Abstract
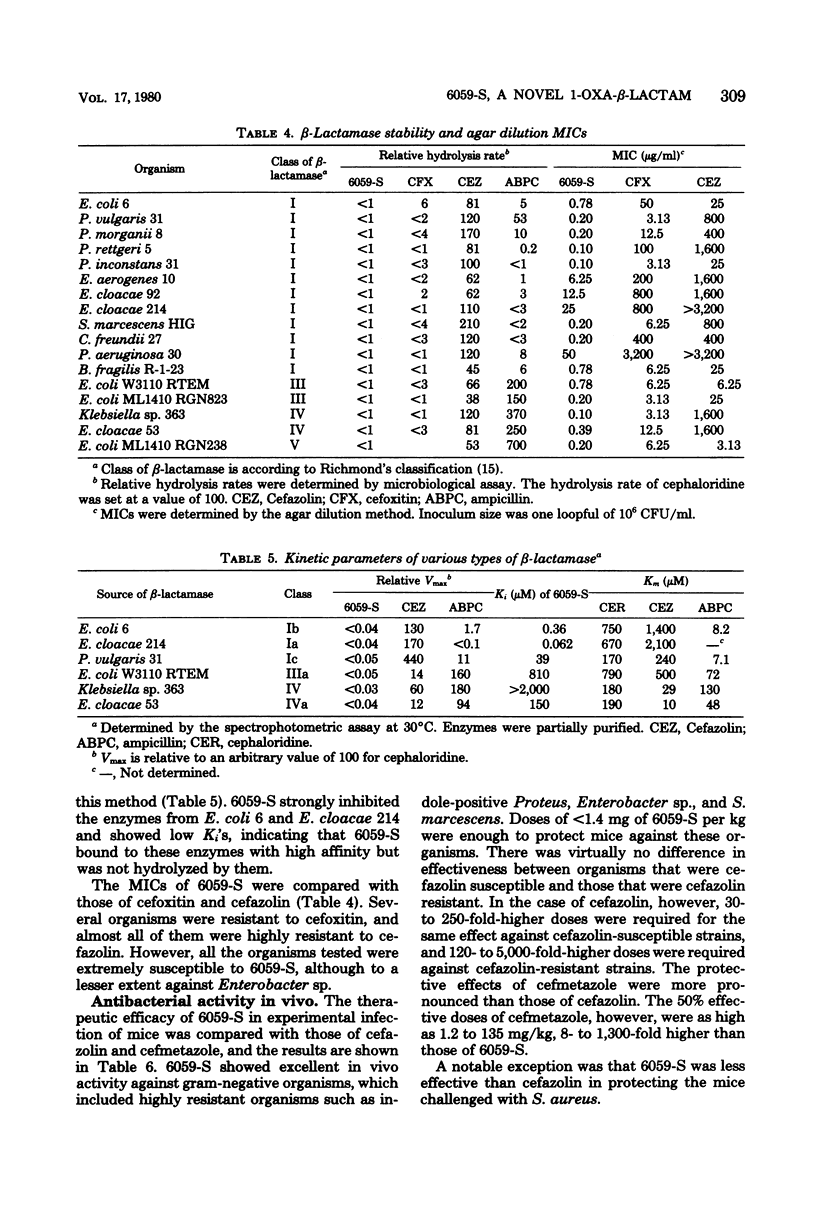
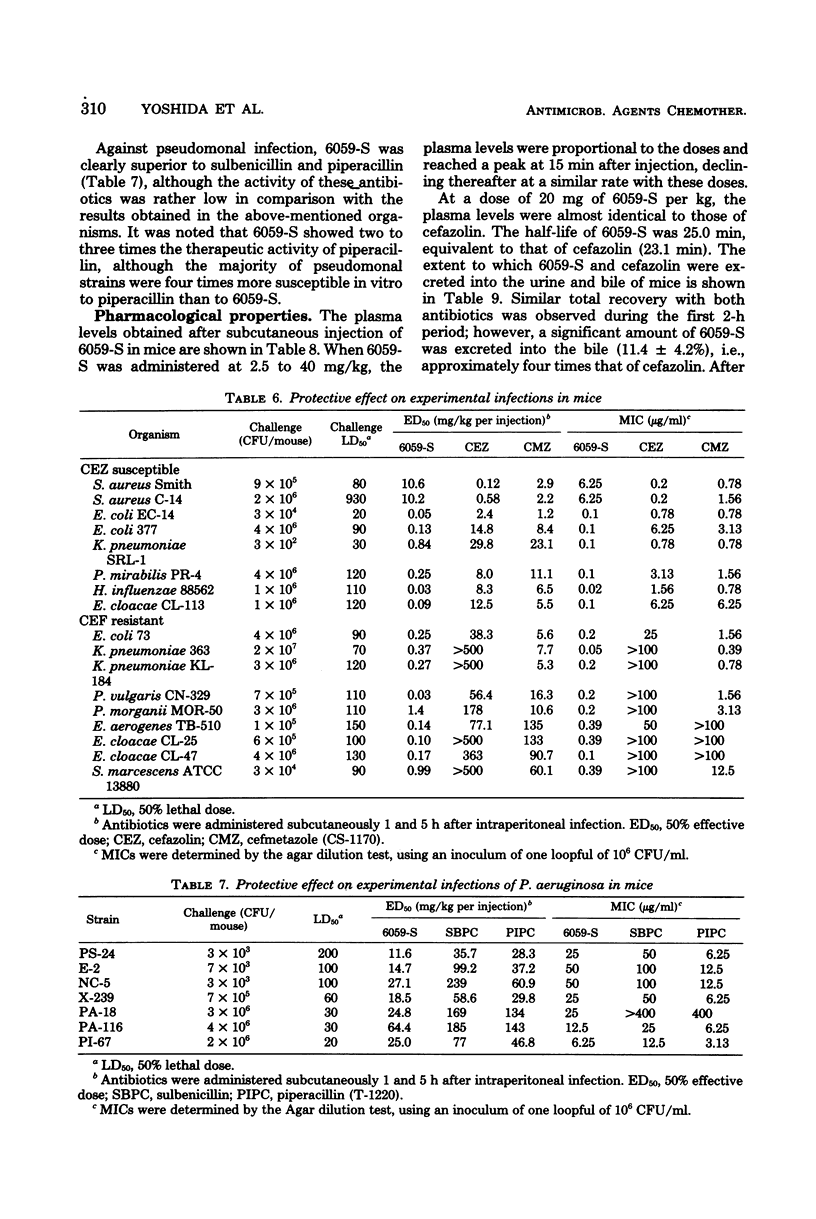
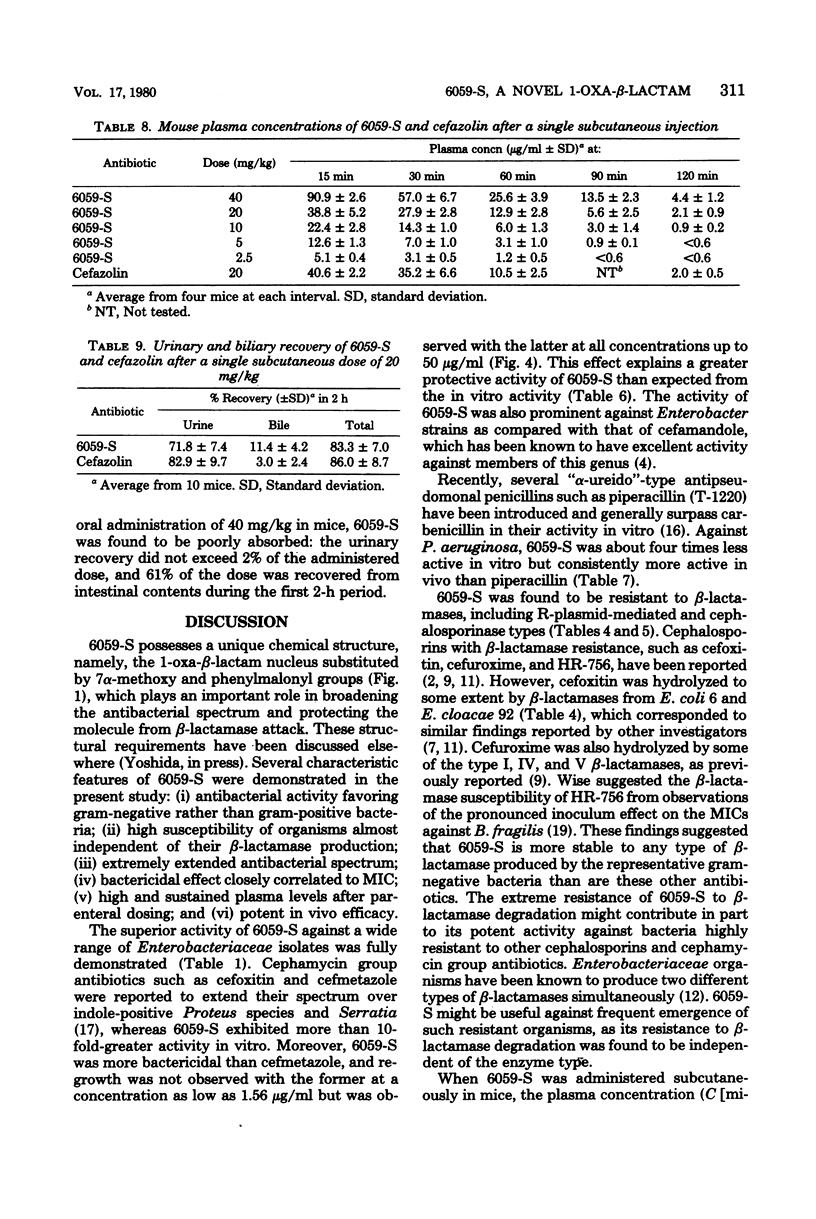
Moxalactam (6059-S) {7β-[2-carboxy-2-(4-hydroxyphenyl)acetamido]-7α-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]-methyl]-1-oxa-1-dethia-3-cephem-4- carboxylic acid disodium salt} is a new semisynthetic 1-oxa-β-lactam derivative for parenteral use. It was highly active against a broad range of gram-negative microorganisms, including those resistant to other cephalosporins. Moreover, it had widely expanded antibacterial spectra which included Haemophilus influenzae, indole-positive Proteus, Enterobacter, Serratia marcescens, Pseudomonas aeruginosa, and Bacteroides fragilis. When a large number of clinical isolates of the above-named bacilli were tested by the agar dilution method, using an inoculum size of one loopful of 106 or 108 organisms or both per ml, the 70% minimal inhibitory concentrations at the lower inoculum were 0.2, 0.2, 0.4, 0.8, 25, and 0.8 μg/ml, respectively. Its activity appeared to be independent of inoculum size and addition of serum. In these organisms, morphological response of the exposed cells revealed that the bacteriolytic effect of 6059-S was initiated by a concentration equivalent to the minimal inhibitory concentration. 6059-S was markedly bactericidal to both β-lactamase-producing and -nonproducing strains of Escherichia coli; this was well reflected by its extraordinary stability to microbial β-lactamase degradation. Administered subcutaneously in mice, 6059-S attained plasma levels and a half-life similar to those of cefazolin and exhibited potent protective efficacy against systemic infections; it also proved to be significantly more effective than either sulbenicillin or piperacillin against Pseudomonas aeruginosa and than either cefazolin or cefmetazole against a variety of other gram-negative bacteria.
Full text
PDF

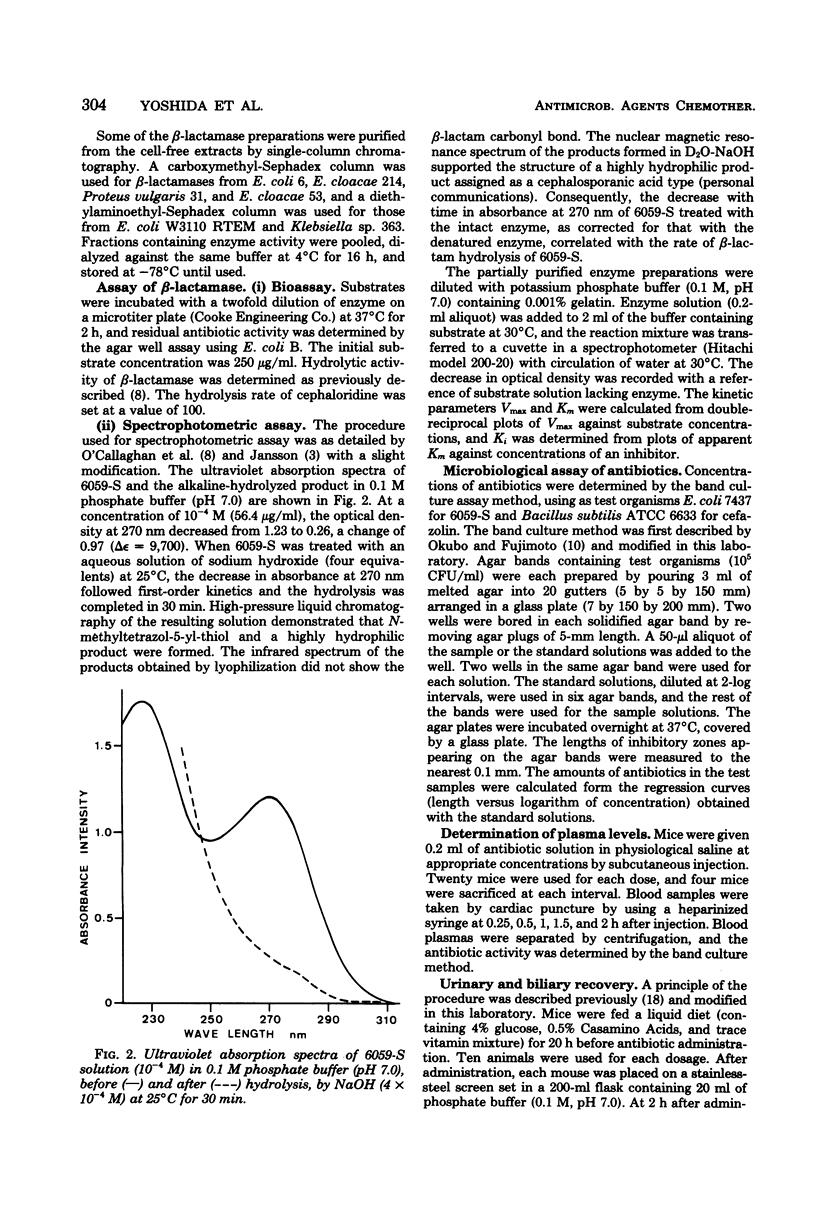

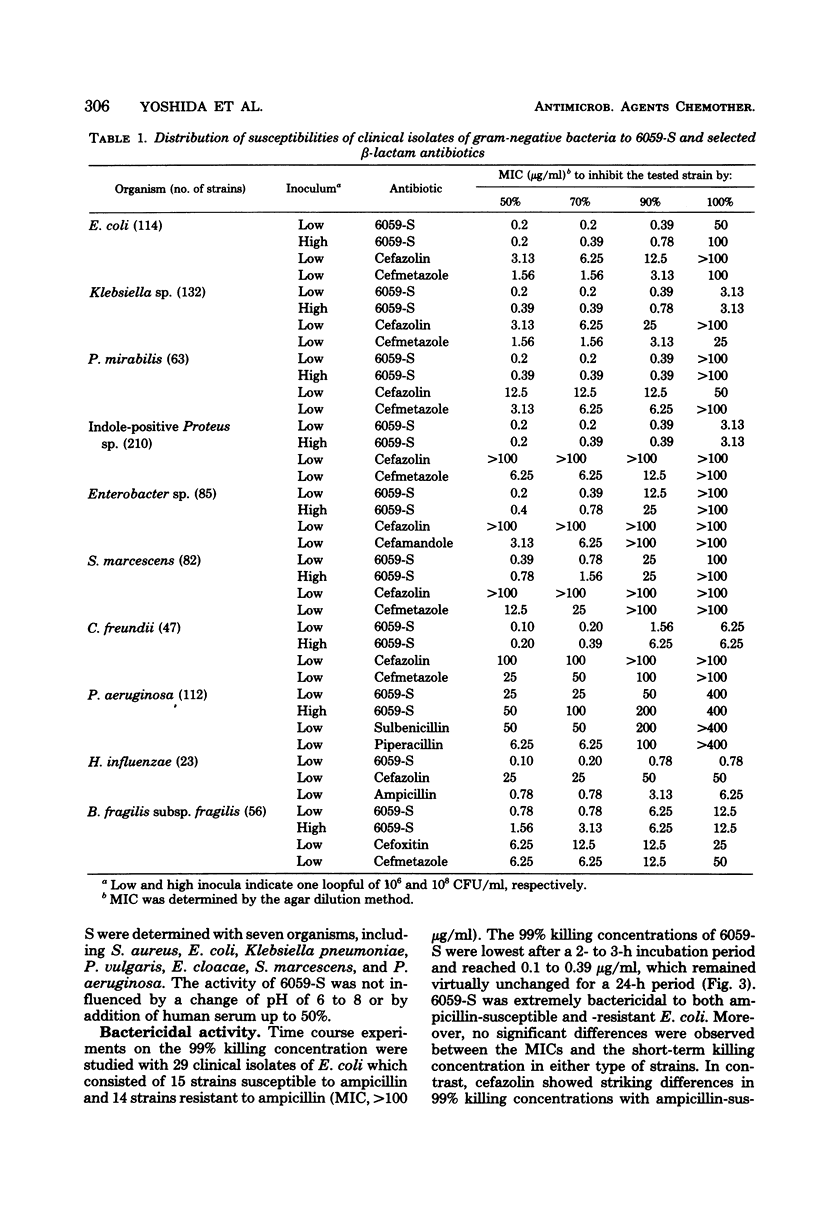
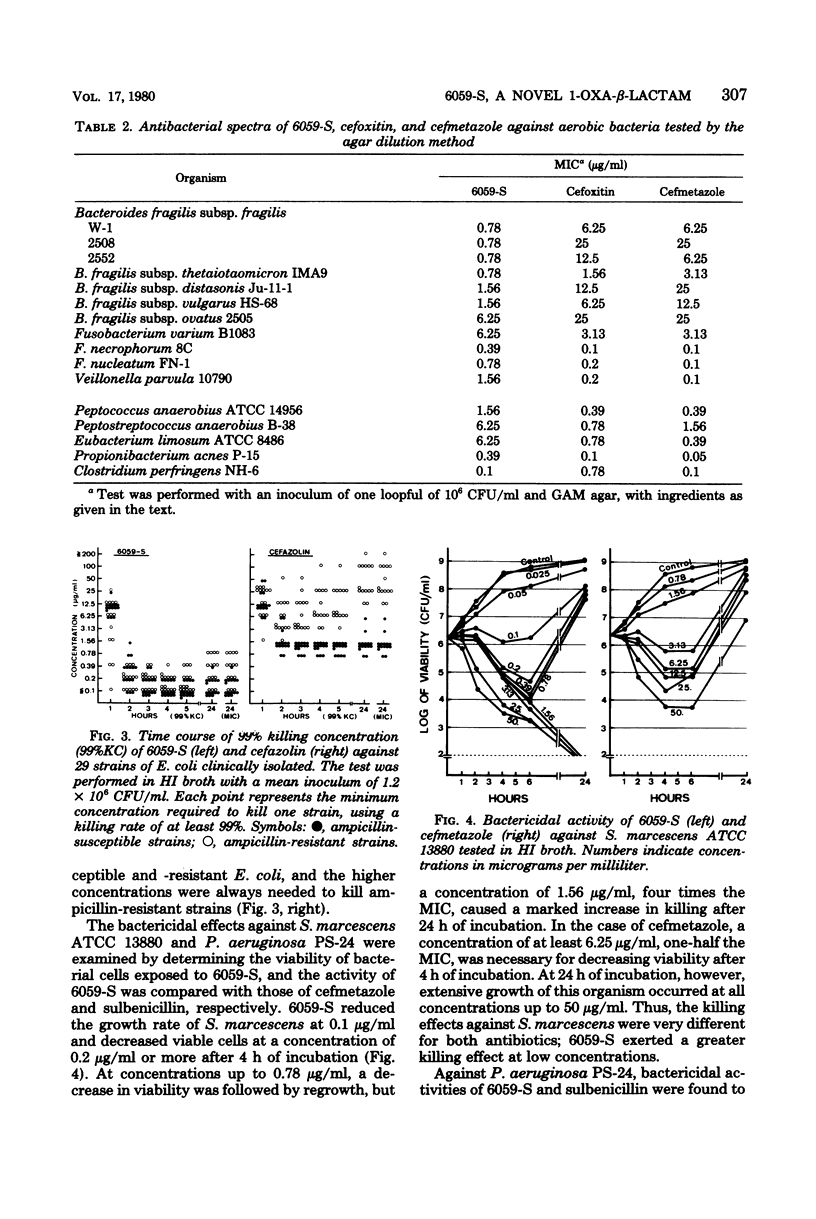
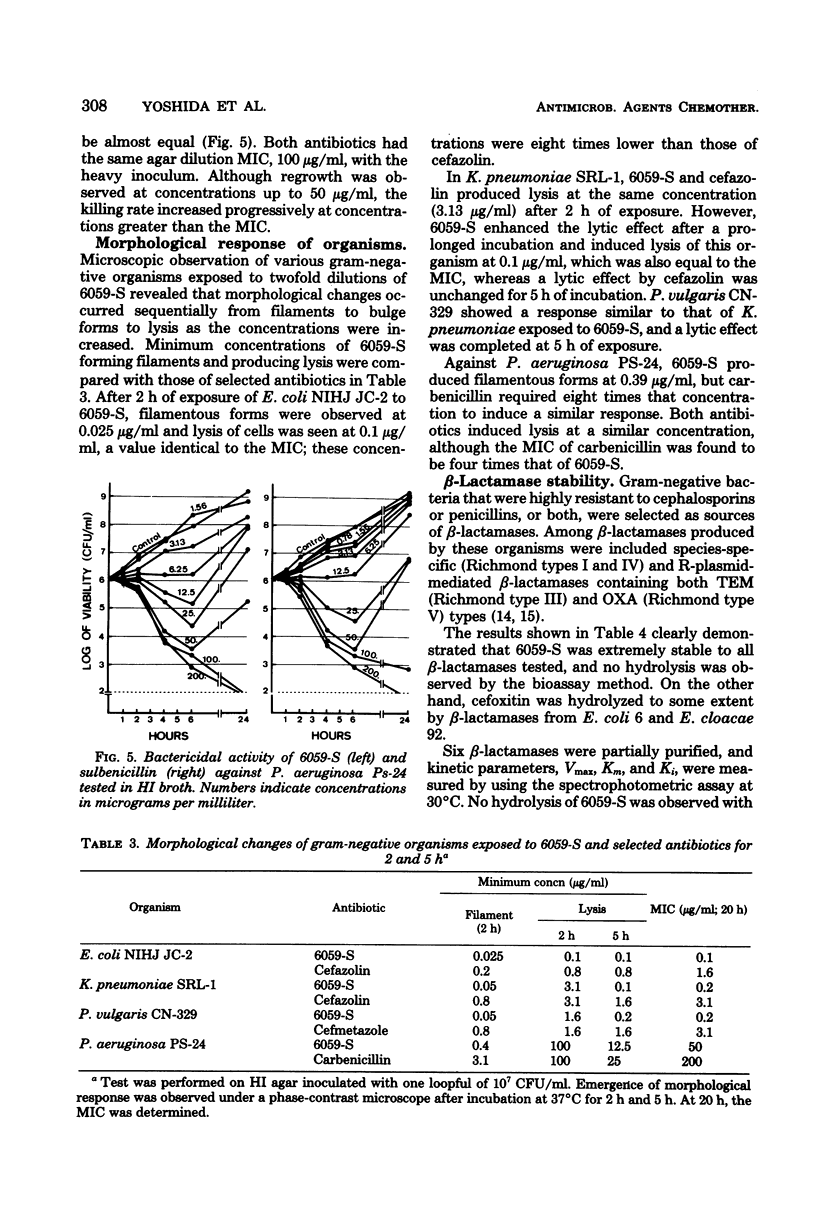

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fare L. R., Actor P., Sachs C., Phillips L., Joloza M., Pauls J. F., Weisbach J. A. Comparative serum levels and protective activity of parenterally administered cephalosporins in experimental animals. Antimicrob Agents Chemother. 1974 Aug;6(2):150–155. doi: 10.1128/aac.6.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. beta-lactamase stability of HR 756, a novel cephalosporin, compared to that of cefuroxime and cefoxitin. Antimicrob Agents Chemother. 1978 Sep;14(3):322–326. doi: 10.1128/aac.14.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSON J. A. A DIRECT SPECTROPHOTOMETRIC ASSAY FOR PENICILLIN BETA-LACTAMASE (PENICILLINASE). Biochim Biophys Acta. 1965 Apr 26;99:171–172. doi: 10.1016/s0926-6593(65)80018-2. [DOI] [PubMed] [Google Scholar]
- Kirby W. M., Regamey C. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis. 1973 Oct;128(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Narisada M., Yoshida T., Onoue H., Ohtani M., Okada T., Tsuji T., Kikkawa I., Haga N., Satoh H., Itani H. Synthetic studies on beta-lactam antibiotics. Part 101. Synthesis of 7beta-[2-carboxy-2-(4-hydroxyphenyl)acetamido]-7alpha-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]-methyl]-1-oxa-1-dethia-3-cephem-4-carboxylic acid disodium salt (6059-S) and its related 1-oxacephems. J Med Chem. 1979 Jul;22(7):757–759. doi: 10.1021/jm00193a001. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Cefoxitin, a semisynthetic cephamycin antibiotic: antibacterial spectrum and resistance to hydrolysis by gram-negative beta-lactamases. Antimicrob Agents Chemother. 1974 Aug;6(2):170–176. doi: 10.1128/aac.6.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Griffiths A., Thornton J. E. Cefuroxime, a new cephalosporin antibiotic: activity in vitro. Antimicrob Agents Chemother. 1976 Mar;9(3):511–519. doi: 10.1128/aac.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. R., Daoust D. R., Zimmerman S. B., Hendlin D., Stapley E. O. Cefoxitin, a semisynthetic cephamycin antibiotic: resistance to beta-lactamase inactivation. Antimicrob Agents Chemother. 1974 Jan;5(1):38–48. doi: 10.1128/aac.5.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Beta-lactamases. Int J Clin Pharmacol Biopharm. 1979 Mar;17(3):135–136. [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Ueo K., Fukuoka Y., Hayashi T., Yasuda T., Taki H., Tai M., Watanabe Y., Saikawa I., Mitsuhashi S. In vitro and in vivo antibacterial activity of T-1220, a new semisynthetic penicillin. Antimicrob Agents Chemother. 1977 Oct;12(4):455–460. doi: 10.1128/aac.12.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uri J. V., Actor P., Guarini J. R., Phillips L., Pitkin D., Demarinis R. M., Weisbach J. A. Biological and chemotherapeutic studies on three semisynthetic cephamycins. J Antibiot (Tokyo) 1978 Jan;31(1):82–91. doi: 10.7164/antibiotics.31.82. [DOI] [PubMed] [Google Scholar]
- Wheeler W. J., Wright W. E., Line V. D., Froggé J. A. Orally active esters of cephalosporin antibiotics. Synthesis and biological properties of acyloxymethyl esters of 7-(D-2-amino-2-phenylacetamido)-3-[5-methyl-(1,3,4-thiadiazol-2-yl)thiomethyl]-3-cephem-4-carboxylic acid. J Med Chem. 1977 Sep;20(9):1159–1164. doi: 10.1021/jm00219a009. [DOI] [PubMed] [Google Scholar]
- Wise R. Bacteroides fragilis and HR 756. J Antimicrob Chemother. 1979 Jan;5(1):115–116. doi: 10.1093/jac/5.1.115. [DOI] [PubMed] [Google Scholar]


