Abstract
Engagement of the T-cell receptor (TCR) in human primary T cells activates a cyclic AMP (cAMP)-protein kinase A (PKA)-Csk inhibitory pathway that prevents full T-cell activation in the absence of a coreceptor stimulus. Here, we demonstrate that stimulation of CD28 leads to recruitment to lipid rafts of a β-arrestin/phosphodiesterase 4 (PDE4) complex that serves to degrade cAMP locally. Redistribution of the complex from the cytosol depends on Lck and phosphatidylinositol 3-kinase (PI3K) activity. Protein kinase B (PKB) interacts directly with β-arrestin to form part of the supramolecular complex together with sequestered PDE4. Translocation is mediated by the PKB plextrin homology (PH) domain, thus revealing a new role for PKB as an adaptor coupling PI3K and cAMP signaling. Functionally, PI3K activation and phosphatidylinositol-(3,4,5)-triphosphate (PIP3) production, leading to recruitment of the supramolecular PKB/β-arrestin/PDE4 complex to the membrane via the PKB PH domain, results in degradation of the TCR-induced cAMP pool located in lipid rafts, thereby allowing full T-cell activation to proceed.
T-cell receptor (TCR) stimulation alone is insufficient for activation of T cells, and sustainable T-cell immune responses require a second signal in addition to the TCR-mediated signal. The second signal is typically elicited by ligands B7-1 or B7-2 on antigen-presenting cells engaging the coreceptor CD28 to prevent anergy and apoptosis and enhancing interleukin-2 (IL-2) production and clonal expansion (4). Although CD28 plays a central role in T-cell activation in vivo (5), relatively little is known about the molecular basis for the increased efficacy of T-cell activation upon TCR and CD28 costimulation. Involvement of Lck, Itk, phosphatidylinositol 3-kinase (PI3K), SLP-76, Vav-1, and phospholipase C-γ (PLC-γ) has, however, been reported (43). CD28-mediated signals are transmitted via a short intracellular stretch in the receptor containing a conserved YMNM motif (32). Phosphorylation of Tyr173 in this motif by Lck and Fyn following CD28 ligation is key to efficient signal transduction (41), generating a binding site for the SH2 domain of the p85 regulatory subunit of PI3K (37, 40). CD28 may also contribute to TCR-dependent PI3K activity without recruiting PI3K directly (18). Whether engagement of CD28 alone can also induce PI3K activity has been a matter of controversy. However, recent reports confirming phosphorylation of the protein kinase B (PKB) substrate glycogen synthase kinase 3 (GSK3) upon CD28 ligation has demonstrated that this is indeed the case (6, 15). In addition, CD28 can recruit growth factor receptor-bound protein 2 (Grb2), and such association of Grb2 occurs via the phosphorylated YMNM motif as well as via the C-terminal PXXP motif (22, 35). The PXXP motif also binds and regulates Src family kinases (SFKs) (21, 47), and knock-in mice mutated in this motif were recently reported to have impaired IL-2 secretion (16).
Ligation of the TCR induces cyclic AMP (cAMP) production (27). However, the significance of this observation is still not fully understood, as it is well established that cAMP potently inhibits T-cell function and proliferation (2, 45, 46, 50). The spatiotemporal dynamics of the activation-induced cAMP gradient also are not completely appreciated. We have previously shown that cAMP is rapidly produced in lipid rafts following engagement of the TCR in primary T cells (3). This activates a pool of PKA type I targeted to rafts by association with the anchoring protein Ezrin, forming part of a supramolecular complex where Ezrin, EBP50, and PAG provide a scaffold that is able to coordinate PKA phosphorylation and activation of Csk, thereby inhibiting T-cell activation (44, 50). In addition, we have demonstrated that CD3/CD28 costimulation leads to recruitment of type 4 phosphodiesterase (PDE4) isoforms to rafts, resulting in degradation of the TCR-induced cAMP pool (3). Thus, we envisage that TCR-induced cAMP production constitutes a negative feedback loop capable of abrogating T-cell activation in the absence of a second signal. In order then to allow full T-cell activation to proceed, cAMP-mediated inhibition must be lifted. This appears to occur in the presence of a costimulus involving CD28 acting to trigger recruitment of PDE4 to lipid rafts, thereby degrading cAMP at this spatially critical location and resulting in an overriding positive feed-forward signal rather than the negative feedback loop activated from the TCR. In addition, a recent publication by Conche et al. has also found a possible stimulatory effect of cAMP, as the paper surprisingly showed that a transient cAMP increase shortly after TCR triggering may potentiate the calcium component of the TCR signaling. This could constitute a positive feed-forward in addition to the negative feedback signal by cAMP (12).
Spatial organization and recruitment of mediators of specific pathways as outlined above are essential to ensure signaling specificity and amplification. Among the many protein scaffolds linking effector molecules into linear pathways, β-arrestins have been reported to confer cross talk with a growing list of molecules important in cellular trafficking and signal transduction, including Src family members and mitogen-activated protein (MAP) kinases (reviewed in reference 14). The arrestins were first identified as having a role in desensitization of G protein-coupled receptors (GPCRs) (9); later, they were discovered to be involved in receptor internalization by interacting with clathrin and AP-2, thereby bringing activated receptors to clathrin-coated pits for endocytosis (19, 26). A role for β-arrestin in the spatially localized degradation of cAMP by scaffolding PDE4 isoforms to the proximity of cAMP generation at the plasma membrane has also been suggested (3, 7, 30, 38).
In the present study, we uncover a novel pathway that defines how T-cell costimulation elicits recruitment of PDE4 to lipid rafts to overcome cAMP-mediated inhibition of T-cell activation. This pathway is initiated by CD28 engagement leading to PI3K activation and phosphatidylinositol-(3,4,5)-triphosphate (PIP3) production and resulting in recruitment of a supramolecular complex of PKB/β-arrestin/PDE4 targeted to the plasma membrane due to sequestration via the PKB plextrin homology (PH) domain. Functionally, this pathway is essential for CD28 costimulation to strengthen and sustain T-cell immune responses.
MATERIALS AND METHODS
Reagents.
Anti-CD3ɛ (OKT3) was affinity purified by Diatec (Oslo, Norway) from supernatants of a hybridoma cell line from ATCC (Manassas, VA), whereas anti-CD28 (clone CD28.2) was purchased from Immunotech (Marseille, France). The AffiniPure F(ab′)2 fragment goat anti-mouse IgG, F(ab′)2, and peroxidase-conjugated secondary reagents were obtained from Jackson ImmunoResearch Europe Ltd. (Newmarket, United Kingdom), and PP2 was bought from Biomol Research Laboratories, Inc (Plymouth Meeting, PA). Flag, glutathione beads, LY294002, rolipram, and His-Select nickel affinity gel were purchased from Sigma-Aldrich. Antibodies toward LAT and PI3K p85 were obtained from Upstate Biotechnology, Inc. (Lake Placid, NY), whereas anti-PKB, anti-phosphoinositide-dependent kinase 1 (anti-PDK1), anti-Flag, and the Alexa488-conjugated hemagglutinin (HA) tag monoclonal antibody (MAb) were purchased from Cell Signaling Technology, Inc. Anticalnexin was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and the glutathione S-transferase (GST)-horseradish peroxidase conjugate was bought from GE Healthcare (United Kingdom). Anti-HA, anti-HA-affinity matrix, and Complete protease inhibitor tablets were purchased from Roche (Basel, Switzerland), and purified human GST-PKBα recombinant protein was obtained from SignalChem (Richmond, Canada). Alexa Fluor 546 goat anti-rabbit IgG and PIP3-labeled beads were from Molecular Probes, Inc. (Invitrogen), and n-octyl-β-d-glucopyranoside was purchased from USB Corporation. The polyclonal antibody toward β-arrestin 1 and 2 was as previously described (38), whereas anti-arrestin 1 and anti-arrestin 2 were obtained from Abcam plc (United Kingdom). The human IL-2 and gamma interferon (IFN-γ) Quantikine enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN), and validated negative-control small interfering RNA (siRNA) and arrestin-specific siRNAs were obtained from Ambion Inc. (Applied Biosystems).
DNA constructs and mutagenesis.
Flag-β-arrestin 1 and Flag-β-arrestin 2 were as previously described (38). The gene encoding human PKBα was subcloned from a T-cell cDNA library into the BamHI/XbaI sites of the pCMV6-HA expression vector. The PKB-Y437A and β-arrestin 1-D377L mutants were generated by site-directed mutagenesis (QuikChange; Stratagene, La Jolla, CA). β-Arrestin 1-GST and β-arrestin 2-GST were constructed using the Gateway system from Invitrogen. Human β-arrestin 1-pENTR221 (Invitrogen) and human β-arrestin 2-pCMV6 (OriGene Technologies Inc.) were used as templates.
Cells and transfections.
Human primary blood CD3+ T cells were purified from healthy blood donors by negative selection (1) and transfected with DNA in accordance with the manufacturer's instructions using the Amaxa Nucleofector kit. The human leukemic T-cell line Jurkat TAg was cultured and transfected by electroporation as previously described (48), and transfections of human embryonic kidney (HEK 293T) cells were performed using Lipofectamine 2000 CD (Invitrogen).
Purification of lipid rafts.
Isolation of Triton X-100-insoluble lipid rafts was performed as previously described (3, 52). After fractionation, all 12 fractions were tested for LAT content by immunoblotting to verify successful separation. As the majority of LAT segregated into fractions 2 to 5, peak raft fractions were prepared by mixing these fractions. Protein contents of all fractions were measured when PDE4 assays were conducted.
Cell stimulation.
Prior to stimulation, cells were preincubated at 37°C for 5 to 10 min. Human primary T cells were stimulated by first adding anti-CD3ɛ (0.3 μg/ml), anti-CD28 (1 μg/ml), or a combination of anti-CD3ɛ and anti-CD28. After 2 min, F(ab′)2 fragment (10 μg/ml) was added for antibody cross-ligation, and stimulation lasted for the indicated times. For inhibition of PI3K, cells were treated with 50 μM LY294002 for 20 min at 37°C prior to stimulation. For Lck/FynT inhibition, cells were pretreated with 10 μM PP2 for 20 min at 37 °C. All reactions were ended by adding ice-cold RPMI, followed by cell pelleting and lysis in ice-cold lysis buffer (50 mM HEPES [pH 7.4], 100 mM NaCl, 5 mM NaF, 10 mM NaPPi, 5 mM EDTA, 1% Triton X-100, 1 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride [PMSF]).
PDE4 activity assay.
After lipid raft separation of CD28-stimulated primary T cells, all fractions were analyzed for PDE activity as described previously (28, 31). Assays were carried out at 1 μM cAMP substrate concentration in duplicate in the presence or absence of the PDE4-selective inhibitor rolipram (10 μM), and thereafter, the fraction of rolipram-dependent PDE4 activity was calculated as a reflection of PDE4 activity. Activity was normalized for protein content, and PDE4 activity was calculated as pmol hydrolyzed cAMP/min/μg protein. Statistical significance was examined using the Student t test.
Immunoprecipitations.
For PIP3 bead precipitations, anti-CD3ɛ- and anti-CD28-stimulated primary T cells (5 × 107) were disrupted in a precipitation buffer containing 10 nM HEPES (pH 7.4), 150 mM NaCl, 0.25% Igepal, 10 mM NaPPi, 5 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, and 60 mM n-octyl-β-d-glucoside (NOG). PIP3-labeled beads were added and incubated overnight at 4°C. Precipitated complexes were subsequently washed in the precipitation buffer and subjected to immunoblotting. For coimmunoprecipitations of Flag-tagged β-arrestin and HA-tagged PKB, transfected primary T cells or Jurkat TAg cells were disrupted 16 to 18 h posttransfection using a lysis buffer containing 25 mM HEPES pH 7.4, 5 mM NaPPi, 0.5% Triton X-100, 25 mM NaCl, 5 mM EDTA, 1 mM PMSF, 1 Na3VO4 and 60 mM n-octyl-β-D-glucoside (NOG). Flag- or HA-beaded agarose was added as recommended by the manufacturer and incubated for 2 to 3 h at 4°C. Immune complexes were subsequently washed and subjected to Western blot analysis.
GST pulldown assay.
Recombinant GST-PKB, His-β-arrestin 1, and maltose binding protein (MBP)-PDE4D were incubated at 1:1:1 ratio (200 nM each) for 30 min at 4°C using a pulldown buffer containing 20 mM Tris-HCl (pH 7.4), 300 mM NaCl, 0.1% Triton X-100, 1 mM PMSF, 1 mM EDTA, 1 mM dithiothreitol (DTT), and protease inhibitors (Complete tablet). Glutathione agarose beads containing 0.1% bovine serum albumin (BSA) were then added, and the samples were incubated for 2 h at 4°C and subsequently washed and subjected to Western blot analysis.
Autospot peptide array.
Peptide arrays were synthesized on nitrocellulose membranes using a MultiPep automated peptide synthesizer (Intavis Bioanalytical Instruments AG) as described previously (24). The peptide membranes were probed with GST (control) and GST fusion proteins (1 μg/ml) and bound proteins subsequently detected using horseradish peroxidase-conjugated anti-GST antibodies and ECL.
Peptide synthesis and loading.
β-Arrestin 1 (LIELDTNDRDFVFEDF) and control (LIELDTNDDLIVFEDF) peptides were synthesized in-house with nine arginine residues at the N termini to make the peptides cell permeable. Purity was analyzed by high-pressure liquid chromatography (HPLC) and mass spectroscopy and peptide concentrations determined using a Biochrom 30 amino acid analyzer (Biochrom, Cambridge, United Kingdom). The peptides were dissolved in dimethyl sulfoxide (DMSO) and added directly to cell cultures at concentrations of up to 30 μM at different time intervals.
Protein expression and purification.
β-Arrestins 1 and 2 were expressed as GST or His fusion proteins in Escherichia coli BL21 at 22°C for 4 h after IPTG (isopropyl-β-d-thiogalactopyranoside) induction (0.1 to 1 mM) and purified overnight on glutathione-agarose beads or by using His-Select Ni affinity gel, respectively, using methods previously described (49). Purified proteins were stored at −80°C in 25% (vol/vol) glycerol.
Immunofluorescence.
Immunofluorescence was performed on human primary T cells attached to polylysine-coated coverslips. Cells were washed in phosphate-buffered saline (PBS), fixed with 3% paraformaldehyde, and permeabilized with 0.1% Triton X-100. Proteins were blocked in 2% BSA-PBS containing Tween 20 before antibody labeling. Primary and secondary antibodies were incubated for 30 min. Cells were subsequently examined using a Zeiss LSM 510 META confocal microscope (63× magnification). Z-line scans and quantification of colocalization were done using the Zeiss Image Browser software. Statistics were applied to the colocalization data in Graphpad Instat.
siRNA duplexes.
siRNA duplexes targeting human β-arrestin 1 (NM_020251) (s1624) and β-arrestin 2 (NM_004313) (s1625) in addition to a negative-control siRNA (AM4611) were obtained from Ambion (Applied Biosystems). The siRNAs used for Fig. 6E were designed and synthesized in-house, and the following siRNA duplexes were used: Arr1 607, 5′ CUG GAU AAG GAG AUC UAU UAC 3′; Arr2 376, 5′ AAG GAC CGC AAA GUG UUU GUG 3′; and negative-control GL3, 5′ CUU ACG CUG AGU ACU UCG AUU 3′. Primary T cells were transfected with 4 to 800 nM siRNA. Jurkat TAg cells were transfected with 800 to 1,000 nM siRNA.
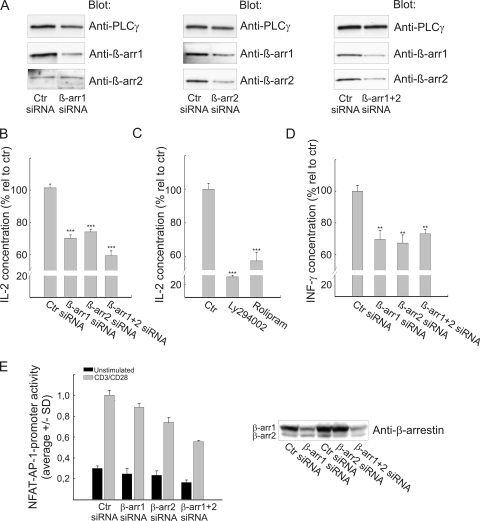
FIG. 6.
Disruption of the PKB/β-arrestin/PDE4 complex by siRNA knockdown reduces IL-2 production in CD3/CD28-costimulated T cells. (A) Human primary T cells were transfected with 400 nM β-arrestin-specific siRNA (s1624 and/or s1635) or validated, negative-control siRNA as indicated. At 48 h posttransfection, cells were lysed on ice and cell extracts analyzed for β-arrestin and PLC-γ content by immunoblotting. The experiment shown is representative of three independent experiments. (B) Human primary T cells were transfected as for panel A, and after 48 h of incubation, the cells were costimulated for 20 h using CD3/CD28-coated beads at a 1:1 bead-to-cell ratio. Supernatants were subsequently analyzed for IL-2 content. The data are presented as means ± SEM and represent 7 to 12 individual cell cultures from three or four transfections. ***, P < 0.001. (C) Human primary T cells were preincubated with DMSO (control), 10 μM LY294002, or 10 μM rolipram for 30 min at 37°C. Stimulation and IL-2 measurements were as for panel B. The data are presented as means ± SEM and represent six individual cell cultures from two different blood donors. ***, P < 0.001. (D) The supernatants described for panel B were also analyzed for IFN-γ content (means ± SEM). **, P < 0.01. (E) Jurkat TAg cells were cotransfected with NFAT-AP-1 luciferase reporter and thymidine kinase (TK)-Renilla-luciferase constructs in addition to control or β-arrestin-specific siRNA (Arr1 607 and Arr2 376) as indicated. At 48 h posttransfection, cells were left unstimulated or costimulated with anti-CD3 (2.5 μg/ml) and anti-CD28 (0.5 μg/ml) for 6 h at 37°C. The cells were subsequently lysed and a dual-luciferase assay performed with triplicate measurements. The data are presented as averages ± standard deviations (SD), and β-arrestin knockdown was verified by immunoblotting.
IL-2 and IFN-γ assays.
For IL-2 and IFN-γ production, human primary T cells were costimulated for 20 h using anti-CD3 and anti-CD28 coated beads at a 1:1 bead-to-cell ratio. Standard IL-2 (D2050) and IFN-γ (DIF50) assays were used to measure secreted IL-2 and IFN-γ (triplicate determinations), respectively.
RESULTS
Recruitment of β-arrestin and PDE4 requires activation of a CD28- and PI3K-dependent pathway.
In the present study, we examined how CD3/CD28 costimulation of primary T cells might elicit recruitment of a β-arrestin/PDE4 complex to lipid rafts. CD28 costimulation is most readily observed in the presence of suboptimal concentrations of CD3. Thus, the concentration of anti-CD3 used in these experiments was insufficient to cause β-arrestin recruitment, and optimal recruitment of β-arrestin to lipid rafts depended on CD3/CD28 costimulation. However, CD28 stimulation alone led to some recruitment of β-arrestin (Fig. 1A), and a rapid increase in the activity of PDE4, which is now well established as being sequestered by β-arrestin (3, 7, 10, 30, 38), was also observed in raft fractions from CD28-stimulated cells (Fig. 1B). In contrast to CD28- and CD3/CD28-stimulated cells, only a minor increase in PDE4 activity was observed in raft fractions from CD3 stimulated T cells (Fig. 1B) (not significant). However, as observed in Fig. 1A and B, stimulation of CD3 acted synergistically with CD28 stimulation in the recruitment of β-arrestin and PDE4. We concluded that the CD28 signal plays a distinct role enhanced by a concomitant TCR signal. To discern the effect of the costimulatory signal we next studied the isolated effect of CD28 stimulation.
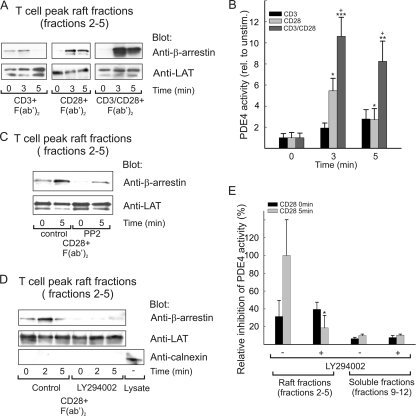
FIG. 1.
β-Arrestin/PDE4 recruitment to T-cell lipid rafts requires activation of a CD28- and PI3K-dependent pathway. (A) Human primary T cells were stimulated with anti-CD3, anti-CD28, or a combination of anti-CD3 and anti-CD28 for 2 min, followed by cross-ligation with F(ab′)2 fragments for the indicated time periods. After lysis on ice, lipid rafts were purified by sucrose gradient ultracentrifugation, and peak raft fractions (fractions 2 to 5) were pooled and analyzed for β-arrestin and LAT content by immunoblotting. The experiment shown is representative of three independent experiments. (B) PDE4 activity was measured in the peak raft fractions of CD3-stimulated, CD28-stimulated, and CD3/CD28-costimulated T cells. Data are presented as means ± standard errors of the means (SEM) relative to the unstimulated control (n = 12). * indicates significance between unstimulated and stimulated samples, whereas + shows significance between CD28 and CD3/CD28 stimulation within the same time point. */+, P < 0.05; **, P < 0.01; ***, P < 0.001. No significant increase in PDE4 activity was observed for cells stimulated with CD3 alone. (C) T cells were preincubated with DMSO (control) or 10 μM PP2 for 20 min at 37°C. The cells were subsequently stimulated with anti-CD28 and rafts purified as described for panel A. Recruitment of β-arrestin to peak raft fractions was examined by immunoblotting. The experiment shown is representative of three independent experiments. (D) T cells were pretreated with DMSO (control) or with 50 μM LY294002 for 20 min at 37°C, and T-cell stimulation, raft preparation, and analysis were performed as described above. Anticalnexin, an endoplasmic reticulum (ER) marker, was used to verify the purity of the rafts. The experiment shown is representative of three independent experiments. (E) LY294002-treated T cells were stimulated and rafts prepared as for panel D. All fractions were subsequently analyzed for PDE4 activity. The data are presented as means ± SEM relative to CD28-stimulated control cells (n = 8). *, P < 0.05.
To establish the pathway involved in CD28 triggered recruitment of the β-arrestin/PDE4 complex, we analyzed how β-arrestin levels in T-cell lipid raft fractions were affected by inhibition of either Src family tyrosine kinases (SFKs) or PI3K, using PP2 and LY294002, respectively. Strikingly, the CD28-induced recruitment of β-arrestin was dramatically reduced upon inhibition of either SFKs (Lck/Fyn in T cells) (Fig. 1C) or PI3K (Fig. 1D). Similar results were obtained using the PI3K inhibitor wortmannin (data not shown). To verify purity of the rafts, blots were also analyzed for the presence of the nonraft protein calnexin (Fig. 1D). Furthermore, PDE4 activity in raft fractions was reduced below basal levels in primary T cells pretreated with LY294002, and no increase in PDE4 activity was observed upon CD28 ligation (Fig. 1E). Taken together, these data suggest a mechanism whereby PI3K can regulate targeted cAMP degradation in lipid rafts via recruitment of a β-arrestin/PDE4 complex.
The β-arrestin/PDE4 complex interacts with both PIP3 and PKB.
Upon activation, class I PI3Ks convert phosphatidylinositol-(4,5)-bisphosphate (PIP2) to phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) in the membrane, and PIP3 subsequently acts as a second messenger by recruiting proteins containing pleckstrin homology (PH) domains to sites of PI3K activation (42). Thus, PIP3 controls both the activity and subcellular localization of a broad range of signal transduction molecules.
In order to test how PI3K mediates the recruitment of the β-arrestin/PDE4 complex, pulldown experiments were performed on detergent-solubilized cell extracts from CD3/CD28-costimulated primary T cells using PIP3-coated beads. These solid-phase adsorption experiments demonstrated that the β-arrestin/PDE4 complex interacts with PIP3 (Fig. 2A). As a positive control, the PH domain-containing proteins PKB and PDK1 were also shown to interact with the PIP3 beads. β-Arrestins do not possess a PH domain, and we could not detect any AP-2 in T-cell rafts, suggesting that β-arrestin recruitment upon CD3/CD28 costimulation does not depend on AP-2 (data not shown). However, we surmised that translocation of the β-arrestin complex to rafts might be mediated by a specifically sequestered PH domain-containing partner protein. In this regard, we observed concurrent high levels of PKB present in lipid raft fractions of TCR- and CD28-costimulated T cells (Fig. 2B).
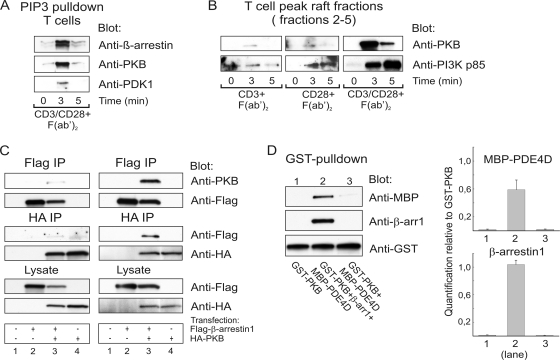
FIG. 2.
The β-arrestin/PDE4 complex interacts with PKB. (A) Human primary T cells stimulated with anti-CD3 and anti-CD28 were subjected to immunoprecipitations using PIP3-coated beads and the precipitates analyzed by immunoblotting with the indicated antibodies. (B) T cells were stimulated and lipid raft fractions prepared as described for Fig. 1A. Recruitment of PKB and PI3K to peak raft fractions (fractions 2 to 5 pooled) was subsequently tested by Western blot analysis. (C) Human primary T cells were transfected with Flag-tagged β-arrestin 1 and/or HA-tagged PKBα as indicated. Prior to lysis and immunoprecipitation (IP), cells were left unstimulated (left panel) or were CD3/CD28 costimulated for 3 min (right panel). Immune complexes were subsequently immunoprecipitated using anti-Flag- or anti-HA-coated beads. The β-arrestin-PKB interaction was examined by immunoblotting. Whole-cell lysates were also tested for Flag-β-arrestin and HA-PKB expression using anti-Flag and anti-HA antibodies, respectively. (D) Recombinant GST-PKB, His-β-arrestin 1, and MBP-PDE4D (200 nM each) were incubated for 30 min at 4°C, followed by addition of reduced glutathione agarose beads. Complex formation was examined by Western blot analysis (left panel). The amounts of β-arrestin 1 and PDE4D pulled down by PKB were calculated by densitometric quantification of Western blots relative to GST-PKB in three individual experiments (right panel). The Western blot experiment shown in each panel is representative of three independent experiments.
This prompted us to examine whether β-arrestin and PKB might interact with each other and whether PKB could thus provide a candidate PH domain for the CD28-triggered, PI3K-dependent recruitment of β-arrestin to lipid rafts. As a first step, we cotransfected Jurkat TAg cells with Flag-tagged β-arrestin constructs and HA-tagged PKB and performed immunoprecipitations on lysates from unstimulated cells using Flag- and HA-coated beads, respectively (data not shown). Interestingly, we found that PKB coimmunoprecipitated with both β-arrestin 1 and β-arrestin 2. However, as Jurkat TAg cells have constitutively high PIP3 levels due to the lack of PTEN, it was not possible to conclude whether a fraction of PKB existed in a preassembled complex with β-arrestin in resting T cells. Next, we therefore repeated the immunoprecipitation studies on lysates from both unstimulated (Fig. 2C, left panel) and CD3/CD28-costimulated (Fig. 2C, right panel) primary T cells. In unstimulated lysates, small amounts of PKB and Flag-β-arrestin 1 were coimmunoprecipitated with Flag-β-arrestin 1 and HA-PKB, respectively. However, significant levels of β-arrestin 1 could be coimmunoprecipitated only with PKB and vice versa in lysates from stimulated cells (Fig. 2C).
While the PKB/β-arrestin and β-arrestin/PDE4 interactions could be demonstrated independently, the possibility that one interaction excluded the other still existed. We therefore next examined the possible presence of a PKB/β-arrestin/PDE4 ternary complex by GST pulldown experiments using equimolar amounts of recombinant GST-PKB, His-β-arrestin 1, and MBP-PDE4D (Fig. 2D). This demonstrated that GST-PKB consistently pulled down both β-arrestin and PDE4, the latter only in the presence of β-arrestin, indicating that trimolecular interactions are permitted but only when β-arrestin is present to contact both PKB and PDE4. Quantitative analyses of the data revealed that somewhat less PDE4 than β-arrestin is pulled down by the glutathione beads interacting with GST-PKB, which presumably reflects the indirect nature of the interaction between PKB and PDE4.
Identification of the β-arrestin and PKB binding motifs.
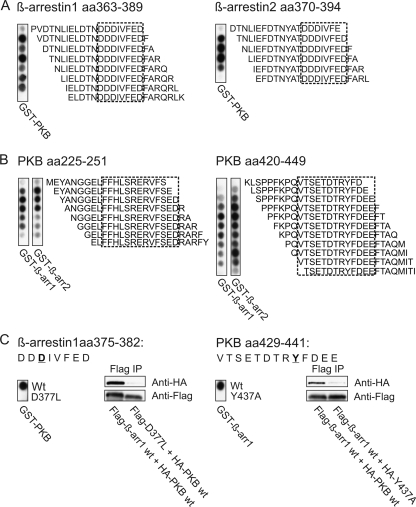
Over the last decade, in addition to their roles in desensitization and internalization, β-arrestins have also been shown to act as signaling scaffolds for a number of pathways (reviewed in references 13, 14, and 20). Recently, β-arrestin 2 was proposed to be involved in the regulation of PKB by dopamine receptors (8). In neurons, dopamine stimulates formation of a signaling complex consisting of β-arrestin 2, PKB, and its negative regulator, protein phosphatase 2A (PP2A). However, it has not been shown whether PKB and/or PP2A binds directly or indirectly to β-arrestin 2. In T cells, we observed assembly of a supramolecular β-arrestin/PDE4/PKB complex. To identify and precisely define the binding motifs in both β-arrestin and PKB, we synthesized libraries of overlapping peptides representing full-length human β-arrestins 1 and 2 (Fig. 3A) and PKBα (Fig. 3B). These peptides were overlaid with recombinant GST fusion proteins of PKB (Fig. 3A) and β-arrestin 1 and 2 (Fig. 3B) in a solid-phase binding experiment employing GST as a control. While no interaction was observed on arrays probed with GST-protein alone (data not shown), PKBα was found to recognize similar regions in the C-terminal parts of both β-arrestins 1 and 2 (Fig. 3A). The PKB-binding regions in β-arrestins 1 and 2 were analyzed further by N- and C-terminal truncations and alanine substitution scans to reveal the boundaries of the interaction sites (amalgamated data are outlined by the dashed boxes in Fig. 3A). By this method a minimal binding motif, DDDIVFED, conserved in both β-arrestins 1 and 2 was identified. β-Arrestin 1 and β-arrestin 2 were found to recognize two regions in the catalytic domain of PKBα (Fig. 3B), both of which are highly conserved in the three human PKB isoforms (α, β, and γ), and minimal binding motifs were defined as described above (dashed boxes, based on amalgamated data from truncation and substitution peptide array studies). Further characterization of the β-arrestin-PKB interaction was accomplished by a two-dimensional (2D) peptide array study. A total of 240 peptide derivatives were generated by replacing the residues in the minimal binding motifs of β-arrestins 1 and 2 with all natural amino acids (data not shown). Subsequently, peptides containing substitutions within the binding motifs of both β-arrestin and PKBα that appeared to reduce binding were spotted on membranes, and interaction was examined using GST-PKBα and GST-β-arrestin as probes, respectively (Fig. 3C). To verify the importance of these amino acid residues in situ, several interaction mutants were prepared based on the 2D peptide array data. HEK 293T cells were subsequently cotransfected with Flag-tagged β-arrestin 1 and HA-tagged PKBα constructs and immunoprecipitations performed. As seen in Fig. 3C, these mutants (β-arrestin 1 D377L and PKB Y437A) displayed a loss of interaction, indicating that motifs mapped in vitro also mediate interactions inside cells (Fig. 3C). Taken together, our data show that the supramolecular complex recruited upon CD3/CD28 stimulation contains β-arrestin 1, PDE4, and PKB and that β-arrestin and PKB interact directly with each other.
FIG. 3.
A conserved amino acid (aa) stretch in β-arrestins 1 and 2 interacts with well-defined and conserved regions in PKB. (A) Human β-arrestins 1 and 2 were spotted as 20-mer peptides with shifts of one amino acid on a cellulose membrane and probed with recombinant GST-PKBα. Binding of PKB to specific peptides was detected by immunoblotting using GST-antibodies, whereas no interaction was observed in overlays using purified GST-protein alone (data not shown). Dashed-line boxes indicate a minimal binding motif conserved in both β-arrestins 1 and 2 as identified by N- and C-terminal truncation studies and double-alanine substitution scans of the PKB-binding sequences. (B) Human PKBα was spotted as described above and the overlay performed using purified GST β-arrestin 1 and 2 recombinant proteins. The minimal binding motifs of each region were identified as described above and are indicated by dashed-line boxes. (C) Peptides containing possible nonbinding substitutions within the minimal binding motifs of both β-arrestin and PKBα were spotted on membranes and the presence or absence of interaction examined using GST-PKBα and GST-β-arrestin in the overlays, respectively. To verify the importance of these amino acid residues, several interaction mutants were prepared and tested in HEK 293T cells. Cells were cotransfected with Flag-tagged β-arrestin 1 and HA-tagged PKBα constructs as indicated and immunoprecipitations performed using Flag-coated beads. Changes in the β-arrestin 1-PKBα interaction were assessed by immunoblotting. The experiment shown in each panel is representative of three similar experiments.
Recruitment of the β-arrestin/PKB complex depends on the PKB PH domain.
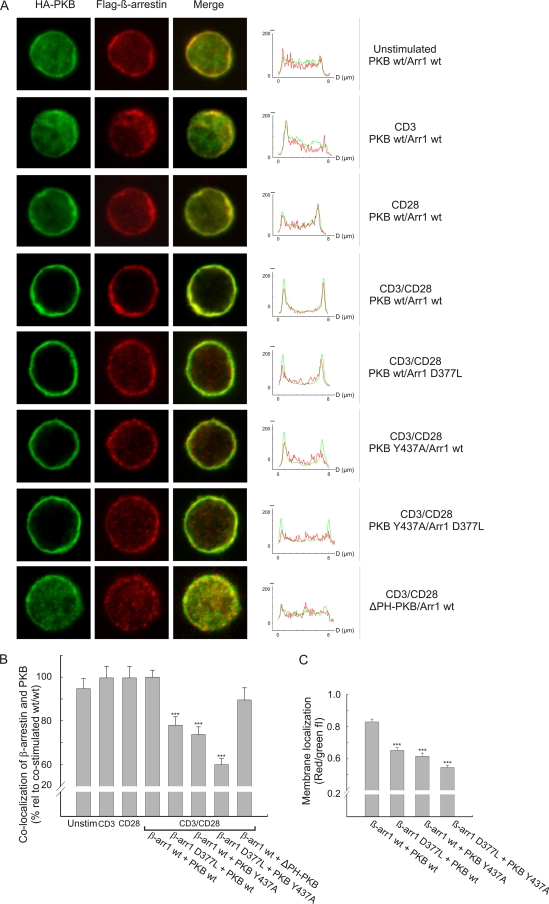
Cytosolic PKB is maintained in its inactive state due to an intrachain interaction between the PH and kinase domains, blocking access to the activation loop (11). Binding of the PKB PH domain to PIP3 directs PKB from the cytosol to the membrane, and this is thought to induce a conformational change that allows the associated PDK1 to phosphorylate Thr308, leading to PKB activation (11, 33). In order to determine how the PKB/β-arrestin/PDE4 complex interacts with the membrane, localizations of Flag-tagged β-arrestin and HA-tagged PKB were examined by dual-immunofluorescence staining of CD3/CD28-costimulated human primary T cells (Fig. 4A, rows 4 to 8). In addition, localizations of β-arrestin and PKB were also examined in resting T cells and separately upon stimulation with anti-CD3 and anti-CD28, which recruited only modest amounts of β-arrestin and PKB to the membrane (Fig. 4A, rows 1 to 3). In contrast, CD3/CD28 costimulation resulted in robust recruitment of both wild-type β-arrestin 1 and wild-type PKBα to the membrane (row 4). The residues in PKB interacting with β-arrestin are located in the C-terminal part of the protein, and mutations here did not interfere with the PIP3-PH interaction in the membrane. Strikingly, mutations in either of the interaction regions affected membrane translocation of β-arrestin 1 but not that of PKBα (rows 5 to 7). This is compatible with the notion that PKB transports β-arrestin to the membrane and not vice versa. In support of PKB-mediated recruitment of β-arrestin 1, neither PKB nor β-arrestin was recruited to the membrane in cells cotransfected with a PKB construct lacking the PH domain (ΔPH-PKBα) and wild-type β-arrestin 1 (Fig. 4A, row 8). We observed that mutation of the PKB and β-arrestin interaction domains did not completely abolish membrane localization of β-arrestin. This is most likely due to β-arrestin association with endogenous PKB but could also be caused by β-arrestin interacting with other partners. Furthermore, when quantifying the effect of the PKB and β-arrestin mutants using confocal images, a 40% loss of colocalization across the cell was observed when analyzing for double-positive pixels, indicating that the mutants disrupted colocalization of both the cytoplasmic and the membrane-associated complex at least partially (Fig. 4B). However, with the submicrometer resolution of the confocal microscope, the apparent colocalization of β-arrestin and PKBα without subcellular colocalization (ΔPH-PKBα) in the cytoplasm and nucleus by no means indicates the existence of an interaction between the two molecules. Furthermore, when analyzing the mean fluorescence intensity ratios, i.e., red (arrestin)/green (PKB), in the membrane areas of the cells, a 35 to 40% reduction in CD28-induced membrane relocalization of β-arrestin with PKB was revealed for cells transfected with both interaction mutants compared to cells expressing the wild-type constructs (Fig. 4C).
FIG. 4.
Recruitment of the β-arrestin/PKB complex depends on the PKB PH domain. (A) Human primary T cells were transfected with Flag-tagged β-arrestin 1 and HA-tagged PKBα constructs as indicated, and the cells were stimulated with anti-CD3, anti-CD28, or a combination of anti-CD3 and anti-CD28 for 2 min, followed by cross-ligation with F(ab′)2-fragments for 3 min as indicated. PKBα was visualized using an Alexa488-conjugated HA-antibody (green), whereas β-arrestin was visualized using an anti-Flag antibody followed by staining with an isotype-specific Alexa546-conjugated secondary antibody (red). In the right panels, z-line scans of the immunofluorescence images show the green and red fluorescence intensity profiles. wt, wild type. (B) Colocalization of β-arrestin and PKB was calculated by quantifying the number of pixels that were both green and red positive throughout the cell. The data are presented as means ± SEM, and 30 to 35 images per group were analyzed. ***, P < 0.001. (C) Membrane localization was measured as red (arrestin)/green (PKB) fluorescence intensity, and this ratio was determined by calculating the areas below the red and green fluorescence intensity profiles, respectively. For each cell image, two z-line scans were performed and four membrane regions examined. The diameter of each region was set to 1 μm, and 10 images per group were analyzed. The data are presented as means ± SEM (n = 40). ***, P < 0.001.
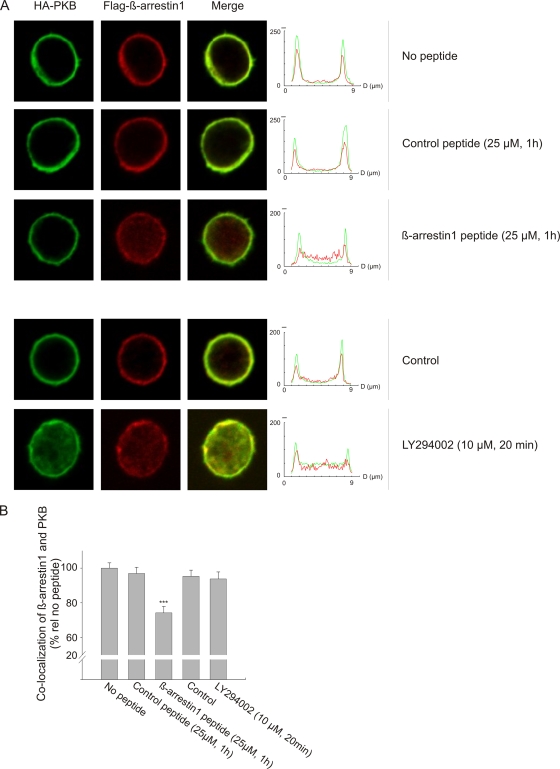
Next, we examined the effect of disrupting the interaction between wild-type β-arrestin and PKB upon CD3/CD28 costimulation. Cell-permeable arginine-coupled peptides were derived from the minimal binding motifs of β-arrestins 1 and 2, and the peptides were designed to bind to PKB with high affinity. Two substitutions (D376R and I378F) were introduced in the β-arrestin 1 peptide (LIELDTNDRDFVFEDF), increasing PKB binding above that of the wild-type sequence. For a control peptide, a D377L substitution (LIELDTNDDLIVFEDF) was inserted to abrogate peptide interaction with PKB. Human primary T cells were transfected with tagged wild-type β-arrestin 1 and PKBα constructs and the disruptor peptides added directly to the cell cultures 1 h prior to stimulation. Following CD3/CD28 costimulation, β-arrestin 1 and PKBα were both colocalized at the membrane in control cells (no peptide) and in cells pretreated with the control peptide (Fig. 5A). Addition of the β-arrestin 1 disruptor peptide affected translocation of β-arrestin 1, but not that of PKBα, to the membrane, confirming the observation made with the interaction mutants (compare Fig. 4A and 5A), indicating that PKB transports β-arrestin to the membrane but not vice versa. Quantitative analyses of the effect of the β-arrestin 1 peptide disruptor revealed a 25% loss of colocalization across the cell (Fig. 5B). In contrast, treating the cells with the PI3K inhibitor LY294002 prior to costimulation prevented both β-arrestin and PKB from being recruited to the membrane but did not affect colocalization of β-arrestin 1 and PKBα in the cytoplasm compared to the peptide disruptor (Fig. 5A and B).
FIG. 5.
Translocation of the β-arrestin/PKB complex is abrogated in the presence of a peptide disruptor of the β-arrestin/PKB interaction or the PI3K inhibitor LY. (A) Human primary T cells were transfected with Flag-tagged β-arrestin 1 and HA-tagged PKBα, and the cells were costimulated as described for Fig. 4. Prior to stimulation, the cells were treated with DMSO (control), 25 μM peptide, or 10 μM LY294002 as indicated. Control and disruptor peptides were designed based on amino acids 368 to 383 of human β-arrestin 1, which were shown in Fig. 3 to interact with PKB. Visualization of HA-PKBα and Flag-β-arrestin 1 was performed as described for Fig. 4. In the right panels, z-line scans of the immunofluorescence images show the green and red fluorescence profiles. (B) Colocalization of β-arrestin 1 and PKB was calculated by quantifying the number of pixels that were both green and red positive throughout the cell. The data are presented as means ± SEM, and 35 to 40 images per group were analyzed. ***, P < 0.001.
siRNA-mediated knockdown of β-arrestin results in reduced cytokine production from primary T cells.
In order to assess the biological importance of the β-arrestin complex in proximal T-cell signaling, small interfering RNA (siRNA)-mediated knockdown of β-arrestin was performed. Different siRNAs were first tested in Jurkat TAg cells (data not shown), and the two most efficient siRNAs resulting in the strongest reduction of β-arrestin levels (s1624 and s1625) were subsequently used in human primary T cells, resulting in knockdown of β-arrestin 1 (Fig. 6A, left panel) as well as β-arrestin 2 (middle panel) or both (right panel). In the case of β-arrestin 2 knockdown, some crossover to β-arrestin 1 was also observed. At 48 hours posttransfection, T cells were costimulated with CD3/CD28 for 20 h and secreted IL-2 subsequently measured in the supernatants (Fig. 6B). Human primary T cells transfected with siRNA targeting both β-arrestins 1 and 2 revealed a 40% reduction in IL-2 production. This reduction is comparable to the decrease observed when treating T cells with the PDE4 inhibitor rolipram (Fig. 6C). No additive effects were observed by combining siRNA and inhibitor, indicating that the effect is saturated by either alone (data not shown). Furthermore, we observed a 30% reduction in IFN-γ production as a result of β-arrestin 1 or 2 knockdown in primary T cells (Fig. 6D). Lastly, a 40% reduction in IL-2 promoter activity was observed after siRNA-mediated knockdown of β-arrestin 1 and 2 in Jurkat TAg cells (Fig. 6E [using a different pair of siRNAs]). Altogether, these data strongly suggest a positive regulatory role of β-arrestin in proximal T-cell activation.
DISCUSSION
cAMP is rapidly produced in lipid rafts upon TCR engagement (3), and this localized cAMP pool inhibits T-cell activation through the PKA-Csk pathway, as demonstrated earlier (44, 50). A full T-cell response is therefore dependent on tight control of the TCR-induced cAMP levels. The cAMP-PKA-Csk negative feedback loop elicited by TCR triggering is overcome by recruitment of a β-arrestin/PDE4 complex upon CD3/CD28 costimulation, breaking the negative feedback loop by delivering an enzyme that actively degrades cAMP to this spatially constrained pool of “inhibitory” cAMP. Thus, a key function of β-arrestin in T cells is the shuttling of PDE4 to the site of cAMP production at this key locale, thereby promoting degradation of the second messenger and relieving the inhibitory constraint on T-cell activation (3, 7, 38). Recently, β-arrestin has been shown to be involved in aiding the reduction of another, very distinct second messenger, diacylglycerol (DAG), by recruiting diacylglycerol kinases (DGKs) to the ligand-activated G-coupled receptor (34). Thus, a critical and novel role of β-arrestin appears to reside in its ability to spatially regulate signaling responses by targeting second-messenger degradation to specific intracellular sites.
Here, we demonstrate that ligation of CD28 elicits recruitment of the β-arrestin/PDE4 complex in human primary T cells, although CD3/CD28 coligation promotes even more potent recruitment. In case of the β2-adrenergic receptor, the cytosolic β-arrestin/PDE4 complex is recruited directly to this G protein-coupled receptor (GPCR) upon agonist occupancy (38). However, as neither the TCR nor CD28 is a GPCR, a novel mechanism must be operating in this instance. Here, we make the novel observation that β-arrestin/PDE4 recruitment to T-cell lipid rafts subsequent to CD28 ligation depends on the activity of both Lck and PI3K. In contrast to the TCR, CD28 can interact directly with PI3K through a YMNM binding motif phosphorylated by Lck, and this interaction has been shown to be critical for cell survival responses mediated by CD28 in vivo (36). CD28 ligation alone has been demonstrated to activate the PI3K pathway in primary T cells (6, 15), and PIP3 is rapidly produced in the membrane upon PI3K activation enabling interaction with effector proteins through their pleckstrin homology (PH) domains. Although β-arrestin does not possess a PH domain, we observed in our study that the β-arrestin complex can interact with PIP3 in human primary T cells in an activation-dependent fashion. While β-arrestin has been reported to interact indirectly with lipids via AP-2 (25) or directly through a high-affinity phosphoinositide (IP6) binding site (17), we found that mutating this site (K232Q/R235Q/K250Q in β-arrestin 1 and K233Q/R237Q/K252Q in β-arrestin 2) did not affect β-arrestin recruitment to lipid rafts (data not shown). In addition, we could not detect any AP-2 in T-cell rafts, indicating that AP-2 is not involved in β-arrestin recruitment upon CD3/CD28 costimulation (data not shown). Thus, we surmised that translocation of the β-arrestin/PDE4 complex upon CD3/CD28 costimulation may be mediated by a PH domain-containing interaction partner that confers binding to PIP3. We were able to demonstrate that the PH domain-containing protein PKB interacts directly with β-arrestin to form a key part of this dynamic signaling complex. The consistent observation of precipitation of the complex by PIP3 beads following CD3/CD28 costimulation was somewhat surprising. The detergent-solubilized extracts used in the PIP3 precipitation experiments would, however, also include the population of the complex that had already bound PIP3 and where PKB is activated. This may suggest that assembly or translocation of the complex is regulated by engagement of the PH domain. In addition, a recent study by Waugh et al. reported that the PDK1 PH domain is required for optimal PKB activation and that PIP3 binding to PDK1 and thereby the strength of PKB activity can determine the nature of the T-cell response (51).
Peptide array analysis and mutational studies allowed us to determine the binding motifs in both β-arrestin and PKB. Interestingly, the core binding sequence in β-arrestin that interacts with PKB (DDDIVFED) is located within a region that has been reported to be involved in both clathrin and AP-2 (β2-adaptin) binding to β-arrestin 2 (23). This suggests that a PDE4/β-arrestin/PKB complex is unlikely to bind clathrin and would thus be excluded from endocytosis upon relocalization to the plasma membrane.
Various studies have implicated β-arrestin in the regulation of PKB activity. Thus, the PI3K/PKB pathway has been reported to be activated in a β-arrestin 1-dependent manner upon stimulation of the insulin-like growth factor (IGF-1) receptor, resulting in increased protection from apoptosis (39). In neurons, β-arrestin 2 has been reported to be involved in PKB signaling downstream of the dopamine receptor, where dopamine stimulates formation of a signaling complex consisting of β-arrestin 2, PKB, and its negative regulator, protein phosphatase 2A (PP2A) (8). Recently, it has also been demonstrated that upon insulin stimulation, β-arrestin 2 mediates PKB activation through Src in a PI3K-independent way (29). Thus, β-arrestin can, seemingly, function as a signaling scaffold by assembling either positive or negative regulators of the PKB pathway. Until now, it has been elusive whether PKB can bind directly or indirectly to β-arrestin. However, here we identify the interacting domains that define the direct interaction surface between PKB and β-arrestin. By means of both β-arrestin-PKB interaction mutants and a β-arrestin-derived, high-affinity disruptor peptide, we also demonstrate that PKB, through its PH domain, is responsible for transporting this supramolecular complex to the membrane upon CD3/CD28 costimulation, thus revealing a novel role for PKB. Further work will be needed to appreciate whether additional signaling molecules may form part of the β-arrestin/PDE4/PKB complex in T cells. The exact role of β-arrestin in proximal T-cell signaling is not fully understood. However, we observed 40% and 30% reductions in IL-2 and IFN-γ production, respectively, after siRNA-mediated knockdown of β-arrestins 1 and 2 in human primary T cells, suggesting a positive regulatory role of β-arrestin in proximal T-cell activation. The magnitudes of these effects parallels those observed by pharmacologically inhibiting PDE4 activity and may indicate that the key role of β-arrestin in proximal T-cell signaling resides in its ability to recruit the signal termination enzyme PDE4 to lipid rafts so as to break the cAMP negative feedback governing T-cell receptor functioning.
Our study also suggests a more general mode of action for the PKB/β-arrestin scaffold, whereby receptor-mediated activation of the PI3K/PKB signal pathway, not only by CD28 ligation but also potentially by other stimuli, may cross talk with GPCR-cAMP signal pathways at the level of the PKB/β-arrestin/PDE4 complex so as to reduce cAMP levels at targeted intracellular sites. This would confer both a spatial regulatory component and a temporal regulatory component on the machinery in the vicinity of the delivery point of β-arrestin with its sequestered PDE cargo. It is interesting to speculate that such cross talk may occur, e.g., in the interplay between insulin and adrenergic signaling in metabolism or in response to IL-4.
In conclusion, our results reveal a novel mechanism whereby CD28 regulates cAMP-degradation in lipid rafts via recruitment of a PKB/β-arrestin/PDE4 complex, with consequences for effector T-cell function. We demonstrate that recruitment depends on both Lck and PI3K activities and the formation of PIP3 and that PKB plays a critical role in mediating the trafficking of this complex. In this regard, we show that β-arrestin interacts directly with PKB and that translocation of this supramolecular complex to the membrane depends on the PKB plextrin homology (PH) domain interacting with PIP3 originating through CD28 ligation (Fig. 7). Thus, we propose a mechanism whereby CD28 engagement supersedes the negative feedback loop provided by TCR-induced cAMP production, consequently allowing full T-cell activation to proceed.
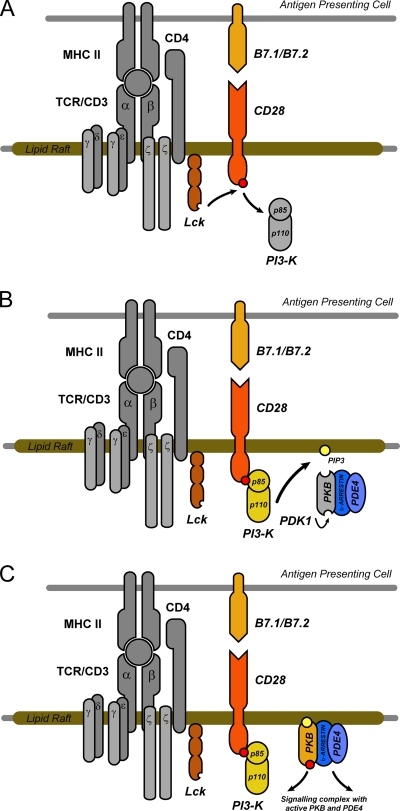
FIG. 7.
Model for a novel mechanism whereby CD28 regulates cAMP degradation in T-cell lipid rafts through recruitment of a PKB/β-arrestin/PDE4 complex. Ligation of CD28 results in phosphorylation of the YMNM motif in the cytoplasmic tail of CD28 by Lck (A), generating a binding site for the SH2 domain of the p85 regulatory subunit of PI3K (B). PI3K activation and PIP3 production serve to recruit a supramolecular PKB/β-arrestin/PDE4 complex to lipid rafts, and recruitment occurs via the PKB plextrin homology (PH) domain (C).
Acknowledgments
We are grateful to J. Solheim, G. Tjørhom, O. Blingsmo, and G. Opsahl for excellent technical assistance.
This work was supported by grants from the Norwegian Functional Genomics Program, the Research Council of Norway, the Norwegian Cancer Society and European Union (grant no. LSHB-CT-2006-037189-thera-cAMP to K.T. and M.D.H.); by the Medical Research Council (United Kingdom) (grant G0600765 to M.D.H. and G.S.B.); and by the Fondation Leducq (grant 06CVD02 to M.D.H. and G.S.B.).
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Aandahl, E. M., P. Aukrust, B. S. Skalhegg, F. Muller, S. S. Froland, V. Hansson, and K. Tasken. 1998. Protein kinase A type I antagonist restores immune responses of T cells from HIV-infected patients. FASEB J. 12:855-862. [DOI] [PubMed] [Google Scholar]
- 2.Aandahl, E. M., W. J. Moretto, P. A. Haslett, T. Vang, T. Bryn, K. Tasken, and D. F. Nixon. 2002. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J. Immunol. 169:802-808. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsen, H., G. Baillie, J. Ngai, T. Vang, K. Nika, A. Ruppelt, T. Mustelin, M. Zaccolo, M. Houslay, and K. Tasken. 2004. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling. J. Immunol. 173:4847-4858. [DOI] [PubMed] [Google Scholar]
- 4.Acuto, O., and F. Michel. 2003. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 3:939-951. [DOI] [PubMed] [Google Scholar]
- 5.Acuto, O., S. Mise-Omata, G. Mangino, and F. Michel. 2003. Molecular modifiers of T cell antigen receptor triggering threshold: the mechanism of CD28 costimulatory receptor. Immunol. Rev. 192:21-31:21-31. [DOI] [PubMed] [Google Scholar]
- 6.Appleman, L. J., A. A. van Puijenbroek, K. M. Shu, L. M. Nadler, and V. A. Boussiotis. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 168:2729-2736. [DOI] [PubMed] [Google Scholar]
- 7.Baillie, G. S., A. Sood, I. McPhee, I. Gall, S. J. Perry, R. J. Lefkowitz, and M. D. Houslay. 2003. Beta-arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. U. S. A. 100:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Beaulieu, J. M., T. D. Sotnikova, S. Marion, R. J. Lefkowitz, R. R. Gainetdinov, and M. G. Caron. 2005. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261-273. [DOI] [PubMed] [Google Scholar]
- 9.Benovic, J. L., H. Kuhn, I. Weyand, J. Codina, M. G. Caron, and R. J. Lefkowitz. 1987. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc. Natl. Acad. Sci. U. S. A. 84:8879-8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolger, G. B., G. S. Baillie, X. Li, M. J. Lynch, P. Herzyk, A. Mohamed, L. H. Mitchell, A. McCahill, C. Hundsrucker, E. Klussmann, D. R. Adams, and M. D. Houslay. 2006. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem. J. 398:23-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calleja, V., D. Alcor, M. Laguerre, J. Park, B. Vojnovic, B. A. Hemmings, J. Downward, P. J. Parker, and B. Larijani. 2007. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS. Biol. 5:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conche, C., G. Boulla, A. Trautmann, and C. Randriamampita. 2009. T cell adhesion primes antigen receptor-induced calcium responses through a transient rise in adenosine 3′,5′-cyclic monophosphate. Immunity 30:33-43. [DOI] [PubMed] [Google Scholar]
- 13.Defea, K. 2008. Beta-arrestins and heterotrimeric G-proteins: collaborators and competitors in signal transduction. Br. J. Pharmacol. 153(Suppl. 1):S298-S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeWire, S. M., S. Ahn, R. J. Lefkowitz, and S. K. Shenoy. 2007. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69:483-510. [DOI] [PubMed] [Google Scholar]
- 15.Diehn, M., A. A. Alizadeh, O. J. Rando, C. L. Liu, K. Stankunas, D. Botstein, G. R. Crabtree, and P. O. Brown. 2002. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc. Natl. Acad. Sci. U. S. A. 99:11796-11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodson, L. F., J. S. Boomer, C. M. Deppong, D. D. Shah, J. Sim, T. L. Bricker, J. H. Russell, and J. M. Green. 2009. Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol. Cell. Biol. 29:3710-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaidarov, I., J. G. Krupnick, J. R. Falck, J. L. Benovic, and J. H. Keen. 1999. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 18:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcon, F., D. T. Patton, J. L. Emery, E. Hirsch, R. Rottapel, T. Sasaki, and K. Okkenhaug. 2008. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood 111:1464-1471. [DOI] [PubMed] [Google Scholar]
- 19.Goodman, O. B., Jr., J. G. Krupnick, F. Santini, V. V. Gurevich, R. B. Penn, A. W. Gagnon, J. H. Keen, and J. L. Benovic. 1996. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383:447-450. [DOI] [PubMed] [Google Scholar]
- 20.Gurevich, V. V., E. V. Gurevich, and W. M. Cleghorn. 2008. Arrestins as multi-functional signaling adaptors. Handb. Exp. Pharmacol. 2008:15-37. [DOI] [PubMed] [Google Scholar]
- 21.Holdorf, A. D., J. M. Green, S. D. Levin, M. F. Denny, D. B. Straus, V. Link, P. S. Changelian, P. M. Allen, and A. S. Shaw. 1999. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 190:375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, H. H., M. Tharayil, and C. E. Rudd. 1998. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J. Biol. Chem. 273:296-301. [DOI] [PubMed] [Google Scholar]
- 23.Kim, Y. M., and J. L. Benovic. 2002. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J. Biol. Chem. 277:30760-30768. [DOI] [PubMed] [Google Scholar]
- 24.Kramer, A., and J. Schneider-Mergener. 1998. Synthesis and screening of peptide libraries on continuous cellulose membrane supports. Methods Mol. Biol. 87:25-39. [DOI] [PubMed] [Google Scholar]
- 25.Laporte, S. A., W. E. Miller, K. M. Kim, and M. G. Caron. 2002. Beta-arrestin/AP-2 interaction in G protein-coupled receptor internalization: identification of a beta-arrestin binging site in beta 2-adaptin. J. Biol. Chem. 277:9247-9254. [DOI] [PubMed] [Google Scholar]
- 26.Laporte, S. A., R. H. Oakley, J. Zhang, J. A. Holt, S. S. Ferguson, M. G. Caron, and L. S. Barak. 1999. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U. S. A. 96:3712-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledbetter, J. A., M. Parsons, P. J. Martin, J. A. Hansen, P. S. Rabinovitch, and C. H. June. 1986. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J. Immunol. 137:3299-3305. [PubMed] [Google Scholar]
- 28.Lobban, M., Y. Shakur, J. Beattie, and M. D. Houslay. 1994. Identification of two splice variant forms of type-IVB cyclic AMP phosphodiesterase, DPD (rPDE-IVB1) and PDE-4 (rPDE-IVB2) in brain: selective localization in membrane and cytosolic compartments and differential expression in various brain regions. Biochem. J. 304:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luan, B., J. Zhao, H. Wu, B. Duan, G. Shu, X. Wang, D. Li, W. Jia, J. Kang, and G. Pei. 2009. Deficiency of a beta-arrestin-2 signal complex contributes to insulin resistance. Nature 457:1146-1149. [DOI] [PubMed] [Google Scholar]
- 30.Lynch, M. J., G. S. Baillie, A. Mohamed, X. Li, C. Maisonneuve, E. Klussmann, G. van Heeke, and M. D. Houslay. 2005. RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with beta arrestin to control the protein kinase A/AKAP79-mediated switching of the beta2-adrenergic receptor to activation of ERK in HEK293B2 cells. J. Biol. Chem. 280:33178-33189. [DOI] [PubMed] [Google Scholar]
- 31.Marchmont, R. J., and M. D. Houslay. 1980. A peripheral and an intrinsic enzyme constitute the cyclic AMP phosphodiesterase activity of rat liver plasma membranes. Biochem. J. 187:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel, F., G. Attal-Bonnefoy, G. Mangino, S. Mise-Omata, and O. Acuto. 2001. CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity 15:935-945. [DOI] [PubMed] [Google Scholar]
- 33.Milburn, C. C., M. Deak, S. M. Kelly, N. C. Price, D. R. Alessi, and D. M. Van Aalten. 2003. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 375:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson, C. D., S. J. Perry, D. S. Regier, S. M. Prescott, M. K. Topham, and R. J. Lefkowitz. 2007. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science 315:663-666. [DOI] [PubMed] [Google Scholar]
- 35.Okkenhaug, K., and R. Rottapel. 1998. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J. Biol. Chem. 273:21194-21202. [DOI] [PubMed] [Google Scholar]
- 36.Okkenhaug, K., L. Wu, K. M. Garza, R. J. La, W. Khoo, B. Odermatt, T. W. Mak, P. S. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2:325-332. [DOI] [PubMed] [Google Scholar]
- 37.Pages, F., M. Ragueneau, R. Rottapel, A. Truneh, J. Nunes, J. Imbert, and D. Olive. 1994. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature 369:327-329. [DOI] [PubMed] [Google Scholar]
- 38.Perry, S. J., G. S. Baillie, T. A. Kohout, I. McPhee, M. M. Magiera, K. L. Ang, W. E. Miller, A. J. McLean, M. Conti, M. D. Houslay, and R. J. Lefkowitz. 2002. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298:834-836. [DOI] [PubMed] [Google Scholar]
- 39.Povsic, T. J., T. A. Kohout, and R. J. Lefkowitz. 2003. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J. Biol. Chem. 278:51334-51339. [DOI] [PubMed] [Google Scholar]
- 40.Prasad, K. V., Y. C. Cai, M. Raab, B. Duckworth, L. Cantley, S. E. Shoelson, and C. E. Rudd. 1994. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc. Natl. Acad. Sci. U. S. A. 91:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raab, M., Y. C. Cai, S. C. Bunnell, S. D. Heyeck, L. J. Berg, and C. E. Rudd. 1995. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc. Natl. Acad. Sci. U. S. A. 92:8891-8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347-8350. [DOI] [PubMed] [Google Scholar]
- 43.Rudd, C. E., and M. Raab. 2003. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol. Rev. 192:32-41. [DOI] [PubMed] [Google Scholar]
- 44.Ruppelt, A., R. Mosenden, M. Gronholm, E. M. Aandahl, D. Tobin, C. R. Carlson, H. Abrahamsen, F. W. Herberg, O. Carpen, and K. Tasken. 2007. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J. Immunol. 179:5159-5168. [DOI] [PubMed] [Google Scholar]
- 45.Skalhegg, B. S., B. F. Landmark, S. O. Doskeland, V. Hansson, T. Lea, and T. Jahnsen. 1992. Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J. Biol. Chem. 267:15707-15714. [PubMed] [Google Scholar]
- 46.Skalhegg, B. S., K. Tasken, V. Hansson, H. S. Huitfeldt, T. Jahnsen, and T. Lea. 1994. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science 263:84-87. [DOI] [PubMed] [Google Scholar]
- 47.Tavano, R., G. Gri, B. Molon, B. Marinari, C. E. Rudd, L. Tuosto, and A. Viola. 2004. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J. Immunol. 173:5392-5397. [DOI] [PubMed] [Google Scholar]
- 48.Torgersen, K. M., T. Vang, H. Abrahamsen, S. Yaqub, V. Horejsi, B. Schraven, B. Rolstad, T. Mustelin, and K. Tasken. 2001. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 276:29313-29318. [DOI] [PubMed] [Google Scholar]
- 49.Vang, T., K. Tasken, B. S. Skalhegg, V. Hansson, and F. O. Levy. 1998. Kinetic properties of the C-terminal Src kinase, p50csk. Biochim. Biophys. Acta 1384:285-293. [DOI] [PubMed] [Google Scholar]
- 50.Vang, T., K. M. Torgersen, V. Sundvold, M. Saxena, F. O. Levy, B. S. Skalhegg, V. Hansson, T. Mustelin, and K. Tasken. 2001. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J. Exp. Med. 193:497-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waugh, C., L. Sinclair, D. Finlay, J. R. Bayascas, and D. Cantrell. 2009. Phosphoinositide (3,4,5)-triphosphate binding to phosphoinositide-dependent kinase 1 regulates a protein kinase B/Akt signaling threshold that dictates T-cell migration, not proliferation. Mol. Cell. Biol. 29:5952-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83-92. [DOI] [PubMed] [Google Scholar]