Abstract
Targeting the estrogen receptor is an important strategy in breast cancer therapy. However, although inhibiting estrogen receptor function with specific estrogen receptor modulators can achieve a primary response in cancer patients, intrinsic or subsequently acquired resistance to the therapy remains a major obstacle in the clinic. Thus, it is critical to gain amore thorough understanding of howestrogen receptor functions are regulated in breast cancer.Here, we demonstrate that the non-receptor tyrosine kinase c-ABL is a functional partner of the estrogen receptor, as expression of c-ABL sustained transcriptional activity of the estrogen receptor. More importantly, inhibition of c-ABL resulted in sensitization to treatment by tamoxifen (TAM) in estrogen receptor-positive breast cancer cells, as manifested by inhibition of cell survival and suppression of anchorage-independent growth. We found that c-ABL interacts with estrogen receptor in breast cancer cells and that expression of c-ABL is a frequent event in primary breast cancer tumor tissues. In estrogen receptor-positive tumors, the expression of c-ABL significantly correlated with disease progression and metastasis. This study shows that c-ABL regulates the cellular response to TAM through functional interaction with the estrogen receptor, which suggests c-ABL as a therapeutic target and a prognostic tumor marker for breast cancer.
Introduction
The steroid hormone estrogen receptor α (ERα, hereafter referred to as ER) is a nuclear receptor that exerts a profound influence on the initiation and progression of breast cancer by regulating cell transformation, proliferation, and metastasis. It is estimated that approximately 70% of breast cancer cases are ER-positive and that their growth and progression are likely dependent on estrogen stimulation. For ER-positive breast cancer patients, systemic antihormonal therapy, such as tamoxifen (TAM), is a first-line targeted treatment. Unfortunately, a substantial proportion of these patients are intrinsically resistant to this therapy and a significant number of patients with advanced disease eventually develop acquired resistance to the treatment, which poses a major clinical problem [1]. Multiple mechanisms including loss of ER expression, endocrine adaptation, altered coregulatory proteins, and deregulated signal transduction can lead to TAM resistance. For example, elevated expression of the receptor tyrosine kinase ErbB-2 (also known as HER-2) has been correlated with the development of TAM resistance in breast tumors and in breast cancer cell line models [2–4]. Overexpression of ErbB-2 has been shown to induce ligand-independent ER phosphorylation and transcriptional activity through the mitogen-activated protein kinase pathway [5].
The non-receptor tyrosine kinase c-ABL is a proto-oncogene with multiple functions. It regulates a variety of cellular activities, including regulation of cell migration, responses to oxidative stress and DNA damage, cell proliferation, and survival [6–10]. Our knowledge of the oncogenic potential of aberrant ABL function in humans is primarily based on the observation that chronic myelogenous leukemia and a subset of acute lymphocytic leukemia are causally associated with the oncogenic BCR-ABL fusion protein created by chromosomal translocation involving the c-ABL and the BCR (break point cluster) genes [11,12]. Recent findings further demonstrated that the oncogenic activity of the ABL protein is not confined to hematopoietic malignancies. Negative regulation of c-ABL kinase activity was found to be impaired in non-small cell lung cancer cells, suggesting that deregulation of c-ABL kinase contributes to the pathogenesis of lung cancer [13]. Furthermore, activation of c-ABL kinase activity promotes invasion of breast cancer cells [14]. Inhibition of c-ABL activity blocks transforming phenotypes, such as cell proliferation and anchorage-independent growth, and sensitizes cancer cells to apoptosis caused by nutrient deprivation [15]. Taken together, these studies have identified c-ABL as a promoting factor in solid tumors and raise the possibility that c-ABL plays an important role in breast carcinogenesis.
In the current study, we identified a previously unrecognized pathway involving the functional interaction between c-ABL and ER that leads to enhanced ER activity and promotes resistance to TAM. In addition, we provide the first evidence that the coexpression of these two proteins may have a prognostic value for breast cancer by examining a cohort of 142 breast cancer primary tumor tissue samples.
Materials and Methods
Cell Culture and Antibodies
The cell lines T47D, BT474, and MDA-MB-231 were purchased from American Type Culture Collection (ATCC, Manassas, VA). All cells were grown in Dulbecco's modified Eagle medium/F12 (1:1) with 10% fetal bovine serum, unless otherwise indicated in experiments requiring phenol red-free medium and charcoal-treated serum to remove estrogenic factors. All the antibodies used in this study were purchased: anti-α-tubulin (Sigma, St Louis, MO); anti-ER-α (Bethyl, Montgomery, TX; LeicaMicrosystems, Bannockburn, IL). The experiments of the anti-c-ABL antibodies and their vendors were as follows: immunoblot analysis (Calbiochem, San Diego, CA), immunohistochemical staining (Abcam, Cambridge, MA), immunofluorescence staining (BD Biosciences, San Jose, CA), and immunoprecipitation (Cell Signaling, Danvers, MA).
Luciferase Assay
Cells (5 x 104) were plated in 24-well plates and steroid-starved for 48 hours. The luciferase reporter controlled by a triplicate of the ER response element (pERE-Luc) was cotransfected with the pRL-TK plasmid, which served as the internal control. Transfections were conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Nine hours after transfection, cells were stimulated with estrogen (1 nM), or an equal amount of control vehicle (ethanol), for 36 hours. Luciferase assays were then carried out using the Dual-Luciferase Assay Kit (Promega, Madison, WI).
Immunoprecipitation Analysis
Cultured cells were washed twice with ice-cold PBS. The cells were then lysed in NETN buffer (150 mM NaCl, 1 mM EDTA pH 8.0, 20 mM Tris pH 8.0, 0.5% NP-40, 25 mM NaF, 2 mM Na3VO4, 20 µl/ml aprotinin, and 0.1 M PMSF) on ice for 30 minutes. For immunoprecipitation, the lysate was incubated with primary antibody at 4°C overnight. The immunocomplexes were then incubated with 50 µl of protein G agarose (Roche, Indianapolis, IN) at 4°C for 2 hours. The beads were washed four times with ice-cold NETN buffer, and the protein complexes were then eluted by boiling the beads in 40 µl of 2x loading buffer.
Anchorage-Independent Growth Assays
Bottom-layer agar was 0.7% agarose in normal medium with 10% FBS. Tumor cells were seeded in top soft agar of 0.35% agarose in normal medium containing 10% FBS. Normal medium containing 10% FBS with and without TAM was then placed on top of the solidified agar in each well. After approximately 3 weeks, tumor cell colonies in an entire well were counted, with three replicate wells per treatment, by using the ImageQuant TL software (GE Health Life Sciences, Piscataway, NJ). Colonies sized 150 µm or greater in diameter were counted.
Confocal Microscopy
Cultured cells were fixed and permeabilized with 4% formaldehyde and 0.2% Triton X-100 for 10 minutes at room temperature. After four washes with PBS, the cells were blocked with 10% normal goat serum for 1 hour at room temperature, and then immunostained with primary antibodies (1:200 dilution in PBS with 0.2% BSA) overnight at 4°C. Cellular c-ABL was detected with mouse monoclonal antibody (BD Biosciences), whereas ER was detected by a rabbit polyclonal antibody (Bethyl). After three washes with PBS, the FITC- or Texas Red-conjugated secondary antibody was applied for 45 minutes at room temperature. Nuclei were stained with TOPRO 3. Images were captured with a Zeiss laser scanning confocal microscope (LSM510).
Tissue Samples and Tumor Microarray
One hundred and forty-two formalin-fixed, paraffin-embedded, archival tumor tissues from breast cancer patients enrolled at the Cancer Center of Kaohsiung Medical University Hospital (Taiwan) from 2005 to 2007 were included in this study (Table 1). The ethics committee approved the study, and informed consent was obtained from each patient. The age at diagnosis ranged from 29 to 81 years (mean, 50.49 years) with 47.9% of the women younger than 50 years. Histologic typing showed 83% invasive ductal carcinomas, 12% infiltrating lobular carcinoma, and 6% carcinoma in situ. The clinicopathologic features of the patients are listed in Table 1. Tumor staging was defined according to the TNM system, and histologic typing was determined according to the guidelines of the World Health Organization. The expression levels of ER, progesterone receptor (PR), and ErbB-2 were obtained from the database of cancer registry from Kaohsiung Medical University Cancer Center. The slides were read by two pathology personnel in a double-blinded manner for c-ABL expression, which was scored by the H-score method (product of intensity and area). The H-score 0.5 was used as a cutoff for c-ABL immunohistochemistry (IHC; H-score < 0.5, low expression; H-score > 0.5, high expression).
Table 1.
Association of c-ABL Expression with Clinicopathological Variables in Breast Cancer Patients (N = 142)
| Characteristics | Total N | ABL+ | ABL- | p* |
| Age (years) | ||||
| <50 | 68 | 28 | 39 | .008 |
| ≥50 | 74 | 48 | 26 | |
| Stage | ||||
| 0/I/II | 108 | 54 | 54 | .072 |
| III/IV | 34 | 23 | 11 | |
| Grade | ||||
| 1 | 8 | 5 | 3 | .597 |
| 2 | 102 | 57 | 45 | |
| 3 | 32 | 15 | 17 | |
| Tumor size (cm) | ||||
| ≤5 | 123 | 70 | 53 | .284 |
| >5 | 19 | 10 | 9 | |
| Lymph node status | ||||
| Negative | 93 | 48 | 45 | .389 |
| Positive | 49 | 29 | 20 | |
| Recurrence† | ||||
| No | 124 | 69 | 55 | .373 |
| Yes | 18 | 8 | 10 | |
| ERα status | ||||
| Positive | 96 | 55 | 41 | .289 |
| Negative | 46 | 22 | 24 | |
| PR status | ||||
| Negative | 65 | 39 | 26 | .204 |
| Positive | 77 | 38 | 39 | |
| ErbB-2 status | ||||
| Negative | 81 | 47 | 34 | .387 |
| Positive | 43 | 25 | 18 | |
| ND | 18 | 5 | 13 | |
ND indicates not determined.
χ2 test or Fisher's exact test.
Median follow-up, 18 months.
Statistical Analysis
Data of each assay were expressed as means ± SD (n = 3). Statistical differences between two groups were determined by Student's t-test. P < .05 was considered significantly different. For the IHC study of tumor tissues, all of the statistical analyses were performed using the statistics program SPSS11.5 for PC (SPSS, Inc, Chicago, IL). Tumors were grouped according to c-ABL expression levels: the high-expression group (ABL+) and the low-expression group (ABL-). Expression levels were correlated with age group, tumor stage, tumor grade, tumor size, lymph node status, ER status, PR status, and ErbB-2 status. The P value was determined by the χ2 test or by Fisher's exact test when the sample size was less than 5. Differences with P ≤ .05 were considered statistically significant.
Gene Silencing by Short Hairpin RNA
To generate lentiviral particles capable of expressing short hairpin RNA (shRNA) against c-ABL, luciferase, and the control of scrambled sequence, the corresponding shRNA was cloned in pLKO.1-hairpin vector and was provided by the National RNAi Core Facility, Academia Sinica, Taiwan. Recombination with the lenti-gag/pol, envelope, and Rev plasmids in 293T cells was conducted at the Viral Vector Core at Cincinnati Children's Hospital. The specific sequences of the shRNA are as follows: shABL-1 CCGGGAGTTCTTGAAGCATTTCAAACTCGAGTTTGAAATGCTTCAAGAACTCTTTTTG; shABL-2 CCGGTAATTACCGTGAG-TGACATAGCTCGAGCTATGTCACTCACGGTAATTATTTTTG; shLuc CCGGCAAATCACAGAATCGTCGTATCTCGAGATACGACGATTCTGTGATTTGTTTTTG. shScramble CCTAAGGTTAAGTCGCCCTCGCTCTAGCGAGGGCGACTTAACCTTAGG generated by Dr. David Sabatini's laboratory [16]. To generate stable cell lines, cells were infected with the corresponding lentivirus and selected with puromycin. The surviving clones were then pooled for further experiments.
Combination Indices and Isobologram Analysis
Data from Cell Titer-Glo assays (Promega) were analyzed using CalcuSyn statistical software (Biosoft, Cambridge, UK). Isobolograms generated by CalcuSyn were based on dose-response curves for both TAM and STI571. Data points below the line represent synergistic drug interactions, whereas points on or above the line indicate additivity or antagonism, respectively. A combination index (CI) value of 1 indicates additivity, a CI value greater than 1 indicates antagonism, and a CI value less than 1 indicates synergism.
Results
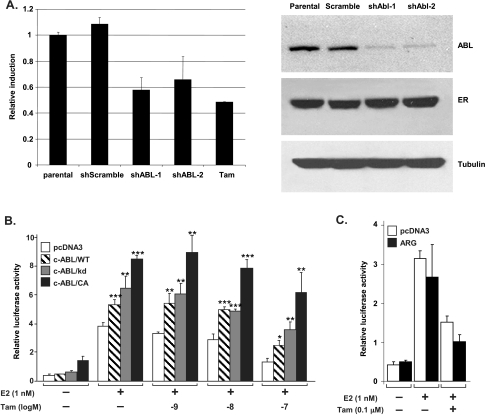
To examine the effect of c-ABL on ER function, we first tested whether c-ABL regulates the transcriptional activity of ER in the breast cancer cell line T47D, which expresses high levels of ER and c-ABL. Cells were infected with lentiviral vectors carrying the shRNA against c-ABL (shABL-1 and shABL-2; two different shRNA clones) or the control shRNA of random sequence (shScramble). Infected cells were selected by puromycin, and the resulting stable clones were pooled for further assays. To determine whether c-ABL regulates the transcriptional function of ER, the shRNA-harboring T47D cells were transiently transfected with a luciferase reporter gene controlled by the estrogen response element (pERE-Luc) and grown in phenol red-free medium with charcoal-treated serum. Cells were then stimulated with 17β-estradiol or the control vehicle followed by luciferase activity assay. Silencing of c-ABL repressed luciferase reporter activity to a level similar to that achieved by TAM (Figure 1A), indicating that c-ABL is required for optimal activation of the reporter gene. The level of c-ABL expression in these engineered clones has no effect on expression of the endogenous ER protein.We then tested whether introducing c-ABL is sufficient for activating the transcriptional function of ER. For this experiment, the breast cancer MCF-7 cells, which are ER-positive but express low level of c-ABL activity [15], grown in phenol red-free medium containing charcoal-treated serum were cotransfected by pERE-Luc with c-ABL complementary DNA (cDNA) or vector control (Figure 1B). As expected, estrogen significantly stimulated the luciferase reporter gene activity in cells cotransfected with the control vector, and treatment with TAM repressed the transactivation in a dose-dependent manner. Cotransfection with the wild-type (WT) c-ABL cDNA resulted in moderate enhancement of estrogen-mediated reporter activation, which sustained the inhibition of up to 10 nM of TAM treatment. The kinase-deficient (kd) mutant (K290R) of c-ABL [17] had a similar effect on the ERE reporter, indicating that a kinase-independent mechanism may be involved in c-ABL-mediated ER activation. Nevertheless, transfection of the constitutively activate (CA) mutant (P242A/P249A) of c-ABL [18] resulted in more dramatic activation by estrogen than the WTand kd c-ABL, even in the presence of TAM (up to 100 nM). Thus, these results support that c-ABL can enhance the transactivation function of ER in both kinase-dependent and -independent manners. This is in contrast to ARG (ABL-related gene; also known as ABL2), the other member of the ABL family. Reporter assay showed that introducing ARG was not able to enhance the estrogen-mediated ER activation in comparison with the vector control (Figure 1C). This result indicates that the effect on ER function is unique to c-ABL in the ABL kinase family.
Figure 1.
Depletion of c-ABL resulted in reduced ER activity in transactivation assays. (A) Parental T47D cells (Parental) and their derivatives harboring shRNA of scrambled control sequence (shScramble) or c-Abl (shABL) were starved in estrogen-free medium for 48 hours then transfected with the ERE-luciferase reporter in the presence or absence of estrogen (1 nM). The fold induction of the reporter was plotted, with the activity of the parental cells set to 1. As a positive control for reduced ER activity, the parental cells were treated with TAM (Tam). The inset shows a Western analysis of c-ABL and ER expression in the cell line used in this experiment. (B) MCF-7 cells (5 x 104) were plated in 24-well plates in phenol red-free medium containing charcoal-treated serum (10%) for 48 hours. The cells were then transfected with ERE-Luc along with a control vector (pcDNA3), or the cDNA of WT c-ABL (c-ABL/WT), or the kd (c-ABL/kd) or CA (c-ABL/CA) c-ABL mutants. The plasmid pRL-TK was cotransfected in each transfection as the internal control. Nine hours after transfection, cells were stimulated with or without estrogen (1 nM) for 2 hours, then treated with different concentrations of TAM as indicated (in the presence of 1 nM estrogen) for 36 hours. Each data point was obtained in triplicate and the experiment was repeated for three times. *P < .05, **P < .01, ***P < .001 (compared with the vector control). Bars, SD. (C) Same as in panel B except that the cells were transfected with ERE-Luc along with the control vector pcDNA3 or the cDNA of WT ARG.
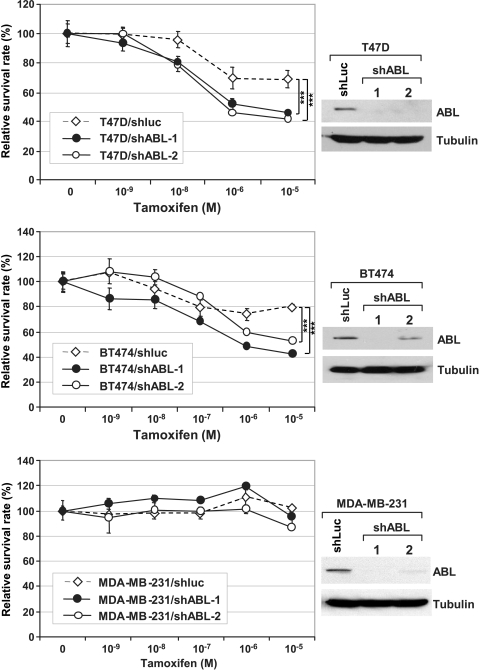
Prompted by these results, we proposed that c-ABL is involved in resistance to TAM-induced growth inhibition in ER-positive breast cancer cells. If this hypothesis is correct, silencing c-ABL expression should result in sensitization of cell to TAM treatment. To test this hypothesis, we compared the T47D/shABL cells and the isogenic control cell line T47D/shLuc, which harbors the shRNA against the luciferase gene introduced by lentiviral vector. In addition to T47D, the same pair was also generated for BT474 (namely BT474/shABL and BT474/shLuc). Like T47D, BT474 is a ER-positive breast cancer cell line expressing high levels of c-ABL (data not shown) [14]. For each cell line, two independent c-ABL shRNA (shABL-1 and shABL-2) were used to generate independent c-ABL-knockdown lines (Figure 2), and there is no significant difference between the c-ABL-silenced lines and the corresponding control lines (Figure W1). The cells were then cultured in medium containing different doses of TAM. Silencing c-ABL in T47D and BT474 cells resulted in sensitization to TAM treatment compared with the corresponding shLuc control. In contrast, down-regulation of c-ABL in the ER-negative c-ABL-expressing breast cancer cell line MDA-MB-231, which is resistant to TAM due to the lack of ER expression, did not sensitize cells to the treatment, suggesting that the c-ABL effect was through a functional ER. In addition to the short-term growth assay, down-regulation of c-ABL resulted in a long-term sensitization to TAM-induced cell growth inhibition as demonstrated by a clonogenic assay in which cells were seeded at low density in medium with or without TAM and grown for 2 weeks (Figure 3A). The effect of c-ABL on long-term growth inhibition by TAM is further supported by comparing the ability of shABL and shLuc cells in anchorage-independent growth as measured by a colony formation assay in soft agar in the presence and absence of TAM (Figure 3B).
Figure 2.
Inhibition of c-ABL resulted in sensitization of breast cancer cells to TAM treatment. Top panel: Pooled clones derived from T47D cells harboring a shRNA against luciferase (T47D/shLuc) and two independent shRNA against c-ABL (T47D/shABL-1 and T47D/shABL-2) were treated with TAM at the indicated concentrations for 1 week. The surviving cells were then assessed by MTT assay, and cell survival was plotted. Western analysis showing the efficiency of shRNA silencing of c-ABL. Middle and lower panels: The same setting for BT474 and MDA-MB-231, respectively. ***P < .001.
Figure 3.
c-ABL is required for long-term TAM resistance. (A) In 24-well plates, 3000 cells of the indicated clones were plated in each well, and the cells were grown for 2 weeks in medium containing 1 nM TAM. The resulting colonies were stained with crystal violet. The experiment was repeated three times, and representative data are shown. (B) Left panel: The indicated T47D sublines were seeded in triplicate in soft agar prepared in phenol red-free medium and layered with medium containing the indicated concentration of TAM and cultured for 3 weeks. Top medium was changed every three days with medium containing the indicated concentration of TAM. The colonies in the well were then stained and counted (right panel; with 1 µM TAM). *P < .05, **P < .01 (compared with the T47D/shLuc control).
These results indicate that c-ABL plays a positive role in maintaining cell growth in response to TAM treatment and that inhibiting c-ABL can sensitize breast cancer cells to treatment of antiestrogens. To test this hypothesis, T47D cells were treated by TAM, the ABL inhibitor imatinib (STI571; Gleevec; LC Laboratories, Woburn, MA) or both in combination (Figure 4A). Treatment with imatinib or TAM alone moderately suppressed the survival of T47D cells. The suppression was significantly enhanced when TAM was combined with imatinib in the treatment (P < .05). To further understand the interaction between imatinib and TAM, we performed an isobologram analysis (Figure 4B). In this analysis, the dose-response curve of TAM alone, imatinib alone, and different doses of TAM combined with 10 µM of imatinib were plotted, and the CI was determined. A CI value less than 1 indicates synergy, a CI value equals to 1 indicates addition, and a CI higher than 1 indicates antagonism [19]. The analysis shows that combination of TAM and imatinib has synergistic effect in T47D cells. Similar results were observed in BT474 cells (Figure W4). Taken together, these results demonstrate a functional interaction between c-ABL and ER in breast cancer cells to develop resistance to TAM and suggest that simultaneously targeting c-ABL and ER can increase therapeutic response in ER-positive breast cancer cells.
Figure 4.
Targeting c-ABL activity sensitizes breast cancer cells to TAM treatment. (A) T47D cells were treated with control vehicle alone (C) (white bars), 1 µM of TAM (T) or 10 µM imatinib (I) alone (gray bars), or in combination (black bars) as indicated for 6 days, and the effect on cytotoxicity was measured by the CellTiter-Glo kit (Promega). The data are derived from three independent experiments. *P < .05, compared with TAM treatment alone. (B) Normalized isobologram produced by the software CalcuSyn in T47D cells. A representative plot is shown. Values below the threshold line indicate synergistic combination. Table shows the CIs of different doses of TAM with 10 µM of imatinib derived from three independent experiments.
To further address the relationship between c-ABL and ER, we examined whether these two proteins interact in vivo. To this end, ER and c-ABL were ectopically coexpressed in HEK293T cells by transient transfection. Pulling down c-ABL by immunoprecipitation with an anti-c-ABL antibody also coprecipitated ER (Figure 5A, left panel ), and vice versa (Figure 5A, right panel), demonstrating that these two proteins form a complex in cells. The interaction between c-ABL and ER was further confirmed in T47D cells by reciprocal immunoprecipitation of the endogenous proteins (Figure 5B). The c-ABL kinase is known to shuttle between the cytoplasm and the nucleus [20]. Under confocal immunofluorescence microscopy colocalization between c-ABL and ER clearly speckled in the nucleus and in the cytoplasm, where most c-ABL resided (Figure 5C). Interestingly, the interaction between c-ABL and ER was enhanced by TAM (Figure 5D), which is in concordance with the notion that c-ABL and ER interact with each other in response to TAM treatment. We observed similar results using the BT474 cell line (Figure W3).
Figure 5.
ER and c-ABL form a protein complex in vivo. (A) Transient transfection in 293T cells. Left panel: 293T cells were cotransfected with cDNA for ER-α and c-ABL. The cell lysates were then immunoprecipitated with an anti-c-ABL antibody or a control immunoglobulin class G. After gel separation, the coprecipitated ER and c-ABL were detected by using the corresponding antibodies. Right panel: The reciprocal approach after immunoprecipitation of ER. (B) Interaction of endogenous ER and c-ABL proteins in T47D cells by immunoprecipitation with anti-c-ABL (left panel) and anti-ER (right panel) antibodies, respectively. HC indicates heavy chain. (C) Colocalization of cellular ER (green) and c-ABL (red) in T47D cells by confocal immunofluorescence. The inset shows the amplified view of the area circled by the dotted square. Arrows point to examples of colocalization indicated by yellow fluorescence. The nuclei were stained blue with TOPRO 3. Bar, 10 µm. (D) The interaction between endogenous ER and c-ABL is enhanced by TAM. T47D cells were treated with TAM(Tam, 1 µM) or ethanol as the control vehicle (Ctrl) for 6 hours. Cell lysates were then subjected to immunoprecipitation with the anti-c-ABL antibody. Left panel: The cellular ER pulled down was detected by Western analysis. Right panel: Data were quantitated and normalized with respect to immunoprecipitated c-ABL.
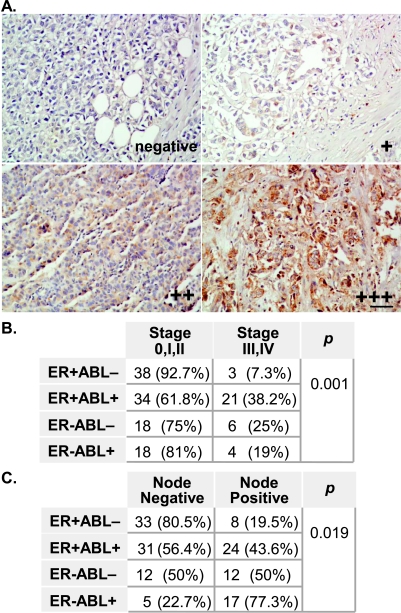
These results also suggest that the expression of c-ABL and ER together may better correlate with breast cancer progression than either marker alone. We evaluated primary tumor tissues derived from 142 archived breast cancer cases (Table 1). IHC analysis was conducted to examine the expression of c-ABL in these tumor tissues (Figure 6). The expression of c-ABL was a frequent event; this can be observed in 54% of the tumors, and immunostaining for c-ABL was visible in both the cytoplasm and the nucleus. Although the expression of c-ABL alone was not correlated with ER, PR, or ErbB-2 expression, or nodal involvement (Table 1), it was marginally correlated with tumor stage in that the c-ABL high-expression group tended to have more advanced disease than the c-ABL low-expression group (30% vs 17%, P = .072). This trend turned into a significant correlation when both c-ABL and ER expression were considered, in which 38.2% of the c-ABL(+)/ER(+) tumors were at an advanced stage, as opposed to only 7.3% of the c-ABL(-)/ER(+) tumors (P = .001; Figure 6B). In addition, 43.6% of the tumors in the c-ABL(+)/ER(+) group had lymph node involvement, as compared with the c-ABL(-)/ER(+) group, which included only 19.5% of the tumors (P = .019; Figure 6C). Collectively, these results suggest that simultaneous expression of c-ABL and ER promotes disease progression and metastasis.
Figure 6.
Expression of c-ABL and ER in primary breast cancer tumors. (A) A representative set of IHC results is shown with the indicated expression status of c-ABL (negative, +, ++, and +++). For details of the tumor microarray and the staining method, refer to the Materials and Methods section. Bar, 50 µm. (B and C) Coexpression of c-ABL and ER is associated with advanced tumor stage (B) and with lymph node involvement (C). P was determined by χ2 test or Fisher's exact test.
Discussion
ER function has a profound impact on the outcome of breast cancer. On one hand, ER expression is correlated with a favorable prognosis, partly because of its potential to confer responsiveness to hormonal therapy. Conversely, ER is a well-established neoplastic factor in promoting proliferation of breast cancer cells. In this study, we showed that c-ABL functionally interacted with ER in regulating the cellular response to TAM and in breast cancer progression. This is based on the observations that c-ABL is required for maintaining ER transcriptional activity and that silencing c-ABL results in the sensitization of ER-positive but not ER-negative breast cancer cells to TAM treatment. In addition, our immunoprecipitation study demonstrated that c-ABL and ER form a complex in breast cancer cells and the association is enhanced by TAM treatment. This observation raises the question of whether the change of c-ABL-ER association is also accompanied with alteration in the complex formation of ER with its other transcriptional coregulators. Previous studies have shown that aberrant regulation of ER resulted in the recruitment of coactivators and converted TAM to an estrogenic rather than antiestrogenic factor in breast cancer cells [5]. In our cohort of human breast cancer cases, positive c-ABL expression was a frequent event. Moreover, coexpression of c-ABL and ER in tumors was significantly correlated with late-stage breast cancer (P = .001) and nodal involvement (P = .019), an indication of cancer cell dissemination. The group of patients included in this study was enrolled within a 2-year period, hence the information on their response to antihormonal therapy would require further follow-up. Nonetheless, our study reveals a novel functional interaction between c-ABL and the nuclear hormonal receptor ER in breast cancer development. This observation is likely to be translated into a therapeutic gain as treatment with the clinically approved ABL inhibitor imatinib sensitizes breast cancer cells to TAM.
Unlike T47D, MCF-7 cells express low levels of c-ABL activity. Although introducing the WT, kd, and CA forms of c-ABL all resulted in enhanced ER activity, the CA c-ABL mediated a more pronounced activation of ER activity compared with the WT and the kd mutant, indicating that the kinase activity of c-ABL plays an important role in ER regulation. The observation that both WT and the kd mutant c-ABL have similar effects on ER activity may be because c-ABL kinase activity is subject to negative regulation by autoinhibition [21,22]. It should be noted that the WT and kd c-ABL cDNA expressed equal levels of c ABL in MCF-7 cells (Figure W2). Thus, the CA mutant, which is not subject to the autoinhibition, exhibited the strongest activation effect on ER through a kinase-dependent mechanism. Conversely, the observation that both the WT and the kd mutant c-ABL induced a moderate increase in ER activity suggests that c-ABL can also activate ER function through a kinase-independent mechanism. Such a mode of c-ABL activity has been documented previously [23]. Consistently, the interaction between c-ABL and ER was independent of the kinase activity of c-ABL because the kd c-ABL mutant interacted with ER as strongly as theWT c-ABL (data not shown).
The functional collaboration between c-ABL and ER resembles the cross talk between ErbB-2 and ER. Coexpression of ErbB-2 and ER tends to occur in high-grade lesions, and elevated ErbB-2 expression correlated with enhanced resistance to antihormonal therapy and a poor outcome in ER-positive patients [4], possibly because the activation of ErbB-2 signaling stimulates the intrinsic activity of ER and activates the nuclear coactivator [5,24]. While this work was in progress, Oh et al. [25] reported that c-ABL physically interacted with and phosphorylated the nuclear receptor coactivator AIB1, resulting in enhanced ER activation and cell growth on estrogen stimulation. This is in agreement with the results of our study in which we further demonstrated that c-ABL interacts with ER, sustains its transcriptional function on TAM treatment, and enhances cellular resistance to TAM. Furthermore, our study identified c-ABL expression as a previously unrecognized tumor marker in breast cancer. Coexpression of c-ABL and ER constitutes a molecular signature that correlates with advanced disease progression and metastasis. Thus, the cellular outcome of ER expression in cancer development seems to depend on the molecular makeup of the tumor, such as the status of its modulator c-ABL, and the pathophysiological consequence of c-ABL expression in breast tumors may also depend on the function of other factors such as ER.
Our study suggests that c-ABL could be used as a prognostic marker and a treatment target of breast cancer. Like ErbB-2, coexpression of c-ABL with ER may render the nuclear receptor under the control of the tyrosine kinase. Thus, markers such as c-ABL and ErbB-2 may also assist in determining appropriate therapeutic approaches targeting tumors resistant to antiestrogen treatments. Further study will be needed to dissect how the c-ABL kinase regulates the tumor's response to hormonal therapy and whether cotargeting of ER and c-ABL can yield improved clinical outcome.
Supplementary Material
Acknowledgments
The authors thank Stephen Goff for the generous gifts of the WT and kd c-ABL cDNA. The authors thank Sohaib Khan for the helpful discussion. The authors also thankMaryellen Daston and GlennDoerman for editing the manuscript and the graphic assistance, respectively. Confocal microscopy was conducted at the Center of Biological Microscopy of the University of Cincinnati with the assistance of Birgit Ehmer.
Abbreviations
- ERα
estrogen receptor α
- ERE
estrogen receptor response element
- WT
wild-type
- kd
kinase-deficient
- CA
constitutively active
- TAM
tamoxifen
- shLuc
short hairpin RNA against luciferase
- shABL
short hairpin RNA against c-ABL
Footnotes
This work was supported in part by the startup fund of the University of Cincinnati Cancer Consortium, the Susan G. Komen Breast Cancer Research Award KG080540, the Department of Defense New Investigator Award PC073951, and the Marlene Harris-Ride Cincinnati Breast Cancer Pilot Grant Program (to S.-C.W.). This project was also supported in part by PHS grant P30 DK078392.
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
- 1.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SAW, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 3.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 4.Dowsett M, Harper-Wynne C, Boeddinghaus I, Salter J, Hills M, Dixon M, Ebbs S, Gui G, Sacks N, Smith I. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res. 2001;61:8452–8458. [PubMed] [Google Scholar]
- 5.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 6.Shaul Y, Ben-Yehoyada M. Role of c-Abl in the DNA damage stress response. Cell Res. 2005;15:33–35. doi: 10.1038/sj.cr.7290261. [DOI] [PubMed] [Google Scholar]
- 7.Pendergast AM. The Abl family kinases: mechanisms of regulation and signaling. Adv Cancer Res. 2002;85:51–100. doi: 10.1016/s0065-230x(02)85003-5. [DOI] [PubMed] [Google Scholar]
- 8.Plattner R, Pendergast AM. Activation and signaling of the Abl tyrosine kinase: bidirectional link with phosphoinositide signaling. Cell Cycle. 2003;2:273–274. [PubMed] [Google Scholar]
- 9.Kharbanda S, Yuan ZM, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;7:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 10.Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000;19:5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- 11.Goldman JM, Melo JV. Chronic myeloid leukemia—advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451–1464. doi: 10.1056/NEJMra020777. [DOI] [PubMed] [Google Scholar]
- 12.Advani AS, Pendergast AM. Bcr-Abl variants: biological and clinical aspects. Leuk Res. 2002;26:713–720. doi: 10.1016/s0145-2126(01)00197-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Sun T, Ji L, Deng W, Roth J, Minna J, Arlinghaus R. Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1. Oncogene. 2007;26:6989–6996. doi: 10.1038/sj.onc.1210500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- 16.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barila D, Superti-Furga G. An intramolecular SH3-domain interaction regulates c-Abl activity. Nat Genet. 1998;18:280–282. doi: 10.1038/ng0398-280. [DOI] [PubMed] [Google Scholar]
- 19.Chou T-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 20.Taagepera S, McDonald D, Loeb JE, Whitaker LL, McElroy AK, Wang JY, Hope TJ. Nuclear-cytoplasmic shuttling of c-ABL tyrosine kinase. Proc Natl Acad Sci USA. 1998;95:7457–7462. doi: 10.1073/pnas.95.13.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang JYJ. Controlling Abl: auto-inhibition and co-inhibition? Nat Cell Biol. 2004;6:3–7. doi: 10.1038/ncb0104-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Zhang J, Lee J, Lin PS, Ford JM, Zheng N, Zhou P. A kinase-independent function of c-Abl in promoting proteolytic destruction of damaged DNA binding proteins. Mole Cell. 2006;22:489–499. doi: 10.1016/j.molcel.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Collins LC, Schnitt SJ. HER2 protein overexpression in estrogen receptor-positive ductal carcinoma in situ of the breast: frequency and implications for tamoxifen therapy. Mod Pathol. 2005;18:615–620. doi: 10.1038/modpathol.3800360. [DOI] [PubMed] [Google Scholar]
- 25.Oh AS, Lahusen JT, Chien CD, Fereshteh MP, Zhang X, Dakshanamurthy S, Xu J, Kagan BL, Wellstein A, Riegel AT. Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol Cell Biol. 2008;28:6580–6593. doi: 10.1128/MCB.00118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.