Abstract
The role of peroxynitrite (PN) as a mediator of nociceptive signaling is emerging. We recently reported that the development of central sensitization that follows the intraplantar injection of carrageenan in rats is associated with spinal PN synthesis. We now demonstrate that a significant pathway through which spinal PN modulates central sensitization is post-translational tyrosine nitration of key proteins involved in the glutamatergic pathway, namely glutamate transporter GLT-1 and glutamine synthetase (GS). We also reveal that spinal activation of the N-methyl-D-aspartate (NMDA) receptor provides a source of PN in this setting. Intraplantar injection of carrageenan led to the development of thermal hyperalgesia as well as nitration of GLT-1 and GS in dorsal horn tissues. Pretreatment with the PN decomposition catalyst FeTM-4-PyP5+ [Fe(III)5,10,15,20-tetrakis(N-methylpyridinium-4-yl)porphyrin] or the NMDA receptor antagonist MK-801 blocked the development of hyperalgesia. Carrageenan-induced hyperalgesia was also associated with nitration and inactivation of spinal mitochondrial superoxide dismutase (MnSOD) known to provide a critical source of PN during central sensitization. Nitration of GLT1 and GS contributes to central sensitization by enhancing glutamateric neurotransmission. Our results support the critical role of nitroxidative stress in the development of hyperalgesia and suggest that post-translational nitration of enzymes and transporters linked to glutamatergic neurotransmission represent a novel mechanism of central sensitization.
Keywords: Peroxynitrite, Hyperalgesia, NMDA, Glutamate transporters, Glutamine synthetase
Introduction
The mechanisms involved in the development of central sensitization are complexed in nature and involve the contribution of many mediators in the spinal cord including the excitatory amino acid, glutamate [16,23,64] and the nitroxidative species, peroxynitrite (PN) [43,44] the reaction product between superoxide (O2•-) and nitric oxide (NO) [2]. Besides its well established role in inflammation [11,42], PN has been recently implicated in the development of thermal hyperalgesia associated with acute and chronic inflammation [3,13,37,54,59,66], in response to spinal activation of the N-methyl-D-aspartate receptor (NMDAR) [34], in the development of orofacial pain [66] and in the development of opiate-induced hyperalgesia and antinociceptive tolerance [1,33,38]. A role for nitroxidative stress (herein defined as stress induced in the presence of (O2•-), NO, PN and related species) was also supported using a variety of non-selective agents in neurogenic pain [8,17,47,48], visceral pain [58] and neuropathic pain [8,39,49,53].
Peroxynitrite contributes to the development of inflammation-derived hyperalgesia by acting peripherally and centrally [37,59] . These studies revealed that peripheral formation of PN contributes to hyperalgesia by favoring production of several proinflammatory cytokines and by increasing the production of prostaglandin E2 [37,59]. The important role of PN as a nociceptive mediator was underscored by findings that intraplantar injection of PN in rats evokes thermal hyperalgesia and inflammation with a rapid onset and long duration of action [37,59]. The mechanisms whereby PN contributes to central sensitization remain largely unknown and form the scope of the current work.
Dysfunction of the glutamatergic pathway is a key component of central sensitization [16,23,29,62,63,64] . Critical to the present study, are the findings that PN nitrates glutamate transporters (GTs), in particular GTL-1 and glutamine synthetase (GS) with nitration being intimately linked to inactivation of the biological function of such proteins [9,32,56,57]. These findings have important ramifications since inactivation of such proteins enhances glutamatergic neurotransmission [see [44] for updated review article]. These finding suggest that these protein are viable targets for PN. In support, we recently reported that nitroxidative alteration of GLT-1 and GS contributes to central sensitization associated with the development of morphine-induced hypersensitivity and antinociceptive tolerance [33].
We now demonstrate for the first time that NMDA receptor activation provides a source of spinal PN that in turn contributes to central sensitization by nitrating GLT-1 and GS. Our results provide the first substantiated mechanism by which spinal PN modulates central sensitization in inflammatory hypersensitivity.
Material and Methods
Animals
Sprague-Dawley rats (male, 175-200 g; Harlan, Indianapolis, IN) were used throughout these studies and housed and cared for using protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Saint Louis University Medical Center, and were in accordance with the NIH Guidelines on Laboratory Animal Welfare. Animal use at the University of Messina likewise complied with Italian regulations for the protection of animals used for experimental and other scientific purpose (D.M. 116192), and with European Economic Community regulations.
Materials
(+)-MK-801 [dizocilpine hydrogen maleate, (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate] and all other drugs were purchased from Sigma (St. Louis, MO). FeTM-4-PyP5+ was obtained from Cayman Chemical Company (Ann Arbor, Michigan). Ionic charges on FeTM-4-PyP5+ have been omitted for clarity in all Figures.
Induction of thermal hyperalgesia by carrageenan
Lightly anesthetized rats [CO2 (80%)/O2 (20%)] received a subplantar injection of carrageenan (0.1 mL of a 1% suspension in 0.85% NaCl) into the right hindpaw. Hyperalgesic responses to heat then were determined at specified time points as described by Hargreaves [10] and using a cutoff latency of 20 s to prevent tissue damage. Rats were individually confined to plexiglass chambers. A mobile unit consisting of a high intensity projector bulb was positioned to deliver a thermal stimulus directly to an individual hindpaw from beneath the chamber. The withdrawal latency periods for the injected paws were determined to the nearest 0.1 s with an electronic clock circuit and thermocouple. Reported results represented the changes at each timepoint in withdrawal latency, calculated as withdrawal latency at different time points after carrageenan minus baseline withdrawal latencies (T=0, pre-injection values). All drugs were given by intraperitoneal injection (1 mL/kg) 30 min before the induction of CO2 anesthesia and the intraplantar injections of carrageenan.
Immunoprecipitation and Western blot analysis
Dorsal horn tissues of the spinal cord lumbar region enlargement (L4-L6) were obtained as described previously [33,59]. The resulting tissue samples were stored immediately at -80°C, until the subsequent immunoprecipitation of tyrosine-nitrated proteins and western blot analyses as previously described [33,59]. For immunoprecipitation of nitrated proteins, an affinity-purified anti-nitrotyrosine monoclonal antibody conjugated to agarose beads from Upstate Biotechnology (Lake Placid, NY) was used according to the manufacturer’s instructions. To determine whether MnSOD, CuZnSOD, GLT-1, and GS were nitrated, western blots of immunoprecipitated protein complex and total lysates were made using antibodies specific to these proteins. Briefly, the immunoprecipitated proteins were resolved in 12% SDS-PAGE mini gels and proteins transferred to nitrocellulose membranes. Membranes were blocked for 1 h at room temperature (RT) using 1% bovine serum albumin (BSA)/0.1%Thimerosal in 50 mM Tris·HCl, (pH 7.4)/150 mM NaCl/0.01%Tween 20 (TBS/T) followed by incubation with rabbit polyclonal antibodies for MnSOD and CuZnSOD (1:2000, Upstate), GLT-1 (1:1000, Alpha Diagnostic Intl., TX) and a monoclonal antibody for GS (1:4000, Transduction Laboratories, KY). Membranes were then washed with TBS/T and incubated with secondary antibodies conjugated to horseradish peroxidase for 1 h at RT. After washes, proteins were visualized by enhanced chemiluminescence (ECL, Amernaive Biosciences, or Femto kit, Pierce). Protein bands of interest were quantified by densitometry using ImageQuant 5.2 software (Molecular Dynamics, CA).
Measurement of Mn and CuZn-SOD activities
Dorsal horn tissues were homogenized with 10 mM phosphate buffered saline (pH 7.4) in a Polytron homogenizer and then sonicated on ice for 1 min (3 × 20 s). Sonicated samples were subsequently centrifuged at 1,100 g for 10 min before SOD activity was measured in the supernatants. In brief, a competitive inhibition assay was performed that used xanthine-xanthine oxidase-generated superoxide to reduce nitroblue tetrazolium (NBT) to blue tetrazolium salt. The reaction was performed in sodium carbonate buffer (50 mM, pH 10.1) containing EDTA (0.1 mM), nitroblue tetrazolium (25 μM), xanthine and xanthine-oxidase (0.1 mM and 2 nM respectively; Boehringer, Germany). The rate of NBT reduction was monitored at 560 nm (Perkin Elmer Lambda 5 Spectrophotometer, Milan, Italy). The amount of protein required to inhibit the rate of NTB reduction by 50% was defined as one unit (U) of enzyme activity. Cu/Zn-SOD activity was determined by performing the assay in the presence of 2 mM NaCN after pre-incubation for 30 min. Enzymatic activity was expressed in U/mg protein [59].
Results
Development of thermal hyperalgesia is accompanied by tyrosine-nitration of GLT-1 and GS: inhibition by FeTMPyP
Intraplantar injection of carrageenan led to a time-dependent development of thermal hyperalgesia that peaked at 3 h and persisted up to 5 h (Fig. 1). At these time points we observed a significant nitration of GLT-1 (from 18±6 to 70±5 densitometry units, DU ± SEM, for vehicle and carrageenan respectively at 3 h, n = 6, P < 0.001, Fig. 2A and from 22±7 to 67±6 DU ± SEM, for vehicle and carrageenan respectively at 5 h, n = 6, P < 0.001, Fig. 2C). In addition to GLT-1, the development of hyperalgesia was also associated with significant nitration of GS (from 13±5 to 65±5 DU for vehicle and carrageenan at 3 h, n = 6, P < 0.001, Fig. 3A and from 15±4 to 84±8 DU for vehicle and carrageenan at 5h, n = 6, P < 0.001, Fig. 3C). These results suggest that nitration of these proteins play a critical role in the initiation and maintenance of hyperalgesia.
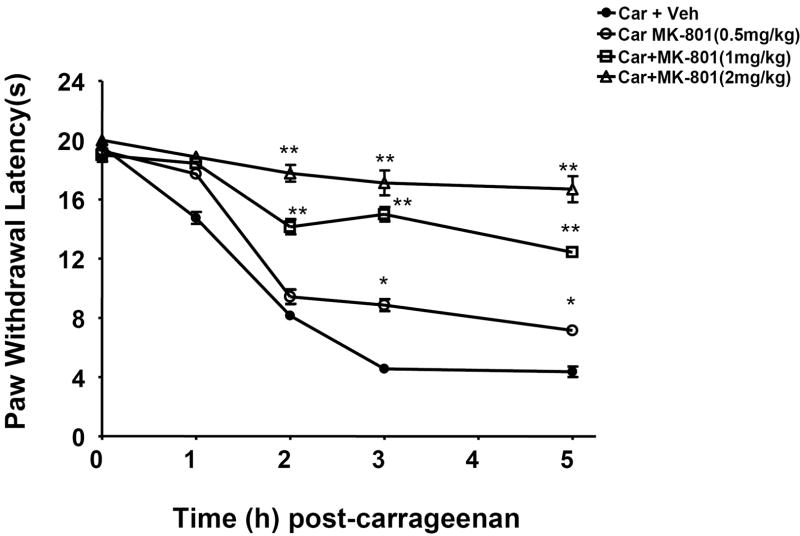
Fig. 1. Inhibition of carrageenan-induced hyperalgesia by MK-801.
The development of thermal hyperalgesia following intraplantar injection of carrageenan was blocked in a dose-dependent manner by MK-801 (0.5-2 mg/kg). MK-801 was given by intraperitoneal injection 30 min before carrageenan. * P<0.05 and * P<0.01 when compared to when compared to responses in the absence of MK-801. Results are expressed as mean ± SEM for 6 rats.
Fig. 2. FeTMPyP blocks nitration of the glutamate transporter GLT-1.
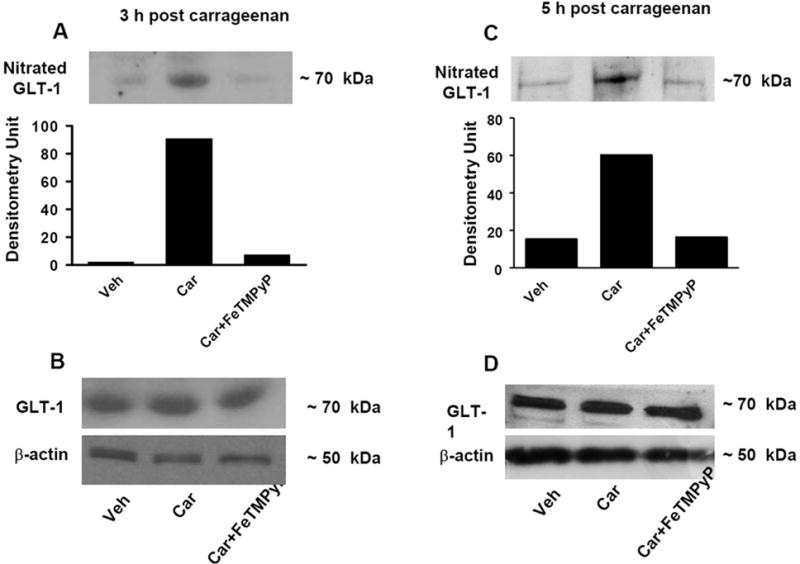
When compared to the vehicle group (Veh), injection of carrageenan (Car) led to significant nitration of GLT-1 at 3 h (A) and 5 h (C). These events were blocked by FeTMPyP (30 mg/kg, n=6) (A, C). When compared to the vehicle group, injection of carrageenan did not change the total amount of GLT-1 (B, D) in dorsal horn tissues as measured by Western blotting analysis. All gels shown are representative from gels obtained in 6 animals. The composite mean ± SEM of the densitometry data resulting from n=6 animals is shown in the result section.
Fig. 3. FeTMPyP blocks nitration of glutamine syntethase (GS).
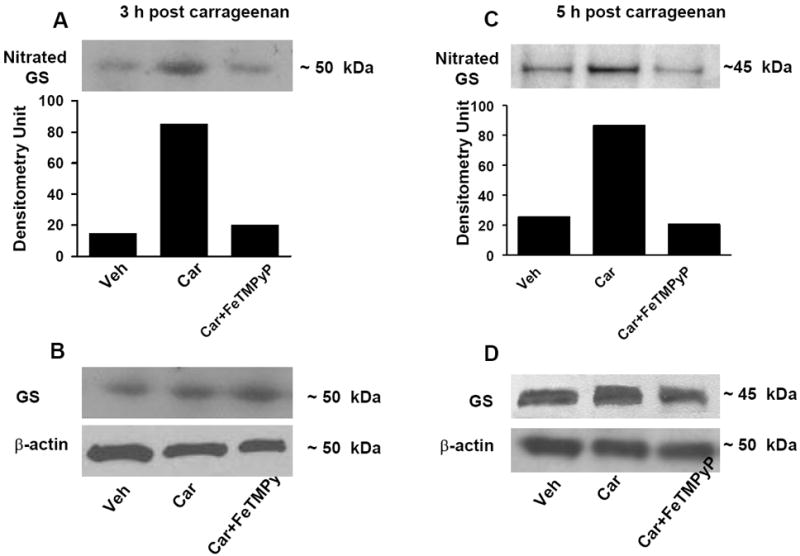
When compared to the vehicle group (Veh), injection of carrageenan (Car) led to significant nitration of GS at 3 h (A) and 5 h (C). These events were blocked by FeTMPyP (30 mg/kg, n=6) (A, C). When compared to the vehicle group, injection of carrageenan did not change the total amount of GS (B, D) in dorsal horn tissues as measured by Western blotting analysis. All gels shown are representative from gels obtained in 6 animals. The composite mean ± SEM of the densitometry data resulting from n=6 animals is shown in the result section.
We previously reported that development of carrageenan-induced edema and hyperalgesia is blocked in a dose-dependent manner by FeTMPyP [37], a well characterized PN decomposition catalyst [42]. FeTMPyP (30 mg/kg, n = 6) blocked hyperalgesia in a time-dependent manner thus supporting our previous findings [37]: in the present study, the inhibition of thermal hyperalgesia by FeTMPyP at 1, 3 and 5 h after carrageenan was 90±5 %, 87±7 %, and 93±8 % (n = 6). As shown in Fig. 2 and 3, removal of PN with FeTMPyP blocked nitration of GLT-1 (from 70±5 to 20±5 DU, for carrageenan and carrageenan plus FeTMPyP respectively at 3 h, n = 6, P < 0.001, Fig. 2A and from 67±6 to 16±5 DU, for carrageenan and carrageenan plus FeTMPyP respectively at 5 h, n = 6, P < 0.001, Fig. 2C). Similar protective effects were seen with GS (from 65±5 to 20±8 DU, for carrageenan and carrageenan plus FeTMPyP respectively at 3 h, n =6, P <0.001, Fig. 3A and from 84±8 to 15±7 DU, for carrageenan and carrageenan plus FeTMPyP respectively at 5 h, n=6, P <0.001, Fig. 3 C]. Total levels of GLT and GS protein did not change among the groups (Fig. 2 and 3; B, D).
These results suggest that PN-mediated nitration of GLT-1 and GS contribute to development of thermal hyperalgesia.
Inhibition of the NMDA receptor with MK-801 blocks nitration of GLT-1 and GS
The development of thermal hyperalgesia was blocked dose-dependently (0.5-2 mg/kg, n = 6) by the NMDA receptor antagonist MK-801 (Fig. 1). MK-801 (2 mg/kg, n = 6) blocked nitration of GLT-1 (from 67±6 to 18±3 DU, for carrageenan and carrageenan plus MK-801 respectively, n = 6, P <0.001; a representative gel of tissue from six animals is shown in Fig. 4A) and GS (from 84±8 to 20±3 DU, for carrageenan and carrageenan plus MK-801 respectively, n = 6, P < 0.001) (Fig. 4C). Total levels of GLT and GS protein did not change among the three groups (Fig. 4 B, D).
Fig. 4. MK-801 blocks nitration of the glutamate transporter GLT-1 and of glutamine syntethase (GS).
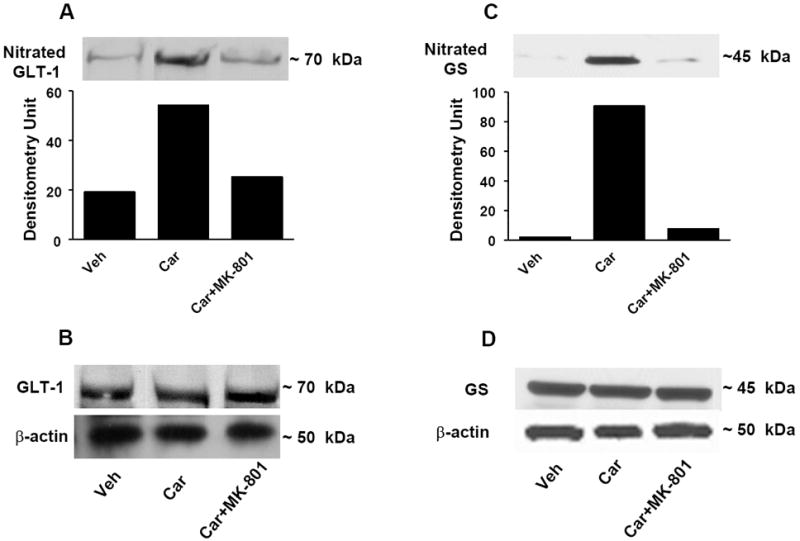
When compared to the vehicle group (Veh), injection of carrageenan (Car) led to significant nitration of GLT-1 (A) and GS (C) and this was blocked by MK-801 (2 mg/kg, n=6) (A, C). When compared to the vehicle group, injection of carrageenan did not change the total amount of GLT-1 or GS (B, D) in dorsal horn tissues as measured by Western blotting analysis. All gels shown are representative from gels obtained in 6 animals. The composite mean ± SEM of the densitometry data resulting from n=6 animals is shown in the result section.
These results suggest that NMDA receptor activation leads to spinal generation of PN which in turn nitrates and inactivates these proteins.
Inhibition of the NMDA receptor with MK-801 blocks nitration and enzymatic inactivation of MnSOD
At 5 h post carrageenan, MnSOD in spinal cord tissues was significantly nitrated (from 70±30 to 1700±100 DU for vehicle and carrageenan respectively, n = 6, P < 0.001, a representative gel of tissues from six animals is shown in Fig. 5A) and inactivated as evidenced by a significant reduction in its ability to dismute superoxide shown by spectrophotometric analysis (Fig. 5B). MK-801 (2 mg/kg, n = 6) blocked PN-mediated nitration (from 1700±100 to 700±88 for carrageenan and carrageenan plus MK-801 respectively, n = 6, P < 0.001) (Fig. 5A) and restored its enzymatic activity in a dose-dependent manner (0.5-2 mg/kg, n = 6) (Fig. 5B). Total levels of MnSOD protein did not change among the three groups (Fig. 5C). The cytosolic form of superoxide dismutase (CuZnSOD) was neither nitrated nor inactivated (data not shown). The finding that hyperalgesia was not associated with nitration or enzymatic inactivation of CuZnSOD is consistent with previous studies that have shown that the interaction of CuZnSOD with peroxynitrite does not affect the catalytic activity of the protein [50].
Fig. 5. MK-801 blocks nitration and enzymatic inactivation of MnSOD.
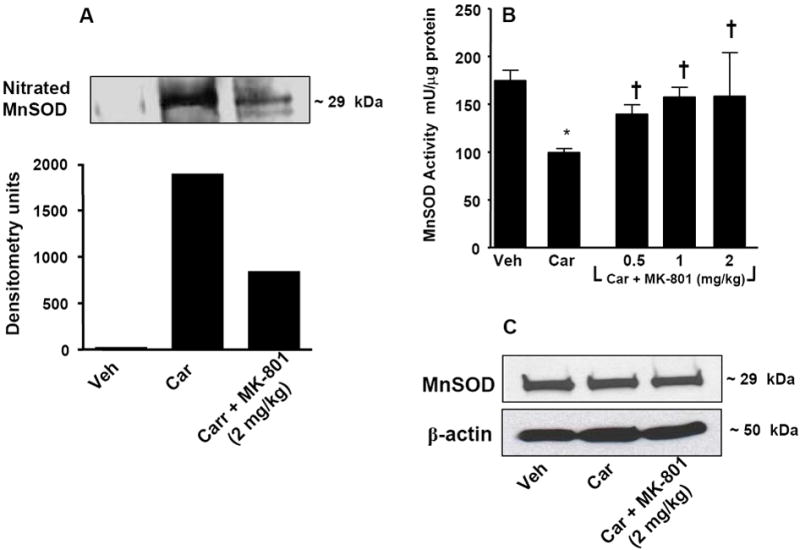
When compared to the vehicle group (Veh, n=6), injection of carrageenan (Car, n=6) led to significant nitration of MnSOD (A). Co-administration of morphine with MK-801 (2 mg/kg, n=6) prevented the nitration of MnSOD (A). Post-translational nitration of MnSOD (A) led to functional enzymatic inactivation as evidenced by loss of its catalytic activity to dismute superoxide as measured spectrophotometrically (B). MK-801 (2 mg/kg, i.p. n=6) restored the enzymatic activity of MnSOD (B). When compared to the vehicle group, injection of carrageenan did not change the total amount of MnSOD (C) in dorsal horn tissues as measured by Western blotting analysis. All gels shown are representative from gels obtained in 6 animals. The composite mean ± SEM of the densitometry data resulting from n=6 animals is shown in the result section. *P<0.05 for carrageenan vs vehicle; †P<0.01 for carrageenan+MK-801 vs carrageenan alone.
These results suggest that NMDA receptor activation generates PN that in turn nitrates and inactivates MnSOD. This pathway must contribute at least in part to central sensitization by fostering the presence of elevated [PN] in spinal cord tissues.
Discussion
Targeting peroxynitrite is an evidence-based approach to develop novel therapeutics for managing acute and chronic inflammatory pain, neuropathic pain as well as to prevent development of opiate induced hypersensitivity and antinociceptive tolerance [reviewed in [43,44,45]]. Understanding the signaling pathways engaged by PN in nociceptive processing is thus of paramount importance. While the molecular pathways affected by PN during peripheral sensitization are emerging, those involved in central sensitization remain largely unknown. During tissue injury and inflammation, hyperalgesia results from persistent peripheral afferent sensitization and then spinal sensitization through release various mediators including glutamate [16,21,22,23,25,28,63] . Glutamate neurotransmission, in particular that mediated via NMDA receptors is key in the development of central sensitization [16,21,22,23,25]. Spinal release of glutamate and subsequent NMDA-receptor activation favors PN accumulation by forming O2•- and NO simultaneously [15,27,28,29,46] . Importantly, formation of NO, O2•- and PN in spinal cord contribute to the development of hyperalgesia resulting from intrathecal delivery of NMDA [14,28,29,33].
The present results extend our previous findings [37,59] to support a plausible mechanism whereby spinally formed PN contributes to central sensitization by post-translational nitration of important glial cells proteins. Released glutamate is not metabolized by extracellular enzymes but must be removed from the synaptic cleft [5]. The homeostasis of extracellular glutamate is tightly regulated by GTs in the plasma membranes of both neurons and glial cells although the bulk (over 90%) of functional glutamate uptake is mediated by the glial transporters GLAST and GLT-1 [31,41]. These transporters prevent overstimulation of glutamate receptors and thus increased neuronal excitability [19,30,57] . If GLAST/GLT-1 function is impacted upon (i.e reduced or eliminated) glutamate can increase in cerebrospinal fluid contributing to rapid alterations in synaptic transmission [24,36,52,65]. In contradistinction to the central role of GTs in regulating the homeostasis of extracellular glutamate, GS plays a pivotal role in its intracellular metabolic fate [51]. In the brain, GS is located mainly in astrocytes and one of the primary roles of these cells is to protect neurons against excitotoxicity by taking up excess ammonia and glutamate, and converting them into glutamine [12,51]. Glutamine is then transported into neurons, where it serves as a precursor for the formation of glutamate and GABA [60,61]. Enzymatic inactivation of GS is expected to facilitate neuronal excitation [35,51]. Furthermore, through feedback regulation, a decrease in the activity of glutamine synthase can reduce the activity of glutamate transporters [51]. Here we show that the development of hyperalgesia was associated with PN-mediated nitration of GLT-1 and GS in dorsal horn tissues and that removal of PN with FeTMPyP blocked nitration of these proteins and attenuated hyperalgesia.
Our results further demonstrate that NMDA receptor activation itself is an important source of PN in this pathway since nitration of GLT-1 and GS was significantly attenuated by MK-801. Although NADPH oxidase is reportedly a primary source of O2•- released by activation of NMDA receptors [4], spinal inactivation of MnSOD, the enzyme that normally keeps [O2•-] under tight control [26], is also important. Indeed, the development of hyperalgesia was associated with nitration and enzymatic of MnSOD and this was blocked by MK-801. Enzymatic inactivation of MnSOD results from nitration of Tyr-34 by PN in a Mn-catalysed process [20]. This process favors the accumulation of PN which in turn, nitrates and alters additional proteins and receptors, thereby perpetuating and extending the initial damage [40]. In support, spinal inactivation of MnSOD has been linked to the development of central sensitization associated with intrathecal injection of NMDA, with inflammation and in the development of opiate-induced hypersensitivity and antinociceptive tolerance [1,33,34,38,47,48,59].
These results suggest that formation of PN in the spinal cord in response to NMDA receptor activation contributes to the development of central sensitization by nitrating key glial cells proteins known to regulate optimal glutamatergic homeostasis and thus optimal neurotransmission. Collectively these results support our general hypothesis that targeting PN should lead to development of novel analgesics for the management of acute and chronic pain.
Acknowledgments
Supported by 1 R01 DA024074-01A1 and 1 R21 DA023056-01A2 (D. Salvemini). We would like to thank Dr Andrew Lechner, Department of Pharmacological and Physiological Science, Saint Louis University School of Medicine for editorial input.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46(2):212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(4):265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- 4.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12(7):857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 6.Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- 7.Fraser CM, Sills GJ, Forrest G, Thompson GG, Brodie MJ. Effects of anti-epileptic drugs on glutamine synthetase activity in mouse brain. Br J Pharmacol. 1999;126(7):1634–1638. doi: 10.1038/sj.bjp.0702472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131(3):262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorg B, Wettstein M, Metzger S, Schliess F, Haussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41(5):1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 11.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4(5):421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AJ, Voaden MJ, Marshall J. Glutamate metabolism in the frog retina. Nature. 1974;252(5478):50–52. doi: 10.1038/252050a0. [DOI] [PubMed] [Google Scholar]
- 13.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548(13):167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett. 1992;148(12):1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- 15.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 16.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133(13):9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine RL. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983;258(19):11828–11833. [PubMed] [Google Scholar]
- 19.Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29(3):222–232. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366(1):82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 21.Mao J. NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Res Brain Res Rev. 1999;30(3):289–304. doi: 10.1016/s0165-0173(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 22.Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann N Y Acad Sci. 2001;933:175–184. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- 23.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62(3):259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 24.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22(18):8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96(14):7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCord JM, Fridovich I. Superoxide dismutase An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 27.McInnis J, Wang C, Anastasio N, Hultman M, Ye Y, Salvemini D, Johnson KM. The role of superoxide and nuclear factor-kappaB signaling in N-methyl-D-aspartate-induced necrosis and apoptosis. J Pharmacol Exp Ther. 2002;301(2):478–487. doi: 10.1124/jpet.301.2.478. [DOI] [PubMed] [Google Scholar]
- 28.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33(11):1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 29.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52(2):127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 30.Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19(21):9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368(6466):59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 32.Minana MD, Kosenko E, Marcaida G, Hermenegildo C, Montoliu C, Grisolia S, Felipo V. Modulation of glutamine synthesis in cultured astrocytes by nitric oxide. Cell Mol Neurobiol. 1997;17(4):433–445. doi: 10.1023/a:1026339428059. [DOI] [PubMed] [Google Scholar]
- 33.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117(11):3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111(12):96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Muscoli C, Visalli V, Colica C, Nistico R, Palma E, Costa N, Rotiroti D, Nistico G, Mollace V. The effect of inflammatory stimuli on NMDA-related activation of glutamine synthase in human cultured astroglial cells. Neurosci Lett. 2005;373(3):184–188. doi: 10.1016/j.neulet.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419(1):39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- 37.Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. Faseb J. 2008;22(9):3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- 38.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J Pharmacol Exp Ther. 2009;329(1):64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391(3):108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 40.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383(34):401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 41.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 42.Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11(4):204–214. [PubMed] [Google Scholar]
- 43.Salvemini D, Neumann W. Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management. Life Sci. 2009a doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Salvemini D, Neumann WL. Peroxynitrite: a strategic linchpin of opioid analgesic tolerance. Trends Pharmacol Sci. 2009b;30(4):194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Salvemini DaT, AA . In: Nitro-oxidative stress and pain. VaW Richardson AV, editor. Book Title∣, Vol. Volume∣. City∣: Publisher∣, Year∣. p.ˆpp. Pages∣. [Google Scholar]
- 46.Savolainen KM, Loikkanen J, Eerikainen S, Naarala J. Glutamate-stimulated ROS production in neuronal cultures: interactions with lead and the cholinergic system. Neurotoxicology. 1998;19(45):669–674. [PubMed] [Google Scholar]
- 47.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29(1):159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138(3):514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55(2):158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Smith CD, Carson M, van der Woerd M, Chen J, Ischiropoulos H, Beckman JS. Crystal structure of peroxynitrite-modified bovine Cu,Zn superoxide dismutase. Arch Biochem Biophys. 1992;299(2):350–355. doi: 10.1016/0003-9861(92)90286-6. [DOI] [PubMed] [Google Scholar]
- 51.Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41(23):123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 52.Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124(12):77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7(8):1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 54.Tang N, Ong WY, Yeo JF, Farooqui AA. Anti-allodynic effect of intracerebroventricularly administered antioxidant and free radical scavenger in a mouse model of orofacial pain. J Orofac Pain. 2009;23(2):167–173. [PubMed] [Google Scholar]
- 55.Tanigami H, Rebel A, Martin LJ, Chen TY, Brusilow SW, Traystman RJ, Koehler RC. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience. 2005;131(2):437–449. doi: 10.1016/j.neuroscience.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2(9):848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- 57.Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271(11):5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 58.Wang J, Cochran V, Abdi S, Chung JM, Chung K, Kim HK. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci Lett. 2008;439(2):216–219. doi: 10.1016/j.neulet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 60.Waniewski RA. Physiological levels of ammonia regulate glutamine synthesis from extracellular glutamate in astrocyte cultures. J Neurochem. 1992;58(1):167–174. doi: 10.1111/j.1471-4159.1992.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 61.Waniewski RA, Martin DL. Exogenous glutamate is metabolized to glutamine and exported by rat primary astrocyte cultures. J Neurochem. 1986;47(1):304–313. doi: 10.1111/j.1471-4159.1986.tb02863.x. [DOI] [PubMed] [Google Scholar]
- 62.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28(12):661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21(2):131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24(8):450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 65.Wen ZH, Wu GJ, Chang YC, Wang JJ, Wong CS. Dexamethasone modulates the development of morphine tolerance and expression of glutamate transporters in rats. Neuroscience. 2005;133(3):807–817. doi: 10.1016/j.neuroscience.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Yeo JF, Ling SF, Tang N, Ong WY. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Exp Brain Res. 2008;184(3):435–438. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]