal of Botany 79: 412–419. [Google Scholar]
- Silva-Hanlin DMW, Hanlin RT (1999). Small subunit ribosomal RNA gene phylogeny of several Loculoascomycetes and its taxonomic implications. Mycological Research 103: 153–160. [Google Scholar]
- Simmons EG (1969). Perfect states of Stemphylium. Mycologia 61: 1–26. [PubMed] [Google Scholar]
- Singh KB, Reddy MV (1993). Resistance to six races of Ascochyta rabiei in the world germplasm collection of chickpea. Crop Science 33: 186–189. [Google Scholar]
- Singh PJ, Pal M, Prakash N (1997). Ultrastructural studies of conidiogenesis of Ascochyta rabiei, the causal organism of chickpea blight. Phytoparasitica 25: 291–304. [Google Scholar]
- Sivanesan A (1990). Mycosphaerella caricae. CMI Descriptions of pathogenic fungi and bacteria No. 984. Mycopathologia 109: 47–48. [Google Scholar]
- Smith IM, McNamara DG, Scott PR, Harris KM (1992). Quarantine Pests for Europe. Data Sheets on Quarantine Pests for the European Communities and for the European and Mediteranean Plant Protection Organization. CABI Publishing, U.K. & OEPP/EPPO, France.
- Sørensen JL, Aveskamp MM, Thrane U, Andersen B (2009). Chemical characterization of Phoma pomorum isolated from Danish maize. International Journal of Food Microbiology, epub. ahead of print, doi 10.1016/j.ijfoodmicro.2009.11.001. [DOI] [PubMed]
- Stamatakis A, Ludwig T, Meier H (2005). RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21: 456–463. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J (2008). A rapid bootstrap algorithm for the RAxML Web-Servers. Systematic Biology 75: 758–771. [DOI] [PubMed] [Google Scholar]
- Stewart RB (1957). Leaf blight and stem dieback of coffee caused by an undescribed species of Ascochyta. Mycologia 49: 430–433. [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, et al. (2009). Molecular systematics of the marine Dothideomycetes. Studies in Mycology 64: 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RF, White Jr JF (2000). Phoma glomerata as a mycoparasite of powdery mildew. Applied and Environmental Microbiology 66: 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC, Lévesque CA, Seifert KA, Bovers M, Fell JW, et al.(2005). Microcoding: the second step in DNA barcoding. Philosophical Transactions of the Royal Society B 360 (1462): 1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC, Moore MK, Starink-Willemse M, Iperen A van (2007). ITS barcodes for Trichophyton tonsurans and T. equinum. Medical Mycology 45: 193–200. [DOI] [PubMed] [Google Scholar]
- Sutton BC (1964). Phoma and related genera. Transactions of the British Mycological Society 47: 497–509. [Google Scholar]
- Sutton BC (1977). Coelomycetes VI. Nomenclature of generic names proposed for coelomycetes. Mycological Papers 141: 1–253. [Google Scholar]
- Sutton BC (1980). The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. 1st edn. Commonwealth Mycological Institute, U.K.
- Sutton BC, Sandhu DK (1969). Electron microscopy of conidium development and secession in Cryptosporiopsis sp., Phoma fumosa, Melanconium bicolor and M. apiocarpum. Canadian Journal of Botany 47: 745–749. [Google Scholar]
- Szentiványi O, Kiss L, Russell JC, Kovács GM, Varga K, Jankovics T, Lesemann S, Xu XM, Jeffries P (2005). Ampelomyces mycoparasites from apple powdery mildew identified as a distinct group based on single-stranded conformation polymorphism analysis of the rDNA ITS region. Mycological Research 109: 429–438. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, et al. (2009). Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs. Studies in Mycology 64: 175–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PWJ, Ford R (2007). Diagnostics, genetic diversity and pathogenic variation of ascochyta blight of cool season food and feed legumes. European Journal of Plant Pathology 119: 127–133. [Google Scholar]
- Trapero-Casas A, Kaiser WJ (1992). Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on chickpea straw. Phytopathology 82: 1261–1266. [Google Scholar]
- Torres MS, White Jr JF, Cazares G, Bergen M, Bischoff JF, Sullivan RF (2005a). A new species and its phylogenetic placement in the Didymella/Phoma complex (Phaeosphaeriaceae, Pleosporales). Mycotaxon 93: 297–308. [Google Scholar]
- Torres MS, Bergen M, Singh S, Bischoff JF, Sullivan RF, White Jr JF (2005b). Plenodomus morganjonesii sp.nov. and a discussion of the genus Plenodomus. Mycotaxon 93: 333–343. [Google Scholar]
- Vegh I, Bourgeois M, Bousquert JF, Velastegui J (1974). Contribution à l'étude du Phoma exigua Desm., champignon pathogène associé au dépérissement de la pervenche mineure (Vinca minor L.) médicinale. Bulletin Trimestriel de la Societe Mycologique de France 90: 121–130. [Google Scholar]
- Verkley GJM, Silva M da, Wicklow DT, Crous PW (2004). Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335. [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt K, Malgorzata J, Wöstemeyer J (2001). Strain typing of Polish Leptosphaeria maculans isolates supports at the genomic level the multispecies concept of aggressive and non-aggressive strains. Microbiological Research 156: 169–177. [DOI] [PubMed] [Google Scholar]
- Voigt K, Cozijnsen AJ, Kroymann J, Pöggeler S, Howlett BJ (2005). Phylogenetic relationships between members of the crucifer pathogenic Leptosphaeria maculans species complex as shown by their mating type (MAT1-2), actin, and β-tubulin sequences. Molecular Phylogenetics and Evolution 37: 541–557. [DOI] [PubMed] [Google Scholar]
- Voronin LV (1989). Phoma Sacc. species from the water and fish of freshwater reservoirs. Mikologia i Fitopatologia 23: 19–27. [Google Scholar]
- White JF, Morgan-Jones G (1987). Studies in the genus Phoma. VI. Concerning Phoma medicaginis var. pinodella. Mycotaxon 28: 241–248. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California, U.S.A.: 315–322.
- Williams RH, Fitt BDL (1999). Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape. Plant Pathology 48: 161–175. [Google Scholar]
- Wilson AD, Kaiser WJ (1995). Cytology and genetics of sexual incompatibility in Didymella rabiei. Mycologia 87: 795–804. [Google Scholar]
- Wirsel SGR, Leibinger W, Ernst M, Mendgen K (2001). Genetic diversity of fungi closely associated with common reed. New Phytologist 149: 589–598. [DOI] [PubMed] [Google Scholar]
- Wollenweber HW, Hochapfel H (1936). Beiträge zur Kenntnis parasitärer und saprophytotischer Pilze. I. Phomopsis, Dendrophoma, Phoma und Ascochyta und ihre Beziehung zur Fruchtfäule. Zeitschrift für Parasitenkunde 8: 561–605. [Google Scholar]
- Woudenberg JHC, Aveskamp MM, Gruyter J de, Spiers AG, Crous PW (2009). Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xianshu Y, Strobel G, Stierle A, Hess WM, Lee J, Clardy J (1994). A fungal endophyte-tree relationship: Phoma sp. in Taxus wallachiana. Plant Science 102: 1–9. [Google Scholar]
- Yarden O, Ainsworth TD, Roff G, Leggat W, Fine M, Hoegh-Guldberg O (2007). Increased prefalence of ubiquitous ascomycetes in an acropoid coral (Acropora formosa) exibiting symptoms of brown band syndrome and skeletal eroding band disease. Applied and Environmental Microbiology 73: 2755–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. (2009). Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]