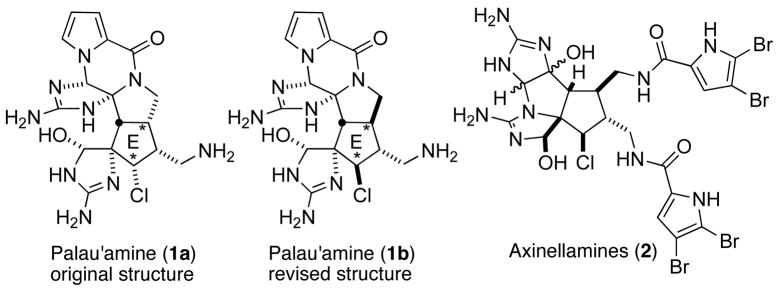
The pyrrole-imidazole alkaloids, which comprise a large family of natural products,1 have received a great deal of attention due to their potent biological activities and tremendous structural diversity. Palau’amine (1a) was originally isolated from a sponge, Stylotella agminata, in 1993 by Scheuer as a novel class of the pyrrole-imidazole alkaloid.2 Since the initial disclosure of its proposed structure (1a), palau’amine (1) has been an attractive synthetic target because of its intriguing molecular architecture and significant biological properties such as antifungal, antitumor, and immunosuppressive activities. However, according to several groups, the originally proposed structure 1a was recently revised as 1b, which possesses the indicated the trans-D/E ring junction and the β-chlorine substituent.3, 5e, 5g
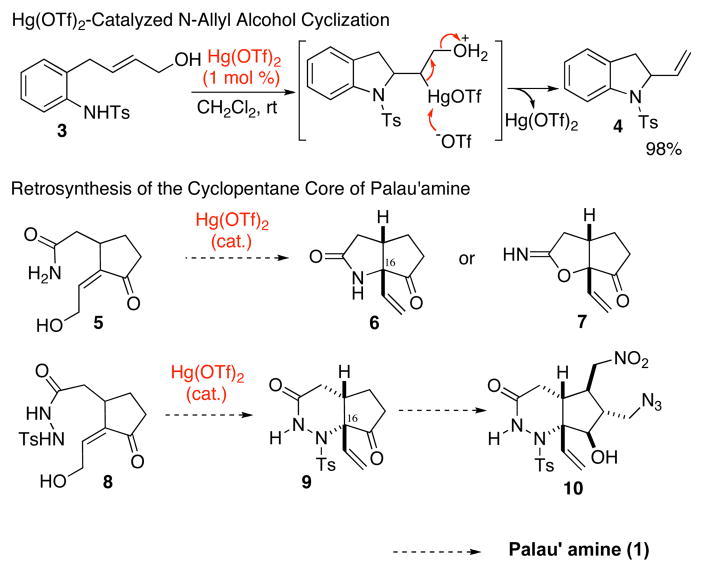
The noteworthy structural features of palau’amine include: two guanidine moieties, fused polycyclic system with a spiro cycle, complex all carbon substituted cyclopentane ring, nitrogen-substituted quaternary carbon center, and eight contiguous stereogenic centers. Not surprisingly, many attempts to synthesize palau’amine and related natural products have been reported so far, 4, 5 and the first total synthesis of the related natural products axinellamines A and B (2) was recently accomplished.6 However, a total synthesis of palau’amine itself has not yet been reported. Efficient construction of the complex cyclopentane core with the correct stereochemistry at each carbon center, including a quaternary carbon center, is definitely one of the most difficult synthetic challenges for the synthesis of palau’ amine. Herein, we describe an efficient synthesis of the cyclopentane core of palau’amine by the application of a highly efficient novel Hg(OTf)2-catalyzed reaction developed in our laboratory.7 In 2008, the Hg(OTf)2-catalyzed alkyne cyclization reactions were expanded to the alkene cyclization reactions by using allylic alcohol or vinyl methyl ether substrates that, after cyclization, undergo a smooth proto-demercuration to give the cyclized products and the regenerated Hg(OTf)2 catalyst.8 For instance, Hg(OTf)2-catalyzed cyclization of N-tosylanilino allylic alcohol 3 provided 2-vinylindolines 4 in high yield (Scheme 1).8b Thus, the cyclization of cyclopentylidene alcohol 5 is expected to give 6 by constructing a quaternary carbon center that corresponds to the C16 of palau’amine. However, the catalytic cyclization of amide 5 is also possible to give the O-cyclized product 7 in preference to the N-cyclized product 6. Indeed, we confirmed this was the case. The conventional methods for N-selective cyclization are limited by the cumbersome substrate modifications, the addition of strong Lewis acids and/or strong base, or the formation of N-radical. 9 Moreover, the catalytic conditions for generating such a quaternary carbon center have not yet to be determined. Therefore, we designed an acylhydrazide 8 as a simple modification of primary amides for Hg(OTf)2-cataylized cyclization. The vinyl lactum 9, prepared by the Hg(OTf)2-catalyzed cyclization of 8, could be an excellent precursor to construct cyclopentane core 10 by introducing two CH2-N groups (Scheme 1).
Scheme 1.
Hg(OTf)2-Catalyzed Cyclization of Allylic Alcohol and Synthetic Design of Palau’amine Cyclopentane Core 10.
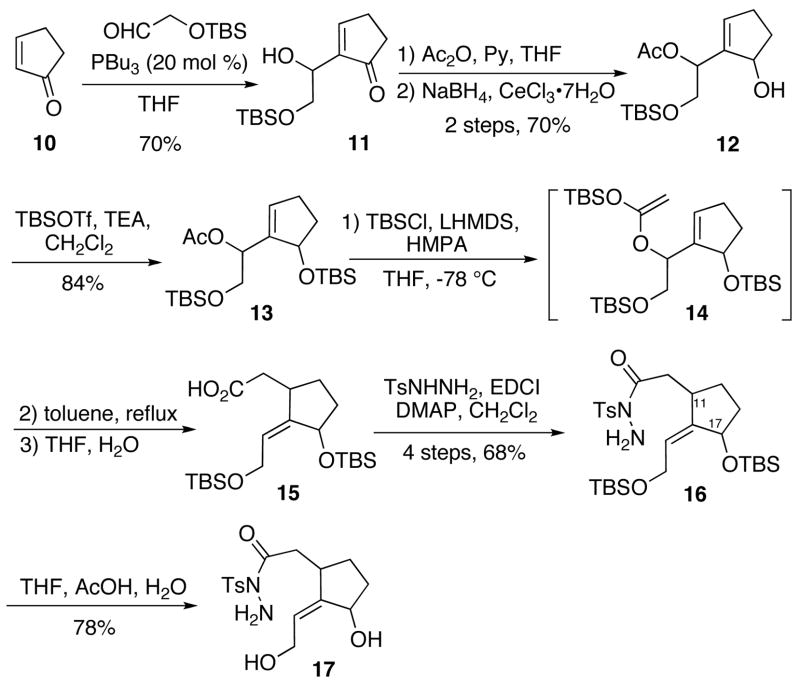
Commercially available 2-cyclopentene-1-one 10 was employed as the starting material. A Morita-Baylis-Hillman reaction of 10 with a commercial preparation of (tert-butyldimethylsilyloxy)-acetaldehyde afforded 11 in 70% yield. 11 was subsequently converted to 13 by a sequential operation of acetylation, a Luche reduction, 10 and a TBS protection. The acetate 13 were then obtained as a 2: 1 diastereomeric mixture, setting the stage for an Ireland-Claisen rearrangement.11, 12 After the treatment of 13 with LHMDS/TBSCl/HMPA in THF at −78 °C, refluxing in toluene induced the desired Ireland-Claisen rearrangement to afford the cyclopentylidene carboxylic acid 15 via 14 in good yield. Next, we attempted to prepare an acylhydrazide by the coupling of 15 with N-tosylhydrazide by the combined action of EDCI and DMAP in dichloromethane. Surprisingly, the nitrogen masked with a tosyl group participated in the condensation to give a 2: 1 diastereomeric mixture of 16 in 68% overall yield after four steps from 13. Presumably, the nucleophilicity of the more basic primary amine was attenuated by protonation with the HCl derived from the used EDCI-HCl salt.13 The double bond geometry of 15 was determined to be Z by the NOE experiment of its amide derivative (Scheme 2). The TBS groups were cleaved under mild acidic conditions to give diol 17.
Scheme 2.
Synthesis of Hydrazide 17
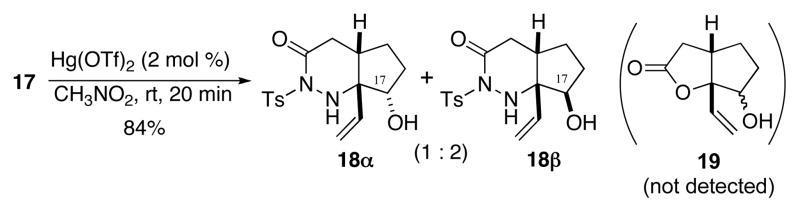
Reaction of 17 with 2 mol % of Hg(OTf)2 took place smoothly in nitromethane at room temperature to afford 18α and 18β in 84% yield as a separable 1:2 diastereomeric mixture. The lactone 19 was not detected (Sheme 3). Stereochemical outcome at the ring junction was completely controlled to cis regardless of the stereocenter of the secondary alcohol at C17. The structures of 18α and 18β were unambiguously confirmed by an X-ray diffraction study and NOE studies (see supporting information). 14 We thus established that the Hg(OTf)2-catalyzed protocol efficiently mediates N-selective cyclizations of amide carbony moieties, which is very difficult to achieve using conventional methodologies.15
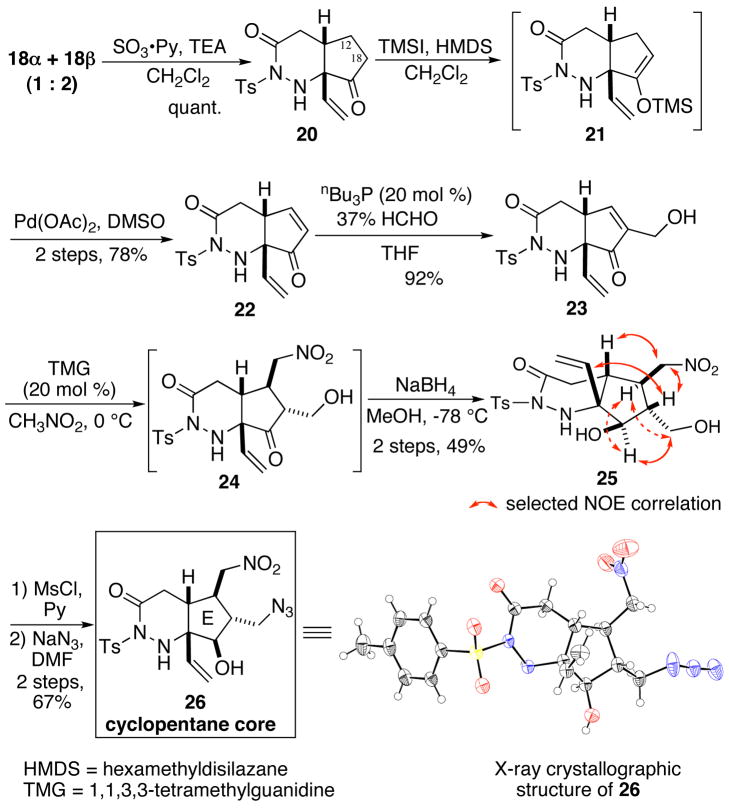
Having prepared a sufficient amount of N-cyclized product 18, we attempted to construct the cyclopentane core of palau’amine. The SO3·pyridine oxidation of the mixture of 18α and 18β gave a single ketone 20 in quantitative yield.16 Direct oxidation of 20 to enone 22, using IBX,17 and selenium dioxide, or enolate oxidation using selenium halide, sulfinimidoyl chloride,18 and NBS, were unsuccessful. Although the preparation of the silyl enol ether was not straightforward, a combination of TMSI and hexamethyldisilazane in dichloromethane was found to give trimethylsilylenolether 21 in quantitative yield.19, 20 Saegusa-Ito oxidation of 21 provided enone 22 in good yield.21 Morita-Baylis-Hillman reaction of 22 with formaldehyde gave alcohol 23 in excellent yield. Subsequent 1, 4-addition of nitromethane in the presence of a catalytic amount of a 1, 1, 3, 3-tetramethylguanidine (TMG) afforded the desired 1, 4-adduct 24.22 We found that 24 was readily converted to the exomethylene product by dehydration during a column chromatography purification on silica gel. Therefore, the crude 24 was directly subjected to reduction with NaBH4 to give 25. The stereochemistry of 25 was confirmed to be as we planned for our palau’amine synthesis by an NOE experiment. Finally, the primary alcohol of 25 was converted to azide 26 that is the targeted cyclopentene core of palau’amine. The structure of 26 was unequivocally established by an X-ray diffraction study (Scheme 6 and supporting information). 23
In summary, we have established an efficient route to the cyclopentane 26, which corresponds to our E ring synthetic intermediate of palau’ amine by the application of novel Hg(OTf)2-catalyzed cyclization. Furthermore, a selective N-cyclization protocol of acylhydrazide for catalytic construction of a quaternary carbon center was also developed. Throughout this investigation, Hg(OTf)2 was shown to be a powerful catalyst for the construction of complex carbon frameworks of the type found in natural products. Total synthesis of palau’amine, one of the most challenging synthetic targets in the last decade, is currently underway in our laboratory.
Experimental Section
Experimental details, full data, 1H and 13C NMR spectra of each intermediate from 11 to 26, and data of X-ray analysis are available in Supporting Information.
Supplementary Material
Figure 1.
Structure of Palau’amine (Original and Revised Structures) and Axinellamines (2).
Scheme 3.
Hg(OTf)2-Catalyzed Allyl Alcohol Cyclization of Acylhydrazide.
Scheme 4.
Synthesis and X-ray crystallographic structure of 26.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culuture, Sports, Science, and Technology of Japanese Government, MEXT. HAITEKU, 2003-2007, and the Global COE Program (Project No. B01: Catalysis as the Basis for Innovation in Materials Science). We acknowledge financial support from the National Institutes of Health (NIH Grant GM068011 to RMW and KN, Colorado State University). K. N. is grateful to Astellas Co., Inc. and JSPS, for a Research Fund for Young Scientists.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.))
References
- 1.For recent reviews on this family of natural products, see: Al Mourabit A, Potier P. Eur J Org Chem. 2001:237–243.Jin Z. Nat Prod Rep. 2006;23:464–496. doi: 10.1039/b502166a.
- 2.a) Kinnel RB, Gehrken HP, Scheuer PJ. J Am Chem Soc. 1993;115:3376–3377. [Google Scholar]; b) Kinnel RB, Gehrken HP, Swali R, Skoropowski G, Scheuer PJ. J Org Chem. 1998;63:3281–3286. [Google Scholar]
- 3.a) Grube A, Köck M. Angew Chem, Int Ed. 2007;119:2320–2324. doi: 10.1002/anie.200604076. [DOI] [PubMed] [Google Scholar]; b) Buchanan MS, Carroll AR, Addelpalli R, Avery VM, Hooper JN, Quinn RJ. J Org Chem. 2007;72:2309–2317. doi: 10.1021/jo062007q. [DOI] [PubMed] [Google Scholar]; c) Kobayashi H, Kitamura K, Nagai K, Nakao Y, Fusetani N, vanSoest RWM, Matsunaga S. Tetrahedron Lett. 2007;48:2127–2129. [Google Scholar]; d) Buchanan MS, Carroll AR, Quinn RJ. Tetrahedron Lett. 2007;48:4573–4574. [Google Scholar]
- 4.For recent reviews of synthetic studies towards palau’ amine, see: Hoffman H, Lindel T. Synthesis. 2003:1753–1783.Jacquot DEN, Lindel T. Curr Org Chem. 2005;9:1551–1565.Arndt HD, Riedrich AM. Angew Chem, Int Ed. 2008;47:4785–4788. doi: 10.1002/anie.200801793.
- 5.For recent advances toward Palau’ amine not cited in ref 4 see: Garrido-Hernandez H, Nakadai M, Vimolratana M, Li QY, Doundoulakis T, Harran PG. Angew Chem, Int Ed. 2005;44:765–769. doi: 10.1002/anie.200462069.Wang S, Dilley A, Poullennec KG, Romo D. Tetrahedron. 2006;62:7155–7161.Tan X, Chen C. Angew Chem, Int Ed. 2006;45:4345–4348. doi: 10.1002/anie.200601208.O’Malley DP, Li K, Maue M, Zografos AL, Baran PS. J Am Chem Soc. 2007;129:4762–4775. doi: 10.1021/ja069035a.Lanman BA, Overman LE, Pulini R, White NS. J Am Chem Soc. 2007;129:12896–12900. doi: 10.1021/ja074939x.Sivappa R, Hernandez NM, He Y, Lovely CJ. Org Lett. 2007;9:3861–3864. doi: 10.1021/ol0711568.Zancanella MA, Romo D. Org Lett. 2008;10:3685–3688. doi: 10.1021/ol801289b.Bultman MS, Ma J, Gin DY. Angew Chem, Int Ed. 2008;47:6821–6824. doi: 10.1002/anie.200801969.Hudon J, Cernak TA, Ashenhurst JA, Gleason JL. Angew, Chem, Int Ed. 2008;47:8885–8888. doi: 10.1002/anie.200803344.
- 6.a) Yamaguchi J, Seiple IB, Young IS, O’Malley DP, Maue M, Baran PS. Angew Chem, Int Ed. 2008;47:3578–3580. doi: 10.1002/anie.200705913. [DOI] [PubMed] [Google Scholar]; b) O’Malley DP, Yamaguchi J, Young IS, Seiple IB, Baran PS. Angew Chem, Int Ed. 2008;47:3581–3583. doi: 10.1002/anie.200801138. [DOI] [PubMed] [Google Scholar]
- 7.a) Nishizawa M, Yadav VK, Skwarczynski M, Takao H, Imagawa H, Sugihara T. Org Lett. 2003;5:1609–1611. doi: 10.1021/ol034201u. [DOI] [PubMed] [Google Scholar]; b) Nishizawa M, Takao H, Yadav VK, Imagawa H, Sugihara T. Org Lett. 2003;5:4563–4565. doi: 10.1021/ol035622e. [DOI] [PubMed] [Google Scholar]; c) Imagawa H, Kurisaki T, Nishizawa M. Org Lett. 2004;6:3679–3681. doi: 10.1021/ol048730p. [DOI] [PubMed] [Google Scholar]; d) Imagawa H, Iyenaga T, Nishizawa M. Org Lett. 2005;7:451–453. doi: 10.1021/ol047472t. [DOI] [PubMed] [Google Scholar]; e) Imagawa H, Iyenaga T, Nishizawa M. Synlett. 2005:703–705. doi: 10.1021/ol047472t. [DOI] [PubMed] [Google Scholar]; f) Yamamoto H, Nishiyama M, Imagawa H, Nishizawa M. Tetrahedron Lett. 2006;47:8369–8373. [Google Scholar]; g) Yamamoto H, Sasaki I, Imagawa H, Nishizawa M. Org Lett. 2007;9:1399–1402. doi: 10.1021/ol070335m. [DOI] [PubMed] [Google Scholar]; h) Yamamoto H, Pandey G, Asai Y, Nakano M, Kinoshita A, Namba K, Imagawa H, Nishizawa M. Org Lett. 2007;9:4029–4032. doi: 10.1021/ol701737x. [DOI] [PubMed] [Google Scholar]
- 8.a) Namba K, Yamamoto H, Sasaki I, Mori K, Imagawa H, Nishizawa M. Org Lett. 2008;10:1767–1770. doi: 10.1021/ol800450x. [DOI] [PubMed] [Google Scholar]; b) Namba K, Nakagawa Y, Yamamoto H, Imagawa H, Nishizawa M. Synlett. 2008:1719–1723. [Google Scholar]
- 9.a) Knapp S, Rodriques KE, Levorse AT, Ornaf RM. Tetrahedron Lett. 1985;26:1803–1806. [Google Scholar]; b) Kitagawa O, Fujita M, Li H, Taguchi T. Tetrahedron Lett. 1997;38:615–618. [Google Scholar]; c) Gaudreault P, Drouin C, Lessard J. Can J Chem. 2005;83:543–545. [Google Scholar]; d) Richardson RD, Zayed JM, Altermann S, Smith D, Wirth T. Angew Chem Int Ed. 2007;46:6259–6532. doi: 10.1002/anie.200702313. [DOI] [PubMed] [Google Scholar]; e) Corey EJ, Yeung YY. Tetrahedron Lett. 2007;48:7567–7570. [Google Scholar]
- 10.Luche JL. J Am Chem Soc. 1978;100:2226–2227. [Google Scholar]
- 11.The direct ortho ester Claisen rearrangement of 11 resulted in a complex mixture formation. For a related successful rearrangement, see: Chapuis C, Büchi GH, Wüest H. Helv Chim Acta. 2005;88:3069–3088.
- 12.Ireland RE, Mueller RH, Willard AK. J Am Chem Soc. 1976;98:2868–2877. [Google Scholar]
- 13.Generalization of this hydrazide formation is under investigation.
- 14.CCDC 711310 contains the supplementary crystallographic data for this These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
-
15.The Hg(OTf)2-catalyzed cyclization of the primary amides i afforded only lactone ii, and lactum iii was not detected at all under a variety of reaction conditions (solvent effect or addition of Lewis acids). Even the reaction of
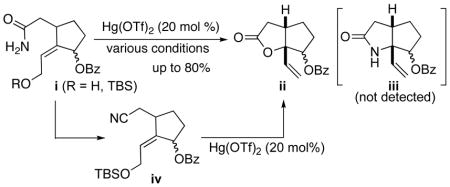 nitrile iv did not afford lactum iii.
nitrile iv did not afford lactum iii.
- 16.Oxidation with Dess-Martin periodinane resulted in modest yield.
- 17.a) Nicolaou KC, Montagnon T, Baran PS. Angew Chem, Int Ed. 2002;41:993–996. doi: 10.1002/1521-3773(20020315)41:6<993::aid-anie993>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Gray DLF, Montagnon T, Harrison S. Angew Chem, Int Ed. 2002;41:996–1000. doi: 10.1002/1521-3773(20020315)41:6<996::aid-anie996>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 18.Mukaiyama T, Matsuo J, Kitagawa H. Chem Lett. 2000;29:1250–1251. [Google Scholar]
- 19.Kuehne ME, Bornmann WG, Parsons WH, Spitzer TD, Blount JF, Zubieta J. J Org Chem. 1988;53:3439–3450. [Google Scholar]
- 20.The other conditions such as LHMDS/TMSCl/THF, large excess of LHMDS/TMSCl/THF, LDA/TMSCl/THF, TEA/TMSCl/CH2Cl2, TEA/TBSOTf/CH2Cl2, TEA/TESOTf/CH2Cl2, and TEA/TMSOTf/benzene reflux did not give any silyl enol ethers.
- 21.Ito Y, Hirao T, Saegusa T. J Org Chem. 1978;43:1011–1013. [Google Scholar]
- 22.Petrignet J, Prathap I, Chandrasekhar S, Yadav JS, Greé R. Angew, Chem, Int Ed. 2007;46:6297–6300. doi: 10.1002/anie.200701663. [DOI] [PubMed] [Google Scholar]
- 23.CCDC 713396 contains the supplementary crystallographic data for this These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.