Abstract
The 32-kDa dopamine- and adenosine 3′,5′-monophosphate-regulated phosphoprotein (DARPP-32) is recognized to be critical to the pathogenesis of drug addiction. Opiates via the μ-receptor act on the dopaminergic system in the brain and modulates the expression of DARPP-32 phosphoprotein which is an important mediator of the activity of the extracellular signal-regulated kinase (ERK) signaling cascades, the activation of which represents an exciting nexus for drug-induced changes in neural long-term synaptic plasticity. Silencing of DARPP-32 using an siRNA against DARPP-32 may provide a novel gene therapy strategy to overcome drug addiction. In this study, we investigated the effect of the opiate (heroin) on D1 receptor (D1R) and DARPP-32 expression and additionally, evaluated the effects of DARPP-32-siRNA gene silencing on protein phosphatase-1 (PP-1), ERK, and cAMP response element-binding (CREB) gene expression in primary normal human astrocytes (NHA) cells in vitro. Our results indicate that heroin significantly upregulated both D1R and DARPP-32 gene expression, and that DARPP-32 silencing in the NHA cells resulted in the significant modulation of the activity of downstream effector molecules such as PP-1, ERK, and CREB which are known to play an important role in opiate abuse-induced changes in long-term neural plasticity. These findings have the potential to facilitate the development of DARPP32 siRNA-based therapeutics against drug addiction.
I. Introduction
Drug addiction is a chronic, relapsing disease, characterized by compulsive drug-seeking behavior that is caused by neurochemical and molecular changes in the brain. Substance abuse is a major public health concern in the United State and several factors contribute to the development and persistence of drug addiction. The key among them is alteration in the individual's neurophysiological functioning induced by the addictive drugs. It is believed that the neurochemical/neurophysiological alterations in the brain caused by addictive drugs have a cellular and molecular basis and may be persistent and contribute to changes in behavior leading to drug addiction. Drugs like opiates induce dopamine-receptor stimulation leading to induction of transcription factors and phosphorylation of many substrate proteins involved in neuronal excitability (Colvis et al., 2005; Fienberg et al., 1998, 2000; Greengard et al., 1998; Guitart-Masip et al., 2006; Nestler, 2005; Nestler et al., 1994; Self et al., 1998). Disruptions in the dynamic balance of dopamine-receptor-mediated phosphorylation and dephosphorylation cascades leads to impaired integration of synaptic inputs that can cause altered neuronal communication and disruption of this balance. This, in turn, may result in the induction of transcription factors and their downstream targets causing long-lasting plastic changes in the brain (Nairn et al., 2004; Takahashi et al., 2005). This process may be the principal molecular mechanism underlying drug addiction. Mechanisms by which neuroimmune signaling affects the brain under normal conditions or during pathologic processes remain largely unexplored.
A. Molecular Mechanisms Underlying Opiate Addiction
Long-term use of opiates leads to tolerance, sensitization, and physical dependence. High rates of relapse to drug use following prolonged withdrawal periods characterize the behavior of experienced heroin users (O'Brien, 1997). In heroin-free individuals, drug craving and relapse to drug use can be triggered by stress (Kreek and Koob, 1998) and by stimuli previously associated with drug use (Carter and Tiffany, 1999; Childress et al., 1992). Many drugs of abuse, administered repeatedly over time, cause tolerance and physical dependence. Tolerance and physical dependence have been correlated with changes in the intracellular cAMP signal transduction cascade. Elements of the cascade found to be altered include G-proteins, adenylate cyclase, protein kinase A (PKA), and its target CREB (cAMP response element-binding) (Nestler et al., 1994; Wang and Gintzler, 1997; Wang et al., 1996). The activity of CREB is regulated by phosphorylation and opiates have been shown to alter phosphorylation of CREB protein. CREB may serve as a final common mediator in the ultimate expression of both positive and negative reinforcing properties of drugs of abuse like opiates (Widnell et al., 1996). Elegant studies by Walters et al. (2001) demonstrated that activation of CREB plays a complex role in the neuroadaptive processes associated with opiate addiction. Repeated opiate exposure induces biochemical changes in specific brain regions, predominantly the mesolimbic dopamine system which mediates the reinforcing actions of the opiates.
B. Opiate-Induced Dopamine-Receptor Stimulation Activates the cAMP-Dependent PKA–DARPP-32 Signaling Pathway
Opiate dependence is characterized by enhanced neuronal excitability associated with upregulation of the cAMP second messenger system. Opiate-induced dopamine-receptor stimulation activates the cAMP-dependent PKA pathway, leading to induction of transcription factors and phosphorylation of many substrate proteins involved in neuronal excitability. Opiate administration can also alter gene expression in different regions of the brain, which may contribute to the plastic changes associated with addictive behavior (Calabresi et al., 2000). An extensive bidirectional communication takes place between the nervous and the immune systems. Both immune cells and neuroreactive molecules may modulate brain function through multiple signaling pathways. DARPP-32 is recognized as critical to the pathogenesis of drug addiction (Greengard et al., 1998, 1999). Opiates act on the dopaminergic system in the brain and perturb DARPP-32 function. DARPP-32 is involved in mediating the actions of virtually all neurotransmitters in all parts of the brain (Greengard et al., 1998, 1999; Nairn et al., 2004; Svenningsson et al., 2004, 2005). Neurotransmitters such as dopamine, through direct or indirect pathways, regulate the phosphorylation of DARPP-32. DARPP-32, when phosphorylated at Thr34, acts as an amplifier of PKA-mediated signaling through its ability to potently inhibit protein phosphatase-1 (PP-1). This amplifying property of DARPP-32 is critical for dopaminergic signaling, but it is also utilized by multiple other neurotransmitters, including glutamate in various regions of the brain. In addition to its role as a PP-1 inhibitor, DARPP-32 when phosphorylated at Thr75 inhibits PKA. Upon dopaminergic neurotransmission, the phosphorylation state at Thr75 is decreased allowing disinhibition of PKA and further increasing phosphorylation at Thr34. This complex positive feedback loop potentiates dopaminergic signaling (Greengard et al., 1998, 1999; Nairn et al., 2004; Nishi et al., 1997; Svenningsson et al., 2004, 2005).
Thus, modulation of the expression of DARPP-32 can cause an inhibition of PP-1 and a concomitant dysregulation of its downstream effector proteins, glycogen synthesis kinase-3 (GSK-3), CREB, and c-Fos. CREB mediates morphine-induced upregulation of specific components of the cAMP pathway that contributes to opiate dependence (Chao and Nestler, 2004; Mahajan et al., 2005b). Thus, DARPP-32, a major regulatory hub of neurosignaling networks, is a unique potential target for new therapeutic approaches to modulate neural plasticity within the brain reward circuitry and break the vicious cycle of addiction.
Studies of the biochemical and molecular basis of drug addiction have several important clinical implications. A better understanding of the neurobiological mechanisms underlying the addictive action of drugs of abuse will help in the development of pharmacological agents that prevent or reverse drug abuse. The availability of such therapeutic agents would represent a significant advancement in our fight against drug addiction. Additionally, these studies will pave the way for further investigations that may ultimately yield designer drugs that may be taken orally that target DARPP-32 for the prevention or therapy of drug addiction.
II. Study Design
In the current study, we used primary normal human astrocytic cell cultures. Interactions between neurons and astrocytes are critical for signaling, energy metabolism, and neuroprotection in the central nervous system (CNS). Astrocytes face the synapses, send end-foot processes that enwrap the brain capillaries, and form an extensive network interconnected by gap junctions. Astrocytes express several membrane proteins and enzymes that are critical for uptake of neurotransmittors such as dopamine, glutamate, etc. at the synapses and participate in detection, propagation, and modulation of excitatory synaptic signals, provide metabolic support to the active neurons. Normal human astrocytes (NHA), 1–3 million cells/ml, were treated with heroin (10−7–10−11) molar concentrations for 48 h, and the effect of heroin on D1 receptor (D1R) and DARPP-32 gene expression was evaluated. An initial gene microarray analysis is done using NHA cells treated with heroin (10−7 M) to identify genes that might play a key role in opiate addiction. Data from our gene microarray analysis of NHA treated with heroin (10−7 M) showed a significant modulation of the DARPP-32 gene and other members of this signaling cascade indicating the involvement and the significance of this pathway in the opiate addiction process. Since, NHA are among these several neuronal cells that expresses DARPP-32, we believe that silencing the expression of DARPP-32 in these cells, may result in the modulation of the expression activation of the extracellular signal-regulated kinase (ERK) mitogen-activated protein (MAP) kinase cascade that causes drug-induced changes in neural plasticity and thereby neurological dysfunction in opiate addicts. Therefore, using commercially available transfection reagent such as siPROT (Ambion Inc., Austin, TX), we did a transient transfection that silenced DARPP-32 gene expression in NHA cells by about 80% for a time period of up to 1 week. Since, optimal transfection efficiency was observed at 48 h, further experiments that evaluated the expression of secondary messengers PP-1, ERK, and CREB in DARPP32-siRNA transfected, scrambled siRNA transfected, and untransfected controls are done at 48 h posttransfection. These transfected cells were treated with/without heroin (10−7 M) for a period of 24 h posttransfection.
III. Material and Methods
A. Cell Culture: In Vitro Treatment of Cells
We will use primary cultures of NHA obtained from Applied Cell Biology Research Institute (ACBRI), Kirkland, WA. Astrocytes were >95% GFAP positive, as characterized by immunocytochemistry, and were >98% viable by trypan blue exclusion criteria. NHA are cultured in Astrocyte Growth Medium kit that includes serum-free basal medium with attachment factors (ABCRI, Cat # 4Z0-210) and Passage Reagent Group™ (ABCRI, Cat # 4Z0-800) and were passaged 1:4 at 80–90% confluence. NHA are obtained at passage 2 for each experiment and are used for all experimental paradigms between within the 8–20 cumulative population doublings. Previous kinetics (12–96 h) and dose (heroin [10−7, 10−9, and 10−11]) response studies indicate that a 48-h time period and a dose of 10−7 M concentration heroin were optimal. The opiate (heroin), 10−7–10−11 M, concentrations that we use in our study are well within the physiological range and have been used by us and other investigators in prior studies (Baritaki et al., 2005; Mahajan et al., 2005a,b; Peterson et al., 2004; Singh et al., 2004). Heroin hydrochloride (Cat # H5144; Sigma, St Loius, MO) is obtained as a 100 mg/ml stock solution.
B. Production of cDNA Microarrays
The microarrays used in this experiment were produced at the Roswell Park Cancer Institute (RPCI) Microarray and Genomics Core Facility and contain approximately 5043 cDNA clones (Research Genetics).
C. Preparation and Hybridization of Fluorescent Labeled cDNA
A total of six RNA samples (i.e., six slides or six cDNA arrays) that included the three separate NHA samples treated with heroin (10−7 M) for 48 h and the corresponding untreated samples were screened for gene expression. cDNA was synthesized from each of those RNA samples and the heroin (10−7 M) treated samples were labeled with Cy5 dye, while the corresponding untreated controls were labeled with the Cy3 dye using the Atlas Powerscript Fluorescent Labeling Kit (BD BioSciences). To minimize intra-array variations, two hybridizations for each sample were performed, details of hybridization procedures have been described earlier (Mahajan et al., 2006). Additionally, the labeling with the Cy dye was interchanged, so as to have technical replicates of the matched pairs of heroin (10−7 M) treated and untreated sample. These standard normalization approaches correct the labeling bias between the Cy3 and Cy5 dyes (Mahajan et al., 2006).
D. Image Analysis
The hybridized slides were scanned using a GenePix 4200A scanner to generate high-resolution (10 μm) images for both Cy3 and Cy5 channels. Image analysis was performed on the raw image files using ImaGene (current version 6.0.1) from BioDiscovery Inc. Each cDNA spot was defined by a circular region. The size of the region was programmatically adjusted to match the size of the spot. Local background for a spot was determined by ignoring a 2–3 pixel buffer region around the spot and then measuring signal intensity in a 2–3 pixel wide area outside the buffer region. Raw signal intensity values for each spot and its background region were segmented using a proprietary optimized segmentation algorithm, which excludes pixels that were not representative of the majority pixels in that region. The background corrected signal for each cDNA spot was the mean signal (of all the pixels in the region)—mean local background. The output of the image analysis was two tab delimited files, one for each channel, containing all of the raw fluorescence data.
E. Microarray Data Processing and Analysis
Expression data extracted from image files were first checked by a M (log2(Cy3/Cy5)) versus A [(log2(Cy3) + log2(Cy5))/2)] plot to see whether intensity-dependent expression bias existed between spots (genes) labeled with Cy3 and Cy5 on each individual slide. After finding that intensity-dependent expression bias existed for all slides, we first performed a Lowess data normalization to correct the observed intensity-dependent expression bias. We then performed a global normalization to bring the median expression values of Cy3 and Cy5 on all three slides to the same scale. This was done by selecting a baseline array (e.g., Cy3) from one of the three slides, followed by scaling expression values of the remaining six arrays to the median value of the baseline array (m̃base):
After data normalization, the average intensity of individual gene from multiple spots on each slide was computed using an in-house developed PERL script. A total of 5043 average expression values were obtained, including empty, dry, null, and DMSO control spots. Paired t-test on normalized intensity with p-values <0.05 was used to generate a list of genes with significant change in expression between normal and heroin (10−7 M)-treated samples. The false positive rate (FDR) of the significant genes was estimated by using the SAM algorithm (Tusher et al., 2001). Quality control measures including ratios of housekeeping genes G3PDH and β-actin, scaling factors, background, and Q-values were within acceptable limits.
F. siRNA Transfection
NHA are transiently transfected with DARPP-32 siRNA for 24–96 h. siRNA (stealthTH siRNA) is obtained from Invitrogen (Carlsbad, CA). The siRNA sequences for DARPP-32 (Accession no. AF464196) are sense—ACA CAC CAC CUU CGC UGA AAG CUG U and antisense—ACA GCU UUC AGC GAA GGU GGU GUG U. The appropriate scrambled control siRNA sequences are sense—ACA CCC AUC CUC GGU AAG ACA CUG U and antisense—ACA GUG UCU UAC CGA GGA UGG GUG U. Twenty-four hours before siRNA transfection, 1 × 104 NHA are seeded onto six-well plates in OPTI minimal essential medium containing 4% FBS with no antibiotics to give 30–50% confluence at the time of transfections. The siRNAs are transfected to a final concentration of 200 pM using siPROT (Ambion Inc.) according to the manufacturer's recommendations. The final concentration of siRNA used for transfections was optimized (data not shown) and are consistent with concentrations used by other investigators (Hassani et al., 2005). We obtained a significant knockdown (~80–90%) of DARPP-32 gene expression using stealthTH siRNA against DARPP-32. Optimal gene knockdown effect is observed up to 1 week posttransfection. The efficiency of gene silencing was determined by measuring the percentage inhibition of the expression of DARPP-32 using quantitative real time Q-PCR. Since these are transient transfections, all experiments using transfected cells are carried out at 48 h posttransfection. Transfected NHA are treated with heroin (10−7 M) for 48 h. Appropriate scrambled siRNA control and a nontransfected control are used. The gene expression levels of DARPP-32, PP-1, ERK, and CREB are determined by real time, quantitative PCR.
G. Cell Viability Assay
Cell viability is measured using the MTT assay, that is, based on the reduction of a tetrazolium component 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into an insoluble formazan product by the mitochondria of viable cells (Promega, Madison, WI). The MTT assay is a quantitative, sensitive detection of cell proliferation since it measures the growth rate of cells by virtue of a linear relationship between cell activity and absorbance.
H. RNA Extraction
Cytoplasmic RNA is extracted using the Trizol reagent (Invitrogen) (Chomczynski and Sacchi, 1987). RNA concentrations are determined using a Nanodrop spectrophotometer. Isolated RNA is stored at −80 °C.
I. Real Time, Quantitative RT-PCR
The relative abundance of each mRNA species is determined by real time, quantitative PCR. To provide precise quantification of initial target in each PCR reaction, the amplification plot is examined at a point during the early log phase of product accumulation. This is accomplished by assigning a fluorescence threshold above background and determining the time point at which each sample's amplification plot reaches the threshold (defined as the threshold cycle number or CT). Differences in threshold cycle number are used to quantify the relative amount of PCR target contained within each tube. Relative expression of mRNA species is calculated using the comparative CT method (Higuchi et al., 1993). All data are controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin. Results on RNA from treated samples are normalized to results obtained on RNA from the control, untreated sample. Briefly, the analysis is performed as follows: for each sample, a difference in CT values (ΔCT) is calculated for each mRNA by taking the mean CT of duplicate tubes and subtracting the mean CT of the duplicate tubes for the reference RNA (β-actin) measured on an aliquot from the same RT reaction. The ΔCT for the treated sample is then subtracted from the ΔCT for the untreated control sample to generate a ΔΔCT. The mean of these ΔΔCT measurements is then used to calculate expression of the test gene relative to the reference gene and normalized to the untreated control as follows: relative expression / transcript accumulation index = 2−ΔΔCT. This calculation assumes that all PCR reactions are working with 100% efficiency. All PCR efficiencies were found to be >95%; therefore, this assumption introduces minimal error into the calculations (Bustin, 2002; Radonic et al., 2004).
J. Immunofluorescent Staining
DARPP-32 siRNA transfected and untranfected (control) NHA were grown to 70% confluence in a Petri dish with a glass bottom. Both transfected and untransfected cells were treated with heroin (10−7 M) for a period of 48 h. Cells were fixed 10 min at 37 °C in 2% formaldehyde followed by permabilization with ice-cold 90% methanol. Cells were then washed in 1× phosphate buffer saline (PBS) and blocking done with 10% normal goat serum and 1% BSA in PBS was followed by incubation with of anti-ERK rabbit polyclonal antibody (Cat # sc-94; Santa Cruz Biotechnologies, Santa Cruz, CA) overnight at 4 °C. Samples were then incubated with a mixture of antirabbit secondary antibodies conjugated to Alexa Fluor® 647 (Cat # A21244; Molecular Probes, Eugene, OR), washed with PBS counter, stained with DAPI, and mounted on glass slides. Standard immunofluoresecent staining procedures were followed. Imaging was done using a Leica Confocal Laser Scanning Microscope (TCS SP2 AOBS, Leica Microsystems Heidelberg GmbH) with an Oil Immersion objective lens 63 . HeNe 633 nm laser was applied to excite Alexa Fluor® 647, argon ion laser was applied to excite DAPI.
IV. Results
A. Microarray Data Preprocessing
To obtain meaningful data from these microarray analyses, preprocessing of the microarray data is very important. This involves data normalization procedures, which corrects systematic differences such as intensity-dependent expression bias and different dye efficiency between and across datasets. Normalization procedures used have been described in details in our previous studies (Mahajan et al., 2006). In microarray studies, a large proportion of genes are usually not expressed across all samples to be compared and, as a common practice, are filtered out before performing statistical analysis. In this study, genes whose expression values were less than 2× the DMSO control expression values across the six array samples were filtered out before performing statistical analysis. These led to an ~13.3% reduction (671 genes) in gene sample size.
To account for variation between replicates, we performed data filtering by measuring the repeatability of gene expression using coefficient of variation (CV). This was done by computing the CV of Cy3/Cy5 ratios for all individual genes from six slides, followed by constructing a 99% confidence interval for CV values of all genes. Genes with CVs outside the upper 99% confidence interval bound were regarded as unreliable measurements and were removed from further analysis. This process eliminated another 3.0% of genes. The remaining 4372 genes were used for the detection of the differentially expressed genes.
B. mRNA Gene Expression Patterns in Heroin-Treated NHA
To compare gene expression levels between heroin (10−7 M)-treated NHA and their respective untreated controls, we used a two-step statistical data analyses. First, we used the regularized paired t-test to detect differentially expressed genes, which resulted in 1403 (p < 0.05) significantly modulated genes. For multiple test correction to control the FDR, these genes were further subjected to SAM analysis, which resulted in a shorter list of 214 genes with an estimated FDR of 4.9%. Of the 214 differentially expressed genes, 173 (81.6%) were upregulated and 41 (18.3%) were downregulated on treatment with heroin (10−7 M). The differentially expressed genes, showing their fold changes with respect to the housekeeping controls were further subdivided into different categories based on their biological function. An interrogation of our microarray datasets of the significantly and reproducibly changed genes showed that many of the genes could be classified into distinct functional groups. Our data (Table I) suggest that heroin (10−7M) treatment significantly modulated genes involved in cytokine and chemokine regulation (INF-γ, MCP-1, TGF-β, v-EGF, IL-1β, TNF-α); signal transduction (IP3, JNK, PI3 kinase, MAPK, DARRP-32, PP-1); cell cycle regulation (Cdk5); and apoptosis regulation (Caspases; Bcl-2). Since several of the molecules implicated in the DARPP-32 signaling pathway were significantly modulated by heroin in NHA as indicated gene microarray data analysis, the current study will focus on evaluating the role of DARPP-32 in the regulation of secondary messengers downstream of DARPP-32 that play a role in transcriptional regulation and consequently bring about changes in neuronal plasticity observed in drug addiction (Fig. 1).
TABLE I.
List of Genes that are Significantly Modulated by Heroin Treatment in NHA
| Gene accession no. | Genes grouped into different functional categories | Fold change | p-Value | |
|---|---|---|---|---|
| 24 h | ||||
| Cytokines and chemokines | ||||
| AA969475 | | Interferon gamma receptor 2 | ▲ | 1.27 | 3.43E–02 |
| AI733305 | ||||
| AA683550 | Interleukin-1 receptor-associated kinase 1 | ▲ | 1.64 | 3.53E–02 |
| AA463497 | MCP | ▲ | 1.4 | 1.36E–02 |
| AA931884 | Small inducible cytokine A1 | ▼ | 0.3 | 1.27E–02 |
| AA487034 | Transforming growth factor, beta | ▲ | 1.25 | 3.47E–02 |
| AA504211 | Tumor necrosis factor (ligand) Superfamily | ▲ | 1.91 | 9.86E–02 |
| AA630120 | Vascular endothelial growth factor B Signaling molecules | ▲ | 1.43 | 3.39E–02 |
| W68281 | Mitogen-activated protein kinase-activated protein kinase 3 | ▲ | 1.37 | 1.09E–01 |
| R50953 | Mitogen-activating protein kinase kinase kinase kinase 2 | ▲ | 1.59 | 7.77E–03 |
| AA018980 | Protein kinase, cAMP dependent | ▲ | 1.37 | 9.92E–03 |
| R26186 | | Protein phosphatase 1, catalytic subunit, beta isoform | ▲ | 1.37 | 1.69E–03 |
| AA460827 | Protein phosphatase 1, regulatory (inhibitor) subunit 1A (DARPP-32) | ▲ | 1.55 | 5.52E–03 |
| R51209 | Protein phosphatase 2A Cell cycle protein | ▲ | 1.33 | 4.67E–02 |
| AA401479 | Cyclin-dependent kinase 5 Apoptosis regulation | ▲ | 1.45 | 4.41E–02 |
| H74208 | BCL2 | ▼ | 0.63 | 2.30E–02 |
| AA459263 | BCL2-related protein A1 | ▼ | 0.24 | 2.54E–02 |
| R42530 | Caspase 3, apoptosis-related cysteine protease | ▲ | 1.46 | 9.45E–03 |
The genes highlighted in boldface are those that are involved in the cAMP-dependent PKA–DARPP-32 pathway.
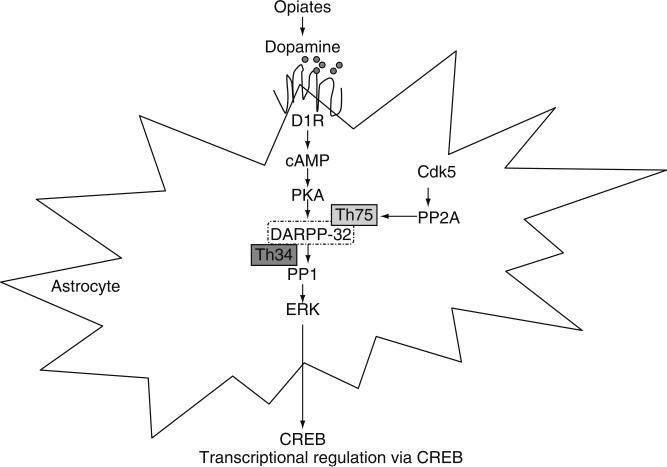
Fig. 1.
Schematic of a signaling cascade activated by opiates. Opiates via the μ-opioid receptor activates the dopiminergic pathway leading to the increased DARPP-32 phosphorylation at Thr34 and increased PP-1 inhibition which modulates ERK activation and consequently the expression of the downstream transcriptional molecule CREB.
C. Opiates Increase D1R Gene Expression by NHA
Opiates activate the dopaminergic system in the brain via dopamine D1Rs. Our results show that heroin significantly increase D1R gene expression by NHA (Fig. 2). These results indicate that the dopaminergic pathway is triggered by opiates. NHA were treated with heroin (10−7 and 10−9 M) for 48 h, RNA was extracted, reverse transcribed, followed by quantitation of gene expression using quantitative, real time PCR. Heroin treatment significantly upregulated D1R gene expression at 10−7 M (TAI = 1.45, 45% increase, p < 0.01) and 10−9 M (TAI = 1.37, 37% increase, p < 0.01). The results are representative of three independent experiments and statistical significance was determined using ANOVA where the comparisons were performed between morphine treated and untreated controls.
FIG. 2.
Effect of heroin on dopamine D1 receptor gene expression by NHA. NHA were treated with 10−7–10−9 M heroin for 48 h. RNA was extracted, reverse transcribed, and the D1 receptor gene expression was quantitated using real time PCR.
D. Opiates Modulate the DARPP-32 Gene Expression in NHA Cells
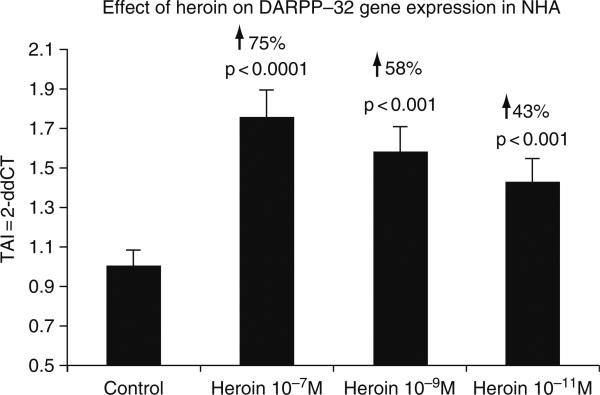
As reported previously by Greengard et al., DARPP-32 occupies a unique position whereby it modulates both dopaminergic and glutamatergic signaling depending upon which Thr residue within the protein is phosphorylated (Greengard et al., 1998, 1999; Nairn et al., 2004; Svenningsson et al., 2004, 2005). Phospho-Thr34-DARPP-32 amplifies the D1/PKA pathway, whereas phospho-Thr75 DARPP-32 inhibits it, thereby shifting the balance toward dephosphorylation of target substrates via PP-1. DARPP-32 thus represents a “molecular switch” that regulates and fine-tunes the phosphorylation state of PP-1 and downstream target proteins. In an in vivo mouse model, acute administration of morphine has been shown to increase the phosphorylation of DARPP-32 at Thr34, without affecting phosphorylation at Thr75 in striatal medium spiny neurons (Borgkvist et al., 2007). NHA were treated with 10−11–10−7 M heroin for 48 h, RNA was extracted followed by quantitation of DARPP-32 gene expression using quantitative, real time PCR. As shown in Fig. 3, heroin at 10−11, 10−9, and 10−7 M concentrations significantly upregulated DARPP-32 gene expression by 43% (TAI = 1.75 ± 0.17, p < 0.0001), 58% (TAI = 1.58 ± 0.08, p < 0.001), and 43% (TAI = 1.43 ± 0.04, p < 0.001), respectively as compared to control cultures (TAI = 1.00 ± 0.03). These results are representative of three separate experiments and statistical significance was determined using Students’ t-test, where the sample comparisons were performed between heroin treated and untreated controls.
FIG. 3.
Effect of heroin on DARPP-32 gene expression by NHA. NHA were treated with 10−7–10−11 heroin for 48 h, RNA was extracted, reverse transcribed, and DARPP-32 gene expression was quantitated using real time PCR. Morphine significantly increased DARPP-32 gene expression in a dose-dependent manner.
We have previously shown that NHA treated with opiate (morphine) significantly downregulated CREB gene expression and also decreased endogenous CREB protein expression (Mahajan et al., 2005). It is believed that morphine activation of the μ-opioid receptor increases intracellular cAMP levels in NHA that triggers the D1/DARPP-32/PP-1 signaling cascade. This, in turn, modulates the activity of CREB, which initiates the transcription of immediate-early genes.
E. Effect of DARPP-32 siRNA Transfection on Cell Viability
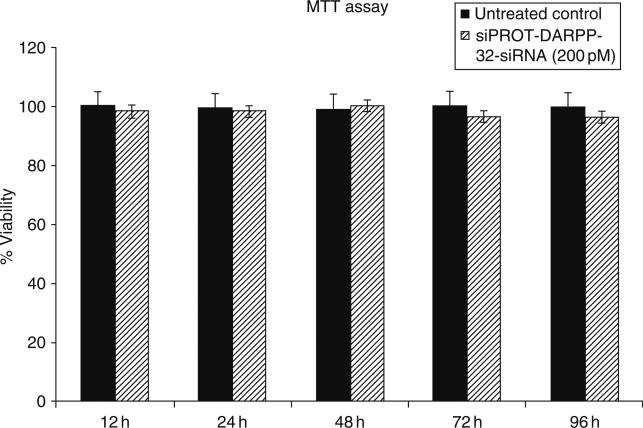
Approximately 10,000 cells/ml transfected NHA cells were incubated with the MTT reagent for approximately 3 h, followed by addition of a detergent solution to lyse the cells and solubilize the colored crystals. The samples are read using an ELISA plate reader at a wavelength of 570 nm. Figure 4 shows >90% viability in both the transfected and untransfected NHA cells up to 96 h posttransfection.
FIG. 4.
Effect of the DARPP-32-siRNA transfection on cell viability in NHA. Cell viability was measured using the MTT assay. NHA were transfected with DARPP-32-siRNA (200 pM) using siPROT transfection reagent from Ambion Inc. (Austin, TX), for a time period of up to 96 h. Data were expressed as a percentage of viable cells calculated with respect to the untreated control and represented as the mean ± S.D. of three separate experiments done in duplicate. Our results showed a >90% viability over the range of time periods tested.
F. Transfection Efficiency of DARPP-32 siRNA
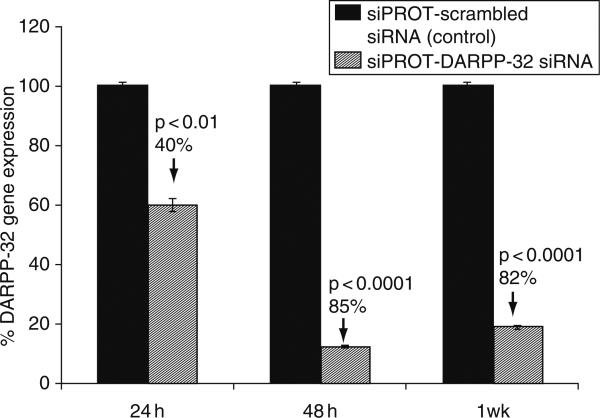
The efficiency of gene silencing was determined by measuring the percentage inhibition of the DARPP-32 gene expression using quantitative real time Q-PCR. The commercially available transfection reagent siPROT was used to transfect the cells using a final concentration of 200 pmol siRNA-DARPP-32 in culture. Our results (Fig. 5) show that the percentage decrease over a time period of 24 h up to 1 week in DARPP-32 gene expression in transfected NHA as compared to the scrambled siRNA used as a control. The percentage decrease in DARPP-32 gene expression was 40% (p < 0.01), 85% (p < 0.0001), and 82% (p < 0.0001) at 24, 48, and 1 week posttransfection, respectively. Significant knockdown of DARPP-32 gene expression was observed even 1 week posttransfection.
FIG. 5.
Effect of the DARPP-32-siRNA transfection on DARPP-32 gene silencing in NHA. NHA were transfected with DARPP-32-siRNA (200 pM) using siPROT transfection reagent from Ambion Inc. (Austin, TX), for a time period of up to 1 week. NHA were treated in vitro with the DARPP-32-siRNA (200 pM) and scrambled-siRNA (transfection control), RNA was extracted, reverse transcribed, cDNA amplified, and the DARPP-32 gene expression was determined by real time quantitative PCR. Relative expression of mRNA species was calculated using the comparative CT method. Data are the mean ± S.D. of three separate experiments done in duplicate. Statistical significance was determined using ANOVA based on comparison between, the positive control, DARPP-32 siRNA and the scrambled-siRNA samples. Our results show a >80% suppression in DARPP-32 gene expression in NHA that were transfected with DARPP-32 siRNA.
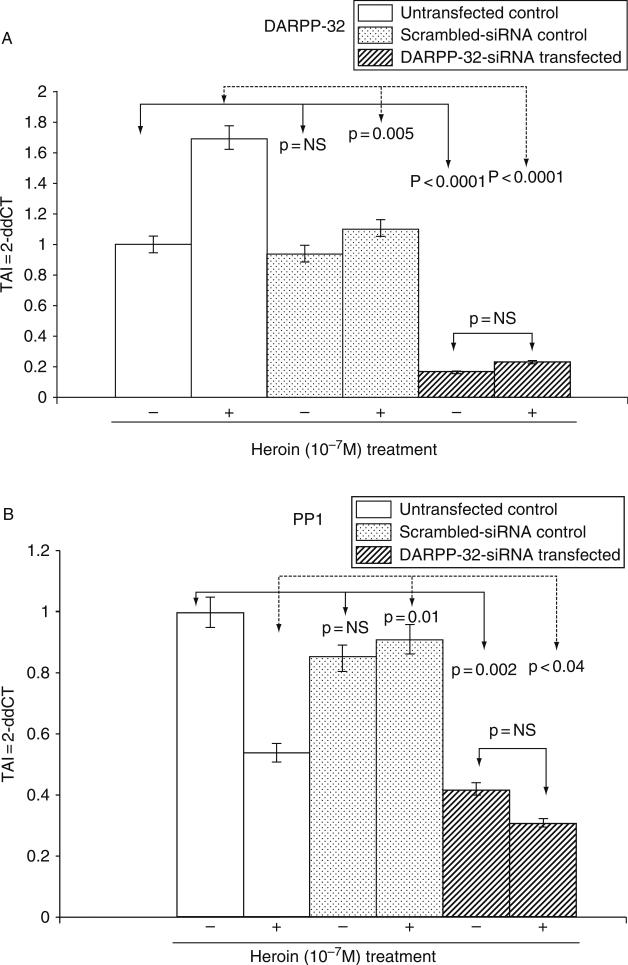
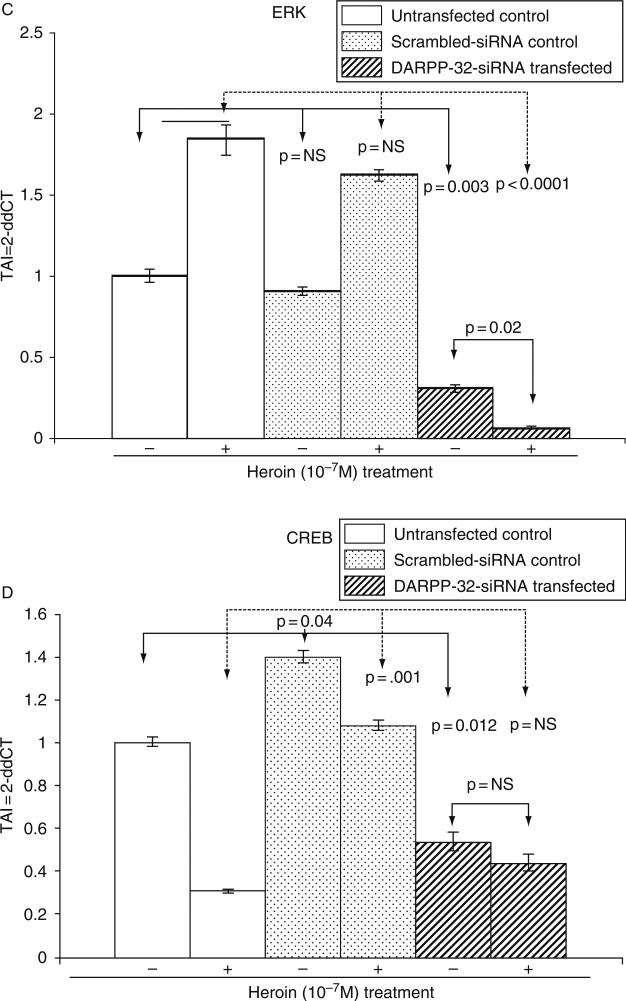
G. Effect of DARPP-32 Silencing on PP-1, ERK, and CREB Gene Expression
Both acute and chronic opiate exposure induce biochemical changes in specific regions in the brain and specifically the dopaminergic pathway which mediates the reinforcing actions of the opiates. We evaluated the effect of DARPP-32 gene silencing on PP1, ERK, and CREB gene expression in both heroin treated and control cultures. Our data (Fig. 6A–D) suggest that DARPP-32 gene silencing resulted in a significant decrease in PP-1, ERK, and CREB gene expression, when comparison were made between the DARPP-32 siRNA transfected cells and the untransfected controls or the scrambled siRNA transfected cells. Heroin treatment of the DARPP-32 siRNA transfected cells resulted in a significant decrease (p = 0.02) in ERK gene expression. Using immunofluoresence techniques, we further examined the effect of heroin on ERK protein expression in DARPP-32 siRNA transfected and untransfected cells. Our results (Fig. 7) show a significant decrease in ERK protein expression in DARPP-32 siRNA transfected cells.
FIG. 6.
Effect of DARPP-32 gene silencing on PP-1, ERK, and CREB gene expression in transfected NHA cells treated with/without heroin. NHA were transfected with DARPP-32-siRNA (200 pM) using siPROT transfection reagent from Ambion Inc. (Austin, TX), for 48 h followed by a 24-h treatment with/without heroin (10−7 M). Appropriate scrambled-siRNA (transfection control) and untransfected cells were used as controls. RNA was extracted, reverse transcribed, cDNA amplified, and the (A) DARPP-32, (B) PP-1, (C) ERK, (D) CREB gene expression was determined by real time quantitative PCR. Relative expression of mRNA species was calculated using the comparative CT method. Data are the mean ± S.D. of three separate experiments done in duplicate. Statistical significance was determined using ANOVA based on comparison between, the DARPP-32 siRNA transfected, scrambled-siRNA transfected, and the untransfected samples in the heroin treated and untreated groups. Our results show a significant decrease in PP-1 and ERK gene expression in heroin treated NHA that were transfected with DARPP-32 siRNA.
FIG. 7.
Immunofluorescent staining for ERK in DARPP-32-siRNA transfected and untransfected NHA cells treated with/without heroin. (A) ERK expression in untransfected NHA cells; (B) ERK expression in heroin (10−7 M) treated untransfected NHA cells; (C) ERK expression in DARPP-32-siRNA transfected NHA cells; (D) ERK expression in heroin (10−7 M)-treated DARPP-32-siRNA transfected NHA cells. Our results show a significant decrease in ERK expression in heroin-treated DARPP-32-siRNA transfected NHA cells. Data shown are representative images from three separate experiments.
V. Discussion
The addictive process occurs as a result of impairments in the three functional systems, namely the motivation-reward, affect regulation, and behavioral inhibition. Nobel laureate Dr. Paul Greengard and his team have extensively studied the phosphoprotein-DARPP-32. DARPP-32 is regulated by many different neurotransmitters and may either inhibit cellular phosphatases or inhibit cAMP-dependent kinases. These opposing activities of DARPP-32 affect the overall sensitivity of the neurons to neurotransmitter signals. The neurotransmitter, dopamine, plays a key role in the development of drug abuse and dependence. Dopamine has been associated with the reinforcing effects of various types of drugs of abuse including opiates.
DARPP-32 is localized in many regions of the brain, and its high enrichment is observed in the dopaminoceptive neurons in the striatum, particularly caudateputamen and nucleus accumbens. DARPP-32 is also localized in the olfactory tubercle, bed nucleus of stria terminalis, and portions of the amygdaloid complex and throughout the neocortex, with particular enrichment in layers II, III, and VI, in the dentate gyrus of the hippocampus, choroid plexus, hypothalamus, and cerebellum. Within the striatum, DARPP-32 is found in medium-sized spiny neurons (Ouimet et al., 1984, 1992; Yoshida et al. 1971). Thus, DARPP32 is widely expressed in both neurons and glial cells. Drug abuse results in damage to brain cells, which is evident long after drug abuse has ceased. The damage to the brain cells, neurodegeneration due to drugs of abuse may be irreversible. In response to neuroinflammation and apoptotic stress as a consequence of chronic opiate abuse, neuropathological abnormalities such as increase in the number of astrocytes or astrogliosis, astrocyte hypertrophy, that involves extension of their processes, and increased production of glial fibrillary acidic protein (GFAP), an intermediate filament protein located within their cytoplasm have been observed. GFAP expression is believed to alter the vulnerability of neurons to excitotoxic and metabolic insults and confer some degree of neuroprotection after an excitotoxic or metabolic insult (Hanbury et al., 2003). Additionally, an association between DARRP-32 expression and astrogliosis after an ischemic insult has also been observed (Han and Holtzman, 2000). These studies lead us to speculate that morphine via the DARPP-32 signaling pathway may alter neuronal plasticity via modulation of signaling intermediates such as ERK and CREB and thereby transcriptional regulation in astrocytes. Astrocytes are important cells in the CNS as they face the synapses, send end-foot processes that enwrap the brain capillaries, and form an extensive network interconnected by gap junctions. Astrocytes express several membrane proteins and enzymes that are critical for uptake of neurotransmittors at the synapses and participate in detection, propagation, and modulation of excitatory synaptic signals and provide metabolic support to the active neurons.
The current treatment regimens in opiate addiction therapy involve the use of drugs such as methadone, naltrexone, and levo-alpha-acetylmethadol (LAAM) and no single treatment is appropriate for all individuals. Relapses to drug use can occur during or after successful treatment episodes. Addicted individuals may require prolonged treatment and multiple episodes of treatment to achieve long-term abstinence and complete cure from drug addiction. Silencing the gene expression of DARPP-32 by RNA interference (RNAi) mechanism may thus be an effective therapeutic strategy toward the treatment of drug addiction.
The results of our study suggest that DARPP-32 gene silencing resulted in a significant decrease in PP-1, ERK, and CREB gene expression in NHA cells. Treatment of NHA with heroin must result in the release of dopamine, via the activation of the D1R, which activates adenylate cyclase resulting in PKA-mediated phosphorylation of DARPP-32 and increased expression of DARPP-32 gene expression levels that we observed in our study (Fig. 6A). DARPP-32 is a potent inhibitor of PP-1, and therefore it is not surprising that we observed a significant decrease of PP-1 gene expression (Fig. 6B). PP-1 is known to play a central role in both dopaminergic and glutamatergic signaling (Greengard et al., 1998, 1999). Our results showed that DARPP-32 gene silencing also resulted in the significant downregulation of ERK and CREB gene expression. ERK activity has been known to be important in neuronal plasticity and its pharmacologic blockade prevents the transcriptional and behavioral effects of various drugs of abuse. The control of the ERK pathway by DARPP-32 is common to most drugs of abuse (Valjent et al., 2001, 2005). ERKs have emerged as an important target in neuronal signal transduction and are believed to participate in diverse processes, including neuronal maturation and survival, synaptic function (Kuroki et al., 2001; Runden, et al., 1998; Xia, et al., 1995). Chronic activation and nuclear retention of ERK may be a critical factor in triggering proapoptotic signals and neuronal cell death. Runden et al. (1998) have shown sustained activation of ERK brought about by PP-1 inhibition induces neuronal cell death via the activation of the caspase-dependent pathway in hippocampal slices (Stanciu, 2000; Stanciu and DeFranco, 2002). Our gene microarray data have shown a significant increase in caspase activity and a significant decrease in the expression of the antiapoptotic protein bcl-2 indicating heroin-induced proapoptotic stimulus in the NHA. The opiate induced increase in ERK expression in NHA and suggests that ERK may be required for activation of proapoptotic pathways.
In the current study, heroin induced D1R-mediated regulation of ERK activity via the DARPP-32 signaling pathway and suggests that the DARPP-32 phosphoprotein may be important in regulating the duration of ERK activation and the subsequent downstream signaling cascades that can modulate the induction of transient immediate early genes and transcription factors such as CREB that modulate transcription and neuronal function and are implicated in the effects of opiates on motor sensitization and reward. The DARPP-32 signaling pathway is thus the central molecular mechanism that underlies the neurobiological alterations due to abuse of opiate drugs. The ERK pathway, as well as DARPP-32 mediated inhibition of PP-1, is critical to the modulation of behavioral responses which are a consequence of changes in neuronal plasticity with structural modification of neural networks in the CNS during drug abuse.
A gene therapy approach such as the silencing of DARPP-32 may provide an effective strategy to overcome drug addiction, particularly because it may also help modify cognitive behavior, thereby preventing a relapse. While we certainly do not anticipate the clinical use of intracerebral DARPP-32 siRNA for the treatment of drug abuse, we expect that results from these studies will support the premise that blocking this pathway can eliminate addictive, drug-seeking behavior, and prevent neurologic dysfunction in drug abusing subjects.
Although chemically synthesized, siRNA can be delivered in vitro and in vivo to achieve therapeutic gene silencing, without interfering with the cell's endogenous microRNA. A major challenge associated with siRNA therapy and its delivery into the cell is the issue of increased cellular toxicity. Innovative methodologies using nanotechnology, that can provide efficient gene delivery that not only provides sustained release of siRNA molecules which can result in effective DARPP-32 gene silencing but also show no significant toxicity over a wide range of dose and time conditions are currently being explored.
Acknowledgments
The authors acknowledge the Cameron Troup Foundation, Kaleida Health, Buffalo General Hospital, Buffalo, NY, for the grant support provided for this research work.
References
- Baritaki S, Dittmar MT, Spandidos DA, Krambovitis E. In vitro inhibition of R5 HIV-1 infectivity by X4 V3-derived synthetic peptides. Int. J. Mol. Med. 2005;16(2):333–336. [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci. Biobehav. Rev. 2007;31(1):79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002;1:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000;20(22):8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu. Rev. Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman R, Rohsenow DJ, Robbins SJ, O'Brien CP. Classically conditioned factors in drug dependence. In: Lowinson JW, Luiz P, Millman RB, Langard G, editors. Substance Abuse: A Comprehensive Textbook. Williams and Wilkins; Baltimore, MD: 1992. pp. 56–69. [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colvis CM, Pollock JD, Goodman RH, Impey S, Dunn J, Mandel G, Champagne FA, Mayford M, Korzus E, Kumar A, Renthal W, Theobald DE, et al. Epigenetic mechanisms and gene networks in the nervous system. J. Neurosci. 2005;25(45):10379–10389. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Greengard P. The DARPP-32 knockout mouse. Brain Res. Rev. 2000;31(2–3):313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, et al. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281(5378):838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Greengard P, Nairn AC, Girault JA, Ouimet CC, Snyder GL, Fisone G, Allen PB, Fienberg A, Nishi A. The DARPP-32/protein phosphatase-1 cascade: A model for signal integration. Brain Res. Brain Res. Rev. 1998;26(2–3):274–284. doi: 10.1016/s0165-0173(97)00057-x. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: The DARPP-32/ protein phosphatase-1 cascade. Neuron. 1999;23(3):435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Johansson B, Fernandez-Teruel A, Canete T, Tobena A, Terenius L, Gimenez-Llort L. Divergent anatomical pattern of D1 and D3 binding and dopamine- and cyclic AMP-regulated phosphoprotein of 32 kDa mRNA expression in the Roman rat strains: Implications for drug addiction. Neuroscience. 2006;142(4):1231–1243. doi: 10.1016/j.neuroscience.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J. Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbury R, Ling ZD, Wuu J, Kordower JH. GFAP knockout mice have increased levels of GDNF that protect striatal neurons from metabolic and excitotoxic insults. J. Comp. Neurol. 2003;461(3):307–316. doi: 10.1002/cne.10667. [DOI] [PubMed] [Google Scholar]
- Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, Behr JP, Demeneix BA. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J. Gene Med. 2005;7(2):198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: Real-time monitoring of DNA amplification reactions. Biotechnology (NY) 1993;11:1026. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward systems. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Neuroprotection by estrogen via extracellular signal-regulated kinase against quinolinic acid-induced cell death in the rat hippocampus. Eur. J. Neurosci. 2001;13:472–476. doi: 10.1046/j.0953-816x.2000.01409.x. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Reynolds JL, Nair BB, Fernandez SF, Schwartz SA, Nair MP. Morphine exacerbates HIV-1 viral protein gp120 induced modulation of chemokine gene expression in U373 astrocytoma cells. Curr. HIV Res. 2005a;3(3):277–288. doi: 10.2174/1570162054368048. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin. Immunol. 2005b;115(3):323–332. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, Nair MPN. Methamphetamine modulates gene expression patterns in Monocyte derived mature Dendritic cells: Implications for HIV-1 pathogenesis. Mol. Diagn. Ther. 2006;10(4):257–269. doi: 10.1007/BF03256465. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: Studies in the rat locus coeruleus. Brain Res. Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci. 1997;17(21):8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr., Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J. Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, LaMantia AS, Goldman-Rakic P, Rakic P, Greengard P. Immunocytochemical localization of DARPP-32, a dopamine and cyclic-AMP-regulated phosphoprotein, in the primate brain. J. Comp. Neurol. 1992;323(2):209–218. doi: 10.1002/cne.903230206. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Cabral G, Lokensgard JR. Cannabinoids and morphine differentially affect HIV-1 expression in CD4(+) lymphocyte and microglial cell cultures. J. Neuroimmunol. 2004;147(1–2):123–126. doi: 10.1016/j.jneuroim.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313(4):856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Runden E, Seglen PO, Haug FM, Ottersen OP, Wieloch T, Shamloo M, Laake JH. Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: Evidence for a MAP kinase-dependent mechanism. J. Neurosci. 1998;18:7296–7305. doi: 10.1523/JNEUROSCI.18-18-07296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: Neural and molecular mechanisms. Drug Alcohol Depend. 1998;51(1–2):49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J. Neurovirol. 2004;10(3):141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Stanciu M, DeFranco DB. Prolonged nuclear retention of activated extracellular signal-regulated protein kinase promotes cell death generated by oxidative toxicity or proteasome inhibition in a neuronal cell line. J. Biol. Chem. 2002;277:4010–4017. doi: 10.1074/jbc.M104479200. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: An integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7(2):E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Ohshima T, Cho A, Sreenath T, Iadarola MJ, Pant HC, Kim Y, Nairn AC, Brady RO, Greengard P, Kulkarni AB. Increased activity of cyclin-dependent kinase 5 leads to attenuation of cocaine-mediated dopamine signaling. Proc. Natl. Acad. Sci. USA. 2005;102(5):1737–1742. doi: 10.1073/pnas.0409456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Caboche J, Vanhoutte J, editors. Mitogen-Extracellular Signal-R/Extra-cellular Signal-Regulated Kinase Induced Gene Regulation in Brain: A Molecular Substrate for Learning and Memory? Vol. 23. Humana Press; Totowa, NJ, USA: 2001. [DOI] [PubMed] [Google Scholar]
- Valjent E, Herve D, Girault JA. Drugs of abuse, protein phosphatases, and ERK pathway. Med. Sci. (Paris) 2005;21:453–454. doi: 10.1051/medsci/2005215453. [DOI] [PubMed] [Google Scholar]
- Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J. Neurosci. 2001;21(23):9438–9444. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gintzler AR. Altered mu-opiate receptor-G protein signal transduction following chronic morphine exposure. J. Neurochem. 1997;68:248–254. doi: 10.1046/j.1471-4159.1997.68010248.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Medina VM, Rivera M, Gintzler AR. Relevance of phosphorylation state to opioid responsiveness in opiate naive and tolerant/dependent tissue. Brain Res. 1996;723:61–69. doi: 10.1016/0006-8993(96)00217-x. [DOI] [PubMed] [Google Scholar]
- Widnell KL, Self DW, Lane SB, Russell DS, Vaidya VA, Miserendino MJ, Rubin CS, Duman RS, Nestler EJ. Regulation of CREB expression: In vivo evidence for a functional role in morphine action in the nucleus accumbens. J. Pharmacol. Exp. Ther. 1996;276(1):306–315. [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Precht W. Monosynaptic inhibition of neurons of the substantia nigra by caudato-nigral fibers. Brain Res. 1971;32:225–228. doi: 10.1016/0006-8993(71)90170-3. [DOI] [PubMed] [Google Scholar]