Abstract
Ginger (Zingiber officinale) supplements are being promoted for arthritis treatment in western societies based on ginger’s traditional use as an anti-inflammatory in Chinese and Ayurvedic medicine. However, scientific evidence of ginger’s antiarthritic effects is sparse, and its bioactive joint-protective components have not been identified. Therefore, the ability of a well-characterized crude ginger extract to inhibit joint swelling in an animal model of rheumatoid arthritis, streptococcal cell wall (SCW)-induced arthritis, was compared to that of a fraction containing only gingerols and their derivatives. Both extracts were efficacious in preventing joint inflammation. However, the crude dichloromethane extract, which also contained essential oils and more polar compounds, was more efficacious (when normalized to gingerol content) in preventing both joint inflammation and destruction. In conclusion, these data document a very significant joint-protective effect of these ginger samples, and suggest that non-gingerol components are bioactive and can enhance the antiarthritic effects of the more widely studied gingerols.
Ginger (Zingiber officinale Roscoe, Zingiberaceae) is one of most commonly used botanicals in the United States.1 Primarily known in western societies for its antiemetic and carminative uses, preparations made from the ground rhizome of ginger have also been used medicinally since antiquity for anti-inflammatory effects.2,3 Phenolic gingerols and related compounds, which are responsible for the pungent taste of ginger, have been a major focus of research related to the anti-inflammatory effects of ginger,3–10 particularly with respect to the ability of the individual, pure compounds to inhibit COX-1 and/or COX-2 activity in vitro. Increasingly, ginger use for arthritis treatment is being promoted in the United States.11–13 However, surprisingly little information is actually available in the scientific literature regarding the antiarthritic efficacy of ginger and we are not aware of any studies aimed at identifying its bioactive, antiarthritic components.14–16
Phenolics in ginger and related plants, such as turmeric [Curcuma longa L. (Zingiberaceae)], have been widely studied for their anti-inflammatory bioactivity.2,3,17 Prior studies by our laboratories have demonstrated non-phenolics in turmeric also have anti-inflammatory properties, but that the phenolic curcuminoids are primarily responsible for the antarthritic properties of turmeric demonstrated in a pre-clinical model of rheumatoid arthritis (RA).18–21
With this as background and using the same pre-clinical arthritis model, we have examined the antiarthritic effects of experimental ginger extracts isolated and characterized chemically and biologically prior to in vivo use. The in vivo model used, streptococcal cell wall (SCW)-induced arthritis, is a well-characterized animal model of RA.22–24 In this model, over a 28 day course, female Lewis rats develop an initial acute phase of joint swelling followed by a chronic phase of inflammation that is associated with actual joint destruction. Joint histopathology in this model is very similar to that seen in RA, an extremely inflammatory type of arthritis that is typified by recurrent cycles of joint inflammation and gradual joint destruction.25,26 In addition, a granulomatous inflammatory response, similar to that responsible for inactivating tuberculosis bacilli by walling off the invading bacteria, occurs in the liver of these animals at sites of SCW deposition.19,22–24 In order to determine whether the antiarthritic efficacy of ginger resides primarily with its phenolic gingerol components, the efficacy of a well-characterized fraction containing only gingerols and their derivatives was compared to that of a more complex extract, which also contained essential oils and more polar compounds.27
Results and Discussion
Chemical and In Vitro Biological Analysis of Ginger
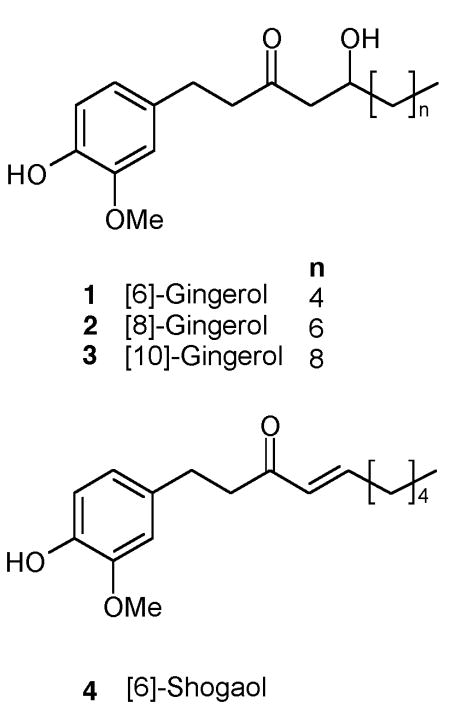
The results of an intensive chemical and biological analysis of a dichloromethane (DCM) extract of dried, powdered ginger rhizome containing all elements (gingerols and their derivatives, essential oils, and polar compounds) has previously been reported by our group (see Figure S1A. Supporting Information for HPLC analysis), including the identification of 31 novel compounds,27 and compared to those compounds present in extracts prepared from fresh ginger rhizome.28 The major components (24% by weight) present in this DCM extract are summarized in Table 1 and include the three most common gingerols (1–3), as well as 6-shogaol (4), a dehydration product of 6-gingerol (1) that is a major component in dried, but not fresh, rhizomes.27,28 This DCM extract, which also contained terpenes such as zingerone and zingerol,27 potently inhibited PGE2 production in vitro (Table 1). Chemical and biological characterization of a gingerol fraction (see Figure S1B. Supporting Information for HPLC analysis), which was composed of fractions 4–9 of eleven sequentially eluted column chromatography fractions, is also indicated in Table 1. Consistent with its 50% yield from the DCM extract, the gingerol fraction was composed of an approximate two-fold increase in content (47% by weight) of [6]-, [8]-, [10]-gingerol and [6]-shogaol (1–4) (Table 1). Examination of in vitro bioactivity of each of the 11 fractions (Table 1) demonstrates that the gingerol- and gingerol derivative-containing fractions (fractions 4–9) were most potent in inhibiting PGE2 production (Table 1). Of note, despite the two-fold lower content of PGE2-active gingerols/gingerol derivatives in the DCM extract, and the absence of potent PGE2 inhibitory effects for other fractions present in this extract, the IC50 for inhibition of PGE2 production by the crude extract was similar to that of the gingerol fraction. This finding suggests that the non-gingerol components of the crude DCM extract, while having no significant effect on PGE2 release when administered in isolation, may synergize with the gingerols to suppress prostaglandin-mediated inflammation.
Table 1.
Ginger Extract Chemical and Biological Characterization
| sample compositiona |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6-,8-, and 10-Gingerol (1–3) and 6-Shogaol (4) (% by weight) | |||||||||
| extracts | CC fractions | % yield from DCM | 1 | 2 | 3 | 4 | Terpenes | in vitro PGE2 inhibition IC50 (μg extract/mL) | |
| DCM Extract | (1–11) | 10.0 | 3.0 | 4.7 | 5.8 | + | 0.06 | ||
| Gingerol Fraction | 4–9 | 42.1 | 21.4 | 6.7 | 10.1 | 9.1 | 0.07 | ||
| 1 | 22.3 | + | >50 | ||||||
| 2 | + | + | 39.00 | ||||||
| 3 | + | + | + | + | + | 0.28 | |||
| 4 | + | + | + | + | 0.07 | ||||
| 5 | + | + | + | 0.07 | |||||
| 6 | + | + | + | 0.08 | |||||
| 7 | + | + | + | 0.07 | |||||
| 8 | + | 0.19 | |||||||
| 9 | + | 0.08 | |||||||
| 10 | 29.8 | 0.38 | |||||||
| 11 | 8.86 | ||||||||
Compounds 1–4 were quantitated in the experimental extracts and assessed qualitatively (+ = present) in the fractions isolated by column chromatograpy (CC) by HPLC.
In vivo Antiarthritic Efficacy of Gingerol Fraction Treatment
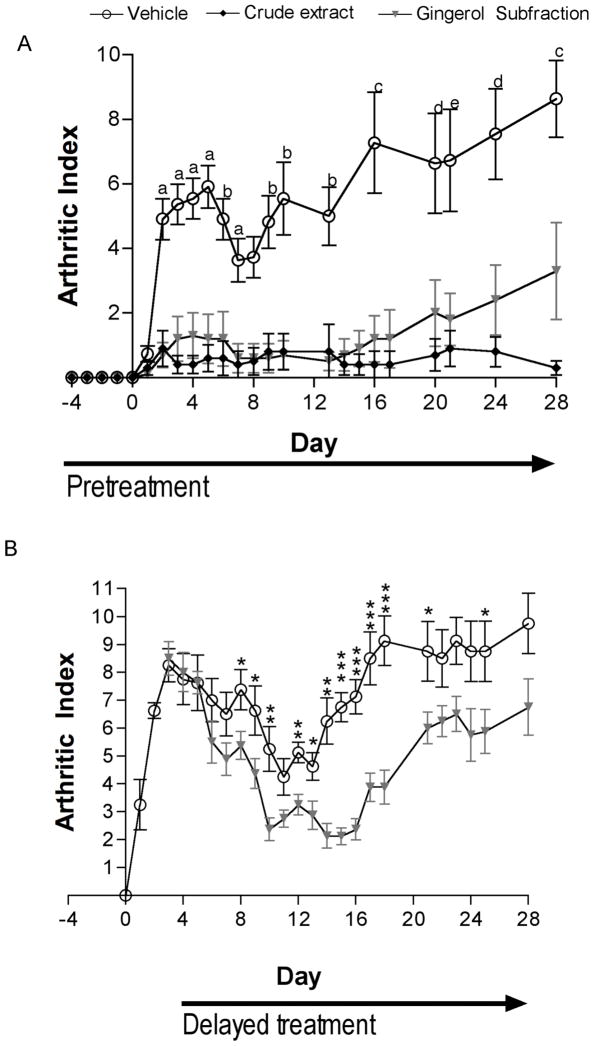
Having previously demonstrated almost complete suppression of SCW-induced joint inflammation in response to turmeric extracts dosed at 23 mg curcuminoids/kg/day,19–21 a similar phenolic dose of the gingerol fraction was administered (26 mg gingerols/kg/day). Intraperitoneal administration of the gingerol fraction, beginning 4 days prior to injection of SCW and continuing for the duration of the experiment, significantly inhibited joint inflammation in both the acute inflammatory and later, chronic joint-destruction phase of SCW-induced arthritis (Figure 1A). Delay of treatment with the essential oil-free gingerol fraction until after the peak in acute inflammation (day 3 post-SCW) also effectively reduced joint inflammation during the chronic joint destructive period phase (Figure 1B). The effects of early vs. delayed treatment with gingerol fraction in the SCW model can also be compared with those reported by Wahl and colleagues in response to specific inhibition of tumor necrosis factor (TNF). 29 Unlike gingerol fraction, delayed treatment with gene therapy to induce the production of a rat homolog of etanercept, a fusion TNF receptor protein currently in clinical use in RA, was without effect, while pre-treatment regimens only reduced chronic swelling while having no effect on acute inflammation. Thus, gingerol fraction treatment was more efficacious than specific TNF blockade in the prevention and treatment of SCW-induced joint swelling.
Figure 1.
Effect of gingerol fraction or DCM extract on joint inflammation. Female Lewis rats were injected on day 0 with SCW (25 μg/g) or vehicle. Joint swelling was assessed by daily calculation of the arthritic index (mean ± SEM) with statistical significance determined by Kruskal-Wallis nonparametric ANOVA with post hoc analysis or Mann Whitney testing as described in the experimental section. (A) Gingerol fraction (26 mg gingerols/kg/day), DCM extract (26 mg gingerols/kg/day) or vehicle alone; i.p. injections were begun 4 days prior to SCW administration (n = 10–11 animals/group) and continued on a daily basis until 14 days after SCW injection at which time treatment frequency decreased to twice weekly. P values (vs. vehicle) are: a p < 0.001, DCM extract and p < 0.01, ginger fraction; b p < 0.01, DCM extract and p < 0.01, ginger fraction; c p < 0.001, DCM extract and p < 0.05, ginger fraction; d p < 0.01, DCM extract and p < 0.05, ginger fraction; e p < 0.01, DCM extract and ns, ginger fraction. (B) Gingerol fraction (26 mg/kg/day) or vehicle alone i.p. injections were begun 3 days after SCW administration (n = 8 animals/group) and continued daily until 10 days post-SCW at which time treatment frequency decreased to twice weekly. P values (vs. vehicle) are: * p < 0.05, ** p < 0.01, *** p < 0.001.
In Vivo Antiarthritic Efficacy of DCM Ginger Extract Treatment
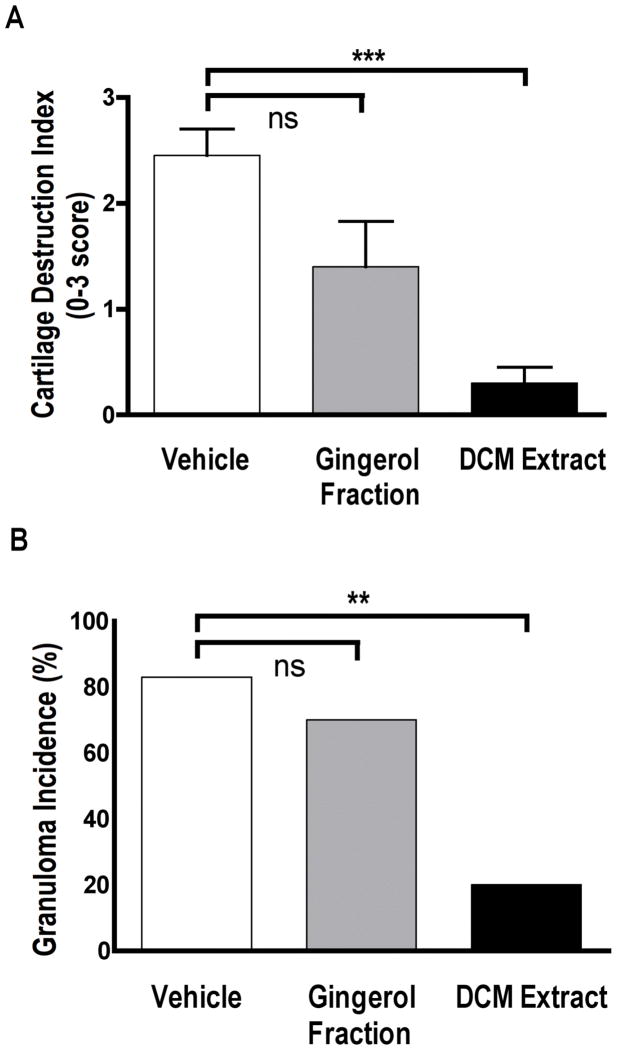
Having previously demonstrated a higher antiarthritic potency for a curcuminoid fraction vs. a complex turmeric extract (normalized to phenolic content) in this arthritis model,19–21 we anticipated a similar result for ginger when comparing the gingerol fraction to a crude ginger extract. In contrast, in a head-to-head comparison of the same phenolic dose (normalized to gingerol content) of the gingerol fraction vs. crude DCM extract, the DCM extract was slightly more effective in reducing joint swelling, particularly at the end of the experimental period when the protective effect of the gingerol fraction appeared to be attenuated. Consistent with this conclusion, only the DCM extract was effective in blocking the joint destruction that accompanies joint inflammation in this model, as measured by destruction of articular cartilage (Figure 2A).
Figure 2.
Comparison of gingerol fraction vs. DCM extract on (A) destruction of articular cartilage and (B) hepatic granulomatous inflammatory response. Female Lewis rats were injected on day 0 with SCW (25 μg/g) or vehicle. Botanical extracts or vehicle alone i.p. injections were begun 4 days prior to SCW administration and continued on a daily basis until 14 days after SCW injection at which time treatment frequency decreased to twice weekly. (A) The degree of cartilage destruction in talo-tibial joints was determined histologically as described in methods (mean ± SEM of score range 0–3) (n = 10–11 animals/group) with statistical significance determined by Kruskal-Wallis nonparametric ANOVA with post hoc analysis. *** p < .001. (B) The incidence of granuloma formation (number of animals with hepatic granuloma out of total in group) was assessed histologically as described in methods (n = 10–11 animals/group). Statistical significance of each treatment (vs. vehicle) was determined by Fisher’s Exact Test. ** p < 0.01.
Effect of Gingerol Fraction vs. DCM Extract on Granuloma Formation
Prevention of granulomatous inflammation by the ginger extracts paralleled their efficacy in preventing joint inflammation; the DCM extract prevented hepatic granuloma formation while the gingerol fraction was without effect (Figure 2B). This result suggests that while gingerols and their derivatives are sufficient for inhibition of joint inflammation, the essential oils and/or polar compounds present only in the DCM extract are required for inhibition of granulomatous inflammation. As reactivation of infectious diseases made quiescent by protective granulomatous responses (e.g., tuberculosis) is a known risk of anti-inflammatory treatments in RA patients,30 these results suggest that ginger extracts depleted of essential oils and polar compounds, while less efficacious in blocking joint inflammation, may have a better safety profile with respect to this potential side effect.
Toxicity of Gingerol Fraction vs. DCM Ginger Extract
Routine toxicity screening in these in vivo bioactivity experiments, which utilized an intraperitoneal (i.p.) mode of administration to maximize absorption of all extract components, were notable for deaths in three animals treated with each of the ginger extracts vs. none in vehicle treatment groups (Table 2). Animals treated with ginger fraction exhibited mild abnormalities in liver and kidney function (Table 2) and also had occult blood in their stool with evidence of hemorrhagic enteritis of the small intestine on necropsy. Thus, consistent with prior reports,2,3 gastrointestinal side effects appear to limit the utility of the gingerol fraction when administered i.p.. In contrast, the cause of increased mortality in the DCM extract group was not evident as necropsies, occult gastrointestinal blood testing, and assessment of liver and renal function in survivors were unremarkable, despite the similar dose of gingerol-related compounds administered.
Table 2.
Toxicity monitoring in ginger extract-treated rats
| Vehicle | Gingerol Fraction | DCM Extract | |
|---|---|---|---|
| mortality, % (number died/total) | 0% (0/25) | 12% (3/25) | 18% (3/17) |
| blood in stool, % (number positive/total tested) | 0% (0/8) | 30% (4/8) | 0% (0/10) |
| alanine aminotransf erase (U/L) | 10.9 ±1.4 | 18.4 ±3.7 | 13.6 ±0.9 |
| % greater than range of normal | 23% (3/13) | 0% (0/13) | |
| creatinine (mg/dL) | 0.20 ±0.01 | 0.25±0.02a | 0.19±0.01 |
| % greater than range of normal | 62% (8/13) | 0% (0/13) | |
p < 0.05 vs. vehicle, by ANOVA with post hoc testing.
Conclusions
Ginger is now the second member of the Zingiberaceae family demonstrated to have profound antiarthritic efficacy in the SCW-induced model of rheumatoid arthritis. The antiarthritic effects of ginger are particularly intriguing as these studies suggest that the antiarthritic bioactivity of this Zingiberaceae involves additive and/or synergistic effects of multiple components, including, but not limited to, the gingerols. Future studies are therefore planned to examine further the efficacy, safety and mechanism of action of complex ginger extracts when administered orally as dietary supplements.
Experimental Section
General Experimental Procedures
Dried ginger rhizome powder was purchased from San Francisco Herb and Natural Food, SF, CA.27 Extract analyses were performed with an Agilent 1100 series HPLC system with a quaternary pump, a degasser, a thermostatted column compartment, a thermostatted autosampler, a diode array detector and a ChemStation for LC 3D, Rev. A.09.03 (1417) software for system control and data acquisition (Agilent Technologies, Inc., Palo Alto, CA).
Sample Preparation
A crude ginger extract was prepared as previously described by extracting ginger powder (627 g) with CH2Cl2 (dichoromethane, DCM) at 25°C for 36 h (6.4% yield). 27 After filtration, washing and work up, 40 g of the resultant extract (“DCM extract’) were applied to a silica gel column and sequentially eluted with solvents of increasing polarity27 to yield 11 fractions. These fractions were screened in vitro for their ability to inhibit in vitro PGE2 production and chemically characterized by HPLC. Fractions 4–9, which each inhibited PGE2 production potently and were composed of gingerols or their derivatives, were combined to create a gingerol/gingerol derivative-enriched fraction (“gingerol fraction”) (approximately 50% yield from crude extract). Fractions 1–3 (essential oils, 22% yield) and 10–11 (polar compounds, 30% yield), which did not potently suppress PGE2 production, were not evaluated further in these studies.
Chemical and Biological Analyses
For HPLC analyses, triplicates of approximately 1 mg samples were weighed using an analytical balance, dissolved in 5 mL MeOH, sonicated for 1 min, and diluted with HPLC grade MeOH in a 10 mL volumetric flask. One mL from each solution was transferred into an amber autosampler vial and 3 × 20 μL were injected onto the HPLC column. Stock solutions of pure 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol reference standards (Dalton Chemical Laboratories, Toronto, Ontario, Canada) were prepared individually and their purity confirmed by HPLC, MS, NMR and elemental analysis.27,28 Samples were injected onto a Synergy, 4μm, Hydro-RP 250 × 4.6 mm column coupled with SecurityGuard AJO-4287 guard column (Phenomenex, Torrance, CA). Mobile phases were as follows: (a) 500 μL HOAc/L of Nanopure H2O; (B) 500 μL HOAc/L of MeCN.27 The eluent (1 μL/min flow rate) was monitored at 210 nm, 230 nm, 280 nm, and 370 nm. 27 In vitro screening for anti-inflammatory activity of extracts, as determined by inhibition of prostaglandin E2 (PGE2) production, was conducted to ensure reproducibility of extract preparation.27 Briefly, U937 cells (ATCC, CRL-1593.2), cultured in RPMI 1640 with 25 mM HEPES, 10 % FBS were plated, differentiated with 10 nM of PMA (Sigma) for 24h, washed with medium, and then treated with LPS (1 μg/mL) with or without varying concentrations of gingerol-containing extracts for an additional 24 h. Culture supernatants were collected and stored at −80°C prior to PGE2 immunoassay (R & D Systems, Minneapolis, MN).
Animal Procedures
Using the same standard protocol previously described for assessment of turmeric extracts,19–21 female Lewis rats (Harlan, Indianapolis, IN) were administered a single intraperitoneal (i.p.) injection of vehicle (normal saline) or peptidoglycan-polysaccharide polymers (25 μg rhamnose/g body weight) isolated from the sonicated cell wall of Group A Streptococcus pyogenes (Lee Laboratories, Grayson, GA). At the indicated times, control and SCW-treated animals received an i.p. injection of botanical sample or vehicle (0.5–1 μL/g DMSO). Intraperitoneal treatments with gingerol fraction or crude ginger extract were administered either (1) beginning 4 days prior to SCW administration and continuing daily until the beginning of the chronic phase (day 14), when treatment frequency was decreased to twice weekly (pretreatment protocol), or (2) delayed until after the peak in acute inflammation (day 3) and continued on a daily basis until the start of the chronic phase of joint swelling (day 10), when treatment frequency was decreased to twice weekly (delayed treatment protocol). For comparison of antiarthritic response to the two samples, dosing was normalized and expressed as mg total gingerols/kg body weight. Joint inflammation was determined in a blinded fashion by daily assessment of arthritic index (AI) in each distal limb using standard criteria (0 = normal; 1 = slight erythema and edema; 2 = increased edema with loss of landmarks; 3 = marked edema; 4 = marked edema with ankylosis on flexion).19,20,23,24 To monitor for possible toxic effects of treatments in normal or SCW-treated animals, daily weights were recorded, and serum creatinine and alanine aminotransferase (ALT) levels in blood samples obtained 28 days after injection of SCW (or vehicle) were determined using a Hemagen Diagnostics Endocheck Plus Chemistry Analyzer to monitor for possible renal- or hepatotoxicity, respectively.20
Histology
Joint and liver specimens obtained 28 days after SCW injection were fixed in 10% formalin; joints were subsequently decalcified in 10% EDTA/pH 7.0; and all tissues were embedded in paraffin. 19,20,23 Granuloma formation in liver was assessed in hematoxylin and eosin (H&E) stained sections of liver using standard criteria.19,22–24 An index of articular cartilage destruction in day 28 hind joint distal tibias was determined, as previously described, using hematoxylin and eosin (H&E) stained sections (0 = normal; 1 = minimal destruction, 2 = at least 50% destroyed, 3 = entirely destroyed).20,22
Statistical Analyses
All values are presented as mean ± the SEM, unless otherwise stated, with statistical significance determined by ANOVA or non-parametric Kruskal-Wallis analysis with post hoc testing, non-parametric Mann Whitney analysis, or Fisher’s Exact Test, as appropriate, using Instat 3.0b software (Graphpad, San Diego, CA).
Supplementary Material
Acknowledgments
This work was supported by the Office of Dietary Supplements (ODS) and the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (NIH) (5 P50 AT000474). We wish to acknowledge the contributions of S. D. Jolad, V. Rodriguez, and A. Solyom (for isolation and identification of compounds) and C. Lantz and G. Chen (for in vitro PGE2 assays). The contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM, ODS or NIH.
Footnotes
Dedicated to Dr. David G.I. Kingston of Virginia Polytechnic Institute and State University for his pioneering work on bioactive natural products.
Supporting Information Available. HPLC chromatograms of ginger extracts. This information is available free-of-charge via the Internet at www.pubs.acs.org.
References and Notes
- 1.Barnes PM, Powell-Griner E, McFann K, Nahin RL. CDC Advance Data Report #343. 2004 May 27; [PubMed] [Google Scholar]
- 2.Ali BH, Blunden G, Tanira MO, Nemmar A. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 3.Chrubasik S, Pittler MH, Roufogalis BD. Phytomedicine. 2005;12:684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Koo KLK, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Thrombosis Res. 2001;103:387–397. doi: 10.1016/s0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 5.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Bioorgan Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 6.Frondoza CG, Sohrabi A, Polotsky A, Phan PV, Hungerford DS, Lindmark L. In vitro Cell Dev Biol-Animal. 2004;40:95–101. doi: 10.1290/1543-706x(2004)040<0095:aivsaf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Kim SO, Chun KS, Kundra JK, Surh YJ. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 8.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Bioorgan Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 9.Phan PV, Sohrabi A, Polotsky A, Hungerford DS, Lindmark L, Frondoza CG. J Altern Comp Med. 2005;11:149–154. doi: 10.1089/acm.2005.11.149. [DOI] [PubMed] [Google Scholar]
- 10.Shen CL, Hong KJ, Kim SW. J Med Food. 2005;8:149–153. doi: 10.1089/jmf.2005.8.149. [DOI] [PubMed] [Google Scholar]
- 11.Foltz-Gray D. Alternative Treatments for Arthritis. Arthritis Foundation; Atlanta: 2005. pp. 231–233. [Google Scholar]
- 12.Weil A. Natural Health, Natural Medicine. Houghton Mifflin; New York: 1995. pp. 381–382. [Google Scholar]
- 13.Underwood A. Newsweek. 2005 Jan 31; [Google Scholar]
- 14.Altman RD, Marcussen KC. Arth Rheum. 2001;44:2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Biddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, Christensen K, Jensen ON, Barslev J. Osteoarth Cartil. 2000;8:9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- 16.Sharma JN, Srivastava KC, Gan EK. Pharmacology. 1994;49:314–318. doi: 10.1159/000139248. [DOI] [PubMed] [Google Scholar]
- 17.Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN. Phytomed. 2005;12:445–52. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Chainani-Wu N. J Altern Comp Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 19.Funk JL, Oyarzo JN, Beischel JA, Chen G, Lantz C, Jolad SD, Sólyom AM, Timmermann BN. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Sólyom AM, Kiela PR, Timmermann BN. Arth Rheum. 2006;54:3452–3464. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 21.Funk JL, Timmermann BN. Nat Prod Comm. 2006;1:1061–1066. [Google Scholar]
- 22.Funk JL, Chen J, Downey KJ, Davee S, Stafford G. Arth Rheum. 2003;48:1721–1731. doi: 10.1002/art.10985. [DOI] [PubMed] [Google Scholar]
- 23.Geratz JD, Tidwell RR, Schwab JH, Anderle SK, Pryzwansky KB. Am J Pathol. 1990;136:909–921. [PMC free article] [PubMed] [Google Scholar]
- 24.Allen JB, Malone DG, Wahl SM, Calandra GB, Wilder RL. J Clin Invest. 1985;76:1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy EH, Panayi GS. New Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 26.Aletaha D, Ward MM. Ann Rheum Dis. 2006;65:227–233. doi: 10.1136/ard.2005.038513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jolad SD, Lantz RC, Solyom AM, Chen CJ, Bates RB, Timmermann BN. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Jolad SD, Lantz RC, Solyom AM, Chen G, Bates RB, Timmerman BN. Phytochemistry. 2004;65:1937–1954. doi: 10.1016/j.phytochem.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Mol Ther: J Amer Soc Gene Ther. 2002;6:727–736. doi: 10.1006/mthe.2002.0808. [DOI] [PubMed] [Google Scholar]
- 30.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. New Eng J Med. 2001;15:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.