Abstract
Mast cells are widely recognized as effector cells of allergic inflammatory reactions. They contribute to the pathogenesis of different chronic inflammatory diseases, wound healing, fibrosis, thrombosis/fibrinolysis, and anti-tumor immune responses. In this paper, we summarized the role of P2X and P2Y receptors in mast cell activation and effector functions. Mast cells are an abundant source of ATP which is stored in their granules and secreted upon activation. We discuss the contribution of mast cells to the extracellular ATP release and to the maintenance of extracellular nucleotides pool. Recent publications highlight the importance of purinergic signaling for the pathogenesis of chronic airway inflammation. Therefore, the role of ATP and P2 receptors in allergic inflammation with focus on mast cells was analyzed. Finally, ATP functions as mast cell autocrine/paracrine factor and as messenger in intercellular communication between mast cells, nerves, and glia in the central nervous system.
Keywords: ATP, Apoptosis, Degranulation, Purinoceptors, Extracellular nucleotides, P2X7 receptor
Introduction
ATP and other nucleotides are present at a high concentration within the cytoplasm of cells and are released actively upon stimulation or by passive leakage from injured or dying cells. The extracellular level of nucleotides is tightly controlled by the ecto-nucleotidases that are expressed on the surface of different cells [1, 2]. The concentration of extracellular nucleotides which is low in healthy tissues may rise in damaged organs and can be sensed by surrounding cells expressing P2 purinoceptors. More than 30 years ago, ATP was identified among the factors inducing mast cell degranulation [3, 4]. Therefore, the existence of a specific receptor for ATP on mast cells was proposed [5, 6]. Years later, families of P2Y and P2X purinergic receptors were cloned and characterized. Mast cells are not only a great source of ATP which is stored in their secretory granules and released upon stimulation [7], but they also express a variety of purinoceptors which trigger mast cell degranulation, cytokine secretion, chemotaxis, and apoptotic cell death [3, 8, 9]. In this review, we discuss the role of the P2 receptors and nucleotide signaling in mast cell biology.
Mast cells
Mast cells were first described by Paul Ehrlich in his Doctoral thesis (1878) based on their unique staining characteristics and large granules [10]. Mast cells originate from CD34+ hematopoietic progenitor cells. They enter the circulation before homing to tissues and acquiring their final effector characteristics under the influence of local tissue microenvironmental factors [11, 12]. Mature mast cells are easily identified in tissues as large granular cells expressing high level of c-Kit (receptor for stem cell factor (SCF)) and FcεRI (high-affinity IgE receptor). The expansion, homing, and maturation of mast cell progenitors are influenced by many cytokines, most important of which are SCF and interleukin (IL)-3 [12]. Mucosal mast cells reside in the respiratory and gastrointestinal tract mucosa. Connective tissue mast cells are found in skin, lung, connective tissue, and submucosa of the gastrointestinal tract. These two subsets differ in granules content and display functional heterogeneity (reviewed in [13]). They are strategically localized in the proximity of epithelia, smooth muscle cells, blood vessels, and nerves where they can execute their function as a first line of defense against allergens/pathogens [11].
Following activation, mast cells release numerous preformed mediators from secretory granules, such as tumor necrosis factor α (TNFα), IL-4, granulocyte/macrophage-colony stimulating factor (GM-CSF), histamine, heparin, serotonin, kinins, and proteases (members of tryptase and chymase families) [11–13]. Upon stimulation, they also synthesize de novo and secrete an array of cytokines (including IL-1 to IL-8, IL-10, IL-12, IL-13, IL-15, IL-18, and IL-21); chemokines (e.g., macrophage inflammatory protein (MIP)-1α; monocyte chemoattractant protein 1 (MCP-1); and regulated on activation, normal T cell expressed and secreted (RANTES)); prostaglandins and leukotrienes; growth and angiogenesis factors, such as platelet-derived growth factor, nerve growth factor (NGF), and vascular endothelial factor [11, 13, 14]. Despite the well-characterized role of FcεRI in mast cell activation [14], a variety of other agonists can activate mast cells. These include complement factors (e.g., C5a), lipid mediators (e.g., prostaglandins, leukotrienes), neuropeptides (e.g., substance P (SP), neurotensin), hormones (e.g., somatostatin), cytokines, chemokines, microbial products (e.g., lipopolysaccharide (LPS) or peptidoglycan), as well as extracellular nucleotides [15–17].
Mast cells are recognized as the key components of allergic inflammatory reactions, but they are also implicated in the pathogenesis of a number of chronic inflammatory diseases, in wound healing, in fibrosis, thrombosis/fibrinolysis, and in innate immunity (reviewed in [11–13, 18–20]). They are described as long-living cells keeping relatively constant numbers in tissues under physiologic conditions. This depends both on the rate of production of mast cell precursors from the bone marrow and the length of survival of mature mast cells within tissues [11–13]. Given, however, their pivotal role in acute allergic reactions, mast cell numbers need to be tightly controlled by a balance between cell proliferation, development, and death. A link between mast cell activation during the early stages of the allergic process and triggering of anti-apoptotic signaling pathways has been suggested as an important factor that contributes to the extended life of mast cells [21]. Anti-apoptotic mechanisms limit the initiation of programmed cell death, thereby contributing to the multiple pathological conditions that involve mast cells’ activities.
Sources of ATP, mechanisms of release, and control of the extracellular pool
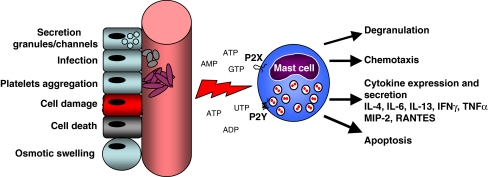
Intracellular ATP is recognized as an energy source for many cellular reactions. The cell utilizes complex mechanisms for synthesizing ATP and maintaining or rapidly restoring the intracellular ATP pool according to cell demands. The cytoplasm contains ∼5 mmol/l (5–10 mM) of ATP [22, 23]. Three general ATP release modes have been described: (1) upon cell damage due to the sheer stress, osmotic swelling, ischemia, inflammation, apoptosis, or necrosis leading to the passive leakage of ATP from the damaged cell; (2) vesicular release; and (3) channel-mediated release (Fig. 1).
Fig. 1.
Sources of extracellular nucleotides and P2 receptor-mediated responses of mast cells. Nucleotides are released from damaged or stressed cells due to osmotic swelling, infection, and cell death. They are also secreted from intracellular granules by exocytosis, from cytoplasm of cell through pannexin channels, or liberated during platelet aggregation. Mast cells sensor extracellular nucleotides via a variety of P2X and P2Y receptors. Nucleotides trigger different responses of mast cells including degranulation, chemotaxis, cytokine expression and release, and apoptosis
Higher ATP levels (up to 100 mmol/l) are present in secretory vesicles of neurons, serotonergic granules of platelets and exocrine glands (e.g., chromaffin granules of adrenal medulla, insulin-containing granules of pancreatic β-cells, and secretory granules of mast cells) [23–25]. The content of these vesicles and granules is released by exocytosis induced by mechanic stimulation or specific stimuli (such as cholinergic stimulation of pancreatic acini or triggering of FcεRI by antigen/IgE cross-linking in mast cells) [23, 25]. The role and fate of mast cell-derived ATP will be discussed below.
Another mechanism of ATP release is via connexin- and pannexin-regulated hemichannels, which, apart from being a building block for the gap junction and allowing the diffusion of ions across the plasma membrane, are implicated in non-vesicular ATP release [25–28]. The connexin-regulated ATP release has been shown for astrocytes [26], while pannexin channels are responsible for ATP secretion from an oocyte heterologous expression system [27], from erythrocytes in response to low oxygen or to sheer stress [28], and from activated T lymphocytes upon T cell receptor stimulation [29].
ATP is acknowledged as a “signal of danger” or damage-associated molecular pattern [22, 30]. Once released from dying or activated cells, it contributes to the efficient triggering of the innate immune system in inflammatory settings together with pathogen-associated molecular patterns (PAMPs) [30]. Under normal conditions, cells are exposed to negligibly low concentrations of extracellular ATP which is readily degraded by the combined action of ubiquitously expressed ecto-nucleotidases. Several families of ecto-nucleotidases were characterized. Ecto-nucleotide triphosphate diphosphohydrolase (E-NTPDase) family with prototype member CD39 (NTPDase1, ecto-apyrase) hydrolyzes ATP to ADP and AMP [1], while CD73 (ecto-5′-nucleotidase) hydrolyzes AMP to adenosine [2]. Ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) family members (NPP1-3) have a broad specificity and hydrolyze pyrophosphate and phosphodiester bonds in ATP and other nucleotides [31]. Mouse bone marrow-derived mast cells (BMMCs) highly express CD39 and CD73 (Bulanova E., unpublished observation) and therefore might contribute to the concentration of ATP and its derivatives, ADP and AMP, in the microenvironment. Importantly, E-NPP3 (CD203c) was recently identified as a novel activation-linked surface marker on human mast cells and basophils [32]. It is upregulated in response to IgE cross-linking and highly expressed on neoplastic mast cells in patients with systemic mastocytosis [32]. The expression and role of these and other ATP-hydrolyzing enzymes for the biology of mast cells remains to be elucidated.
P2 receptors
Purinergic receptors (purinoceptors) are classified according to Burnstock [33]. Two primary classes of purinoceptors are recognized, P1 (receptors for adenosine) and P2 (receptors for extracellular nucleotides). There are four existing types of P1 receptors (A1, A2A, A2B, and A3) [34]. The P2 receptors are subdivided in two subclasses, P2X and P2Y. P2Y receptors are seven membrane-spanning, G protein-coupled receptors [35]. This family includes eight members: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 [35, 36]. UTP serves as a high potency agonist for human P2Y2, P2Y4, and P2Y6 receptors [35, 36]. ADP activates P2Y1, P2Y12, and P2Y13 and was reported to be equipotent or even more potent then ATP for P2Y1, while for P2Y2 and P2Y11, ATP is more potent than ADP [36]. The pharmacological profile of the recently cloned P2Y14 (GPR105) receptor differs from the other P2Y receptors; it recognizes UDP-glucose, UDP-galactose, UDP-glucuronic acid, and UDP-N-acetylglucosamine [37].
P2X receptors are ligand-gated ion channels activated by extracellular ATP and selective for monovalent and divalent cations (Na+, K+, and Ca2+) [36, 37]. They are two membrane-spanning receptors with cytoplasmic amino- and carboxyl-terminal domains. The unique, naturally occurring agonist of all P2X receptors is ATP. P2X subunits aggregate to form homo- or heteromultimers [38]. Upon agonist activation, some receptors (P2X1 and P2X3) desensitize rapidly; others show little or no desensitization at all [38, 39].
The P2X7 receptor is unique among other members of the P2X receptor family. It conveys a longer C-terminal domain (∼200 amino acids longer) [38]. In contrast to the other P2X receptors, the P2X7 receptor requires millimolar levels of ATP to achieve activation, which leads to the formation of a nonselective cationic channel with low affinity for ATP and increased permeability to Ca2+ [39, 40]. This permeabilizing receptor, expressed on mast cells and lymphocytes, which responds to the fully dissociated form of ATP (ATP4−) [5], was initially named P2Z (for alphabetic consistency) by Gordon [24]. In 1996, the first P2Z receptor was cloned from the rat brain and designated as P2X7 [41].
P2X7 has a prominent expression in many immune cells, particularly lymphocytes, monocytes, macrophages, mast, dendritic, mesangial, and microglial cells as well as on a limited number of other cell types including parotid acinar cells, testis, and fibroblasts [17, 38, 40]. In addition to the cationic channel, the P2X7 receptor may also induce the opening of a nonselective pore permeable to large organic molecules up to 900 Da [17, 40]. Continuous activation of the receptor and the formation of a large transmembrane pore cause perturbations in the ion homeostasis and finally result in cell death [40]. Remarkably, there is an alternative pathway of P2X7 receptor activation in the mouse system: by the nicotinamide adenine dinucleotide (NAD)-dependent ADP ribosylation. This pathway was initially shown for mouse T lymphocytes which constitutively express one of the ADP-ribosyltransferase (ART) enzymes, ART2, that catalyzes ADP ribosylation of the P2X7 receptor using NAD as a substrate. Such ATP-independent activation leads to Ca2+ influx, membrane pore formation, CD62L shedding, and apoptosis of T cells [42]. Murine mast cells do not express ART2 and do not respond to the NAD-mediated P2X7 activation (Bulanova E., unpublished observation). However, recent work shows that expression of ART2 on mouse macrophages could be induced by pro-inflammatory stimuli (LPS and IFNγ) [43]. While NAD alone does not activate P2X7 in macrophages, it has a synergistic effect with extracellular ATP [43]. Such activation mode could be applicable to mast cells as well and remains to be elucidated. Moreover, expression of ARTs on the membrane of other cells (T cells or macrophages) might lead to the cross-activation of P2X7 receptors present on mast cells.
Extracellular ATP and other nucleotides regulate a broad range of cellular responses, such as smooth muscle contractility, neurotransmission, multinuclear giant cell formation, vascular tone, mucociliary clearance, mitogenic stimulation, apoptosis, or necrosis of many cell types [17, 38, 40, 44]. Extracellular ATP and ADP induce platelet aggregation via P2Y1 and P2Y12 receptors [45]. ATP induces phospholipase D-mediated shedding of L-selectin (CD62L) and CD23 from the surface of lymphocytes [46, 47]; chemotaxis of neutrophils, eosinophils, and lymphocytes [48]; and triggers release of TNFα, IL-1β, IL-18, and IL-33 in macrophages, dendritic, and glial cells via P2X7 receptor activation [49–52]. P2X7-mediated synthesis and release of TNFα by glial cells requires the activation of MAPK pathways [52]. Recent study also shows that P2X7 receptor mediates activation of TNFα-converting enzyme (TACE/ADAM17) [53]. IL-1β is packaged into small plasma membrane blebs that are released into the cellular space as microvesicles of a size of 200 nm–1 µm [50, 51]. Maturation and release of IL-1β, IL-18, and IL-33 requires the activation of caspase-1 (IL-1β-converting enzyme) [30, 49] in an assembly of cytosolic protein complex called inflammasome [30, 50, 51]. P2X7-activated inflammasome comprises NLRP3 (NACHT, LRR, and pyrin domain containing protein 3), adapter protein ASC (apoptosis-associated speck-like protein containing a CARD), and caspase-1 [30]. It has been postulated that inflammasome components could also be loaded into P2X7-derived microvesicles together with IL-1β and IL-18 [49]. However, experimentally, only IL-1β could be detected in microvesicles [50, 51].
Effects of P2 receptor stimulation in mast cells are summarized in Fig. 1 and will be discussed below.
Expression of P2 receptors on mast cells
Although experiments employing different P2 receptor agonists and antagonists have clearly suggested the presence of various purinoceptor subtypes in mast cells, only few studies have investigated P2 receptors expression on mRNA and protein levels (Table 1). We have shown that P815 mastocytoma cells (derived from DBA/2 mice) express mRNA for all P2X and P2Y receptors, while BMMCs derived from C57BL/6 mice express mRNA for P2X1–4, P2X6–7, and all P2Y receptors with the exception of P2Y2 [9]. MC/9 cells (derived from fetal liver of a (B6 X A/J)F1 mouse) express P2X1–2, P2X4, P2X6–7, P2Y6, P2Y13, and P2Y14 receptors [9]. The expression of P2X7 receptor protein on BMMCs, P815, and MC/9 cells was shown by immunoblotting analysis [9] and fluorescence-activated cell sorter (FACS) [54]. Expression of P2X1 receptor was confirmed by FACS while expression of P2X3 receptor by immunoblotting [54]. Interestingly, BMMCs do not express the P2X4 receptor on the surface, as was shown by FACS analysis and confirmed by immunoblotting, despite the presence of mRNA transcript [9, 54]. This could be explained by the recent finding that P2X4 receptor contains a non-canonical tyrosine-based sorting motif which leads to constitutive endocytosis and recycling of the P2X4 receptor [55].
Table 1.
Expression of P2 receptor subtypes by mast cells
| Mast cell type | Receptors | Method of detection | References |
|---|---|---|---|
| Human | |||
| CBMCs | P2X1, P2X4, P2Xa7, P2Y1, P2Y2 | GeneChip arrays | [57, 59] |
| P2Y11, P2Y12, P2Y13 | RT-PCR | ||
| HLMCs | P2X1, P2X4, P2Y1, P2Y2 | GeneChip arrays, RT-PCR | [57, 58] |
| HMC-1 cell line | P2X7 | RT-PCR | [58] |
| Skin mast cells | P2X1, P2X4 | GeneChip arrays | [57] |
| CD34+ progenitors | P2X1, P2X4, P2Y1, P2Y2, P2Y11, P2Y12, P2Y13 | RT-qPCR | [56] |
| Mouse | |||
| BMMCs | P2X1-4, P2X6, P2X7, P2Y1, P2Y6, P2Y12-14 | RT-PCR, IB, FACS | [9, 54] |
| MC/9 | P2X1-2, P2X4, P2X6–7, P2Y6, P2Y13, P2Y14 | RT-PCR, IB | [9] |
| P815 | P2X1–7, P2Y1–14 | RT-PCR, IB | [9] |
| Rat | |||
| RBL-2H3, peritoneal mast cells | P2Y, P2X1, P2Xb7 | Pharmacol. assays | [7, 60–64] |
IB immunoblotting
aUpon IgE activation
bFunctional and pharmacological evidence strongly suggest the expression of P2X7 receptor
The fact that BMMCs, MC/9, and P815 mast cells are derived from different sources (e.g., fetal liver versus bone marrow), distinguished morphologically and functionally, and require individual culture protocols (e.g., requirement of growth factors) could explain, in part, the deviation in P2X and P2Y receptor expression by these cells.
Expression of P2 receptors was also analyzed in human mast cells derived from different sources and in CD34+ progenitors at mRNA level. Recent studies show that human CD34+ progenitor cells express several P2 receptors (namely P2X1, P2X4, P2Y1, P2Y2, and P2Y11–13) [56]. Notably, the expression of the P2X1 and P2X4 receptors was significantly higher than the expression of P2X7 [56]. Human lung mast cells (HLMCs) express mRNA transcripts for P2X1 and P2X4 and P2Y1 and P2Y2, but not for P2X7 [57, 58]. In contrast, the human mast cell line HMC-1 highly expresses the P2X7 receptor which is further upregulated upon PMA or ionomycin stimulation [58]. Human cord blood mast cells (CBMCs) express mRNA for P2X1 and P2X4 as well as for P2Y1, P2Y2, P2Y11, P2Y12, and P2Y13 [57, 59]. P2X7 is expressed on CBMCs only upon IgE cross-linking or dexamethasone stimulation [57]. Mast cells isolated from human skin express P2X1 and only show a marginal P2X4 receptor expression [57].
As mentioned above, the heterogeneity of human mast cells is well documented [13]. Moreover, following transfer from one anatomic site to another, ex vivo isolation and in vitro culture, mast cells change their phenotype [12]. That underlines the importance of microenvironment for mast cell differentiation and could explain the differences in P2 receptor expression by these cells. The fact that human primary mast cells isolated from lung and skin do not express P2X7 while this receptor is highly expressed by cells cultured in vitro (HMC-1 and activated CBMC) is interesting and deserves further investigation. The question whether human mast cells in vivo express P2X7 receptor and whether P2X7 expression could be induced upon stimulation and under certain pathologic conditions remains open.
P2X and P2Y receptor expression profile of the rat basophilic leukemia cell line RBL-2H3 or primary rat mast cells was never analyzed in detail. RBL-2H3 and rat peritoneal mast cells indeed express functional P2Y, P2X1, and P2X7 receptors as was shown indirectly by pharmacological studies [7, 60–64].
Thus, mast cells derived from distinct species and sources express different P2X and P2Y receptors, which might be responsible for heterogeneous effects in response to stimulation with extracellular ATP and other nucleotides.
P2 receptor-mediated responses of mast cells
Membrane permeabilization and Ca2+ influx
First observations of extracellular ATP effects on mast cells reported an increase in intracellular Ca2+ [3, 61, 65, 66]. Such elevation in the intracellular Ca2+ concentration is a result of Ca2+ influx across the cellular membrane (feature of P2X receptors) or Ca2+ release from the intracellular stores (linked to the P2Y receptors) [34, 38]. Activation of P2X receptors leads to the formation of a nonselective cationic channel with increased permeability to Ca2+ within milliseconds of activation, intracellular depolarization, and equilibration of Na+ and K+ gradients [39]. Recent studies from our laboratory demonstrated that ATP in a millimolar range was capable of inducing P2X7-dependent Ca2+ influx in mouse mast cells, while this effect was inhibited by a selective P2X7 receptor antagonist, 1[N, O-bis(5-isoquinolinesulphonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazine (KN-62), or oxidized ATP (oATP) [9]. In the P2X7 receptor knockout (P2X−/−7) BMMCs, ATP triggered P2X1 and P2X3 receptor activation. Specific P2X1 and P2X3 receptors agonist, α, β-methylene-ATP (αβmeATP), induced transient Ca2+ influx in these cells which was blocked by specific antagonist, 2′,3′-O-(2,4,6-trinitrophenyl)-ATP [54]. Stimulation of P2Y receptors generally leads to the activation of phospholipase C that catalyzes the hydrolysis of phosphatidylinositol 4,5-biphosphate into the inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), leading to the mobilization of Ca2+ from intracellular stores [36]. In accordance with this, ADP induced Ca2+ influx in CBMCs via P2Y1 receptor stimulation [59].
Importantly, P2X7 receptor activation leads to the opening of a nonselective pore permeable to large organic molecules (choline (100 Da), ethidium bromide (314 Da), YO-PRO-1 (376 Da), propidium iodide (414 Da), Lucifer Yellow (457 Da), and ATP itself (605 Da)) [5, 40, 60]. The ability to open such pore is an exclusive feature of the P2X7 receptor. ATP-induced membrane permeabilization for Lucifer Yellow was inhibited by KN-62 in mouse BMMCs [9]. Moreover, BMMCs derived from P2X−/−7 mice were resistant to ATP-mediated permeabilization [54]. The size of this pore increased gradually with the ATP concentration. The largest molecules that have been loaded into mast cells using ATP have molecular weights of about 600–1,000 Da [5]. Recent studies have shown the importance of the pannexin-1 protein in the P2X7-mediated large pore formation [67, 68]. Pannexin-1 co-associates with P2X7, and this complex is not only essential for P2X7-induced dye uptake without altering Ca2+ influx but also for the caspase-1 processing and release of mature IL-1β [67, 68].
Degranulation
ATP has long been known to induce histamine release from mast cells [3–5, 60–63]. In 1979, Cockcroft and Gomperts have shown that ATP caused Ca2+-dependent histamine release and striking morphological changes in rat mast cells [5, 60]. ATP4− induced histamine release at a concentration of ∼3 μM, while at higher concentrations (>5 mM), degranulation was inhibited due to increased membrane permeabilization and leakage of larger molecules, including nucleotides [5]. Several groups reported ATP-dependent degranulation in different mast cell models. Rat peritoneal mast cells released histamine and generated prostaglandin D2 after ATP stimulation [63]. Degranulation of these mast cells was mediated by protein kinase C (PKC) [69]. A link between ATP-triggered degranulation and phospholipase A2-mediated arachidonic acid (AA) metabolism was shown. Inhibitors of the AA cascade also blocked ATP-induced histamine release in these cells [69]. ATP caused an increase in intracellular Ca2+ and β-hexosaminidase release by MC/9 cells [70]. These effects were dependent on the P2X7 receptor but did not require PKC activity [70]. Recent data from our laboratory also demonstrated the requirement of the P2X7 receptor for ATP-mediated degranulation. ATP failed to induce the β-hexosaminidase release in BMMCs derived from P2X−/−7 mice [54]. However, Saito et al. [66] have shown that not only ATP but also ADP and AMP are able to induce Ca2+-dependent histamine and leukotriene C4 release from mouse BMMCs. Neither ADP nor ATP directly induced exocytosis, whereas simultaneous stimulation at concentrations of 50 μM (much lower than required for P2X7 receptor activation) triggered β-hexosaminidase release from human CBMCs [59]. Therefore, additional P2 receptors could be involved in the regulation of mast cell degranulation.
ATP not only induces degranulation per se but also effectively modulates exocytosis mediated by other stimuli. ATP stimulation potentiates degranulation of rat mast cells induced by FcεR cross-linking [7], compound 48/80, or by Ca2+ ionophore A23187 [71, 72]. This cumulative effect required low ATP concentrations (10–20 μmol), while higher concentrations (20–80 μmol) of ATP exhibited inhibitory effect [71]. Remarkably, in HLMCs, neither ATP nor other nucleotides or adenosine directly induced histamine release [58]. However, ATP, UTP, and other adenine nucleotides enhanced IgE- but not ionophore A23187-mediated histamine release in these cells. Other nucleotides or ATP analogues also increased histamine release induced by IgE cross-linking. Together with the analysis of the P2X and P2Y receptor expression on HLMCs, these data reveal the engagement of P2Y1 and P2Y2 receptors but not P2X7 [58].
Thus, extracellular nucleotides trigger and modulate degranulation of mast cells via multiple P2Y and P2X receptors.
Intracellular signaling
P2Y receptor signaling is linked to phospholipase C (PLC) and/or adenylate cyclase activation [36]. In turn, PLC activation triggers generation of inositol 1,4,5-triphosphate (IP3), a mediator of Ca2+ release from intracellular stores, and diacylglycerol, an activator of PKC, whereas adenylate cyclase generates cyclic adenosine monophosphate (cAMP), an activator of protein kinase A [36]. Unfortunately, these signaling pathways in mast cells were not analyzed in detail. Sudo et al. [70] demonstrated that ATP did not induce phosphorylation of PLCγ in MC/9 cells and still was able to increase Ca2+ influx when PKC was already activated by PMA. Moreover, ATP-induced degranulation of MC/9 cells was not mediated by PKC [70]. P2Y11 receptor seems the only P2Y receptor that is linked to adenylate cyclase/cAMP signaling in the human system [73]. The impact of P2Y11 receptor activation on cytokine release will be discussed below.
One of the early effects of P2X receptors activation is Ca2+ influx across the plasma membrane [38]. Such ionic perturbations are sufficient to trigger activation of a number of intracellular signaling molecules, including MAP kinases [74]. It has been shown that ATP can induce Ca2+-dependent phosphorylation of ERK in PC12 cells and fetal astrocytes [74, 75] and of JNK in mouse macrophages [76]. In addition, we have demonstrated that stimulation with extracellular ATP mediates activation of p56lck, ERK1/2, and JNK in Jurkat T cells [77].
In mouse BMMCs and MC/9 cells, high concentrations of ATP (≥3 mM) induced transient tyrosine phosphorylation of several proteins [9]. Among these phosphorylated proteins, STAT6, ERK1/2, and Jak2 were identified, while other Jaks (Jak1, Jak3, and Tyk2) and STATs (STAT1, STAT3, and STAT5) as well as p38 kinase, JNK1, and JNK2 were not affected [9]. Sudo et al. [70] also reported tyrosine phosphorylation of several intracellular proteins (44 and 110 kDa) in MC/9 cells upon ATP treatment. Importantly, the pattern of ATP-induced protein phosphorylation varied from the IgE-mediated phosphorylation detected in MC/9 cells (e.g., ATP did not induce PLCγ1 phosphorylation in these cells, as was mentioned above) [70]. Interestingly, KN-62 and oATP inhibited ATP-induced tyrosine phosphorylation in BMMCs and MC/9 cells, but did not completely abrogate this effect, suggesting that other purinoceptors might be involved in this process [9]. Indeed, ATP preserved the ability of stimulating ERK1/2 phosphorylation in P2X7−/− BMMCs [54]. This effect is likely mediated by P2X1 and P2X3 receptors according to the pharmacological profile and desensitization properties [54]. In favor of the other P2 receptors’ involvement, it was shown that ADP in low concentrations (5 μM) induces phosphorylation of ERKs and p38 MAPK in human CBMCs, which is effectively blocked by simultaneous action of P2Y1- and P2Y12-selective antagonists [59].
Cytokine/chemokine expression and secretion
Triggering of intracellular signaling cascades (such as MAP kinases or Jaks/STATs) is linked to cytokine expression and release. Indeed, extracellular nucleotides are powerful inducers of pro-inflammatory cytokine secretion. ATP mediates secretion of TNFα, IL-1β, IL-18, and IL-33 from different cell types, including macrophages, dendritic, and glial cells via the P2X7 receptor [49–52, 78]. ADP and ATP modulate IL-12/IL-23 secretion by dendritic cells via P2Y11 receptor [73]; UTP induces IL-8 production by monocytes via P2Y6 receptor [79]. Synthesis and intracellular storage of cytokines/chemokines and subsequent release are the major mast cell functions [11, 12]. ATP enhanced the expression of several pro-inflammatory cytokines and chemokines, including IL-4, IL-6, IL-13, TNFα, IFNγ, MIP-2, and RANTES and induced secretion of IL-6 and IL-13 in supernatants of stimulated mouse mast cells (BMMCs and MC/9) [9, 54]. Initially, it was suggested that this effect is mediated solely by the activation of the P2X7 receptor since KN-62 inhibited this effect [9]. However, P2X−/−7 BMMCs still preserved the ability to upregulate the expression of these cytokines and chemokines [54]. Using the panel of specific agonists and antagonists and analyzing the receptor repertoire expression on P2X−/−7 BMMCs, we have recently demonstrated that this effect is mediated in part by P2X1 and P2X3 on BMMCs [54].
Despite induction of protein phosphorylation in CBMCs, ATP and ADP in concentrations below 100 μM failed to induce cytokine generation by these cells [58, 59]. However, they dose-dependently decreased TNFα and IL-5 secretion in response to the TLR2 ligand, peptidoglycan and blocked the TNFα, IL-5, IL-8, and MIP-1β release in response to leukotriene D4 via activation of P2Y1, P2Y11, and P2Y12 receptors, but did not affect the IgE-mediated cytokine release [59]. IgE cross-linking induces release of pre-formed and pre-stored cytokines through degranulation, while other stimuli (such as TLRs ligands or leukotrienes) induce cytokine expression and subsequent release without degranulation. Feng et al. [59] suggested that P2Y receptors activate inhibitory pathways that suppress the transcription of cytokines. In accordance with this hypothesis, several publications have demonstrated that Gs protein-coupled receptors which interfere with activation of immune cells (e.g., receptors for PGE2 and β2 adrenergic receptor) activate adenylate cyclase and initiate cAMP-dependent inhibitory signaling pathways [80, 81]. Indeed, ATP and ADP triggered cAMP accumulation, phosphorylation of CREB, and the upregulation of CREB-dependent inducible cAMP early repressor, an inhibitor of NF-AT and AP-1-mediated cytokine transcription, presumably via P2Y11 receptor [59].
Chemotaxis
Extracellular nucleotides are powerful chemoattractants for neutrophils, eosinophils, and dendritic cells (reviewed in [48]). The data on chemotactic activity of nucleotides in mast cells are rare. McCloskey et al. [8] showed that ATP, ADP, and UTP are indeed efficient chemoattractants for rat BMMCs. This process was dependent on Ca2+ influx, required the existence of a refillable intracellular Ca2+ depot, and was mediated by the P2Y2 receptor, previously known as P2U [8]. Recent work also demonstrated that UTP is a potent chemoattractant for CD34+ stem cells [82].
Apoptosis
A major effect of extracellular ATP is the ability to induce apoptosis via P2X7 receptor [40]. This phenomenon was initially documented in P815 mastocytoma and YAC lymphoid cells [83, 84] and subsequently extended to thymocytes [83], macrophages [85], and dendritic cells [86]. Activation of the P2X7 receptor induces typical features of apoptosis: phosphatidylserine externalization and membrane blebbing, cytoplasm condensation, and DNA fragmentation [40, 83]. P2X7-mediated blebbing, membrane permeabilization, and phosphatidylserine flip are reversible when the receptor activation is relatively brief (<10–15 min) [87]. Recent data from our laboratory demonstrated that millimolar concentrations of ATP induced apoptosis in mouse BMMCs as well as in MC/9 and P815 mast cells [9]. Interestingly, stimulation with high doses of ATP induced apoptosis very rapidly, within a few minutes, and ATP removal did not result in cell recovery, thereby indicating that such treatment triggered irreversible apoptotic signals [9]. Apoptosis of mouse mast cells is caspase-dependent (ATP induced activation of caspase-3 and -8 and poly(ADP-ribose)polymerase cleavage) and is mediated by the P2X7 receptor since both apoptosis and caspases activation were abrogated by KN-62 [9]. Moreover, P2X−/−7 BMMCs are resistant to ATP-induced apoptosis [54]. Remarkably, wild-type BMMCs show a higher rate of spontaneous cell death in comparison to P2X−/−7, more likely due to ATP leakage and P2X7 receptor-mediated apoptosis [54].
Functional effects of P2X and P2Y receptors triggering in different mast cells are summarized in Table 2.
Table 2.
Effects of P2 receptor activation in mast cells
| Effect | Mast cell types | Responsible receptors | References |
|---|---|---|---|
| Ca2+ influx | Mouse BMMCs | P2X1, P2X3, P2X7 | [9, 54] |
| CBMCs | P2Y1 | [59] | |
| Rat peritoneal mast cells | ND | [3, 61–63] | |
| Membrane permeabilization and nonselective pore formation | Mouse BMMCs | P2X7 | [9, 54] |
| MC/9 | P2X7 | [70] | |
| Rat peritoneal mast cells | ND | [61, 62, 65, 69] | |
| Degranulation | Rat peritoneal mast cells | ND | [5, 6, 60–63, 65, 69] |
| MC/9 | P2X7 | [70] | |
| Mouse BMMCs | P2X7, ND | [54, 66] | |
| Human CBMCs | ND | [59] | |
| HLMCs | P2Y1, P2Y2 | [58] | |
| Phosphorylation of intracellular proteins | Mouse BMMCs, MC/9, P815 | P2X1, P2X3, P2X7 | [9, 54, 70] |
| Human CBMCs | P2Y1, P2Y12 | [59] | |
| Cytokine/chemokine expression/secretion | Mouse BMMCs, MC/9 | P2X7, P2X1, P2X3 | [9, 54] |
| Human CBMCs | P2Y1, P2Y11, P2Y12 | [59] | |
| Chemotaxis | Rat BMMCs | P2Y2 | [8] |
| CD34+ progenitors | P2Y2 | [82] | |
| Apoptosis | Mouse BMMCs, P815, MC/9, | P2X7 | [9, 54, 83] |
ND not detected
Purinergic signaling in allergic airway inflammation: focus on mast cells
Asthma is a chronic airway inflammatory disease which involves episodes of airway obstruction, wheezing, and coughing [88]. Pathophysiologically, asthma is characterized by bronchial hyperreactivity, goblet cells hyperplasia, mucus overproduction, smooth muscle cell hypertrophy, and increased contractility, submucosal infiltration by eosinophils, neutrophils, mast cells, and lymphocytes [88]. Dendritic cells play a key role in initiating and maintaining allergic inflammation. These cells uptake and process antigen/allergen and present it in the context of MHC to Th0 T lymphocytes [89]. Antigen presentation, together with co-stimulatory ligand–receptor interaction and cytokine production, guides lymphocytes to a Th2 phenotype (Fig. 2). Moreover, dendritic cells produce cytokines that mediate activation and expansion of Th2 cells, mast cells, basophils, etc. [89]. Th2 cells, in turn, secrete a number of mediators and provide additional co-stimulatory signals that induce B cells to the production of allergen-specific IgE [90].
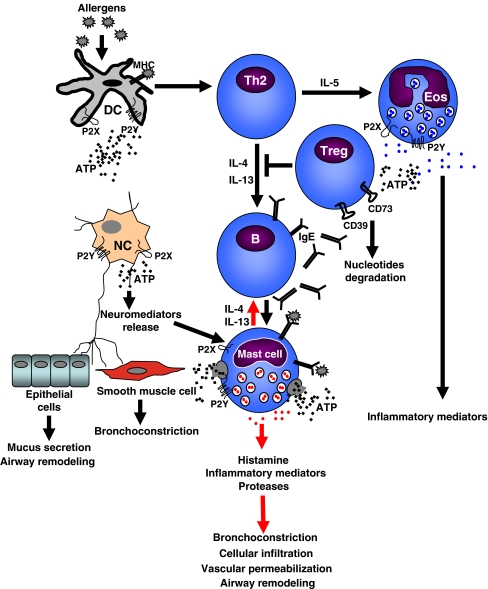
Fig. 2.
Purinergic regulation of airway inflammation. Dendritic cells uptake and process allergen. In parallel with allergen presentation to Th0 cells, they drive Th0 cells toward Th2 phenotype. Th2 cells release IL-4 and IL-13 that activate B lymphocytes to produce allergen-specific IgE and IL-5 that induces proliferation and recruitment of eosinophils to the site of inflammation. IgE binds to the Fc receptors on mast cells. Upon allergen/antigen IgE cross-linking, mast cells degranulate with release of histamine and pro-inflammatory mediators and cytokines, including IL-4 and IL-13. Nerves carry out autonomous control of epithelial and smooth muscle cell functions and release neuropeptides that modulate mast cell response. ATP and other nucleotides stimulate different P2 receptors on the surface of dendritic, mast, nerve cells, eosinophils, inducing additional cytokines and chemokines release, recruiting more cells, mediating IgE-induced degranulation of mast cells, and amplifying bronchoconstriction. Tregs degrade ATP due to the high expression of ATP-converting enzymes (CD39 and CD73) and inhibit Th2-mediated response. DC dendritic cell, NC nerve cell, Eos eosinophil, Treg T regulatory cell
Mast cells play an important role in the pathophysiology of asthma as effector cells through their chronic activation by IgE and allergens [13, 15, 16]. To fulfill this role, mast cells not only secrete a plethora of bronchospastic and pro-inflammatory mediators, shift Th1/Th2 balance toward the differentiation and expansion of Th2 cells, modulate antigen presentation by dendritic cells, and govern proliferation and activation of eosinophils [13, 88, 90] but also infiltrate the airway smooth muscle layer where they contribute to the increased ATP concentration in airways (Fig. 2). In fact, recent observation shows that ATP levels are significantly elevated in the airways of asthmatic patients after allergen challenge and in mice in a model of ovalbumin (OVA)-induced asthma [91]. Aerosolized ATP triggers bronchoconstriction in healthy and asthmatic individuals [92]. ATP induces release of neuropeptides (neurokinin A, SP) from adjacent nerve terminals. These neuropeptides exert pro-inflammatory effects, inducing submucosal gland secretion, smooth muscle contraction, and migration of eosinophils and neutrophils, increasing vascular permeabilization, and triggering degranulation of mast cell and chemokine release [93, 94]. There is a tight cross-communication between neurons and mast cells which will be discussed below. ATP not only directly induces degranulation of mast cells but also modulates IgE/antigen-mediated degranulation [60–62, 70]. Moreover, ATP and other nucleotides are potent chemoattractants [48]. Secreted from mast cells, they might contribute to the recruitment of other cells (e.g., eosinophils, macrophages, dendritic cells, etc.), increasing cellular infiltration in the airways and maintaining asthmatic airway inflammation. ATP induces expression and release of many pro-inflammatory mediators from mast cells, including IL-4 and IL-13 [9, 54], which are essential for Th2 cells effector functions, creating in that way a vicious circle maintaining allergic inflammation. The role of other mast cell-derived cytokines (TNFα, IL-1β, IL-18, IL-25, and IL-33) in asthma pathogenesis was recently reviewed in detail [88, 89]. It was also reported that ATP induces the release of matrix metalloproteinase-9 via a P2X7-dependent mechanism [95]. Mast cells are a rich source of this enzyme that participates in extracellular matrix remodeling by catalyzing the degradation of type IV collagen, the main component of the basement membrane [96].
Allergic inflammation includes a variety of signaling events and negative feedback loops that serve to terminate the activation process. Rapid desensitization of some of P2 receptors (e.g., P2X1 and P2X3) [38, 39] might represent one of such mechanisms. ATP at high concentration induces P2X7-mediated apoptosis of dendritic cells and mast cells [9, 54, 86]. This could represent a protective mechanism that limits the number of hyperactivated cells during diverse inflammatory processes. Mast cell-secreted inhibitory cytokines (e.g., IL-10) or mediators that shift the Th1/Th2 balance to Th1 (IFNγ and IL-12) could contribute to the downregulation of the allergic response [88, 90]. Another inhibitory mechanism is provided by T regulatory cells (Tregs). Several possible scenarios by which Tregs could inhibit allergic inflammation are discussed to date. Tregs reduce eosinophil infiltration, Th2 cytokine production, and airway hyperreactivity (reviewed in [88, 97]). ATP-degrading enzymes (CD39 and CD73) highly expressed on the membrane on Tregs are recognized recently as their activation markers [98, 99]. Tregs exert immune suppression by increasing the concentration of intracellular cAMP in target cells [98]. It was suggested that Tregs first convert ATP to adenosine which, in turn, triggers adenyl cyclase by activating Gs protein-coupled A2A receptor [98]. As was noted above, mast cells are also equipped with a number of ATP-converting enzymes (CD39, CD73, and CD203c) that degrade nucleotides and diminish their hazardous concentration.
Thus, ATP and other nucleotides modulate mast cell responsiveness to allergic stimuli and regulate the function of other players in allergic airway inflammation (dendritic cells, eosinophils, nerve cells, etc.), supporting a crucial role of purinergic signaling in the acute hypersensitivity phase of asthma and opening new perspectives for pharmaceutical intervention.
ATP in intercellular communication: role of mast cells
There is increasing evidence that communication between certain cell types occurs via ATP secretion and activation of purinoceptors on target cells. Mechanic stimulation of mouse fibroblasts, hepatocytes, or glial cells evokes an increase in Ca2+ not only in stimulated cells but also in distant cells [100–102]. This remote signaling is mediated by ATP or other nucleotides. Initially, it was postulated that such way of cell-to-cell communication occurs exclusively via gap junctions which control the release of ATP in the extracellular space [27]. However, Osipchuk and Cahalan [7] showed that ATP liberated from one mast cell could diffuse several tens of millimeters to elicit a rise in Ca2+ in distant cells. This ATP release is triggered either by FcεR cross-linking, by extracellular nucleotide application (ATP or GTP), or by mechanic stimulation and is independent on gap–junctional coupling [7]. A local extracellular ATP concentration (1–10 μM) is sufficient to activate Ca2+ responses mediated by high-affinity P2 receptors in neighboring cells. Moreover, extracellular ATP stimulation synergizes with FcεR signaling, and it is reasonable to assume that the release of ATP can introduce an element of positive feedback in the initial secretory response (Fig. 3). Extracellular ATP may accelerate the release of granules containing ATP in intact neighboring cells or in cells that have begun to degranulate. At the same time, ATP undergoes subsequent degradation due to the enzymatic activity of extracellular ATP-ases, CD39, and CD73. ATP derivatives, ADP, AMP, and adenosine could bind to the P2X, P2Y, or P1 receptors on the surface of the same or adjacent mast cell (Fig. 3). Thus, ATP functions as an important autocrine/paracrine mediator for mast cells.
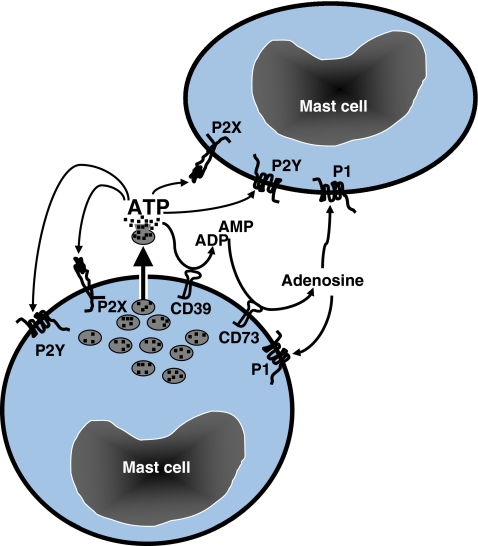
Fig. 3.
ATP is an autocrine/paracrine factor for mast cells. ATP is released from mast cell granules and could induce activation of the same or neighbor mast cell via P2X and P2Y receptors. Simultaneously, ectonucleotidases, CD39 and CD73, catalyze the degradation of extracellular ATP to ADP and AMP and, subsequently, to adenosine. ADP and AMP could activate mast cell via P2X and P2Y receptors, while adenosine triggers P1 receptors
ATP acts as an autocrine as well as a paracrine factor, mediating in that way intercellular communication. For instance, ATP mediates neuron–glia or neuron–keratinocyte cross-communication [103, 104]. The mast cell–nerve relationship has served as a prototypic demonstration of neuroimmune interaction and has provided substantial evidence for bidirectional communication between nerves and immune cells (Fig. 4). Mast cell products (neuropeptides, cytokines (e.g., TNFα), growth factors (e.g., NGF), and arachidonic acid metabolites) could directly activate nerves [11, 12, 25]. Tryptase, for example, directly triggers proteinase-activated receptors on neurons [105]. Electrical nerve stimulation causes mast cell degranulation in the dura mater [106]. Elegant studies have shown that nerve–mast cell interaction is direct and does not require the presence of intermediate cells and that ATP, released from mast cells, plays an essential role in this interaction. Recently, Suzuki et al. [107] demonstrated that activated rat mast cells cause ATP-dependent Ca2+ mobilization in adjacent superior cervical ganglia neurites, suggesting that bidirectional signaling exists between mast cells and neurons via ATP. ATP is released from mast cell granules upon IgE-mediated degranulation and stimulates adjacent neurites via P2X and P2Y receptors (Fig. 4). The neuropeptide SP was found among other important intermediates of nerve–mast cell communication which is released from nerve terminals upon bradykinin or scorpion venom stimulation [108]. SP operates via the neurokinin-1 (NK-1) receptor on mast cells and in high concentration could induce mast cell degranulation, evoking Ca2+ mobilization and priming mast cells for activation at a low dose [108]. Moreover, recent observations demonstrated that close, synapse-like contact exists between nerve and mast cells, and N-cadherin and cell adhesion molecule 1 play an essential role in this contact [109]. These molecules possibly not only provide a molecular platform for nerve–mast cell interaction but also promote the development of neuroimmunological synapses where both types of cells have enhanced susceptibility to bidirectional activation.
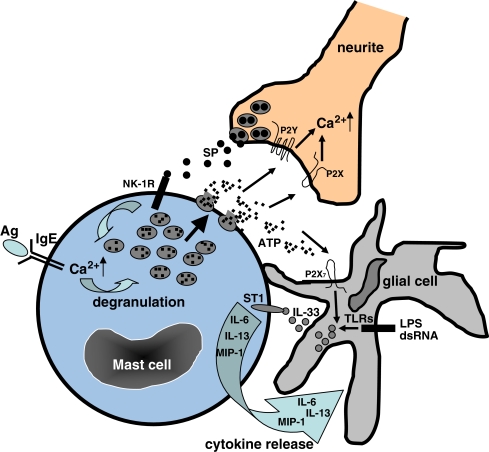
Fig. 4.
Model of intercellular communication in CNS (role of mast cells and ATP). Mast cells release ATP from intracellular granules upon specific (IgE+Ag) and non-specific (stress, mechanic stimulation, osmotic swelling, etc.) stimulation. Extracellular ATP triggers P2Y and P2X receptor in neurites, evoking Ca2+ currents. Neurites release SP from nerve terminals. Activation of NK-1R by SP leads to the Ca2+ influx and, at high concentrations of SP, to degranulation of mast cells. Extracellular ATP also stimulates the release of IL-33 from glial cells which were pre-activated with PAMPS (LPS or dsRNA) via TLRs (in order to increase IL-33 expression). IL-33 binds to the ST-1 (IL-33R) receptor on mast cell and induces the secretion of IL-6, IL-13, and MCP-1 which, in turn, could modulate the activity of glial cell
It has been recently shown that there is another active participant in neuroimmune cross-communication, the glial cell (Fig. 4). ATP has long been known to induce IL-1β and TNFα release from glial cells [49, 51, 52]. Treatment of cells with PAMPs (dsRNA or LPS) that was followed by ATP pulse significantly induced the release of IL-1β and IL-33 by the glial cells [110]. IL-33 acts via the ST1 receptor which is highly expressed on mast cells and induces release of TNFα, IL-1β, IL-6, IL-13, RANTES, GM-CSF, MCP-1, MIP1α, and MIP1β by BMMCs [90]. Supernatants from ATP and PAMPs-treated glial cells induced secretion of IL-6, IL-13, and MCP-1 by MC/9 cells similar to the effect of the recombinant IL-33 [110]. These pro-inflammatory cytokines could stimulate the glial cells in return. In vivo ATP might come from mast cells and induce the secretion of IL-1β and IL-33 by glial cells. Taken together, ATP released from mast cells is an important autocrine/paracrine/exocrine factor that mediates cross-communication between different cells, mast, neurons, and glial cells.
Giving the pivotal role of ATP and mast cells in intercellular cross-communication in CNS, it is extremely important to highlight their possible role in the pathophysiology of CNS. It has been shown that the concentration of mast cells is relatively high in the cerebral cortex and thalamus, hypothalamus, and hippocampus [111]. These cells are constitutively present in the brain. Moreover, pharmacologically induced mast cell degranulation regulates neuronal activity of thalamus [112]. Importantly, tryptase, a mast cell-specific protease, is elevated in the cerebrospinal fluid of patients with multiple sclerosis [113]. In the experimental autoimmune encephalomyelitis (EAE) model, increased numbers of degranulated mast cells were found in the brain of afflicted animals [114]. It was suggested that mast cell activation is important for EAE disease onset [115]. Brain-resident mast cells affect inflammatory response associated with EAE in different ways. The P2 receptor-mediated degranulation and release of cytokines from mast cells (IL-4, IL-13, IFNγ, and TNFα) increases the permeabilization of vascular endothelium, influences immune cells trafficking to the inflamed sites, and guides differentiation of CD4+ T cells to Th1 or Th2 subsets. Mast cell proteases degrade myelin, and myelin could, in turn, stimulate degranulation of mast cells [116, 117]. As was noted above, ATP mediates release of IL-33 from glial cells. While IL-33 governs Th2 immune response, it also mediates secretion of IL-13 by mast cells [90, 110], which could further amplify the Th2-mediated response. Therefore, purine/cytokine-mediated cross-communication between mast–nerve–glial cells represents a key neural–immune interaction in CNS inflammation and autoimmune diseases.
Conclusions and perspectives
We reviewed selected examples in which both mast cells and extracellular nucleotides play a recognized role in physiological and pathophysiological processes. The ability of nucleotides to mediate mast cell responses such as chemotaxis, proliferation, cytokine secretion, cell death, and to participate in the intercellular communication opens entirely new perspectives for the development of anti-inflammatory drugs. The pharmacological modulation of nucleotide-mediated signaling in mast cells, as well as in other cell types, represents a desirable new therapeutic approach for the treatment of acute and chronic inflammatory diseases. During the last few years, several P2X receptor agonists were implicated in the therapy of inflammatory diseases. The usefulness of oATP (nonselective P2X receptors antagonist) was proposed for the treatment rheumatoid arthritis and multiple sclerosis after promising use in a mouse model of arthritic pain and EAE [118]. oATP was successfully applied to limit T cell-mediated inflammation in mouse models of type 1 diabetes and inflammatory bowel disease [29, 118]. oATP, suramine, and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid significantly reduced allergic airway inflammatory response in the OVA-mediated model of asthma [91]. Thus, purinergic receptor blockade might represent a promising therapeutic strategy for the treatment of a number of inflammatory diseases including allergy in which mast cells are recognized to play an effector role.
Acknowledgments
We are grateful to Dr. Zane Orinska for critical reading of the manuscript and to Annette Wallisch for editorial assistance.
Abbreviations
- ART
ADP-ribosyltransferase
- BMMC
Bone marrow-derived mast cell
- CBMC
Cord blood mast cell
- CNS
Central nervous system
- HLMC
Human lung mast cell
- IL
Interleukin
- MIP
Macrophage inflammatory protein
- NAD
Nicotinamide adenine dinucleotide
- NGF
Nerve growth factor
- oATP
Oxidized ATP
- OVA
Ovalbumin
- PAMPS
Pathogen-associated molecular patterns
- PKC
Protein kinase C
- RBL
Rat basophilic leukemia
- RANTES
Regulated on activation, normal T cell expressed and secreted
- SCF
Stem cell factor
- SP
Substance P
- TNFα
Tumor necrosis factor α
References
- 1.Dwyer KM, Deaglio S, Gao W, et al. CD39 and control of cellular immune responses. Purinergic Signalling. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colgan SP, Eltzschig HK, Eckle T, et al. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signalling. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahlquist R, Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharm Toxicol (Copenh) 1974;34:368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama K. Calcium-dependent histamine release with degranulation from isolated rat mast cells by adenosine 5′-triphosphate. Jpn J Pharmacol. 1971;21:209–226. doi: 10.1254/jjp.21.209. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft S, Gomperts BD. ATP induces nucleotide permeability in rat mast cells. Nature. 1979;279:541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft S, Gomperts BD. The ATP4− receptor of rat mast cells. Biochem J. 1980;188:789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 8.McCloskey M, Fan Y, Luther S. Chemotaxis of rat mast cells towards adenine nucleotides. J Immunol. 1999;163:970–977. [PubMed] [Google Scholar]
- 9.Bulanova E, Budagian V, Orinska Z, et al. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174:3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich P (1878) Beiträge zur Theorie und Praxis der histologischen Färbung. Dissertation, Leipzig University
- 11.Galli SJ, Tsai M. Mast cells: versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J Dermatol Sci. 2008;49:7–19. doi: 10.1016/j.jdermsci.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heib V, Becker M, Taube C, et al. Advances in the understanding of mast cell function. Br J Haematol. 2008;142:683–694. doi: 10.1111/j.1365-2141.2008.07244.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann NY Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- 14.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stelekati E, Orinska Z, Bulfone-Paus S. Mast cells in allergy: innate instructors of adaptive responses. Immunobiology. 2007;212:505–519. doi: 10.1016/j.imbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Galli S, Maurer M, Lantz C. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53–59. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 17.Virgilio F, Chiozzi P, Ferrari D, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 18.Sayed BA, Christy A, Quirion MR, et al. The master switch: the role of mast cells in autoimmunity and tolerance. Ann Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 19.Bankl HC, Valent P. Mast cells, thrombosis, and fibrinolysis: the emerging concept. Thromb Res. 2002;105:359–365. doi: 10.1016/s0049-3848(02)00016-6. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Piliponsky AM, Levi-Schaffer F. Regulation of apoptosis in mast cells. Apoptosis. 2000;5:435–441. doi: 10.1023/a:1009680500988. [DOI] [PubMed] [Google Scholar]
- 22.Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signalling. 2005;1:205–209. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak I. ATP as a signaling molecule: the exocrine focus. News Physiol Sci. 2003;18:12–17. doi: 10.1152/nips.01409.2002. [DOI] [PubMed] [Google Scholar]
- 24.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakanishi M, Furuno T. Molecular basis of neuroimmune interaction in an in vitro coculture approach. Cell Mol Immunol. 2008;5:249–259. doi: 10.1038/cmi.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotrina ML, H-C LJ, Alves-Rodrigues A, et al. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber MT, Monyer H, Bruzzone R. Cell–cell communication beyond connexins: pannexin channels. Physiology. 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 28.Locovei A, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenk U, Westendorf AM, Radaelli E, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:85–97. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 30.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 31.Stefan C, Jansen S, Bollen M. Modulation of purinergic signaling by NPP-type ectophosphodiesterases. Purinergic Signalling. 2006;2:361–370. doi: 10.1007/s11302-005-5303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauswirth AW, Escribano L, Pardos A, et al. CD203c is overexpressed on neoplastic mast cells in systemic mastocytosis and is upregulated upon IgE receptor cross-linking. Int J Immunopathol Pharmacol. 2008;21:797–806. doi: 10.1177/039463200802100404. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. New York: Raven; 1978. pp. 107–118. [Google Scholar]
- 34.Fredholm BB, Ijzerman AP, Jacobson KA, et al. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 35.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolen K, Slegers H. Integration of P2Y receptor-activated signal transduction pathways in G protein-dependent signaling networks. Purinergic Signalling. 2006;2:451–469. doi: 10.1007/s11302-006-9008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbracchio MP, Boeynaems JM, Barnard EA, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 39.Markwardt F. Activation kinetics of single P2X receptors. Purinergic Signalling. 2007;3:249–253. doi: 10.1007/s11302-007-9070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Virgilio F, Ferrari D. P2X7 receptor: death or life? Purinergic Signalling. 2005;1:219–227. doi: 10.1007/s11302-005-6322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surprenant A, Rassendren F, Kawashima E, et al. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 42.Seman M, Adriouch S, Scheuplein F, et al. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/s1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Schwarz N, Brass A, et al. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J Immunol. 2009;183:578–592. doi: 10.4049/jimmunol.0900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgilio F. Purinergic signalling in the immune system: a brief update. Purinergic Signalling. 2007;3:1–3. doi: 10.1007/s11302-006-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 46.Jamieson GP, Snook MB, Thurlow PJ, et al. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinoceptors. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 47.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–951. [PubMed] [Google Scholar]
- 48.Myrtek D, Idzko M. Chemotactic activity of extracellular nucleotides on human immune cells. Purinergic Signalling. 2007;3:5–11. doi: 10.1007/s11302-006-9032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 50.MacKenzie A, Wilson HL, Kiss-Toth E, et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 51.Bianco F, Pravettoni E, Colombo A, et al. Astrocyte-derived ATP induces vesicles shedding and IL-1β release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gall SM, Bobe P, Reiss K, et al. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor α, L-selectin, and tumor necrosis factor α. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bulanova E, Budagian V, Orinska Z, et al. ATP induces P2X7 receptor-independent cytokine and chemokine expression through P2X1 and P2X3 receptors in murine mast cells. J Leukoc Biol. 2009;85:692–702. doi: 10.1189/jlb.0808470. [DOI] [PubMed] [Google Scholar]
- 55.Royle SJ, Bobanovic LK, Murrell-Lagnado RD. Identification of non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Jakobsen SEW, Bengtsson A, et al. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16–22. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradding P, Okayama Y, Kambe N, et al. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol. 2003;73:614–620. doi: 10.1189/jlb.1202602. [DOI] [PubMed] [Google Scholar]
- 58.Schulman ES, Glaum MC, Post T, et al. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–537. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- 59.Feng C, Mery AG, Beller EM, et al. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 60.Cockcroft S, Gomperts BD. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tatham PER, Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen. Physiol. 1990;95:459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaffar ZH, Pearce FL. Some characteristics of the ATP-induced histamine release from and permeabilization of rat mast cells. Agents Actions. 1993;40:18–27. doi: 10.1007/BF01976747. [DOI] [PubMed] [Google Scholar]
- 63.Izushi K, Tasaka K. Essential role of ATP and possibility of activation of protein kinase C in Ca2+-dependent histamine release from permeabilized rat peritoneal mast cells. Pharmacology. 1991;42:297–308. doi: 10.1159/000138812. [DOI] [PubMed] [Google Scholar]
- 64.Buell G, Michel AD, Lewis C, et al. P2X1 receptor activation in HL60 cells. Blood. 1996;87:2659–2664. [PubMed] [Google Scholar]
- 65.Qian YX, McCloskey MA. Activation of mast cells K+ channels through multiple G protein-linked receptors. Proc Natl Acad Sci USA. 1993;90:7844–7848. doi: 10.1073/pnas.90.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito H, Ebisawa M, Reason DC, et al. Extracellular ATP stimulates interleukin-dependent cultured mast cells and eosinophils through calcium mobilization. Int Arch Allergy Appl Immunol. 1991;94:68–70. doi: 10.1159/000235327. [DOI] [PubMed] [Google Scholar]
- 67.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1β release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 69.Lee YH, Lee SJ, Seo MH, et al. ATP-induced histamine release is in part related to phospholipase A2-mediated arachidonic acid metabolism in rat peritoneal mast cells. Arch Pharm Res. 2001;24:552–556. doi: 10.1007/BF02975164. [DOI] [PubMed] [Google Scholar]
- 70.Sudo N, Tanaka K, Koga Y, et al. Extracellular ATP activates mast cells via a mechanism that is different from the activation induced by the cross-linking of Fc receptors. J Immunol. 1996;156:3970–3979. [PubMed] [Google Scholar]
- 71.Chakravarty N. The role of plasma membrane Ca++–Mg++ activated adenosine triphosphatase of rat mast cells on histamine release. Acta Pharm Toxicol (Copenh) 1980;47:223–225. doi: 10.1111/j.1600-0773.1980.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarty N, Nielesen EH. Adenosine triphosphatase in non-secreting and secreting mast cells. Agents Actions. 1981;11:67–69. doi: 10.1007/BF01991458. [DOI] [PubMed] [Google Scholar]
- 73.Schnurr M, Toy T, Schin A, et al. Extracellular nucleotides signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 74.Swanson KD, Reigh C, Landreth GE. ATP-stimulated activation of the mitogen-activated protein kinases through ionotrophic P2X2 purinoreceptors in PC12 cells: difference in purinoreceptor sensitivity in two PC12 cell lines. J Biol Chem. 1998;273:19965–19971. doi: 10.1074/jbc.273.32.19965. [DOI] [PubMed] [Google Scholar]
- 75.Neary JT, McCarthy M, Kang Y, et al. Mitogenic signaling from P1 and P2 purinergic receptors to mitogen-activated protein kinase in human fetal astrocyte cultures. Neurosci Lett. 1998;242:159–162. doi: 10.1016/s0304-3940(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 76.Humphreys BD, Rice J, Kertesy SB, et al. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem. 2000;275:26792–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- 77.Budagian V, Bulanova E, Brovko L, et al. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-κB. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- 78.Gabel CA. P2 purinergic receptor modulation of cytokine production. Purinergic Signalling. 2007;3:27–38. doi: 10.1007/s11302-006-9034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cox MA, Gomes B, Plamer K, et al. The pyrimidinergic P2X6 receptor mediates a novel release of proinflammatory cytokines and chemokines in monocytic cells stimulated with UDP. Biochem Biophys Res Commun. 2005;330:467–473. doi: 10.1016/j.bbrc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Takayama K, Garsia-Gardena G, Sukhova GK, et al. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002;277:44147–44154. doi: 10.1074/jbc.M204810200. [DOI] [PubMed] [Google Scholar]
- 81.Okamoto T, Murayama Y, Hayashi Y, et al. Identification of a Gs activator region of the β2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell. 1991;67:723–730. doi: 10.1016/0092-8674(91)90067-9. [DOI] [PubMed] [Google Scholar]
- 82.Rossi L, Manfredini R, Bertolini F, et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
- 83.Zheng LM, Zychlinsky A, Liu C-C, et al. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zanovello P, Bronte V, Rosato A, et al. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- 85.Pfeiffer ZA, Aga M, Prabhu U, et al. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leuk Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- 86.Nihei OK, Carvalho AC, Salvino W, et al. Pharmacologic properties of P2Z/P2X7 receptor characterized in murine dendritic cells: role in the induction of apoptosis. Blood. 2000;96:996–1005. [PubMed] [Google Scholar]
- 87.Mackenzie AB, Young MT, Adinolfi E, et al. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem. 2005;280:33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- 88.Shum BOV, Rolph MS, Sewell WA. Mechanisms in allergic airway inflammation—lessons from studies in the mouse. Expert Rev Mol Med. 2008;10:e15. doi: 10.1017/S1462399408000707. [DOI] [PubMed] [Google Scholar]
- 89.Long AA. Immunomodulators in the treatment of asthma. Allergy Asthma Proc. 2009;30:109–119. doi: 10.2500/aap.2009.30.3203. [DOI] [PubMed] [Google Scholar]
- 90.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Idzko M, Hammad H, Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Immunol. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 92.Pellegrino R, Wilson O, Jenairi G, et al. Lung mechanics during induced bronchoconstriction. J Appl Physiol. 1996;81:964–975. doi: 10.1152/jappl.1996.81.2.964. [DOI] [PubMed] [Google Scholar]
- 93.Pelleg A, Schulman ES. Adenosine 5′-triphosphate axis in obstructive airway disease. Am J Ther. 2002;9:454–464. doi: 10.1097/00045391-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 94.Kulka M, Sheen CH, Tancowny BP, et al. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2009;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107:4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 96.Kanbe N, Tanaka A, Kanbe M, et al. Human mast cells produce matrix metalloproteinase 9. Eur J Immunol. 1999;29:2645–2649. doi: 10.1002/(SICI)1521-4141(199908)29:08<2645::AID-IMMU2645>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 97.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 98.Borsellino G, Kleinewietfeld M, Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 99.Kobie JJ, Shah PR, Yang L, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 100.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guthrie PB, Knappenberger J, Segal M, et al. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grierson JP, Meldolesi J. Shear stress-induces [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995;270:4451–4456. doi: 10.1074/jbc.270.9.4451. [DOI] [PubMed] [Google Scholar]
- 103.Deitmer JW. Strategies for metabolic exchange between glial cells and neurons. Respir Physiol. 2001;129:71–81. doi: 10.1016/s0034-5687(01)00283-3. [DOI] [PubMed] [Google Scholar]
- 104.Koizumi S, Fujishita K, Inoue K, et al. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 106.Dimitriadou V, Buzzi MG, Moskowitz MA, et al. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;41:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki R, Furuno T, Okamoto K, et al. ATP plays a role in neurite stimulation with activated mast cells. J Neuroimmunol. 2007;192:49–56. doi: 10.1016/j.jneuroim.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki R, Furuno T, McKay DM, et al. Direct neurite–mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol. 1999;163:2410–2415. [PubMed] [Google Scholar]
- 109.Suzuki A, Suzuki R, Furuno T, et al. N-cadherin plays a role in the synapse-like structures between mast cells and neuritis. Biol Pharm Bull. 2004;27:1891–1894. doi: 10.1248/bpb.27.1891. [DOI] [PubMed] [Google Scholar]
- 110.Hudson CA, Christophi GP, Gruber RC, et al. Induction of IL-33 expression and activity in central nervous system glia. J Leuk Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dropp JJ. Mast cells in mammalian brain. Acta Anal (Basel) 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- 112.Kovacs P, Hernadi I, Wilhelm M. Mast cells modulate maintained neuronal activity in the thalamus in vivo. J Neuroimmunol. 2006;171:1–7. doi: 10.1016/j.jneuroim.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 113.Rozniecki JJ, Hauser SL, Strein M, et al. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 114.Bo L, Olsson T, Nyland H, et al. Mast cells in brain during experimental allergic encephalomyelitis in Lewis rats. J Neurol Sci. 1991;105:135–142. doi: 10.1016/0022-510x(91)90136-u. [DOI] [PubMed] [Google Scholar]
- 115.Brown MA, Tanzola MB, Robbie-Ryan M. Mechanisms underlying mast cell influence on EAE disease course. Mol Immunol. 2001;38:1373–1378. doi: 10.1016/s0161-5890(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 116.Dietsch GN, Hinrichs DJ. Mast cell proteases liberate stable encephalitogenic fragments from intact myelin. Cell Immunol. 1991;135:541–548. doi: 10.1016/0008-8749(91)90297-o. [DOI] [PubMed] [Google Scholar]
- 117.Johnson D, Seeldrayers PA, Weiner HL. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988;44:195–198. doi: 10.1016/0006-8993(88)90929-8. [DOI] [PubMed] [Google Scholar]
- 118.Ferrero ME. A new approach to the inflammatory/autoimmune diseases. Recent Pat Antiinfect Drug Discov. 2009;4:108–113. doi: 10.2174/157489109788490343. [DOI] [PubMed] [Google Scholar]