Abstract
The Gram-negative type II secretion (T2S) system is a multiprotein complex mediating the release of virulence factors from a number of pathogens. While an understanding of the function of T2S components is emerging, little is known about what identifies substrates for export. To investigate T2S substrate recognition, we compared mutations affecting the secretion of two highly homologous substrates: heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli (ETEC) and cholera toxin (CT) from Vibrio cholerae. Each toxin consists of one enzymatic A subunit and a ring of five B subunits mediating the toxin's secretion. Here, we report two mutations in LT's B subunit (LTB) that reduce its secretion from ETEC without global effects on the toxin. The Q3K mutation reduced levels of secreted LT by half, and as with CT (T. D. Connell, D. J. Metzger, M. Wang, M. G. Jobling, and R. K. Holmes, Infect. Immun. 63:4091-4098, 1995), the E11K mutation impaired LT secretion. Results in vitro and in vivo show that these mutants are not degraded more readily than wild-type LT. The Q3K mutation did not significantly affect CT B subunit (CTB) secretion from V. cholerae, and the E11A mutation altered LT and CTB secretion to various extents, indicating that these toxins are identified as secretion substrates in different ways. The levels of mutant LTB expressed in V. cholerae were low or undetectable, but each CTB mutant expressed and secreted at wild-type levels in ETEC. Therefore, ETEC's T2S system seems to accommodate mutations in CTB that impair the secretion of LTB. Our results highlight the exquisitely fine-tuned relationship between T2S substrates and their coordinate secretion machineries in different bacterial species.
Gram-negative bacteria have evolved a number of methods to secrete proteins into the extracellular milieu, with at least six specific secretion systems currently described (14, 30). Type II secretion (T2S), or the main terminal branch of the general secretory pathway, is a feature of a number of proteobacteria and has been shown to be required for pathogenesis and maintenance of environmental niches in a large number of species (5). The T2S system is a multiprotein complex of 12 to 15 components that spans the inner and outer membranes, allowing for the controlled release of certain folded proteins that have been directed to the periplasm through the Sec or Tat machinery (21). Aside from providing a means of exporting freely released virulence factors from plant, animal, and human pathogens (5), the T2S system has been shown to export surface-associated virulence factors (18), fimbrial components (46), outer membrane cytochromes (36), and a surfactant required for sliding motility in Legionella pneumophila (39), among other substrates.
While an increasing number of studies have focused on understanding the structure and function of the components of the T2S system itself, little is known about what identifies a periplasmic protein as a substrate for secretion (21, 32). Because proteins secreted from the same bacterial species need not share any obvious structural homology, it is not even clear how much of a T2S substrate interacts with the secretion machinery (32). Analysis of two similar substrates that can each be secreted by the T2S systems of two distinct species would provide information about species-specific identification of T2S substrates and, by extension, the nature of the “secretion motif” identifying those substrates. Heat-labile enterotoxin (LT) from enterotoxigenic Escherichia coli (ETEC) and cholera toxin (CT) from Vibrio cholerae represent one such pair of substrates.
ETEC and V. cholerae are enteric pathogens causing significant morbidity and mortality worldwide (33). The causative agents of traveler's diarrhea and cholera, respectively, these two pathogens share a number of similarities, including the nature of their disease symptoms (38). Each pathogen secretes an AB5 toxin important for colonization and the induction of water and electrolyte efflux from intestinal epithelial cells (1, 29). These toxins, LT and CT, are both encoded by two-gene operons. After sec-dependent transport to the periplasm, holotoxin formation occurs spontaneously (13), with one catalytic A subunit (LTA or CTA) assembling with five B subunits (LTB or CTB), which are responsible for the binding properties of the toxins. Export of fully folded and assembled LT or CT is then accomplished by the T2S system (34, 40). In ETEC, this system is encoded by gspC to -M (40), while in V. cholerae, these genes are found in the eps operon (34).
LT and CT are very similar in structure, sharing approximately 80% sequence homology and 83% identity in the mature B subunit (16, 24). ETEC is thought to have acquired the genes for CT through horizontal transfer, with the toxins evolving over time to possess slight differences (45). As such, these toxins share the same primary host receptor, the monosialoganglioside GM1, and catalyze the same ADP-ribosylation reaction within host cells (38). However, LT is able to bind other host sphingolipids in addition to GM1 and to interact with sugar residues from the A-type blood antigen, which CT cannot bind (16, 41). Both LT and CT are able to associate with sugar residues in lipopolysaccharide (LPS) on the surface of E. coli cells (17). Binding to each of these substrates can be impaired by point mutation (26, 43).
In this study, we report point mutations impairing the release of LT from ETEC and CT from V. cholerae. We analyzed the specificity of the defects in substrate recognition by comparing the effects of substituting charged and neutral residues in key regions of LTB and CTB. To confirm that the identified mutations resulted specifically in a secretion defect, we tested the effect of the mutations on (i) ligand binding by each toxin, (ii) toxin stability, and (iii) formation of secretion-competent B-subunit pentamers. By introducing comparable mutations into both toxins, including one previously reported to impair the secretion of CT (6), and exchanging toxin substrates between the two species, we have revealed species-dependent differences in T2S substrate recognition. Although wild-type LT and CT can be heterologously expressed and secreted from V. cholerae and ETEC, respectively, the substrate residues identified by the secretion machinery in each species are distinct. Together, our results demonstrate that highly homologous T2S substrates are recognized in different ways when secreted by two distinct systems.
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. Strains were grown at 37°C in LB or CFA (1% Casamino Acids, 0.15% yeast extract, 0.005% MgSO4, 0.005% MnCl2) and maintained on LB plates (LB with 15 g/liter agar; Genesee Scientific). When appropriate, antibiotics (Sigma) were added at the following concentrations: 100 μg/ml ampicillin, 25 μg/ml chloramphenicol, and 50 μg/ml kanamycin. E. coli transformations were carried out using a CaCl2 protocol as described previously (18). Plasmids were conjugated into V. cholerae by triparental mating as described previously (12), using DH5α (Invitrogen) containing the appropriate plasmid as the donor strain and MT616 carrying pRK2013 as the helper strain. Selection of transconjugants was carried out on LB agar plates supplemented with kanamycin and ampicillin. Similar levels of expression of wild-type and mutant LT, LTB, or CTB constructs were detected after induction with a final concentration of 200 μM isopropyl-1-thio-β-d-galactopyranoside (IPTG; Sigma), except for the CTB[Q3K] construct, which required induction with 50 μM IPTG. Y1 adrenal cells (ATCC CCL-79) were maintained in Kaighn's medium supplemented with 10% fetal calf serum (Sigma). Unless specified, reagents were purchased from VWR.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype | Relevant characteristics | Reference or source |
|---|---|---|---|
| Strains | |||
| MK233 | MT616/pRK2013 | Helper strain for triparental mating; Cmr | 9 |
| jf570 | H10407 ΔeltA | ETEC strain with polar insertion in eltA (LT deficient) | 8 |
| MK1052 | H10407 ΔeltA/pILT | jf570 carrying inducible LT plasmid; Ampr | 26 |
| MK1053 | H10407 ΔeltA/pTrc99A | jf570 carrying empty vector; Ampr | This work |
| MK1202 | H10407 ΔeltA/pILT[L25E] | As MK1052, with inducible L25E LT mutant; Ampr | This work |
| MK1201 | H10407 ΔeltA/pILT[Q3K] | As MK1202, with LT[Q3K]; Ampr | 26 |
| MK1239 | H10407 ΔeltA/pILT[Q3A] | As MK1201, with LT[Q3A]; Ampr | This work |
| MK1231 | H10407 ΔeltA/pILT[E11K] | As MK1201, with LT[E11K]; Ampr | This work |
| MK1240 | H10407 ΔeltA/pILT[E11A] | As MK1201, with LT[E11A]; Ampr | This work |
| MK741 | DH5α degP::Tn5/pDsbA/pILT | E. coli K-12 degP knockout, carrying a plasmid copy of dsbA and an inducible LT plasmid; Kanr Cmr Ampr | 26 |
| MK1101 | DH5α degP::Tn5/pDsbA/pILTB | As MK741, with pILTB; Kanr Cmr Ampr | This work |
| P4 | P27459 ΔctxAB | CT-deficient El Tor V. cholerae strain; Strr Kanr | 10, 11 |
| MK1123 | P4/pTrc99A | P4 carrying empty vector; Strr Kanr Ampr | This work |
| MK1225 | P4/pICTB | P4 carrying inducible CTB plasmid; Strr Kanr Ampr | This work |
| MK1236 | P4/pICTB[Q3K] | As MK1225, with inducible Q3K CTB mutant; Strr Kanr Ampr | This work |
| MK1232 | P4/pICTB[E11K] | As MK1225, with CTB[E11K]; Strr Kanr Ampr | This work |
| MK1233 | P4/pICTB[E11A] | As MK1225, with CTB[E11A]; Strr Kanr Ampr | This work |
| MK1242 | DH5α degP::Tn5/pDsbA/pICTB | As MK741, with pICTB; Kanr Cmr Ampr | This work |
| MK1223 | H10407 ΔeltA/pICTB | jf570 with inducible CTB plasmid; Ampr | This work |
| MK1224 | H10407 ΔeltA/pICTB[Q3K] | As MK1223, with inducible Q3K CTB mutant; Ampr | This work |
| MK1234 | H10407 ΔeltA/pICTB[E11K] | As MK1224, with CTB[E11K]; Ampr | This work |
| MK1235 | H10407 ΔeltA/pICTB[E11A] | As MK1224, with CTB[E11A]; Ampr | This work |
| MK1122 | P4/pILTB | P4 carrying inducible LTB plasmid; Strr Kanr Ampr | This work |
| MK1237 | P4/pILTB[Q3K] | As MK1122, with inducible Q3K LTB mutant; Strr Kanr Ampr | This work |
| MK1243 | P4/pILTB[Q3A] | As MK1122, with LTB[Q3A]; Strr Kanr Ampr | This work |
| MK1244 | P4/pILTB[E11K] | As MK1122, with LTB[E11K]; Strr Kanr Ampr | This work |
| MK1238 | P4/pILTB[L25E] | As MK1122, with LTB[L25E]; Strr Kanr Ampr | This work |
| Plasmids | |||
| pILT | IPTG-inducible LT holotoxin; Ampr | 26 | |
| pILTB | IPTG-inducible LTB pentamer; Ampr | This work | |
| pICTB | IPTG-inducible CTB pentamer; Ampr | This work |
Plasmid construction.
An IPTG-inducible LTB plasmid (pILTB) was generated by cloning the coding sequence of eltB from pILT (26). The gene was amplified by PCR using primers eltb-bamh1 and eltABreverse, which include restriction sites for BamHI and PstI, respectively (Table 2). PCR products were digested with BamHI and PstI (New England Biolabs) and then ligated into a similarly digested pTrc99A vector to form pILTB.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′→3′)a |
|---|---|
| eltb-bamh1 | CGGGATCCCGGAATTCGGGATGAATTATGAATAAAG |
| eltABreverse | AACTGCAGAACCAATGCATTGGCTAGTTTCCATACTGATTG |
| ltbL25Esense | CGATAAATGACAAGATAGAATCATATACGGAATCG |
| ltbL25Eantisense | CGATTCCGTATATGATTCTATCTTGTCATTTATCG |
| ltbQ3Ksense | CACACGGAGCTCCTAAATCTATTACAGAACTATG |
| ltbQ3Kantisense | CATAGTTCTGTAATAGATTTAGGAGCTCCGTGTG |
| ltbQ3Asense | CACACGGAGCTCCTGCATCTATTACAGAACTATG |
| ltbQ3Aantisense | CATAGTTCTGTAATAGATGCAGGAGCTCCGTGTG |
| ltbE11Ksense | CAGAACTATGTTCGAAATATCACAACACAC |
| ltbE11Kantisense | GTGTGTTGTGATATTTCGAACATAGTTCTG |
| ltbE11Asense | CAGAACTATGTTCGGCATATCACAACACAC |
| ltbE11Aantisense | GTGTGTTGTGATATGCCGAACATAGTTCTG |
| CTB_Q3K_sense | GGCGCATGCAACCCCTAAAAATATTACTGATTTG |
| CTB_Q3K_antisense | CAAATCAGTAATATTTTTAGGGGTTGCATGCGCC |
| CTBE11Ksense | CTGATTTGTGTGCAAAATACCACAACACAC |
| CTBE11Kantisense | GTGTGTTGTGGTATTTTGCACACAATCAG |
| CTB_E11A_sense | CTGATTTGTGTGCAGCATACCACAACACAC |
| CTB_E11A_anti | GTGTGTTGTGGTATGCTGCACACAAATCAG |
Restriction sites are italic; mutagenic codons are bold.
An IPTG-inducible CTB plasmid based on the pTrc99A vector (pICTB) was generated by cloning the ctxB gene from pLMP1 (20). After restriction with SacI and KpnI (New England Biolabs), the digested DNA fragment containing ctxB was gel purified using the QIAquick gel extraction kit (Qiagen) and ligated into similarly digested pTrc99A (GE Healthcare) to form pICTB.
Mutagenesis.
Site-directed mutagenesis was performed on pILT, pILTB, and pICTB using the QuikChange kit (Qiagen), according to the manufacturer's instructions. Primers used are listed in Table 2. All mutations were verified by sequencing at the Duke University DNA Analysis Facility or Eton Biosciences (Durham, NC) (data not shown). Mutants are named by combining the original amino acid residue, its position in the primary sequence of LTB or CTB after cleavage of the signal sequence, and the new substituted amino acid.
Cell fractionation.
Strains were diluted 1:50 from overnight cultures, grown for 3 to 4 h at 37°C to an optical density at 600 nm (OD600) of ∼0.8, and induced 3 h (for CTB constructs) or overnight (for LT or LTB constructs). For some experiments, one Mini Complete protease inhibitor pellet (Roche) was dissolved per 10 ml of culture at the time of induction. Following induction and growth, a sample of total culture was precipitated with 20% trichloroacetic acid (TCA). Each strain was serially plated for CFU, and the remaining cells were pelleted at 10,000 × g for 1 min. Supernatant was collected, and periplasm was isolated by polymyxin B treatment essentially as described previously (19). Both supernatant and periplasmic fractions were passed through Durapore microcentrifuge filter devices (Millipore; 0.45 μm for ETEC fractions, 0.22 μm for V. cholerae fractions). For some experiments, a sample of cell-free supernatant was also precipitated with 20% TCA. Periplasmic alkaline phosphatase activity was measured using the SensoLyte kit (AnaSpec) according to the manufacturer's instructions.
Immunoblotting.
To assess the expression of wild-type and mutant constructs, CFU-matched total culture samples were TCA precipitated, boiled in sample buffer, applied to 15% SDS-polyacrylamide gels, and immunoblotted with anti-CT antibody (1:15,000; Sigma), which is cross-reactive with LT, as described previously (19).
Toxin purification.
Wild-type and mutant LT, LTB, and CTB were purified from strains MK741, MK1101, and MK1242 and derivatives thereof expressing the appropriate mutant constructs as described previously (7, 26). Briefly, periplasm was isolated using osmotic shock after growth in CFA media with ampicillin, and toxins or pentamers were purified by binding to immobilized d-galactose beads (Pierce). For the LT E11K and E11A mutants, 4-(2-aminoethyl)benzenesulfonyl fluoride (Sigma) was added during purification at a final concentration of 1 mM to prevent degradation. Toxins and pentamers were assessed for purity by SDS-PAGE and protein staining with Ruby Red (Molecular Probes).
ELISAs.
Enzyme-linked immunosorbent assays (ELISAs) for binding to the monosialoganglioside GM1 (Sigma) and blood group A terminal trisaccharide (GalNAcα3[Fucα2]Gal) conjugated to bovine serum albumin (V-Labs) were performed as previously described (26). Purified toxin or pentamer was tested at a final concentration of 1 nM, and a different batch was used for each ELISA. Cell-free supernatant was diluted 1:3 (for ETEC expressing LT or LT mutants) or 1:20 (for ETEC or V. cholerae expressing CTB or CTB mutants); periplasm samples were diluted 1:20 after adjustment for levels of alkaline phosphatase activity.
Toxin stability assay.
Purified toxin or pentamer was added at a final concentration of 50 nM to 1.5 ml of cell-free supernatant from a log-phase culture of toxin-deficient ETEC (MK1053) or V. cholerae (MK1123), each carrying the empty pTrc99A vector. One 0.5-ml aliquot was immediately frozen, one incubated at 37°C for 1 h (for LT and LT mutants) or 2 h (for CTB and CTB mutants), and one incubated at 25°C for 4 days. Levels of assembled toxin or pentamer remaining after the incubation were compared by GM1 ELISA, with data normalized to the levels detected in the aliquot that had immediately been frozen. Each experiment was performed with a separate batch of purified pentamer and a fresh preparation of cell-free supernatant.
Pronase sensitivity assay.
Purified wild-type or mutant LT or CTB (500 ng) was incubated with water or Pronase (Fluka) at the indicated final concentration and incubated for 1 h at 37°C. Afterward, samples were boiled for 10 min and subjected to SDS-PAGE, followed by staining with Ruby Red. Densitometry of the band corresponding to LTB or CTB was performed in the linear range using ImageJ software (NIH). Data are normalized to the intensity of the untreated LTB or CTB band.
Toxicity assay.
A bioassay on Y1 cells was performed in duplicate as described previously (19), with at least two independent trials for each mutant toxin.
In vitro pentamer reassembly assay.
Wild-type and mutant LTB pentamers were assessed for reassembly in vitro essentially as previously described (31). Briefly, purified wild-type or mutant LTB (5 μg) was treated with 0.1 M HCl for 1 min in a final volume of 20 μl to induce dissociation into monomers and then neutralized with 80 μl of McIlvaine's buffer (31). After incubation for the indicated times at 4°C, samples were diluted 1:50 into phosphate-buffered saline (pH 7.4) to halt reassembly and immediately frozen. Pentamer reassembly was assessed by a GM1 binding ELISA. Each trial was carried out with a separate batch of pentamers.
Cell surface binding assay.
Assessment of the binding of purified toxins to DH5α cells was performed using an immunoblot-based assay as previously described (26). Independent assays were carried out with fresh batches of purified toxin.
Growth of V. cholerae on lipid agar and skim milk agar.
Overnight cultures of MK1225, MK1232, and MK1233 were matched for optical density at 600 nm, diluted 1:1,000, and plated on lipid agar (22) containing ampicillin and 200 μM IPTG.
After induction for 3 h as described above, 100 μl of 10−7 dilutions of MK1225, MK1232, and MK1233 were plated on LB-skim milk agar (42) containing ampicillin and 200 μM IPTG. All plates were incubated for 36 h at 37°C.
Statistical analysis.
Results were analyzed by Student's t test; two-tailed P values less than 0.05 were considered statistically significant. Error bars in the figures represent standard errors of the mean (SEM).
RESULTS
Mutations affecting the secretion of LT from ETEC.
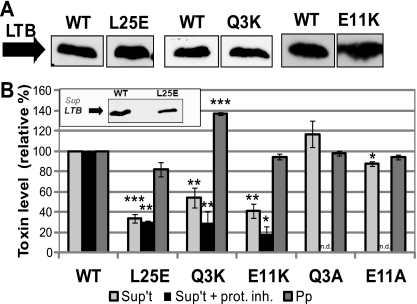
In a previous study, we identified two mutations in LTB (L25E and Q3K) that independently reduced bacterial cell surface binding of the toxin (26). Upon further examination, when mutants LT[L25E] and LT[Q3K] were expressed in an LT-deficient ETEC strain (jf570), they were detected at wild-type levels in total culture samples but showed reduced levels in the cell-free supernatant (Fig. 1A and B). Consistent with a secretion defect, LT[Q3K] was found at increased levels in the periplasm (Fig. 1B). In contrast, periplasmic LT[L25E] was found at a level close to that of the wild type, suggesting that it did not accumulate in a presecreted form.
FIG. 1.
Three mutations impair the secretion of LT from ETEC. (A) Representative immunoblots of TCA-precipitated total culture samples, adjusted for CFU, showing induced expression of wild-type LT (WT), LT[L25E], LT[Q3K], and LT[E11K] in strain jf570. Blots were probed with cross-reactive anti-CT antibody. (B) Strains expressing wild-type (WT) and the indicated mutant toxins were fractionated to isolate cell-free supernatant (Sup't) and periplasm (Pp). Each fraction was tested for toxin levels by GM1 ELISA, with wild-type levels set to 100%. Supernatant levels were normalized to CFU, and periplasm levels were normalized to alkaline phosphatase activity. *, P < 0.01; **, P < 0.005; ***, P < 10−6 compared to wild-type (n ≥ 3); n.d., not determined. For some experiments, cultures were grown in the presence of a protease inhibitor cocktail (+ prot. inh.) (n ≥ 2). Inset: representative immunoblot showing levels of wild-type LT (WT) and LT[L25E] secreted from jf570. Cell-free supernatant fractions were precipitated with TCA, and samples were adjusted for CFU. Blots were probed with cross-reactive anti-CT antibody.
Since levels of the toxins in the supernatants were measured by a GM1 binding ELISA, we examined whether these mutations affected the toxin's ability to bind GM1 (Table 3). Purified LT[Q3K] showed wild-type levels of GM1 binding. LT[L25E] bound GM1 approximately 30% less efficiently than wild-type LT, which, although significant, could not be fully responsible for the observed 65% defect in secretion. To verify the apparent decrease in secretion of LT[L25E] with a GM1-independent assay, we immunoblotted cell-free supernatant. The results were consistent with a secretion defect for LT[L25E] (Fig. 1B, inset).
TABLE 3.
Binding properties of LT and CTB mutants
| Mutant | GM1 bindinga |
Blood sugar bindingc |
E. coli surface bindingd |
|||
|---|---|---|---|---|---|---|
| % | P valueb | % | P value | % | P value | |
| LT[L25E] | 69.3 ± 5.3e | <0.005 | 64.2 ± 7.2 | <0.01 | 35.2 ± 10.9 | <0.005 |
| LT[Q3K]f | 96.0 ± 4.6 | 0.43 | 0.67 ± 0.43 | <0.005 | 33.8 ± 8.8 | <0.005 |
| LT[Q3A] | 95.5 ± 14.4 | 0.77 | 4.0 ± 4.0 | 2.3 × 10−6 | 36.5 ± 4.7 | <0.0005 |
| LT[E11K] | 90.9 ± 4.6 | 0.08 | 109.6 ± 17.8 | 0.65 | 19.6 ± 9.3 | <0.001 |
| LT[E11A] | 81.5 ± 6.5 | 0.07 | ||||
| CTB[Q3K] | 93.1 ± 1.9 | <0.05 | ||||
| CTB[E11K] | 94.7 ± 2.5 | 0.08 | ||||
| CTB[E11A] | 106.7 ± 1.8 | <0.05 | ||||
Purified toxin binding to GM1 was determined by ELISA; values are expressed as mean percentages of wild-type LT or CTB binding ±SEM (n ≥ 3).
P values are for comparison to wild-type binding as determined by Student's t test.
Purified toxin binding to blood group A trisaccharide conjugated to BSA was determined by ELISA; values are expressed as mean percentages of wild-type binding ±SEM (n ≥ 2).
Purified toxin binding to the surface of DH5α E. coli cells was determined by a previously described immunoblot-based binding assay (26); values are expressed as mean percentages of wild-type LT binding ±SEM (n ≥ 3).
Significant differences from wild-type (P < 0.05) are in bold.
Data for this mutant were reported previously (26).
The substitution of lysine for glutamine in LT[Q3K] results in a relatively drastic amino acid change, and therefore, we wondered whether the observed secretion deficiency was due to the addition of a positive charge at that site in the protein. We made other mutations of Gln-3 to explore the impact of the character of this residue on the secretion phenotype. LT[Q3L] was undetectable when expressed in jf570 (26); however, LT[Q3A] was stable when expressed in ETEC and could be studied (data not shown). LT[Q3A] was detected in the cell-free supernatant at levels slightly higher than those of the wild type, but the difference was not statistically significant, and periplasmic levels of the mutant were equivalent to those of wild-type LT (Fig. 1B). These results suggest that the presence of a charged amino acid at residue 3 impedes the secretion of LTB.
LT secretion mutants show wild-type stability and pentamer assembly.
Degradation could have been responsible for the decreased detection of mutant LT in the cell-free supernatant, and therefore, we investigated the stability of the mutant toxins in that milieu using several methods. The addition of protease inhibitors to the culture media during growth did not change the observed secretion defect of LT[L25E] or LT[Q3K] (Fig. 1B). We also performed a stability assay similar to one described in a study of secretion-impaired mutants of Pseudomonas aeruginosa exotoxin A (23) and observed that both mutants were stable over time when purified and incubated with sterile-filtered supernatant from a log-phase ETEC culture (data not shown).
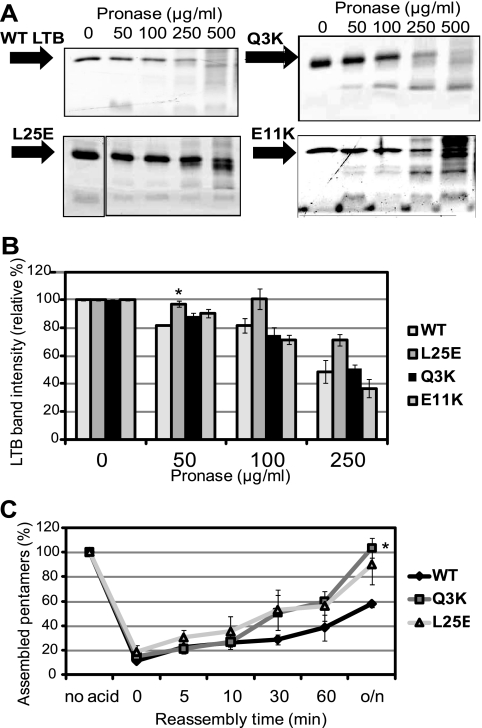
To further assess the stability of these mutants, they were tested for sensitivity to pronase, a protease previously reported to degrade LT (19). Wild-type and mutant LT were equally sensitive to the enzyme, with degradation products visible beginning at a pronase concentration of 50 μg/ml and much of the full-length monomer broken down at a concentration of 500 μg/ml (Fig. 2A and B). Thus, proteolytic degradation was not responsible for reduced detection of the LT[L25E] or LT[Q3K] mutant in the ETEC supernatant.
FIG. 2.
LT secretion mutants show WT protease sensitivity and pentamer formation. (A) Representative protein gels showing degradation of WT or the indicated mutant toxin. Purified toxin (500 ng) was incubated with pronase at the indicated final concentration for 1 h at 37°C. Following the incubation, samples were boiled and separated by 15% SDS-PAGE, and the gel was stained with Ruby Red. (B) Densitometric measurements of the intensities of the bands corresponding to LTB on the protein gels described in the legend for panel A. The intensity of the band corresponding to untreated WT LTB or the indicated mutant was set to 100%. *, P < 0.05 compared to WT at the same concentration (n ≥ 2) (C) Purified WT LTB and the indicated mutants were dissociated into monomers by treatment with acid, and then neutralized and allowed to reassemble into pentamers for the indicated number of minutes or overnight (o/n). Pentamer reformation was assessed by GM1 ELISA, with values normalized to levels of each pentamer without acid treatment (no acid). *, P < 0.01, compared to WT at the same time point (n ≥ 3).
The first 10 amino acids of LTB have previously been reported to be involved in pentamer assembly, and pentamer assembly is critical for secretion (4, 15). Therefore, we determined whether the effect of the Q3K or L25E mutation on LT secretion was due to a decreased efficiency of pentamer assembly. LT's toxic effects require the interaction of LTA with the LTB pentamer, and thus, we used a toxicity assay to determine whether LTA associates with mutant LTB in a manner equivalent to that of wild-type LT. The toxicity of purified mutant LT was measured using an in vitro cell culture assay that is based on a morphological change in Y1 adrenal cells induced by LTA. Both mutants were as toxic as wild-type LT (8 ng of each elicited maximal rounding of 4 × 105 cells), suggesting that periplasmic holotoxin formation was not affected by the L25E or Q3K mutation. Furthermore, each mutant behaved similarly to or better than wild-type LT in an established in vitro cell-free assay measuring pentamer reassembly after dissociation at extremely low pH (Fig. 2C) (31). Together, these assays indicated that the observed secretion defects for LT[L25E] and LT[Q3K] were not due to defects in their stability or assembly.
Despite the fact that the secretion-defective mutant toxins were wild type in their stability and assembly, we were interested in determining if the mutant toxins displayed more subtle differences in structure. We reasoned that such differences could be detected by measuring the ability of the mutants to bind each of the three major ligands of LT. Therefore, purified mutant toxins were assessed for binding to blood group A sugars and the surface of E. coli, in addition to GM1, and compared to wild-type LT (Table 3). LT[Q3A] showed wild-type binding to GM1 but impaired binding to blood antigen and E. coli, consistent with the previously reported phenotypes of LT[Q3K] (26) (Table 3).
LT[L25E] was found to be impaired for binding to all substrates of LTB tested (Table 3). Therefore, although it is stable and assembles into a holotoxin-like wild-type toxin, LT[L25E] appears to have an external interaction surface that does not permit normal substrate recognition. Because of the potential for more global defects that might confound our results, L25E was not further considered in this study.
The E11K mutation, originally characterized with CT, also impairs the secretion of LT from ETEC.
An E11K mutation in the B subunit of CT reduces its secretion from V. cholerae to approximately 60% of wild-type levels (6). Because LT and CT are highly similar secretion substrates, we wondered whether this mutation would affect the secretion of LT from ETEC, and so we introduced the E11K mutation into LTB. Indeed, the levels of LT[E11K] found in ETEC supernatant were substantially reduced, despite a wild-type level of expression (Fig. 1A and B). Periplasmic levels of LT[E11K] were nearly wild type, as was the case for LT[L25E] (Fig. 1B). GM1 binding of purified LT[E11K] was slightly reduced, although not significantly (Table 3). Like the Q3K and L25E mutants, LT[E11K] was stable when incubated in ETEC culture supernatant (data not shown). Additionally, supernatant levels of LT[E11K] did not increase upon addition of protease inhibitor to the growth medium (Fig. 1B). LT[E11K] also behaved similarly to wild-type LT in the pronase sensitivity assay (Fig. 2A and B). While purified LT[E11K] is as toxic as wild-type LT, indicating proper holotoxin formation in vivo, the mutation appears to impede the reformation of LTB pentamers in vitro after dissociation with acid (data not shown).
To explore the characteristics of residue 11 that are critical for secretion, we tested a more neutral mutation, LT[E11A]. Detected levels of LT[E11A] in the supernatant were decreased only approximately 12% compared to wild-type LT (Fig. 1B), but this value may be the result of its slightly decreased ability to bind GM1 (Table 3). Periplasmic levels of LT[E11A] were normal (Fig. 1B). These data suggest that introduction of a charge at residue 11 is critical for LT[E11K]'s observed secretion defect in ETEC.
Consistent with its location outside the blood antigen binding pocket, LT[E11K] was fully capable of binding blood group A trisaccharide, but intriguingly, this mutant showed a strong impairment in binding to the surface of E. coli K-12 cells (Table 3). As such, Glu-11 represents a residue that is located outside the peripheral blood sugar binding pocket but is nevertheless critical for binding to LPS on the surface of E. coli.
The Q3K mutation does not alter the secretion of CTB from V. cholerae.
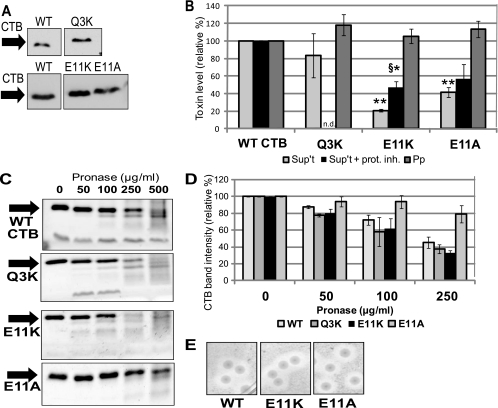
Because the E11K mutation affected LT's secretion from ETEC similarly to its impairment of CT secretion from V. cholerae, we were curious whether the Q3K mutation that affected LT secretion from ETEC would also affect CTB secretion from V. cholerae. To that end, an inducible plasmid expressing wild-type or mutant CTB carrying the Q3K mutation (CTB[Q3K]) was introduced into the CT-deficient V. cholerae strain P4 (10). B-subunit pentamers, without the catalytically active A subunit, were used for reasons of biosafety. Interestingly, a fourfold lower concentration of IPTG was needed to induce CTB[Q3K] to levels matching those of induced wild-type CTB (Fig. 3A).
FIG. 3.
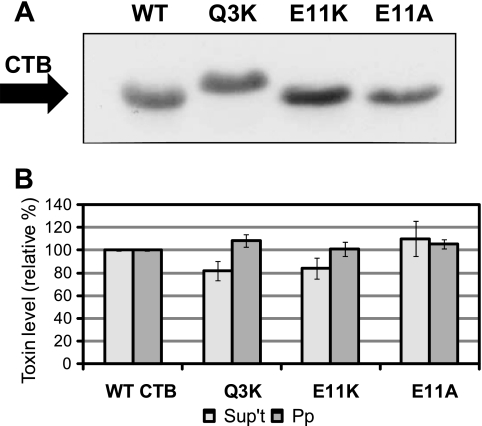
Mutation of Glu-11 impairs CTB secretion from V. cholerae. (A) Representative immunoblot of TCA-precipitated total culture samples, adjusted for CFU, showing induced expression of WT CTB, CTB[Q3K], CTB[E11K], and CTB[E11A] in strain P4. Blots were probed with anti-CT antibody. (B) Strains expressing WT CTB and the indicated CTB mutants were fractionated to isolate cell-free supernatant (Sup't) and periplasm (Pp). Each fraction was tested for pentamer levels by GM1 ELISA, with WT levels set to 100%. Supernatant levels were normalized to CFU, and periplasm levels were normalized to alkaline phosphatase activity. *, P < 0.05; **, P < 0.0005 compared to wild-type (n ≥ 3). For some experiments, cultures were grown in the presence of a protease inhibitor cocktail (+ prot. inh.). §, P < 0.05 compared to E11K supernatant levels without protease inhibitor (n ≥ 2). (C) Representative protein gels showing degradation of WT CTB or the indicated mutant pentamer. Purified pentamer (500 ng) was incubated with pronase at the indicated final concentration for 1 h at 37°C. Following the incubation, samples were boiled and separated by 15% SDS-PAGE, and the gel was stained with Ruby Red. (D) Densitometric measurements of the intensities of the CTB bands on the protein gels described in the legend for panel C. The intensity of the band corresponding to untreated WT or the indicated mutant CTB was set to 100% (n ≥ 2). (E) Zones of clearance formed by V. cholerae on skim milk agar. Strains expressing WT CTB and the indicated mutant pentamers were plated on skim milk agar and incubated for 36 h at 37°C.
Unlike LT[Q3K] in ETEC, CTB[Q3K] levels were only slightly reduced in the supernatant of V. cholerae P4 (83.3%), and the difference was not significant (Fig. 3B). The GM1 binding levels of purified CTB[Q3K] were slightly reduced compared to wild-type CTB (Table 3); therefore, the level of secreted CTB[Q3K] may be even closer to that of wild-type CTB than our assay indicates. Periplasmic levels of CTB[Q3K] were close to those of wild-type CTB (Fig. 3B). These results suggest a difference in substrate selectivity between the T2S systems of ETEC and V. cholerae.
An inducible plasmid expressing CTB[E11K] was also cloned and utilized for comparison studies of our assay system. CTB[E11K] expressed at levels similar to those of wild-type CTB (Fig. 3A), and we could recapitulate its secretion impairment, albeit to an even greater extent than was previously reported (20.9 ± 1.7% of that of the wild type, Fig. 3B). However, in contrast to the near-wild-type secretion phenotype of LT[E11A] from ETEC, alteration of Glu-11 in CTB to alanine (CTB[E11A]) resulted in significantly reduced CTB levels in the cell-free supernatant, despite wild-type levels of expression (Fig. 3A and B). Although addition of protease inhibitor to the growth medium raised the levels of CTB[E11K] detected, proteolytic degradation cannot account for the full secretion defect (Fig. 3B). The presence of protease inhibitor did not alter the amount of CTB[E11A] detected in the supernatant (Fig. 3B). Like the LT mutants incubated in ETEC supernatant, these CTB mutants were not degraded in V. cholerae culture supernatant in our stability assay (data not shown). Moreover, purified wild-type and mutant CTB pentamers behaved similarly in the pronase sensitivity assay (Fig. 3C and D), with CTB[E11A] showing a trend toward increased stability. Despite their secretion defect, periplasmic forms of CTB[E11K] and CTB[E11A] did not accumulate to a level higher than that of wild-type CTB (Fig. 3B).
The V. cholerae T2S system is not blocked by secretion-impaired CTB mutants.
We wondered if nonsecreted CTB mutants would block the T2S system, preventing the export of other T2S substrates. In V. cholerae, the T2S system secretes a lipase required for acquisition of olive oil as a carbon source, and degradation of casein occurs in a T2S-dependent manner (35, 37). To test for mutant-dependent blockage of the T2S system, we examined whether V. cholerae strains expressing wild-type CTB, CTB[E11K], and CTB[E11A] were able to grow on lipid agar containing olive oil as the sole carbon source and found that their growth was not inhibited (data not shown). Furthermore, these strains were tested for generating zones of clearance on LB-skim milk plates. All strains formed clear haloes of similar sizes (Fig. 3E). Taken together, these results suggest that the T2S system is not completely blocked by mutant CTB, although our data do not rule out the possibility of partial blockage. ETEC did not grow on lipid agar or create a zone of clearance on skim milk plates (data not shown).
CTB mutants are secreted efficiently from ETEC.
In order to further investigate the common factors in the recognition of T2S substrates, we measured the secretion of CTB[Q3K], CTB[E11K], and CTB[E11A] through a heterologous T2S system in ETEC. In all cases, the mutant toxins expressed at wild-type levels (Fig. 4A). A slight but insignificant impairment in secretion was seen for CTB[Q3K], and this could be partly explained by the mutant's reduced GM1 binding (Fig. 4B; Table 3). Surprisingly, however, whereas CTB[E11K] and CTB[E11A] had strong defects in secretion from V. cholerae, these CTB mutants had no significant reduction in secretion from ETEC (Fig. 4B). The modest impairment seen for CTB[E11K] (Fig. 4B) was again accompanied by a slight reduction in GM1 binding of the purified pentamer (Table 3) and could be responsible for the apparent reduction in secreted levels. Intriguingly, the E11A mutation generated a GM1 binding capacity slightly higher than that of wild-type CTB (Table 3), and it is likely that this increased binding is reflected in the slightly elevated levels of CTB[E11A] detected in the supernatant (Fig. 4B). In all cases, periplasmic pentamer levels were close to that of wild-type CTB (Fig. 4B). These data demonstrate that recognition of CT as a secretion substrate occurs differently in the ETEC and V. cholerae T2S systems.
FIG. 4.
Secretion of CTB mutants is not impaired in ETEC. (A) Representative immunoblot of TCA-precipitated total culture samples, adjusted for CFU, showing induced expression of WT CTB, CTB[Q3K], CTB[E11K], and CTB[E11A] in strain jf570. The blot was probed with anti-CT antibody. (B) Strains expressing WT CTB and the indicated mutant pentamers were fractionated to isolate cell-free supernatant (Sup't) and periplasm (Pp). Each fraction was tested for pentamer levels by GM1 ELISA, with WT levels set to 100%. Supernatant levels were normalized to CFU (n ≥ 3), and periplasm levels were normalized to alkaline phosphatase activity (n ≥ 2).
LTB mutants are unstable in V. cholerae.
In order to complete our cross-species analysis of the effects of the T2S substrate mutations, wild-type and mutant LT B subunits were expressed in V. cholerae strain P4. Again, LTA-deficient constructs were tested for reasons of safety. Although wild-type LTB was readily produced in V. cholerae, the Q3K and E11K LTB mutants were detected in small amounts, if at all (Fig. 5), and thus, we were unable to measure the effect of Q3K or E11K on LTB secretion from V. cholerae. LTB[L25E] showed significant levels of expressed protein, indicating that some LTB mutations are tolerated by V. cholerae (Fig. 5), and LTB[L25E] in V. cholerae supernatant was readily detectable by immunoblotting (data not shown). However, the expression of LTB[L25E] was not uniform enough to allow for quantification of its secreted levels. Taken together, our results provide evidence that, despite a high degree of conservation between the substrates, LTB and CTB are recognized and dealt with in different ways during secretion from ETEC and V. cholerae (Table 4).
FIG. 5.
LTB mutants express poorly in V. cholerae. Representative Ruby Red protein stain (top) and immunoblot (bottom) of TCA-precipitated total culture samples, adjusted for CFU, showing induced expression of a vector control (Vec), WT LTB, LTB[Q3K], LTB[Q3A], LTB[E11K], and LTB[L25E] in strain P4. The blot was probed with cross-reactive anti-CT antibody.
TABLE 4.
Summary of the effects of the mutations investigated in this studya
| Mutationb | Secretion from ETEC |
Secretion from V. cholerae |
||
|---|---|---|---|---|
| LT | CTB | CTB | LTB | |
| Q3K | Reduced | Wild type | Wild type | Not detectedd |
| Q3A | Wild type | —c | — | Not detected |
| E11K | Reduced | Wild type | Reduced | Little detected |
| E11A | Near wild type | Wild type | Reduced | — |
| L25E | Reduced | — | — | Wild typee |
Data shown characterize levels in cell-free supernatant, as measured by GM1 ELISA.
All mutations are in the B subunit; the LT holotoxin was expressed in ETEC.
—, not tested.
No mutant LTB could be detected in total culture samples by immunoblotting.
Secreted LTB[L25E] is readily detectable in the cell-free supernatant by immunoblotting.
DISCUSSION
We have identified three point mutations of LTB (L25E, Q3K, and E11K) that impair LT secretion from ETEC. These mutants behaved like wild-type LT in assays measuring stability (Fig. 2 and data not shown), indicating that the reduced levels found in ETEC cell-free supernatants are due to an effect on secretion and not increased rates of degradation. In contrast to mutants with a substituted lysine residue, LT[Q3A] and LT[E11A] were secreted at essentially wild-type levels from ETEC. Our results are in line with previous research demonstrating that alteration of a single amino acid can impair or block the secretion of other T2S substrates, including CT from V. cholerae, aerolysin from Aeromonas hydrophila, and Cel5 endogluconase from Dickeya dadantii (formerly Erwinia chysanthemi) (3, 6, 44).
The similar natures of LT and CT led us to explore whether mutations affecting the secretion of one substrate might affect the secretion of the other. The ability of V. cholerae to secrete LT has been recognized for years (27), and our group has reported that E. coli K-12 carrying a plasmid-borne T2S operon is able to secrete CTB (17). In this study, we have now shown that the native secretion apparatus of ETEC is also able to secrete CTB. Whereas the E11K mutation impairs the secretion of both toxins from their native bacterial species, other mutations affecting the secretion of each toxin are not identical. The Q3K mutation prevents LT secretion and causes LT to back up in the periplasm of ETEC, but the same mutation has no significant effect on the secretion of CT from V. cholerae. Furthermore, while the E11A mutation does not strongly impair the secretion of LT from ETEC, CTB[E11A] behaves like the poorly secreted CTB[E11K] when expressed in V. cholerae. These results demonstrate that LT and CT are identified as secretion substrates based on distinct sets of residues and/or that mutations of substrates are tolerated to different extents by the T2S systems of ETEC and V. cholerae.
Our results investigating the effects of expressing CTB[E11K] or CTB[E11A] on the secretion of other T2S substrates from V. cholerae suggest that recognition of the toxin is impaired by these mutations, not that the mutations cause the toxin to remain bound to the secretion machinery, blocking the export of other proteins. However, these assays were not highly quantitative, and therefore, partial blockage may not have been detected. Further studies with a quantitative system will be needed to address this question. As ETEC could not be used in these assays, it is not yet clear whether LT mutants are impaired at the point of being recognized or transiting the outer membrane.
Thus far, there is very little knowledge of how T2S substrates are recognized by the complex T2S apparatus, and an obvious amino acid sequence motif in T2S substrates is lacking. A study of PehA polygalacturonase secretion has provided some of the only direct evidence in support of a hypothetical three-dimensional recognition motif. In that study, one mutation in PehA that reduced the levels detected in Erwinia carotovora supernatant was determined to be internal to folded protein and thus to be responsible for impairing the secretion-competent conformation of the substrate (28). However, the stability of the mutant seemed to be reduced. Our data with the L25E mutation provide more definitive evidence for a three-dimensional “structural motif” in T2S substrates. In fact, secretion of the CT/LT B-subunit pentamer may require the simultaneous recognition of multiple subunits in three-dimensional space. The binding of LT to GM1, blood group sugars, and the surface of E. coli occurs at the interface between two adjacent B subunits (16, 25, 26). It is particularly notable that the L25E mutation affects the binding of the toxin to all three substrates, each of which binds regions of the B pentamer that do not include the Leu-25 residue (16, 25, 26). Because LT[L25E] did not show any differences from wild-type LT in terms of protease sensitivity or pentamer reassembly, we propose that altered spacing of the monomeric B subunits within the pentamer underlies these results. Therefore, this is strong evidence that the portion of LTB that is recognized by the secretion apparatus spans two adjacent subunits and that L25E alters the three-dimensional organization of the residues in this region.
The determination that CTB[E11K] and CTB[E11A] have two different secretion phenotypes in V. cholerae and ETEC allows for future research to determine the portion(s) of the T2S system involved in the recognition of CTB for secretion in these two pathogens. The GspC/EpsC and GspD/EpsD proteins are good candidates for the portions of the T2S apparatus that confer substrate selectivity. Elegant research carried out with two Erwinia species determined that the N-terminal portion of OutD (the GspD homolog in Erwinia) and a PDZ domain in OutC (GspC) were involved in species-specific substrate recognition (2). Unfortunately, we were unable to assess such roles in ETEC or V. cholerae T2S components because we were not able to establish functional secretion of CTB or LTB in a gspD knockout strain of ETEC complemented with epsD from V. cholerae, even in the presence of epsC (our unpublished observations). These results are in line with a previous study reporting that the entire V. cholerae Eps system was not functional when expressed in E. coli (34).
Our results reveal the exquisitely controlled coordination between the T2S machinery and its substrates. Despite the high degree of homology between LTB and CTB, we have discovered that mutations affect their secretion differently in distinct T2S systems, indicating that each substrate has evolved closely with its T2S apparatus to enable its secretion. A number of previous studies have attempted to identify the domain (or domains) present in T2S substrates that mark them for secretion. Our data identifying distinct mutations that affect the secretion of highly similar substrates like LT and CT raise the possibility that it will not be possible to find a common recognition motif among T2S substrates. However, the use of these CT and LT mutants could help elucidate the portions of the T2S complex that are involved in substrate recognition.
Acknowledgments
We thank A. Kulp for technical assistance and other members of the Kuehn lab for helpful discussions and support. We also thank J. Fleckenstein for providing strain jf570, K. Fullner Satchell for strain P4, and R. Holmes for pLMP1.
This work was supported by RO1AI064464 and R21AI063239 from the NIAID and a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease Award (to M.J.K.).
Footnotes
Published ahead of print on 22 January 2010.
REFERENCES
- 1.Allen, K. P., M. M. Randolph, and J. M. Fleckenstein. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 74:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouley, J., G. Condemine, and V. E. Shevchik. 2001. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J. Mol. Biol. 308:205-219. [DOI] [PubMed] [Google Scholar]
- 3.Chapon, V., H. D. Simpson, X. Morelli, E. Brun, and F. Barras. 2000. Alteration of a single tryptophan residue of the cellulose-binding domain blocks secretion of the Erwinia chrysanthemi Cel5 cellulase (ex-EGZ) via the type II system. J. Mol. Biol. 303:117-123. [DOI] [PubMed] [Google Scholar]
- 4.Chung, W. Y., R. Carter, T. Hardy, M. Sack, T. R. Hirst, and R. F. James. 2006. Inhibition of Escherichia coli heat-labile enterotoxin B subunit pentamer (EtxB5) assembly in vitro using monoclonal antibodies. J. Biol. Chem. 281:39465-39470. [DOI] [PubMed] [Google Scholar]
- 5.Cianciotto, N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581-588. [DOI] [PubMed] [Google Scholar]
- 6.Connell, T. D., D. J. Metzger, M. Wang, M. G. Jobling, and R. K. Holmes. 1995. Initial studies of the structural signal for extracellular transport of cholera toxin and other proteins recognized by Vibrio cholerae. Infect. Immun. 63:4091-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland, B. R., R. J. Richter, and C. E. Furlong. 1982. Renaturation and identification of periplasmic proteins in two-dimensional gels of Escherichia coli. J. Biol. Chem. 257:15065-15071. [PubMed] [Google Scholar]
- 8.Dorsey, F. C., J. F. Fischer, and J. M. Fleckenstein. 2006. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell. Microbiol. 8:1516-1527. [DOI] [PubMed] [Google Scholar]
- 9.Finan, T. M., B. Kunkel, G. F. De Vos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fullner, K. J., W. I. Lencer, and J. J. Mekalanos. 2001. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 69:6310-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg, I., and J. J. Mekalanos. 1986. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J. Bacteriol. 165:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg, J. B., and D. E. Ohman. 1984. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J. Bacteriol. 158:1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy, S. J., J. Holmgren, S. Johansson, J. Sanchez, and T. R. Hirst. 1988. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 85:7109-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson, I. R., J. P. Nataro, J. B. Kaper, T. F. Meyer, S. K. Farrand, D. L. Burns, B. B. Finlay, and J. W. St. Geme III. 2000. Renaming protein secretion in the gram-negative bacteria. Trends Microbiol. 8:352. [PubMed] [Google Scholar]
- 15.Hirst, T. R., J. Sanchez, J. B. Kaper, S. J. Hardy, and J. Holmgren. 1984. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 81:7752-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmner, A., G. Askarieh, M. Okvist, and U. Krengel. 2007. Blood group antigen recognition by Escherichia coli heat-labile enterotoxin. J. Mol. Biol. 371:754-764. [DOI] [PubMed] [Google Scholar]
- 17.Horstman, A. L., S. J. Bauman, and M. J. Kuehn. 2004. Lipopolysaccharide 3-deoxy-d-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins. J. Biol. Chem. 279:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horstman, A. L., and M. J. Kuehn. 2002. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J. Biol. Chem. 277:32538-32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horstman, A. L., and M. J. Kuehn. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275:12489-12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobling, M. G., L. M. Palmer, J. L. Erbe, and R. K. Holmes. 1997. Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of Escherichia coli. Plasmid 38:158-173. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, T. L., J. Abendroth, W. G. Hol, and M. Sandkvist. 2006. Type II secretion: from structure to function. FEMS Microbiol. Lett. 255:175-186. [DOI] [PubMed] [Google Scholar]
- 22.Kagami, Y., M. Ratliff, M. Surber, A. Martinez, and D. N. Nunn. 1998. Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol. Microbiol. 27:221-233. [DOI] [PubMed] [Google Scholar]
- 23.McVay, C. S., and A. N. Hamood. 1995. Toxin A secretion in Pseudomonas aeruginosa: the role of the first 30 amino acids of the mature toxin. Mol. Gen. Genet. 249:515-525. [DOI] [PubMed] [Google Scholar]
- 24.Mekalanos, J. J. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:253-263. [DOI] [PubMed] [Google Scholar]
- 25.Merritt, E. A., T. K. Sixma, K. H. Kalk, B. A. van Zanten, and W. G. Hol. 1994. Galactose-binding site in Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT). Mol. Microbiol. 13:745-753. [DOI] [PubMed] [Google Scholar]
- 26.Mudrak, B., D. L. Rodriguez, and M. J. Kuehn. 2009. Residues of heat-labile enterotoxin involved in bacterial cell surface binding. J. Bacteriol. 191:2917-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neill, R. J., B. E. Ivins, and R. K. Holmes. 1983. Synthesis and secretion of the plasmid-coded heat-labile enterotoxin of Escherichia coli in Vibrio cholerae. Science 221:289-291. [DOI] [PubMed] [Google Scholar]
- 28.Palomaki, T., R. Pickersgill, R. Riekki, M. Romantschuk, and H. T. Saarilahti. 2002. A putative three-dimensional targeting motif of polygalacturonase (PehA), a protein secreted through the type II (GSP) pathway in Erwinia carotovora. Mol. Microbiol. 43:585-596. [DOI] [PubMed] [Google Scholar]
- 29.Pierce, N. F., J. B. Kaper, J. J. Mekalanos, and W. C. Cray, Jr. 1985. Role of cholera toxin in enteric colonization by Vibrio cholerae O1 in rabbits. Infect. Immun. 50:813-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pukatzki, S., S. B. McAuley, and S. T. Miyata. 2009. The type VI secretion system: translocation of effectors and effector-domains. Curr. Opin. Microbiol. 12:11-17. [DOI] [PubMed] [Google Scholar]
- 31.Ruddock, L. W., J. J. Coen, C. Cheesman, R. B. Freedman, and T. R. Hirst. 1996. Assembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. Kinetics and molecular basis of rate-limiting steps in vitro. J. Biol. Chem. 271:19118-19123. [DOI] [PubMed] [Google Scholar]
- 32.Russel, M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279:485-499. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez, J., and J. Holmgren. 2005. Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr. Opin. Immunol. 17:388-398. [DOI] [PubMed] [Google Scholar]
- 34.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, and V. J. DiRita. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott, M. E., Z. Y. Dossani, and M. Sandkvist. 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc. Natl. Acad. Sci. U. S. A. 98:13978-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi, L., S. Deng, M. J. Marshall, Z. Wang, D. W. Kennedy, A. C. Dohnalkova, H. M. Mottaz, E. A. Hill, Y. A. Gorby, A. S. Beliaev, D. J. Richardson, J. M. Zachara, and J. K. Fredrickson. 2008. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J. Bacteriol. 190:5512-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sikora, A. E., S. R. Lybarger, and M. Sandkvist. 2007. Compromised outer membrane integrity in Vibrio cholerae type II secretion mutants. J. Bacteriol. 189:8484-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart, C. R., O. Rossier, and N. P. Cianciotto. 2009. Surface translocation by Legionella pneumophila: a form of sliding motility that is dependent upon type II protein secretion. J. Bacteriol. 191:1537-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teneberg, S., T. R. Hirst, J. Angstrom, and K. A. Karlsson. 1994. Comparison of the glycolipid-binding specificities of cholera toxin and porcine Escherichia coli heat-labile enterotoxin: identification of a receptor-active non-ganglioside glycolipid for the heat-labile toxin in infant rabbit small intestine. Glycoconj. J. 11:533-540. [DOI] [PubMed] [Google Scholar]
- 42.Teo, J. W., L. H. Zhang, and C. L. Poh. 2003. Cloning and characterization of a metalloprotease from Vibrio harveyi strain AP6. Gene 303:147-156. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji, T., T. Honda, T. Miwatani, S. Wakabayashi, and H. Matsubara. 1985. Analysis of receptor-binding site in Escherichia coli enterotoxin. J. Biol. Chem. 260:8552-8558. [PubMed] [Google Scholar]
- 44.Wong, K. R., and J. T. Buckley. 1991. Site-directed mutagenesis of a single tryptophan near the middle of the channel-forming toxin aerolysin inhibits its transfer across the outer membrane of Aeromonas salmonicida. J. Biol. Chem. 266:14451-14456. [PubMed] [Google Scholar]
- 45.Yamamoto, T., T. Gojobori, and T. Yokota. 1987. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J. Bacteriol. 169:1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zalewska-Piatek, B., K. Bury, R. Piatek, P. Bruzdziak, and J. Kur. 2008. Type II secretory pathway for surface secretion of DraD invasin from the uropathogenic Escherichia coli Dr+ strain. J. Bacteriol. 190:5044-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]