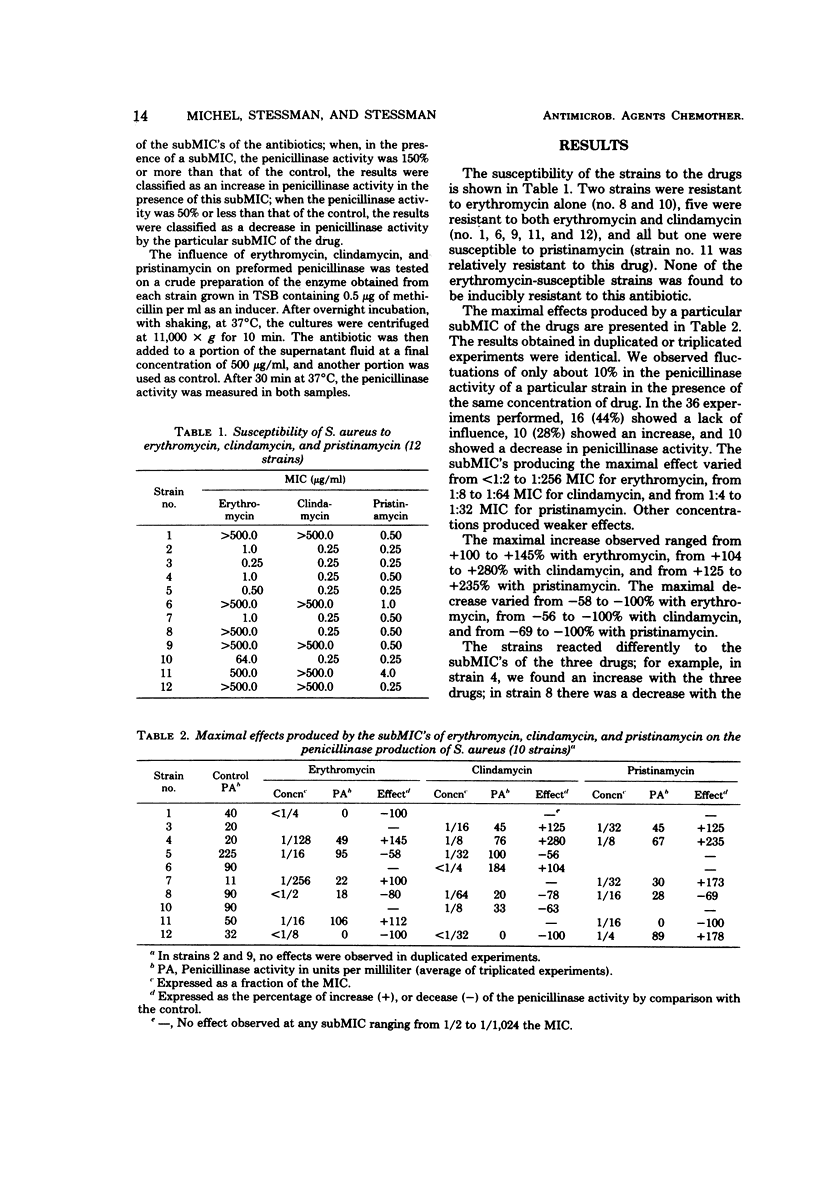
Abstract
The influence of subminimal inhibitory concentrations of erythromycin, clindamycin, and pristinamycin on the penicillinase production of Staphylococcus aureus was tested in 12 strains. Of the 36 experiments performed, 16 (44%) showed a lack of influence, 10 (28%) displayed an increase, and 10 revealed a decrease in penicillinase activity. The maximal effect produced was generally induced by concentrations ranging from 1/4 to 1/32 the minimal inhibitory concentration, irrespective of the susceptibility of the strain to the drug. In spite of the fact that the drugs are closely related, they sometimes produced opposite effects on the same strain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Epp J. K. Mechanism of penicillin-erythromycin synergy on antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1978 May;13(5):849–853. doi: 10.1128/aac.13.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIAK J., HAHN F. E. Mechanisms of action of antibiotics. I. Additive action of chloramphenicol and tetracyclines on the growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):125–129. doi: 10.1128/jb.75.2.125-129.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITRI N. DETERMINATION OF PENICILLINASE ACTIVITY. Methods Med Res. 1964;10:221–232. [PubMed] [Google Scholar]
- Greenwood D. Differentiation of mechanisms responsible for inoculum effects in the response of Escherichia coli to a variety of antibiotics. J Antimicrob Chemother. 1976 Mar;2(1):87–95. doi: 10.1093/jac/2.1.87. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M. Nitrofurans, a group of synthetic antibiotics, with a new mode of action: discrimination of specific messenger RNA classes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3386–3390. doi: 10.1073/pnas.73.10.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorain V., Sabath L. D. Penicillins and cephalosporins: differences in morphologic effects on Proteus mirabilis. J Infect Dis. 1972 May;125(5):560–564. doi: 10.1093/infdis/125.5.560. [DOI] [PubMed] [Google Scholar]
- Lorian V., Atkinson B. Abnormal forms of bacteria produced by antibiotics. Am J Clin Pathol. 1975 Nov;64(5):678–688. doi: 10.1093/ajcp/64.5.678. [DOI] [PubMed] [Google Scholar]
- Lorian V., Popoola B. The effect of nitrofurantoin on the morphology of gram-negative bacilli. J Infect Dis. 1972 Feb;125(2):187–189. doi: 10.1093/infdis/125.2.187. [DOI] [PubMed] [Google Scholar]
- Lorian V. Some effect of subinbilitory concentrations of penicillin on the structure and division of staphylococci. Antimicrob Agents Chemother. 1975 Jun;7(6):864–867. doi: 10.1128/aac.7.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V. Some effects of subinhibitory concentrations of antibiotics on bacteria. Bull N Y Acad Med. 1975 Oct;51(9):1046–1055. [PMC free article] [PubMed] [Google Scholar]
- Michel J., Bornstein H., Luboshitzky R., Sacks T. Mechanis of chlorampenicol-cephalordine synergism on Enerobacteiaeae. Antimicrob Agents Chemother. 1975 Jun;7(6):845–849. doi: 10.1128/aac.7.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J., Jacobs J., Sacks T. Bactericidal synergistic effect due to chloramphenicol-induced inhibition of staphyloccal penicillinase. Chemotherapy. 1977;23(1):32–36. doi: 10.1159/000221968. [DOI] [PubMed] [Google Scholar]
- Rolinson G. N. Subinhibitory concentrations of antibiotics. J Antimicrob Chemother. 1977 Mar;3(2):111–113. doi: 10.1093/jac/3.2.111. [DOI] [PubMed] [Google Scholar]
- Sacks T., Michel J., Durst A., Stessman J. Inhibition of beta-lactamase: synergistic bactericidal effect of combination of chloramphenicolcephaloridine. J Antimicrob Chemother. 1977 Sep;3(5):525–527. doi: 10.1093/jac/3.5.525. [DOI] [PubMed] [Google Scholar]
- Shah P. M., Heetderks G., Stille W. Activity of amikacin at sub-inhibitory levels. J Antimicrob Chemother. 1976 Mar;2(1):97–100. doi: 10.1093/jac/2.1.97. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969 May;98(2):447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman R. G., Farrar W. E., Jr Activity of penicillinase in Staphylococcus aureus as studied by the iodometric method. J Infect Dis. 1970 Apr;121(4):433–437. doi: 10.1093/infdis/121.4.433. [DOI] [PubMed] [Google Scholar]


