Abstract
Neutrophils (PMNs) are a vital part of host defense and are the principal leukocyte in innate immunity. Interleukin (IL)-18 is a proinflammatory cytokine with roles in both innate and adaptive immunity. We hypothesize that PMNs contain preformed IL-18, which is released in response to specific inflammatory stimuli. Isolated PMNs were stimulated with a battery of chemoattractants (5 min to 24 h), and IL-18 release was measured. PMNs were also separated into subcellular fractions and immunoblotted with antibodies against IL-18 or were fixed and probed with antibodies to IL-18 as well as to the contents of granules, intracellular organelles, and filamentous actin (F-actin), incubated with fluorescent secondary antibodies, and examined by digital microscopy. Quiescent PMNs contained IL-18 in the cytoplasm, associated with F-actin, as determined by positive fluorescence resonance energy transfer (FRET+). In turn, TNF-α stimulation disrupted the association of IL-18 with F-actin, induced a FRET+ interaction of IL-18 with lipid rafts, and elicited IL-18 release. Manipulation of F-actin status confirmed the relationship between IL-18 and F-actin in resting PMNs. Consequently, incubation with monomeric IL-18 binding protein inhibited TNF-α-mediated priming of the PMN oxidase. We conclude that human PMNs contain IL-18 associated with F-actin in the cytoplasm and TNF-α stimulation causes dissociation of IL-18 from F-actin, association with lipid rafts, and extracellular release. Extracellular IL-18 participates in TNF-α priming of the PMN oxidase as demonstrated by inhibition with the IL-18 binding protein.
Keywords: F-actin, fluorescent resonance energy transfer, lipid rafts
neutrophils (PMNs) are a vital part of host defense, especially against bacterial and fungal pathogens (43). Normal PMN physiology requires the emigration of PMNs from the vasculature to the tissues, and this process involves adhesion of the PMN to the vascular endothelium, also known as priming, before exiting the vessel (1, 12, 27, 55, 62). In the tissues, PMNs phagocytize and eradicate microbial invaders through both oxidative and nonoxidative mechanisms (1, 12, 27, 55, 62). In response to physiological stress, PMNs synthesize and release various proinflammatory mediators, including interleukin (IL)-8 and other effective proinflammatory molecules (13).
Tumor necrosis factor (TNF)-α is a pleiotropic cytokine that produces varied effects including proinflammatory regulation of the immune system, cellular proliferation and differentiation, and cellular death through necrosis and apoptosis (4, 6, 42, 65). Mainly produced by activated macrophages, TNF-α is the ligand for two specific receptors, TNF receptor (TNFR)-1 and TNFR-2, which are expressed on a number of cell types including vascular endothelium, lymphocytes, monocytes/macrophages, and PMNs (4, 42). TNF-α is a known PMN priming agent, because it induces adhesion and chemotaxis, and augments the release of the microbicidal arsenal in response to a subsequent stimulus (24, 33).
Formerly known as interferon-γ producing factor, IL-18 is a proinflammatory cytokine that plays important roles in both adaptive and innate immunity, including the activation of natural killer cells and cytotoxic T cells, as well as directly affecting PMN function (20, 21, 50, 62). A number of cell types have been reported to produce IL-18, including monocytes and macrophages, epithelial cells, astrocytes, and microglia (11, 17, 20, 50). Similar to IL-1β, IL-18 may be released under conditions of physiological stress, and increased plasma levels, which may be found in patients with infection, correlate with disease severity (18, 31, 63). Because of its described proinflammatory effects, IL-18 may act as a bridging molecule that coordinates both innate and adaptive immunity to more effectively respond to invasive infection and inflammation (21, 31, 41, 62). Therefore, because of its effects on innate immunity and its synthesis by leukocytes, we hypothesize that PMNs contain IL-18 and release it in response to specific proinflammatory stimuli.
MATERIALS AND METHODS
Materials.
All chemicals, unless otherwise specified, were purchased from Sigma (St. Louis, MO). All solutions and buffers were made from sterile water for human injection, United States Pharmacopeia (USP), or sterile 0.9% saline for intravenous administration, USP, purchased from Baxter Healthcare (Deerfield, NY) as reported previously, followed by sterile filtering with Nalgene MF75 series disposable sterilization filter units purchased from Fisher Scientific (Pittsburgh, PA) (62). Images were acquired with a Leica DRM mechanized fluorescence microscope equipped with a movable stage (Leica Microsystems, Exton, PA) and four epifluorescence cubes (Cy-3, Cy-5, FITC, and AMCA) with dichroic filters (Chroma Technology, Brattleboro, VT). The microscope and a cooled charge-coupled device (CCD) camera (Cooke, Tonawanda, NY) were controlled by Slidebook (Intelligent Imaging Innovations, Denver, CO). A polyclonal antibody to IL-18 from rabbit whole sera was obtained as described previously (34), and this polyclonal antibody recognizes both the pro-IL-18 polypeptide and active, cleaved IL-18 (34). In addition, two different commercial antibodies to active, cleaved IL-18 and two different antibodies to pro-IL-18 were purchased from R&D Systems (Minneapolis, MN). Antibodies to the phosphorylated (S 315) 40-kDa phagocyte oxidase protein (p-p40phox), p47phox, p67phox, Cdc42, and the β-coatomer protein-1 (βCOP-1) were obtained from Santa Cruz (Santa Cruz, CA). A polyclonal antibody to βCOP-1 also was the kind gift of Dr. Katherine Howell (Department of Cell and Ultrastructural Biology, University of Colorado, Boulder, CO), and the antibody to lysosome-associated membrane glycoprotein-1 (LAMP-1) was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). An antibody to F-actin and wheat germ agglutinin (WGA) linked to Alexa 488 and all secondary antibodies were purchased from Abcam (Cambridge, MA) and Invitrogen/Molecular Probes (Eugene, OR), respectively. All other antibodies to intracellular granule proteins and lysosomes were purchased from Accurate Chemical (Westbury, NY). Jasplakinolide and latrunculin B were obtained from Calbiochem (La Jolla, CA) and Biomol (Exton, PA), respectively. A Vybrant lipid raft labeling kit was purchased from Molecular Probes (Eugene, OR).
Labeling of primary antibodies.
Antibodies were fluorescently labeled with a previously published protocol (45). The labeling efficiency was from 70% to 95% as determined by spectrofluorimetry at UV wavelengths depending upon the fluorophore conjugated per the Molecular Probes Protein Labeling Kit instructions (45).
PMN isolation.
PMNs were isolated from whole blood drawn after informed consent from healthy donors under a protocol approved by the Colorado Multiple Institutional Review Board at the University of Colorado at Denver School of Medicine. The isolation employed standard techniques including dextran sedimentation, Ficoll-Hypaque gradient centrifugation, and hypotonic lysis of contaminating red blood cells (62). Subcellular fractionations were performed on PMNs that were sonicated (30% of maximal power) at 4°C in the presence of 100 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mg/ml leupeptin or disrupted by nitrogen cavitation for 30 min at 4°C with the addition of 2 μM di-isopropyl fluorophosphates to inhibit proteolysis, the nucleus was removed by centrifugation, and the plasma membrane, cytosol, and granule fractions were separated by discontinuous sucrose gradient centrifugation, as previously described (2).
Digital microscopy.
Isolated PMNs were incubated with buffer or 1–10 ng/ml TNF-α for 1–10 min, fixed with 4% paraformaldehyde, and smeared onto slides. For selected experiments, PMNs were pretreated for 5 min with 0.1% DMSO control, 10 μM jasplakinolide, or 5 μM latrunculin B before fixation. All manipulations, unless indicated, were at room temperature, and digital microscopy was performed as previously described, including staining of the nuclei with bis-benzimide (blue) and localization of the membrane employing WGA tagged with Alexa 488 (green) (53). Lipid rafts were visualized with a Vybrant lipid raft labeling kit. Briefly, PMNs were incubated with cholera toxin subunit B (CT-B) conjugated to Alexa 555 at a concentration of 1 μg/ml for 10 min at 4°C. The cells were then washed twice in PBS (pH 7.0) at 4°C and permeabilized with acetone:methanol, and then the CT-B was cross-linked to the lipid rafts with the antibody at a concentration of 5 mg/ml for 15 min at 4°C. The cells were fixed, smeared onto slides, and examined by digital microscopy.
Images were acquired with a Zeiss Axiovert fitted with a Cooke CCD SensiCam using a Chroma Sedat Multiple Bandpass filter wheel and Sutter filter control using Intelligent Imaging Inovations Slidebook software, and images compared within a single figure were acquired as Z stacks in 0.2-μm intervals, with the planes themselves described within the figure legends as described previously (45, 46). All Z-stack images were deconvolved by applying constrained iterative deconvolution and Gaussian noise smoothing from system-specific point spread functions. After deconvolution, images were cropped to represent the middlemost planes (center ± 10 planes) and the proteins in question were masked to represent zero fluorescence in IgG negative controls.
Fluorescence resonance energy transfer.
Briefly, fixed, permeabilized PMNs were incubated with serum of the species of the secondary antibodies for 1 h and then incubated with primary antibodies, isotypic controls, or buffer overnight at 4°C. After extensive washing, the PMNs were incubated with fluorescently labeled secondary antibodies for 1 h at room temperature and washed. To negate nonspecific binding of the primary or secondary antibodies, the highest fluorescence emitted from PMNs treated with buffer or with isotypic primary antibody controls followed by incubations with fluorescently labeled secondary antibodies, using all fluorochromes for each experiment, was used as the zero value for fluorescence detection. Therefore, all fluorescence from nonspecific antibody binding was negated. After a digital image of the fixed PMNs is taken, the lower-energy, acceptor, fluorochrome is bleached to <10% of the original intensity. A second image is then taken and the higher-energy, donor, fluorochrome channel is overlaid, pixel by pixel, on the first image. Fluorescence resonance energy transfer (FRET) positive (FRET+) pixels are those in which an increase in intensity of the donor can be calculated after bleaching of the acceptor (45, 46). The fluorescence emitted by a donor dye may only be absorbed and reemitted provided that the two fluorochrome dipoles fulfill the Forster criteria of spectral overlap, orientation, and distance (see Refs. 45, 46). Since energy transfer decreases as the sixth power of radius, the maximal distance between two proteins of interest for a positive FRET with labeled secondary antibodies is <30 nm, when one takes into account the freedom of movement coefficient of (52). As further controls we performed experiments with known cytosolic proteins that bind to one another, Rab5a and its dissociation inhibitor RabGDP dissociation inhibitor (RabGDI), as well as phospho-p40phox and Gαi-1, two proteins that are not known to demonstrate a physical association (57). In the case of Rab5a and the RabGDI, both primary and secondary antibodies were labeled with the identical acceptor:donor fluorochromes and FRET analyses were performed as described previously (45, 46). Quantification of cellular pixels or voxels of IL-18 or of the FRET+ interactions between F-actin + IL-18 or lipid rafts + IL-18 was performed as previously described (45, 46).
Release of IL-18 from isolated PMNs.
PMNs (1.25 × 106 at a density of 2.5 × 107 PMNs/ml) were warmed to 37°C in a shaking water bath or, in selected experiments, pretreated with 5 μM cytochalasin B or DMSO (control), and stimulated with buffer, 2 μM platelet-activating factor (PAF), 1 μM N-formylmethionyl-leucyl-phenylalanine (fMLP), or 200 ng/ml phorbol 12-myristate 13-acetate (PMA) for 5 min at 37°C. Additionally, PMNs primed with PAF for 3 min followed by fMLP for 5 min were also assayed for IL-18 release. Separate experiments examined incubations of isolated PMNs from 1 to 24 h with 0.2–2 μg/ml LPS and stimulation of PMNs with 1–10 ng/ml TNF-α for 1–15 min. After incubation, the PMNs were pelleted and the supernatants removed. IL-18 concentration was measured in the supernatants by commercial ELISA or by bead capture assay, both of which employed antibodies specific for the cleaved form of IL-18 (22).
Colocalization of IL-18 with F-actin.
IL-18 was immunoprecipitated from resting PMNs as described previously (32). Briefly, 5 × 107 PMNs were placed into ice-cold relaxation buffer (in mM: 10 PIPES, pH 7.0, 3 NaCl, 100 KCl, and 3.5 MgCl2 with a protease inhibitor mix: 40 mM sodium orthovanadate, 1 M nitrophenyl phosphate, and 100 μM PMSF) and sonicated for 30 s at 30% of maximal power. The whole cell lysates were incubated at 4°C overnight with 40 μg of anti-IL-18 agarose conjugate, agarose alone, isotypic antibody-agarose conjugates, or anti-p67phox-agarose conjugates. The lysates were centrifuged at 10,000 g for 5 min, the supernatant was removed, and the pellet was washed three times with relaxation buffer. After the final wash, the pellet was resuspended in 70 μl of SDS-digestion buffer with 10 μl of protease inhibitor mix, and the proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and immunoblotted with a monoclonal antibody to F-actin.
PMN priming assays.
Isolated PMNs were preincubated with buffer or 500 ng/ml of monomeric IL-18 binding protein for 5 min at 37°C. After this preincubation these PMNs were primed with buffer or 10 ng/ml of TNF-α for 15 min at 37°C and activated with 1 μM fMLP, and the maximal rate of superoxide dismutase-inhibitable superoxide anion production was measured as the reduction of cytochrome c at 550 nm as previously described (62).
Statistics.
Statistical differences among groups were determined by a paired or an independent analysis of variance (ANOVA) followed by either a Bonferroni or a Newman-Keuls post hoc test for multiple comparisons depending upon the equality of variance. Statistical significance was determined at the P < 0.05 level.
RESULTS
PMNs contain IL-18, and TNF-α causes its release.
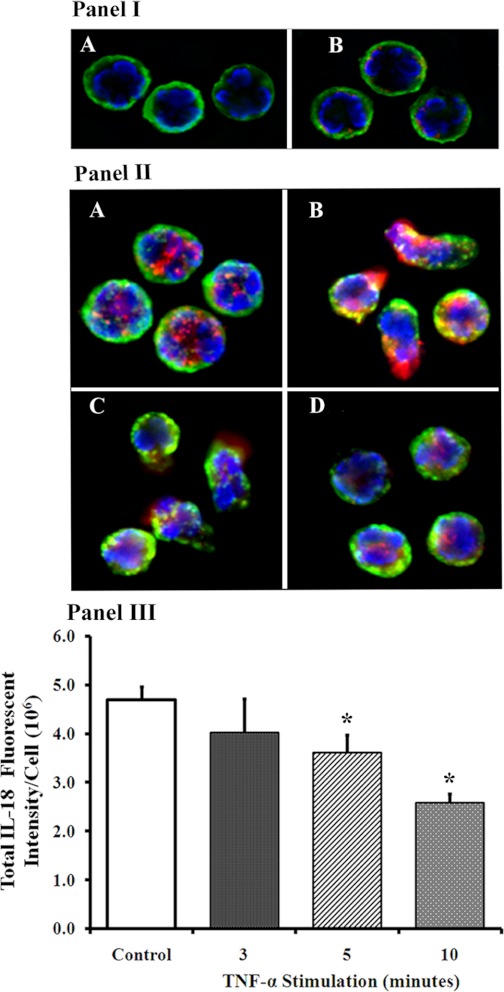
Buffer- or TNF-α-treated PMNs (10 ng/ml for 1–10 min) were incubated with an antibody to IL-18, the nucleus was stained with bis-benzimide (blue), the plasma membrane was localized by WGA conjugated to Alexa 488 (green), and these PMNs were analyzed by digital microscopy (Fig. 1). The negative controls for these images are shown in Fig. 1I, A and B, and demonstrate the staining of the nucleus by bis-benzamide and of the membrane by WGA conjugated to Alexa 488, because WGA binds to the sialoproteins in the membrane (40, 61). Moreover, the PMNs in Fig. 1I, A and B, were both incubated with isotypic primary antibody controls for the IL-18 antibodies, but only the PMNs in Fig. 1IB were incubated with fluorescently labeled secondary antibodies. The faint red color observed in Fig. 1IB, which is due to nonspecific binding of the labeled secondary, is used as the baseline fluorescence such that all of the fluorescence acquired must be greater than that of this negative control.
Fig. 1.
Interleukin (IL)-18 is present in neutrophils (PMNs) and is released by tumor necrosis factor (TNF)-α incubation. I: representative images of the negative controls used for these studies. A: fixed and permeabilized PMNs that have been incubated with bis-benzimide, which stains the nucleus (blue), wheat germ agglutinin (WGA)-Alexa 488, which binds to the sialoproteins in the membrane and renders a green color, and the isotypic control primary antibodies. B: background red fluorescence as the result of incubation with bis-benzimide, WGA, an isotypic control antibody, and a fluorescently labeled (Cy3) secondary antibody. This background fluorescence is used as the baseline such that no nonspecific red fluorescence is acquired; thus the most intense red color acquired is used as the zero value for the next set of images in B. II: isolated PMNs were treated with buffer (controls) or 10 ng/ml of TNF-α for 3–10 min at 37°C and fixed, smeared onto slides, and permeabilized with acetone:methanol. The membranes were localized with WGA linked to Alexa 488 and the nucleus with bis-benzimide. IL-18 immunoreactivity is red and appears to be in the cytosol or possibly in the granule fraction in the controls (A). TNF-α treatment results in release of IL-18 at 3 min from cellular projections with the appearance of pseudopodia (B), with some extracellular IL-18 still present at 5 min (C). At 10 min (D) the PMNs have morphology similar to the quiescent controls but with decreased amounts of intracellular IL-18. III: quantification of the pixels/cell of IL-18 immunoreactivity for each of the conditions in II. *Statistical significance from the buffer-treated controls (P < 0.05). Figure, including all panels, is representative of 3 identical experiments, which used 10 cells/treatment from these 3 different donors.
PMNs contained IL-18 immunoreactivity that was punctate in appearance, and this immunoreactivity was found with the use of two distinct antibodies against IL-18 (results not shown) (Fig. 1IIA). Within 3 min TNF-α elicited an apparent increase (2.1 ± 0.2-fold) in IL-18-immunoreactive fluorescent intensity in newly formed cellular projections, presumably pseudopodia, compared with buffer-treated PMNs (Fig. 1IIA control PMNs vs. Fig. 1IIB PMNs treated with TNF-α for 3 min). This increase was transient, because the majority of PMNs demonstrated TNF-α-mediated release of IL-18 immunoreactivity into the extracellular environment as visualized by a diffuse red glow on the outside of the PMNs, although the cellular IL-18 immunoreactivity was still visible in the pseudopodia (Fig. 1II, B and C). At 10 min, the PMNs had virtually regained their “resting” morphology and IL-18 immunoreactivity was still present in the cytosol but diminished visually (Fig. 1IID) compared with controls (Fig. 1IA). These data were confirmed through quantification of the IL-18 pixels/cell as shown in Fig. 1III. The control had the most IL-18 immunoreactivity that significantly decreased with TNF-α stimulation such that the intracellular amounts were less beginning at 3 min and reaching the lowest levels at 10 min (Fig. 1III).
Because PMNs release serine proteases and other proteins in response to inflammatory stimuli, we investigated IL-18 release by a number of proinflammatory mediators including the effects of cytochalasin B on the release of granule constituents. IL-18 release into the supernatant was quantified by both commercial ELISA and bead capture assay, and these two different methods used disparate antibodies that recognize different epitopes on the polypeptide (22). Unlike other mediators, TNF-α (10 ng/ml) caused rapid release of IL-18 into the supernatant that was statistically different from buffer-treated controls at 3 min (Table 1) but returned to baseline levels at 10 min (92 ± 14 pg/ml). Conversely, the chemoattractants fMLP (1 μM), PAF (2 μM), and PMA (200 ng/ml) did not cause significant release of IL-18 from PMNs after a 5-min activation period compared with controls (results not shown). Furthermore, PAF-primed PMNs (5 min) activated with fMLP (5 min) did not elicit IL-18 release compared with buffer-treated controls. In addition, cytochalasin B pretreatment had no effect on control cell release of granule proteins or IL-18 and was able to augment the release of elastase (2.3 ± 0.3-fold) and lactoferrin (2.1 ± 0.4-fold), but not IL-18, compared with DMSO-pretreated controls (results not shown). PMNs were then incubated with endotoxin (LPS; 0.2–2 μg/ml) or buffer for 1, 12, and 24 h and assayed for IL-18 release into the supernatant. Compared with controls, LPS did not cause release of IL-18 at any concentration or incubation time employed (results not shown).
Table 1.
TNF-α-mediated IL-18 release from human PMNs
| Agonist | Time, min | IL-18 in Supernatant, pg/ml |
|---|---|---|
| Buffer | 3 | 89.4 ± 16 |
| TNF-α (1 ng/ml) | 1 | 136.1 ± 21.5 |
| 3 | 120.8 ± 20.3 | |
| TNF-α (10 ng/ml) | 1 | 128.0 ± 36.0 |
| 3 | 158.8 ± 32.7* |
Data represent means ± SE for 5 separate experiments. IL-18, interleukin-18. Isolated human neutrophils (PMNs; 1.25 × 106, at a density of 2.5 × 107/ml) were treated with buffer or tumor necrosis factor (TNF)-α at 37°C with constant agitation. Statistical differences among groups were determined by a paired analysis of variance (ANOVA) followed by a Bonferroni post hoc test for multiple comparisons.
Statistical significance (P < 0.05) vs. buffer-treated control PMNs.
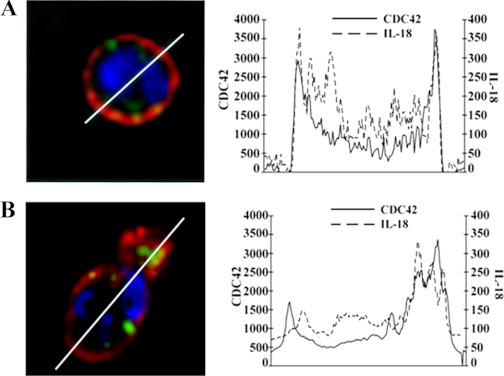
To further characterize the pseudopodia from which IL-18 was visually released we investigated the presence of the small GTP-binding protein Cdc42 in these TNF-α-induced projections. In controls Cdc42 (red) and IL-18 (green) did not evidence high areas of colocalization (lack of yellow color) for IL-18 residing in the cytoplasm, whereas Cdc42 demonstrated primacy in the plasma membrane (Fig. 2A). Compared with control PMNs, TNF-α (10 ng/ml) stimulation at 3 min (Fig. 2B) increased the membrane-associated Cdc42 (red) in the pseudopodia where IL-18 (green) localized before release (Fig. 2).
Fig. 2.
TNF-α mediated IL-18 release from pseudopodia. Isolated PMNs were fixed, smeared onto slides, and incubated with primary antibodies to IL-18 and Cdc42 and with bis-benzimide, a nuclear stain (blue), followed by exposure to fluorochrome-labeled secondary antibodies, such that IL-18 is green and the Rho family GTPase Cdc42 is red (left). A: resting PMN. B: PMN that has been treated with TNF-α (10 ng/ml) for 3 min. Diameters are drawn under the control of Slidebook software and compare similar diameters in resting PMNs and in TNF-α-stimulated PMNs, with the latter line drawn through the cellular projection (B). Graphic representation of the amount of immunoreactivity (pixels) is shown for both IL-18 and Cdc42 on right. The resting (control) PMNs demonstrate that the IL-18 reactivity resides in the cytosol and Cdc42 is in the periphery. With TNF-α stimulation the PMNs rapidly change shape and the IL-18 immunoreactivity moves to the periphery with Cdc42 (B). The largest amount area under the curve is found in the pseudopodia for both IL-18 and Cdc42, which coincides with polarization of the PMNs (B), and these cellular projections contain increased amounts of Cdc42 immunoreactivity (B). In addition, these cellular projections are in the locality of IL-18 release and have the appearance of pseudopodia (see Fig. 1). Figure is representative of 3 separate experiments.
Subcellular localization of IL-18.
Because of the punctate appearance of the IL-18 immunoreactivity and the capacity of PMNs to store many proteases, adhesion molecules, and proteins within their granules (7), we explored the subcellular location of IL-18 employing antibodies to known granule proteins: lactoferrin, myeloperoxidase, alkaline phosphatase, and gelatinase [matrix metallopeptidase (MMP)-9], as well as to other subcellular structures including LAMP-1, a component of human lysosomes, and βCOP-1, a component of the human Golgi apparatus (7, 15, 28). These studies did not demonstrate significant amounts of IL-18 colocalization (<2%) with any of these proteins, indicating that IL-18 does not reside in the azurophilic (primary), specific (secondary), gelatinase-containing granules or phosphosomes (data not shown). Moreover, IL-18 does not appear to be concentrated in the Golgi apparatus, as demonstrated by the lack of colocalization with βCOP-1, or in lysosomal structures because of a lack of colocalization with LAMP-1 (data not shown).
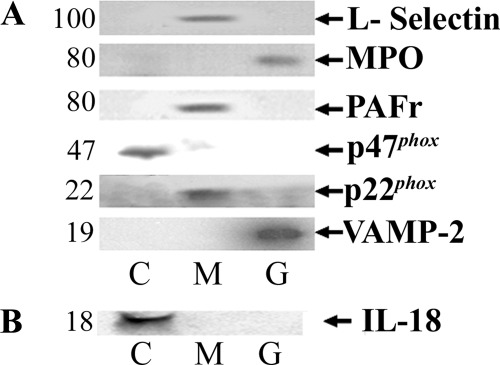
Unable to localize IL-18 to the granules, lysosomes, endoplasmic reticulum, or the Golgi apparatus, we divided 108 PMNs into subcellular fractions, separated the proteins by SDS-polyacrylamide gel electrophoresis, transferred them to a nitrocellulose membrane, and probed them with a monoclonal antibody to IL-18 (2). Two separate methods of cellular lysis were employed, nitrogen cavitation and sonication, which demonstrated identical results for the subcellular restriction of soluble granule protein, elastase to the granules, and markers of the Golgi apparatus (βCOP-1) and endoplasmic reticulum (calnexin) to the nuclear fraction without nonspecific “leak” to other subcellular compartments (data not shown). In addition, control PMNs documented that only the membrane fraction contained the PAF receptor and L-selectin immunoreactivity, two known membrane-restricted proteins (Refs. 8, 47, 48; data not shown), the cytosolic fraction of resting PMNs only demonstrated positivity for p47phox, and the granule fraction had immunoreactivity to myeloperoxidase (MPO) and vesicle-associated membrane protein (VAMP)-2, identical to previous results (Fig. 3A) (7, 16, 47, 48). Thus these results demonstrate that the employed method of sonication was not different from nitrogen cavitation and did not cause nonspecific release of IL-18 into the cytoplasm from either granules or intracellular organelles. IL-18 immunoreactivity was present in the cytosol but could not be detected in the nuclear, membrane, or granule fractions (Fig. 3B).
Fig. 3.
IL-18 immunoreactivity in subcellular fractions from isolated, resting human PMNs. A: immunoreactivity of a number of proteins in discrete subcellular fractions from whole cells that were sonicated. All of these control proteins are restricted to 1 subcellular fraction except for p22phox, which has a small amount of reactivity in the specific granules. C, cytosolic fraction; M, membrane fraction; G, granule fraction; MPO, myeloperoxidase; PAFr, platelet-activating factor receptor; p47phox, 47-kDa cytosolic phagocyte oxidase protein; p22phox, 22-kDa cytosolic phagocyte oxidase protein; VAMP-2, vesicle-associated membrane protein-2. In B, IL-18 immunoreactivity is restricted to the cytosolic fraction. Immunoblots are representative of 3 separate experiments; B employed 2 dissimilar antibodies to IL-18 and yielded identical results.
FRET analysis of IL-18 and F-actin.
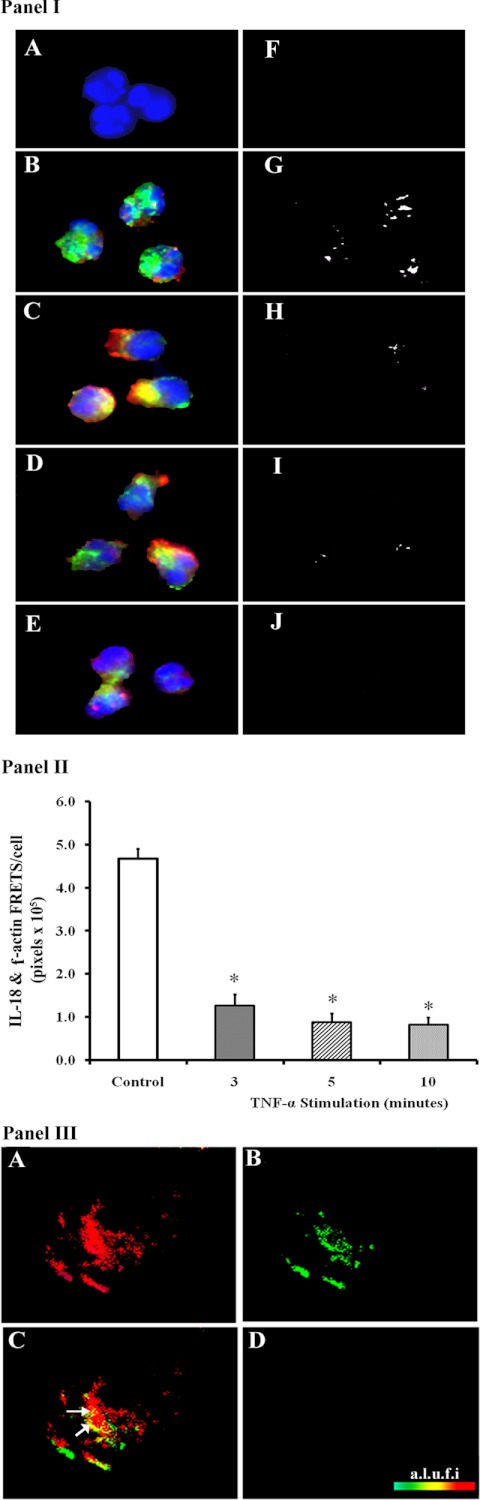
IL-18 immunoreactivity (red) demonstrated colocalization (yellow) with F-actin (green) in control PMNs (Fig. 4IB) and demonstrated a FRET+ with an efficiency of 34 ± 4% (Fig. 4IG). IL-18 did not demonstrate FRET positivity with F-actin along the cell periphery, the region rich in cortical F-actin (Fig. 4IG). TNF-α (10 ng/ml) at 3 min caused a decrease in cytosolic IL-18 (loss of red color) (Fig. 4IC) with a concomitant increase in IL-18 immunoreactivity in the pseudopodia and a decrease in the FRET+ interaction between IL-18 and F-actin (Fig. 4IH). At 5 min of TNF-α stimulation (Fig. 4ID) the amount of intracellular IL-18 immunoreactivity at the cell periphery is less than the immunoreactivity at 3 min (Fig. 4IC), and becomes even further decreased at 10 min. Moreover, the FRET+ association between F-actin and IL-18 is further diminished at 5 min of TNF-α stimulation compared with controls and completely disappears at 10 min (Fig. 4, I, I and J). These data were quantified as the amount of FRET+ (IL-18 and F-actin) pixels/cell (Fig. 4II). The untreated control PMNs contained the most FRET+ pixels, and the amount of intracellular IL-18 steadily diminished, with TNF-α stimulation becoming significant at 3 min of stimulation and remaining diminished through 10 min (Fig. 4II).
Fig. 4.
Association of IL-18 with F-actin by fluorescence resonance energy transfer (FRET). I: control (buffer) and TNF-α-treated PMNs were fixed and incubated with primary antibodies to F-actin and IL-18 followed by fluorescently labeled secondary antibodies such that the F-actin immunoreactivity is green, the IL-18 immunoreactivity is red, and the nuclei are blue (bis-benzimide). Negative controls (no primary antibodies) are shown in A and demonstrate that there is no significant cellular fluorescence from incubation with the 2 fluorescently labeled secondary antibodies, and a FRET+ interaction was not observed (F). In B the buffer-treated PMNs demonstrate colocalization of the IL-18 and F-actin immunoreactivity, which also demonstrated a FRET+ interaction between F-actin and IL-18 in control PMNs (G) with a FRET efficiency of 34%. After 3 min of stimulation with TNF-α, the IL-18 immunoreactivity (red) moved to the cell periphery (C), with a decrease in the FRET+ interaction between IL-18 and F-actin (H) with a FRET efficiency of 8%. Moreover, the decrease in IL-18 immunoreactivity continues at 5 and 10 min of TNF-α stimulation (D and E) with a concomitant decrease in FRET-positive pixels (I and J). I, A–J, are representative of 2 identical experiments with different donors and FRET efficiencies of 34 ± 4% between IL-18 and F-actin in control PMNs. II: quantification of the number of FRET+ (IL-18 + F-actin) pixels per cell. *Statistical significance between buffer-treated controls and TNF-α treatments (P < 0.05). The quantification employed 10 separate cells per treatment for each donor. III: further controls that examine the colocalization of phospho-p40phox and Gαi-1 that do not demonstrate a FRET+ interaction. Isolated PMNs were treated for 3 min with TNF-α (10 ng/ml), and the PMNs were fixed, permeabilized, and incubated with primary antibodies to phospho-p40phox (S 315) and the heterotrimeric G protein subunit Gαi-1 followed by incubation with labeled secondary antibodies such that the phospho-p40phox is red (A) and Gαi-1 is green (B). The colocalization (yellow demarcated by white arrows) is seen in C; however, there was not a FRET+ association between these 2 proteins as demonstrated by the absence of color in D, using pseudocolor with arbitrary linear units of fluorescent intensity (a.l.u.f.i.).
As additional controls, we performed FRET analysis of known binding partners, namely, the small GTPase Rab5a and its regulatory protein partner RabGDI, and performed FRET analysis on two proteins not known to associate with one another: phospho-p40phox and the G protein subunit Gαi-1 (57). The positive FRET between Rab5a and RabGDI demonstrated an efficiency of 49.3 ± 4.8% when the fluorochromes were conjugated to the primary antibodies and an efficiency or 42.3 ± 5.2% when the fluorochromes were conjugated to the secondary antibodies (30 PMNs in each group, P = 0.84; FRETs not shown). Second, although the immunoreactivity of phospho-p40phox (Fig. 4IIIA) and Gαi-1 (Fig. 4IIIB) appeared to colocalize (Fig. 4IIIC), there was not a FRET+ association with calculated FRET efficiencies of 0% (Fig. 4IIID).
To further investigate the relationship of IL-18 with F-actin and to determine whether IL-18 colocalized with cortical F-actin in control or TNF-α-stimulated PMNs, we analyzed the FRET-positive areas in the cell with computer-generated, randomly drawn diameters (Slide Book, Intelligent Imaging Innovations, Denver, CO), which fairly represent cross sections of the cell, removing investigator bias. This technique showed the coincidence of the fluorescent intensity on the different channels pixel by pixel (Fig. 5) and demonstrated that the FRET positivity between IL-18 with F-actin is not at the regions of cortical F-actin near the plasma membrane irrespective of TNF-α treatment (Fig. 5).
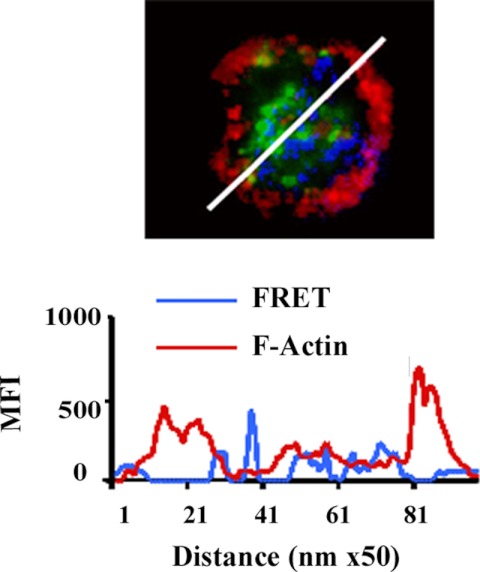
Fig. 5.
Cellular localization of IL-18 association with F-actin. In the micrograph IL-18 (green) does not appear to associate with the dense cortical F-actin (red) in resting neutrophils, as assessed by the lack of a FRET+ between F-actin and IL-18 (blue) in the cellular periphery. To better demonstrate the locality of IL-18 and dense cortical actin, a line scan was performed through the cell (white line), and below it is a graphic representation of the pixels per nanometer of the FRET between IL-18 and F-actin (blue) demonstrating that this interaction appeared in the cytosol and did not localize with the dense cortical F-actin immunoreactivity (red) present at the cell periphery. Multiple random diameters were examined (n = 9) on PMNs from at least 3 donors with similar results and FRET efficiencies of 32–33% MFI, mean fluorescence intensity. Figure is representative of 3 identical experiments.
To confirm whether IL-18 is associated with F-actin in resting cells, PMNs were sonicated and immunoprecipitated with an antibody to IL-18 (Fig. 6). The proteins from this immunoprecipitate were separated, transferred to nitrocellulose, and immunoblotted for F-actin. The IL-18 pulldowns demonstrated colocalization with F-actin but not other proteins in resting PMNs; moreover, F-actin was not precipitated by the beads themselves or by isotypic control antibodies including an antibody to the cytosolic oxidase component p67phox (Fig. 6).
Fig. 6.

Immunoprecipitation of IL-18 and the supernatant from resting PMNs immunoblotted for F-actin. No F-actin immunoreactivity (47 kDa) was present in the beads (B) or from an immunoprecipitation of p67phox, an antibody that is isotypically identical to the antibody employed for IL-18. F-actin immunoreactivity was precipitated by antibodies to IL-18 and was also present in the whole cell lysates (WC), which were used as a positive control MW, molecular weight. Figure is representative of 3 identical experiments with different donors.
Modulation of F-actin content and its effects on IL-18 release.
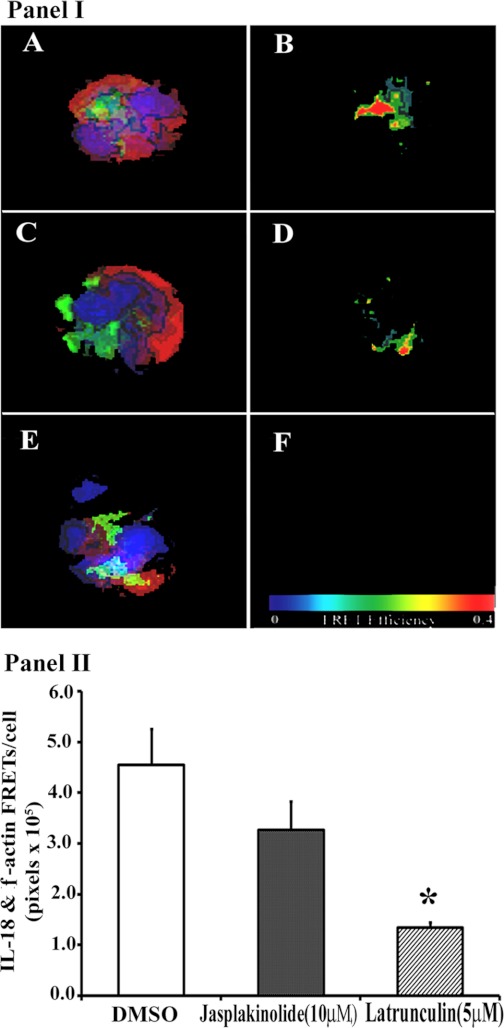
Because of the observed association of F-actin with IL-18 in resting PMNs, we modulated the F-actin content by incubating PMNs with jasplakinolide, which stabilizes the F-actin content, or with latrunculin B, which destabilizes F-actin and increases the amount of cellular G-actin (3, 9, 10, 19, 49). FRET analysis of the different groups suggested that, compared with DMSO-treated controls, jasplakinolide did not seem to affect the amount of IL-18 immunoreactivity associated with F-actin (Fig. 7I, A–D). In contrast, latrunculin B (Fig. 7I, E and F) decreased the amount of IL-18 that colocalized with F-actin in buffer-treated controls (Fig. 7I, A and B), specifically by a decrease in FRET positivity (Fig. 7I, F vs. B). These data were reinforced by quantification of the FRET+ (IL-18 and F-actin) pixels per cell, which demonstrated that DMSO-treated (control) PMNs had the highest amount of IL-18:F-actin FRET, which was slightly decreased by jasplakinolide pretreatment and significantly inhibited by latrunculin treatment (Fig. 7II).
Fig. 7.
Manipulation of cellular F-actin and changes in the physical association (FRET) between IL-18 and F-actin. I: isolated PMNs were treated with 0.1% DMSO (A and B), 10 μM jasplakinolide (C and D), which stabilizes F-actin, for 5 min, or 5 μM latrunculin B (E and F), which destabilizes F-actin. IL-18 immunoreactivity is green, F-actin immunoreactivity is red, and nuclei are blue. B, D, and F illustrate the presence of FRET+ between F-actin and IL-18 in a.l.u.f.i., such that red = most FRET and blue = least. The FRET+ seen in control cells (B) is modestly affected by jasplakinolide (D) and is abrogated by latrunculin B (F). II: quantification of the FRET+ (IL-18 + F-actin) pixels/cell for each of the treatments that appear in I. *Statistical differences between among the groups (P < 0.05). Figure is representative of 3 identical experiments with dissimilar donors and virtually identical results with FRET efficiencies of 30–38%. The quantification employed 10 cells per experimental treatment per donor.
TNF-α causes a FRET+ association between IL-18 and lipid rafts.
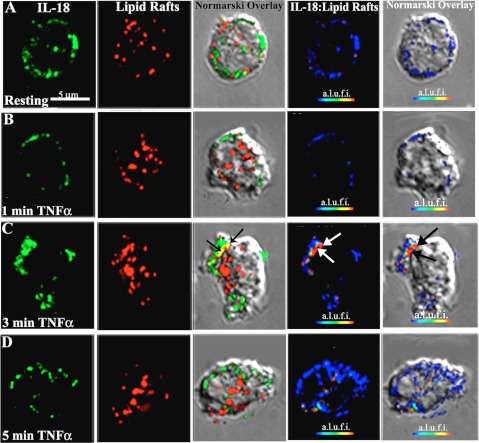
Because lipid rafts offer a possible method for release of proinflammatory proteins from cells, we examined whether TNF-α induced an association of IL-18 with lipid rafts. Figure 8 illustrates that TNF-α (10 ng/ml) for 3 min caused significant colocalization (yellow color demarcated by arrows, Fig. 8C, 3rd column), which was not present in the controls or at any other time points. Furthermore, this association between IL-18 immunoreactivity and lipid rafts demonstrated a FRET+ interaction, which was not present in control PMNs or PMNs treated with TNF-α (10 ng/ml) for 1 min and decreased at 5 min. This FRET+ association of IL-18 and lipid rafts occurred at the “pseudopod” as demonstrated in the bright field Nomarski images (Fig. 8C, 5th column).
Fig. 8.
TNF-α causes IL-18 to dissociate from F-actin and to colocalize with lipid rafts as it is being released from PMNs. Isolated PMNs were stimulated with buffer or 10 ng/ml TNF-α for 1–5 min, fixed, smeared onto slides, permeabilized, and incubated with a primary antibody (conjugated to Alexa 488 = green, 1st column) against IL-18, and lipid rafts were visualized via the affinity of cholera toxin B (conjugated to Alexa 555 = red, 2nd column). Colocalization of IL-18 with the lipid rafts is shown in a Nomarski overlay (3rd column) and is yellow. FRET+ interactions between IL-18 and the lipid rafts are demonstrated in color (blue = least, red = most) in a.l.u.f.i. (4th column), and cellular location of the FRET+ interaction is demonstrated as a Nomarski overlay (5th column). In control PMNs (A) the IL-18 immunoreactivity does not colocalize with lipid rafts as visualized by the lack of yellow color in the 3rd column. There is negligible FRET+ interaction between IL-18 and lipid rafts as demonstrated in 4th and 5th columns. Similarly, TNF-α (10 ng/ml) stimulation for 1 min did not change the interaction of IL-18 with lipid rafts (B). However, at 3 min (C), the time of maximal TNF-α-mediated release, there is colocalization (yellow color, 3rd column) demarcated by black arrows and a FRET+ interaction between IL-18 and the lipid rafts (4th column, white arrows) that localizes to pseudopods, as demonstrated by the bright field (Nomarski) image with black arrows (5th column). This FRET+ interaction is decreased at 5 min (D, 4th and 5th columns). When the FRET+ (IL-18 + lipid rafts) voxels were quantified per cell 0.8% of IL-18 were physically associated with lipid rafts in controls, and after TNF-α stimulation 4.5% of the FRET+ voxels were present at 1 min, 14.5% at 3 min, and 8.4% at 5 min. These experiments are representative of the data from 2 separate experiments with disparate PMN donors. In addition, for the quantification of the FRETs 25 cells were used per treatment group and the number of Fret+ voxels was divided by the number of voxels of intracellular IL-18 immunoreactivity.
TNF-α priming of the PMN oxidase: effects of IL-18 binding protein.
To determine whether released IL-18 was required for TNF-α priming of the PMN oxidase, we incubated PMNs with a monomeric IL-18 binding protein (500 ng/ml), which is known to inhibit IL-18 activity, before (5 min) priming with TNF-α (35). Importantly, IL-18 binding protein did not affect the fMLP-activated respiratory burst (Table 2). Conversely, incubation of the PMNs with IL-18 binding protein significantly decreased TNF-α priming of the PMN oxidase by 77 ± 8% (Table 2).
Table 2.
IL-18 binding protein inhibits TNF-α priming of PMN oxidase
| Preincubation | Priming Agent | fMLP Activation of Oxidase |
|---|---|---|
| Buffer | Buffer | 1.0 ± 0.2 |
| IL-18 binding protein | Buffer | 0.7 ± 0.2 |
| Buffer | TNF-α | 2.0 ± 0.5* |
| IL-18 binding protein | TNF-α | 1.2 ± 0.3 |
Data are means ± SE of the maximal rate of O2− production (nmol/min) from PMNs isolated from 5 disparate donors. fMLP, N-formylmethionyl-leucyl-phenylalanine. PMNs were incubated with the priming agent for 15 min at 37°C ± IL-18 binding protein.
Statistical difference (P < 0.05) between TNF-α-primed PMNs and all other groups. Statistical differences among groups were determined by a paired ANOVA followed by a Newman-Keuls post hoc test for multiple comparisons.
DISCUSSION
The data presented demonstrate that PMNs contain IL-18 in the cytosol, with negligible IL-18 immunoreactivity in the nucleus, membrane, granules, lysosomes, Golgi apparatus, or the endoplasmic reticulum. In control PMNs, IL-18 colocalized with cytoplasmic F-actin, demonstrated by a FRET+ between the fluorochrome-tagged antibodies bound to the proteins but not with the dense cortical actin fibers. IL-18 release from PMNs appears to be specific to TNF-α priming, in contrast to the release of serine proteases and other granule contents elicited by a number of proinflammatory agents, none of which induced IL-18 release, nor was release affected by treatment with cytochalasin B (7). IL-18 also colocalized with Cdc42 at 3 min of TNF-α stimulation at the time of release in cellular structures that visually appear to be pseudopodia such that this colocalization is consistent with pseudopod formation (56). Furthermore, the released IL-18 is the cleaved active protein, and not the propeptide, because the epitope recognized by the antibodies employed is masked in the propeptide and only becomes accessible after cleavage (21). TNF-α also caused dissociation of IL-18 from F-actin, which was confirmed by pretreatment with latrunculin B, an agent that causes actin depolymerization (3, 19). Conversely, increasing cellular F-actin content with jasplakinolide did not augment the association of IL-18 with F-actin compared with DMSO-treated controls; however, these results were expected, for jasplakinolide stabilizes F-actin by direct insertion into the actin filament, which may inhibit IL-18 colocalization because of its presence (9, 10, 49). Immunoprecipitation of IL-18 also demonstrated colocalization with F-actin in control, quiescent PMNs that was disrupted by treatment with TNF-α. The TNF-α-mediated (10 ng/ml) release of IL-18 also caused a FRET+ association of IL-18 with lipid rafts at 3 min of stimulation, the time of its cellular release for this concentration of TNF-α (23, 30, 51). In addition, TNF-α (10 ng/ml) induced the release of active, cleaved IL-18 at 3 min and primed the fMLP-activated respiratory burst; moreover, the concentration of IL-18 released has been shown to prime the PMN oxidase in vitro, with lesser TNF-α concentrations not causing IL-18 release or priming of the oxidase (62). Finally, the addition of monomeric, recombinant IL-18 binding protein to the reaction buffer significantly reduced TNF-α priming of the PMN oxidase without affecting fMLP activation of the PMN oxidase. These data imply that TNF-α priming of PMNs may require the release of IL-18, which then engages the IL-18 receptor on the PMN membrane.
TNF-α causes the synthesis and release of IL-18 from cardiac myocytes and adipocytes, from which IL-18 is postulated to be involved with the formation of atherosclerotic plaques and in the pathogenesis of insulin resistance, respectively (38, 58, 59). However, in these studies TNF-α-induced IL-18 synthesis and release was 6–12 h, which required protein synthesis, dissimilar to the presented data that describe the rapid release of preformed IL-18 in 3 min (38, 58, 59). In addition, IL-18 augments and prolongs TNF-α signaling by stabilizing mRNA transcripts for c-apoptosis inhibitor-2 TNF-α receptor-associated factor-1, resulting in prolonged survival of natural killer cells (29). In the presented study, TNF-α-induced IL-18 release causes priming of the PMN oxidase that could be specifically inhibited by extracellular IL-18 binding protein. These data imply a direct effect of IL-18 in TNF-α priming of the PMN oxidase and provide an explanation as to the rapid kinetics of IL-18 release and reuptake, presumably by receptor engagement, quantified in the ELISA data. As mentioned above, recombinant human IL-18 (rhIL-18) rapidly primes the oxidase (15 min), temporally congruous with the presented data (62).
The presented FRET data, excluding Fig. 8, resulted from fluorescent probes conjugated to the secondary rather than the primary antibodies, a technique that is not novel (37, 60, 64). Compared with FRET analyses with primary antibodies on the known binding pair of Rab5a and Rab5-GDI the actual FRET efficiencies were not statistically different for the FRETs between the fluorochromes conjugated to primary or secondary antibodies, respectively; however, the FRET efficiencies were decreased when the fluorochromes were tagged to the secondary antibodies. These results may be explained by the increased distance, spatial dilution, between fluorochromes on the secondary antibodies making the FRET interaction less common compared with an interaction of fluorochromes on the primary antibodies, which are not separated by this greater distance. In addition, although proteins may appear to colocalize, the presented data with p-p40phox and Gαi-1 demonstrated that such a colocalization does not imply a FRET+ interaction that has stringent physical limitations. Importantly, the colocalization of IL-18 with lipid rafts also demonstrated a positive FRET interaction in the identical cells with labeled primary antibodies. With FRET efficiencies of 52%, these proteins would be <5 nm from one another, and such a close spatial interaction would imply a physical association and implicate lipid rafts as one method of TNF-α-mediated cellular release. Quantification of these data demonstrated that at 3 min of TNF-α stimulation 11% of the IL-18 was associated with lipid rafts.
IL-18 is present in areas of PMN-mediated inflammation, especially in the synovial fluid of patients with rheumatoid arthritis and in the bronchoalveolar lavage fluid of patients with acute lung injury, and it may be released from isolated PMNs into the extracellular milieu; moreover, IL-18 accumulates in the plasma fraction of packed red blood cells, released from PMNs and other leukocytes (26, 31, 41, 44, 54). Although previous data demonstrated that PMNs contain active IL-18 and IL-18 may be released in response to LPS in an NF-κB-dependent process over 1–2 h, this report is novel for it shows that 1) PMNs contain active IL-18 in the cytosol associated with F-actin, 2) that proinflammatory (TNF-α) stimulation causes the rapid (minutes) release of active IL-18, 3) that this release from the cytosol involves lipid rafts, and 4) that the released IL-18 is associated with TNF-α priming of these PMNs (23a, 30a, 50a). Moreover, processing of the propeptide to the active form requires activation of caspase-1/ICE, an activity that is TNF-α mediated in many cell lines including PMNs (14). There are recent data that chicken heterophils, which are analogous to human PMNs, synthesize and release IL-18 in response to IL-2, but these experiments did not investigate the possibility that heterophils contain preformed IL-18 (36). Although the ability of IL-18 to associate with F-actin, as demonstrated by FRET+ and coimmunoprecipitation, may support the idea that IL-18 has an actin-binding domain, there is no such domain from the published primary structure of IL-18 despite its structural similarity to human fascin, an actin cross-linking protein (25). The possibility exists that an intermediary, actin-binding protein may bind both actin and IL-18 in such a manner as not to disrupt the colocalization of Il-18 with F-actin in the resting state; however, further work is required to delineate this interaction. In addition, TNF-α induces a decrease in F-actin in PMNs, providing indirect evidence that a TNF-α-mediated decrease in F-actin may result in the release of a proinflammatory cytokine associated with F-actin in the quiescent PMN (5, 39). PMNs also contain lipid rafts, which have been shown to be important for the release of cellular proteins in response to LPS stimulation; therefore, the implication that TNF-α causes IL-18 release via lipid rafts appears plausible (23).
In summary, the presented data have demonstrated that PMNs may contain cytosolic mediators that are not stored in the granular compartments, and in the case of IL-18 it may serve as a bridging molecule to activate both innate and adaptive immunity at the site of infection/inflammation (21, 31, 41, 62). These studies also demonstrated that released mediators may rapidly impact their cells of origin, and such findings are novel for PMNs. Moreover, the presence of IL-18 in PMNs may represent a mechanism by which acute infection/inflammation that induces activation of PMNs, innate immunity, may cause recruitment/activation of adaptive immunity at this specific site/nidus. These data also demonstrate the complexity of immunity and mechanistic links between what have been termed distinct systems. Additional studies are required to deduce the role of IL-18 in host defense, its importance in the eradication of pathogenic organisms, its precise role in both the acute and chronic phases of inflammation, and its ability to activate or bridge both adaptive and innate immunity.
GRANTS
This work was funded by National Institutes of Health Grant HL-59355 (C. C. Silliman), P50-GM-49222 (C. C. Silliman, A. Banerjee), and AI-15614 (C. A. Dinarello).
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors' academic institutions or employers.
Supplementary Material
REFERENCES
- 1.Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J 8: 504–512, 1994 [PubMed] [Google Scholar]
- 2.Ambruso DR, Bolscher BG, Stokman PM, Verhoeven AJ, Roos D. Assembly and activation of the NADPH:O2 oxidoreductase in human neutrophils after stimulation with phorbol myristate acetate. J Biol Chem 265: 924–930, 1990 [PubMed] [Google Scholar]
- 3.Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol 137: 399–416, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 334: 1717–1725, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Berkova N, Gilbert C, Goupil S, Yan J, Korobko V, Naccache PH. TNF-induced haptoglobin release from human neutrophils: pivotal role of the TNF p55 receptor. J Immunol 162: 6226–6232, 1999 [PubMed] [Google Scholar]
- 6.Beutler B, Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med 316: 379–385, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89: 3503–3521, 1997 [PubMed] [Google Scholar]
- 8.Borregaard N, Kjeldsen L, Sengelov H, Diamond MS, Springer TA, Anderson HC, Kishimoto TK, Bainton DF. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol 56: 80–87, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869–14871, 1994 [PubMed] [Google Scholar]
- 10.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275: 5163–5170, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Burbach GJ, Naik SM, Harten JB, Liu L, Dithmar S, Grossniklaus H, Ward SL, Armstrong CA, Caughman SW, Ansel JC. Interleukin-18 expression and modulation in human corneal epithelial cells. Curr Eye Res 23: 64–68, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood 84: 2068–2101, 1994 [PubMed] [Google Scholar]
- 13.Cassatella MA, Bazzoni F, Ceska M, Ferro I, Baggiolini M, Berton G. IL-8 production by human polymorphonuclear leukocytes. The chemoattractant formyl-methionyl-leucyl-phenylalanine induces the gene expression and release of IL-8 through a pertussis toxin-sensitive pathway. J Immunol 148: 3216–3220, 1992 [PubMed] [Google Scholar]
- 14.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA. Molecular cloning of the interleukin-1beta converting enzyme. Science 256: 97–100, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol 101: 85–95, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA, Volpp BD, Leidal KG, Nauseef WM. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J Clin Invest 85: 714–721, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res 67: 46–52, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Corvino CL, Mamoni RL, Fagundes GZ, Blotta MH. Serum interleukin-18 and soluble tumour necrosis factor receptor 2 are associated with disease severity in patients with paracoccidioidomycosis. Clin Exp Immunol 147: 483–490, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett 213: 316–318, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 27: 98–114, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol 103: 11–24, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest 104: 761–767, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, Nick JA, Bratton DL, Malcolm KC, Worthen GS. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J Biol Chem 279: 39989–39998, 2004 [DOI] [PubMed] [Google Scholar]
- 23a.Fortin CF, Ear T, McDonald PP. Autocrine role of endogenous interleukin-18 on inflammatory cytokine generation by human neutrophils. FASEB J 23: 194–203, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Gibbs LS, Lai L, Malik AB. Tumor necrosis factor enhances the neutrophil-dependent increase in endothelial permeability. J Cell Physiol 145: 496–500, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Gibrat JF, Madej T, Bryant SH. Surprising similarities in structure comparison. Curr Opin Struct Biol 6: 377–385, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest 104: 1393–1401, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol 55: 662–675, 1994 [PubMed] [Google Scholar]
- 28.Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 360: 352–354, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Hodge DL, Subleski JJ, Reynolds DA, Buschman MD, Schill WB, Burkett MW, Malyguine AM, Young HA. The proinflammatory cytokine interleukin-18 alters multiple signaling pathways to inhibit natural killer cell death. J Interferon Cytokine Res 26: 706–718, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun 324: 511–517, 2004 [DOI] [PubMed] [Google Scholar]
- 30a.Jablonska E, Puzewska W, Grabowska Z, Jablonski J, Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine 30: 93–99, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J Immunol 167: 7060–7068, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Kelher MR, Ambruso DR, Elzi DJ, Anderson SM, Paterson AJ, Thurman GW, Silliman CC. Formyl-Met-Leu-Phe induces calcium-dependent tyrosine phosphorylation of Rel-1 in neutrophils. Cell Calcium 34: 445–455, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kharazmi A, Nielsen H, Bendtzen K. Modulation of human neutrophil and monocyte chemotaxis and superoxide responses by recombinant TNF-alpha and GM-CSF. Immunobiology 177: 363–370, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Azam T, Yoon DY, Reznikov LL, Novick D, Rubinstein M, Dinarello CA. Site-specific mutations in the mature form of human IL-18 with enhanced biological activity and decreased neutralization by IL-18 binding protein. Proc Natl Acad Sci USA 98: 3304–3309, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SH, Eisenstein M, Reznikov L, Fantuzzi G, Novick D, Rubinstein M, Dinarello CA. Structural requirements of six naturally occurring isoforms of the IL-18 binding protein to inhibit IL-18. Proc Natl Acad Sci USA 97: 1190–1195, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kogut MH, Rothwell L, Kaiser P. Priming by recombinant chicken interleukin-2 induces selective expression of IL-8 and IL-18 mRNA in chicken heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enterica serovar enteritidis. Mol Immunol 40: 603–610, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Koksch M, Rothe G, Kiefel V, Schmitz G. Fluorescence resonance energy transfer as a new method for the epitope-specific characterization of anti-platelet antibodies. J Immunol Methods 187: 53–67, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab 291: E108–E114, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Kutsuna H, Suzuki K, Kamata N, Kato T, Hato F, Mizuno K, Kobayashi H, Ishii M, Kitagawa S. Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: the role of MAP kinases. Am J Physiol Cell Physiol 286: C55–C64, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Latt SA, Gerald PS. Staining of human metaphase chromosomes with fluorescent conjugates of polylysine. Exp Cell Res 81: 401–406, 1973 [DOI] [PubMed] [Google Scholar]
- 41.Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, Cunha F, Liew FY, McInnes IB. A role for IL-18 in neutrophil activation. J Immunol 167: 2879–2886, 2001 [DOI] [PubMed] [Google Scholar]
- 42.MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 14: 477–492, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Malech HL, Gallin JI. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med 317: 687–694, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Mathiak G, Neville LF, Grass G, Boehm SA, Luebke T, Herzmann T, Kabir K, Rosendahl R, Schaefer U, Mueller C, Bohlen H, Wassermann K, Hoelscher AH. Chemokines and interleukin-18 are up-regulated in bronchoalveolar lavage fluid but not in serum of septic surgical ICU patients. Shock 15: 176–180, 2001 [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol 176: 7039–7050, 2006 [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol 180: 8192–8203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Flaherty JT, Jacobson DP, Redman JF. Regulation of platelet-activating-factor receptors and the desensitization response in polymorphonuclear neutrophils. Biochem J 288: 241–248, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Flaherty JT, Surles JR, Redman J, Jacobson D, Piantadosi C, Wykle RL. Binding and metabolism of platelet-activating factor by human neutrophils. J Clin Invest 78: 381–388, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posey SC, Bierer BE. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J Biol Chem 274: 4259–4265, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA 96: 2256–2261, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a.Robertson SE, Young JD, Kitson S, Pitt A, Evans J, Roes J, Karaoglu D, Santora L, Ghayur T, Liew FY, Gracie JA, McInnes IB. Expression and alternative processing of IL-18 in human neutrophils. Eur J Immunol 36: 722–731, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic 5: 255–264, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholes GD. Long-range resonance energy transfer in molecular systems. Annu Rev Phys Chem 54: 57–87, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol 73: 511–524, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Silliman CC, Kelher M, Hess JR, England KM, Gorman J, McLaughlin N, Greenwalt TJ. Experimental additive solution-64 inhibits the accumulation of neutrophil priming activity and interleukin-18 during storage of packed red blood cells (Abstract). Blood 100, Suppl: 286a, 2002 [Google Scholar]
- 55.Springer TA. Adhesion receptors of the immune system. Nature 346: 425–434, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol 160: 375–385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature 368: 157–160, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem 284: 7853–7865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun 337: 422–429, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Wouters FS, Bastiaens PI, Wirtz KW, Jovin TM. FRET microscopy demonstrates molecular association of non-specific lipid transfer protein (nsL-TP) with fatty acid oxidation enzymes in peroxisomes. EMBO J 17: 7179–7189, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright CS. Structural comparison of the two distinct sugar binding sites in wheat germ agglutinin isolectin II. J Mol Biol 178: 91–104, 1984 [DOI] [PubMed] [Google Scholar]
- 62.Wyman TH, Dinarello CA, Banerjee A, Gamboni-Robertson F, Hiester AA, England KM, Kelher M, Silliman CC. Physiological levels of interleukin-18 stimulate multiple neutrophil functions through p38 MAP kinase activation. J Leukoc Biol 72: 401–409, 2002 [PubMed] [Google Scholar]
- 63.Yang YJ, Chen SH, Ge XR. Role of interleukin-18 in the development of acute pulmonary injury induced by intestinal ischemia/reperfusion and its possible mechanism. J Gastroenterol Hepatol 22: 253–260, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol 23: 5409–5420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler EJ. Tumor necrosis factor in humans. N Engl J Med 318: 1533–1535, 1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.