Abstract
ClpS is an adaptor protein that interacts with ClpA and promotes degradation of proteins with N-end rule degradation motifs (N-degrons) by ClpAP while blocking degradation of substrates with other motifs. Although monomeric ClpS forms a 1:1 complex with an isolated N-domain of ClpA, only one molecule of ClpS binds with high affinity to ClpA hexamers (ClpA6). One or two additional molecules per hexamer bind with lower affinity. Tightly bound ClpS dissociates slowly from ClpA6 with a t½ of ∼3 min at 37 °C. Maximum activation of degradation of the N-end rule substrate, LR-GFPVenus, occurs with a single ClpS bound per ClpA6; one ClpS is also sufficient to inhibit degradation of proteins without N-degrons. ClpS competitively inhibits degradation of unfolded substrates that interact with ClpA N-domains and is a non-competitive inhibitor with substrates that depend on internal binding sites in ClpA. ClpS inhibition of substrate binding is dependent on the order of addition. When added first, ClpS blocks binding of both high and low affinity substrates; however, when substrates first form committed complexes with ClpA6, ClpS cannot displace them or block their degradation by ClpP. We propose that the first molecule of ClpS binds to the N-domain and to an additional functional binding site, sterically blocking binding of non-N-end rule substrates as well as additional ClpS molecules to ClpA6. Limiting ClpS-mediated substrate delivery to one per ClpA6 avoids congestion at the axial channel and allows facile transfer of proteins to the unfolding and translocation apparatus.
Keywords: Protease/ATP-dependent, Adaptor Proteins, Chaperone Chaperonin, Protein Degradation, Protein-Protein Interactions, AAA+ Protein, Adaptor, Clp Chaperones, N-end Rule, Protein Unfoldase
Introduction
ClpA and ClpX, the regulatory components of the ATP-dependent ClpAP and ClpXP proteases, are members of the Clp/Hsp100 family of molecular chaperones, which play important roles in protein quality control and regulatory protein degradation (1, 2). Specific proteins targeted for regulatory unfolding or degradation are recognized by virtue of sequence or structural motifs located in accessible regions near their amino or carboxyl termini. Clp chaperones can also target aberrant or altered forms of virtually any cellular protein either through weak interaction with unfolded regions of the protein or by more specific recognition of motifs that become exposed in the protein or are added to the protein as degradation tags by other enzymatic systems. The specific motifs or degradation tags are either directly recognized by sites in ClpA or ClpX or recognized by one of several adaptor proteins that associate with ClpA or ClpX, and, in some instances, different parts of the tag can be recognized by the chaperone and the adaptor (3–5).
Among the substrates for ClpAP in Escherichia coli are proteins degraded by the N-end rule pathway (6). The N-end rule relates the stability of a protein to its N-terminal residue. A destabilizing N-terminal residue is referred to as an N-degron (7) when it is recognized by a component of a degradative system and is sufficient to target the protein for degradation. In E. coli, the N-degrons include phenylalanine, tyrosine, tryptophan, and leucine (6). Arginine and lysine serve as secondary destabilizing residues, because proteins with these residues at the N terminus are modified by Leu/Phe-tRNA protein transferase to generate an N-degron by post-translational attachment of phenylalanine or leucine (8, 9). Destabilizing residues are usually not found at the N terminus of newly synthesized proteins in vivo, because protein biosynthesis usually begins with methionine, and the N-terminal methionine is not removed by methionine aminopeptidase when one of these residues is in the second position (10). Initially, components of the N-end rule pathway in E. coli were identified using proteins engineered to display N-degrons (6, 8). Recently, however, endogenous substrates degraded by the N-end rule have been identified in E. coli (9, 11). In at least one case, an aminopeptidase generates a protein with an N-degron that is degraded by ClpAP (9, 11, 12), but the mechanisms by which N-end rule substrates are formed and the frequency with which they occur are largely unknown.
In E. coli, proteins with N-degrons are recognized by a small protein, ClpS, which binds the N-degron and delivers the protein to ClpAP (5). ClpS is a highly conserved and has sequence homology to a domain found in the E3 ligases of yeast and mammals, which recognize N-degrons (13). ClpS is expressed in an operon with ClpA and is required for rapid degradation of proteins with N-degrons in vivo. Purified ClpS is a monomer with a single binding site for an N-degron (12, 14–16). Peptides with Phe, Tyr, Trp, or Leu at the N terminus bind to ClpS, and similar peptides with other N-terminal residues bind with substantially lower affinity (5). The crystal structure of ClpS with a bound peptide bearing an N-degron shows the hydrophobic side chain of the N-degron pointing into a deep hydrophobic pocket in ClpS and the α-amino group of the peptide forming an ion pair with an acidic residue on the rim of the hydrophobic pocket (12, 16). Other contributions from hydrogen bonding between the main chain carbonyls and amides are limited and nonspecific. In vitro, ClpS increases the catalytic efficiency of ClpAP against proteins with N-degrons (5) while inhibiting the degradation of proteins with other degradation motifs (14, 15). Details of how ClpS interacts with the ClpAP holoenzyme complex and performs its functions are not well understood.
The holoenzyme complex of ClpAP is a barrel-like complex. The center has two heptameric rings of ClpP subunits stacked face to face. The ClpP rings are flanked on either side by axially aligned hexameric rings of ClpA (17, 18). ATP binding to ClpA is required to stabilize the ClpA hexamers and to maintain the ClpAP complex. ClpA subunits contain three tandem domains: an N-domain and two non-identical ATPase domains (NBD1 and NBD2) (19). In the ClpA hexamer, NBD1 and NBD2 form separate homomeric rings superimposed on each other. The NBD2 ring makes the contact with ClpP, whereas NBD1 forms the distal ring. The N-domain folds independently and is connected to NBD1 by a flexible linker (19). Electron microscopic studies suggest that the N-domains are mobile and occupy multiple positions above surface of the NBD1 ring (20). The N-domain binds unfolded proteins, which serves to recruit substrates to NBD1 and to slow the release of proteins unfolded by ClpA. The N-domain also binds ClpS. Substrates bound to the N-domain or to ClpS are handed off to the ATPase domains, unfolded, and translocated via an axial pathway into ClpP.
Both ClpS and the isolated ClpA N-domain are monomers and form a tight 1:1 complex when mixed in vitro (15, 21). Thus, up to six molecules of ClpS might be expected to bind to assembled ClpA hexamers. The binding of multiple molecules of an adaptor and its substrate cargo raises a question as to the efficiency of substrate delivery and processing by hexameric ClpA. The ClpA axial channel appears too narrow to allow translocation of more than one protein at a time, which suggests that a mechanism to limit the binding or transfer of bound substrate to the unfolding and translocation apparatus would be needed to prevent steric clashes between multiple bound substrates and to improve overall efficiency. In fact, several observations suggest that the geometry of interaction of ClpS with assembled ClpA or structural changes subsequent to binding might affect the binding stoichiometry and activity. In the crystal, the mobile N-terminal 17 amino acids of ClpS (ClpS-N1–17) extend out from the complex, and residues 3–8 interact with another ClpA N-domain. ClpS-N1–17 is not needed for binding to N-domains but is required for inhibition of activity against substrates, such as SsrA-tagged proteins (15). ClpS-N1–17 is also needed for activation of N-end rule degradation (22), and it has been proposed that ClpS-N1–17 interacts within or near the axial channel of ClpA. To learn more about the interaction of ClpS with ClpA and the mechanisms by which it affects ClpA activity, we investigated whether ClpS can bind to all six N-domains in a ClpA hexamer and how many ClpS molecules are needed for optimal activation or inhibition of protein degradation. Here we show that one molecule of ClpS binds to a ClpA hexamer with high affinity and that only one ClpS molecule is required for maximum stimulation or inhibition of degradation. We propose that ClpS allosterically affects the structure of ClpA hexamers and reorganizes the N-domains to reduce the number of ClpS molecules that can bind.
MATERIALS AND METHODS
Plasmid Clones and Protein Expression
ClpA, ClpP, and ClpS were expressed and purified as described (15, 23, 24). ClpA-Δ153 (25), the phage P1 RepA protein (26), and GFP2-SsrA (27) were expressed and purified as described. To clone the SUMO-LR-GFPVenus fusion protein, we first amplified the GFPVenus coding region in plasmid pVenus-N1-NPY (28) with primers (5′-cttagaaaaggagaagaacttgttactggagttgtggtgagcaagggcgaggagctg and 3′-ttacttgtacagctcgtccatgccgagagtgatc). The forward primer added the coding region for 11 amino acids (LRKGEELVTGV) to the coding region of GFPVenus. The product was ligated to the Champion SUMO expression vector (Invitrogen) by TA cloning. The new ORF encoded a fusion protein with His6-SUMO linked to LR-GFPVenus. The plasmid was transformed into E. coli BL21-DE3 cells. Cells were grown in LB plus ampicillin, and isopropyl 1-thio-β-d-galactopyranoside was added at midexponential phase to induce expression for 4 h. Cells were harvested and frozen until used. After suspension in 50 mm Tris/HCl, pH 7.5, with 10% (v/v) glycerol, the thawed cells were lysed by a single pass through a French pressure cell at 20,000 p.s.i. Cell debris was removed by centrifugation at 30,000 × g for 60 min, and the clear supernatant solution was passed over a 10-ml bed of Talon metal chelate affinity resin. After washing the column with 5 column volumes of buffer, the bound protein was eluted with 10 aliquots of 2 ml each of 0.2 m imidazole in 50 mm Tris/HCl, pH 7.5, with 10% (v/v) glycerol. Fractions with the SUMO-LR-GFPVenus fusion protein were pooled and dialyzed against buffer without imidazole. The fusion protein was cleaved with 50 μg of SUMO hydrolase (29) per mg of SUMO-LR-GFPVenus by incubation for 6 h at 4 °C. The solution was passed through a 10-ml bed of Talon resin, and the unbound LR-GFPVenus was collected.
[3H]ClpS was prepared by reductive methylation. [3H]formaldehyde (350 μCi; 5 μmol) (PerkinElmer Life Sciences) was added to 1 mg of purified ClpS in 0.2 ml of 25 mm Hepes/KOH, pH 7.5, 0.1 m KCl, and 10% (v/v) glycerol, followed by the addition of 5 μl of 20 mm NaCNBH3. The mixture was placed on ice for 16 h. The protein was precipitated with 60% saturated ammonium sulfate, dissolved in 100 μl of 50 mm Tris/HCl, pH 7.5, 0.2 m KCl, and desalted by passing over a 2-ml column of Sephadex G25 (Amersham Biosciences) in the same buffer. [3H]RepA was prepared by a similar procedure.
Protein and Enzymatic Assays
Protein concentrations were measured by absorbance using either experimentally determined absorption coefficients (23, 29) or absorption coefficients calculated from the aromatic amino acid content using the Pepstats program (available on the World Wide Web). For routine assays or in the presence of substances that interfere with absorbance measurements, a dye-binding assay (Bio-Rad) was used after calibration with known stock solutions of the particular protein. Protein concentrations are expressed in molar units of the native solution structure of the protein: hexamers of ClpA, tetradecamers of ClpP, monomers of ClpS or isolated ClpA-N-domain, and so on. Fluorescence measurements for LR-GFPVenus were made using an Aminco Bowman spectrofluorometer with excitation at 490 nm and emission at 528 nm (2–4-nm bandwidth). For GFP-SsrA fluorescence measurements, excitation was at 394 nm and emission was at 509 nm with a 2-nm bandwidth.
Degradation of radioactively labeled proteins was measured by release of trichloroacetic acid-soluble counts as described previously (23). LR-GFPVenus degradation was determined in a continuous assay by monitoring the decrease in fluorescence in an assay solution containing excess ClpP (30 μg) in 50 mm Tris/HCl, pH 8.0, 0.1 m KCl, 20 mm MgCl2, 2–4 mm ATP, and varying concentrations of ClpA, ClpS, and the substrate. GFP-SsrA degradation was assayed under similar conditions without ClpS.
Peptide Binding and Inhibition
The peptides, YLFVQ, LRKGE, MYLFVQ, SLRKGE, and MLRKGE, were obtained from Biomatik Corp. USA, LLC (Wilmington, DE). The peptide, FKTA, was synthesized in house from FMOC-amino acids on an ABI431 peptide synthesizer following standard procedures and purified by reverse phase chromatography (HPLC). Peptide purity was confirmed by HPLC and mass spectroscopic analysis; all peptides had free amino and carboxyl termini. Peptides were dissolved in water for use. To measure inhibition of LR-GFPVenus degradation, peptides were added to assay solutions prior to the addition of ClpA to initiate the reaction. Binding measurements were made by titration calorimetry using a VP-ITC microcalorimeter (Microcal).
Ultrafiltration Assays for Binding
ClpA hexamers were assembled in 50 mm Tris/HCl, pH 7.5, 0.2 m KCl, and 10% (v/v) glycerol with 25 mm MgCl2 and 1 mm ATPγS. [3H]ClpS was added, and, after 5 min at room temperature, the mixture was centrifuged through a Microcon100 ultrafiltration membrane. To avoid excessive concentration of the ClpA during ultrafiltration and to minimize dissociation of the complex, centrifugation time was limited to 30 s to allow less than half of the solution to pass through the membrane. The filtrate was collected and counted, and the amount of [3H]ClpS bound was calculated from the decrease in counts between the filtrate and an equivalent volume of the mixture before filtration.
Other Analytical Procedures
Analytical gel filtration was performed at room temperature using a Superdex200 or Superdex75 column (3 mm × 25 cm) (Amersham Biosciences). Columns were run in 50 mm Tris/HCl, pH 7.5, 0.1 m KCl, and 10% (v/v) glycerol at a flow rate of 0.04–0.08 ml/min. ATPγS (1 mm) and MgCl2 (20 mm) were added to buffers as required for specific experiments. Protein absorbance was monitored at 235 and 280 nm in the absence of nucleotide and at 295 nm when nucleotide was present. When needed, fractions were collected manually at 1-min intervals. SDS-PAGE was performed using precast Novex 4–20% polyacrylamide gels in the BisTris/MES buffer system as described by the manufacturer. Proteins were stained with Coomassie Blue. To quantitate proteins in gel filtration fractions, equal aliquots were run in each lane, digital images of the gels were recorded, and the intensities of the stained protein bands were measured using NIH Image software. To compare the distribution of ClpS in different experiments, the ClpS intensities in the fractions within a given run were summed and normalized to the amount of ClpS loaded on the column.
RESULTS
ClpS Mediates Interaction of N-end Rule Proteins with ClpA
Based on the reported specificity of ClpS in targeting proteins for degradation by ClpAP, we constructed the substrate, LR-GFPVenus, which has an N-terminal leucine followed by arginine. LR-GFPVenus was degraded by ClpAP in the presence of ClpS but was not degraded in the absence of ClpS (Table 1 and supplemental Fig. S1). To confirm the importance of the N-terminal amino acid in allowing ClpS to target LR-GFPVenus to ClpAP, we synthesized several peptides with N-degrons and determined their ability to bind to ClpS and inhibit degradation of LR-GFPVenus. The peptides, LRKGE and FKTA, displayed monotonic binding isotherms with one molecule of peptide bound per ClpS (supplemental Fig. S2, A and B). The Kd for binding was 3.0 μm for LRKGE and 3.5 μm for FKTA. Virtually identical data were obtained for binding of the peptides to the heterodimeric complex of ClpS and isolated ClpA N-domain (supplemental Fig. S2, C and D). Variants of the peptides with N-terminal serine or methionine residues (SLRKGE, MLRKGE, and MYVLVQ) showed no evidence of binding at concentrations of ≤30 μm (data not shown).
TABLE 1.
Effects of ClpS and peptides with N-degrons on protein degradation by ClpAP
| Addition | Activity | Ka (or Kd) | Ki |
|---|---|---|---|
| μmol/min/μmol ClpA6 | μm | μm | |
| Degradation of LR-GFPVenus by ClpAPa | |||
| Without ClpS | 0 | NAb | NA |
| With ClpSc | 5.0 | 0.05 | NA |
| With ClpS + LRKGEd | <0.1 | 3.8 | 1.0 |
| With ClpS + FKTAd | <0.1 | 3.0 | 0.9 |
| With ClpS + YLFVQd | <0.1 | NDe | 0.8 |
| With ClpS + SLRKGE, MFKTA, MLRKGE, or MYLFVQd | d∼5.0 | ND | >30 |
| Degradation of GFP-SsrA by ClpAPf | |||
| None | 8.2 | 1.5 | NA |
| With ClpS | <0.1 | NA | 0.035 |
a 0.4 μm ClpA6 and 1.0 μm ClpP14 were present in standard assay mixture with 11 μm LR-GFPVenus (see “Materials and Methods”).
b NA, not applicable.
c ClpS was added at 1.0 μm in the assay.
d Peptide concentrations were 30 μm; LR-GFPVenus concentration was 3 μm.
e ND, no binding detected.
f 0.4 μm ClpA6 and 1.0 μm ClpP14 were present in standard assay mixture with 10 μm GFP-SsrA (see “Materials and Methods”).
When added at saturating levels, LRKGE, FKTA, and YVLVQ completely inhibited ClpS-mediated degradation of LR-GFPVenus by ClpAP (Table 1); half-maximal inhibition occurred at ∼1 μm peptide (Table 1), consistent with the dissociation constants measured by direct binding. None of the peptides inhibited degradation of the non-N-end rule substrate, GFP-SsrA, by ClpAP (Table 1). Peptide variants with N-terminal serine or methionine residues had no inhibitory activity in either assay at concentrations up to 30 μm. We conclude that degradation of LR-GFPVenus by ClpAP is dependent on recognition of the N-degron by ClpS.
Non-equivalent Binding of ClpS to ClpA Hexamers
If ClpS binds to the N-domains in ClpA6 in the same manner as it binds to the isolated N-domain, each hexamer of ClpA could bind up to six ClpS monomers. However, neither the substrate saturation curve for LR-GFPVenus nor the peptide inhibition curves gave indications of multiple binding sites (supplemental Fig. S1). To address the question of how many ClpS molecules bind to assembled ClpA hexamers and are required for activity, we measured binding of ClpS to ClpA6 and then determined the binding stoichiometry needed for expression of ClpS activities.
To isolate tightly binding complexes of ClpS and ClpA6, we initially assembled ClpA6 in the presence of ATPγS, incubated it with increasing amounts of ClpS, and passed the mixtures over a gel filtration column under conditions in which ClpA remained hexameric. When one equivalent of ClpS was added per ClpA6 (Fig. 1A, circles), essentially all of the ClpS co-migrated with ClpA6, indicating that the complex was very stable. When 2 eq of ClpS were added (Fig. 1A, triangles), only about two-thirds of the ClpS remained bound to ClpA6, and a significant amount was found as free ClpS. The addition of more than 3 eq of ClpS (Fig. 1A, squares) did not increase the amount of ClpS bound to ClpA6, and the excess ClpS migrated as the unbound protein. These data suggested that only 1–2 molecules of ClpS bind tightly enough to ClpA6 to remain bound during gel filtration, and any additional molecules that bind do so with lower affinity and dissociate during gel filtration.
FIGURE 1.
ClpS binding to ClpA hexamers. Binding of ClpS or ClpS-Δ17 to ClpA6 was measured by co-migration during gel filtration (A and C) and by retention of the complex following ultrafiltration (B and D). For the co-migration assays, ClpS or ClpS-Δ17 was added in various stoichiometric ratios to 1 μm ClpA6 in 50 mm Tris/HCl, pH 7.5, 0.1 m KCl, 10% (v/v) glycerol, 2 mm ATPγS, and 25 mm MgCl2. The mixtures were run over a Superdex200 column in the same solution. Equal aliquots of fractions were subjected to SDS-PAGE, digital images of the Coomassie Blue-stained gels were recorded, and the distribution of ClpS or ClpS-ΔN17 was determined (see “Materials and Methods”). A, ClpS and ClpA6 in a ratio of 1:1 (circles), 3:1 (triangles), and 6:1 (squares); fraction sizes were 80 μl each. C, ClpS-Δ17 and ClpA6 at a ratio of 1:1 (circles), 3:1 (triangles), 6:1 (squares), and 12:1 (inverted triangles); fraction sizes were 60 μl each. For the ultrafiltration assays, ClpA was mixed with increasing amounts of [3H]ClpS (B) or [3H]ClpS-Δ17 (D) in the presence of 2 mm ATPγS and 25 mm MgCl2 in 50 mm Tris/HCl, pH 7.5, 0.1 m KCl, and 10% (v/v) glycerol. After 5 min at room temperature, the mixture was subjected to ultrafiltration through a Microcon 100 membrane (see “Materials and Methods”). Bound [3H]ClpS or [3H]ClpS-Δ17 was calculated from the difference in free radioactive protein recovered in the filtrate and the total radioactive protein in the original mixture. The data are displayed in a Scatchard plot (bound/free versus bound), which highlights the tight binding of one ClpS and the difference in binding affinity (proportional to the slope) between ClpS and ClpS-ΔN17.
To quantitate binding between ClpS and ClpA6 directly, we measured [3H]ClpS bound to ClpA6 following ultrafiltration through 30,000 Mr cut-off membranes. To minimize dissociation of the complex, the samples were centrifuged for only 30 s. A Scatchard plot of the binding data is shown in Fig. 1B. Binding was biphasic, indicating more than one binding interaction. Extrapolation of the steep portion of the line to the abscissa showed that ∼1 molecule of ClpS per ClpA6 was bound with high affinity, and the Kd, obtained from the slope of the line, was ∼40 nm. The slope of the shallower portion of the line indicates the presence of weaker binding sites with Kd of >700 nm. Extrapolation of this segment to the axis indicated two or more additional ClpS molecules bound, but the exact number of ClpS molecules binding with low affinity cannot be determined by this method, because rapid dissociation prevents retention of some of the weakly bound species. These data indicate that the mode of binding of the first molecule of ClpS to ClpA6 differs from the binding of subsequent molecules.
The N-terminal Portion of ClpS Is Needed for Asymmetric Binding of ClpS to ClpA6
The N-terminal portion of ClpS (residues 1–17) is an extended, mobile polypeptide that is important for inhibitory activity (15, 22) and is required for efficient degradation of proteins bearing N-degrons (5, 22). To determine if the ClpS N-terminal region affects binding to ClpA6, we generated ClpS-Δ17 by tryptic cleavage and removed the N-terminal peptide by gel filtration (15). Co-migration of ClpS-ΔN17 with ClpA6 during gel filtration indicated that binding of ClpS-ΔN17 was significantly weaker than binding of intact ClpS. Very little ClpS-ΔN17 co-migrated with ClpA6 when added at a 3:1 ratio (Fig. 1C, circles), and at 6:1, about two molecules of ClpS-ΔN17 were found with ClpA6 (Fig. 1C, triangles). Approximately four molecules of ClpS-ΔN17 co-migrated with ClpA6 when excess ClpS-ΔN17 was added (Fig. 1C, squares and inverted triangles). Trailing of the ClpS-ΔN17 in the fractions after the complex of ClpA6 and ClpS suggested that more than four molecules might be bound under equilibrium conditions.
Binding measurements using the ultrafiltration assay confirmed that [3H]ClpS-ΔN17 binds more weakly to ClpA6 than does intact ClpS. In a Scatchard plot (Fig. 1D), the data were fit by a simple linear regression, consistent with a single type of binding site, which gave a Kd of ∼1.0 μm. Thus, ClpS-ΔN17 binds with about one-tenth the affinity of ClpS. Extrapolation of the line to the axis indicated that four or more molecules of ClpS-ΔN17 can bind per ClpA6. The difference in binding between ClpS and ClpS-ΔN17 suggests that some part of the N terminus of ClpS contributes directly or indirectly to ClpS binding to a high affinity site on ClpA6; this ClpA site is occupied by only one ClpS molecule at a time.
Competition between ClpS and ClpS-ΔN17 for Binding to ClpA6
Weak binding of ClpS-ΔN17 should make it a poor competitive inhibitor of ClpS binding to ClpA6. To measure competition between ClpS and ClpS-ΔN17, we mixed [3H]ClpS with increasing amounts of non-labeled ClpS-ΔN17 and measured the amount of [3H]ClpS bound to ClpA6 by the ultrafiltration assay. Under the conditions of the assay (4 μm ClpA6), all of the [3H]ClpS was bound, and none was in the filtrate (Fig. 2A). The addition of an equal amount of non-labeled ClpS blocked binding of ∼40% of the [3H]ClpS that was released in the filtrate, and excess ClpS inhibited [3H]ClpS binding by ≥85%. In contrast, equimolar amounts of ClpS-ΔN17 had negligible effect on binding of [3H]ClpS (Fig. 2A), and up to a 24-fold excess of ClpS-ΔN17 caused <30% inhibition. A 60-fold excess of ClpS-ΔN17 was needed to achieve 80% inhibition of binding. In separate experiments, we also found that ClpS-ΔN17 competes poorly with ClpS under assay conditions and that >10-fold excess of ClpS-ΔN17 was needed to block ClpS activity in either activating LR-GFPVenus degradation or inhibiting GFP-SsrA degradation (supplemental Fig. S3). Together, these data confirm that, under exactly the same conditions, ClpS-ΔN17 cannot compete with ClpS for the same high affinity site in ClpA6. Displacement of nearly half of the [3H]ClpS by 1 eq of non-labeled ClpS also confirms that the first ClpS goes to a high affinity site and that competition for that site occurs at low concentrations of ClpS insufficient to saturate the binding sites on the other N-domains of ClpA6.
FIGURE 2.
Binding competition between ClpS and ClpSΔN17 or isolated N-domain of ClpA and slow dissociation of the complex. A, a fixed amount (0.4 μm) of [3H]ClpS was mixed with variable amounts of non-radioactive ClpS (light bars) or ClpSΔN17 (dark bars) in the presence of 0.4 μm ClpA6 in 50 mm Tris/HCl, pH 7.5, 0.1 m KCl, 25 mm MgCl2, 2 mm ATPγS, and 10% (v/v) glycerol. After 5 min at room temperature, bound and free [3H]ClpS were separated by ultrafiltration through Microcon 100 membranes, and bound [3H]ClpS was calculated as described in Fig. 1. B, the conditions were the same as in A except that isolated ClpA N-domain was added to compete with ClpA6 for binding of [3H]ClpS. C, complexes of 1 eq of [3H]ClpS per ClpA hexamer (0.5 μm each) were assembled in the buffer described above. After incubation for ≥5 min at room temperature, a 30-fold excess of either non-radioactive ClpS to replace [3H]ClpS as it dissociated from ClpA6 (open diamonds) or ClpA N-domain to trap [3H]ClpS as it dissociated from ClpA6 (closed circles) was added. At each interval, the bound and free ClpS were separated by ultrafiltration through Microcon 100 membranes. The ClpS remaining bound to ClpA6 was calculated as the difference between the original [3H]ClpS and the radioactivity released into the filtrate (open diamonds). D, LR-GFPVenus degradation was initiated in the presence of ClpAP and ClpS in a standard degradation reaction mixture. Excess purified ClpA N-domain was used to trap any ClpS that dissociated from the degradative complexes, thus slowing the reaction until all ClpS had dissociated and the reaction stopped altogether. The degradation reaction was linear with time without the addition of N-domain. The dissociation of ClpS was determined from the instantaneous decrease in the degradation rate after the addition of N-domain.
ClpS Binds with Higher Affinity to the Tight Site on ClpA6 than to Free ClpA N-domain
Binding competition assays were conducted as above, except that [3H]ClpS was mixed with free ClpA N-domains and then added to ClpA6. With free N-domains equimolar with N-domains in ClpA6, ∼15% inhibition of ClpS binding was observed (Fig. 2B) rather than the 50% expected if free N-domains bound with the same affinity as the N-domains in ClpA6. With N-domains in 24-fold excess over [3H]ClpS (4-fold excess over the N-domains in ClpA6), only 25% inhibition was seen, and a 60-fold excess (10-fold over total N-domains in ClpA6 was needed to competitively bind >80% of the [3H]ClpS (Fig. 2B). We also tested the ability of free N-domains to block the interaction between ClpS and ClpA6 under assay conditions. In degradation reactions in which ClpS was limiting, a large excess of free N-domains over the total N-domains in ClpA6 was needed to block ClpS-mediated degradation of LR-GFPVenus or inhibition of GFP-SsrA degradation (supplemental Fig. S3).
One ClpS Molecule Has a Low Off-rate from ClpA6
Retention of one tightly bound ClpS during gel filtration and ultrafiltration suggested that this ClpS molecule dissociates slowly from the complex with ClpA6. To confirm the low off-rate, one equivalent of [3H]ClpS was allowed to bind to ClpA6 in the presence of ATPγS, and then an excess of non-radioactive ClpS sufficient to block >90% of [3H]ClpS binding was added. At intervals, aliquots were centrifuged through 30,000 Mr cut-off membranes, and the amount of [3H]ClpS exchanged into the filtrate was determined. ClpS dissociated from ClpA6 with a half-time of ∼5 min (Fig. 3B). To ensure that ClpS binding to other N-domains did not affect the dissociation of the tightly bound [3H]ClpS, we used another method to trap [3H]ClpS as it dissociated from the complex. After binding [3H]ClpS to ClpA6, an excess of free ClpA N-domains was added to bind [3H]ClpS and prevent its reassociation with ClpA6. The half-time of dissociation of [3H]ClpS from ClpA6 was the same as observed in the previous experiment (Fig. 3C). To confirm that ClpS dissociation from ClpA6 is also slow under assay conditions, we initiated a ClpS-dependent degradation reaction and added excess free ClpA N-domain to trap any ClpS that dissociated, which was measured by the decrease in the rate of degradation of LR-GFPVenus. The degradation reaction slowed with first order kinetics, and the half-time for dissociation of ClpS was ∼2 min (Fig. 3D). The slightly faster off-rate probably reflects the higher temperature under assay conditions (37 versus 25 °C) and the presence of hydrolyzable ATP, which renders ClpA hexamers more dynamic than when non-hydrolyzable ATPγS is present. The similarity in dissociation rates measured by direct binding and by loss of function suggested that activity of ClpS is dependent on one tightly bound molecule.
FIGURE 3.
One ClpS molecule is sufficient for activation of N-end rule degradation and inhibition of non-N-end rule degradation by ClpAP. A, four series of reactions were conducted to measure degradation of LR- GFPVenus by ClpAP in the presence of ClpS. In each series, the LR-GFPVenus concentration was held constant at 11 μm, and the ClpS concentrations (expressed as equivalents of ClpA6) were varied. The observed specific activities (μmol of substrate/μmol of ClpA6/min) versus ClpS concentration are plotted. At ClpA concentrations below the Kd for dissociation with ClpS, higher amounts of ClpS are required to saturate the ClpA and achieve maximum specific activity. At 400 nm ClpA6, only 1 eq of ClpS is needed to achieve maximum activity of ClpA6, and higher concentrations begin to show inhibitory effects, presumably because, at free ClpS concentrations >1 μm, competition with the ClpA-ClpS complex for substrate binding becomes significant. Circles, 100 nm ClpA6; squares, 200 nm ClpA6; diamonds, 300 nm ClpA6; inverted triangles, 400 nm ClpA6. B, degradation of LR-GFPVenus by ClpAP was measured with different ratios of ClpA and ClpS. Circles, 100 nm ClpA6 and 100 nm ClpS; squares, 100 nm ClpA6 and 200 nm ClpS; diamonds, 100 nm ClpA6 and 600 nm ClpS; inverted triangles, 400 nm ClpA6 and 400 nm ClpS. In each series, the specific activity (μmol of substrate/μmol of ClpA6/min) is plotted as a function of substrate (LR-GFPVenus) concentration. The apparent Km for LR-GFPVenus did not change, suggesting that the active species is the same with different ratios of ClpS and ClpA6. The minimum value for Km/kcat is achieved with a 1:1 stoichiometry of ClpS to ClpA6.
Maximum Degradation of N-end Rule Proteins Occurs with One ClpS per ClpA6
To determine the optimum amount of ClpS needed to promote degradation of the N-end rule substrate, LR-GFPVenus, ClpP was kept in excess so that activity was limited by ClpA or ClpS. Assay sets contained a fixed concentration of ClpA6 and varying amounts of ClpS. Plots of specific activity (μmol of substrate/min/μmol of ClpA6) versus ClpS with ClpA6 present at 100–300 nm gave the same specific activity of ClpA6 at saturating ClpS (Fig. 4A). Several kinetic models were tested, but good fits were obtained only by fitting the data to a model of simple saturation binding to a single binding site. The Kd for activation by ClpS varied between 10 and 40 nm in assays with different concentrations of ClpA6. When lower concentrations of ClpA6 were used, the concentration of ClpS required to reach saturation was higher than that of ClpA6 (Fig. 4A, circles, squares, and diamonds), which reflects the need for free ClpS to be in ∼10-fold excess of the Kd to reach 90% saturation and does not indicate higher stoichiometry of binding. To confirm that a 1:1 stoichiometric ratio of ClpS to ClpA6 is sufficient for activation, 0.4 μm ClpA6 was used in the assays, a concentration that is >20 times the Kd for ClpS binding to the high affinity site, so that essentially all of the ClpS would be bound to the high affinity sites. Under these conditions, maximal activity was obtained with one equivalent of ClpS (Fig. 4A, inverted triangles). The addition of more ClpS did not stimulate activity further, and higher amounts began to inhibit the reaction, presumably as free ClpS competed with ClpA-bound ClpS for the substrate. For assays conducted in this manner, the kinetics of the reaction were analyzed during the linear range only (first 30 s), before excess depletion of ATP or the substrate.
FIGURE 4.
Degradation of LR-GFPVenus by ClpA hexamers with only one N-domain. Intact ClpA and ClpA-Δ153 were combined in different stoichiometric ratios and added to standard LR-GFPVenus degradation assay solutions at a combined concentration of 0.2 μm. The solutions contained 0.5 μm ClpP and either no ClpS or 0.4 μm ClpS. A, the activities of the hybrid hexamers in the absence (light bars) and in the presence (dark bars) of ClpA plus ClpA-Δ153 are plotted for each mixture. Activity is expressed as a fraction of the specific activity of the intact ClpA hexamers. B, the ClpS-dependent activity, calculated as the difference between the assays with and without ClpS, is plotted for each molar ratio of the two proteins. The dotted line shows the fraction of hexamers with at least one N-domain; the dashed line shows the fraction with at least two N-domains. The number of intact and N-terminally deleted ClpA subunits among the hexamers in each case was calculated assuming a binomial distribution.
ClpS binds a single molecule of N-end rule substrate (12, 16). To test whether the efficiency of substrate delivery was dependent on the number of ClpS bound to ClpA6, we measured degradation as a function of LR-GFPVenus concentration with different stoichiometric ratios of ClpS to ClpA6. The Vmax obtained was the same for one ClpS bound to ClpA6 as for higher ratios of ClpS, and the apparent Km for LR-GFPVenus was essentially the same with different amounts of ClpS present (Fig. 4B).
One N-domain of ClpA per Hexamer Is Sufficient for Maximum ClpS-dependent Degradation of N-end Rule Substrates
ClpA-Δ153, which lacks the entire N-terminal domain, assembles into hexamers, interacts with ClpP, and activates ATP-dependent protein degradation by ClpP (24, 25). Because ClpS binds to the N-domain of ClpA, ClpA-Δ153 is insensitive to ClpS as an inhibitor of non-N-end rule substrate degradation (14). As a measure of the efficiency of N-end rule substrate delivery by ClpS, we assembled mixed hexamers containing intact ClpA and ClpA-Δ153 and compared degradation activity against LR-GFPVenus with and without ClpS. As expected, hexamers composed entirely of intact ClpA had no activity in the absence of ClpS but showed high activity when ClpS was present (Fig. 5A). Hexamers composed of only ClpA-Δ153 had some activity against LR-Venus, but ClpS has no effect on that activity (Fig. 5A). Mixed hexamers containing 1–4 ClpA-Δ153 subunits had almost no activity in the absence of ClpS but were highly activated by ClpS. Even with at a ratio of 1 ClpA to 11 ClpA-Δ153, when most hexamers would have no more than one N-domain, degradation was highly activated by ClpS (Fig. 5A). In Fig. 5B, the ClpS-dependent activities, calculated from the difference between the activities with and without ClpS in Fig. 5A, are plotted and compared with activities expected when either one (dotted line) or two (dashed line) N-domains per hexamer are required for ClpS-mediated degradation. For this analysis, the subunits were assumed to assume a binomial distribution, and the positions within the hexamers were assumed to have no influence on activity. The experimental data fall between the two calculated curves but closer to that predicted if only one N-domain were required. The calculated specific activity for hexamers with one N-domain was the same as that of hexamers containing six N-domains, consistent with a single N-domain with ClpS bound being sufficient to promote maximum activity in degrading N-end rule substrates.
FIGURE 5.
One ClpS molecule is sufficient to inhibit degradation of non-N-end rule substrate by ClpAP. GFP-SsrA degradation by ClpAP was measured in standard degradation assay solutions with limiting ClpA and excess ClpP. To measure inhibition of degradation by ClpS under steady state conditions, ClpS was added together with the substrate to assays containing either 100 μm (open circles) or 400 μm ClpA6 (open, closed, and inverted triangles). At 400 μm ClpA6 ClpS was added either at the same time as GFP-SsrA (inverted triangles) or was preincubated with ClpA6 for 15 (open triangles) or 30 s (closed triangles) before the addition of GFP-SsrA. Assay times were restricted to 45 s to avoid significant dissociation of ClpS, which has a slow off-rate from ClpA. The specific activity of GFP-SsrA degradation is plotted versus the molar ratio of ClpS to ClpA6.
One ClpS per ClpA6 Is Sufficient to Inhibit Degradation of Non-N-end Rule Proteins
To determine the binding stoichiometry needed for maximum inhibition by ClpS, we needed to consider the degree of saturation with ClpS and the possibility of competition between ClpS and substrates (30). We assayed GFP-SsrA degradation, which is inhibited by ClpS with a KI of ∼30 nm (Table 1). With a moderate amount (100 nm) of ClpA in the assay and substrate added in excess of its Km, the apparent KI was ∼60 nm, and the amount of ClpS required to completely inhibit degradation was in excess of the ClpA6 (Fig. 6, open circles). To achieve nearly complete inhibition with a stoichiometric ratio of ClpS to ClpA6 required adding ClpA6 far in excess of the Kd for the interaction of ClpS with ClpA6. With 400 nm ClpA6 and 400 nm ClpS, inhibition was only partial when the substrate and ClpS were added simultaneously (Fig. 6, open triangles). However, when we preincubated ClpS and ClpA to allow the tight complex to form before adding substrate, the activity against GFP-SsrA was completely inhibited (Fig. 6, closed symbols). For this determination, we also restricted the assay time to 30 s to avoid significant dissociation of the ClpS from ClpA6 during the reaction. Thus, the complex with one molecule of ClpS bound to ClpA6 is not only sufficient to activate N-end rule protein degradation but also blocks heterologous protein substrates from being degraded.
FIGURE 6.
ClpS displays mixed kinetics of inhibition of ClpAP activity against non-N-end rule substrates. Steady state kinetics of degradation of [3H]α-casein (A) and GFP-SsrA (B) were measured as described under “Materials and Methods.” Solutions with different concentrations of substrate protein and ClpS and all other assay components except ClpA were preincubated for 2 min at 37 °C before reactions were initiated by the addition of ClpA. Results of a single set of experiments are shown, but two other independent experiments showed the same inhibition kinetics.
Kinetics of Inhibition by ClpS Is Dependent on the Substrate
Because the ClpA N-domains affect the kinetics of unfolding or degradation of different substrates in different ways (24) (see below), we considered that ClpS, which exerts its effects in part by binding to the N-domains, might also display variable kinetics of inhibition depending on the substrate. ClpS acted as a mixed competitive inhibitor of casein degradation (Fig. 7A). Both ClpS and casein bind to the ClpA N-domains, and mixed inhibition could result from partial overlap of the binding sites that allows either protein to weaken the binding of the other. Alternatively, ClpS and casein could bind to one or more of the six N-domains in the same hexamer and sterically interfere with formation of a productive complex of the other protein. ClpS acted as a non-competitive inhibitor of GFP-SsrA degradation (Fig. 7B). This finding was somewhat surprising because ClpS can block the binding of SsrA-tagged proteins (see below). Non-competitive inhibition implies that ClpS can interact with the GFP-SsrA-ClpA complex to prevent processing of the substrate. This mechanism is possible, because GFP-SsrA does not appear to interact with the N-domains (24), which are the major entry site for binding of ClpS to ClpA hexamers. As shown below, ClpS remains bound to ClpA6 in the presence of excess GFP-SsrA.
FIGURE 7.
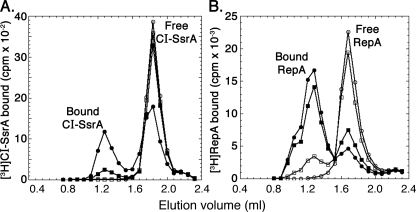
ClpS inhibition of binding of non-N-end rule substrates to ClpA. Binding of substrates to ClpA6 was measured by co-migration of the proteins with ClpA6 during gel filtration on a Superdex 200 column. The buffer used for assembly of the complexes and for equilibration of the column was 50 mm Tris/HCl, pH 7.5, 0.1 m KCl, 10% (v/v) glycerol, 2 mm ATPγS, and 25 mm MgCl2. A, [3H]CI-SsrA (0.2 μm) binding to ClpA6 (0.2 μm) with and without ClpS (1 μm). Closed triangles, [3H]CI-SsrA alone; open triangles, [3H]CI-SsrA and ClpA6, no ClpS; closed circles, [3H]CI-SsrA incubated with ClpA6 for 5 min prior to the addition of ClpS; open circles, ClpS incubated with ClpA6 for 5 min prior to the addition of [3H]CI-SsrA. B, [3H]RepA (0.2 μm) binding to ClpA6 (0.2 μm) with and without ClpS (1 μm); closed triangles, [3H]RepA alone; open triangles, [3H]RepA and ClpA6; closed circles, [3H]RepA incubated with ClpA6 for 5 min prior to the addition of ClpS; open circles, ClpS incubated with ClpA6 for 5 min prior to the addition of [3H]RepA.
To directly determine competition between ClpS and non-N-end rule proteins for binding to ClpA6, we measured binding by retention of the different proteins in the ClpA6 fractions after gel filtration. Binding of CI-SsrA and RepA (Fig. 8) as well as α-casein (data not shown) was diminished or completely blocked in the presence of ClpS. With CI-SsrA, binding was inhibited >80% by ClpS present at one molecule per ClpA6 (Fig. 8A). ClpS blocked CI-SsrA binding whether it was added before (open triangles), after (closed triangles), or simultaneously with (closed circles) CI-SsrA. Interestingly, when ClpS is bound to ClpA6, the addition of excess GFP-SsrA did not displace the ClpS (data not shown). The binding of ClpS in the presence of an excess of GFP-SsrA is consistent with its acting as a non-competitive inhibitor of GFP-SsrA degradation (Fig. 7B). Competition between ClpS and RepA was dependent on the order of addition (Fig. 8B). The addition of ClpS prior to (open circles) or simultaneously with (open triangles) RepA blocked binding of RepA, but when RepA was incubated with ClpA prior to the addition of ClpS, RepA was retained with ClpA. RepA binding to ClpA is known to undergo a change from a reversible mode to a “committed” mode in which RepA has a much reduced off-rate from ClpA. ClpS apparently cannot disrupt the committed mode of RepA binding, although it can prevent its formation. ClpS does not bind well to the committed complex of RepA and ClpA (data not shown), probably because, although the N-domains are not needed for RepA binding, once RepA is bound, it interacts with the N-domains of ClpA and reduces their availability to ClpS.
FIGURE 8.
Schematic drawing of the interaction between ClpS and ClpA. A molecule of ClpS (red) binds to one of the six N-domains on ClpA using the interface observed in the co-crystal of ClpS and the isolated ClpA N-domain (15, 21). Substrates with N-degrons (gold) interact with a specific binding site on ClpS and are delivered to ClpA for processing. ClpS excludes substrates lacking N-degrons (brown) by blocking access to other sites within ClpA. The flexible N-terminal portion of ClpS also contributes to binding; however, only one ClpS-N-terminal peptide can utilize this site at a time, thus restricting ClpS to one high affinity binding interaction per hexamer of ClpA. The region around residue 17 of ClpS appears to be needed for this secondary interaction, although it is not known whether it interacts on the surface of ClpA-D1 near the axial channel (upper path) or with other ClpA N-domains (lower path).
On the basis of the inhibition data, we propose that ClpS binding to the N-domains allows the N-terminal peptide to engage an additional site. Once tightly bound, ClpS prevents substrates from entering the axial channel and accessing protein interaction sites required for tight binding of substrates, such as GFP-SsrA and RepA. Inhibition of casein degradation is partially competitive because both proteins bind to the N-domains of ClpA, whereas competition is non-competitive with GFP-SsrA because GFP-SsrA cannot block ClpS from binding the N-domains and gaining access to its tight binding site. Competition between RepA and ClpS is complicated because RepA binding proceeds in multiple steps. ClpS competes effectively with the fast reversible step in RepA binding but cannot reverse the committed binding mode once it has occurred. Consistent with this model, ClpS blocks RepA degradation very effectively when added to assay mixtures simultaneously with RepA; however, ClpS allows the degradation of most of the RepA preloaded onto ClpA prior to the addition of ClpS (supplemental Fig. S4). ClpS binds weakly to committed ClpA-RepA complexes (data not shown), most likely because RepA in such complexes engages the ClpA N-domains and prevents ClpS from binding and forming the inhibitory complex.
In the Absence of ClpS, the ClpA N-domains Have Variable Effects on Protein Degradation
In the absence of ClpS, ClpAP degrades various proteins with accessible N-terminal or C-terminal degradation signals as well as unfolded proteins or proteins with unstructured regions. In a previous study, we showed that, when the N-domain of ClpA was deleted, the Vmax for degradation of both folded and unfolded proteins was higher, and the Km for the unfolded substrate, α-casein, was also 10-fold higher (24). To address the effects of the N-domains in more detail, we compared the kinetics of degradation of three different substrates by ClpP complexed with ClpA or ClpA-Δ153. When RepA was used as the substrate, the Km and Vmax were essentially the same as with wild type ClpA and ClpA-Δ153 (Table 2 and supplemental Fig. S5). Consistent with our previous study (24), activity against casein and GFP-SsrA was 2–3 times higher with ClpA-Δ153 than with wild type ClpA (supplemental Fig. S5). The Km for casein was increased by a factor of ≥10 in the absence of the N-domains, whereas the Km for GFP-SsrA was unaffected (Table 2). The Km values in this work were slightly lower than reported previously, because the current assays were done in the absence of detergent, which affects substrate binding. To confirm that the change in Km for casein when the N-domains are missing reflects reduced binding affinity, we compared the ability of casein to inhibit GFP-SsrA degradation by ClpAP with or without N-domains. Casein competitively inhibited GFP-SsrA degradation by full-length ClpAP with an apparent KI of 0.9 μm, close to its Km value as a substrate; the KI for casein increased to ∼10 μm when ClpA-Δ153 was used, close to the observed Km for casein degradation in the absence of the N-domains (Table 2 and supplemental Fig. S4).
TABLE 2.
Substrate interactions and activities of ClpA lacking its N-terminal domain
0.2 μm ClpA6 and 0.4 μm ClpP14 were present in standard assay mixtures (see “Materials and Methods”).
| Substrate | Parametera | ClpA | ClpA-Δ153 |
|---|---|---|---|
| α-Casein | Km | 0.3 | 6.0 |
| Vmax | 10 | 27 | |
| GFP-SsrA | Km | 1.5 | 1.5 |
| Vmax | 8–11 | 18–22 | |
| RepA | Km | 4.0 | 4.0 |
| Vmax | 3.5 | 3.5 |
a Km values are expressed as μm substrate; Vmax values are expressed as μmol of substrate hydrolyzed/min/μmol of ClpA6.
To determine whether the N-domains act in concert or individually during protein degradation, we formed mixed hexamers of full-length ClpA and ClpA-Δ153 and measured activity against casein and GFP-SsrA. The Vmax for degradation of both substrates increased as the number of ClpA-Δ153 subunits increased (Table 3), and, for casein, the Km showed an incremental increase as N-domains were removed. Because the kinetic properties changed in proportion to the number of N-domains removed, we propose that the N-domains each can bind unfolded proteins, which serves to recruit unfolded substrates, but they also bind proteins as they are unfolded by ClpA, resulting in slower release to the translocation apparatus.
TABLE 3.
Protein degradation promoted by mixed hexamers of ClpA and ClpA-Δ153
0.2 μm ClpA6 and 0.4 μm ClpP14 were present in standard assay mixtures (see “Materials and Methods”).
| [ClpA]/[ClpA-Δ153] | Casein degradationa |
GFP-SsrA degradation (Vmaxb) | |
|---|---|---|---|
| Kma | Vmaxb | ||
| μmol | μmol/min/μmol | μmol/min/μmol | |
| 0/6 | 0.33 | 10 | 10 |
| 2/4 | 0.84 | 15 | 14 |
| 3/3 | 1.2 | 18 | 17 |
| 4/2 | 2.6 | 20 | 18 |
| 0/6 | 3.5 | 25 | 22 |
a Km values are expressed as μm substrate.
b Vmax values are expressed as μmol of substrate hydrolyzed/min/μmol of ClpA6.
DISCUSSION
ClpS is a specific adaptor protein that redirects the activity of ClpAP toward proteins that contain exposed N-terminal leucine, phenylalanine, tyrosine, or tryptophan residues (N-degrons) (5, 11, 12). In our study, LR-GFPVenus, which carries an N-degron but no other recognition motif, was degraded by intact ClpAP in the presence of ClpS but not by ClpAP alone. Short peptides with leucine or phenylalanine at the N terminus were effective inhibitors of LR-GFPVenus degradation, but the presence of a single hydrophilic residue at the N terminus of the peptides rendered them completely ineffective as inhibitors. These data are consistent with other studies showing that ClpS is needed to mediate degradation of proteins targeted by virtue of an N-degron (5, 11, 12, 16).
ClpAP is not restricted to degrading proteins with N-degrons and will degrade unfolded proteins and proteins with unstructured polypeptide regions near their N or C termini (31–33) or, less efficiently, internal regions, as in unstructured linkers between protein domains (34). Degradation based on accessible hydrophobic regions is dependent on the binding of substrates to the N-domains of ClpA or to sites within the axial channel of ClpA. Axial binding sites in ClpA have been shown to bind hydrophobic peptides (35, 36), and they serve by unknown mechanisms in formation of committed substrate enzyme complexes, possibly by capturing proteins with unstructured N- or C-terminal regions that can extend into the axial channel as suggested for similar loops in ClpX (37). ClpS inhibits degradation of substrates with these more general hydrophobic motifs by competitively blocking substrate binding to the ClpA N-domains or by blocking direct access to binding sites within the axial channel. Thus, ClpS acts as a gatekeeper for ClpAP, making it highly specific for proteins with N-degrons. Some proteins possess both an N-degron and an unstructured and hydrophobic N- or C-terminal region. ClpAP will degrade such proteins in the absence or the presence of ClpS using either mode of substrate recognition (30).
Our data show that when ClpS binds to ClpA hexamers, one molecule of ClpS binds more tightly than was observed for ClpS binding to isolated N-domains. Additional molecules of ClpS can bind to ClpA hexamers, but they bind less tightly, and total binding appears to be limited to ≤3 molecules/hexamer. Binding of a single molecule of ClpS allows the maximum rate of degradation of N-end rule proteins by ClpAP and is sufficient to inhibit degradation of proteins lacking N-degrons. High affinity binding of the first ClpS molecule is lost when the first 17 amino acids of ClpS are removed, suggesting that part or all of the ClpS N-terminal peptide interacts with an additional site in ClpA hexamers. Although the N terminus of ClpS contributes to the interaction between the first ClpS molecule and ClpA6, a peptide consisting of residues 1–22 of ClpS is a weak inhibitor of casein and GFP-SsrA degradation (data not shown) and LR-GFPVenus degradation (supplemental Fig. S6), indicating that it has low intrinsic affinity for ClpA.
Where on ClpA the ClpS N-terminal peptide binds is not known. One possibility, also suggested by others (22), is near the entrance or within the axial channel of the ClpA ring (Fig. 8). Binding to a centrally located site would limit the number of tightly binding ClpS molecules to one or two, because steric clashes would prevent multiple ClpS molecules from binding. Axial binding of the N-terminal peptide of ClpS should also tether the mobile N-domain to which ClpS is bound and position the N-recognin to which the substrate binds over the axial channel. Recent studies show that a segment of ≥4 amino acids between the N-degron and the folded domain of a protein is necessary for efficient degradation by ClpAP and ClpS (5, 38), and it is plausible that the positioning of ClpS with its bound substrate over the axial allows the unstructured portion of the substrate to be brought close to the surface of ClpA and thus facilitates its transfer to the interior unfolding apparatus of ClpA (Fig. 8). The presence of the ClpS N-terminal peptide in the axial channel would also sterically block terminal peptide extensions or unstructured loops from other protein substrates from entering the channel and accessing the binding sites within ClpA. An alternative model is that the N terminus of ClpS interacts with one or more of the other N-domains. In the co-crystal of ClpS and the ClpA N-domain, the ClpS N-terminal peptide was bound in a hydrophobic pocket of another N-domain molecule. Even transient interactions with the ClpS N-terminal peptide could bring the N-domains together sufficiently to limit their accessibility to additional ClpS molecules and to substrates.
Truncations of the N-terminal 17 amino acids of ClpS are not tolerated for degradation of N-end rule proteins or for inhibition of non-N-end rule protein degradation; however, alanine scanning mutagenesis did not identify specific residues in the N terminus that are essential for these functions (22). Thus, it appears that, once ClpS is anchored to an N-domain, the presence of a relatively flexible N-terminal extension is sufficient to promote capture and uptake of the substrate bound to ClpS and to impede uptake of non-N-end rule substrates. Deletions beyond residue 17 eliminate both activities (15, 22). Curiously, when 16 residues are deleted, the presence of a single bulky amino acid (methionine) at the N terminus is sufficient to provide inhibitory activity, but a smaller residue (valine) is not (22), implying that the portion of the N terminus of ClpS closer to the globular domain is essential for interaction with another site on ClpA.
Positioning substrates for efficient translocation through the axial channel of ClpA needs to occur in a manner that avoids steric clashes between substrates and avoids clogging the channels with multiple polypeptides seeking to enter at the same time. Evidence is accumulating that these conditions are met in ClpA and related ATPases, such as ClpX, in part by locating the high affinity binding sites for substrates within the axial channels themselves, where only a single polypeptide can have access (35–37, 39–41). Substrate adaptors present potential problems, because the adaptors bind to the N-domains and in principle could deliver as many as six substrates at once. However, this dilemma is resolved for ClpAP and ClpXP by adaptor binding geometries that limit the number of substrate molecules that can be delivered simultaneously. SspB binds asymmetrically to ClpX hexamers despite its ability to bind stoichiometrically to isolated ClpX N-domain dimers (42). The two interaction domains of the SspB dimer are bound to separate ClpX N-domain dimers, permitting one SspB dimer to occupy four of the six ClpX N-domains and allowing only one substrate molecule to be positioned close to the axial channel (42, 43). As we discussed above, ClpS binding stoichiometry is limited by the N-terminal peptide, which interacts with a centrally located site in assembled ClpA. Thus, occlusion of the channel by bound substrate or a portion of the adaptor-substrate complex appears to be a common mechanism for limiting the number of substrates processed at any one time.
The contributions of the N-domains to binding and degradation of proteins, apart from serving as the docking site for adaptors, remain somewhat ill defined. ClpA lacking N-domains carries out most enzymatic functions quite efficiently and in some cases faster than the wild type enzyme, but the influence of the N-domains on degradation is distinctly substrate-dependent. N-domains reduce the degradation rate for both casein, which does not require unfolding, and GFP-SsrA, which does require unfolding, suggesting that the rate-limiting step in degradation when the N-domains are present is not substrate unfolding. The lack of an effect on the Km for GFP-SsrA or RepA indicates that the N-domains do not affect the on-rate for either substrate, and thus initial substrate binding does not appear to be rate-limiting. We favor a model in which the N-domains interact with proteins as they are unfolded on ClpA and retard the hand-off from D1 to D2 and thus reduce the overall rate of translocation. With RepA, the N-domains appear to have no effect on the rate of degradation, although it has been reported that RepA can interact with the N-domains. However, RepA binding to ClpA proceeds in two steps, a fast reversible step and a slower “committed” step (44). In separate experiments, we have found that RepA also forms committed complexes with ClpA-Δ153, and the latter step is most likely rate-limiting in RepA degradation. From the above discussion, it is clear that that the N-domains have multiple modes of interaction with substrates and that their effects on substrate binding, unfolding, and degradation vary with the nature of the protein substrate.
In E. coli cells, ClpS restricts the activity of ClpAP to protein substrates with an accessible N-degron. Measurements of the intracellular levels of ClpA and ClpS and the stability of ClpA in vivo suggest that ClpA is not saturated with ClpS under all conditions. ClpA hexamers and ClpS are present in exponentially growing cells at concentrations in the range of 100–200 nm (45, 46), which would mean that ∼75% of ClpA hexamers will have ClpS bound. ClpS protects ClpA from autodegradation, but more than half of the ClpA is degraded per generation in cells expressing endogenous levels of ClpA and ClpS (22), suggesting that not all of the ClpA has ClpS bound in vivo. Thus, it is likely that both ClpAP and ClpAP-ClpS complexes are available to target their selective substrates and that at least part of the time both systems operate simultaneously. Direct measurement of the effects of ClpS on degradation of non-N-end rule substrates is consistent with this model (46, 47). Although substrates specifically regulated by ClpS-mediated degradation via the N-end rule have recently been identified (9, 11), endogenous substrates that are primarily targeted by ClpAP are yet unknown.
ClpA and ClpX levels are nearly the same in E. coli cells (45), and both enzymes have similar activity in vitro, so the expectation is that they would have similar numbers of substrates in vivo. It is not known whether N-end rule substrates are only generated by specific regulatory processes or can also arise as a result of damage or errors in transcription or translation. It seems plausible that N-end rule proteins might occur at least as often as SsrA-tagged proteins and that the levels would reflect particular stress conditions or nutritional deficiencies. Whether there are regulatory targets for ClpAP, independent of ClpS, is still an open question.
Supplementary Material
Acknowledgments
We thank Dr. Grzegorz Piszczek (Biophysics Unit of NHLBI, National Institutes of Health) for advice and use of the microcalorimeter and Drs. Jan Rozycki and Agnieszka Szyk (Laboratory of Cell Biology, NCI, National Institutes of Health) for synthesis of several peptides.
This work was supported, in whole or in part, by the National Institutes of Health, NCI, Center for Cancer Research, Intramural Research Program.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- GFP
- green fluorescent protein
- HPLC
- high pressure liquid chromatography
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Schirmer E. C., Glover J. R., Singer M. A., Lindquist S. (1996) Trends Biochem. Sci. 21, 289–296 [PubMed] [Google Scholar]
- 2.Gottesman S., Maurizi M. R., Wickner S. (1997) Cell 91, 435–438 [DOI] [PubMed] [Google Scholar]
- 3.Levchenko I., Seidel M., Sauer R. T., Baker T. A. (2000) Science 289, 2354–2356 [DOI] [PubMed] [Google Scholar]
- 4.Sauer R. T., Bolon D. N., Burton B. M., Burton R. E., Flynn J. M., Grant R. A., Hersch G. L., Joshi S. A., Kenniston J. A., Levchenko I., Neher S. B., Oakes E. S., Siddiqui S. M., Wah D. A., Baker T. A. (2004) Cell 119, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erbse A., Schmidt R., Bornemann T., Schneider-Mergener J., Mogk A., Zahn R., Dougan D. A., Bukau B. (2006) Nature 439, 753–756 [DOI] [PubMed] [Google Scholar]
- 6.Tobias J. W., Shrader T. E., Rocap G., Varshavsky A. (1991) Science 254, 1374–1377 [DOI] [PubMed] [Google Scholar]
- 7.Varshavsky A. (1991) Cell 64, 13–15 [DOI] [PubMed] [Google Scholar]
- 8.Shrader T. E., Tobias J. W., Varshavsky A. (1993) J. Bacteriol. 175, 4364–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ninnis R. L., Spall S. K., Talbo G. H., Truscott K. N., Dougan D. A. (2009) EMBO J. 28, 1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirel P. H., Schmitter M. J., Dessen P., Fayat G., Blanquet S. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 8247–8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt R., Zahn R., Bukau B., Mogk A. (2009) Mol. Microbiol. 72, 506–517 [DOI] [PubMed] [Google Scholar]
- 12.Schuenemann V. J., Kralik S. M., Albrecht R., Spall S. K., Truscott K. N., Dougan D. A., Zeth K. (2009) EMBO Rep. 10, 508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lupas A. N., Koretke K. K. (2003) J. Struct. Biol. 141, 77–83 [DOI] [PubMed] [Google Scholar]
- 14.Dougan D. A., Reid B. G., Horwich A. L., Bukau B. (2002) Mol. Cell 9, 673–683 [DOI] [PubMed] [Google Scholar]
- 15.Guo F., Esser L., Singh S. K., Maurizi M. R., Xia D. (2002) J. Biol. Chem. 277, 46753–46762 [DOI] [PubMed] [Google Scholar]
- 16.Román-Hernández G., Grant R. A., Sauer R. T., Baker T. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8888–8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessel M., Maurizi M. R., Kim B., Kocsis E., Trus B. L., Singh S. K., Steven A. C. (1995) J. Mol. Biol. 250, 587–594 [DOI] [PubMed] [Google Scholar]
- 18.Beuron F., Maurizi M. R., Belnap D. M., Kocsis E., Booy F. P., Kessel M., Steven A. C. (1998) J. Struct. Biol. 123, 248–259 [DOI] [PubMed] [Google Scholar]
- 19.Guo F., Maurizi M. R., Esser L., Xia D. (2002) J. Biol. Chem. 277, 46743–46752 [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T., Maurizi M. R., Steven A. C. (2004) J. Struct. Biol. 146, 180–188 [DOI] [PubMed] [Google Scholar]
- 21.Zeth K., Ravelli R. B., Paal K., Cusack S., Bukau B., Dougan D. A. (2002) Nat. Struct. Biol. 9, 906–911 [DOI] [PubMed] [Google Scholar]
- 22.Hou J. Y., Sauer R. T., Baker T. A. (2008) Nat. Struct. Mol. Biol. 15, 288–294 [DOI] [PubMed] [Google Scholar]
- 23.Maurizi M. R., Thompson M. W., Singh S. K., Kim S. H. (1994) Methods Enzymol. 244, 314–331 [DOI] [PubMed] [Google Scholar]
- 24.Xia D., Esser L., Singh S. K., Guo F., Maurizi M. R. (2004) J. Struct. Biol. 146, 166–179 [DOI] [PubMed] [Google Scholar]
- 25.Singh S. K., Rozycki J., Ortega J., Ishikawa T., Lo J., Steven A. C., Maurizi M. R. (2001) J. Biol. Chem. 276, 29420–29429 [DOI] [PubMed] [Google Scholar]
- 26.DasGupta S., Mukhopadhyay G., Papp P. P., Lewis M. S., Chattoraj D. K. (1993) J. Mol. Biol. 232, 23–34 [DOI] [PubMed] [Google Scholar]
- 27.Singh S. K., Grimaud R., Hoskins J. R., Wickner S., Maurizi M. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8898–8903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. (2002) Nat. Biotechnol. 20, 87–90 [DOI] [PubMed] [Google Scholar]
- 29.Levine R. L., Federici M. M. (1982) Biochemistry 21, 2600–2606 [DOI] [PubMed] [Google Scholar]
- 30.Wang K. H., Sauer R. T., Baker T. A. (2007) Genes Dev. 21, 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gottesman S., Clark W. P., Maurizi M. R. (1990) J. Biol. Chem. 265, 7886–7893 [PubMed] [Google Scholar]
- 32.Hoskins J. R., Kim S. Y., Wickner S. (2000) J. Biol. Chem. 275, 35361–35367 [DOI] [PubMed] [Google Scholar]
- 33.Hoskins J. R., Singh S. K., Maurizi M. R., Wickner S. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8892–8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoskins J. R., Yanagihara K., Mizuuchi K., Wickner S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11037–11042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinnerwisch J., Fenton W. A., Furtak K. J., Farr G. W., Horwich A. L. (2005) Cell 121, 1029–1041 [DOI] [PubMed] [Google Scholar]
- 36.Farbman M. E., Gershenson A., Licht S. (2008) Biochemistry 47, 13497–13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin A., Baker T. A., Sauer R. T. (2008) Nat. Struct. Mol. Biol. 15, 1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K. H., Oakes E. S., Sauer R. T., Baker T. A. (2008) J. Biol. Chem. 283, 24600–24607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piszczek G., Rozycki J., Singh S. K., Ginsburg A., Maurizi M. R. (2005) J. Biol. Chem. 280, 12221–12230 [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui S. M., Sauer R. T., Baker T. A. (2004) Genes Dev. 18, 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin A., Baker T. A., Sauer R. T. (2008) Mol. Cell 29, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolon D. N., Wah D. A., Hersch G. L., Baker T. A., Sauer R. T. (2004) Mol. Cell 13, 443–449 [DOI] [PubMed] [Google Scholar]
- 43.Wah D. A., Levchenko I., Rieckhof G. E., Bolon D. N., Baker T. A., Sauer R. T. (2003) Mol. Cell 12, 355–363 [DOI] [PubMed] [Google Scholar]
- 44.Pak M., Wickner S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4901–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega J., Lee H. S., Maurizi M. R., Steven A. C. (2004) J. Struct. Biol. 146, 217–226 [DOI] [PubMed] [Google Scholar]
- 46.Farrell C. M., Grossman A. D., Sauer R. T. (2005) Mol. Microbiol. 57, 1750–1761 [DOI] [PubMed] [Google Scholar]
- 47.Lies M., Maurizi M. R. (2008) J. Biol. Chem. 283, 22918–22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.