Abstract
Nrf2:INrf2 acts as a sensor for oxidative/electrophilic stress. INrf2 serves as an adaptor to link Nrf2 to the ubiquitin ligase Cul3-Rbx1 complex that ubiquitinate and degrade Nrf2. Under basal conditions, cytosolic INrf2/Cul3-Rbx1 is constantly degrading Nrf2. When a cell encounters stress Nrf2 dissociates from the INrf2 and translocates into the nucleus. Oxidative/electrophilic stress induced modification of INrf2Cysteine151 and/or protein kinase C (PKC)-mediated phosporylation of Nrf2Serine40 controls Nrf2 release from INrf2 followed by stabilization and nuclear translocation of Nrf2. Nrf2 binds to the antioxidant response element (ARE) and activates a myriad of genes that protect cells against oxidative/electrophilic stress and neoplasia. A delayed response of oxidative/electrophilic stress activates GSK-3β that phosphorylates Fyn at unknown threonine residue(s). Phosphorylated Fyn translocates to the nucleus and phosphorylates Nrf2Tyrosine568 that leads to nuclear export and degradation of Nrf2. Prothymosin-α mediated nuclear translocation of INrf2 also degrades nuclear Nrf2. The degradation of Nrf2 both in cytosol and nuclear compartments rapidly brings down its levels to normal resulting in suppression of Nrf2 downstream gene expression. An autoregulatory loop between Nrf2 and INrf2 controls their cellular abundance. Nrf2 regulates INrf2 by controlling its transcription, and INrf2 controls Nrf2 by degrading it. In conclusion, switching on and off of Nrf2 combined with promoting an autoregulatory loop between them regulates activation/deactivation of defensive genes leading to protection of cells against adverse effects of oxidative and electrophilic stress and promote cell survival.
Keywords: Nrf, INrf2 (keap1), Oxidative/electrophilic stress, Defensive gene expression, Cell signaling, Cell survival
Introduction
Reactive oxygen species (ROS) cause oxidative stress and have a profound impact on the survival and evolution of all living organisms (Breimer, 1990; Meneghini, 1997). Endogenous and exogenous reactions/pathways generate ROS (Grisham and McCord, 1986; Thelen et al., 1993; Kerr et al., 1996; Wei, 1998; Kasprzak, 1995; Suzuki et al., 1997; Kim et al., 2008; Breen et al., 1995; Last et al., 1994; Goetz and Luch, 2008; Ward, 1994). Therefore, it is clear that all cells must continuously strive to keep the levels of ROS in check. ROS attack DNA and other cellular macromolecules causing oxidative stress and many other physiological and pathological conditions. These conditions include aging, neurodegenerative diseases, arthritis, arteriosclerosis, inflammatory responses, and tumor induction and promotion (Grisham and McCord, 1986; Ward, 1994; Kim et al., 2008; Rosen et al., 1995). Many of these conditions, including cancer, are often preceded by damage or mutation of genomic DNA (Ames et al., 1995). Much of the research on ROS has been centered on the damaging effects of oxidative stress. However, it is now apparent that ROS activate a battery of cellular enzymes that either prevent the generation of ROS or detoxify ROS and thereby protect the cell against damage caused by oxidative stress. Prokaryotic cells utilize transcription factors OxyR and SoxRS to sense the redox state of the cell, and in times of oxidative stress these factors induce the expression of about eighty defensive genes (Bauer et al., 1999; Zheng and Storz, 2000). Similar mechanisms of protection against oxidative stress have been found in eukaryotic cells that activate at least one hundred genes (Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008). Their products regulate a wide variety of cellular activities including signal transduction, proliferation, and immunologic defense reactions. There is a great variety of factors involved in the cellular response to oxidative stress. For instance, activation of heat shock response activator protein 1, NF-E2 related factor 2 (Nrf2), extracellular signal related kinases (ERK1/2), protein kinase B (Akt), and NF-kB promote cell survival, whereas prolonged activation of c-jun, N-terminal kinases (JNK), p38 kinase, and TP53 may lead to cell cycle arrest and apoptosis (Halliwell and Gutteridge, 2007). The Nrf2 pathway is presumably the most important for the cell to deal with oxidative/electrophilic stress generated from exposure to exogenous and endogenous chemicals, metals and radiation (Dhakshinamoorthy et al., 2000).
Antioxidant Response Element and NF-E2 Related Factors
Exposure of cells to antioxidants and xenobiotics leads to the induction of a battery of defensive genes encoding detoxifying enzymes [NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GST), heme oxygenase 1 (HO-1)], antioxidant and related proteins [thioredoxins, γ-glutamyl cysteinyl synthetase (γ-GCS), glutathione peroxidase, glutathione reductase], ubiquitination enzymes and proteasomes, and drug transporters (MRPs) (reviewed in Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto,2006; Copple et al., 2008; Kwak et al., 2007; Hayashi et al. 2003; Maher et al., 2005; Slitt et al., 2006; Maher et al., 2007; Maher et al., 2008). NQO1 is a flavoprotein that competes with cytochrome P450 reductase and catalyzes two-electron reduction and detoxification of quinones and other redox cycling compounds (Joseph and Jaiswal, 1994). GST Ya conjugates hydrophobic electrophiles with glutathione, aiding in their excretion (Pickett and Lu, 1989; Tsuchida and Sato, 1992). HO-1 catalyzes the first and rate-limiting step in heme catabolism (Choi and Alam, 1996). glutamate cysteine ligase is a rate limiting step in the synthesis of glutathione (Kretzschmar et al., 1992). Glutathione peroxidase and reductase are glutathione metabolism enzymes (Pickett and Lu, 1989; Tsuchida and Sato, 1992). Ubiquitination enzymes and proteasomes mediate ubiquitination and degradation of proteins including oxidized proteins (Kwak et al., 2007). Drug transporters play an important role in drug intake and efflux (Hayashi et al., 2003; Maher et al., 2005; Slitt et al., 2006; Maher et al., 2007; Maher et al., 2008).
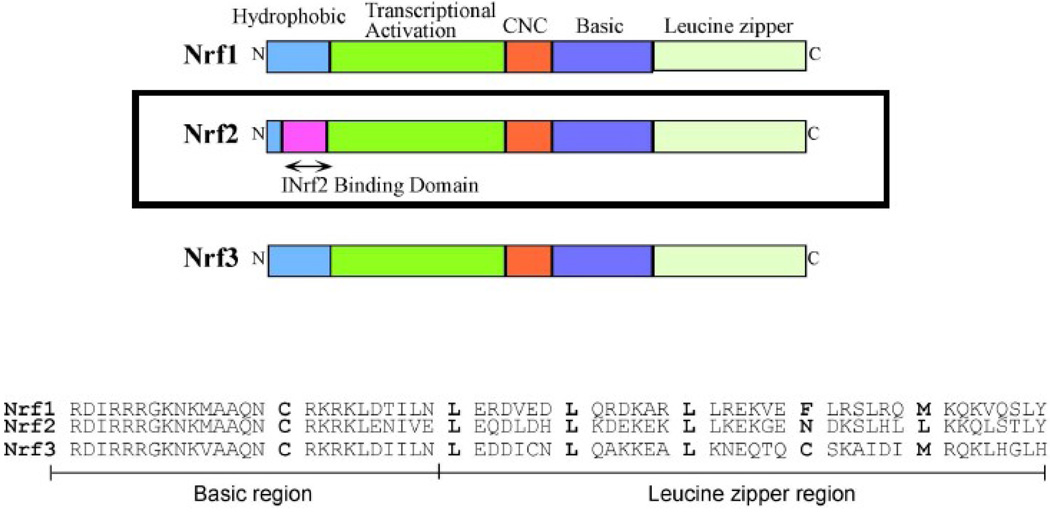
Deletion mutagenesis and transfection studies identified an element designated as antioxidant response element (ARE) in the promoter regions of Nrf2 downstream genes that regulates expression and coordinated induction of these genes in response to antioxidants and xenobiotics (reviewed in Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008). Mutational analysis revealed GTGACA***GC as the core sequence of the ARE (reviewed in Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008; Li and Jaiswal, 1992; Prestera et al., 1993; Wasserman and Fahl, 1997). NF-E2 related factors are known to bind to the ARE and regulate ARE-mediated antioxidant enzyme genes expression and induction in response to a variety of stimuli including antioxidants, xenobiotics, metals and UV irradiation (Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008). NF-E2 (p45) binds to an Activator Protein 1 (AP1) like, NF-E2 recognition site (GCTGAGTCA), and regulates tissue specific expression of the globin genes (Andrews et al., 1993; Ney et al., 1990; Moi and Kan, 1990; Liu et al., 1992; Mignotte, et al., 1989). NF-E2 functions as a heterodimer with the ubiquitously expressed small Maf proteins (Igtarashi et al., 1994). NF-E2−/− mice have no circulating platelets, and most die due to hemorrhage (Shivdasani and Stuart.1995). NF-E2 related factors Nrf2 and Nrf1, both 66–68 kDa proteins, were cloned using a yeast complementation assay (Chan et al., 1993; Moi et al., 1994). Both display a significant amount of homology to NF-E2, but unlike NF-E2 both are ubiquitously expressed. A third family member of the Nrfs, Nrf3, was also cloned and sequenced (Kobayashi et al., 1999). The Nrfs belong to the family of basic leucine zipper proteins (bZIP) (Fig. 1). The basic region, just upstream of the leucine zipper region, is responsible for DNA binding. The acidic region is required for transcriptional activation. The cap’n’collar region, so called because of its homology to the Drosophila cap’n’collar protein, is highly conserved among the Nrfs, but the function of this region remains unknown. Nrf1 −/− mice die in utero due to a decreased number of enucleated red blood cells and severe anemia (Chan et al., 1998). Nrf2−/− mice are viable and live to adulthood (Chan et al., 1996). Nrf2 is therefore not required for erythropoeisis, development, or growth (Chan et al., 1996).
Fig. 1. NF-E2 related factors.
Protein domains of three NF-E2 related factors are shown. INrf2 binding site in Nrf2 is also shown. Nrf1 and Nrf3 lack INrf2 binding domain and do not bind with INrf2. CNC, Cap'n'Collar region. Amino acid sequence of basic and leucine zipper regions from three factors are aligned to demonstrate conservation of cysteine (C) and leucines (L). Please note that the three proteins and its domains are not drawn on scale.
The evidence demonstrating the role of Nrf2 and Nrf1 in protection against oxidative and/or electrophilic stress came from studies on the role of Nrf2 in ARE-mediated regulation of NQO1 gene expression (Venugopal and Jaiswal, 1996). Overexpression of Nrf2 and Nrf1 cDNA was shown to upregulate the expression and induction of the NQO1 gene in response to antioxidants and xenobiotics (Venugopal and Jaiswal, 1996). In addition, Nrf2-null mice exhibited a marked decrease in the expression and induction of NQO1, indicating that Nrf2 plays an essential role in the in vivo regulation of NQO1 in response to oxidative stress (Itoh et al., 1997). Further studies have shown that Nrf2 is also a prevailing factor in the regulation of ARE-mediated activation of other defensive genes including GST Ya, γ-GCS, HO-1 antioxidants, proteasomes, drug transporters etc (Kobyashi and Yamamoto, 2006; Copple et al., 2008; Kwak et al., 2007; Hayashi et al., 2003; Maher et al., 2005; Slitt et al., 2006; Maher et al., 2007; Maher et al., 2008). Studies have also demonstrated that Nrf2 is the most prominent factor in activation of ARE-mediated genes expression and induction as compared with Nrf1 and Nrf3 (Venugopal and Jaiswal, 1996; 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008).
INrf2, a Cytosolic Inhibitor of Nrf2
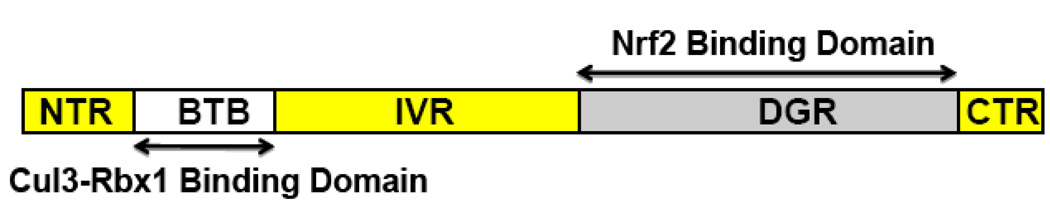
INrf2 (Inhibitor of Nrf2) or KEAP1 (Kelch-like ECH-associated protein1), protein retains Nrf2 in the cytoplasm (Dhakshinamoorthy and Jaiswal, 2001; Itoh et al., 1999) (Fig. 2). Analysis of the INrf2 amino acid sequence revealed a protein-protein interaction domain BTB/POZ BTB (broad complex, tramtrack, bric-a-brac)/POZ (poxvirus, zinc finger) and a Kelch domain (Itoh et al., 1999). In the Drosophila Kelch protein, and in PIP, the Kelch domain binds to actin (Albagli et al., 1995; Kim et al., 1999). Therefore, it was expected that INrf2 bind to actin in the cytoskeleton. Indeed, a report showed that INrf2 binds to actin of cytoskeleton (Kang et al., 2004). The same report also showed that scaffolding of INrf2 to the actin cytoskeleton plays an important role in retention of Nrf2 in the cytosol. INrf2 exists as dimers inside the cells (Zipper and Mulcahy, 2002). INrf2 functions as an adapter for Cul3/Rbx1 mediated degradation of Nrf2 (Kobayashi et al., 2004; Cullinan et al., 2004; Zhang et al., 2004; Fig. 2). INrf2 with its N-terminal BTB/POZ domain binds to Rbx1 bound Cul3 and with C-terminal Kelch domain binds to Nrf2. This leads to ubiquitination and degradation of Nrf2. Under basal/normal conditions, cytosolic INrf2/Cul3-Rbx1 is constantly degrading Nrf2. When a cell encounters stress Nrf2 dissociates from INrf2, stabilized and translocates into the nucleus leading to activation of ARE-gene expression (reviewed in Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto,2006; Copple et al., 2008). It is noteworthy that INrf2 is specific to Nrf2 and does not interact with Nrf1 or Nrf3 (Jain and Jaiswal, Unpublished).
Fig. 2. Schematic presentation of the various domains of INrf2.
Nrf2, NF-E2 Related Factor 2; INrf2, Inhibitor of Nrf2; NTR, N-Terminal Region; BTB, Broad complex, Tramtrack, Bric-a-brac; IVR, Intervening/linker Region; DGR, Kelch domain/diglycine repeats; CTR, C-Terminal Region.
Studies have shown that INrf2 (Keap1)-null mice are not viable and die shortly after birth, probably from malnutrition resulting from hyperkeratosis in the esophagus and forestomach (Wakabayashi et al., 2003). However, mating of heterozygous INrf2+/− mice with Nrf2−/− mice led to generation of double knockout (INrf2−/− and Nrf2−/−) mice that did not express both INrf2 and Nrf2 (Kwak et al., 2003). The double knockout mice survived. These results indicated that Nrf2 accumulation in the absence of INrf2 in the nucleus might be sensitive for esophagus and forestomach tissues due to unknown reasons. INrf2 floxed mice were generated (Okawa et al., 2006). Hepatocyte-specific deletion of the Keap1 (INrf2) gene led to activation of Nrf2 that conferred potent resistance to acetaminophen toxicity (Okawa et al., 2006).
Reports have suggested that any mechanism that modifies INrf2, and/or Nrf2, allowing it to disrupt the Nrf2/INrf2 complex will result in the upregulation of ARE-mediated gene expression. Because the metabolism of both antioxidants and xenobiotics results in the generation of superoxides and electrophiles (De Long et al., 1987), it is thought that these molecules might act as second messengers, activating ARE-mediated gene expression. Several protein kinases such as protein kinase C (PKC) (Huang et al., 2002; Bloom and Jaiswal, 2003), extracellular signal-regulated kinases (ERK) (Buckley et al., 2003), MAPK and p38 MAP kinase (Yu et al., 1999; Zipper et al., 2000), and PKR-like endoplasmic reticulum kinase (PERK) (Cullinan et al., 2003) are known to modify Nrf2 and hence activate its release from INrf2. Among these mechanisms, oxidative/electrophilic stress mediated phosphorylation of Nrf2 at serine40 by PKC is a very well-studied and accepted model for activation mechanism of Nrf2 (Huang et al., 2002; Bloom and Jaiswal, 2003). In addition to post-translational modification in Nrf2 resulting in ARE-induction, several crucial residues in INrf2 have also been proposed to be important for activation of Nrf2. Studies based on the electrophile mediated modification, location and mutational analyses revealed that three cysteine residues, Cys151, Cys273 and Cys288 are crucial for INrf2 activity (Zhang and Hannink, 2003). INrf2 itself undergoes ubiquitination by the Cul3 complex which was markedly increased in response to phase II inducers such as t-BHQ (t-butylhydroquinone) (Zhang et al., 2005). It has been suggested that, under physiological conditions INrf2 targets Nrf2 for ubiquitin mediated degradation but electrophiles may trigger a switch of Cul3 dependent ubiquitination from Nrf2 to INrf2 resulting in ARE gene induction. Recently, Eggler at al. (2005) demonstrated that modifying specific cysteines of the electrophile-sensing human INrf2 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Upon introduction of electrophiles, modification of INrf2 C151 leads to a change in the conformation of the BTB domain by means of perturbing the homodimerization site, disrupting Neh2 ubiquitination, and causing ubiquitination of INrf2. Modification of INrf2 cysteines by electrophiles does not lead to disruption of the INrf2–Nrf2 complex. Rather, the switch of ubiquitination from Nrf2 to INrf2 leads to Nrf2 nuclear accumulation.
The redox modulation of cysteines in INrf2 might be a mechanism redundant to the phosphorylation of Nrf2 by PKC, or that the two mechanisms work in concert. In addition to cysteine151 modification, phosphorylation of Nrf2 has also been shown to play a role in INrf2 retention and release of Nrf2. Serine104 of INrf2 is required for dimerization of INrf2 (Zipper and Mulcahy, 2002). Mutation of Serine104 led to disruption of INrf2 dimer and release of Nrf2 (Zipper and Mulcahy, 2002). Recently, we demonstrated that phosphorylation and dephosphorylation of INrf2tyrosine141 regulates stability and degradation of INrf2 (Jain et al., 2008). Phosphorylation of tyrosine141 is required for stability of INrf2. The treatment of cells with hydrogen peroxide led to dephosphorylation of tyrosine141 and destabilization/degradation of INrf2. This led to release of Nrf2. More recently, we showed that prothymosin-α mediates nuclear import of INrf2/Cul3-Rbx1 complex (Niture and Jaiswal, 2009). Antioxidant treatment increases nuclear import of INrf2/Cul3-Rbx1 complex. The INrf2/Cul3-Rbx1 complex inside the nucleus exchanges prothymosin-α with Nrf2 resulting in degradation of Nrf2. These results led to the conclusion that prothymosin-α mediated nuclear import of INrf2/Cul3-Rbx1 complex leads to ubiquitination and degradation of Nrf2 inside the nucleus presumably to regulate nuclear level of Nrf2 and rapidly switch off the activation of Nrf2 downstream gene expression.
Mechanism of Signal Transduction and Regulation of Nrf2 Activation and Degradation
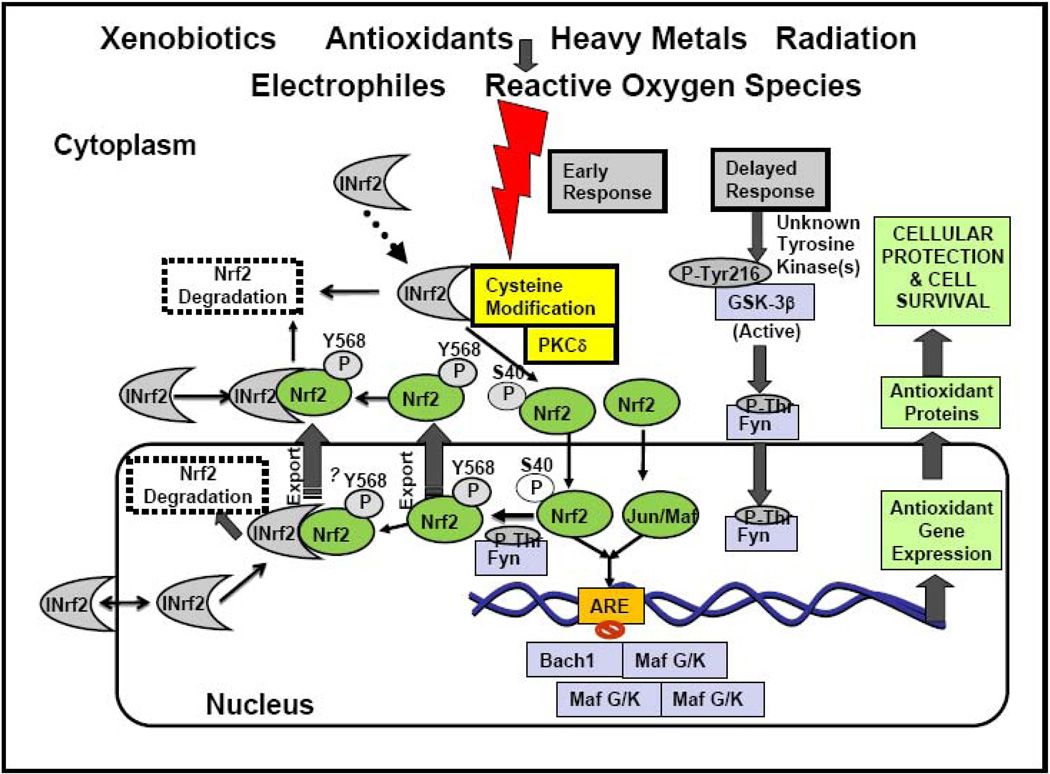
A hypothetical model illustrating the role of INrf2, Nrf2 and other ARE-binding factors in activation of antioxidant genes by antioxidants and xenobiotics is shown in Fig. 3. Nrf2:INrf2 acts as a sensor for oxidative/electrophilic stress. In response to oxidative/electrophilic stress, Nrf2 is switched on (separation from INrf2 and stabilization of Nrf2) and then off (ubiquitination and degradation of Nrf2) by distinct early and delayed mechanisms. Oxidative/electrophilic modification of INrf2cysteine151 and/or PKC phosphorylation of Nrf2serine40 results in the escape or release of Nrf2 from INrf2. Nrf2 is stabilized and translocates to the nucleus, heterodimerizes with small Maf, Jun or yet unknown proteins and binds to the ARE that leads to coordinated activation of gene expression (Jaiswal, 2004; Kobyashi and Yamamoto, 2006; Copple et al., 2008). It takes close to two hours from the time of exposure to optimally switch on nuclear import of Nrf2 (Jain et al., 2005). This is followed by activation of a delayed mechanism involving GSK3β that controls switching off of Nrf2 activation of gene expression. GSK3β phosphorylates Fyn at unknown threonine residue(s) leading to nuclear localization of Fyn (Jain and Jaiswal, 2007). Fyn phosphorylates Nrf2tyrosine568 resulting in nuclear export of Nrf2, binding with INrf2 and degradation of Nrf2 (Jain and Jaiswal, 2006). Bach1 is a nuclear protein that competes with Nrf2 for binding to ARE leading to suppression of Nrf2 downstream genes (Dhalshinamoorthy et al., 2005). The negative regulation of Nrf2 through Fyn and Bach1 pathways are important in switching off the induction of Nrf2 downstream genes that were switched on in response to oxidative stress. The switching on and off of Nrf2 protect cells against free radical damage, prevents apoptosis and promotes cell survival (Dhakshinamoorthy et al., 2000; Jaiswal, 2004; Zhang, 2006; Kobyashi and Yamamoto, 2006; Copple et al., 2008; Giudice and Montella, 2006).
Fig. 3. Oxidative/electrophilic stress and Nrf2:INrf2 signaling.
S40 is serine 40 phosphorylation in Nrf2. Y568 is tyrosine 568 phosphorylation in INrf2.
Auto-regulatory Loop Between INrf2 and Nrf2
An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance and ARE-mediated gene expression and induction (Lee et al., 2007). An ARE in the reverse strand of the proximal INrf2 promoter that binds to Nrf2 regulates expression and antioxidant induction of the INrf2 gene expression. The induction of INrf2 followed ubiquitination and degradation of Nrf2 and suppression of INrf2 gene expression. In other words, Nrf2 regulates INrf2 by controlling its transcription, and INrf2 controls Nrf2 by degrading it.
Nrf2:INrf2 in Cell Survival, Cancer and Chemoprevention, and Drug Resistance
Nrf2 is considered a major mechanism of protection against chemical and radiation capable of damaging DNA integrity and initiating carcinogenesis (Giudice and Montella, 2006). It protects cells against chemical and radiation stress and promotes cell survival. The Nrf2-knockout mouse was prone to acute damages induced by acetaminophen, ovalbumin, cigarette smoke, pentachlorophenol and 4-vinylcyclohexene diepoxide and had increased tumor formation when they were exposed to carcinogens such as benzo[a]pyrene, diesel exhaust and N-nitrosobutyl (4-hydroxybutyl) amine (Enomoto et al., 2001; Chan et al., 2001; Rangasamy et al., 2005; Iizuka et al., 2005; Hu et al., 2006; Aoki et al., 2001; Ramos-Gomez et al., 2001; Iida et al., 2004).
A role of Nrf2 in drug resistance is suggested based on its property to induce detoxifying and antioxidant enzymes (Vollrath et al., 2006; Kim et al., 2007; Okawa et al., 2006; Wang et al., 2008; Padmanabhan et al., 2006; Singh et al., 2006). The loss of INrf2 (Keap1) function is shown to lead to nuclear accumulation of Nrf2, activation of metabolizing enzymes and drug resistance (Wang et al., 2008). Studies have reported mutations resulting in dysfunctional INrf2 in lung, breast and bladder cancers (Padmanabhan et al., 2006; Singh et al., 2006; Ohta et al., 2008; Nioi and Nguyen, 2007; Shibata et al., 2008A). A recent study reported that somatic mutations also occur in the coding region of Nrf2, especially in cancer patients with a history of smoking or suffering from squamous cell carcinoma (Shibata et al., 2008B). These mutations abrogate its interaction with INrf2 and nuclear accumulation of Nrf2. This gives advantage to cancer cell survival and undue protection from anti-cancer treatments. However, the understanding of the mechanism of Nrf2 induced drug resistance remains in its infancy. In addition, the studies on Nrf2 regulated downstream pathways that contribute to drug resistance remain limited. Furthermore, it is unknown if the inhibition of Nrf2 or activation/increased expression of INrf2 and Bach1 could reverse the drug resistance.
Future Perspectives
Further studies are required to completely understand the mechanism of signal transduction from chemicals and radiation to Nrf2:INrf2 complex leading to the release of Nrf2 from INrf2 and activation of a battery of defensive genes. Preliminary studies demonstrate that deactivation of Nrf2 is equally important as activation of Nrf2. Therefore, studies must also explore negative regulation of Nrf2. Future studies should also address the role of Nrf2 and INrf2 in cell survival and drug resistance and cancer.
Acknowledgements
This work was supported by NIGMS grant RO1 GM047466 and NIEHS grant RO1 ES012265.
Abbreviations
- ROS
Reactive oxygen species
- ARE
Antioxidant response element
- Nrf2
NF-E2 related factor 2
- NQO1
NAD(P)H:quinone oxidoreductase1
- PKC
Protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albagli O, Dhordain P, Deweindt C, Lecocq G, Leprince D. The BTB/POZ domain: a new protein-protein interaction motif common to DNA- and actin-binding proteins. Cell Growth & Differentiation. 1995;6:1193–1198. [PubMed] [Google Scholar]
- Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews NC, Erdjument-Bromage H, Davidson MB, Tempst P, Orikin SH. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993;339:722–727. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharm. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- Bauer CE, Elsen S, Bird TH. Mechanisms for redox control of gene expression. Annu. Rev. Microbiol. 1999;53:495–523. doi: 10.1146/annurev.micro.53.1.495. [DOI] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser(40) by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Rad. Biol. Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol. Carcinogenesis. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- Chan JY, Han X, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc. Natl. Acad. Sci. USA. 1993;90:11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci USA. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell. Mol. Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannik M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 2004;24:8477–8486. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long MJ, Santamaria AB, Talalay P. Role of cytochrome P1-450 in the induction of NAD(P)H:quinone reductase in a murine hepatoma cell line and its mutants. Carcinogenesis. 1987;8:1549–1553. doi: 10.1093/carcin/8.10.1549. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Jaiswal AK. Functional characterization and role of INrf2 in antioxidant response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. Oncogene. 2001;20:3906–3917. doi: 10.1038/sj.onc.1204506. [DOI] [PubMed] [Google Scholar]
- Dhakshinamoorthy S, Long DJ, II, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Current Topics in Cellu. Regulation. 2000;36:201–206. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophilie-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc. Natl. Acad. SCi. USA. 2005;102:10070–10075. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–181. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- Goetz ME, Luch A. Reactive species: a cell damaging rout assisting to chemical carcinogens. Cancer Letters. 2008;266:73–83. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Grisham MB, McCord JM. Physiology of Oxygen Radicals. Baltimore: Waverly Press; 1986. Chemistry and cytotoxicity of reactive oxygen metabolites; pp. 1–18. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. fourth ed. Oxford: University Press; 2007. [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem. Biophys. Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Helen M, Dewald B, Baggiolini M. Neutrophil signal transduction and activation of the respiratory burst. Physiol. Rev. 1993;73:797–821. doi: 10.1152/physrev.1993.73.4.797. [DOI] [PubMed] [Google Scholar]
- Hu X, Roberts JR, Apopa PL, Kan YW, Ma Q. Accelerated ovarian failure induced by 4-Vinyl Cyclohexane diepoxide in Nrf2-null mice. Mol Cell Biol. 2006;26:940–954. doi: 10.1128/MCB.26.3.940-954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kataoka K, Nishizawa M, Yamamoto M. Regulation of transcription of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994;367:568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2 deficient mice are highly susceptible to cigarette smoke-induced emphyseema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh K, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Mahajan S, Jaiswal AK. Phosphorylation and dephosphorylation of tyrosine 141 regulate stability and degradation of INrf2: A novel mechanism in Nrf2 activation. J. Biol. Chem. 2008;283:17712–17720. doi: 10.1074/jbc.M709854200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J. Biol. Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- Jain AK, Jaiswal AK. Phosphorylation of tyrosine 568 controls nuclear export of Nrf2. J. Biol. Chem. 2006;281:12132–12142. doi: 10.1074/jbc.M511198200. [DOI] [PubMed] [Google Scholar]
- Jain AK, Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Rad. Biol. Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Joseph P, Jaiswal AK. NAD(P)H:Quinone oxidoreductase1 (DT diaphorase) specifically prevents the formation of benzo(a)pyrene quinone-DNA adducts generated by cytochrome P4501A1 and P450 reductase. Proc. Natl. Acad. Sci. USA. 1994;91:8413–8417. doi: 10.1073/pnas.91.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Kobayashi A, Wakabayashi N, Kim S, Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak KS. Possible role of oxidative damage in metal-induced carcinogenesis. Cancer Invest. 1995;13:411–430. doi: 10.3109/07357909509031921. [DOI] [PubMed] [Google Scholar]
- Kerr ME, Bender CM, Monti EJ. An introduction to oxygen free radicals. Heart Lung. 1996;25:200–209. doi: 10.1016/s0147-9563(96)80030-6. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug. Dis. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- Kim IF, Mohammadi E, Huang RCC. Isolation and characterization of IPP, a novel human gene coding an actin-binding, KELCH-like protein. Gene. 1999;228:73–83. doi: 10.1016/s0378-1119(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Ito E, Toki T, Kogame K, Takahashi S, Igarashi K, Hayashi N, Yamamoto M. Molecular cloning and functional characterization of a new cap’n’collar family transcription factor Nrf3. J. Biol. Chem. 1999;274:6443–6452. doi: 10.1074/jbc.274.10.6443. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Reinhardt D, Schlechtweg J, Machnik G, Klinger W, Schirrmeister W. Glutathione homeostasis in rats chronically treated with ethanol. Evidence for an increased hepatic GSH export in vivo. Exp Toxicol Pathol. 1992;44:344–348. doi: 10.1016/S0940-2993(11)80225-3. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Cho JM, Huang B, Shin S, Kensler TW. Role of increased expression of the proteasome in the protective effects of sulforaphane against hydrogen peroxide-mediated cytotoxicity in murine neuroblastoma cells. Free Radic. Biol. Med. 2007;43:809–817. doi: 10.1016/j.freeradbiomed.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Kwak M, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway: identification of novel gene clusters for cell survival. J. Biol. Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- Last JA, Sun WM, Witschi H. Ozone, NO, and NO2: oxidant air pollutants and more. Environ. Health Perspect. 1994;102 Suppl 10:179–184. doi: 10.1289/ehp.94102s10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee O, Jain AK, Papusha V, Jaiswal AK. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J Biol Chem. 2007;282:36412–36420. doi: 10.1074/jbc.M706517200. [DOI] [PubMed] [Google Scholar]
- Li Y, Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene:role of AP1 binding site contained within human antioxidant response element. J. Biol. Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- Liu D, Chang JC, Moi P, Liu W, Kan YW, Curtin PT. Dissection of the enhancer activity of beta-globin 5' DNase I-hypersensitive site 2 in transgenic mice. Proc. Natl. Acad. Sci. USA. 1992;89:3899–3903. doi: 10.1073/pnas.89.9.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transportersby chemical activators of receptor-mediated pathways in mouse liver. Drug Metab. Dispos. 2005;33:956–962. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- Maher J, Aleksunes L, Dieter M, Tanaka Y, Peters J, Manautou J, Klaassen C. Nrf2 and PPAR{alpha}-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol. Sci. 2008;106:319–328. doi: 10.1093/toxsci/kfn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, Dalton TP, Yamamoto M, Klaassen CD. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Mignotte V, Eleouet JF, Raich N, Romeo PH. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc. Natl. Acad. Sci. USA. 1989;86:6548–6522. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2 like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of β-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moi P, Kan YW. Synergistic enhancement of globin gene expression by activator protein-1-like proteins. Proc. Natl. Acad. Sci. USA. 1990;87:9000–9004. doi: 10.1073/pnas.87.22.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney PA, Sorrentino BP, McDonaugh KT, Nienhuis AW. Tandem AP-1-binding sites within the human beta-globin dominant control region function as an inducible enhancer in erythroid cells. Genes Dev. 1990;4:993–1006. doi: 10.1101/gad.4.6.993. [DOI] [PubMed] [Google Scholar]
- Nioi P, Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochem Biophys Res Commun. 2007;362:816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- Niture SK, Jaiswal AK. Prothymosin-a mediates nuclear import of INrf2/Cul3-Rbx1 complex to degrade nuclear Nrf2. J. Biol. Chem. 2009 doi: 10.1074/jbc.M808084200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the Keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provided by its point mutations in lung cancer. Mol Cell. 2006;21:689–734. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Pickett C. Glutathione S-transferases: gene structure, regulation and biological function. Ann. Rev. Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. USA. 1993;90:2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme induccers is lost in Nrf2 transcription factor deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GM, Pou S, Ramos CL, Cohen MS, Britigan BE. Free radicals and phagocytic cells. FASEB J. 1995;9:200–209. doi: 10.1096/fasebj.9.2.7540156. [DOI] [PubMed] [Google Scholar]
- Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008A;135:1358–1368. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in Nrf2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. USA. 2008B;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani R, Stuart HO. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc. Natl. Acad. Sci. USA. 1995;92:8690–8694. doi: 10.1073/pnas.92.19.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional Keap1-Nrf2 interaction in non-small-cell lung cancer. PLos Med. 2006;3:1865–1876. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Dieter MZ, Aleksunes LM, Scheffer GL, Huang W, Moore DD, Klaassen CD. trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors. Mol. Pharmacol. 2006;69:1554–1563. doi: 10.1124/mol.105.014571. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida S, Sato K. Glutathione transferases and cancer. Critical Rev. Biochem. Mol. Biol. 1992;27:337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath V, Wielandt AM, Iruretagoyena M, Chianale M. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem. J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng YX, Wondrack GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JF. The complexity of DNA damage: relevance to biological consequences. Int. J. Rad. Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- Wasserman W, Fahl WE. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc. Soc. Exp. Biol. Med. 1998;217:53–63. doi: 10.3181/00379727-217-44205. [DOI] [PubMed] [Google Scholar]
- Yu R, Mandlekar S, Weber MJ, Der CJ, Wu J, Tony-Kong AN. Role of mitogen-activated protein kinase pathway in the induction of phaseII detoxifying enzymes by chemicals. J. Biol. Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23:8137–815. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD, Lo S, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J. Biol. Chem. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Storz G. Redox sensing by prokaryotic transcription factors. Biochem. Pharm. 2000;59:1–6. doi: 10.1016/s0006-2952(99)00289-0. [DOI] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem. Biophys. Res. Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002;277:36544–36552. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]