Abstract
The increased prevalence of overweight adults has serious health consequences. Epidemiological studies suggest an association between low activity and being over-weight; however, few studies have objectively measured activity during a period of weight gain, so it is unknown whether low activity is a cause or consequence of being overweight. To determine whether individual differences in adult weight gain are linked to an individual's activity level, we measured activity, via accelerometry, over a prolonged period (9 mo) in 18 adult female rhesus monkeys. Weight, food intake, metabolic rate, and activity were first monitored over a 3-mo period. During this period, there was mild but significant weight gain (5.5 ± 0.88%; t =−6.3, df = 17, P < 0.0001), whereas caloric intake and activity remained stable. Metabolic rate increased, as expected, with weight gain. Activity level correlated with weight gain (r = −0.52, P = 0.04), and the most active monkeys gained less weight than the least active monkeys (t = −2.74, df = 8, P = 0.03). Moreover, there was an eightfold difference in activity between the most and least active monkeys, and initial activity of each monkey was highly correlated with their activity after 9 mo (r = 0.85, P < 0.0001). In contrast, food intake did not correlate with weight gain, and there was no difference in weight gain between monkeys with the highest vs. lowest caloric intake, total metabolic rate, or basal metabolic rate. We conclude that physical activity is a particularly important factor contributing to weight change in adulthood and that there are large, but stable, differences in physical activity among individuals.
Keywords: exercise, obesity, weight gain, energy balance
Epidemiological studies indicate that body weight and body fat increase through early and middle adulthood such that by late middle age there is an increased percentage of overweight and obese individuals compared with the early adult period (33, 57, 63, 108, 115). Weight gain over the adult years has escalated over the past two decades such that 65% of adults in the United States have a body mass index (BMI) above the healthy range (>25.0 kg/m2) (32). Weight gain and obesity in adulthood have been associated with overall increases in morbidity and mortality (82) and in the risk of diabetes mellitus (23, 33, 53, 115a), gall bladder disease (115a), coronary heart disease (41, 49, 53, 69, 86, 111, 115a), hypertension (53, 115a), stroke (115a), dyslipidemia (115a), osteoarthritis (115a), gout (115a), pulmonary diseases (115a), colon cancer (35), and breast cancer (118).
A large body of epidemiologic data shows an association between low levels of physical activity and a higher rate of adult weight gain, and a greater increase in percent body fat, throughout adulthood (14, 24, 44, 51, 54, 65, 87, 97, 114). However, most of these studies rely on self-reporting of physical activity, and several studies have shown that self-reporting of activity can be inaccurate and problematic (50, 72, 75, 105). Accelerometry, an objective way to monitor physical activity, recently has been used to show that obese children and adults have lower activity levels than their lean counterparts (1, 28, 45, 96, 105, 106). Similarly, studies that objectively measured activity in rodents also showed that obese individuals have lower activity levels compared with lean individuals (21, 60). However, it is unclear from these studies whether low activity is a cause or consequence of obesity. Accelerometry would be ideal for measuring the contribution of individual differences in activity to adult weight gain but would require the wearing of activity monitors during the prolonged periods over which adult weight gain takes place. In fact, two studies in mice that objectively measured activity found that weight gain in adulthood was negatively correlated with activity level (15, 26), supporting the notion that low activity is a cause of obesity.
In the current study, we maintained adult monkeys wearing accelerometers over a prolonged period (i.e., 9 mo), allowing us to determine whether individual differences in adult weight gain differed in monkeys exhibiting low activity vs. high physical activity. Food intake and metabolic rate were measured to allow assessment of the relative contribution of physical activity level to adult weight gain. Results of this study show that of all of the parameters that we measured, an individual's level of physical activity is the strongest predictor of weight gain in ovariectomized female monkeys.
MATERIALS AND METHODS
Animals
Eighteen adult female rhesus monkeys (Macaca mulatta) 9–13 yr of age, weighing between 4.7 and 11.1 kg, and living in individual stainless steel cages (32 × 24 × 27 or 32 × 34 × 27 in.) in a temperature-controlled room (24 ± 2°C) with lights on for 12 h per day (0700–1900) were studied. Approximately 1 yr before initiation of this study, the monkeys had been ovariectomized and maintained on a high-fat diet (35% fat) to approximate the conditions experienced by many postmenopausal women in the Western world (112). The high-fat diet was formulated according to the recipe developed by Clarkson and colleagues (94, 112) to study diet-induced atherosclerosis. Monkeys were fed ad libitum with meals provided at ~0915 and 1515. All aspects of the study were reviewed and approved by the Oregon National Primate Research Center Animal Care and Use Committee and were performed according to federal guidelines.
Experimental Design
The goal of this experiment was to determine whether the activity level of an individual is predictive of weight gain over a period of time in adulthood during which food intake is stable and there is slow progressive weight gain. In addition, other parameters known to influence weight gain, such as food intake and metabolic rate, were measured. The experimental period was 9 mo in duration, during which time the activity level of each monkey was measured continuously using a three-way accelerometer. During the first 3 mo of the study, the weight of each monkey was measured weekly, food intake was quantified at each meal, and percent body fat was determined at the beginning and end of the study. Metabolic rate was measured over a 4-h period at the beginning of the study and for 24 h at the end of the first 3 mo. Morning metabolic rate during fasting was compared between the two time points. During the last 6 mo, activity was continuously monitored to allow assessment of the stability of this physiological measure.
Experimental Measures
Body weight
Body weight was measured weekly at ~0800, before the morning meal.
Dual-energy X-ray absorptiometry scans
Percent body fat was determined using dual-energy X-ray absorptiometry. Animals were sedated with Telazol (3 mg/kg im; Fort Dodge Animal Health, Fort Dodge, IA) supplemented with ketamine HCl (10 mg/kg im Ketaset; Fort Dodge Animal Health) and were positioned supine on the bed of a Lunar DPX scanner (Lunar, Madison, WI). Total body scans were done in the “pediatric medium” scan mode with a voltage of 76 kV. Lunar software version 3.4 was used to calculate body composition. Two or three scans at each time period were performed per monkey, and body fat was calculated as a percentage of total body mass.
Calorie intake
Each monkey was fed more food than she routinely consumed at each meal to ensure ad libitum food intake. Total food consumption at each meal was recorded daily throughout the study by quantifying the amount of food remaining before the next meal. On 1 day during the study, the total amount of stool excreted in a 24-h period was collected from each monkey by placing a metal pan covered with wire mesh under each monkey's cage for 24 h. The amount of stool was weighed, and a representative sample was collected at 0900 the next morning and immediately frozen at −20°C. The caloric content of a sample of stool from the two monkeys that consumed the most calories and the two monkeys that consumed the least number of calories was determined using bomb calorimetry (Kinetica, Franklin, OH) to quantify differences in calories excreted vs. calories absorbed.
Metabolic rate
Metabolic rate of each monkey was measured by placing the monkey in a sealed Lexan and stainless steel metabolic chamber (Columbus Instruments, Columbus, OH) and measuring the amount of carbon dioxide produced and oxygen consumed with a computer-controlled indirect open-circuit calorimeter (Oxymax system; Columbus Instruments). The metabolic chamber was approximately the same size as the monkey's home cage (inside dimensions: 30 × 24 × 24 in.). To prevent social isolation during metabolic testing, we placed two monkeys familiar with the test monkey in cages across from and in clear view of the animal in the metabolic testing chamber at all times. The familiar monkeys were animals that were housed in the same room as the test monkey before and after metabolic chamber test periods. Before each recording session, the oxygen and carbon dioxide sensors were calibrated with a standard mixture of gases (20.5% oxygen, 0.5% carbon dioxide, and nitrogen balance). Fresh air was pumped into the chamber (12–40 l/min) with an external fresh air pump controlled by a flowmeter (Columbus Instruments) and was circulated within the chamber with a 4-in. fan. The flow rate into the chamber was adjusted for each monkey so that the difference in oxygen concentration between the chamber and the room air was >0.2% and the carbon dioxide level in the chamber was <0.6%. The chamber air was sampled at a rate of 0.5 l/min and was circulated over a water-absorbent (Drierite) column before passing through the oxygen and carbon dioxide sensors. The oxygen and carbon dioxide concentrations of the ambient air and chamber air were recorded every 4 min. Oxygen consumption, carbon dioxide production, and total energy expenditure (kcal) were calculated using Oxymax software version 2.3 (Columbus Instruments). The Oxymax system calculated oxygen consumption (V̇O2) by taking the difference between input oxygen flow and output oxygen flow. Similarly, carbon dioxide production (V̇CO2) was calculated by taking the difference between output and input carbon dioxide flows. To determine energy expenditure, we calculated the respiratory exchange ratio (RER), the ratio of V̇CO2 to V̇O2, and the energy expenditure (EE) using the following equation: EE = (3.82 + 1.23 × RER) × V̇O2 × 0.001. Upon study initiation, monkeys were individually placed in the metabolic chamber at 0900 and remained in the chamber until 1300. The day before testing, the monkey was fed its standard meal, and at 1700, all food was removed from the monkey's cage and the monkey was fasted until completion of metabolic testing. After 3 mo, 24-h metabolic rate of the monkeys was assessed. Monkeys (n = 16) were placed in the metabolic chamber at 1000 and remained in the chamber until 0900 the next morning. Before placement in the chamber, monkeys were fed a standard meal at 0915 and were fed a 114 ± 1-g banana at 1515 while in the chamber. Basal metabolic rate was calculated as the average number of kilocalories expended per hour from 2300 to 0300. This time period was selected because this is when monkeys typically sleep, and this is when heart rate is typically slowest (Cameron J, unpublished observations). In addition, this was the time when monkeys exhibited the lowest number of activity counts in this study. The thermic effect of an isocaloric meal (the banana fed at 1515) was calculated by subtracting basal metabolic rate and activity-associated energy expenditure from total energy expenditure for the 4 h after the banana was consumed. Studies have shown that the majority of energy expended due to meal digestion and processing is within the first 4 h after a meal is eaten (12, 84, 98).
Activity
The naturally occurring activity level of each monkey was assessed using triaxial Actical accelerometers (MiniMitter, Bend, OR). The Actical monitor contains an omnidirectional sensor capable of detecting acceleration in all directions. The sensor integrates the speed and distance of acceleration and produces an electrical current that varies in magnitude depending on a change in acceleration. An increased speed or distance of the acceleration, or a change in direction, produces an increase in electrical current. The activity monitors store this information as activity counts.
Each monkey was fitted with a loose-fitting metal collar (Primate Products, Immokalee, FL) with an activity monitor mounted on it, housed in a snug, protective stainless steel box. The monitor was programmed to store the total number of activity counts per minute. These monitors are capable of storing data for up to 45 days. During the study period, monkeys were sedated with ketamine HCl (10–20 mg/kg im Ketaset; Fort Dodge Animal Health), and the data from each activity monitor were downloaded at least every 45 days. After the data were downloaded and saved, the activity monitor was reprogrammed and replaced on the collar. Activity counts recorded from 0700 to 1900 (when lights were on) were considered daytime activity, and activity counts recorded from 1900 to 0700 (when lights were off) were considered nighttime activity. Activity-associated energy expenditure was calculated by determining the energy expended (in kcal) per activity count. This was calculated by measuring total energy expenditure at times of day in which there would be little thermic effect of food contributing to the metabolic rate (from 1400 to 1500 and 1800 to 1900), subtracting basal metabolic rate, and dividing the remaining energy expenditure by the number of activity counts occurring during this time period. The number of calories expended per activity count was multiplied by total daily activity counts to determine daily activity-associated energy expenditure.
The duration of time that the monkeys were sedentary was assessed on a representative day from the week of initial activity measurement by determining how many minutes the monkeys had no activity counts.
Data Analysis
Activity was analyzed during a representative week at the beginning of the study, after 3 mo, and again after 9 mo. Body weight, average weekly food intake, and fasted morning metabolic rate were compared upon study initiation and after 3 mo. The associations between all measurements (food intake, total energy expenditure, basal metabolic rate, thermic effect of food, and activity) and weight gain were determined. Regression analysis demonstrated that lean body mass was the best predictor of total energy expenditure (R2 = 0.52) and basal metabolic rate (R2 = 0.58); thus, after the raw values were analyzed, the adjusted residuals also were analyzed for their association with weight gain.
For all analyses, normality and homoscedasticity were initially tested. Initially, multivariate regression analysis was used to determine which variable (basal metabolic rate, activity, and food intake) was best able to predict weight gain. If data were normally distributed, paired t-tests were used to evaluate differences between measures made at two different time points, and repeated-measures ANOVA was used to evaluate differences in activity measurements made upon initiation of the study, after 3 mo, and after 9 mo. Independent t-tests were utilized to assess differences in amount of weight gained by monkeys divided into groups of the highest and lowest quartiles based on food intake, activity, and metabolic rate. Correlations between measurements were determined using Pearson product moment correlation. If data were not normally distributed and could not be transformed (using a square root or log transformation), then nonpara-metric tests were utilized. The Wilcoxon signed ranks test was used to assess differences in nonnormally distributed data over time. Spear-man's Rho correlation was used to analyze correlations between parameters that were not normally distributed. Linear regression analysis was performed to develop an equation for predicting the energy expenditure in kilocalories per activity count. Data are presented as means ± SE. Alpha values were considered significant if P ≤ 0.05. Statistical analyses were performed with SPSS software, version 13.0 (SPSS, Chicago, Illinois).
RESULTS
During the first 3 mo of the study, the group showed a small but significant gain in body weight (5.5 ± 0.88%, t = −6.3, df = 17, P < 0.0001; Table 1). There were large differences in weight gain between individual monkeys, with several monkeys gaining no weight during this experimental period, whereas others gained up to 13% of their initial weight in 3 mo. Body fat also increased significantly in the group, from 15.9 ± 3.0 to 18.87 ± 3.4% of total body mass during this time period (z = −3.17, P = 0.002; Table 1). Initial body fat was not correlated with weight (r = −0.19, P = 0.45) or fat gain (r = −0.17, P = 0.51), and there was no difference in weight gain or fat gain between the monkeys who were in the leanest vs. fattest quartile at the beginning of the study (t = 0.33, df = 8, P = 0.75; t =−1.3, df = 8, P = 0.24 for weight and fat gain, respectively). In addition, the age of the monkeys was not correlated with weight gain (r = −0.25, P = 0.33) or fat gain (r = 0.05, P = 0.86), and there were no differences in the amount of weight or fat gained between the youngest and oldest monkeys (t = 0.04, df = 8, P = 0.97; t = −0.11, df = 8, P = 0.92 for weight and fat gain, respectively).
Table 1.
Metabolic parameters across 3 mo of weight gain
| Initial | Final | |
|---|---|---|
| Body weight, kg | 7.54±0.48 | 7.94±0.49* |
| body fat, % | 15.9±3.0 | 18.8±3.5* |
| Food intake, kcal | 1,188±129 | 1,113±105 |
| Energy expenditure, kcal/h | 21.3±2.3 | 28.7±2.9* |
| Activity, counts/day | 291,296±35,805 | 233,651±37,983 |
Values are means ± SE. Asterisks indicate a significant difference from initial measures.
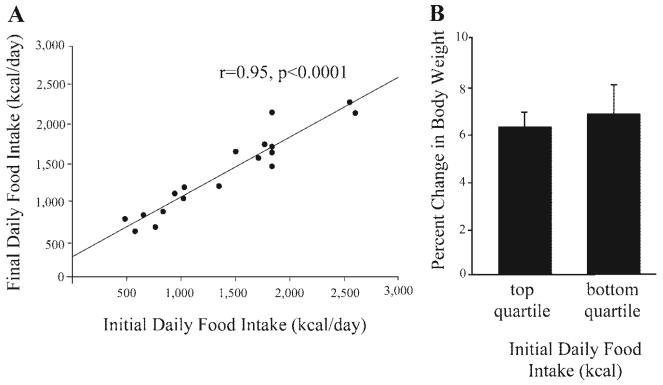
Initially, a multivariate regression analysis determined that when food intake, basal metabolic rate, and activity were used as independent variables, body weight gain could not be significantly predicted (R2 = 0.27, F3,12 = 1.50, P = 0.27). However, activity was a better predictor of weight gain (P = 0.07) than food intake (P = 0.73) and basal metabolic rate (P = 0.87). Food intake was not significantly changed during the 3-mo period (t = 1.04, df = 17, P = 0.31; Table 1). Although there were considerable individual differences in the amount of calories consumed by individual animals (a 5-fold difference, ranging from 411 to 2,210 kcal/day), the food intake of individual monkeys was consistent over this 3-mo time period, such that the initial food intake of each individual was highly correlated with food intake after 3 mo (r = 0.95, P < 0.0001; Fig. 1A). However, food intake did not correlate with the amount of weight gained by each monkey during this time period (r = 0.15, P = 0.56). In addition, there was no difference in the weight gain of the quartile of monkeys that ate the most compared with that of the quartile of monkeys that ate the least (t = −0.20, df = 8, P = 0.85; Fig. 1B). To begin to look at whether food intake accurately predicts calorie absorption, we measured the caloric content of the stool in the two monkeys eating the most and the two eating the least calories. Mean caloric content of the four stool samples was 1.70 ± 0.28 kcal/g, and the caloric content of the stool samples was correlated with food intake such that the monkeys that ate the most excreted more calories per gram of stool (r = 0.95, P = 0.046, 2.1-fold difference between monkeys with the highest and lowest food intake).
Fig. 1.
A: food intake for individual monkeys remained stable. There was a correlation between food intake at study initiation and after 3 mo (r = 0.95, P < 0.0001). B: however, the quartile of monkeys eating the most food showed the same percent change in body weight as the quartile that ate the least amount of food (t =−0.20, df = 8, P = 0.85).
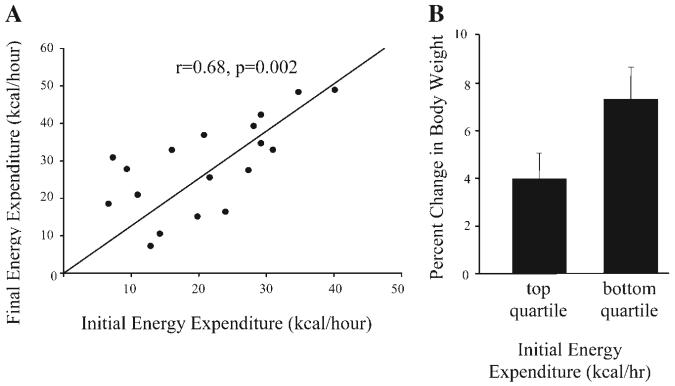
Daily energy expenditure significantly increased from 21.3 ± 2.3 to 28.7 ± 2.9 kcal/h (t = −3.46, df = 17, P = 0.003; Table 1) over the first 3 mo of the experimental period. Initial daily energy expenditure was correlated with final daily energy expenditure (r = 0.68, P = 0.002; Fig. 2A). Moreover, the change in daily energy expenditure correlated with weight gain (r = 0.47, P = 0.05) such that the monkeys that gained the most weight increased their daily energy expenditure the most. There was a sixfold difference in daily energy expenditure between individual monkeys. However, initial daily energy expenditure did not correlate with weight gain (r = −0.35, P = 0.16), and although percent weight gain was somewhat higher in the quartile of monkeys with the lowest daily energy expenditure (7.4% weight gain) compared with the quartile of monkeys with the highest daily energy expenditure (4.0% weight gain), this was not a significant difference (t = 2.08, df = 8, P = 0.07; Fig. 2B). Once total energy expenditure was adjusted for lean body mass by regression analysis, there was only a 2.6-fold difference in energy expenditure between individual monkeys. However, adjusted daily energy expenditure did not correlate with weight gain (r = −0.04, P = 0.89), and there was no difference in weight gain between monkeys in the quartile with the highest adjusted daily energy expenditure compared with the quartile of monkeys with the lowest adjusted daily energy expenditure (t = 0.04, df = 6.1, P = 0.97).
Fig. 2.
A: correlation between daily energy expenditure at study initiation and after 3 mo (r = 0.68, P = 0.002). B: the change in body weight over 3 mo between the monkeys in the top and bottom quartiles of energy expenditure was not significantly different (t = 2.08, df = 8, P = 0.07).
The average basal metabolic rate was 291 ± 19 kcal/day and ranged from 172 to 406 kcal/day. On average, basal metabolic rate accounted for 61% of total daily energy expenditure, ranging from 47 to 83% of total energy expenditure in individual monkeys. Basal metabolic rate did not correlate with weight gain (r = 0.08, P = 0.75), and weight gain was not different between the quartile of monkeys with the highest basal metabolic rate and the quartile of monkeys with the lowest basal metabolic rate (t = 0.40, df = 8, P = 0.70). In addition, basal metabolic rate adjusted for lean body mass with the use of regression analysis was not correlated with weight gain (r = −0.04, P = 0.89), and there was no difference in weight gain between the quartile that had the highest adjusted basal metabolic rate and the quartile with the lowest adjusted basal metabolic rate (t = 0.04, df = 6.1, P = 0.97).
The mean thermic effect of a 108-calorie meal was 19.9 ± 3.2 kcal and ranged from 8.5 to 59.3 kcal (a 7-fold difference) between individual monkeys. There was no significant difference in the weight gain in the monkeys with the highest thermic effect of the meal and the monkeys with the lowest thermic effect of the meal (t = −1.81, df = 8, P = 0.11).
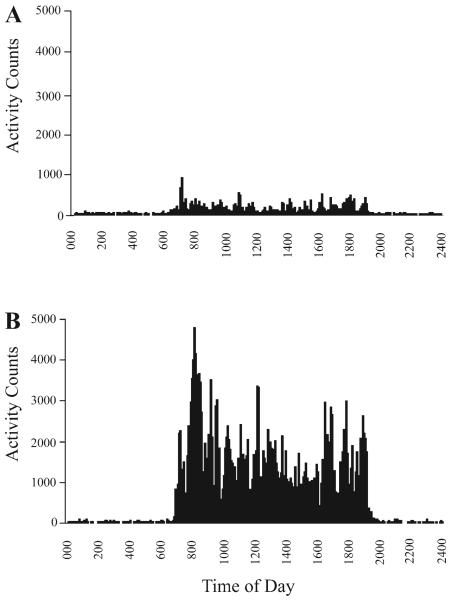
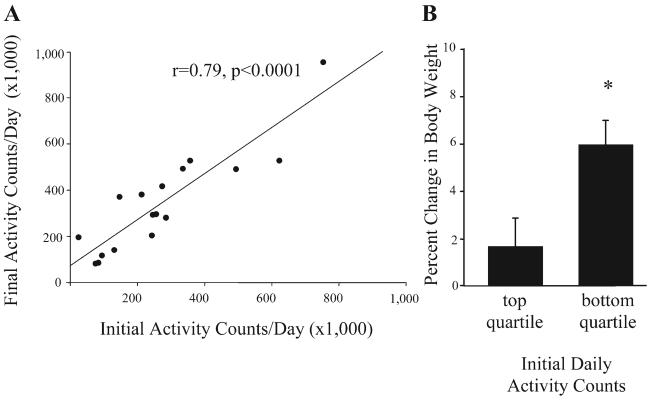
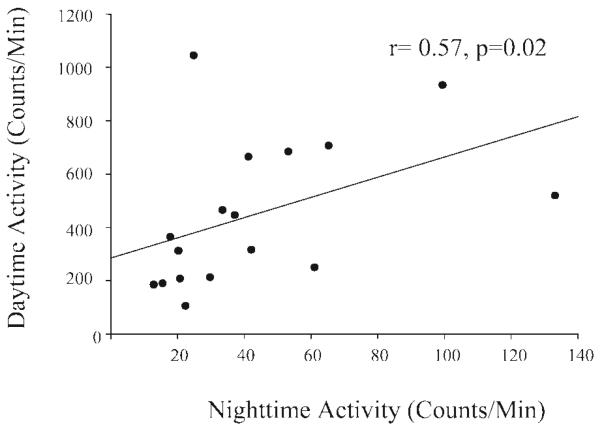
There was an eightfold difference in activity between the most active and most sedentary monkey (Fig. 3), with the most sedentary monkey displaying a mean of 92,110 ± 7,873 activity counts per day and the most active monkey displaying 770,446 ± 110,476 activity counts per day. The number of activity counts per day did not change significantly during the 3-mo period (t = 1.15, df = 15, P = 0.27; Table 1), and each monkey's daily activity level (counts/day) was consistent over time such that the number of activity counts per day initially recorded for each monkey was highly correlated with the number of activity counts per day recorded after 3 mo (r = 0.79, P < 0.0001; Fig. 4A). There was a significant correlation between the number of daily activity counts and weight gain such that the most active monkeys gained less weight than the least active monkeys (r = −0.52, P = 0.04). The quartile of monkeys that were most active gained significantly less weight during the 3-mo period than the quartile of monkeys that were least active (t = −2.7, df = 8, P = 0.03; Fig. 4B). To follow up this initial finding, we measured activity over an additional 6 mo and found that the number of activity counts per day remained stable (F1,16 = 1.13, P = 0.30) and that the number of activity counts initially recorded for each monkey was highly correlated with the number of activity counts recorded for that monkey after 9 mo (r = 0.85, P < 0.0001). There was a 10-fold difference in the number of activity counts recorded during the day between individual monkeys, and 96% of total daily activity occurred during daylight hours. Interestingly, although nighttime activity accounted for only 4% of total daily activity, there also was a 10-fold difference in nighttime activity. Nighttime activity was positively correlated with daytime activity such that the monkeys that were the most active during the day were also the most active at night (r = 0.57, P = 0.02; Fig. 5).
Fig. 3.
The most sedentary monkey (A) was 8 times less active than the most active monkey (B).
Fig. 4.
A: there was a significant correlation between physical activity at study initiation and after 3 mo (r = 0.79, P < 0.0001). B: the quartile of monkeys that had the lowest physical activity had significantly greater weight gain than the quartile of monkeys that had the highest physical activity (t = −2.7, df = 8, P = 0.03). *Significant difference in percent change in body weight between groups.
Fig. 5.
Nighttime activity was significantly correlated with daytime activity (r = 0.57, P = 0.02).
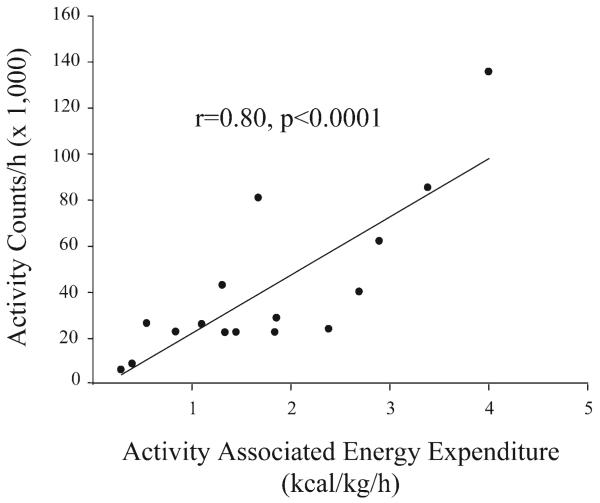
Activity counts correlated strongly with activity-associated energy expenditure (adjusted for body mass) during the time periods from both 0200 to 0300 (r = 0.80, P < 0.0001; Fig. 6) and 0600 to 0700 (r = 0.74, P = 0.001; data not shown). The regression equation for calculation of activity-associated energy expenditure (AEE) was similar at both times of day [0200–0300: AEE = (number of activity counts × 0.000025) + 0.71; 0600–0700: AEE = (number of activity counts × 0.000021) + 0.54]. On average, 0.045 ± 0.006 kcal were expended per kilogram of body weight per 1,000 activity counts. Average activity-associated energy expenditure was 109 ± 14 kcal/day and ranged from 24 to 206 kcal/day. On average, 18 ± 3% of total energy was expended by physical activity, with physical activity accounting for 8–43% of total daily energy expenditure in individual monkeys. The quartile of monkeys that expended the most calories due to activity gained significantly less weight than the quartile of monkeys that expended the least amount of energy due to activity (t = −2.85, df = 4.6, P = 0.04).
Fig. 6.
Correlation between activity counts and activity-associated energy expenditure measured simultaneously from 0200 to 0300 (r = 0.80, P < 0.0001).
Further analysis of the activity data revealed that there was an inverse correlation between the number of daily activity counts and the number of minutes that the monkeys were completely inactive such that the least active monkeys were inactive more than the most active monkeys (r = −0.51, P = 0.046). However, there was no correlation between the number of minutes that the monkeys were inactive and weight gain (r = 0.39, P = 0.13).
DISCUSSION
In this study, we objectively measured individual monkeys' levels of physical activity, as well as other components of energy balance (caloric intake, metabolic rate) over a period of weight gain in adulthood when monkeys were eating a stable diet. There was a slow but significant increase in body weight (5.5%) during the experimental period. However, calorie in-take and physical activity level remained stable during this period. The amount of weight gained was predicted by physical activity level such that the most active monkeys gained significantly less weight than the least active monkeys. This finding shows a very strong relationship between an individual's physical activity level and its tendency to gain weight and suggests that physical activity is an important determinant of body weight gain in adulthood. Activity level differed eightfold between monkeys, but the activity level of an individual was remarkably consistent throughout the 9-mo experimental period, suggesting that activity level is an intrinsic property of an individual.
In this study, the most active individuals were less likely to gain weight than the most sedentary individuals during a period of stable dietary intake in adulthood. This finding supports the epidemiologic data showing that low levels of physical activity predict greater increases in body weight and body fat and that high levels of physical activity prevent or limit weight and fat gain (14, 24, 44, 54, 65, 87, 97, 114). Most of these reports relied on self-reporting of physical activity. However, Levine et al. (62) directly assessed posture allocation in 10 obese and 10 lean individuals and found that obese individuals were seated significantly more than lean individuals and that lean individuals were standing more than obese individuals. Our findings also support findings reporting that activity level in mice is negatively correlated with weight gain (15, 26). Our study represents the first direct measure of activity during a period of adult weight gain in a primate species and strongly indicates that inactivity is an important factor contributing to adult weight gain.
Our findings also are supported by studies that used accelerometry to objectively monitor physical activity and found that obese individuals have lower activity levels than individuals of normal weight (1, 28, 29, 45, 96, 105, 106) and by studies in rodents showing that obese mice have lower activity levels than their lean counterparts (21, 60). However, it has been unclear from these studies whether low activity is a cause or consequence of obesity. Our findings suggest that individual differences in physical activity levels play an important causal role in adult weight gain.
Interestingly, we found large individual difference in nighttime activity (10-fold). This finding is supported by Mehlman et al. (74), who also found a 10-fold range in duration of nighttime activity in free-ranging male rhesus monkeys. This study also showed that nighttime activity was correlated with daytime activity, suggesting that the mechanisms controlling activity at these two time periods are similar. A positive correlation between daytime and nighttime activity is supported by studies in children with attention-deficit hyperactivity disorder, showing that these children are more active than controls during both the day and the night (22). The mechanisms that regulate physical activity are currently not well understood. However, studies have shown that peptides that regulate food intake, such as leptin (76, 83, 89, 90), ghrelin (17, 70), pancreatic polypeptide (58, 77, 107), cholecystokinin (92), the neurotransmitters serotonin (11, 42, 79, 80), glutamate (25, 101), dopamine (30, 89, 100, 117), norepinephrine (19, 89), nitric oxide synthase (27, 47), and β-endorphin (43), and the hormone estrogen (104, 109), all play potential roles in the regulation of physical activity. In addition, several brain regions have been implicated in the regulation of activity, predominantly the reticular activating formation (89). Also, lesion studies have shown that areas of the basal forebrain, ventromedial hypothalamus, paraventricular nucleus, amygdala, and thalamus all play possible roles in activity regulation (89). The mechanisms that regulate nighttime activity have received much less attention. However, two recent studies began to address this issue. Orexin A injection in the paraventricular nucleus increases activity during both the day and night in rats, suggesting that orexin A may play a role in regulating both daytime and nighttime activity (48). Also, central nervous system serotonin turnover has been shown to be inversely correlated with nighttime activity in male rhesus monkeys (74).
Activity-associated energy expenditure was associated with weight gain, and monkeys that expended the most energy by being physically active gained significantly less weight than monkeys that expended the least energy by being physically active. On average, 18 ± 3% of total energy was expended by physical activity; however, individual differences in the percentage of total energy expenditure due to activity ranged from 8 to 43% of total energy expenditure. This is similar to findings in humans showing that the amount of total energy expenditure due to activity averages 30% (110) and ranges from 21 to 51% (61).
Individual differences in the number of calories consumed per day were great and ranged from 411 to 2,210 kcal/day. The number of calories consumed by each monkey remained stable during the experimental period; however, food intake was not predictive of weight gain. This parallels previous studies in humans that failed to find an association between individual caloric intake and individual weight gain or body fat gain (3, 5, 7, 38, 46, 66, 71, 73, 85, 95, 116). This frequent failure to find an association between weight gain and caloric intake may reflect the large role that individual differences in activity level play in regulating body weight. Interestingly, in our study the number of calories excreted per gram of stool was correlated with energy intake such that the individuals with the highest caloric intake excreted twice as many calories per gram of stool. The fact that individuals absorb fewer calories when they consume more calories is well documented in the animal literature (10, 13, 91) but is not generally considered in human studies. Individual differences in energy absorption may contribute to the lack of association between caloric intake and weight gain.
Measurements of energy expenditure in this study were similar to what has been previously reported in rhesus monkeys (9, 55). Daily energy expenditure increased over the period of weight gain and was correlated with change in body weight such that the monkeys that gained the most weight increased their energy expenditure the most. The finding that energy expenditure increases with increased body weight has been documented in studies with humans (4, 16, 36, 78, 88), so it is not surprising that energy expenditure would increase as the volume of metabolically active tissue increases. Although there was a change in energy expenditure, the initial energy expenditure of each individual monkey was correlated with that monkey's final energy expenditure. However, we found that the weight gain of monkeys with the highest energy expenditure did not differ from the weight gain of the monkeys with the lowest energy expenditure. In addition, when energy expenditure was normalized for lean body mass with the use of regression analysis, there was still no difference in weight gain between the monkeys with the highest adjusted energy expenditure and the lowest adjusted energy expenditure. We note that in no monkey did energy balance (intake minus expenditure) equal zero. Energy that was excreted in the stool and thus not absorbed would account in part for this discrepancy. This has been reported to be 6.3% in rhesus monkeys (56). Energy excreted in urine and in skin cells, hair, and nails also was not accounted for.
We determined that the average basal metabolic rate accounted for 61% of total daily energy expenditure and ranged from 47 to 83% of total energy expenditure in individual monkeys. Similarly, the basal metabolic rate of humans accounts for 60% of total energy expenditure (78, 110), ranging from 22 to 83% (8, 61). Basal metabolic rate also did not predict weight gain in this study. In addition, when basal metabolic rate was adjusted for lean body mass with the use of regression analysis, there was still no difference in weight gain between the monkeys with the highest adjusted basal metabolic rate and the monkeys with the lowest adjusted basal metabolic rate. These findings are supported by previous studies in both humans and mice showing that individuals with a low metabolic rate are not more susceptible to weight gain than individuals with a high metabolic rate (40, 93). However, there are certain populations in which low metabolic rate predicts weight gain. For example, in Pima Indians, low metabolic rate is a risk factor for weight gain (102). In addition, children with stunted growth because of poor nutrition (37) and children with Down syndrome (68) have a lower basal metabolic rate and are more prone to obesity and weight gain than individuals in a control population. Basal metabolic rate accounts for the largest proportion of energy expenditure; however, the lack of relationship between low basal metabolic rate and weight gain suggests that differences in basal metabolic rate within the normal range are not as likely to underlie weight gain as differences in activity.
Ovariectomy (surgical menopause) is associated with changes in energy balance. We have previously shown that ovariectomy is associated with a rapid increase in caloric intake (29%) and weight (~3%) in female rhesus monkeys (99). In addition, it is well documented in small animals (mice, rats, cats; Refs. 2, 20, 31, 34) that ovariectomy leads to a 14–21% increase in weight within several weeks of ovariectomy. Also, it is well documented that along with weight gain, an increase in BMI and an increase in adiposity occur during the menopausal transition in women (18, 39, 52, 64, 67, 103, 113). Thus it is important to note that the monkeys in this study were ovariectomized. It is possible that the relationships between energy balance parameters are different in the ovariectomized vs. the ovary-intact state. Thus caution should be used in extending the findings we report in this study to all weight gain over adulthood. Future studies are needed to objectively measure activity in gonad-intact females and males over periods of adult weight gain.
In conclusion, this study shows that physical activity level is the best predictor of weight gain in adulthood in ovariectomized female monkeys consuming a diet typical of that consumed in the Western world. This finding suggests that the best way to prevent weight gain over adulthood is to focus on living an active lifestyle, as opposed to only dieting. However, even though a high percentage of adults in Western countries are overweight and/or obese, there is little evidence that people are routinely opting for a more active lifestyle. More than 60% of Americans do not participate in the recommended amount of physical activity, and 25% are inactive (59, 81). Although physicians routinely advocate that obese patients adopt a more active lifestyle, the results of this study suggest that this strategy deserves greater emphasis.
ACKNOWLEDGMENTS
We are grateful to the Division of Animal Resources at the Oregon National Primate Research Center for expert care of the monkeys used in this study and to Darla Kneeland, Diana Takahashi, Lindsay Pranger, and Meghan Martin for technical assistance.
GRANTS
This work was supported by National Institutes of Health Grants DK-55819, HD-18185, and RR-00163.
REFERENCES
- 1.Abbott RA, Davies PS. Habitual physical activity and physical activity intensity: their relation to body composition in 5.0–10.5-y-old children. Eur J Clin Nutr. 2004;58:285–291. doi: 10.1038/sj.ejcn.1601780. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 3.Baecke JA, van Staveren WA, Burema J. Food consumption, habitual physical activity, and body fatness in young Dutch adults. Am J Clin Nutr. 1983;37:278–286. doi: 10.1093/ajcn/37.2.278. [DOI] [PubMed] [Google Scholar]
- 4.Bandini LG, Schoeller DA, Edwards J, Young VR, Oh SH, Dietz WH. Energy expenditure during carbohydrate overfeeding in obese and nonobese adolescents. Am J Physiol Endocrinol Metab. 1989;256:E357–E367. doi: 10.1152/ajpendo.1989.256.3.E357. [DOI] [PubMed] [Google Scholar]
- 5.Bellisle F, Rolland-Cachera MF, Deheeger M, Guilloud-Bataille M. Obesity and food intake in children: evidence for a role of metabolic and/or behavioral daily rhythms. Appetite. 1988;11:111–118. doi: 10.1016/s0195-6663(88)80010-2. [DOI] [PubMed] [Google Scholar]
- 7.Birkbeck JA. Obesity, socioeconomic variables and eating habits in New Zealand. J Biosoc Sci. 1981;13:299–307. doi: 10.1017/s002193200001350x. [DOI] [PubMed] [Google Scholar]
- 8.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 9.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 10.Blaxter KL. Energy Metabolism in Animals and Man. Cambridge University Press; New York: 1989. [Google Scholar]
- 11.Borer KT, Bonna R, Kielb M. Hippocampal serotonin mediates hypoactivity in dietarily obese hamsters: a possible manifestation of aging? Pharmacol Biochem Behav. 1988;31:885–892. doi: 10.1016/0091-3057(88)90400-5. [DOI] [PubMed] [Google Scholar]
- 12.Brehm BJ, Spang SE, Lattin BL, Seeley RJ, Daniels SR, D'Alessio DA. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J Clin Endocrinol Metab. 2005;90:1475–1482. doi: 10.1210/jc.2004-1540. [DOI] [PubMed] [Google Scholar]
- 13.Brody S. Bioenergetics and Growth. Reinhold; New York: 1945. [Google Scholar]
- 14.Brown WJ, Williams L, Ford JH, Ball K, Dobson AJ. Identifying the energy gap: magnitude and determinants of 5-year weight gain in midage women. Obes Res. 2005;13:1431–1441. doi: 10.1038/oby.2005.173. [DOI] [PubMed] [Google Scholar]
- 15.Brownlow BS, Petro A, Feinglos MN, Surwit RS. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996;60:37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Lifetime exercise activity and breast cancer risk among post-menopausal women. Br J Cancer. 1999;80:1852–1858. doi: 10.1038/sj.bjc.6690610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- 18.Chang CJ, Wu CH, Yao WJ, Yang YC, Wu JS, Lu FH. Relationships of age, menopause and central obesity on cardiovascular disease risk factors in Chinese women. Int J Obes Relat Metab Disord. 2000;24:1699–1704. doi: 10.1038/sj.ijo.0801457. [DOI] [PubMed] [Google Scholar]
- 19.Christin L, O'Connell M, Bogardus C, Danforth E, Jr, Ravussin E. Norepinephrine turnover and energy expenditure in Pima Indian and white men. Metabolism. 1993;42:723–729. doi: 10.1016/0026-0495(93)90239-k. [DOI] [PubMed] [Google Scholar]
- 20.Chu SC, Chou YC, Liu JY, Chen CH, Shyu JC, Chou FP. Fluctuation of serum leptin level in rats after ovariectomy and the influence of estrogen supplement. Life Sci. 1999;64:2299–2306. doi: 10.1016/s0024-3205(99)00181-2. [DOI] [PubMed] [Google Scholar]
- 21.Clark LD, Gay PE. Activity and body-weight relationships in genetically obese animals. Biol Psychiatry. 1972;4:247–250. [PubMed] [Google Scholar]
- 22.Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): a review of naturalistic and stimulant intervention studies. Sleep Med Rev. 2004;8:379–402. doi: 10.1016/j.smrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, Speizer FE. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 24.Di Pietro L, Dziura J, Blair SN. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord. 2004;28:1541–1547. doi: 10.1038/sj.ijo.0802821. [DOI] [PubMed] [Google Scholar]
- 25.Donzanti BA, Uretsky NJ. Effects of excitatory amino acids on locomotor activity after bilateral microinjection into the rat nucleus accumbens: possible dependence on dopaminergic mechanisms. Neuropharmacology. 1983;22:971–981. doi: 10.1016/0028-3908(83)90213-7. [DOI] [PubMed] [Google Scholar]
- 26.Dunnington EA, White JM, Vinson WE. Genetic parameters of serum cholesterol levels, activity and growth in mice. Genetics. 1977;85:659–668. doi: 10.1093/genetics/85.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzoljic E, De Vries R, Dzoljic MR. New and potent inhibitors of nitric oxide synthase reduce motor activity in mice. Behav Brain Res. 1997;87:209–212. doi: 10.1016/s0166-4328(97)02281-x. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U, Aman J, Yngve A, Renman C, Westerterp K, Sjostrom M. Physical activity but not energy expenditure is reduced in obese adolescents: a case-control study. Am J Clin Nutr. 2002;76:935–941. doi: 10.1093/ajcn/76.5.935. [DOI] [PubMed] [Google Scholar]
- 29.Ekelund U, Sardinha LB, Anderssen SA, Harro M, Franks PW, Brage S, Cooper AR, Andersen LB, Riddoch C, Froberg K. Associations between objectively assessed physical activity and indicators of body fatness in 9- to 10-y-old European children: a population-based study from 4 distinct regions in Europe (the European Youth Heart Study) Am J Clin Nutr. 2004;80:584–590. doi: 10.1093/ajcn/80.3.584. [DOI] [PubMed] [Google Scholar]
- 30.Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 31.Fettman MJ, Stanton CA, Banks LL, Hamar DW, Johnson DE, Hegstad RL, Johnston S. Effects of neutering on bodyweight, metabolic rate and glucose tolerance of domestic cats. Res Vet Sci. 1997;62:131–136. doi: 10.1016/s0034-5288(97)90134-x. [DOI] [PubMed] [Google Scholar]
- 32.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 33.Folsom AR, Jacobs DR, Jr, Wagenknecht LE, Winkhart SP, Yunis C, Hilner JE, Savage PJ, Smith DE, Flack JM. Increase in fasting insulin and glucose over seven years with increasing weight and inactivity of young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144:235–246. doi: 10.1093/oxfordjournals.aje.a008918. [DOI] [PubMed] [Google Scholar]
- 34.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 36.Goran MI, Figueroa R, McGloin A, Nguyen V, Treuth MS, Nagy TR. Obesity in children: recent advances in energy metabolism and body composition. Obes Res. 1995;3:277–289. doi: 10.1002/j.1550-8528.1995.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 37.Grillol LP, Siqueira AF, Silva AC, Martins PA, Verreschi IT, Sawaya AL. Lower resting metabolic rate and higher velocity of weight gain in a prospective study of stunted vs nonstunted girls living in the shantytowns of São Paulo, Brazil. Eur J Clin Nutr. 2005;59:835–842. doi: 10.1038/sj.ejcn.1602150. [DOI] [PubMed] [Google Scholar]
- 38.Guillaume M, Lapidus L, Lambert A. Obesity and nutrition in children. The Belgian Luxembourg Child Study IV. Eur J Clin Nutr. 1998;52:323–328. doi: 10.1038/sj.ejcn.1600532. [DOI] [PubMed] [Google Scholar]
- 39.Haffner SM, Katz MS, Dunn JF. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes. 1991;15:471–478. [PubMed] [Google Scholar]
- 40.Hambly C, Adams A, Fustin JM, Rance KA, Bunger L, Speak-man JR. Mice with low metabolic rates are not susceptible to weight gain when fed a high-fat diet. Obes Res. 2005;13:556–566. doi: 10.1038/oby.2005.59. [DOI] [PubMed] [Google Scholar]
- 41.Hamm P, Shekelle RB, Stamler J. Large fluctuations in body weight during young adulthood and twenty-five-year risk of coronary death in men. Am J Epidemiol. 1989;129:312–318. doi: 10.1093/oxfordjournals.aje.a115135. [DOI] [PubMed] [Google Scholar]
- 42.Heisler LK, Kanarek RB, Homoleski B. Reduction of fat and protein intakes but not carbohydrate intake following acute and chronic fluoxetine in female rats. Pharmacol Biochem Behav. 1999;63:377–385. doi: 10.1016/s0091-3057(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 43.Hill C, Lapanowski K, Dunbar JC. The effects of β-endorphin (β-END) on cardiovascular and behavioral dynamics in conscious rats. Brain Res Bull. 2002;59:29–34. doi: 10.1016/s0361-9230(02)00834-1. [DOI] [PubMed] [Google Scholar]
- 44.Hunter GR, Byrne NM. Physical activity and muscle function but not resting energy expenditure impact on weight gain. J Strength Cond Res. 2005;19:225–230. doi: 10.1519/14123.1. [DOI] [PubMed] [Google Scholar]
- 45.Janz KF, Levy SM, Burns TL, Torner JC, Willing MC, Warren JJ. Fatness, physical activity, and television viewing in children during the adiposity rebound period: the Iowa Bone Development Study. Prev Med. 2002;35:563–571. doi: 10.1006/pmed.2002.1113. [DOI] [PubMed] [Google Scholar]
- 46.Johnson ML, Burke BS, Mayer J. Relative importance of inactivity and overeating in the energy balance of obese high school girls. Am J Clin Nutr. 1956;4:37–44. doi: 10.1093/ajcn/4.1.37. [DOI] [PubMed] [Google Scholar]
- 47.Khedara A, Goto T, Morishima M, Kayashita J, Kato N. Elevated body fat in rats by the dietary nitric oxide synthase inhibitor, l-Nω nitroarginine. Biosci Biotechnol Biochem. 1999;63:698–702. doi: 10.1271/bbb.63.698. [DOI] [PubMed] [Google Scholar]
- 48.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–E559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 49.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 50.Klesges RC, Eck LH, Mellon MW, Fulliton W, Somes GW, Hanson CL. The accuracy of self-reports of physical activity. Med Sci Sports Exerc. 1990;22:690–697. doi: 10.1249/00005768-199010000-00022. [DOI] [PubMed] [Google Scholar]
- 51.Klesges RC, Klesges LM, Haddock CK, Eck LH. A longitudinal analysis of the impact of dietary intake and physical activity on weight change in adults. Am J Clin Nutr. 1992;55:818–822. doi: 10.1093/ajcn/55.4.818. [DOI] [PubMed] [Google Scholar]
- 52.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–202. [PubMed] [Google Scholar]
- 53.Kujala UM, Kaprio J, Taimela S, Sarna S. Prevalence of diabetes, hypertension, and ischemic heart disease in former elite athletes. Metabolism. 1994;43:1255–1260. doi: 10.1016/0026-0495(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 54.Kyle UG, Gremion G, Genton L, Slosman DO, Golay A, Pichard C. Physical activity and fat-free and fat mass by bioelectrical impedance in 3853 adults. Med Sci Sports Exerc. 2001;33:576–584. doi: 10.1097/00005768-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lane MA, Baer DJ, Tilmont EM, Rumpler WV, Ingram DK, Roth GS, Cutler RG. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995;50:B295–B302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- 57.Lara-Castro C, Weinsier RL, Hunter GR, Desmond R. Visceral adipose tissue in women: longitudinal study of the effects of fat gain, time, and race. Obes Res. 2002;10:868–874. doi: 10.1038/oby.2002.119. [DOI] [PubMed] [Google Scholar]
- 58.Lassmann V, Vague P, Vialettes B, Simon MC. Low plasma levels of pancreatic polypeptide in obesity. Diabetes. 1980;29:428–430. doi: 10.2337/diab.29.6.428. [DOI] [PubMed] [Google Scholar]
- 59.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and coronary heart disease risk in men: does the duration of exercise episodes predict risk? Circulation. 2000;102:981–986. doi: 10.1161/01.cir.102.9.981. [DOI] [PubMed] [Google Scholar]
- 60.Levin BE. Spontaneous motor activity during the development and maintenance of diet-induced obesity in the rat. Physiol Behav. 1991;50:573–581. doi: 10.1016/0031-9384(91)90548-3. [DOI] [PubMed] [Google Scholar]
- 61.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 62.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 63.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 64.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 65.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes Relat Metab Disord. 2005;29:524–533. doi: 10.1038/sj.ijo.0802886. [DOI] [PubMed] [Google Scholar]
- 66.Lorenzo V, Martin M, Rufino M, Sanchez E, Jimenez A, Hernandez D, Torres A. High prevalence of overweight in a stable Spanish hemodialysis population: a cross sectional study. J Ren Nutr. 2003;13:52–59. doi: 10.1053/jren.2003.50004. [DOI] [PubMed] [Google Scholar]
- 67.Lovejoy JC. The influence of sex hormones on obesity across the female life span. J Womens Health. 1998;7:1247–1256. doi: 10.1089/jwh.1998.7.1247. [DOI] [PubMed] [Google Scholar]
- 68.Luke A, Roizen NJ, Sutton M, Schoeller DA. Energy expenditure in children with Down syndrome: correcting metabolic rate for movement. J Pediatr. 1994;125:829–838. doi: 10.1016/s0022-3476(94)70087-7. [DOI] [PubMed] [Google Scholar]
- 69.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 70.Matsuda K, Miura T, Kaiya H, Maruyama K, Uchiyama M, Kangawa K, Shioda S. Stimulatory effect of n-octanoylated ghrelin on locomotor activity in the goldfish, Carassius auratus. Peptides. 2006;27:1335–1340. doi: 10.1016/j.peptides.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 71.Matter S, Weltman A, Stamford BA. Body fat content and serum lipid levels. J Am Diet Assoc. 1980;77:149–152. [PubMed] [Google Scholar]
- 72.Matthews CE, Freedson PS. Field trial of a three-dimensional activity monitor: comparison with self report. Med Sci Sports Exerc. 1995;27:1071–1078. doi: 10.1249/00005768-199507000-00017. [DOI] [PubMed] [Google Scholar]
- 73.Maxfield E, Konishi F. Patterns of food intake and physical activity in obesity. J Am Diet Assoc. 1966;49:406–408. [PubMed] [Google Scholar]
- 74.Mehlman PT, Westergaard GC, Hoos BJ, Sallee FR, Marsh S, Suomi SJ, Linnoila M, Higley JD. CSF 5-HIAA and nighttime activity in free-ranging primates. Neuropsychopharmacology. 2000;22:210–218. doi: 10.1016/S0893-133X(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 75.Melanson EL, Jr, Freedson PS. Validity of the Computer Science and Applications, Inc (CSA) activity monitor. Med Sci Sports Exerc. 1995;27:934–940. [PubMed] [Google Scholar]
- 76.Nagy TR, Gower BA, Shewchuk RM, Goran MI. Serum leptin and energy expenditure in children. J Clin Endocrinol Metab. 1997;82:4149–4153. doi: 10.1210/jcem.82.12.4435. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima M, Inui A, Teranishi A, Miura M, Hirosue Y, Okita M, Himori N, Baba S, Kasuga M. Effects of pancreatic polypeptide family peptides on feeding and learning behavior in mice. J Pharmacol Exp Ther. 1994;268:1010–1014. [PubMed] [Google Scholar]
- 78.Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–856. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen T, Porter J, Svec F. Dehydroepiandrosterone (DHEA) decreases open-field spontaneous activity of Zucker rats. Physiol Behav. 1999;67:725–731. doi: 10.1016/s0031-9384(99)00132-8. [DOI] [PubMed] [Google Scholar]
- 80.Nonogaki K, Abdallah L, Goulding EH, Bonasera SJ, Tecott LH. Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT2C receptor mutant mice. Diabetes. 2003;52:315–320. doi: 10.2337/diabetes.52.2.315. [DOI] [PubMed] [Google Scholar]
- 81.Oguma Y, Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and all cause mortality in women: a review of the evidence. Br J Sports Med. 2002;36:162–172. doi: 10.1136/bjsm.36.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 83.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 84.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63:164–169. doi: 10.1093/ajcn/63.2.164. [DOI] [PubMed] [Google Scholar]
- 85.Ries W. Feeding behaviour in obesity. Proc Nutr Soc. 1973;32:187–193. doi: 10.1079/pns19730038. [DOI] [PubMed] [Google Scholar]
- 86.Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, Willett WC. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 87.Rissanen AM, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Determinants of weight gain and overweight in adult Finns. Eur J Clin Nutr. 1991;45:419–430. [PubMed] [Google Scholar]
- 88.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–R192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 89.Rowland TW. The biological basis of physical activity. Med Sci Sports Exerc. 1998;30:392–399. doi: 10.1097/00005768-199803000-00009. [DOI] [PubMed] [Google Scholar]
- 90.Salbe AD, Nicolson M, Ravussin E. Total energy expenditure and the level of physical activity correlate with plasma leptin concentrations in five-year-old children. J Clin Invest. 1997;99:592–595. doi: 10.1172/JCI119200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scotellaro PA, Ji LL, Gorski J, Oscai LB. Body fat accretion: a rat model. Med Sci Sports Exerc. 1991;23:275–279. [PubMed] [Google Scholar]
- 92.Sei M, Sei H, Shima K. Spontaneous activity, sleep, and body temperature in rats lacking the CCK-A receptor. Physiol Behav. 1999;68:25–29. doi: 10.1016/s0031-9384(99)00146-8. [DOI] [PubMed] [Google Scholar]
- 93.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–674. [PubMed] [Google Scholar]
- 94.Shadoan MK, Anthony MS, Rankin SE, Clarkson TB, Wagner JD. Effects of tibolone and conjugated equine estrogens with or without medroxyprogesterone acetate on body composition and fasting carbohydrate measures in surgically postmenopausal monkeys. Metabolism. 2003;52:1085–1091. doi: 10.1016/s0026-0495(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 95.Stefanik PA, Heald FP, Jr, Mayer J. Caloric intake in relation to energy output of obese and non-obese adolescent boys. Am J Clin Nutr. 1959;7:55–62. doi: 10.1093/ajcn/7.1.55. [DOI] [PubMed] [Google Scholar]
- 96.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP., Jr Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc. 2005;37:1195–1202. doi: 10.1249/01.mss.0000170083.41186.b1. [DOI] [PubMed] [Google Scholar]
- 97.Sternfeld B, Wang H, Quesenberry CP, Jr, Abrams B, Everson-Rose SA, Greendale GA, Matthews KA, Torrens JI, Sowers M. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 98.St-Pierre DH, Karelis AD, Cianflone K, Conus F, Mignault D, Rabasa-Lhoret R, St-Onge M, Tremblay-Lebeau A, Poehlman ET. Relationship between ghrelin and energy expenditure in healthy young women. J Clin Endocrinol Metab. 2004;89:5993–5997. doi: 10.1210/jc.2004-0613. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res. 2005;13:2072–2080. doi: 10.1038/oby.2005.257. [DOI] [PubMed] [Google Scholar]
- 100.Swanson CJ, Heath S, Stratford TR, Kelley AE. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 101.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–414. [PubMed] [Google Scholar]
- 102.Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord. 2003;27:1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 103.Tchernof A, Poehlman ET. Effects of the menopause transition on body fatness and body fat distribution. Obes Res. 1998;6:246–254. doi: 10.1002/j.1550-8528.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 104.Thorburn AW, Proietto J. Biological determinants of spontaneous physical activity. Obes Rev. 2000;1:87–94. doi: 10.1046/j.1467-789x.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 105.Treuth MS, Sherwood NE, Baranowski T, Butte NF, Jacobs DR, Jr, McClanahan B, Gao S, Rochon J, Zhou A, Robinson TN, Pruitt L, Haskell W, Obarzanek E. Physical activity self-report and accelerometry measures from the Girls health Enrichment Multi-site Studies. Prev Med. 2004;38(Suppl):S43–S49. doi: 10.1016/j.ypmed.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord. 2003;27:834–839. doi: 10.1038/sj.ijo.0802311. [DOI] [PubMed] [Google Scholar]
- 107.Uhe AM, Szmukler GI, Collier GR, Hansky J, O'Dea K, Young GP. Potential regulators of feeding behavior in anorexia nervosa. Am J Clin Nutr. 1992;55:28–32. doi: 10.1093/ajcn/55.1.28. [DOI] [PubMed] [Google Scholar]
- 108.Vardi P, Pinhas-Hamiel O. The young hunter hypothesis: age-related weight gain—a tribute to the thrifty theories. Med Hypotheses. 2000;55:521–523. doi: 10.1054/mehy.2000.1112. [DOI] [PubMed] [Google Scholar]
- 109.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 110.Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 111.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, Hennekens CH. Weight, weight change, and coronary heart disease in women. Risk within the “normal” weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 112.Williams JK, Kaplan JR, Suparto IH, Fox JL, Manuck SB. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:864–871. doi: 10.1161/01.ATV.0000067934.12783.6A. [DOI] [PubMed] [Google Scholar]
- 113.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 114.Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a US national cohort. Int J Obes Relat Metab Disord. 1993;17:279–286. [PubMed] [Google Scholar]
- 115.Wilson PW, Kannel WB. Obesity, diabetes, and risk of cardiovascular disease in the elderly. Am J Geriatr Cardiol. 2002;11:119–123,125. doi: 10.1111/j.1076-7460.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 115a.World Health Organization Obesity: preventing, and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 116.Yearick ES. Nutritional status of the elderly: anthropometric and clinical findings. J Gerontol. 1978;33:657–662. doi: 10.1093/geronj/33.5.657. [DOI] [PubMed] [Google Scholar]
- 117.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 118.Ziegler RG, Hoover RN, Nomura AM, West DW, Wu AH, Pike MC, Lake AJ, Horn-Ross PL, Kolonel LN, Siiteri PK, Fraumeni JF., Jr Relative weight, weight change, height, and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1996;88:650–660. doi: 10.1093/jnci/88.10.650. [DOI] [PubMed] [Google Scholar]