Abstract
Objective
This study was undertaken to test injectible surgical sealants that are biocompatible with fetal membranes, eventually for closure of iatrogenic membrane defects.
Study Design
Dermabond, Histoacryl, Tissucol fibrin glue, and three types of in situ forming poly(ethylene glycol)-based polymer hydrogels were tested for acute toxicity upon direct contact with fetal membranes for 24h. For determination of elution toxicity, extracts of sealants were incubated on amnion cell cultures for 72h. Bonding and toxicity was assessed through morphological and/or biochemical analysis.
Results
Extracts of all adhesives were non-toxic for cultured cells. However, only Tissucol and one type of poly(ethylene glycol)-based hydrogel, mussel-mimetic tissue adhesive, showed efficient, non-disruptive, non-toxic bonding to fetal membranes. Mussel-mimetic tissue adhesive applied over membrane defects created with a 3.5 mm trocar accomplished leak-proof closure that withstood membrane stretch in an in vitro model.
Conclusion
A synthetic hydrogel-type tissue adhesive emerged as potential sealing modality for iatrogenic membrane defects that merits further evaluation in vivo.
Keywords: fetal membranes, fetoscopy, iatrogenic PPROM, membrane sealing, tissue adhesive, fetoscopic access, fibrin, DOPA
Introduction
Invasive diagnostic and therapeutic interventions with large diameter fetoscopes are frequently complicated by amniotic fluid leakage, separation of amnion and chorion, or even frank iatrogenic preterm premature rupture of the fetal membranes (iPPROM). For fetoscopic procedures, rates of iPPROM range between 6 to 45%,1 but in a trial of fetal endoscopic tracheal occlusion for severe congenital diaphragmatic hernia a 100% rate was reported.2 Since these procedures are usually performed in the second trimester of pregnancy, iPPROM usually occurs at an early gestational age. Hence, the associated morbidity and mortality may compromise the expected benefits of the intervention. iPPROM is therefore a potentially serious complication for prenatal fetal surgery. Clinically, measures of plugging membranes after established rupture as well as of preventive plugging of fetoscopic access sites have been undertaken, as reviewed previously.3,4 For closure after obvious iatrogenic rupture, intra-amniotic injection at the puncture site of maternal platelets mixed with fibrin cryoprecipitate ('amniopatch') has evolved as promising route to seal.5,6 However, the sudden activation of a large number of platelets in the amniopatch was accounted for otherwise unexplained fetal demise in some cases.5 But increasing efforts have been concentrated on taking prophylactic measures prior to rupture rather than therapy after established or symptomatic rupture of the membranes. Several preventive plugging methods using dry collagen and gelatin plugs or liquid blood-derived sealants have already been clinically investigated.7,8 Preliminary experience supports this prophylactic intervention for prevention of iPPROM. A 2006 report on a 27 patient cohort found a 4.2% rate of postoperative PPROM upon gelatin plug (Gelfoam) insertion upon port retrieval in endoscopic fetal surgery.7 In another small clinical study, sequential injection of platelets, fibrin glue and powdered collagen slurry directly to the puncture site successfully prevented amniotic fluid loss after endoscopic procedure.8 Still, the positive outcome with these methods await to be reproduced in other centers. Of note, collagen fleece plugs (Lyostypt) are now routinely used for prophylactic plugging of iatrogenic membrane defects following fetoscopic endoluminal tracheal occlusion for in utero therapy of congenital diaphragmatic hernia in one center of the authors, in Leuven. Other prophylactic plugging techniques such as scaffold-type plugs manufactured directly from decellularized amnion tissue have been so far only evaluated in animal models.9,10 Further, also laser welding, pre-emptive placement of synthetic surgical sealants before fetoscopic access, direct injection into amnotic fluid of fibrinogen/thrombin-based tissue sealant, and sealing with platelet-rich plasma were evaluated in laboratory settings.11,12,13,14 An emerging notion is that spontaneous healing appears slow, if not absent in human fetal membranes. Histological follow-up of fetoscopic puncture defects in membranes of human patients several months after the procedure showed that the defects did not close by growth of new tissue.15 The amnion layer contains few cells and does not contain blood vessels, which makes healing response in this layer unlikely. Trials in rabbits of prophylactic plugging of membrane defects with decellularized amnion scaffolds showed effective sealing without detectable signs of biological repair after a 1-week period,9 which is the maximum achievable in this experimental model. Recent studies in the midgestational rabbit observed signs of early healing of membrane defects upon addition of platelets or amniotic fluid cells to collagen plugs;16,17 it is unclear whether this effect could assume relevant degrees of healing long-term. The criteria for a prophylactic plug material may be to present an immediate, non-toxic and ideally, durable physical barrier to amniotic fluid, and not necessarily induction of biological healing. With this strategy in mind, we examined five liquid synthetic sealants, namely two types of cyanoacrylate glues and three poly (ethylene glycol-based) hydrogel-type polymers, for their principal aptitude for fetal membrane repair. Repair of defective tissue in moist/wet conditions or even underwater presents a particular challenge. In the present study, we addressed adhesion to moist, intact fetal membranes. Alkyl-cyanoacrylate glues were chosen on the basis of their well-known strong bonding to tissue, and their use as tissue adhesives in surgical and traumatic wound repair.18 Our choice of three synthetic poly (ethylene glycol) (PEG)-based hydrogel sealants, photopolymerizable gel, mussel-mimetic adhesive and commercial SprayGel was based on data showing their interfacial bonding to various tissues, and the possibility to deliver them in minimally invasive liquid form for gelation in situ.19–21 Two types of PEG-based hydrogels under present study, SprayGel and photopolymerized PEG, were already used clinically. SprayGel has been clinically used as bioabsorbable anti-adhesion barrier in patients undergoing myomectomy.22 A clinically approved formulation of photopolymerized PEG hydrogel sealant, FocalSeal-L sealant, proved successful for closure of pulmonary air leaks in the lung occuring at cardiac operations.23 For the experimental mussel-mimetic adhesive hydrogel formulation of the present study, no clinical data exist yet. Here we estimated applicability of these synthetic polymers as sealants on fetal membranes based on their bonding to fetal membranes and toxicity in vitro, using the biosurgical Tissucol fibrin glue sealant as internal reference.
Material and methods
Membrane collection and amnion cell isolation
The Ethical Committee of the District of Zurich approved the protocol (study Stv22/2006). A total of 15 fetal membranes were collected with written patient consent from elective caesarean sections. Mean gestational age was 38 ±1 weeks in the absence of labor, preterm rupture of membranes, chorioamnionitis, or chromosomal abnormalities. Fetal membrane pieces of 150–200 cm2 were collected. The fetal membanes were cut approximately 2 cm from the placental disc to avoid the 'zone of altered morphology' overlying the cervix that is considered to be a naturally predefined breaking site of the membranes.24 Human amnion epithelial (hAEC) and amnion mesenchymal cells (hAMC) were isolated and cultured as described previously.9
Sealants
Alkyl-cyanoacrylate glue sealants: Dermabond (Ethicon Inc., Norderstedt, Germany) and Histoacryl (B. Braun GmbH, Tuttlingen, Germany) are 2-octyl cyanoacrylate monomer and n-butyl-2-cyanoacrylate monomer, respectively. The formulations possess syrup-like viscosity. These glue act through anionic polymerization of hydroxyl groups from the minute amounts of moisture normally present on actual surfaces that are glued, including biological surfaces. Indeed, cyanoacrylate glues are known to be extremely adhesive to tissue.18 Water act as a catalyst to accelerate this polymerization. The polymerization oocurs within minutes after application to tissue. The resulting resin is water resistant. Both Dermabond and Histoacryl are marketed as topical skin adhesives to hold skin edges of wounds from surgical incisions. As specified by the manufacturer of Dermabond, it is not for application on wet wounds.
Hydrogel sealants
SprayGel (Confluent Surgical, Inc., Waltham, MA) is a sprayable anti-adhesion barrier polymer that consists of two synthetic liquid precursors that when mixed together, rapidly cross-link to form a solid absorbable hydrogel in situ. The first precursor is a modified polyethylene glycol (PEG) with terminal electrophilic esters groups while the other precursor solution contains PEG that has nucleophilic amine groups.25 SprayGel is marketed outside the US for use in abdominal and pelvic surgical procedures. It has been clinically also tried to reduce adhesion formation after ovarian surgery.21 SprayGel was deposited at the fetal membranes through the air pump-assisted SprayGel Laparoscopic Sprayer. The gel is formulated to remain adherent on the site of application for approximately five days whereafter it is absorbed by way of gradual hydrolysis.
Photopolymerized PEG hydrogel sealant (pPEG) was formed via in situ interfacial photopolymerization of PEG diacrylate precursor of average molecular weight 700 Da (Sigma) according to a previously described gelation protocol.20 Fetal membranes were flushed with a tissue adsorbing photoinitiator eosin Y (1mM in 10mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, pH 7.4, 0.15M sodium chloride; (HEPES-buffered saline). Then solution containing 10% PEG diacrylate and the co-catalysts triethanolamine (13.2 µL/mL) and 1-vinyl-2- pyrrolidine (3.5µL/mL) in HEPES-buffered saline was applied to the membranes and photopolymerized by irradiation at 480–520 nm and 75mW/cm2 for 1 min from a portable Cermax xenon fiber optic light source, CXE300 (ILC Technology Inc., USA).
The mussel-mimetic tissue sealant is a catechol-functionalized poly(ethylene glycol) (cPEG) whose molecules crosslink into a hydrogel by way of oxidation after addition of sodium periodate.26 The composition and synthesis of cPEG is described elsewhere.27 For gelation, equal volumes of the polymer precursor solution (300 mg/mL in phosphate-buffered saline (PBS)) and the cross-linking solution (12 mg/mL sodium periodate in water) were mixed using a dual syringe applicator device equipped with a blending connector with mixer (FibriJet; Micromedics, Inc., St. Paul, MN). Hydrogels prepared from cPEG polymer and its derivatives are expected to possess the ability to secure very strong adhesion to almost any surface, even under wet conditions. The presence of catechol in cPEG sealant was inspired by the wet adhesive properties conferred by the catechol side chain of 3,4-dihydroxyphenylalanine (DOPA) amino acid, which is found in high concentrations in the foot proteins of marine or freshwater mussels.28,29
Tissucol Duo S fibrin glue (Baxter AG, Volketwil, Switzerland) is a biological two-component adhesive that forms by mixing of human plasma cryoprecipitate solution with thrombin solution. The chemical and physical polymerization of the main component of fibrin glue sealant, fibrinogen, mimics the last step of the natural blood clot formation; Fibrin glue is clinically widely applied as hemostatic surgical sealant or adjunct to suture.30
Toxicity tests
Toxicity of sealants for fetal membrane cells was evaluated using direct contact and elution tests, as per International Organization for Standardization (ISO) 10993-5 guidelines.
Direct contact cytotoxicity
Direct contact studies were performed with term fetal membranes obtained from three cases. The amniotic layer was chosen for sealant application (Fig. 1A) because this layer was proposed to be the strength-bearing layer of fetal membranes and major determinant for PPROM.31 2×1cm patches of freshly harvested fetal membranes were placed into wells of 6-well plates with amnion layer up. The sealants were applied at 50µl and 200µl volumes except cPEG adhesive which was only tested at 200µl volume because of limited material. Membranes covered with sealant were covered with 3 mL culture medium (Ham’s F-12/DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin) and cultured for 24h at 37°C. Controls were untreated membranes that were immediately processed for histology (control '0') or cultured for 24h (control '24'). After 24h, the treated membranes were fixed in 4% formaline, embedded in paraffin and sectioned for histology. Deparaffinized sections were either stained with hematoxylin-eosin (H/E), or stained for apoptotic cells using TUNEL technology (Terminal deoxynucleotidyl transferase dUTP nick end labeling; In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics GmbH, Mannheim, Germany). For total cell counts, all cell nuclei were counterstained with 4',6-diamidin-2'-phenylindol-dihydrochlorid (DAPI; Sigma, Buchs, Switzerland). The histologic images were taken with a Zeiss Axiovert 200M fluorescent microscope (Carl Zeiss, Goettingen, Germany) equipped with an Zeiss AxioCam MRc digital camera and analysed with AxioVision Rel. 4.5 software (Carl Zeiss). Apoptotic and total cell counts were acquired from fluorescence micrographs using automated image analysis software ImageJ 1.34s (National Institute of Health, Bethesda, ML). One tissue section per case was analysed, taking four optical fields per section for analysis.
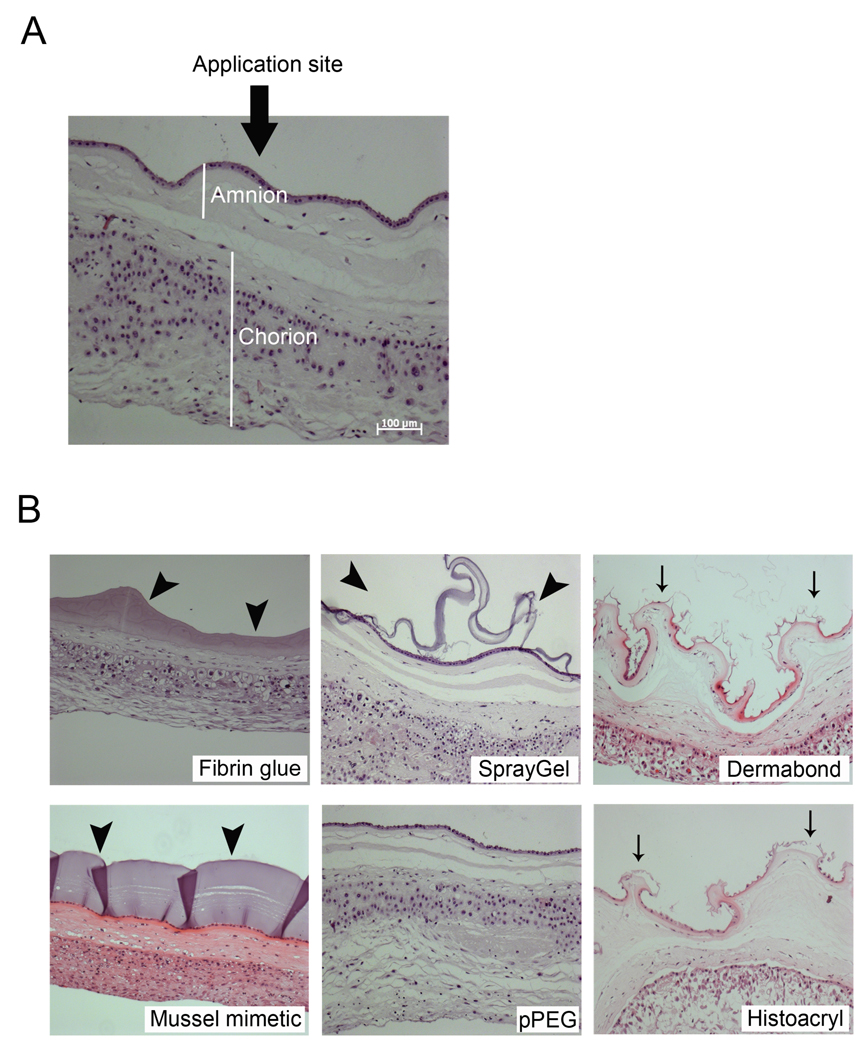
Figure 1.
Histologic assessment of bonding properties and effect for membrane morphology of bioadhesives. (A) Sealants were applied on the amniotic site of fetal membranes. (B) Images of hematoxylin/eosin-stained cross-sections of fetal membranes that were incubated with sealants for 24h. Fat arrows mark the hydrogels, thin arrows mark the damage to the amnion layer by Dermabond and Histoacryl. Bar size: 100 µm.
Elution toxicity
To test potential toxicity of soluble compounds released from the sealants for cultured amnion cells, two types of extractions were performed: First, extracts from sealant alone. For that, 0.2 mL of glue/hydrogel were incubated for 24h in 3 mL Ham's-F12/DMEM/FCS culture medium. Second, extracts from sealants applied to membranes. The second method was to resolve whether treatment of membranes could result in production of cytokines by hAECs and hAMCs that add to induction of apoptosis. For that 0.2 mL glue /hydrogel sealant were applied to 2×1 cm pieces of fetal membranes and incubated for 24h in 3 mL Ham's-F12/DMEM/FCS culture medium. The extracts were collected and stored at −80°C until use for culture. Amnion cells from four human cases were subjected for assay of toxicity, and for each sealant the extraction test was evaluated in triplicate. 2×104 hAECs or hAMSCs were seeded per well of 48 well plates and cultured in Ham's/F12/DMEM/FCs standard medium near to confluence. Then medium was removed, and cells overlaid with 0.4 mL of extracts from either sealant alone, or extract from membranes+sealant. Extracts from untreated membrane samples from the same patients in standard culture medium served as controls. The cells were cultured for 72h. Cell morphology was assessed microscopically, and degree of cell detachment and lysis was judged qualitatively. Following evaluations were performed: (i) For total cell count, hAECs and hAMSCs were stained with DAPI (ii) Apoptotic cells were detected with in situ Cell Death Detection Kit (Roche Diagnostics GmbH). Total cell counts and apoptotic cell counts were acquired from fluorescence fluorescence micrographs using automated image analysis software ImageJ 1.34s (National Institute of Health, Bethesda, ML). (iii) Areas of individual cells were measured using LeicaQ Win Image Analysis software (Leica Imaging System Ltd, Cambridge, UK). (iv) Live/dead cell staining was performed. For that, amnion cell cultures were incubated for 3 min with a mix of calcein to detect live cells and ethidiumbromide homodimer to detect dead cells at 1µM and 2µg/ml, respectively. All experiments were performed in triplicates and four optical fields were analysed for each sample.
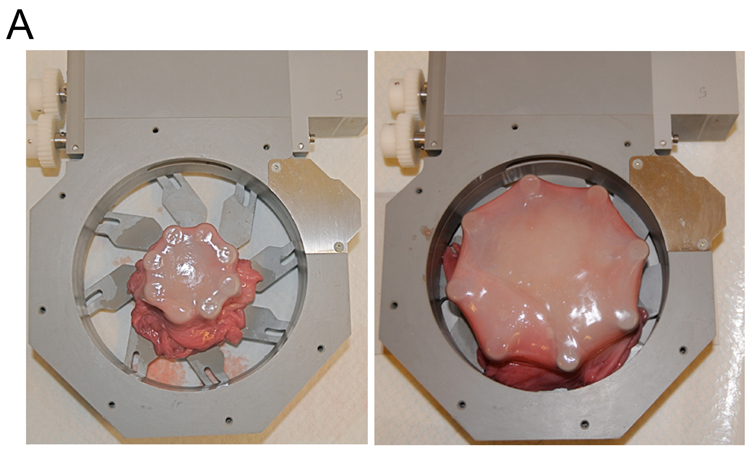
Sealing of fetal membranes lesion in vitro
Sealing performance of cPEG adhesive was tested on trocar punctures through fresh fetal membranes. For that, wet fetal membranes were flat-mounted with the amnion side up on a commercial motorized mechanical stretch device named 'The Cellerator' (Cytomec GmbH, Switzerland; http://www.cytomec.com/) that we further adapted for use in fetal membrane studies (Fig. 3A). The Cellerator device permits expansion of fetal membranes expansion in a quasi-isotropic fashion, with points of attachment distributed in a near-circular pattern around the mounted membrane. While mounted in this device, membranes were continually kept moist with PBS. Puncture lesions were created with a three-side pointed Ø 3.5mm trocar (Richard Wolf GmbH, Knittlingen, Germany), and approximately 0.5 mL cPEG adhesive was applied over the membrane defect. Two minutes after treatment, membranes were further stretched by about 30% of their original area. To demonstrate leak-proof sealing, the stretched membranes, still mounted in the device, were overlaid for 10 min with 0.3 L water. After the leak-proof test, the area of the treated membrane defect was excised and processed for standard histology. Histologic sealing was estimated microscopically from hematoxylin/eosin stained sections by the ability of the sealant to form a continuous bridge between the wound edges.
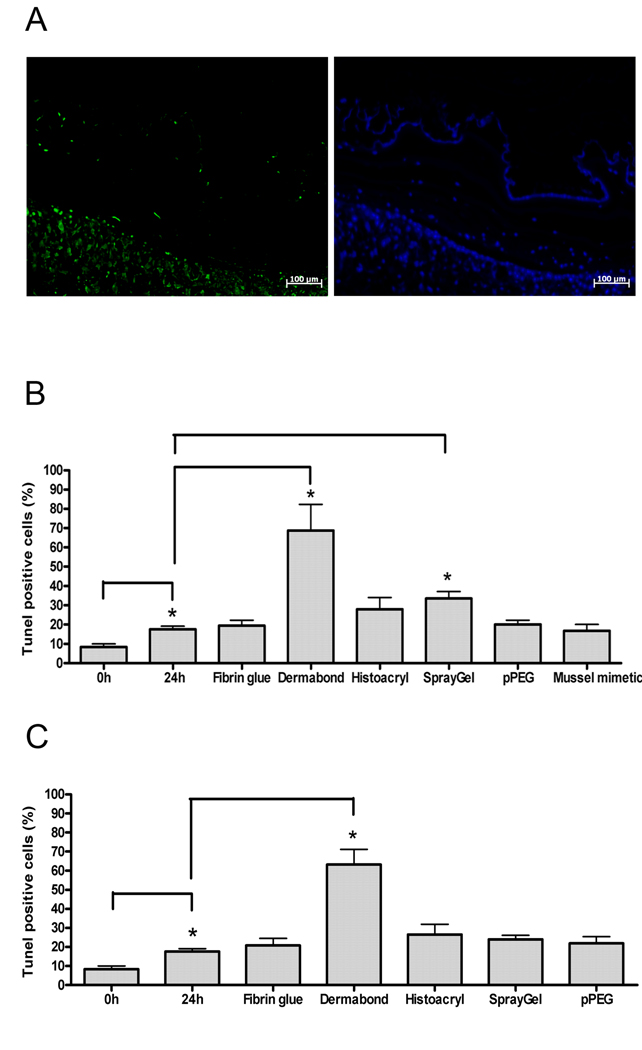
Figure 3.
Direct contact-mediated cytotoxic effect of sealants for fetal membranes. (A) Fluorescence micrographs of apoptotic cells (green) and total cells (DAPI). The example shows a section of a membrane treated with Dermabond. (B) Apoptosis rates in membranes treated with 200µl sealant volumes. (C) Apoptosis rates in membranes treated with 50µl sealant volumes. Values are mean ± SEM. * indicates p< 0.05.
Statistical analysis
Data are shown as mean ± SEM. Two-tailed unpaired t test was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA). Significance level was set at p< 0.05.
Results
Contact-mediated effect of sealants for membrane morphology
Histology in figure 1 illustrates the effect of treatment for overall membrane morphology for the six bioadhesives under test. Fibrin glue and cPEG adhesive formed a continuous layer tightly bound to tissue (Figure 1B, arrow heads). The normal membrane morphology appeared maintained, with the amnion epithelial layer intact. SprayGel and pPEG exhibited partial or no binding to tissue, respectively. In the case of pPEG, we found the hydrogel layer sloughed into the culture medium shortly after immersion of the membranes in culture medium. Binding of Dermabond and Histoacryl to fetal membranes resulted in disruption of the amnion layer and change of overall membrane morphology, which was more pronounced for Dermabond. The effects of 50µl treatment volumes were very similar (not shown).
Direct contact-induced apoptosis
We estimated acute toxic effects of sealants by measuring apoptosis in fetal membranes after 24h of direct contact with sealants. Fig. 2A depicts fluorescence micrographs of apoptotic cells (TUNEL) and all cell nuclei in tissue (DAPI) in fetal membranes treated with Dermabond. Fig. 2B gives the apoptosis rates for the 200µl test series. In untreated reference membranes, the apoptosis rate increased to 17±2% during the 24h incubation. Treatment with fibrin glue, cPEG adhesive, and pPEG did not enhance apoptosis over control. Dermabond and SprayGel treatment significantly (p< 0.05) enhanced apoptosis by 3.9-fold to 69±13 % and by 1.9-fold to 34±3% over the control, respectively. Histoacryl treatment produced a 1.6-fold increase of apoptosis rate to 28±6%, which was not significant over control. The outcome in the 50µl test series was similar, except that at lower dose, the apoptosis rate by SprayGel was not significantly over control (Fig. 2C).
Figure 2.
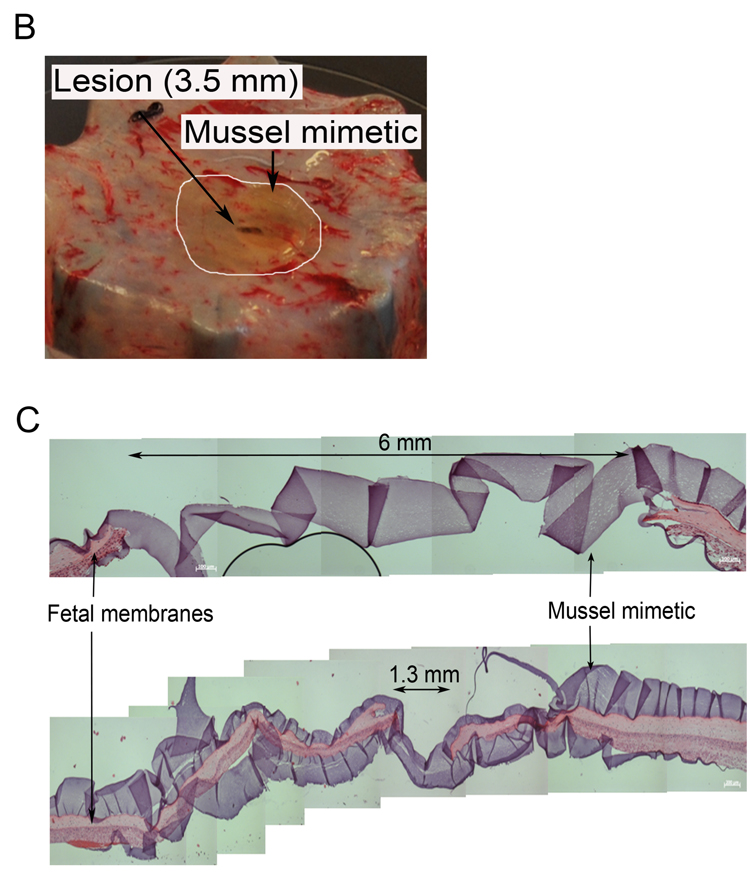
Ex vivo sealing of fetal membrane defects with mussel-mimetic adhesive. (A) Fetal membranes mounted in a computerized radial stretch device before and after stretch. (B) Through-thickness puncture wounds (arrow) were created on fresh fetal membranes with a Ø 3.5mm trocar. Approximately 0.5 mL of adhesive was applied over the defect. The white line marks the area of sealant. (C) Hematoxylin/eosin stained cross-section of a trocar puncture treated with cPEG adhesive. The hydrogel appears as ribbon-like structure that bridges the puncture edges. The bottom image shows a cross-section of the same lesion at a narrow location.
Elution toxicity of sealants for primary cultures of amnion cells
To test for toxicity from compounds released from the sealants, we investigated cell lysis, cell detachment and change of cell shape in hAECs and hAMCs that were grown in extracts of sealants in culture medium. None of the cultures, except those grown in extracts of Dermabond, appeared affected by toxic compounds after 24h and 72h. There was no difference between extracts prepared from sealant alone, or from sealants applied to membranes. hAMSC were not affected in any condition as estimated by cell size and cell number. Only in the condition of hAECs grown in Dermabond, we observed modest, insignificant reduction of cell size and number. Cell size of hAECs cultured in Dermabond extracts were 1138±166 µm2 (n=193 cells) versus 1423±196 µm2 (n=168 cells); cell numbers in the Dermabond condition were lower (323±31 cells/optical field) compared to control cultures (424±47 cells/optical field). TUNEL staining of hAEC and hAMC cultures did not show any induction of apoptosis, and live/dead staining with calcein and ethidium bromide showed that practically all cells in culture were alive. Overall, extracts of sealants behaved non-toxic for amnion primary cultures.
Sealing of puncture lesions in vitro
Our stratification revealed that cPEG adhesive and Tissucol fibrin glue both show strong bonding to fetal membranes and behave non-toxic, which are two basic prerequisites for prospective application for repair. cPEG adhesive is a new formulation that has never been tested for sealing of membrane defects before. We tested 0.5 mL cPEG adhesive for closure of Ø 3.5mm trocar puncture wounds in fetal membranes mounted in a biomechanical test device (Fig. 3). Successful closure was achieved in all three test cases. Application of cPEG tissue adhesive over the defect resulted in an immediate leak-proof membrane seal that remained functional upon further radial stretch of the membranes. Fig. 3C shows representative histologic images from two locations of a puncture lesion sealed with cPEG tissue adhesive. Histology confirmed that the cPEG tisssue adhesive connected the wound edges over a distance of approximately 6 mm, which was the maximum diameter in such lesions. cPEG tissue adhesive was found adhered to both the amnion side, the application side in these experiments, and also to the chorionic side of the membranes, Thus, sealant apparently passed through the lesion and spread underneath the membranes.
Comment
Three commercial and two experimental synthetic sealants were tested along with Tissucol fibrin glue for applicability on fresh, moist human fetal membranes, using interfacial bonding and cytotoxicity after a 24h direct contact duration in vitro for performance assessment. Four of the five synthetic sealants failed to meet the combined requirements of membrane bonding and non-toxicity which excludes them for this type of repair. Our screen identifies one synthetic hydrogel, cPEG tissue adhesive, that exhibits bonding to membranes and non-toxic characteristics that favorably compare to fibrin glue. cPEG adhesive demonstrated repair capacity for 3.5 mm trocar punctures, accomplishing immediate leak-proof sealing, which may warrant further evaluation in vivo.
Membrane bonding properties of the six bioadhesives under this study demonstrated large variability. Cyanoacrylate-based glues seem inappropriate for application on fetal membranes. The observation of their strong bonding to fetal membrane tissue was accompanied by obvious damage to the amnion epithelial layer and disruption of membrane structure, especially the amnion layer. Amniotic integrity is considered more important than chorionic integrity because the amnion is thought to have greater tensile strength.31 In addition, Dermabond, but not Histoacryl, exhibited significant cytotoxicity. Two of the PEG-based hydrogel polymers, photopolymerizable PEG hydrogel and SprayGel, failed to bond to fetal membranes sufficiently. Photopolymerized PEG-diacrylate hydrogels were previously used to create thin intravascular barriers to block thrombus deposition after balloon-induced arterial injuries in animal models, and firm adhesion of the PEG-diacrylate hydrogel to arterial walls was reported.20,32 Although the pPEG and SprayGel hydrogels could be polymerized on fetal membranes, they sloughed off from the membranes quickly after the immersion of the membranes in culture medium. Neither the hydrogel itself nor the one minute laser irradiation required for hydrogel curing produced adverse effects for membrane integrity. In the case of SprayGel, the resulting polymer layer was discontinuous, weakly bonded to membranes, and cytotoxic.
cPEG adhesive, on the other hand, displayed membrane bonding and compatibility comparable to that of Tissucol fibrin glue. cPEG adhesive is a two-component, self-crosslinking polymer with the remarkable property to form strong and durable bonds to many surfaces even in wet environment. Creation of cPEG adhesive has been inspired by the composition of liquid adhesives secreted by marine mussels, which allow these organisms to firmly anchor themselves to any surface. The wet adherence of native mussel adhesive proteins rests on the unusual amino acid residue 3,4 dihydroxyphenylalanine (DOPA) that is present in high concentrations in the foot proteins of mussels.28,33,34 Work in the group of one of the authors (P.B.M.) and other laboratories has demonstrated that the wet adherence ability of mussel foot proteins can be conferred onto synthetic polymers by way of incorporating DOPA and DOPA analogues.19,26,35,36 Indeed, previous work has demonstrated that DOPA-functionalized PEG precursors cross-link via sodium periodate-mediated oxidation to form adhesive hydrogels with high rigidity.26 In the present study, the cPEG polymer contains a simplified mimic of DOPA in the form of a reactive catechol group, generating a new variation of the adhesive that can be used under the same preparative conditions.27 This formulation possesses both appealing and potentially problematic characteristics for use as fetal membrane sealant. Properties that we consider favorable are fast gelation (under a minute); very slow hydrolysis over several months, allowing for durable sealing; and excellent tissue adhesion. Recent analysis revealed that bonds formed by a mussel-mimetic adhesive between porcine dermal tissues were several times stronger than those formed by fibrin glue.19 Possible disadvantages of the present formulation are the use of the strong oxidizing reagent sodium periodate as trigger of polymerization, which is known to be strongly irritating. However, a rapid chemical reaction ensues upon contact of periodate with pPEG, which ultimately gives rise to chemical crosslinking of the polymer but also results in reduction of periodate to less harmful oxidative species.19 Our estimates of direct contact cytoxicity were obtained after 24h, which is the minimal contact duration for assessment according to the ISO 10993-5 test guidelines. Additional studies with extended contact between fetal membranes and cPEG sealant in organ culture in vitro37 and in animal models will be necessary to establish long-term safety for membrane sealing. Issues of in vivo irritation and inflammatory tissue response to cPEG adhesive are currently addressed in another study involving the use of cPEG for transplantation of mouse islets.27 While the results of that study in mice, like the in vitro data reported herein, reveal favorable biocompatibility, ultimately the full effects of cPEG on uterine contractions and fetal survival and integrity will require further evaluation in the pregnant rabbit model. Of further note, the present study was performed with membranes from the third trimester of pregnancy, while operative fetoscopy is usually performed in the second trimester. Membranes of earlier gestation could exhibit different reactivity to sealants and further testing on such membranes will be necessary.
In summary, this study points to a new synthetic hydrogel formulation, mussel-mimetic sealant, and the biological Tissucol fibrin glue with appealing properties for membrane sealing. Still, we are aware that such data in vitro are limited in their ability to predict success in the in vivo situation.
Acknowledgements
We thank Esther Kleiner for assistance with histology.
This work was supported by the Swiss National Science Foundation grant no. 31000-108270; by the European Commission in its 6th Framework Programme ('EuroSTEC' (European program for soft tissue engineering for children (http://eurostec.tv.nl); LIFESCIHEALTH-2006-37409); and the Zurich Centre for Integrative Human Physiology. Portions of this work were supported by National Institues of Health (NIH, USA) grant DE014193 to P.B.M. C.B. was supported by a NIH Regenerative Medicine training grant (5 T90 DA022881)
Footnotes
Presented at the 27th Annual International Fetal Medicine Surgery Society (IFMSS) meeting, Athenia Rivera, Greece, September 12 –16, 2008.
Conflict of interest:
One of the authors of this paper, P.B. Messersmith, holds equity in Nerites Corporation, a company that develops surgical sealants and adhesives.
One test device in this study, the Cellerator, is marketed by Cytomec GmbH in which one of the authors (T.M. Quinn) holds equity.
References
- 1.Deprest J, Lewi L, Devlieger R, et al. Enrichment of collagen plugs with platelets and amniotic fluid cells increases cell proliferation in sealed iatrogenic membrane defects in the fetal rabbit model. Prenat Diagn. 2008;28:878–880. doi: 10.1002/pd.2010. [DOI] [PubMed] [Google Scholar]
- 2.Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- 3.Devlieger R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: A review of current evidence. Am J Obstet Gynecol. 2006;195:1512–1520. doi: 10.1016/j.ajog.2006.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zisch A, Zimmermann R. Bioengineering of foetal membrane repair. Swiss Med Wkly. 2008;138:596–601. doi: 10.4414/smw.2008.11997. [DOI] [PubMed] [Google Scholar]
- 5.Quintero RA. Treatment of previable premature ruptured membranes. Clin Perinatol. 2003;30:573–589. doi: 10.1016/s0095-5108(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 6.Quintero RA. New horizons in the treatment of preterm premature rupture of membranes. Clin Perinatol. 2001;28:861–875. doi: 10.1016/s0095-5108(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Tracy TF, Jr, Carr SR, Sorrells DL, Jr, Luks FI. Port insertion and removal techniques to minimize premature rupture of the membranes in endoscopic fetal surgery. J Pediatr Surg. 2006;41:905–909. doi: 10.1016/j.jpedsurg.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Young BK, Roman AS, MacKenzie AP, et al. The closure of iatrogenic membrane defects after amniocentesis and endoscopic intrauterine procedures. Fetal Diagn Ther. 2004;19:296–300. doi: 10.1159/000076715. [DOI] [PubMed] [Google Scholar]
- 9.Mallik AS, Fichter MA, Rieder S, et al. Fetoscopic closure of punctured fetal membranes with acellular human amnion plugs in a rabbit model. Obstet Gynecol. 2007;110:1121–1129. doi: 10.1097/01.AOG.0000284624.23598.7c. [DOI] [PubMed] [Google Scholar]
- 10.Ochsenbein-Kolble N, Jani J, Lewi L, et al. Enhancing sealing of fetal membrane defects using tissue engineered native amniotic scaffolds in the rabbit model. Am J Obstet Gynecol. 2007;196:263, e1–e7. doi: 10.1016/j.ajog.2006.10.904. [DOI] [PubMed] [Google Scholar]
- 11.Petratos PB, Baergen RN, Bleustein CB, Felsen D, Poppas DP. Ex vivo evaluation of human fetal membrane closure. Lasers Surg Med. 2002;30:48–53. doi: 10.1002/lsm.1140. [DOI] [PubMed] [Google Scholar]
- 12.Cortes RA, Wagner AJ, Lee H, et al. Pre-emptive placement of a presealant for amniotic access. Am J Obstet Gynecol. 2005;193:1197–1203. doi: 10.1016/j.ajog.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 13.Reddy UM, Shah SS, Nemiroff RL, et al. In vitro sealing of punctured fetal membranes: potential treatment for midtrimester premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1090–1093. doi: 10.1067/mob.2001.117685. [DOI] [PubMed] [Google Scholar]
- 14.Louis-Sylvestre C, Rand JH, Gordon RE, Salafia CM, Berkowitz RL. In vitro studies of the interactions between platelets and amniotic membranes: a potential treatment for preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;178:287–293. doi: 10.1016/s0002-9378(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Gratacos E, Sanin-Blair J, Lewi L, et al. A histological study of fetoscopic membrane defects to document membrane healing. Placenta. 2006;27:452–456. doi: 10.1016/j.placenta.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Liekens D, Lewi L, Jani J, et al. Enrichment of collagen plugs with platelets and amniotic fluid cells increases cell proliferation in sealed iatrogenic membrane defects in the foetal rabbit model. Prenat Diagn. 2008;28:503–507. doi: 10.1002/pd.2010. [DOI] [PubMed] [Google Scholar]
- 17.Papadopulos NA, Klotz S, Raith A, et al. Amnion cells engineering: a new perspective in fetal membrane healing after intrauterine surgery? Fetal Diagn Ther. 2006;21:494–500. doi: 10.1159/000095660. [DOI] [PubMed] [Google Scholar]
- 18.Leggat PA, Smith DR, Kedjarune U. Surgical applications of cyanoacrylate adhesives: a review of toxicity. ANZ J Surg. 2007;77:209–213. doi: 10.1111/j.1445-2197.2007.04020.x. [DOI] [PubMed] [Google Scholar]
- 19.Burke SA, Ritter-Jones M, Lee BP, Messersmith PB. Thermal gelation and tissue adhesion of biomimetic hydrogels. Biomed Mater. 2007;2:203–210. doi: 10.1088/1748-6041/2/4/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West JL, Hubbell JA. Separation of the arterial wall from blood contact using hydrogel barriers reduces intimal thickening after balloon injury in the rat: the roles of medial and luminal factors in arterial healing. Proc Natl Acad Sci U S A. 1996;93:13188–13193. doi: 10.1073/pnas.93.23.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns DA, Ferland R, Dunn R. Initial feasibility study of a sprayable hydrogel adhesion barrier system in patients undergoing laparoscopic ovarian surgery. J Am Assoc Gynecol Laparosc. 2003;10:334–338. doi: 10.1016/s1074-3804(05)60257-5. [DOI] [PubMed] [Google Scholar]
- 22.Mettler L, Audebert A, Lehmann-Willenbrock E, Schive-Peterhansl K, Jacobs VR. A randomized, prospective, controlled, multicenter clinical trial of a sprayable, sitespecific adhesion barrier system in patients undergoing myomectomy. Fertil Steril. 2004;82:398–404. doi: 10.1016/j.fertnstert.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 23.Gillinov AM, Lytle BW. A novel synthetic sealant to treat air leaks at cardiac reoperation. J Card Surg. 2001;16:255–257. doi: 10.1111/j.1540-8191.2001.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 24.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–241. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 25.Ferland R, Mulani D, Campbell PK. Evaluation of a sprayable polyethylene glycol adhesion barrier in a porcine efficacy model. Hum Reprod. 2001;16:2718–2723. doi: 10.1093/humrep/16.12.2718. [DOI] [PubMed] [Google Scholar]
- 26.Lee BP, Dalsin JL, Messersmith PB. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules. 2002;3:1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 27.Brubaker CE, Kissler H, Wang L, Kaufman DB, Messersmith PB. Biocompatibility of mussel-inspired adhesive in murine extrahepatic beta-islet transplantation. doi: 10.1016/j.biomaterials.2009.09.062. To be submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Lee BP, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448:338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 29.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MG, Jones D. Applications of fibrin sealant in surgery. Surg Innov. 2005;12:203–213. doi: 10.1177/155335060501200304. [DOI] [PubMed] [Google Scholar]
- 31.Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195:510–515. doi: 10.1016/j.ajog.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Hill-West JL, Chowdhury SM, Slepian MJ, Hubbell JA. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc Natl Acad Sci U S A. 1994;91:5967–5971. doi: 10.1073/pnas.91.13.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waite JH. Reverse engineering of bioadhesion in marine mussels. Ann N Y Acad Sci. 1999;875:301–309. doi: 10.1111/j.1749-6632.1999.tb08513.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamada K, Chen T, Kumar G, et al. Chitosan based water-resistant adhesive. Analogy to mussel glue. Biomacromolecules. 2000;1:252–258. doi: 10.1021/bm0003009. [DOI] [PubMed] [Google Scholar]
- 36.Deming TJ. Mussel byssus and biomolecular materials. Curr Opin Chem Biol. 1999;3:100–105. doi: 10.1016/s1367-5931(99)80018-0. [DOI] [PubMed] [Google Scholar]
- 37.Devlieger R, Gratacos E, Wu J, et al. An organ-culture for in vitro evaluation of fetal membrane healing capacity. Eur J Obstet Gynecol Reprod Biol. 2000;92:145–150. doi: 10.1016/s0301-2115(00)00439-5. [DOI] [PubMed] [Google Scholar]