Abstract
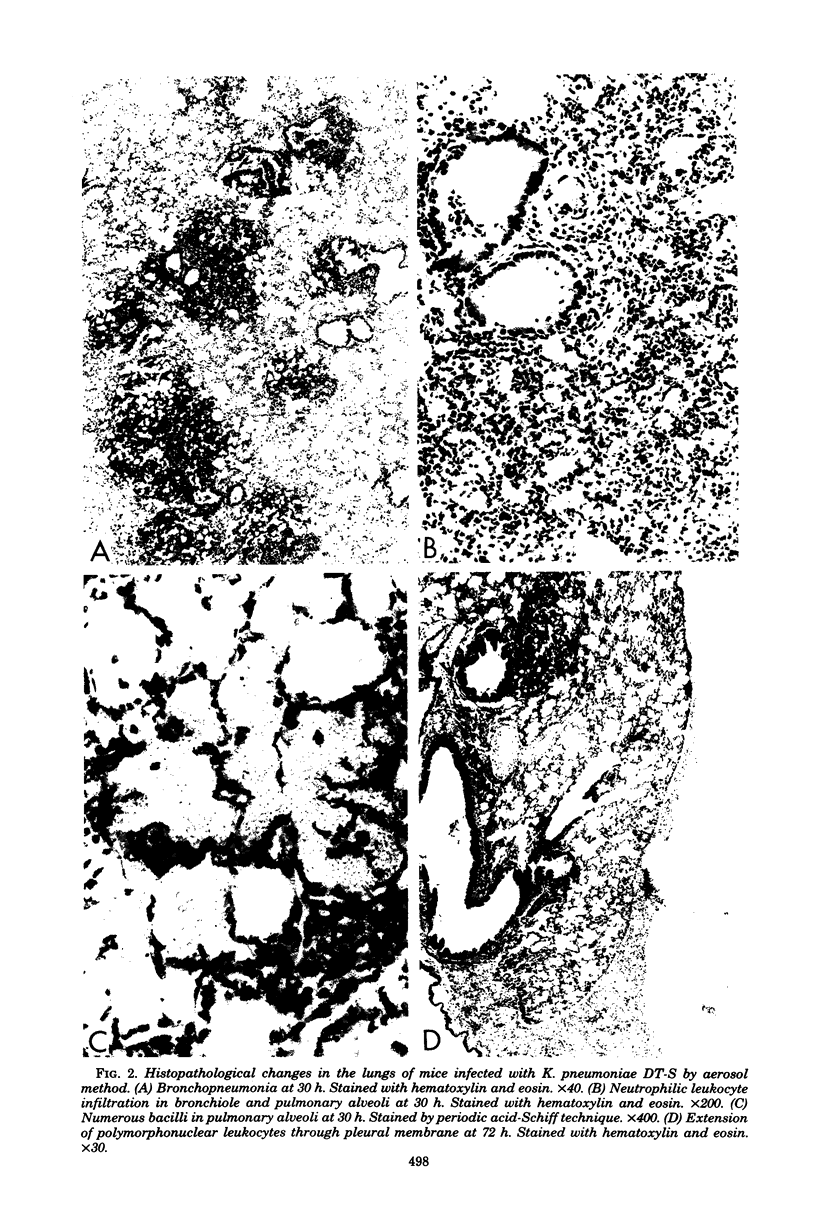
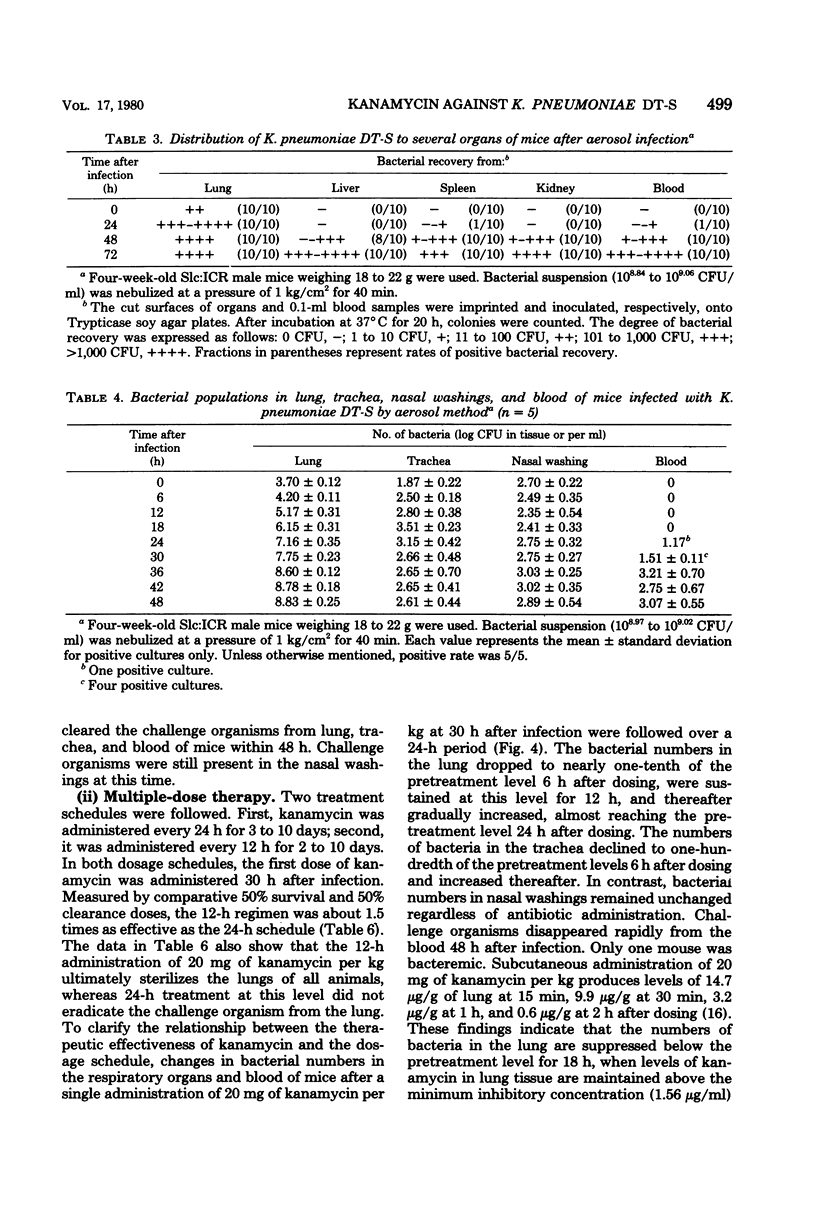
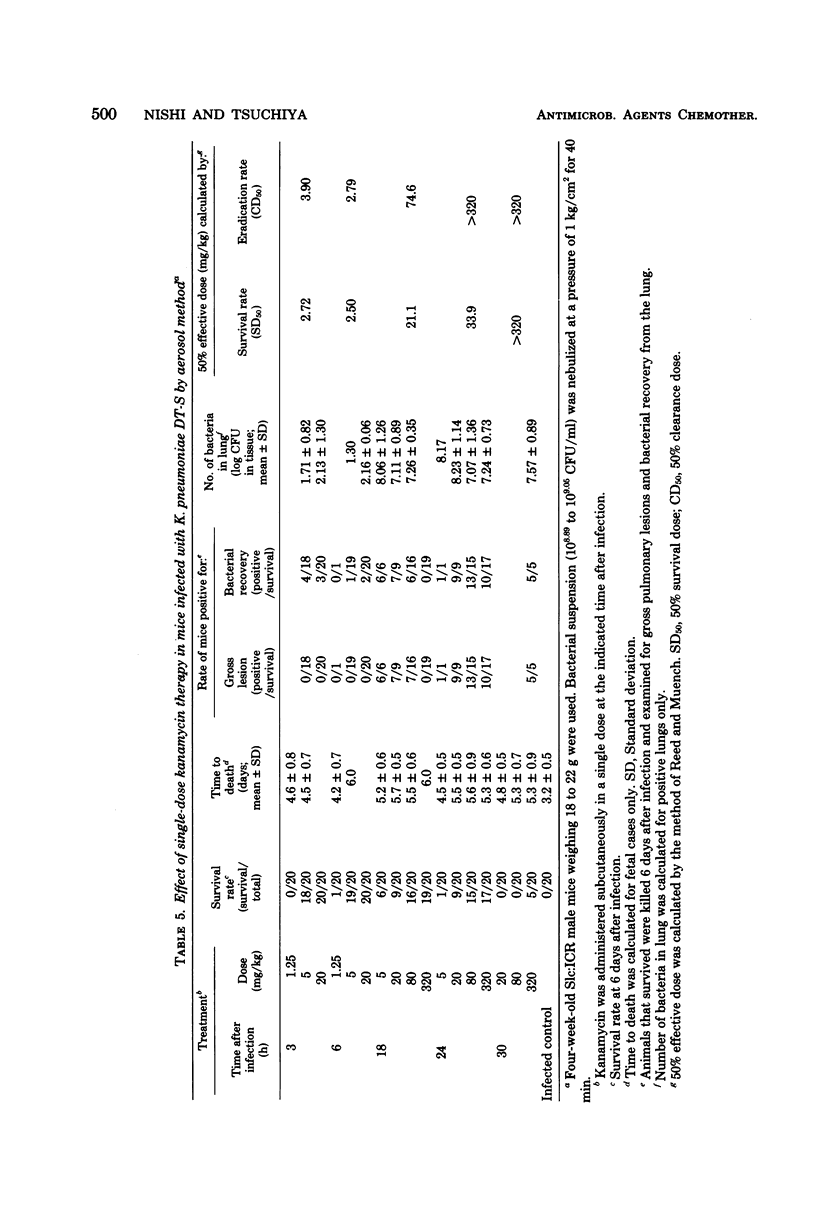
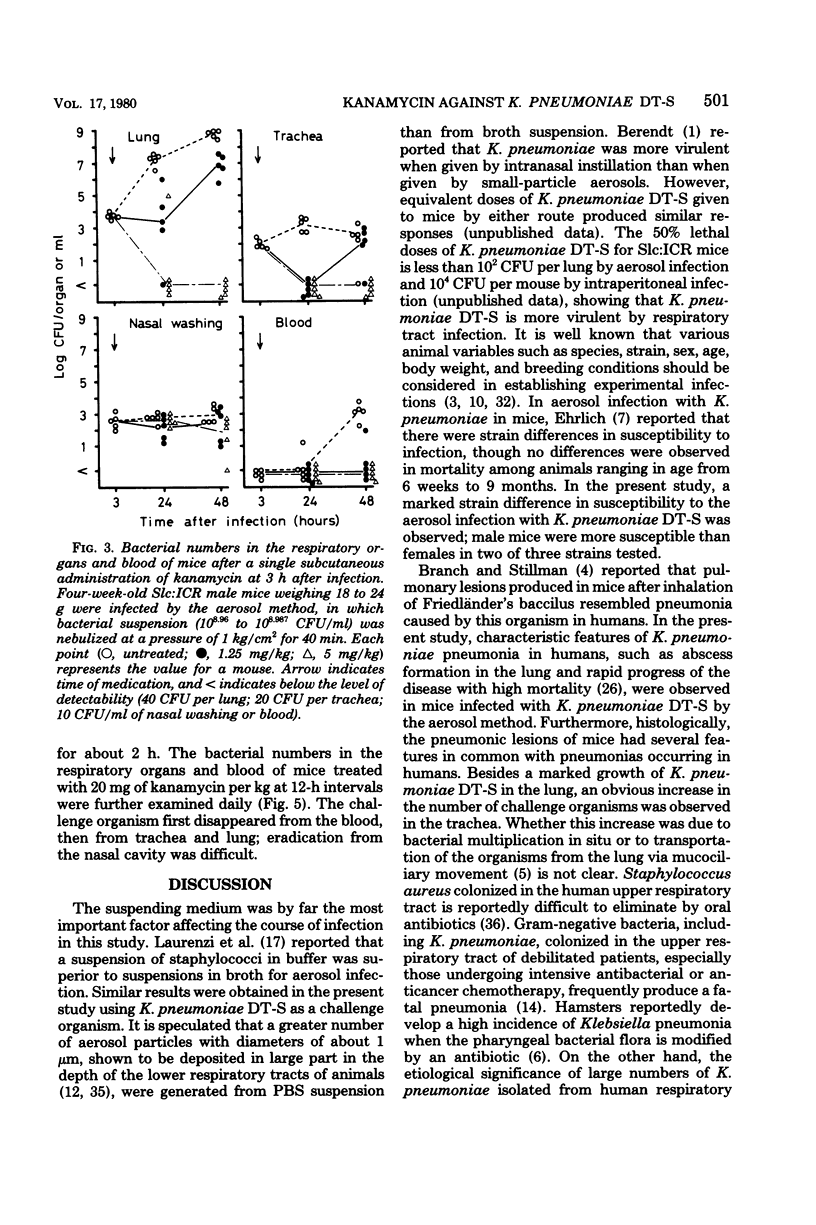
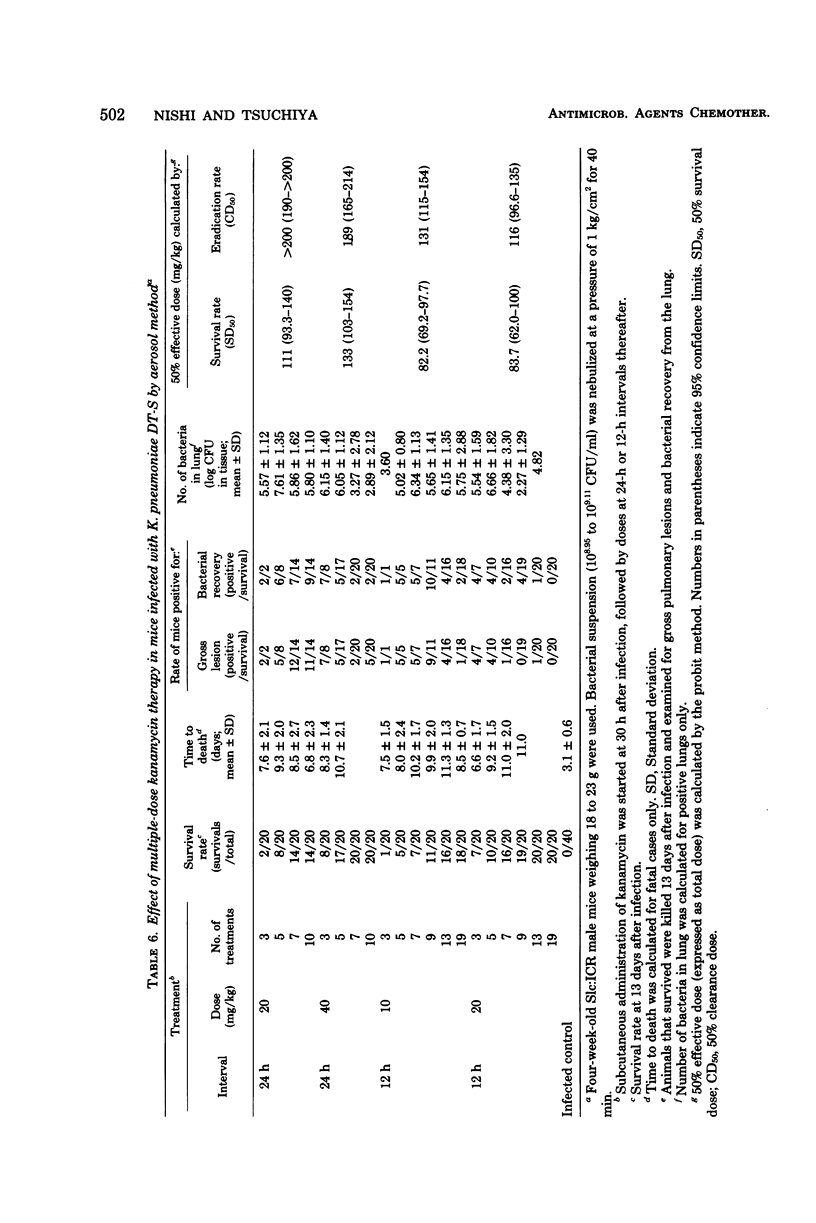
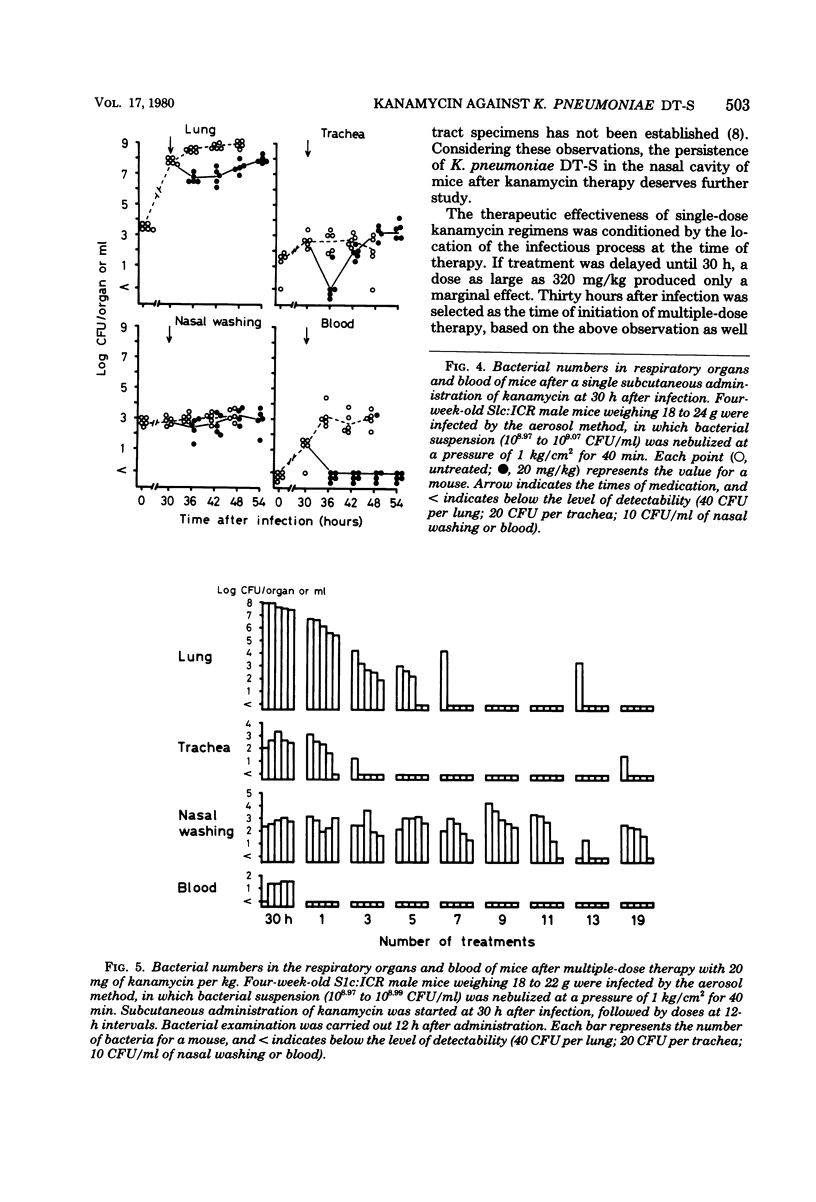
Such factors as suspending medium, operating pressure, exposure time, inoculum size, and strain, sex, age, and weight of the animals were examined for their effects on the development of respiratory tract infection with Klebsiella pneumoniae DT-S in mice. The suspending medium was one of the most important factors. Aerosol challenge with a 10(9) colony-forming units per ml resulted in deposition of 10(4) colony-forming units of the organisms in the lung. The numbers of organisms in the lung increased rapidly, and by 30 h, a well-developed pneumonia was apparent. All the mice died within 4 days after infection. The therapeutic effectiveness of single-dose kanamycin regimens decreased markedly with a delay in administration. The effectiveness of multi-dose kanamycin regimens was influenced by the frequency of dosage. Thus a 12-h dosage schedule was superior to a 24-h regimen. Administration of 20 mg of kanamycin per kg at 12-h intervals for 10 days, initiated 30 h after infection, provided a complete cure. The infecting organisms in the lung, trachea, and blood were eradicated by the kanamycin therapy, but those in the nasal cavity were difficult to eliminate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt R. F., Long G. G., Walker J. S. Treatment of respiratory Klebsiella pneumoniae infection in mice with aerosols of kanamycin. Antimicrob Agents Chemother. 1975 Nov;8(5):585–590. doi: 10.1128/aac.8.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt R. F. Relationship of method of administration to respiratory virulence of Klebsiella pneumoniae for mice and squirrel monkeys. Infect Immun. 1978 May;20(2):581–583. doi: 10.1128/iai.20.2.581-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouley G., Dubreuil A., Prulière D., Bouley M. Sex factors in airborne infection: comparative susceptibility of male and female non-immunized mice to aerosolized Pasteurella multocida. Lab Anim. 1974 Jan;8(1):9–12. doi: 10.1258/002367774780943814. [DOI] [PubMed] [Google Scholar]
- Dalton H. P., Muhovich M., Escobar M. R., Allison M. J. Pulmonary infection due to disruption of the pharyngeal bacterial flora by antibiotics in hamsters. Am J Pathol. 1974 Sep;76(3):469–480. [PMC free article] [PubMed] [Google Scholar]
- Ehrlich R. Effect of nitrogen dioxide on resistance to respiratory infection. Bacteriol Rev. 1966 Sep;30(3):604–614. doi: 10.1128/br.30.3.604-614.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. J. The relationship between the biotype of Klebsiella species and their pathogenicity. J Clin Pathol. 1973 Jul;26(7):523–528. doi: 10.1136/jcp.26.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG L. J., WATKINS H. M., DOLMATZ M. S., SCHLAMM N. A. Studies on the experimental epidemiology of respiratory infections. VI. The relationship between dose of microorganisms and subsequent infection or death of a host. J Infect Dis. 1954 Jan-Feb;94(1):9–21. doi: 10.1093/infdis/94.1.9. [DOI] [PubMed] [Google Scholar]
- GOWEN J. W. Genetic effects in nonspecific resistance to infectious disease. Bacteriol Rev. 1960 Mar;24(1):192–200. doi: 10.1128/br.24.1.192-200.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Marshall L. W., Charache P., Wallace C. K., Melvin V. B. Nosocomial pneumonia. A continuing major problem. Am Rev Respir Dis. 1973 Nov;108(5):1130–1140. doi: 10.1164/arrd.1973.108.5.1130. [DOI] [PubMed] [Google Scholar]
- Johanson W. G., Pierce A. K., Sanford J. P. Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N Engl J Med. 1969 Nov 20;281(21):1137–1140. doi: 10.1056/NEJM196911202812101. [DOI] [PubMed] [Google Scholar]
- LAURENZI G. A., BERMAN L., FIRST M., KASS E. H. A QUANTITATIVE STUDY OF THE DEPOSITION AND CLEARANCE OF BACTERIA IN THE MURINE LUNG. J Clin Invest. 1964 Apr;43:759–768. doi: 10.1172/JCI104960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V., Topf B. Microbiology of nosocomial infections. Arch Intern Med. 1972 Jul;130(1):104–110. [PubMed] [Google Scholar]
- MILLER S., EHRLICH R. Susceptibility to respiratory infections of animals exposed to ozone. I. Susceptibility to Klebsiella pneumoniae. J Infect Dis. 1958 Sep-Oct;103(2):145–149. doi: 10.1093/infdis/103.2.145. [DOI] [PubMed] [Google Scholar]
- Miller A. K. In vivo evaluation of antibacterial chemotherapeutic substances. Adv Appl Microbiol. 1971;14:151–183. doi: 10.1016/s0065-2164(08)70543-4. [DOI] [PubMed] [Google Scholar]
- PURVIS M. R., MILLER S., EHRLICH R. Effect of atmospheric pollutants on susceptibility to respiratory infection. I. Effect of ozone. J Infect Dis. 1961 Nov-Dec;109:238–242. doi: 10.1093/infdis/109.3.238. [DOI] [PubMed] [Google Scholar]
- Pierce A. K., Sanford J. P. Aerobic gram-negative bacillary pneumonias. Am Rev Respir Dis. 1974 Nov;110(5):647–658. doi: 10.1164/arrd.1974.110.5.647. [DOI] [PubMed] [Google Scholar]
- SCHMIDT L. H., WALLEY A. The influence of the dosage regimen on the therapeutic effectiveness of penicillin G in experimental lobar pneumonia. J Pharmacol Exp Ther. 1951 Dec;103(4):479–488. [PubMed] [Google Scholar]
- TAYLOR M. J., KENNEDY G. H., BUNDELL G. P. Experimental anthrax in the rat. I. The rapid increase of natural resistance observed in young hosts. Am J Pathol. 1961 Apr;38:469–480. [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kida M., Kondo M., Ono H., Takeuchi M., Nishi T. SCE-963, a new broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Oct;14(4):557–568. doi: 10.1128/aac.14.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. Z., Martin R. R., Putman M., Greenberg S. B., Wallace R. J., Jr, Jemsek J. G. Quantitative nasal cultures from carriers of Staphylococcus aureus: effects of oral therapy with erythromycin, rosamicin, and placebo. Antimicrob Agents Chemother. 1979 Mar;15(3):379–383. doi: 10.1128/aac.15.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]