Abstract
A large percentage of current drugs target G-protein-coupled receptors, which couple to well-known signaling pathways involving cAMP or calcium. G-proteins themselves may subserve a second messenger function. Here, we review the role of tubulin and microtubules in directly mediating effects of heterotrimeric G-proteins on neuronal outgrowth, shape and differentiation. G-protein-tubulin interactions appear to be regulated by neurotransmitter activity, and, in turn, regulate the location of Gα in membrane microdomains (such as lipid rafts) or the cytosol. Tubulin binds with nanomolar affinity to Gsα, Giα1 and Gqα (but not other Gα subunits) as well as Gβ1γ2 subunits. Gα subunits destabilize microtubules by stimulating tubulin's GTPase, while Gβγ subunits promote microtubule stability. The same region on Gsα that binds adenylyl cyclase and Gβγ also interacts with tubulin, suggesting that cytoskeletal proteins are novel Gα effectors. Additionally, intracellular Giα-GDP, in concert with other GTPase proteins and Gβγ, regulates the position of the mitotic spindle in mitosis. Thus, G-protein activation modulates cell growth and differentiation by directly altering microtubule stability. Further studies are needed to fully establish a structural mechanism of this interaction and its role in synaptic plasticity.
Key Words: G-protein, Tubulin, Lipid rafts, Microtubule-associated protein, Gs, Gβγ, Mitosis, Neurite outgrowth, GPCR, Synaptogenesis
Introduction
The classical G-protein-signaling pathway involves receptor activation leading to functional Gα and Gβγ dissociation, causing activation of effectors such as adenylyl cyclase, phospholipase C, and ion channels. Our understanding of this pathway has become refined by such concepts as RGS proteins, receptor coupling, G-protein-coupled receptor (GPCR) dimerization, and potentially novel guanine-nucleotide exchange factors. The ultimate effect is to promote alterations in cellular processes such as neurite outgrowth or formation, cellular differentiation, vesicle release and cell division. Many of these processes involve the interface between G-protein and the microtubule cytoskeleton.
Cytoskeletal elements – microtubules, microfilaments and intermediate filaments – play a role in determining cell shape, and processes such as neuronal outgrowth and formation involve alterations of these structures. There is a symbiotic relationship between GPCR signaling and cytoskeletal elements, as microtubules and microfilaments may help to ‘corral’ signaling molecules in membrane microdomains [reviewed in [2]] and G-proteins can directly modulate microtubule function. Note that G12α and G13α indirectly, through rho, alter microfilament stability [see Suzuki et al., this issue]. The focus of this review will be the direct effects of heterotrimeric G-proteins on microtubules, and the resulting changes in cellular morphology and physiology.
A brief introduction to the cytoskeleton, and especially microtubules, is in order. Microtubules are hollow cylinders of tubulin about 20 nm in diameter, and up to hundreds of micrometers long, and are concentrated in axonal and dendritic shafts of neurons. Dendritic spines, and the growth cone itself, do not contain long microtubules, but are richly endowed with heterodimeric tubulin [67]. Tubulin is a physiologically inseparable heterodimer (α and β subunits), each of which bind GTP. Whereas α-tubulin binds GTP irreversibly, β-tubulin shows both reversible binding and intrinsic GTPase activity. β-Tubulin does not require a guanine exchange factor (it binds cytosolic GTP), but its intrinsic GTP hydrolysis rate is very slow [13,14,15]. This rate is accelerated by another tubulin molecule (during the microtubule polymerization process), as well as myriad microtubule-disrupting agents or proteins (e.g., colchicine, taxol, vinblastine and G-proteins) [19, 42, 43, 62]. Tubulin-GTP binds to, and dissociates from, microtubules much faster than tubulin-GDP. This difference establishes an inherent polarity in microtubules that affects their polymerization. The minus end of a microtubule (composed of tubulin-GDP) is typically oriented towards the soma of a neuron, while the more dynamic plus end (containing tubulin-GTP) is located towards the growth cone [58]. Thus, microtubules undergo active polymerization and depolymerization in regions of active synaptic plasticity, and this is modulated by tubulin's GTP hydrolysis rate.
Heterotrimeric G-Proteins Bind Tubulin: A Regulated Process
The first evidence of functional interaction between cytoskeleton and G-proteins came about 25 years ago. Initial studies showed that microtubule disruption by colchicine or vinblastine potentiated isoproterenol- or fluoride-stimulated adenylyl cyclase activity [29, 41, 50]. Later, tubulin was shown to specifically and tightly bind to Gsα, Gqα and Giα1 (KD = 115–130 nM), but not Gtα or Giα2 [60]. Moreover, tubulin and Gsα co-immunoprecipitate from rat brain synaptic membranes [63]. Consistent with Gα-tubulin association, tubulin specifically binds some GPCRs (metabotropic glutamate receptors and melatonin receptors) [12, 27]. More recent studies have identified the molecular interface involved in Gα-tubulin interactions. Initial studies using Giα-Gtα chimeras revealed a requirement for residues 219–295 on Giα in binding tubulin, and residues 237–270 for functional Giα-tubulin interactions [11]. Peptide array studies suggested that homologous regions on Gsα were involved in the interaction with tubulin as well [31]. Functional studies (see below) indicated the involvement of β-tubulin, rather than α-tubulin.
To further identify interacting regions, the Gsα-tubulin complex structure was computationally modeled [31]. Processed PDB structure files of Gsα (‘ligand’) and β-tubulin (‘receptor’) were entered into ZDOCK software [76], resulting in 2,000 complexes. Using ClusPRO software [77], these complexes were grouped into similar sets based on three-dimensional structural similarity (pairwise RMSD criterion), and the largest sets were retained. Thirty final complexes were analyzed for interface regions, interacting residues, electrostatic charge, hydrophobicity and shape fit, and the best 5 complexes were retained. The complex that best fit biochemical data is shown in figure 1 (see legend and Layden et al. [31] for details).
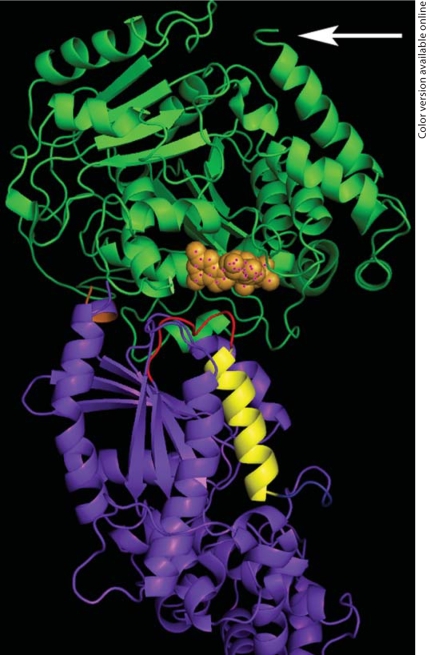
Fig. 1.
Molecular model of Gsα complexed to tubulin. Molecular modeling was used to visualize the Gsα-GTP · tubulin-GDP complex. Tubulin is in green (top); Gsα is in blue (bottom). The C-terminus of tubulin is indicated by an arrow, and N-terminal residues on Gsα are shown in orange. Note that the guanine nucleotide on tubulin (orange spheres) is located near Gsα, thereby allowing Gsα stimulation of tubulin GTPase activity. Also, the α3-β5 loop (red), but not the α3 helix (yellow), on Gsα is in close proximity to tubulin. The former region undergoes a large conformational change upon G-protein activation, and is also involved in the interface with adenylyl cyclase and Gβγ subunits. This model was generated using ZDOCK and ClusPro [31] . The crystal structure of bovine Gsα-GTPγS was solved as a dimer (resolution 2.30 Å, short form) [78]. One Gsα was deleted along with its corresponding ligands. In the remaining Gsα molecule, the Mg2+ and PO4−4 molecules were deleted while GTPγS was included for the final docking structure. Since the sulfur of GTPγS is not included in the GNP (part of the CHARM file), the parameters for the sulfur in Cys were used. ClusPro parameters were 9 Å radius and 1,500 electrostatic hits. Interface residues were defined as any two residues (on different proteins) being within 5 Å of each other. Interacting residues were less than 4 Å apart.
Consistent with the chimera studies, this model of the Gsα-tubulin complex demonstrated that the interaction regions include the adenylyl cyclase/Gβγ interaction regions of Gsα and the exchangeable nucleotide-binding site of tubulin [31]. Specifically, a portion of the amino terminus, α2-β4 (the region between switch II and switch III) and α3-β5 (just distal to the switch III region) domains of Gsα are important for interaction with tubulin (fig. 1). The interaction of Gsα at the exchangeable nucleotide-binding site of tubulin explain the ability of Gα to increase intrinsic tubulin GTPase activity and increasing microtubule dynamics [47].
In order to regulate microtubules, G-proteins must translocate from the membrane to cytosol. NGF treatment of PC-12 cells promotes colocalization of Gsα and tubulin in the cytosol [52]. Similarly, agonist activation causes Gsα internalization, and possibly microtubule association, via lipid raft-derived vesicles [3, 66]. Moreover, Gsα-tubulin interactions may occur in lipid rafts (see below). Consistent with these results, the Gsα-tubulin interaction is sensitive to nocodazole (depolymerizes microtubules) but not latrunculin (depolymerizes microfilaments) [52]. Additionally, functional Gsα-tubulin interactions appear to involve activated Gsα [46]. Note that although Giα, Gsα and Gqα bind tubulin, Gsα is the only heterotrimeric G-protein known to internalize. Therefore, Gsα-tubulin interactions are likely regulated by receptor activation, which causes Gsα to internalize and interact with microtubules. Gβγ subunits also translocate and associate with microtubules subsequent to agonist activation [40].
Lipid Rafts: Potential Sites of Tubulin-Gsα Interactions
There is mounting proteomic [1, 18, 32, 65], biochemical [22, 33, 35, 36], and fluorescent [22, 26] evidence for the localization of G-proteins and tubulin to detergent-resistant lipid raft membrane domains both in vivo and in vitro. Lipid rafts have been shown to be scaffolds for many cell-signaling molecules [for reviews, see [2, 56]]. Recent studies have focused on the function of raft-associated tubulin and microtubules [6, 22, 26, 28, 30, 35, 36].
Proteomics data from a variety of cell types including HeLa cells [18], monocytes [32], Akt-1 cells [1], and neonatal mouse brain neurons [65] have clearly demonstrated the presence of both tubulin and G-proteins in lipid rafts using different analytical techniques. In fact, in these membrane microdomains, G-proteins may be concentrated up to 10-fold compared to the rest of the plasma membrane [18]. Furthermore, proteomic studies have revealed that many other tubulin-binding proteins (including tubulin-specific chaperone A, KIF13, MAPs 1A, 1B, and 2, and stathmin) have been found in lipid rafts via proteomic analysis [32, 65]. One possibility is that tubulin scaffolds Gsα in lipid rafts.
A subpopulation of tubulin is localized to lipid rafts, but its role there is unclear. Currently, there are a number of ongoing studies to determine the function/s of lipid raft localized tubulin. Neuronal tissue and cell lines have been a great source for studying tubulin/raft associations. In fact, there is evidence for two distinct types of detergent-insoluble raft-like domains in myelin with only one of them containing tubulin [6]. The neuron-specific stathmin, SCG10, is a neuronal growth-associated, microtubule-destabilizing factor that has been localized to lipid rafts in postnatal rat brain [33]. It has been shown that SCG10 binds to tubulin heterodimers and plays a role in microtubule dynamics [17, 44]. The fact that SCG10 has been shown to localize to rafts suggests that neuronal rafts are potential sites of cytoskeletal and membrane reorganization. Other studies in an oligodendrocyte model of multiple sclerosis have shown that antibody crosslinking of myelin oligodendrocyte glycoprotein (MOG) leads to repartitioning of MOG into lipid rafts, decreased Fyn signaling, and dephosphorylation of raft-associated β-tubulin [35, 36]. This sequence of events resulted in retraction of oligodendrocytic processes and loss of myelination [35, 36]. These data suggest an organizational role for raft-associated tubulin, but also allows for a role in a MOG-induced signaling cascade. Another potential role for raft tubulin may be in G-protein signaling associated with antidepressant action. It has been shown in C6 glioma cells that the microtubule-disrupting agent colchicine decreases Gsα raft localization similar to chronic antidepressants and either treatment augments the coupling between Gsα and adenylyl cyclase [16].
Non-neuronal cell types have also been a good medium for studying tubulin and lipid raft associations. A CLIP-170-related protein, CLIPR-59, localizes to lipid rafts in mouse embryonic fibroblasts and is the first raft-associated CLIP to be identified [30]. CLIPR-59 has a microtubule-binding domain (MTB) similar to CLIP-170, however this MTB preferentially binds unpolymerized tubulin or small tubulin oligomers and actually prevents microtubule polymerization, again suggesting a role in microtubule dynamics and reorganization at the raft domain [30]. Lipid rafts have also been implicated in the spread of HIV type 1 and it has recently been shown that disruption of the microtubule cytoskeleton with colchicine or nocodazole can disrupt the spread of HIV-1 in T cells [28]. HIV-1 assembly and budding occurs at lipid raft domains on the T-cell plasma membrane and the polarization of the Gag and Env proteins at the rafts is crucial to this process. Disruption of microtubules inhibits the incorporation of Env into virions and viral assembly and budding is blocked [28]. Finally, cardiac myocytes have been used to study the association of adenylyl cyclase-signaling components with rafts [22]. In that study, the microtubule cytoskeleton has been shown to maintain raft/caveolae structure, which serves to inhibit cAMP signaling via the activation adenylyl cyclase. Disruption of the microtubule cytoskeleton with colchicine or the raft structure with methyl-β-cyclodextrin increases β-adrenergic-stimulated cAMP production, suggesting that microtubules and rafts act in concert to tonically inhibit β-adrenergic signaling [22]. Other studies have also implicated lipid rafts as inhibitors of G-protein signaling by demonstrating increased cAMP activity after raft disruption [37, 51] and internalization of activated Gsα via lipid rafts [3]. From all of the studies mentioned it is clear that tubulin and microtubules play a role in maintaining lipid raft structure and function.
Heterotrimeric G-Proteins Modulate Microtubule Dynamics
Although initial studies showed alterations in adenylyl cyclase activity in response to microtubule-disrupting agents, it remained unclear whether this was due to a direct interaction between Gsα and tubulin, or the result of disruption of cellular architecture [41]. In vitro, and in permeabilized cells, tubulin-GPPNHP (tubulin covalently liganded to a non-hydrolyzable GTP analogue) activates Gsα independently of receptor [64]. However, this has not been seen in living cells. Rather, microtubule-disrupting agents may affect a scaffolding or organizing role of membrane tubulin, which can alter the stability of G-protein-signaling complexes. Indeed, treatment of cardiomyocytes with colchicine causes AC activation and promotes translocation of Gsα and its effector adenylyl cyclase into similar membrane domains (non-lipid raft membrane fractions) [22]. Similar effects were seen in S49 cells, which lack lipid rafts, suggesting that Gsα activation of AC occurs outside of these regions. In summary, alterations in cAMP formation due to microtubule-disrupting agents may be a result of alterations in cytoskeletal organization of the membrane rather than direct Gsα-tubulin interactions.
What are the functional consequences of direct Gα-tubulin interactions? Giα was observed to destabilize microtubules by stimulating the tubulin-GTP hydrolysis rate [47]. This effect persisted even if a GTPase-deficient GiαQ204L mutant was used to prevent Giα from hydrolyzing GTP on tubulin. This could not be reversed by addition of exogenous non-hydrolyzable GTP analogues. Consistent with the effect on tubulin GTPase activity, Giα affected microtubule dynamics by increasing the frequency of catastrophes (number of rapid microtubule shortening per second) without affecting rescue frequency or growing or shortening rate [47, 49]. This has the net effect of converting long microtubules into a greater number of shorter microtubules.
Gsα appears to work by a similar mechanism. In response to agonist, Gsα internalizes through caveolae/lipid raft-derived vesicles, thereby facilitating interaction with the plus ends of microtubules that are rich in tubulin-GTP [3]. There, active Gsα stimulates hydrolysis of tubulin-GTP, and likely increases microtubule dynamics and decreases microtubule stability [31, 46]. Moreover, active Gsα may sequester newly released tubulin-GDP to prevent repolymerization. After some time, Gsα hydrolyzes its own GTP to GDP, adopts the inactive conformation, and releases tubulin. In cells, the result is an increase in process formation in response to Gα-activation [11].
One issue that arises is why Gsα and Giα have similar, rather than opposing, effects on microtubule polymerization. One must keep in mind that the designations ‘stimulatory’ and ‘inhibitory’ are somewhat simplistic, and were generated to refer to the activities of these G-proteins on adenylyl cyclase. Indeed, Gsα- and Giα-mediated signaling pathways interact fruitfully with each other in a complex manner. For example, the β2-AR can couple to both Gsα and Giα to regulate airway reactivity in asthma, and Gsα-coupled β-ARs can heterodimerize with Giα-coupled opioid receptors [69,70,71]. Furthermore, while Giα and Gsα interact with different surfaces of adenylyl cyclase, the two proteins probably interact with a similar surface on tubulin [31]. The presumed interface of both Giα and Gsα with tubulin on the G-protein includes the region between switch II and switch III, a region also involved in binding Gβγ and effectors such as adenylyl cyclase (fig. 1). Finally, the effects of Gα subunits on tubulin are direct and independent of adenylyl cyclase.
Recent studies have revealed a role for Gβγ subunits in modulating microtubule polymerization as well. Gβ1γ2, but not Gβ1γ1 or a prenylation-deficient Gβ1γ2 mutant, promotes microtubule polymerization, both in vitro and in cells [40, 46]. This occurs even in the absence of microtubule associated proteins, suggesting a direct interaction between Gβγ and tubulin. Gqα-agonist stimulation of cells causes receptor, Gβγ, and tubulin (but not Gqα) to internalize in clathrin-coated vesicles [68]. Once inside the cell, Gβγ binds along the length of microtubules (but not to dimeric tubulin) to increase microtubule stability [38, 46, 48]. Giα also interferes with the ability of Gβγ to stabilize microtubules, as the latter protein is inactive when preincubated with heterotrimeric Giαβγ [46]. Since the active tubulin-binding interfaces for Gα and Gβγ are probably occluded in the heterotrimer, the heterotrimer may bind tubulin via an alternate binding site on Gα or Gβγ [53]. In conclusion, Gβγ and Gα subunits have opposite effects on microtubules through distinct mechanisms.
Gqα also binds to tubulin with 130 nM affinity, but its effects on tubulin are very different from Gsα and Giα. Stimulation of Gqα-coupled receptors recruits tubulin to the membrane [12,72,73,74]. This interaction involves GTP-tubulin, and occurs on a time course similar to PLC-β1 activation [40]. Microtubule stabilization appears to inhibit this process and microtubule depolymerization mimics it. Activation of Gqα-coupled mGluRs promotes microtubule depolymerization in cells. This may be due to Ca2+ released from intracellular stores as a result of IP3 generation.
Another relationship between G-proteins and cytoskeleton is the role of microtubule and actin filament on translocation of transducin or Gtα translocation in rod photoreceptor cells. Analogous to Gsα, Gtα undergoes a light (‘agonist’)-dependent translocation from the rod outer segment to the inner segment within minutes, and the reverse slowly occurs in the dark [9]. The two segments of rods are connected by a non-motile cilium. Although initial studies proposed the translocation to occur via diffusion, the cytoskeleton also plays a role in this process [44]. Gtα colocalizes with microtubules in dark-adapted retinas. Light-dependent translocation of Gtα did not depend on microfilaments (cytochalasin-D independent) or microtubules (thiabendazole treatment). In contrast, the reverse translocation of Gtα in the dark depends on both microfilaments and microtubules [57]. Note, however, that Gtα does not bind tubulin or microtubules directly [60]. Thus, the mechanisms of ‘agonist’-induced Gsα and Gtα translocation are likely divergent.
Interaction of G-Protein with Microtubules and Cellular Morphology
It seems that G-proteins exert different roles in cells when they associate with specific binding partners. While the interplay of G-proteins with microtubules and tubulin have been studied for decades, its biologic role is not clear. However, recent studies have indicated a role in cellular morphologic change and neuronal differentiation. Overexpression of cytosolic His6-Gi1α in COS-1 cells increases the number and length of cellular processes. Conversely, overexpression of a Giα mutant dominant negative for functional tubulin interactions inhibits process formation in COS-1 cells [11]. In PC-12 cells, NGF treatment promotes translocation of Gα subunits in a microtubule-dependent manner, and promotes colocalization of Gsα with microtubules [52]. Gα and Gβγ subunits have opposing effects on microtubule stability, and both localize to cytosol upon agonist treatment [23, 24, 40, 66]. These observations raise the possibility that, in response to agonist, Gsα internalizes to destabilize microtubules to promote process formation.
Activation of Goα- and Gsα-coupled receptors promote neurite outgrowth [4, 5, 10, 21]. This process may be Goα-dependent, as activated Goα can promote neurite outgrowth, perhaps by releasing Gβγ subunits that stabilize microtubules [25]. Conversely, destabilization of microtubules by Gα subunits may increase microtubule dynamics and may be permissive for the formation of new processes. Indeed, the microtubule-destabilizing proteins, stathmin and SCG10, appear to be required for neurite outgrowth [34, 39]. It has been proposed that microtubule destabilization may be necessary for growth cone guidance and neuronal pathfinding [8]. In conclusion, a dynamic interaction between heterotrimeric G-proteins (both α and βγ subunits) and microtubules alters microtubule stability and may be involved in neuronal differentiation, outgrowth and plasticity.
G-Protein-Microtubule Interactions Modulate Cell Division
In addition to the conventional effect of G-proteins that play a role in signal transduction of extracellular signals from GPCR to their effectors, some G-proteins appear to play important roles in cell division. Various studies in Caenorhabditis elegans, Drosophila and mammalian cells demonstrated the importance of intracellular functions of G-proteins, such as spindle positioning and microtubule pulling force generation. These functions have been found to be independent of GPCRs. GoLoco-containing proteins such as GPR1/2 (in C. elegans), Pins and Loco (in Drosophila) and LGN (in mammalian cells) bind to Gα-GDP, promote release of Gβγ and act as GDIs for Gα, therefore stabilizing Gα-GDP [54, 61]. The complex of Giα-GDP and GoLoco protein bind to the microtubule-binding protein such as Lin5 (in C. elegans), Mud (in Drosophila), and NuMA (in mammalian cells). This trimeric complex binds astral microtubules to orient the mitotic spindle [55]. Ric-8 (also known as synembryn) is an intracellular protein that can activate the G-protein α subunit independently of GPCR. Like GPCRs, Ric-8 behaves as a guanine exchange factor for the Gα subunit that promotes the association of Giα with GTP when it is complexed with GoLoco proteins and microtubule-binding proteins (Lin5, NuMA and Mud) [59]. Since Giα-GTP does not bind NuMA, this complex becomes destabilized and the effects on microtubule stabilization are prevented. Conversely, RGS protein (RGS-7 in C. elegans) acts as a GTPaseactivating protein which catalyzes the conversion of Giα-GTP to Giα-GDP. The net effect is an oscillation of Giα between GTP and GDP states, leading to cycles of astral microtubule stabilization and destabilization that orient the mitotic spindle during mitosis [75].
In addition to Gα, Gβγ has also been reported to play a role in these processes. Studies in C. elegans show that Gβγ regulates migration of the centrosome around the nucleus and the orientation of the mitotic spindle [20]. GPB-1 (a Gβ subunit) is required for the positioning of early cell division axes in C. elegans embryos, while GPC-2 (a Gγ subunit) is required for spindle orientation in the early embryo. Depleting both subunits (by RNAi) results in defective spindle orientation. In human cell lines, Gβγ colocalizes with centrosomes and γ-tubulin in living cells [38]. This association is resistant to nocodoazole, and Gβγ subunits may play a role in stabilizing microtubules at centrosomes during mitosis [38, 48].
Conclusion: A Biologic Rationale for Functional Tubulin-G-Protein Interactions
In this article, we have delineated a novel role for heterotrimeric G-proteins in regulating microtubule stability, process outgrowth and cellular division. These effects appear to be independent of classical effectors – such as adenylyl cyclase, phospholipase C, or ion channels – and appear to involve direct interactions between Gα and Gβγ subunits with tubulin and microtubules. Thus, it should not be surprising that different classes of Gα subunits have similar effects on microtubules, while Gβγ and Gα appear to have opposing effects on microtubule stability.
Upon activation of a GPCR, Gsα functionally dissociates from Gβγ subunits, and internalizes in lipid raft-derived vesicles to interact with tubulin at the plus end of a microtubule (fig. 2). Tubulin may play a scaffolding role, as Gsα-tubulin interactions may occur in lipid rafts. Activated Gsα promotes GTP hydrolysis on tubulin, causing destruction of the microtubule GTP-cap, and microtubule destabilization. This process is likely limited by the autohydrolysis of GTP on Gsα. In contrast, some Gβγ subunits stabilize microtubules by binding along the filament. The interplay between active Gα and Gβγ remains unclear, and, under physiologic conditions, it is possible that these two subunits act on different subsets of microtubules. In vitro, inactive Gsα in a heterotrimer interferes with functional Gβγ-tubulin interactions. The end result is an increase in microtubule dynamics, resulting in process outgrowth in multiple cell types, including PC-12 cells and primary hippocampal neurons. Cytosolic Giα behaves similarly to Gsα, but internalization of Giα has not been observed [6]. A corollary of these studies is that the Gsα-tubulin interface may be a novel target to modulate synaptic plasticity and neuronal morphology. Towards this end, Gsα mimetic peptides have been developed and the functional effects of these peptides in neurons are currently being evaluated [31]. At a structural level, current work is directed towards crystallizing the Gsα-tubulin complex, as well as establishing a mechanism for effects of Gsα on neuronal morphology in living cells.
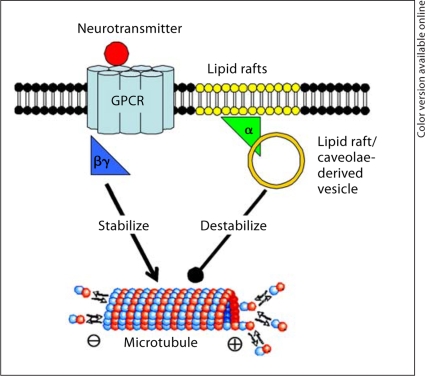
Fig. 2.
Model for G-protein modulation of microtubule dynamics. Upon neurotransmitter stimulation, Gα-GTP internalizes by ‘coating’ caveolar/lipid raft-derived vesicles. The internalized Gα-GTP interacts with either microtubules or tubulin, and stimulates tubulin GTPase activity at the plus end of microtubules. This increases microtubule dynamic instability, and allows for neurotransmitter-dependent plasticity. Conversely, Gβγ subunits stabilize microtubules after internalizing.
Thus, heterotrimeric G-proteins have physiologic roles in cells distinct from their canonical signaling pathways. These processes are regulated by the G-protein activation state and modulated by differential location in membrane or subcellular compartments such as lipid rafts. We are just beginning to appreciate this added complexity to G-protein-signaling pathways, and eagerly anticipate exciting new results that we hope will prove illuminating and insightful.
Acknowledgements
Studies described herein were supported, in part, by grants from the US Public Health Service (MH 39595, DA 20568 and MH78200) and the US Department of Defense Breast Cancer program (W81XWH-07-1-0670). R.H.D. was supported by T32 HL-07692 and the UIC Medical Scientist Training Program. We also thank Sonya Dave for technical contributions.
References
- 1.Adam RM, Yang W, Di Vizio D, Mukhopadhyay NK, Steen H. Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis. BMC Cell Biol. 2008;9:30. doi: 10.1186/1471-2121-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 3.Allen JA, Yu JZ, Donati RJ, Rasenick MM. β-Adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol Pharmacol. 2005;67:1493–1504. doi: 10.1124/mol.104.008342. [DOI] [PubMed] [Google Scholar]
- 4.Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun. 2006;347:678–682. doi: 10.1016/j.bbrc.2006.06.141. [DOI] [PubMed] [Google Scholar]
- 5.Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA. 2005;102:19138–19143. doi: 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitis DN, Min W, Gong Y, Heng YM, Boggs JM. Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin. J Neurochem. 2005;94:1696–1710. doi: 10.1111/j.1471-4159.2005.03331.x. [DOI] [PubMed] [Google Scholar]
- 7.Bünemann M, Frank M, Lohse MJ. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc Natl Acad Sci USA. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnette DT, Schaefer AW, Ji L, Danuser G, Forscher P. Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of aplysia neuronal growth cones. Nat Cell Biol. 2007;9:1360–1369. doi: 10.1038/ncb1655. [DOI] [PubMed] [Google Scholar]
- 9.Calvert PD, Strissel KJ, Schiesser WE, Pugh EN, Jr, Arshavsky VY. Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol. 2006;16:560–568. doi: 10.1016/j.tcb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Cazillis M, Gonzalez BJ, Billardon C, Lombet A, Fraichard A, Samarut J, Gressens P, Vaudry H, Rostène W. VIP and PACAP induce selective neuronal differentiation of mouse embryonic stem cells. Eur J Neurosci. 2004;19:798–808. doi: 10.1111/j.0953-816x.2004.03138.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen NF, Yu JZ, Skiba NP, Hamm HE, Rasenick MM. A specific domain of Giα required for the transactivation of Giα by tubulin is implicated in the organization of cellular microtubules. J Biol Chem. 2003;278:15285–15290. doi: 10.1074/jbc.M300841200. [DOI] [PubMed] [Google Scholar]
- 12.Ciruela F, Robbins MJ, Willis AC, McIlhinney RA. Interactions of the C-terminus of metabotropic glutamate receptor type 1α with rat brain proteins: evidence for a direct interaction with tubulin. J Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- 13.David-Pfeuty T, Laporte J, Pantaloni D. GTPase activity at ends of microtubules. Nature. 1978;272:282–284. doi: 10.1038/272282a0. [DOI] [PubMed] [Google Scholar]
- 14.David-Pfeuty T, Simon C, Pantaloni D. Effect of antimitotic drugs on tubulin GTPase activity and self-assembly. J Biol Chem. 1979;254:11696–11702. [PubMed] [Google Scholar]
- 15.Davis A, Sage CR, Dougherty CA, Farrell KW. Microtubule dynamics modulated by guanosine triphosphate hydrolysis activity of β-tubulin. Science. 1994;264:839–842. doi: 10.1126/science.8171338. [DOI] [PubMed] [Google Scholar]
- 16.Donati RJ, Rasenick MM. Chronic antidepressant treatment prevents accumulation of Gs in cholesterol-rich, cytoskeletal-associated plasma membrane domains (lipid rafts) Neuropsychopharmacology. 2005;30:1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- 17.Fleury D, Grenningloh G, Lafanechene L, Antonsson B, Job D, Cohen-Addad C. Preliminary crystallographic study of a complex formed between the α/β-tubulin heterodimer and the neuronal growth-associated protein SCG10. J Struct Biol. 2000;131:156–158. doi: 10.1006/jsbi.2000.4283. [DOI] [PubMed] [Google Scholar]
- 18.Foster LJ, de Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435:519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 20.Gotta M, Ahringer J. Axis determination in C. elegans: initiating and transducing polarity. Curr Opin Genet Dev. 2001;11:367–373. doi: 10.1016/s0959-437x(00)00206-9. [DOI] [PubMed] [Google Scholar]
- 21.He JC, Neves SR, Jordan JD, Iyengar R. Role of the Go/i signaling network in the regulation of neurite outgrowth. Can J Physiol Pharmacol. 2006;84:687–694. doi: 10.1139/y06-025. [DOI] [PubMed] [Google Scholar]
- 22.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem. 2006;281:26391–26399. doi: 10.1074/jbc.M602577200. [DOI] [PubMed] [Google Scholar]
- 23.Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor. J Biol Chem. 2004;279:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- 24.Hynes TR, Tang L, Mervine SM, Sabo JL, Yost EA, Devreotes PN, Berlot CH. Visualization of G-protein βγ dimers using bimolecular fluorescence complementation demonstrates roles for both β and γ in subcellular targeting. J Biol Chem. 2004;279:30279–30286. doi: 10.1074/jbc.M401432200. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi M, Strittmatter SM, Vartanian T, Fishman MC. Mediation by G-proteins of signals that cause collapse of growth cones. Science. 1993;259:77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- 26.Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581:1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 27.Jarzynka MJ, Passey DK, Ignatius PF, Melan MA, Radio NM, Jockers R, Rasenick MM, Brydon L, Witt-Enderby PA. Modulation of melatonin receptors and G-protein function by microtubules. J Pineal Res. 2006;41:324–336. doi: 10.1111/j.1600-079X.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 28.Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy MS, Insel PA. Inhibitors of microtubule assembly enhance β-adrenergic and prostaglandin E1-stimulated cyclic AMP accumulation in S49 lymphoma cells. Mol Pharmacol. 1979;16:215–223. [PubMed] [Google Scholar]
- 30.Lallemand-Breitenbach V, Quesnoit M, Braun V, El Marjou A, Pous C, Goud B, Perez F. CLIPR-59 is a lipid raft-associated protein containing a cytoskeleton-associated protein glycine-rich domain (CAP-Gly) that perturbs microtubule dynamics. J Biol Chem. 2004;279:41168–41178. doi: 10.1074/jbc.M406482200. [DOI] [PubMed] [Google Scholar]
- 31.Layden BT, Saengsawang W, Donati RJ, Yang S, Mulhearn DC, Johnson ME, Rasenick MM. Structural model of a complex between the heterotrimeric G-protein, Gsα, and tubulin. Biochim Biophys Acta. 2008;1783:964–973. doi: 10.1016/j.bbamcr.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Shaw AR, Zhang N, Mak A, Li L. Lipid raft proteomics: analysis of in-solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins by liquid chromatography-matrix-assisted laser desorption/ionization tandem mass spectrometry. Proteomics. 2004;4:3156–3166. doi: 10.1002/pmic.200400832. [DOI] [PubMed] [Google Scholar]
- 33.Maekawa S, Morii H, Kumanogoh H, Sano M, Naruse Y, Sokawa Y, Mori N. Localization of neuronal growth-associated, microtubule-destabilizing factor SCG10 in brain-derived raft membrane microdomains. J Biochem. 2001;129:691–697. doi: 10.1093/oxfordjournals.jbchem.a002908. [DOI] [PubMed] [Google Scholar]
- 34.Manna T, Grenningloh G, Miller HP, Wilson L. Stathmin family protein SCG10 differentially regulates the plus and minus end dynamics of microtubules at steady state in vitro: implications for its role in neurite outgrowth. Biochemistry. 2007;46:3543–3552. doi: 10.1021/bi061819d. [DOI] [PubMed] [Google Scholar]
- 35.Marta CB, Montano MB, Taylor CM, Taylor AL, Bansal R, Pfeiffer SE. Signaling cascades activated upon antibody cross-linking of myelin oligodendrocyte glycoprotein: potential implications for multiple sclerosis. J Biol Chem. 2005;280:8985–8993. doi: 10.1074/jbc.M413174200. [DOI] [PubMed] [Google Scholar]
- 36.Marta CB, Taylor CM, Coetzee T, Kim T, Winkler S, Bansal R, Pfeiffer SE. Antibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphology. J Neurosci. 2003;23:5461–5471. doi: 10.1523/JNEUROSCI.23-13-05461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura Y, Hanada K, Jones TL. G(s) signaling is intact after disruption of lipid rafts. Biochemistry. 2001;40:15418–15423. doi: 10.1021/bi015574a. [DOI] [PubMed] [Google Scholar]
- 38.Montoya V, Gutierrez C, Najera O, Leony D, Varela-Ramirez A, Popova J, Rasenick MM, Das S, Roychowdhury S. G-protein βγ subunits interact with αβ- and γ-tubulin and play a role in microtubule assembly in PC12 cells. Cell Motil Cytoskeleton. 2007;64:936–950. doi: 10.1002/cm.20234. [DOI] [PubMed] [Google Scholar]
- 39.Morii H, Shiraishi-Yamaguchi Y, Mori N. SCG10, a microtubule-destabilizing factor, stimulates the neurite outgrowth by modulating microtubule dynamics in rat hippocampal primary cultured neurons. J Neurobiol. 2006;66:1101–1114. doi: 10.1002/neu.20295. [DOI] [PubMed] [Google Scholar]
- 40.Popova JS, Rasenick MM. Gβγ mediates the interplay between tubulin dimers and microtubules in the modulation of Gq signaling. J Biol Chem. 2003;278:34299–34308. doi: 10.1074/jbc.M301748200. [DOI] [PubMed] [Google Scholar]
- 41.Rasenick MM, Stein PJ, Bitensky MW. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981;294:560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- 42.Rasenick MM, Donati RJ, Popova JS, Yu J-Z. Tubulin as a regulator of G-protein signaling. Meth Enzymol. 2004;390:389–403. doi: 10.1016/S0076-6879(04)90024-9. [DOI] [PubMed] [Google Scholar]
- 43.Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 44.Reidel B, Goldmann T, Giessl A, Wolfrum U. The translocation of signaling molecules in dark adapting mammalian rod photoreceptor cells is dependent on the cytoskeleton. Cell Motil Cytoskeleton. 2008;65:785–800. doi: 10.1002/cm.20300. [DOI] [PubMed] [Google Scholar]
- 45.Riederer BM, Pellier V, Antonsson B, Di Paolo G, Stimpson SA, Lutjens R, Catsicas S, Grenningloh G. Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proc Natl Acad Sci USA. 1997;94:741–745. doi: 10.1073/pnas.94.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roychowdhury S, Martinez L, Salgado L, Das S, Rasenick MM. G-protein activation is prerequisite for functional coupling between Gα/Gβγ and tubulin/microtubules. Biochem Biophys Res Commun. 2006;340:441–448. doi: 10.1016/j.bbrc.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 47.Roychowdhury S, Panda D, Wilson L, Rasenick MM. G-protein α subunits activate tubulin GTPase and modulate microtubule polymerization dynamics. J Biol Chem. 1999;274:13485–13490. doi: 10.1074/jbc.274.19.13485. [DOI] [PubMed] [Google Scholar]
- 48.Roychowdhury S, Rasenick MM. G-protein β1γ2 subunits promote microtubule assembly. J Biol Chem. 1997;272:31576–31581. doi: 10.1074/jbc.272.50.31576. [DOI] [PubMed] [Google Scholar]
- 49.Roychowdhury S, Rasenick MM. Submembraneous microtubule cytoskeleton: regulation of microtubule assembly by heterotrimeric G-proteins. FEBS J. 2008;275:4654–4663. doi: 10.1111/j.1742-4658.2008.06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudolph SA, Hegstrand LR, Greengard P, Malawista SE. The interaction of colchicine with hormone-sensitive adenylate cyclase in human leukocytes. Mol Pharmacol. 1979;16:805–812. [PubMed] [Google Scholar]
- 51.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 52.Sarma T, Voyno-Yasenetskaya T, Hope TJ, Rasenick MM. Heterotrimeric G-proteins associate with microtubules during differentiation in PC12 pheochromocytoma cells. FASEB J. 2003;17:848–859. doi: 10.1096/fj.02-0730com. [DOI] [PubMed] [Google Scholar]
- 53.Sato M, Cismowski MJ, Toyota E, Smrcka AV, Lucchesi PA, Chilian WM, Lanier SM. Identification of a receptor-independent activator of G-protein signaling (AGS8) in ischemic heart and its interaction with Gβγ. Proc Natl Acad Sci USA. 2006;103:797–802. doi: 10.1073/pnas.0507467103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaefer M, Petronczki M, Dorner D, Forte M, Knoblich JA. Heterotrimeric G-proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 55.Siller KH, Cabernard C, Doe CQ. The NuMA-related MUD protein binds PINS and regulates spindle orientation in Drosophila neuroblasts. Nat Cell Biol. 2006;8:594–600. doi: 10.1038/ncb1412. [DOI] [PubMed] [Google Scholar]
- 56.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 57.Slepak VZ, Hurley JB. Mechanism of light-induced translocation of arrestin and transducin in photoreceptors: interaction-restricted diffusion. IUBMB Life. 2008;60:2–9. doi: 10.1002/iub.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas CJ, Tall GG, Adhikari A, Sprang SR. Ric-8A catalyzes guanine nucleotide exchange on Gαi1 bound to the GPR/GoLoco exchange inhibitor AGS3. J Biol Chem. 2008;283:23150–23160. doi: 10.1074/jbc.M802422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang N, Yan K, Rasenick MM. Tubulin binds specifically to the signal-transducing proteins, Gsα and Giα1. J Biol Chem. 1990;265:1239–1242. [PubMed] [Google Scholar]
- 61.Willard FS, Kimple RJ, Siderovski DP. Return of the GDI: the GoLoco motif in cell division. Annu Rev Biochem. 2004;73:925–951. doi: 10.1146/annurev.biochem.73.011303.073756. [DOI] [PubMed] [Google Scholar]
- 62.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by taxol. Proc Natl Acad Sci USA. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan K, Green E, Belga F, Rasenick MM. Synaptic membrane G-proteins are complexed with tubulin in situ. J Neurochem. 1996;66:1489–1495. doi: 10.1046/j.1471-4159.1996.66041489.x. [DOI] [PubMed] [Google Scholar]
- 64.Yan K, Popova JS, Moss A, Shah B, Rasenick MM. Tubulin stimulates adenylyl cyclase activity in C6 glioma cells by bypassing the β-adrenergic receptor: a potential mechanism of G-protein activation. J Neurochem. 2001;76:182–190. doi: 10.1046/j.1471-4159.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Wakim B, Li M, Halligan B, Tint S, Patel SB. Quantifying raft proteins in neonatal mouse brain by ‘tube-gel’ protein digestion label-free shotgun proteomics. Proteome Sci. 2007;5:17. doi: 10.1186/1477-5956-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu JZ, Rasenick MM. Real-time visualization of a fluorescent Gαs: dissociation of the activated G-protein from plasma membrane. Mol Pharmacol. 2002;61:352–359. doi: 10.1124/mol.61.2.352. [DOI] [PubMed] [Google Scholar]
- 67.Van Rossum D, Hanisch UK. Cytoskeletal dynamics in dendritic spines: direct modulation by glutamate receptors? Trends Neurosci. 1999;22:290–295. doi: 10.1016/s0166-2236(99)01404-6. [DOI] [PubMed] [Google Scholar]
- 68.Popova JS, Rasenick MM. Clathrin-mediated endocytosis of M3 muscarinic receptors. Roles for Gβγ and tubulin. J Biol Chem. 2004;279:30410–30418. doi: 10.1074/jbc.M402871200. [DOI] [PubMed] [Google Scholar]
- 69.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with β2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci USA. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G-proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 71.McGraw DW, Elwing JM, Fogel KM, Wang WC, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest. 2007;117:1391–1398. doi: 10.1172/JCI30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Popova JS, Rasenick MM. Muscarinic receptor activation promotes the membrane association of tubulin for the regulation of Gq-mediated phospholipase Cβ1 signaling. J Neurosci. 2000;20:2774–2782. doi: 10.1523/JNEUROSCI.20-08-02774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Popova JS, Greene AK, Wang J, Rasenick MM. Phosphatidylinositol 4,5-bisphosphate modifies tubulin participation in phospholipase Cβ1 signaling. J Neurosci. 2002;22:1668–1678. doi: 10.1523/JNEUROSCI.22-05-01668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciruela F, McIlhinney RA. Metabotropic glutamate receptor type 1α and tubulin assemble into dynamic interacting complexes. J Neurochem. 2001;76:750–758. doi: 10.1046/j.1471-4159.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- 75.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G-proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 76.Chen R, Tong W, Mintseris J, Li L, Weng Z. ZDOCK predictions for the CAPRI challenge. Proteins. 2003;52:68–73. doi: 10.1002/prot.10388. [DOI] [PubMed] [Google Scholar]
- 77.Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004;32:W96–W99. doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsα. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]