Abstract
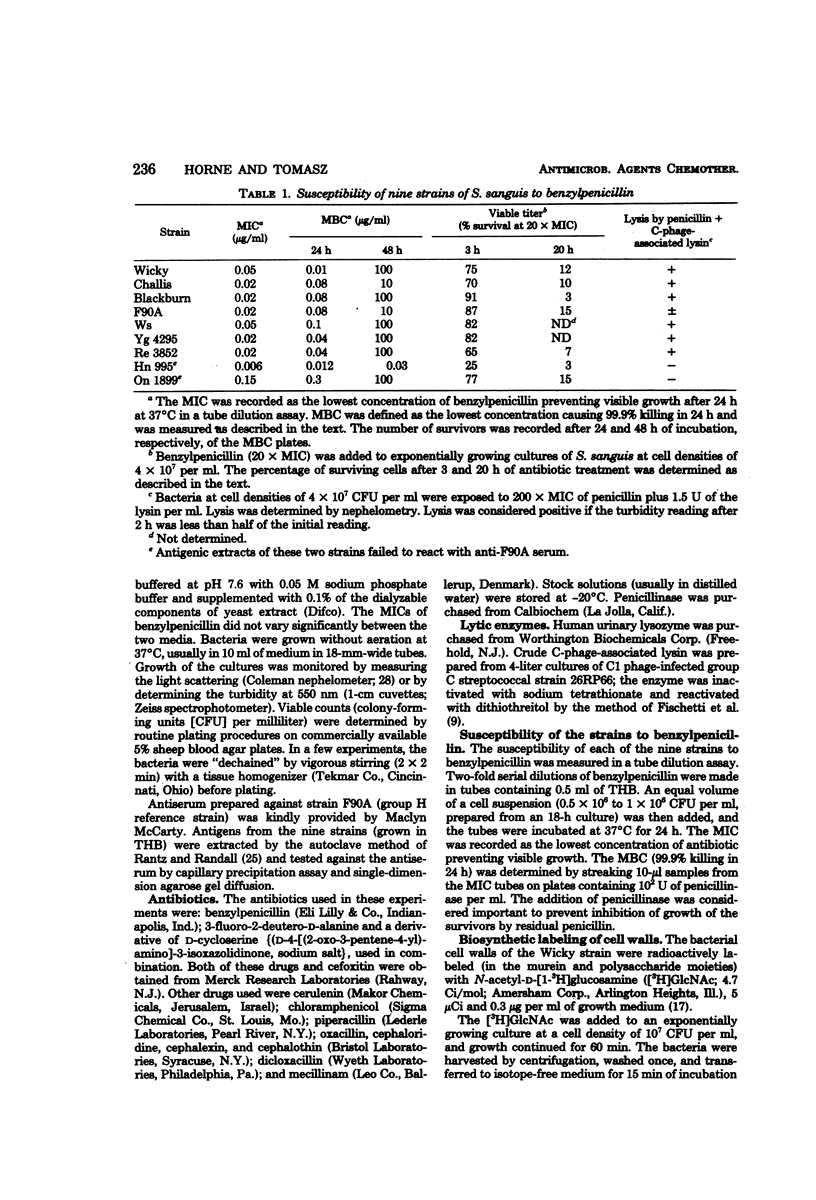
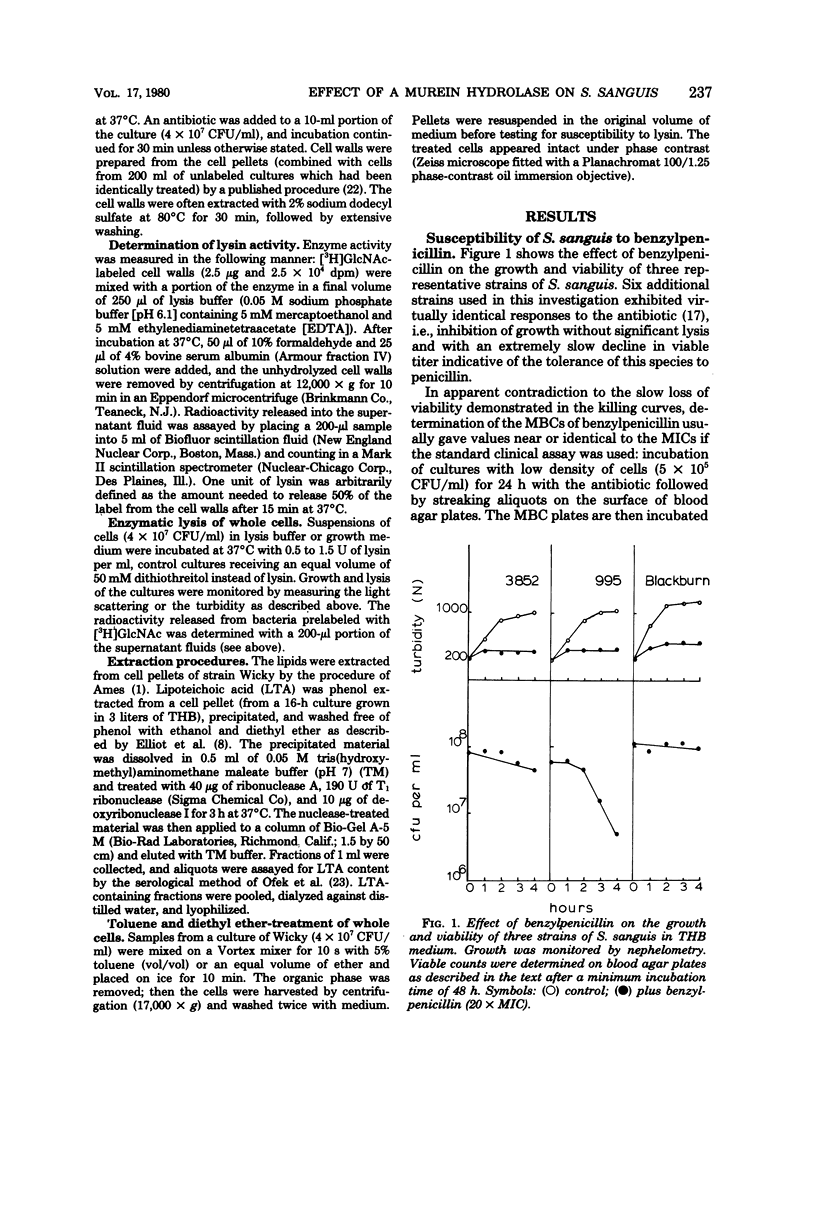
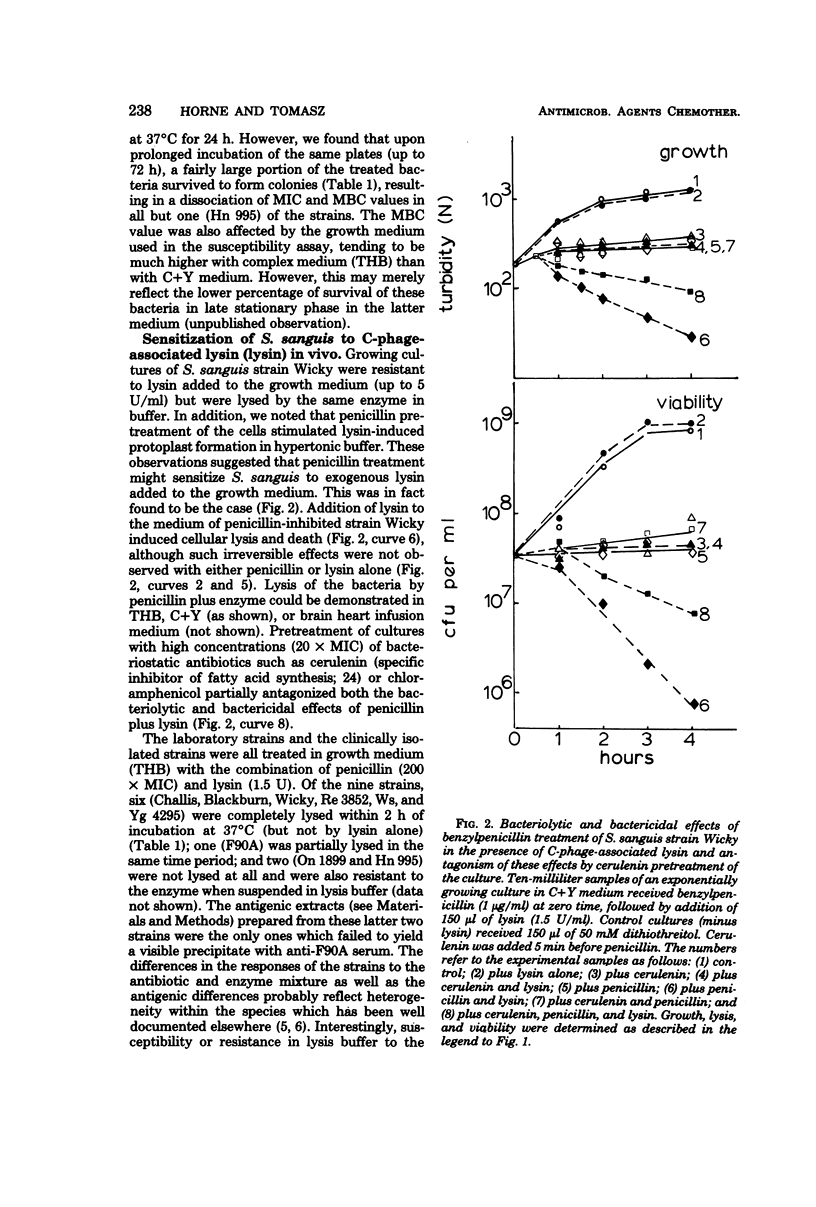
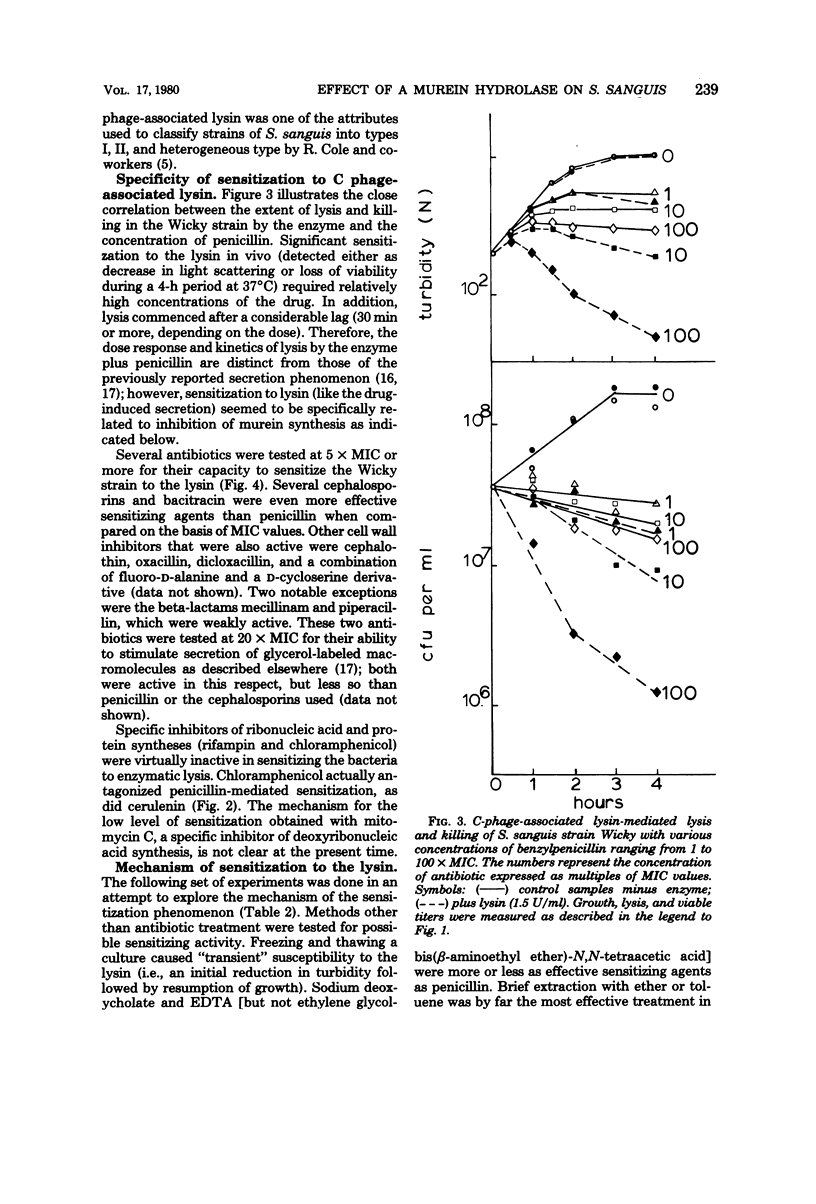
Nine strains of Streptococcus sanguis exhibited tolerance to benzylpenicillin: the growth of each strain was susceptible to penicillin with minimal inhibitory concentrations of 0.1 μg/ml or lower, but the bacteriolytic and bactericidal effects were limited in each case. The tolerance of these bacteria was also reflected in the large discrepancies between the minimal inhibitory and minimal bactericidal concentrations for benzylpenicillin. The hypothesis that a natural deficiency of endogenous murein hydrolase (autolysin) in this species accounts for the penicillin tolerance was tested by using a heterologous murein hydrolase, the C-phage-associated lysin. In seven of the strains, addition of the lysin to the culture together with penicillin or other cell wall inhibitors resulted in lysis and rapid loss of viability. The enzyme alone did not appreciably affect normally growing cultures. The irreversible effects of penicillin plus lysin were drastically reduced in the presence of the bacteriostatic agents chloramphenicol and cerulenin. Speculations based on experiments are presented for the mechanisms by which penicillin treatment sensitizes these bacteria to an exogenous lytic enzyme. Similar phenomena requiring cooperation of host factors and penicillin may occur during infection, since somewhat similar although less pronounced results were obtained by addition of human lysozyme to penicillin-treated S. sanguis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra G. B., Nugent K. M., Cole R. M. Preparation of protoplasts of group H streptococci (Streptococcus sanguis). Appl Microbiol. 1975 Jan;29(1):90–93. doi: 10.1128/am.29.1.90-93.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Calandra G. B., Huff E., Nugent K. M. Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res. 1976 Jan;55:A142–A153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- Elliott S. D., McCarty M., Lancefield R. C. Teichoic acids of group D streptococci with special reference to strains from pig meningitis (Streptococcus suis). J Exp Med. 1977 Mar 1;145(3):490–499. doi: 10.1084/jem.145.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H., Warren G. H. Antibody-mediated bacteriolysis: enhanced killing of cyclacillin-treated bacteria. Proc Soc Exp Biol Med. 1976 Nov;153(2):301–304. doi: 10.3181/00379727-153-39533. [DOI] [PubMed] [Google Scholar]
- Friedman H., Warren G. H. Enhanced susceptibility of penicillin-resistant staphylococci to phagocytosis after in vitro incubation with low doses of nafcillin. Proc Soc Exp Biol Med. 1974 Jul;146(3):707–711. doi: 10.3181/00379727-146-38177. [DOI] [PubMed] [Google Scholar]
- Glaser L., Lindsay B. Relation between cell wall turnover and cell growth in Bacillus subtilis. J Bacteriol. 1977 May;130(2):610–619. doi: 10.1128/jb.130.2.610-619.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lopez R., Ronda-Lain C., Tapia A., Waks S. B., Tomasz A. Suppression of the lytic and bactericidal effects of cell wallinhibitory antibiotics. Antimicrob Agents Chemother. 1976 Oct;10(4):697–706. doi: 10.1128/aac.10.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]



