Abstract
Recent studies have implicated Toll-like receptor 2 (TLR2) and TLR4 signaling in delimiting liver and brain injury following ischemia-reperfusion (I/R). To determine whether TLR2 and TLR4 conferred cytoprotection in the heart, we subjected hearts of wild-type (WT) mice and mice deficient in TLR2 (TLR2D), TLR4 (TLR4D), and TIR domain-containing adapter protein (TIRAP-D) to ischemic preconditioning (IPC). Langendorff-perfused hearts were subjected to 30 min ischemia and 60 min reperfusion with or without IPC. IPC resulted in a significant increase (P < 0.05) in the percent recovery of left ventricular developed pressure (%LVDP) in WT mouse hearts (54.4 ± 2.7% of baseline), whereas there was no significant increase in %LVDP (P > 0.05) in TIRAP-D mouse hearts (43.8 ± 1.9%) after I/R injury. IPC also resulted in a significant (P < 0.05) decrease in I/R-induced creatine kinase release and Evans blue dye uptake in WT but not TIRAP-D hearts. Interestingly, IPC resulted in a significant (P < 0.05) increase in %LVDP in TLR4-deficient hearts (52.7 ± 3%) but not in TLR2D hearts (39.3 ± 1.5%). Pretreatment with a specific TLR2 ligand (Pam3CSK) protected WT hearts against I/R-induced left ventricular dysfunction. The loss of IPC-induced cardioprotection in TIRAP-D mouse hearts was accompanied by a decreased translocation of protein kinase C-ε and decreased phosphorylation of GSK-3β. Taken together, these data suggest that the cardioprotective effect of IPC is mediated, at least in part, through a TLR2-TIRAP-dependent pathway, suggesting that the modulation of this pathway represents a viable target for reducing I/R injury.
Keywords: myocardial dysfunction, ischemic preconditioning
the classical view of the immune system is that it evolved to enable the host organism to discriminate “self” from “nonself” (17). Traditionally, the immune system has been divided into innate and adaptive components, each of which has a different role in helping the host to differentiate self from nonself. The innate immune system is activated by a family of pattern recognition receptors that reside in a variety of cell types, including cardiac myocytes. These pattern recognition receptors recognize invariant patterns (so-called pathogen-associated molecular patterns) that are shared by groups of microorganisms but not by host tissues (17, 25, 27). Whereas the concept of an immune system that is designed to discriminate between self and nonself works well for classical immunological disorders involving T cells and B cells, it has been difficult heretofore to apply these concepts to the heart, wherein tissue injury is encountered more frequently than invading pathogens. Recently, Matzinger (26) has suggested an alternative view of the role of the immune system that lends itself more readily to understanding the role of innate immunity in the heart. The so-called “Danger Model” of immunity suggested by Matzinger proposes that cell damage, rather than foreignness, is what initiates an immune response. The Danger Model further proposes that what really matters from an evolutionary perspective is whether a given entity causes cell damage or not. Consistent with this model, recent studies have shown that injured and/or stressed tissues release a variety of distinct mediators that serve as potent effectors of the innate immune system. Indeed, molecules released by stressed cells [heat shock protein 60 and 70, and high-mobility group box protein 1 (HMGB1)] and/or injured tissue (fibronectin) activate signaling through Toll-like receptor 2 (TLR2) and TLR4, which serve as classic pattern recognition receptors for gram-positive and gram-negative organisms, respectively.
Germane to the present discussion, recent studies suggest that TLR signaling may play an important homeostatic role by delimiting tissue injury following ischemia-reperfusion (I/R) injury. For example, Izuishi et al. (21) showed that pretreatment with HMGB1 (a TLR4 ligand) conferred cytoprotection in liver I/R injury in wild-type but not in TLR4 mutant mice (21). Furthermore, treatment with a TLR2-specific ligand decreased brain infarct size in mice that were subjected to focal cerebral I/R injury (20). Viewed together, these observations suggested the interesting possibility that TLR2 and/or TLR4 signaling might also play an important homeostatic role in the heart by upregulating cytoprotective signaling in response to ischemic injury. Accordingly, to begin to test this hypothesis, we subjected the hearts of mice that were deficient in TLR2, TLR4, and TIR domain-containing adapter protein (TIRAP), an adaptor molecule necessary and specific for TLR2 and TLR4 signaling (18, 19), to ischemic preconditioning followed by I/R injury. Here we show for the first time that ischemic preconditioning confers cytoprotection in the heart through TLR2-TIRAP-dependent signaling.
METHODS
Mice.
The mutant mice deficient in TIRAP (TIRAP-D; 129SV × C57BL/6), TLR2 (TLR2D; 129SV × C57BL/6), TLR4 (TLR4D; C57BL/6), and IL-1 receptor-associated kinase-1 (IRAK1) (IRAK1D; C57BL/6) were generated by gene targeting, as described previously (7, 18, 22, 37). The TIRAP-D mice were a gift from Dr. Ruslan Medzhitov (Yale University, New Haven, CT), whereas the TLR2D and TLR4D mice were a gift from Shizuo Akira (Osaka University, Osaka, Japan) (37). For the purpose of these studies, we established colonies of TIRAP-D, TLR2D, TLR4D, IRAK1D mice, as well as respective colonies of wild-type control mice that were bred on identical genetic backgrounds. Male mice (10–12 wk of age) used in this study were maintained in specific pathogen-free conditions and were fed pellet food and water ad libitum. All studies were performed with the approval of the Institutional Animal Care and Use Committee at Baylor College of Medicine. These investigations conform to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health.
Isolated heart perfusion studies.
Hearts from wild-type, TIRAP-D, TLR2D, TLR4D, and IRAK-1D mice were isolated and perfused in the Langendorff mode as previously described (34). Isolated hearts were perfused at a constant pressure of 80 mmHg with modified Krebs-Henseleit buffer containing (in mmol) 118 NaCl, 24 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.2 CaCl2, 10 glucose, and 2 pyruvate (pH 7.4; 37°C), equilibrated with 95% O2-5% CO2 to yield a pH of 7.4. The perfusate was gassed with 95% O2-5% CO2. A handmade balloon connected to a polyethylene tube was inserted into the left ventricle through the mitral valve via an incision in the left atrium and was connected to a pressure transducer (ML844, AD Instruments, Colorado Springs, CO). The balloon was inflated with water to adjust left ventricular (LV) end-diastolic pressure (LVEDP) at 7–10 mmHg.
I/R and ischemic preconditioning protocols.
Figure 1 summarizes the I/R and the ischemic preconditioning protocols used for these studies. After a 30-min stabilization period, hearts from wild-type, TIRAP-D, TLR2D, TLR4D, and IRAK1D mice and their respective wild-type controls were subjected to 30 min of zero-flow ischemia (t = −30 min) followed by reperfusion (t = 0) for 30 to 60 min (Fig. 1). All hearts were paced at 420 beats/min with pacing electrodes placed on the right atrium. Pacing was interrupted during ischemia and resumed 3 min after the start of reperfusion. Functional data were recorded at 1 KHz on a data acquisition system (PowerLab, AD Instruments). LV developed pressure was calculated as the difference between peak-systolic pressure and LVEDP, and the resulting LV functional recovery data were expressed as the percentage of baseline LVEDP. For ischemic preconditioning, the hearts from the TIRAP-D, TLR2D, TLR4D, and IRAK1D mice and their respective wild-type control mice were subjected to three episodes of 2 min of global ischemia and 5 min of reperfusion, followed by 30 min of zero-flow ischemia (t = −30 min) and reperfusion (t = 0) for 30 to 60 min (Fig. 1). Hemodynamic parameters were recorded as described above. At the end of each experiment, hearts were frozen in liquid nitrogen for later use.
Fig. 1.
Ischemic preconditioning (IPC) and ischemia-reperfusion (I/R) protocols. Mice were subjected to 3 episodes of 2 min of global ischemia and 5 min of reperfusion followed immediately by no-flow ischemia (30 min) and reperfusion (60 min) or were subjected to 30 min of perfusion at 80 mmHg followed by no-flow ischemia (30 min) and reperfusion (60 min).
Creatine kinase assay.
Coronary effluent was collected during the first 30 min after ischemia. Creatine kinase (CK) activity was measured with a commercially available CK assay kit (Diagnostic Chemical, Charlottetown, PE, Canada) according to the manufacturer's recommendations. CK activity was normalized for frozen-dry heart weight. Data are expressed as units per gram of cardiac tissue.
Evans blue staining.
Because triphenyltetrazolium chloride staining may underestimate the true extent of tissue injury within the first 3 h of cardiac injury (4), we used Evans blue dye uptake to assess the degree of myocardial tissue injury following I/R injury. Evans blue is a cell-impermeable diazo dye that has been used to study the integrity/permeability of blood vessels and cell membranes that become injured. In muscle cells with permeable membranes, Evans blue dye crosses into the cell and accumulates in myofibrils, where it emits red autofluorescence when examined using fluorescence microscopy (16). At the end of the reperfusion protocol (i.e., 60 min of reperfusion), the hearts were perfused (1 ml/min) first with 3 ml of ice-cold 0.1% diluted in 1 × PBS, followed by perfusion with 10 ml of ice-cold 1 × PBS, which was used to clear Evans blue dye from the interstitium. The hearts were then subsequently perfused with 10 ml of Z-fix fixative (1 ml/min), paraffin-embedded, and sectioned (0.5 μm). To quantify the extent of Evans blue dye uptake, 10 predetermined radially arranged transmural LV sections were chosen for evaluation in each heart. Each transmural slice through the LV wall was further divided into thirds to allow for an analysis of the endocardium, the midwall. Fluorescence microscopy (×200) was performed using a filter set with an excitation of 510–560 nm and an emission of 590 nm to assess the amount of Evans blue dye uptake in the myocardium following I/R injury with or without ischemic preconditioning. Hearts were examined at the level of the papillary muscle, using a total of 10 microscopic fields per heart. Data are expressed as the percent area of the myocardium with red fluorescence.
Pam3CSK4 treatment.
Isolated hearts from wild-type and TLR2D mice were treated with the TLR2-specific agonist Pam3CSK4 (5 μg/ml; Invivogen, San Diego, CA) for 5 min. The hearts were then subjected to the I/R protocol (Fig. 1). Wild-type and TLR2D hearts were perfused for 5 min with Pam3CSK4 (0.5 μg/ml), and cytosolic and membrane proteins were isolated as described in Isolation of cytoplasmic and membrane proteins. These samples were examined for protein kinase C-ε (PKCε) translocation as described in Immunoprecipitation and Western blot analysis.
Isolation of cytoplasmic and membrane proteins.
LV tissue was homogenized in isotonic sucrose buffer A containing (in mM) 20.0 Tris · HCl, 250.0 sucrose, 1 Na3VO4, 2.0 MgCl2, 2.0 EDTA, 0.5 EGTA, 2.0 PMSF, and 1.0 DTT and 0.02% (vol/vol) protease inhibitor cocktail (pH 7.4). The homogenates were centrifuged at 100,000 g for 60 min at 4°C to separate the particulate fraction from the cytosolic fraction. The resulting supernatant was labeled as the cytosolic fraction, whereas the pellet was resuspended with 0.1% Triton X-100 in buffer A and incubated on ice for 30 min. The resuspended pellet was then centrifuged at 100,000 g for 60 min at 4°C, and this supernatant was labeled as the particulate fraction. The protein concentration was determined using the bicinchoninic assay with bovine serum albumin as a standard (Pierce, Life Science; Rockford, IL). All samples were frozen in aliquots and stored at −80°C.
Immunoprecipitation and Western blot analysis.
Extracts with equal amount of proteins were used for immunoprecipitation studies. A total of 1 mg of whole cell extract was incubated overnight at 4°C on a rotator with 2 μg of anti-TLR2 (Santa Cruz) or anti-TLR4 antibody (Santa Cruz). Protein G agarose beads (50 μl) were then added to each sample, followed by incubation for an additional 2 h at 4°C. The samples were spun briefly in a microcentrifuge and washed for four times in lysis buffer. Samples were subsequently solubilized by SDS sample buffer containing 80 mM Tris · HCl (pH 6.8), 2% SDS, 50% glycerol, 0.05% bromphenol blue, and 0.2 M DTT, separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories), probed with an anti-PKCε antibody (BD Biosciences), and detected by enhanced chemiluminescence reagent.
Whole cell lysates were prepared using heart from wild-type and TIRAP-D mice subject to I/R or ischemic preconditioning + I/R. The protein concentration was determined using the bicinchoninic assay with bovine serum albumin as a standard (Pierce, Life Science). Protein was separated on percent SDS-polyacrylamide gel under denaturing conditions and was transferred onto a polyvinylidene difluoride membrane (Bio-Rad). The membrane was immunoblotted with rabbit anti-phosphorylated Akt (1:000; Cell Signaling, Beverly, MA) or anti-pGSK-β antibody (1:1,000; Cell Signaling). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit polyclonal antibody (1:1,000; Cell Signaling). The levels of phosphorylated Akt and phosphorylated GSK3-β were normalized to the levels of total Akt (1:1,000; Cell Signaling) and GSK3-β (1:1,000; Cell Signaling), respectively.
Statistical analysis.
All data are expressed as means ± SE. Two-way analysis (ANOVA) was used to determine differences between groups of mice following I/R injury. A post hoc test of least significant differences (Fisher protected least significant difference test) was used to determine differences at specific time points. A Student's paired t-test was used to determine differences in Evans blue uptake, CK release, PKCε translocation, AKT phosphorylation, and GSK3-β phosphorylation. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
TIRAP deficiency abolishes the cardioprotective effects of ischemic preconditioning.
To determine whether TIRAP was necessary to mediate preconditioning, we subjected wild-type and TIRAP-D hearts to preconditioning (Fig. 1) followed by I/R injury. The salient finding shown by Fig. 2A is that the cardioprotective effects of preconditioning were abolished in the TIRAP-D mice. As shown in Fig. 2A, the recovery of LV function (60 min) was significantly greater (P > 0.05) in the wild-type mice that underwent preconditioning compared with the wild-type mice that were subjected to I/R injury alone (54.4 ± 2.7% vs. 26.2 ± 1.3% of baseline). In contrast, the recovery of LV function was not significantly different (P > 0.05) in the TIRAP-D mice that underwent preconditioning compared with the TIRAP-D mice that were subjected to I/R injury alone (43.8 ± 1.9% vs. 41.8 ± 1.5% of baseline, respectively). Moreover, when we compared the degree of functional recovery in the wild-type and TIRAP-D mice that underwent preconditioning (Fig. 2, A and B), the degree of functional recovery was significantly greater (P < 0.05) in the wild-type mice than in the TIRAP-D mice (54.4 ± 2.7% vs. 43.8 ± 1.9% of baseline). Consistent with the LV functional recovery data shown in Fig. 2A, preconditioning resulted in a significant decrease (P < 0.05) in Evans blue dye uptake, as well as a significant decrease in CK release in the wild-type mice. In contrast, preconditioning had no effect (P = 0.42) on Evans blue dye uptake or CK release (P = 0.4) in the TIRAP-D mice compared with wild-type control mice. Importantly, the degree of Evans blue dye uptake and CK release following preconditioning was significantly greater (P < 0.05) in the TIRAP-D mice compared with the wild-type mice.
Fig. 2.
Effect of TIR domain-containing adapter protein (TIRAP) deficiency on IPC. A: percent recovery of left ventricular developed pressure (%LVDP) in hearts from wild-type (WT) mice. B: TIRAP-deficient (TIRAP-D) mice during I/R injury or IPC + I/R injury. C: representative photomicrograph of Evans blue dye uptake in hearts from WT and TIRAP-D mice. D: group data of Evans blue dye uptake in hearts from WT and TIRAP-D mice during I/R injury or I/R + I/R injury (n = 5–7 hearts/group). E: group data of creatine kinase release in hearts from WT and TIRAP-D mice during I/R injury or I/R + I/R injury (n = 5–8 hearts/group). *P ≤ 0.05 for WT vs. TIRAP-D; **P ≤ 0.05 WT I/R vs. WT IPC + I/R.
Interestingly, the degree recovery of functional LV function following I/R injury was 1.6-fold greater (P < 0.05) in the TIRAP-D mice (Fig. 2B) compared with the wild-type mice (Fig. 2A). Similarly, the extent Evans blue dye uptake (Fig. 2D) and CK release (Fig. 2E) were significantly greater (P < 0.05) in the wild-type mice with intact TIRAP signaling compared with mice lacking TIRAP signaling. Taken together, these data suggest that TIRAP signaling plays both beneficial and deleterious roles in the heart following I/R injury, the former by upregulating cardioprotective pathways following repetitive bouts of ischemic injury (i.e., preconditioning) and the latter by amplifying cell injury following an abrupt onset of ischemic injury.
The cardioprotective effects of ischemic preconditioning are mediated via TLR2.
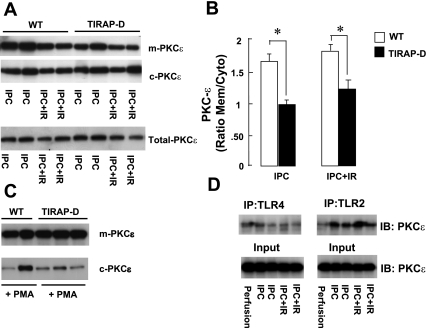
Given the specificity of TIRAP for TLR2 and TLR4 signaling, we next performed studies to determine the contribution of TLR2 and TLR4 to ischemic preconditioning. Ischemic preconditioning in wild-type hearts resulted in a significant (P < 0.05) recovery of LV function 60 min following I/R injury (Fig. 3, A and B) compared with that in wild-type hearts subjected to I/R injury alone. However, the major finding shown by Fig. 3 is that the cardioprotective effect of ischemic preconditioning was abolished in the TLR2D mice (Fig. 3B), whereas the cardioprotective effect of ischemic preconditioning was intact in the TLR4D mice (Fig. 3D). Figure 4B shows that that recovery of LV function (60 min) following I/R injury was not significantly different (P > 0.05) in the TLR2D hearts subjected to ischemic preconditioning compared with hearts that were subjected to I/R injury alone (39.3 ± 1.5% vs. 35.5 ± 1.3%, respectively). In contrast, Fig. 3D shows that recovery of LV function (60 min) following I/R injury was significantly greater (P < 0.05) in the TLR4D hearts subjected to ischemic preconditioning, compared with TLR4D hearts subjected to I/R injury alone (52.7 ± 3% vs. 35.3 ± 1.7%, respectively). Taken together, these data suggest that the protective effect of ischemic preconditioning is mediated primarily through a TLR2-TIRAP-dependent pathway.
Fig. 3.
Effect of Toll-like receptor 2 (TLR2) and TLR4 deficiency on IPC. A: %LVDP in hearts isolated from WT mice. B: TLR2-deficient (TLR2D) mice following I/R or IPC + I/R. C: %LVDP in hearts isolated from WT mice. D: TLR4D mice following I/R or IPC + I/R. *P ≤ 0.05 I/R vs. IPC + I/R.
Fig. 4.
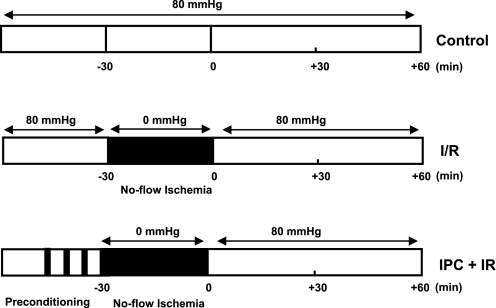
PKCε translocation in TIRAP-, TLR2-, and TLR4-deficient mouse hearts following IPC. A: representative Western blot showing PKCε translocation in hearts from WT and TIRAP-D mice following I/R injury or IPC + I/R injury. B: group data of PKCε translocation in hearts from WT and TIRAP-D mice following I/R injury and IPC + I/R injury. Data are expressed as the ratio of membrane (Mem) to cytosolic (Cyto) PKCε. C: PKCε translocation in WT and TIRAP-D hearts after administration of 200 nM PMA, which was used as a positive control. D: representative Western immunoblots (IB) illustrating the association of PKCε with TLR2 or TLR4. All Western immunoblots shown are representative of 3 experiments with similar results. *P ≤ 0.05 I/R vs. IPC + I/R.
In agreement with a previous report from this laboratory, TLR2D mice were protected from myocardial I/R injury when compared with wild-type mice that were subjected to I/R injury alone (34); that is, the recovery of postischemic contractile performance was significantly greater (P < 0.05) in the hearts of TLR2D mice subjected to I/R injury (Fig. 3, A and B) compared with wild-type mice with intact TLR2 signaling (35.5 ± 1.3%. vs. 27.2 ± 2.2% of baseline, respectively). Interestingly, when the degree of functional LV recovery was compared in hearts from wild-type TLR4D and TLR4 wild-type mice that were subjected to I/R injury alone (Fig. 3C compared with Fig. 3D), there was no significant difference (P > 0.05) in the recovery of postischemic contractile performance at 60 min (35.3 ± 1.7% vs. 31.8 ± 1.8% of baseline, respectively).
TIRAP-dependent ischemic preconditioning involves translocation of PKCε.
Recent studies have shown that several PKC isoforms are essential components of the TLR signaling pathway (2, 23). PKCε is known to play an important role in ischemic preconditioning, insofar as selective PKCε antagonists inhibit the cardioprotective effects induced by ischemic preconditioning (13, 29). To determine whether the cardioprotective effects conferred by TIRAP were mediated, at least in part, through PKCε, we measured the translocation of PKCε in hearts of wild-type and TIRAP-D mice following ischemic preconditioning, as well as ischemic preconditioning followed by I/R injury. In preliminary control experiments (see supplemental data supplement Fig. S1; note: supplemental material may be found posted with the online version of this article), we observed that there was no significant PKCε translocation in wild-type and TIRAP-D hearts at baseline in the absence of ischemic preconditioning and/or I/R injury.
Representative Western blot data are depicted in Fig. 4A, whereas group data are summarized in Fig. 4B. As shown, the translocation of PKCε was significantly (P < 0.05) greater in the wild-type hearts than in the TIRAP-D hearts following ischemic preconditioning. Similar findings were observed with respect to the translocation of PKCε following ischemic preconditioning followed by I/R injury. The differences in PKCε translocation in the wild-type and TIRAP-D mice were not secondary to inherent differences in PKCε translocation in TIRAP-D mouse hearts, insofar as the administration of the pan-PKC agonist PMA (200 nM) induced similar PKCε translocation in the hearts of wild-type and TIRAP-D mice.
Insofar as the ischemic preconditioning studies suggested that PKCε-mediated translocation was decreased in the hearts of TIRAP-D mice, we next asked whether there was an association between PKCε and TLR2 and TLR4. As shown in Fig. 4D, PKCε was detected primarily in the Western blots from protein extracts that were immunoprecipitated with an anti-TLR2 antibody. Although PKCε was also detected in the Western blots of extracts that were immunoprecipitated with an anti-TLR4 antibody, the amount of PKCε detected was comparatively less. Taken together, these data suggest that the protective cardioprotective effects of ischemic preconditioning are primarily mediated through a TLR2-TIRAP-PKCε-dependent pathway.
Treatment with Pam3CSK4 mimics ischemic preconditioning.
Since the studies above suggested that TLR2 was necessary for ischemic preconditioning, we sought to determine whether a TLR2 agonist, Pam3CSK4, would mimic the effects of ischemic preconditioning in terms of recovery of LV function following I/R injury. In preliminary control experiments (see supplemental data Fig. S1), we observed that there was no significant PKCε translocation in wild-type and TLR2-D hearts at baseline in the absence of Pam3CSK4 treatment. As shown in Fig. 5A, pretreatment with Pam3CSK4 (5 min) resulted in a significant increase (P < 0.05) in the recovery of postischemic contractile performance in the hearts of wild-type mice compared with hearts subjected to I/R injury alone (44.7 ± 2.6% vs. 28.3 ± 1.5%, respectively). The degree of LV recovery following ischemic preconditioning was significantly greater (P < 0.006) than that achieved with pretreatment with Pam3CSK4 (Fig. 5A) alone. Pretreatment with Pam3CSK4 pretreatment did not result in a significant increase in the recovery of postischemic LV recovery (P = 0.38) in the hearts of TLR2D mice compared with vehicle-treated hearts TLR2D mice (34.2 ± 2% vs. 35.5 ± 1.2%, respectively; Fig. 5B). Given that the immunoprecipitation studies suggested that PKCε translocation was TIRAP dependent (Fig. 3, A and B), we also examined the translocation of PKCε in the hearts of wild-type and TLR2D mice after treatment with Pam3CSK4. As shown by the representative Western blot in Fig. 5C and the group data summarized in Fig. 5D, the translocation of PKCε was significantly (P < 0.05) decreased in TLR2D hearts following the administration of Pam3CSK4.
Fig. 5.
Effect of TLR2 stimulation on left ventricular functional recovery following I/R injury in WT and TLR2-deficient mice. A: percent recovery of %LVDP in hearts isolated from WT mice. B: TLR2D mice following I/R injury, IPC + I/R injury, or Pam3CSK4 + I/R injury. *P ≤ 0.05 vs. I/R injury. C: representative Western blots of PKCε translocation in hearts from WT and TLR2D mice treated with Pam3CSK4. Con, control. D: group data of PKCε translocation in hearts from WT and TLR2D mice treated with Pam3CSK4 injury. Data are expressed as the ratio of membrane to cytosolic PKCε (n = 5 hearts/group). *P ≤ 0.05 WT vs. TLR2D.
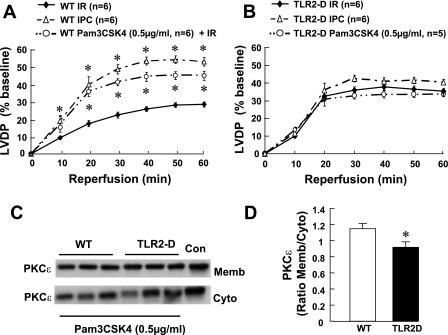
IRAK1 deficiency does not abolish myocardial ischemic preconditioning.
IRAK1 is an important signaling molecule that is downstream of TIRAP-myeloid differentiation factor-88 signaling. IRAK1 has been suggested to play an important role in endotoxin tolerance, which has also been implicated in preconditioning (3). To determine whether IRAK1 signaling contributed to ischemic preconditioning, we subjected wild-type and IRAK1D mouse hearts to ischemic preconditioning followed by I/R injury. Figure 6 shows that loss of IRAK1 signaling did not abrogate ischemic preconditioning. The recovery of LV function following ischemic preconditioning was not significantly different (P > 0.05) in wild-type mice (Fig. 6A) compared with IRAK1D mice (Fig. 6B; 51.6 ± 2.2% vs. 51 ± 3%, respectively). Moreover, the translocation of PKCε following ischemic preconditioning and I/R injury was similar in wild-type and IRAK1D mice (Fig. 6, C and D).
Fig. 6.
Effect of IL-1 receptor-associated kinase-1 deficiency (IRAK1-D) on IPC. A: %LVDP in hearts isolated from WT mice. B: IRAK1-D mice following I/R injury or IPC + I/R injury. C: representative Western blot of PKCε translocation in hearts from WT and IRAK1D mice following IPC + I/R injury. D: group data of PKCε translocation in hearts from WT and IRAK1D mice following IPC + I/R injury. Data are expressed as the ratio of membrane to cytosolic PKCε (n = 3–6 hearts/group). *P < 0.05 compared with I/R + IPC.
TIRAP-dependent ischemic preconditioning involves phosphorylation of GSK3-β.
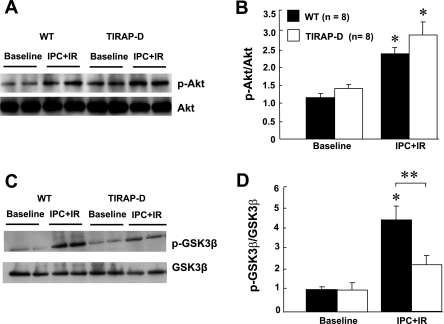
Given the central role of Akt and GSK-3β in mediating ischemic preconditioning, we examined the phosphorylation of Akt and GSK-3β in wild-type and TIRAP-D mice. Figure 7A depicts representative Western blot data for Akt phosphorylation, whereas Fig. 7B summarizes the results of group data. As shown in Fig. 7B, preconditioning followed by I/R injury resulted in a significant (P < 0.05) increase in Akt phosphorylation in both the wild-type and TIRAP-D mice compared with their respective values at baseline. There was, however, no significant difference (P > 0.05) in Akt phosphorylation between the wild-type and TIRAP-D mice that underwent ischemic preconditioning followed by I/R injury, suggesting that the lack of TIRAP signaling does not impair Akt phosphorylation. Figure 7C depicts representative Western blot data for GSK-3β phosphorylation, whereas Fig. 7D summarizes the results of group data. As shown, there was a significant (P < 0.05) increase in GSK-3β phosphorylation in wild-type mice relative to baseline values following ischemic preconditioning and I/R injury. In contrast, the increase in GSK-3β phosphorylation following ischemic preconditioning and I/R injury was not significant (P > 0.05) statistically in the mice lacking TIRAP signaling, relative to baseline values. Importantly, GSK-3β phosphorylation was significantly less (P < 0.05) in the TIRAP-D mice relative to wild-type mice, following preconditioning and I/R injury.
Fig. 7.
Effect of TIRAP deficiency on Akt and GSK3-β phosphorylation. A: representative Western blot of total and phosphorylated (p) Akt in hearts from WT and TIRAP-D mice at baseline and following IPC + I/R injury. B: group data of the ratio of p-Akt to total Akt in hearts from WT and TIRAP-D mice. C: representative Western blot of total and p-GSK3-β in hearts from WT and TIRAP-D mice at baseline and following IPC + I/R injury. D: group data of the ratio of p-GSK3-β to total GSK3-β in hearts from WT and TIRAP-D mice. *P < 0.05 compared with baseline; **P < 0.05 WT vs. TIRAP-D.
DISCUSSION
Here we show for the first time that repetitive injury to the heart, in the form of short bouts of ischemia followed by reperfusion (a.k.a., ischemic preconditioning), confers cytoprotection through TLR2-TIRAP dependent signaling pathways. The following lines of evidence support this statement. First, whereas ischemic preconditioning resulted in a significant 2.1-fold recovery in LV function in wild-type mice (Fig. 2A), ischemic preconditioning had no significant effect on LV functional recovery following I/R injury in mice in lacking TIRAP signaling (Fig. 2B). Moreover, preconditioning resulted in a significant (P < 0.05) 2.0-fold decrease in Evans blue dye uptake and a 1.7-fold decrease in CK release in wild-type mice, whereas preconditioning had no significant (P > 0.05) effect on Evans blue dye uptake or CK release in TIRAP-D mice (Fig. 2, D and E). Second, when we examined the receptors that were coupled to TIRAP-dependent signaling, namely, TLR2 and TLR4, we observed that the cardioprotective effect of ischemic preconditioning was abolished in the TLR2D mice (Fig. 3B), whereas the cardioprotective effect of ischemic preconditioning was intact in the TLR4D mice (Fig. 3D). Furthermore, the TLR2 agonist, Pam3CSK4, mimicked the effects of ischemic preconditioning in wild-type mice (Fig. 5A). Interestingly, the degree of LV recovery following ischemic preconditioning was significantly greater (P < 0.006) than that with Pam3CSK4 (Fig. 5A), suggesting that preconditioning works through TLR2-independent and TLR2-dependent pathways. Pam3CSK4 had no effect on recovery of contractile performance in the hearts of TLR2D mice following I/R injury (Fig. 5B). Taken together, these observations suggest that the protective effect of ischemic preconditioning is mediated, at least in part, through a TLR2-TIRAP-dependent pathway.
To delineate the mechanisms that were responsible for the cytoprotective effects of TLR2-TIRAP-dependent signaling, we measured the translocation of PKCε in hearts of wild-type and TIRAP-D mice following ischemic preconditioning. Consistent with the functional recovery data in wild-type (Fig. 2A) and TIRAP-D mice (Fig. 2B), PKCε translocation was significantly (P < 0.05) greater in the wild-type hearts than in the TIRAP-D hearts (Fig. 4B). Immunoprecipitation studies showed that PKCε was readily detected in the Western blots from protein extracts that were immunoprecipitated with an anti-TLR2 antibody, whereas PKCε was barely detectable in the extracts immunoprecipitated with an anti-TLR4 antibody. To determine whether the activation and subsequent translocation of PKCε occurred proximal or distal to the phosphorylation of IRAK1, we examined ischemic preconditioning in IRAK1-deficient mice. Interestingly, the loss of IRAK1 signaling did not abrogate ischemic preconditioning (Fig. 6B) or attenuate PKCε translocation (Fig. 6D). Taken together, these observations suggest that the signaling protein complex that is necessary for PKCε activation/translocation is assembled proximal to IRAK-1.
Given that Akt and of GSK-3β have been implicated in the ischemic preconditioning signaling cascade (41), we examined Akt and GSK-3β phosphorylation in the hearts of wild-type and TIRAP-D mice. Although we observed a significant increase in both Akt and GSK-3β phosphorylation in wild-type and TIRAP-D mice following ischemic preconditioning and I/R injury (Fig. 7B), there was no significant difference in the degree of Akt phosphorylation between wild-type and TIRAP-D mice, suggesting that Akt phosphorylation was not TIRAP dependent. However, the phosphorylation of GSK-3β was significantly attenuated in the TIRAP-D mice following ischemic preconditioning and I/R injury (Fig. 7D), suggesting that the mechanism for TIRAP-mediated cytoprotection may involve, at least in part, an inhibition of the formation of mitochondrial permeability transition pores that develop following reperfusion injury.
Innate immunity in the heart.
Since the original description of TLRs in the heart (12), there has been a growing appreciation that these receptors play an important role in modulating tissue injury in the heart (6, 14, 32, 39). For example, mice with targeted disruption of TLR4 (32), TLR2 (9), or myeloid differentiation factor 88 (10), as well as mice with a missense mutation of TLR4 (6, 24), have reduced infarct sizes compared with wild-type controls. Similarly, mice pretreated with a TLR4 antagonist (Eritoran) (35) had smaller infarct sizes compared with vehicle-treated animals. Furthermore, mortality and postinfarction LV remodeling was reduced in mice with targeted disruption of TLR4 or TLR2 (33, 36). Although the mechanism(s) for the deleterious effects of TLR signaling following I/R injury and/or myocardial infarction has not been established, a recent study suggests that TLRs may serve to modulate the recruitment of bone marrow-derived hematopoietic cells to the myocardium, with subsequent tissue damage and worsening cardiac remodeling (38). Moreover, our data suggest that TLR2-TIRAP signaling may play a deleterious role in the heart in the absence of preconditioning by amplifying the degree of LV dysfunction (Fig. 3, A and B) when the heart is subjected to the abrupt onset of ischemic injury.
In contrast to the aforementioned studies, which suggest a deleterious role for TLR signaling, there is a large body of literature that suggests that the systemic activation of TLR4 by sublethal injections of lipopolysaccharide (LPS) results in a reduced infarct size and an improved LV function in mice, rats, and rabbits (reviewed in Ref. 5). The cytoprotective effect of LPS, which occurs between 12–24 h, was sensitive to the inhibition with cycloheximide (28), suggesting that de novo protein synthesis was required for the “late” LPS-induced preconditioning. Subsequent studies have suggested that the beneficial effects of LPS-TLR4 preconditioning are mediated by inducible nitric oxide synthase (42) and the phosphatidylinositol 3 kinase/Akt pathway (15).
Viewed within the context of the above discussion, the results of the present study both expand and extend these important prior studies by demonstrating that the activation of TLR2-TIRAP-dependent signaling by repetitive bouts of ischemia and reperfusion confers “early” cytoprotection. The observation that TLR2-TIRAP cytoprotective pathway involves the translocation of PKC is in keeping with the “multiple trigger theory” for preconditioning, which suggests that all triggers converge on a common target: PKC (13). Although we did not identify the mechanism for PKCε activation, it is worth noting that a recent study showed that stimulation TLRs by their cognate ligands result in the recruitment of PKCε to the TLR signaling complex, resulting in an increased phosphorylation of PKCε and an increased binding of 14-3-3β (8).
Although, we did not identify the ligand(s) that was responsible for activating TLR2-TIRAP-dependent preconditioning, it is worth noting that so-called danger-associated molecular patterns that are released by stressed cells (e.g., heat shock protein 60 and 70, HMGB1, S100, adenosine, and ATP) or injured tissue (fibronectin) have been shown to activate TLR2 and TLR4 signaling with a resultant activation of NF-κB, which has been implicated in cytoprotective signaling (1, 30). Recent evidence suggests that reactive oxygen intermediates are sufficient to activate TLRs through an as-yet unknown mechanism. Indeed, oxidative stress has been shown to activate NF-κB via TLR2 in neonatal rat cardiac myocytes, suggesting that TLRs may be capable of sensing the reactive oxygen intermediates generated by repetitive bouts of ischemia followed by reperfusion (11). One potential limitation of the present study is that it was performed ex vivo, which allowed us to uncouple the innate and adaptive components of the immune system following I/R injury. Accordingly, we cannot exclude the formal possibility that the beneficial effects of TLR2-TIRAP-dependent preconditioning might be less evident in vivo, wherein the effects on innate immunity on leukocyte recruitment to the myocardium might result in excessive tissue injury that outweighs the beneficial effects conferred by TLR2-TIRAP cytoprotective signaling.
Conclusion.
The results of this study suggest that TLR2-TIRAP-dependent signaling comprises part of an evolutionarily conserved “early warning system” in the heart that is activated in response to tissue injury. When viewed within the broader context of prior studies, the findings presented herein suggest that the innate immune system confers short-term beneficial effects in the heart by activating cytoprotective signaling pathways but also has the capacity to induce long-term detrimental effects if sustained TLR signaling engages the adaptive immune system, with a subsequent recruitment of leukocytes to sites of tissue injury (31). A second interesting aspect of this study is that the cytoprotective arm of TLR2-TIRAP-dependent signaling was upstream of IRAK1, which is the responsible for NF-κB activation and subsequent recruitment of leukocytes to the heart. This observation suggests the interesting possibility that it may be possible to develop therapeutic strategies that activate the proximal “cytoprotective” arm of TLR2-TIRAP-dependent signaling without engaging the distal and potentially maladaptive “inflammatory” arm of this signaling cascade. Future studies will be necessary to address this interesting, if not important, question.
GRANTS
This research was supported by National Institutes of Health Grants HL-58081, HL-42250, HL-073017, GM-62474, and HL-083426.
DISCLOSURES
The authors have no conflicts of interest to disclose.
Supplementary Material
REFERENCES
- 1.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6: 435–442, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal 17: 385–394, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Belosjorow S, Schulz R, Dorge H, Schade FU, Heusch G. Endotoxin and ischemic preconditioning: TNF-α concentration and myocardial infarct development in rabbits. Am J Physiol Heart Circ Physiol 277: H2470–H2475, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum Y, Hale SL, Kloner RA. Differences in reperfusion length following 30 minutes of ischemia in the rabbit influence infarct size, as measured by triphenyltetrazolium chloride staining. J Mol Cell Cardiol 29: 657–666, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Chao W. Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Radu C, Diab A, Tsen MF, Hussain R, Cowdery JS, Racke MK, Thomas JA. IL-1 receptor-associated kinase 1 regulates susceptibility to organ-specific autoimmunity. J Immunol 170: 2833–2842, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Faisal A, Saurin A, Gregory B, Foxwell B, Parker PJ. The scaffold MyD88 acts to couple protein kinase Cepsilon to Toll-like receptors. J Biol Chem 283: 18591–18600, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol 27: 1064–1071, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U, Chao W. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol 295: H1311–H1318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J Biol Chem 276: 5197–5203, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res 77: 611–621, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb RA, Kitsis RN. Seeing death in the living. Nat Med 7: 1277–1278, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res 78: 546–553, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200: 69–79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann JA, Kafatos FC, Janeway CA, Jr, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science 284: 1313–1318, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420: 329–333, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol 2: 835–841, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. J Neuroimmunol 199: 75–82, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, Demarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR. High-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol 176: 7154–7158, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kalb A, Bluethmann H, Moore MW, Lesslauer W. Tumor necrosis factor receptors (Tnfr) in mouse fibroblasts deficient in Tnfr1 or Tnfr2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J Biol Chem 271: 28097–28104, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Kim DC, Kim SH, Jeong MW, Baek NI, Kim KT. Effect of rottlerin, a PKC-delta inhibitor, on TLR-4-dependent activation of murine microglia. Biochem Biophys Res Commun 337: 110–115, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, Meyer R, Grohe C, Knowlton AA, Baumgarten G. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol 7: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mann DL. Tumor necrosis factor and viral myocarditis: the fine line between innate and inappropriate immune responses in the heart. Circulation 103: 626–629, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell 91: 295–298, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Meng X, Ao L, Brown JM, Meldrum DR, Sheridan BC, Cain BS, Banerjee A, Harken AH. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-α. Am J Physiol Heart Circ Physiol 273: H1894–H1902, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Miki T, Cohen MV, Downey JM. Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem 186: 3–12, 1998 [PubMed] [Google Scholar]
- 30.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17: 359–365, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D, Pauschinger M, Schultheiss HP, Tschope C. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol 180: 6954–6961, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H503–H509, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 114: I270–I274, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108: 2905–2910, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res 95: 700–707, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Timmers L, Sluijter JP, Van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-Like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function following myocardial infarction. Circ Res 102: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.