Abstract
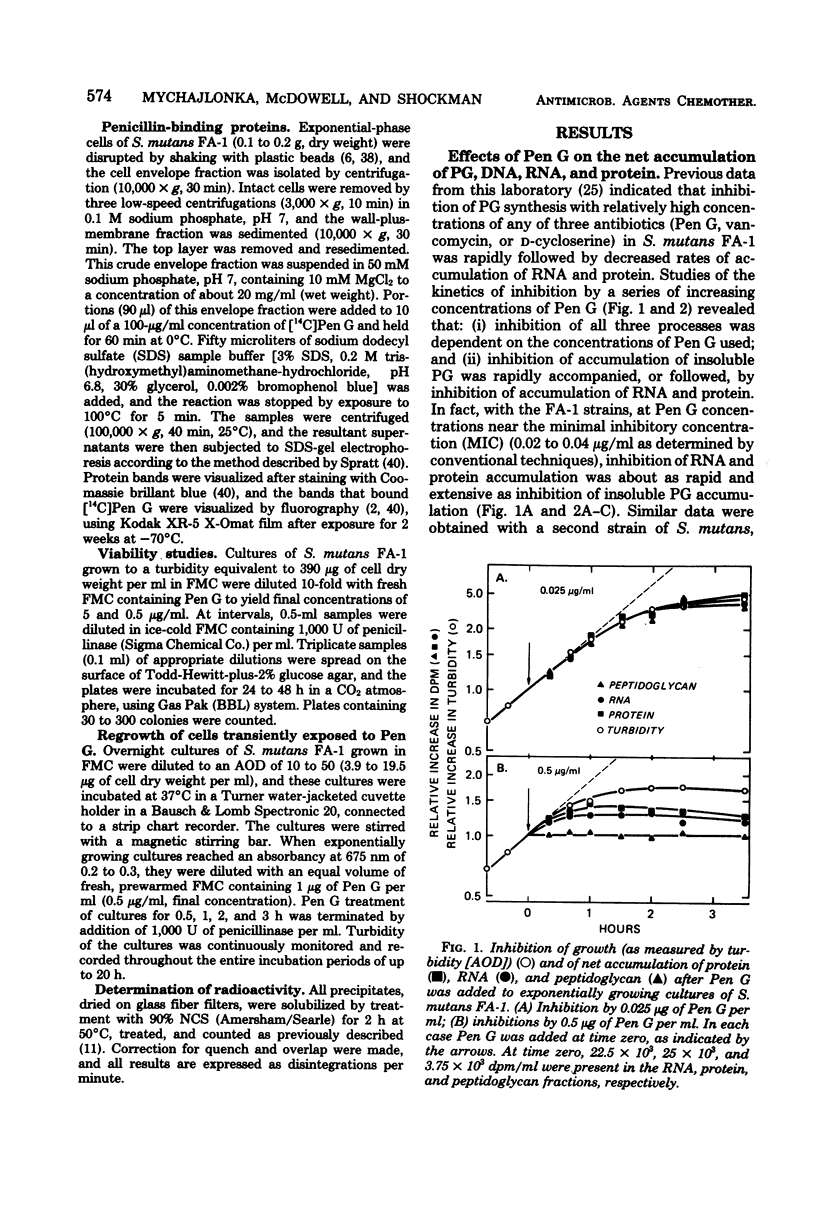
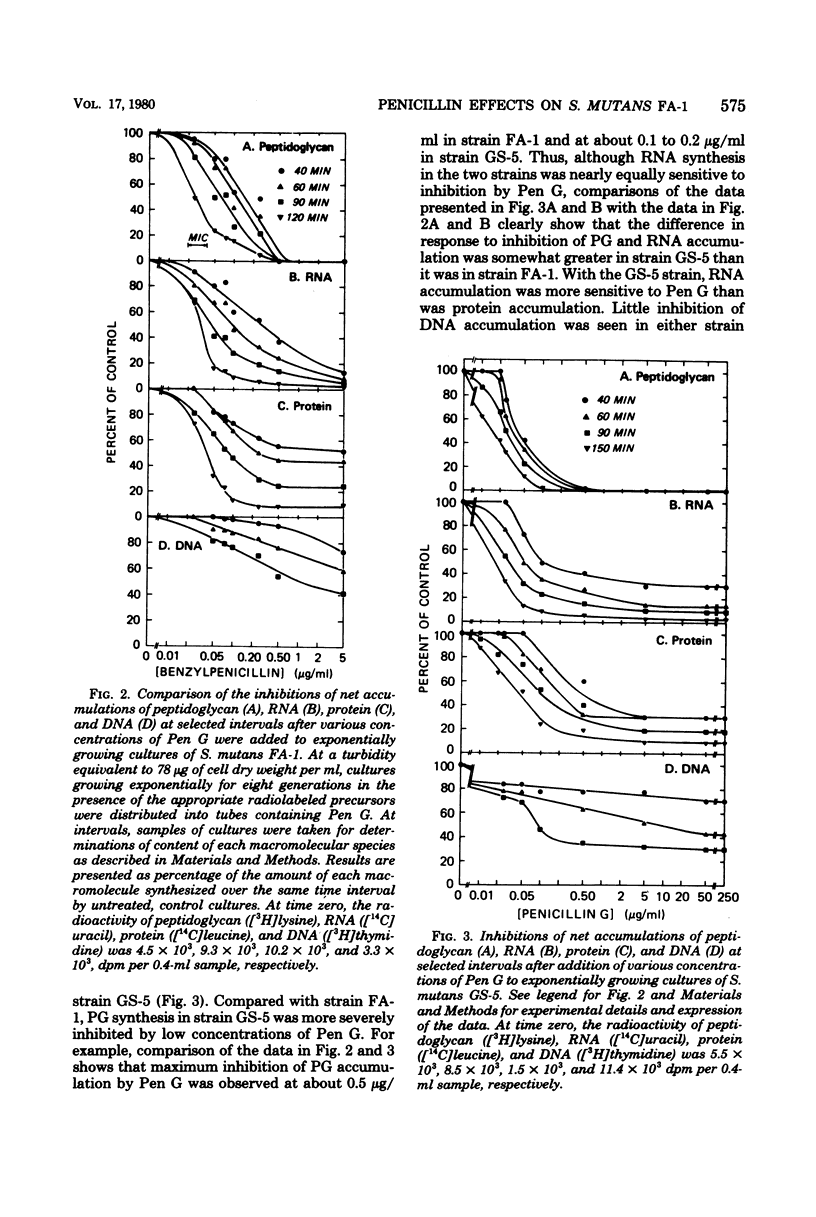
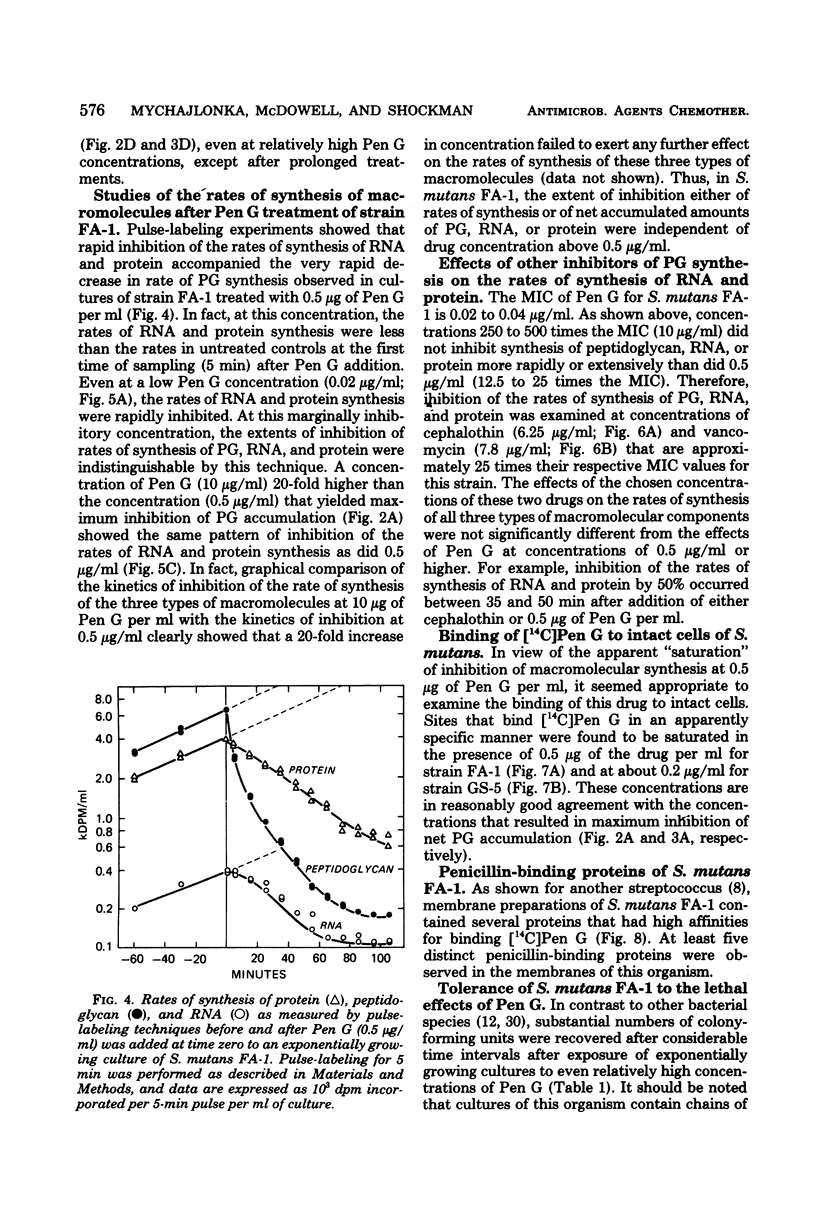
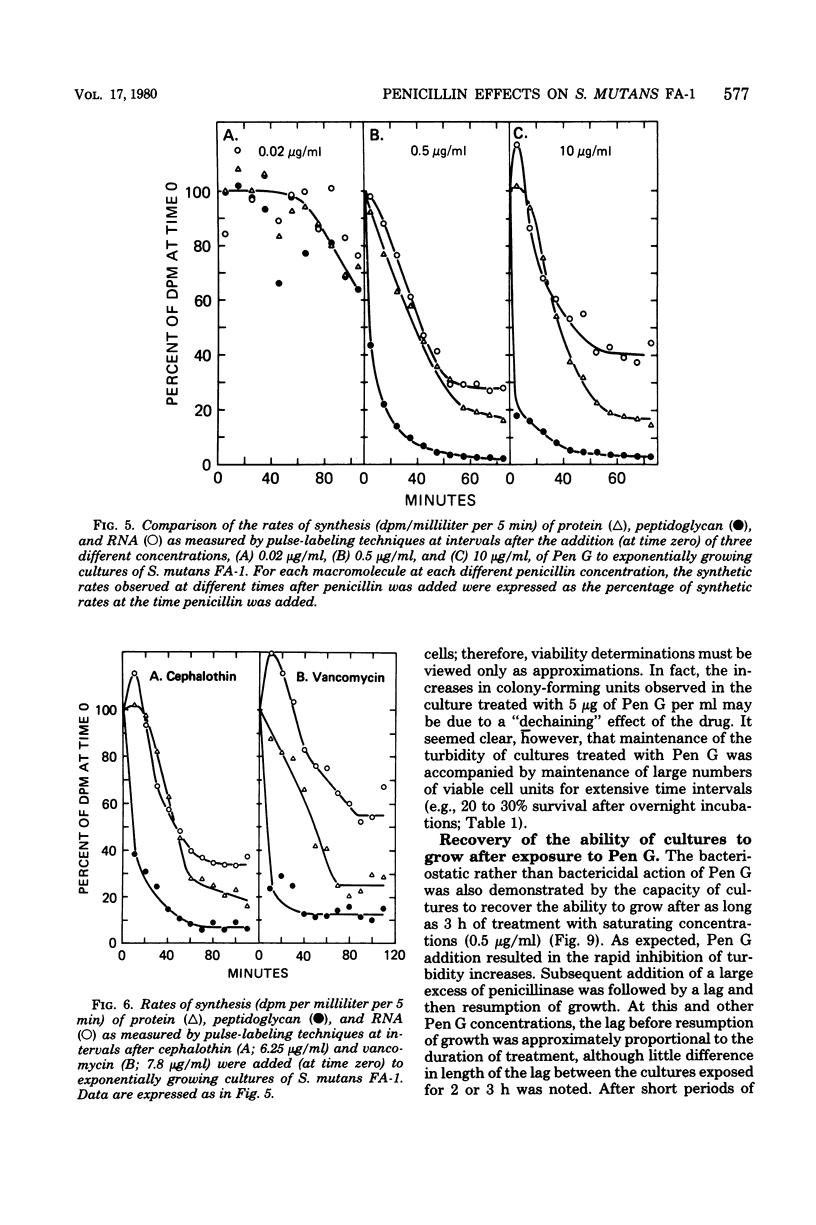
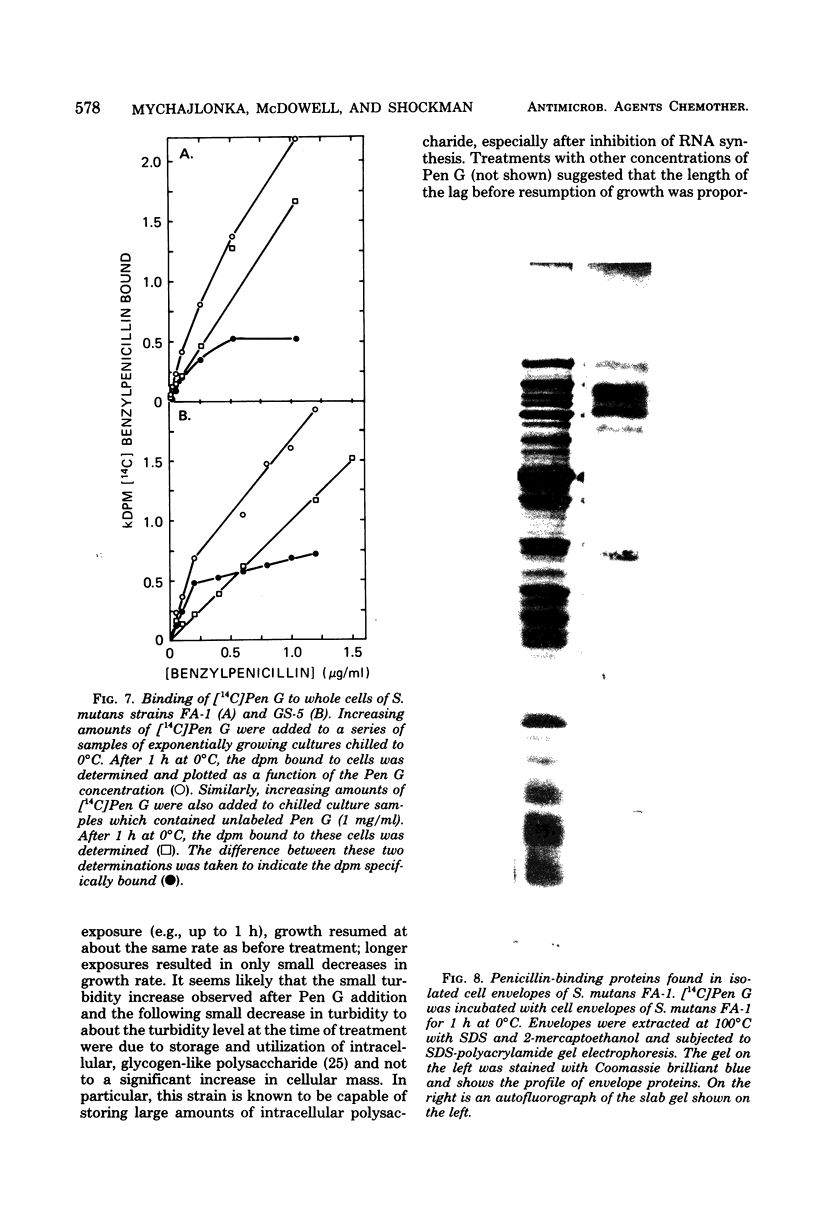
Exposure of exponentially growing cultures of Streptococcus mutans strains FA-1 and GS-5 to various concentrations of benzylpenicillin (Pen G) resulted in inhibition of turbidity increases at low concentrations (0.02 to 0.04 μg/ml). However, in contrast to some other streptococcal species, growth inhibition was not accompanied by cellular lysis or by a rapid loss of viability. In both strains, synthesis of insoluble cell wall peptidoglycan was very sensitive to Pen G inhibition and responded in a dose-dependent manner to concentrations of about 0.2 and 0.5 μg/ml for strains GS-5 and FA-1, respectively. Higher Pen G concentrations failed to inhibit further either growth or insoluble peptidoglycan assembly. Somewhat surprisingly, Pen G also inhibited both ribonucleic acid (RNA) and protein syntheses, each in a dose-dependent manner. Compared with inhibition of peptidoglycan synthesis, inhibition of RNA and protein syntheses by Pen G was less rapid and less extensive. Maximum amounts of radiolabeled Pen G were specifically bound to intact cells upon exposure to about 0.2 and 0.5 μg/ml of Pen G for strains GS-5 and FA-1, respectively, concentrations consistent with those that resulted in maximum or near-maximum inhibitions of the synthesis of cellular peptidoglycan, RNA, and protein. Five polypeptide bands that had a very high affinity for [14C]Pen G were detected in a crude cell envelope preparation of strain FA-1. After exposure of cultures of strain FA-1 to the effects of saturating concentrations of the drug for up to 3 h, addition of penicillinase was followed by recovery of growth after a lag. The length of the lag before regrowth depended on both Pen G concentration and time of exposure. On the basis of these and other observations, it is proposed that the secondary inhibitions of cellular RNA or protein synthesis, or both, are involved in the tolerance of these organisms to lysis and killing by Pen G and other inhibitors of insoluble peptidoglycan assembly.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Daneo-Moore L., Coyette J., Sayare M., Boothby D., Shockman G. D. Turnover of the cell wall peptidoglycan of Lactobacillus acidophilus. The presence of a fraction immune to turnover. J Biol Chem. 1975 Feb 25;250(4):1348–1353. [PubMed] [Google Scholar]
- Daneo-Moore L., Terleckyj B., Shockman G. D. Analysis of growth rate in sucrose-supplemented cultures of Streptococcus mutans. Infect Immun. 1975 Nov;12(5):1195–1205. doi: 10.1128/iai.12.5.1195-1205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Ward M. Susceptibility of Streptococcus mutans to antimicrobial agents. Antimicrob Agents Chemother. 1976 Aug;10(2):274–276. doi: 10.1128/aac.10.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Lopez R., Tomasz A. Suppression of lytic effect of beta lactams on Escherichia coli and other bacteria. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3293–3297. doi: 10.1073/pnas.73.9.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. E., Lawrence P. J. The formation of functional penicillin-binding proteins. J Biol Chem. 1975 Aug 25;250(16):6578–6585. [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Delbos F., Chabbert Y. A. Caractéristiques des souches de Streptococcus mutans isolées d'endocardites subaigues et sensibilité aux antibiotiques. Ann Microbiol (Paris) 1977 Feb-Mar;128(2):205–216. [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Synthesis and excretion of glycerol teichoic acid during growth of two streptococcal species. Infect Immun. 1975 Aug;12(2):333–338. doi: 10.1128/iai.12.2.333-338.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. J. Penicillin: reversible inhibition of forespore septum development in Bacillus megaterium cells. Antimicrob Agents Chemother. 1974 Dec;6(6):815–820. doi: 10.1128/aac.6.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Daneo-Moore L., Shockman G. D. Factors regulating cell wall thickening and intracellular iodophilic polysaccharide storage in Streptococcus mutans. Infect Immun. 1977 Jun;16(3):967–973. doi: 10.1128/iai.16.3.967-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Dipersio J. R., Higgins M. L., Shockman G. D. Unbalanced growth and macromolecular synthesis in Streptococcus mutans FA-1. Infect Immun. 1976 Mar;13(3):941–948. doi: 10.1128/iai.13.3.941-948.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D., LAMPEN J. O. Inhibition by antibiotics of the growth of bacterial and yeast protoplasts. J Bacteriol. 1962 Sep;84:508–512. doi: 10.1128/jb.84.3.508-512.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMINGER J. L. Microbial uridine-5'-pyrophosphate N-acetylamino sugar compounds. II. Incorporation of uracil-2-C14 into nucleotide and nucleic acid. J Biol Chem. 1957 Jan;224(1):525–532. [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Higgins M. L., DaneoMoore L., Mattingly S. J., Diersio J. R., Terleckyj B. Studies of balanced and unblaanced growth of Streptococcus mutans. J Dent Res. 1976 Jan;55:A10–A18. doi: 10.1177/002203457605500101011. [DOI] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. Replacement of Lysine by Hydroxylysine and Its Effects on Cell Lysis in Streptococcus faecalis. J Bacteriol. 1965 Sep;90(3):575–588. doi: 10.1128/jb.90.3.575-588.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Autolysis in strains of viridans streptococci. J Gen Microbiol. 1976 Sep;96(1):87–94. doi: 10.1099/00221287-96-1-87. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]



