Abstract
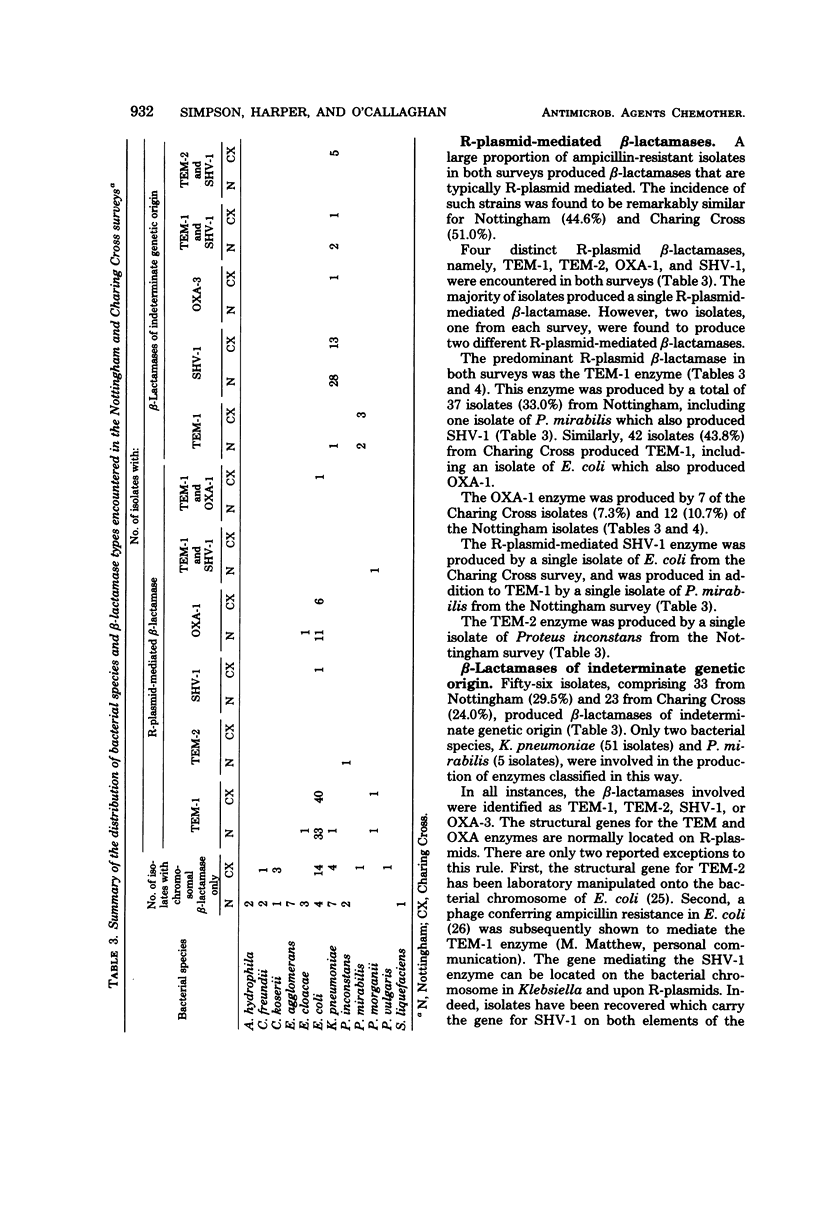
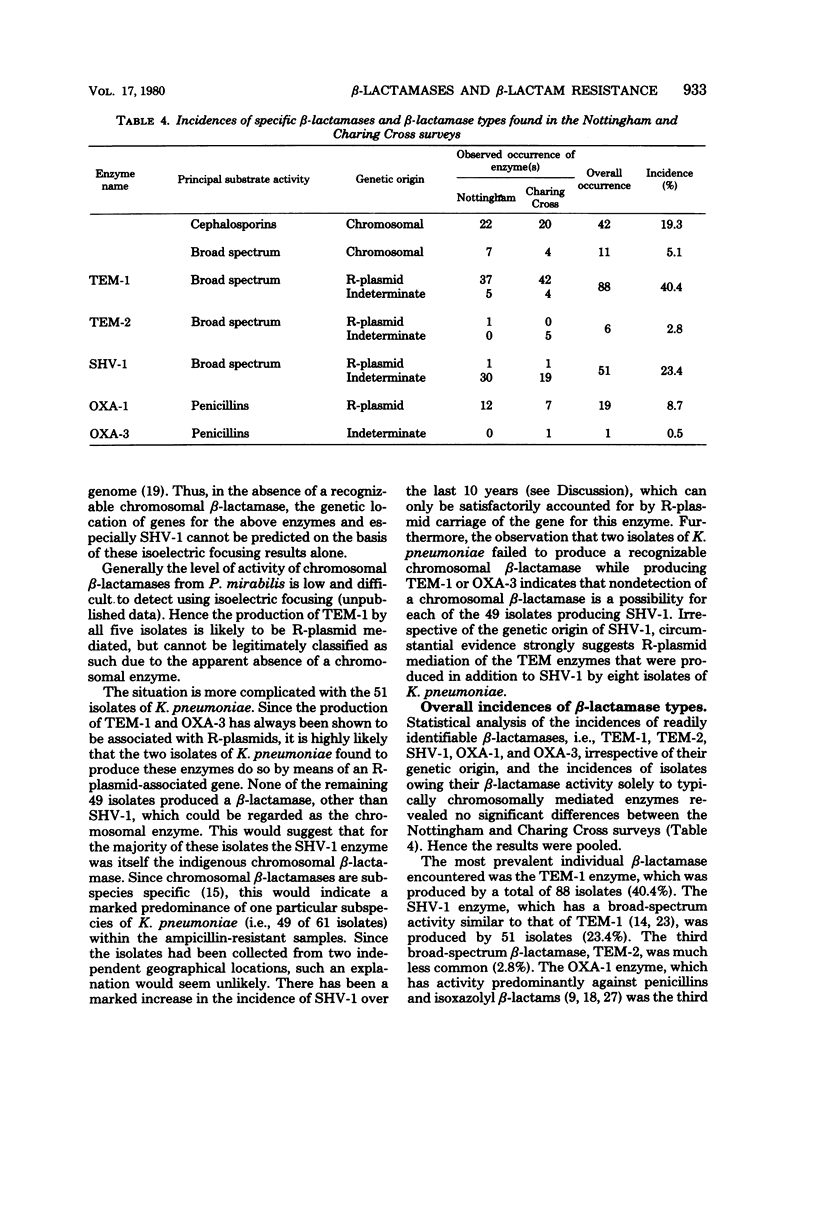
Two independent surveys have been conducted to determine the prevalent bacterial species and beta-lactamase types present in clinical populations of gram-negative, ampicillin-resistant isolates. A total of 208 isolates (112 from Nottingham Hospital and 96 from Charing Cross Hospital), all of which had been collected from out-patients suffering from urinary tract infections, were investigated. The incidence of ampicillin-resistant isolates (minimum inhibitory concentrations, 8 micrograms/ml) was 24.1% and 18.8% within the Nottingham and Charing Cross samples, respectively. The surveys gave similar results within the ampicillin-resistant samples. Escherichia coli was the prevalent bacterial species (52.9%), followed by Klebseilla pneumoniae (30.3%). The majority of isolates, at least 54.8% and possibly as high as 74.5%, owed their principal beta-lactamase activity to enzymes mediated by R-plasmids. The most prevalent beta-lactamases were TEM-1 (53.3%), SHV-1 (30.9%), and OXA-1 (11.5%). Positive associations were found between E. coli and TEM-1 or OXA-1 and between K. pneumoniae and SHV-1.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G., Nordström K., Normark S. Penicillin resistance in Escherichia coli K12: synergism between penicillinases and a barrier in the outer part of the envelope. Ann N Y Acad Sci. 1974 May 10;235(0):569–586. doi: 10.1111/j.1749-6632.1974.tb43291.x. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. The molecule of infectious drug resistance. Sci Am. 1973 Apr;228(4):19–27. [PubMed] [Google Scholar]
- Dale J. W. Characterization of the -lactamase specified by the resistance factor R-1818 in E. coli K12 and other Gram-negative bacteria. Biochem J. 1971 Jul;123(4):501–505. doi: 10.1042/bj1230501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Gavan T. L., Gerlach E. H., Barry A. L., Thornsberry C. Cefuroxime, a new parenteral cephalosporin: collaborative in vitro susceptibility comparison with cephalothin against 5,887 clinical bacterial isolates. Antimicrob Agents Chemother. 1977 Jul;12(1):47–50. doi: 10.1128/aac.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Marshall M. J., Ross G. W., Chanter K. V., Harris A. M. Comparison of the substrate specificities of the -lactamases from Klebsiella aerogenes 1082E and Enterobacter cloacae P99. Appl Microbiol. 1972 Apr;23(4):765–769. doi: 10.1128/am.23.4.765-769.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W., Smith J. T. Types of beta-lactamase determined by plasmids in gram-negative bacteria. J Bacteriol. 1979 Jun;138(3):657–662. doi: 10.1128/jb.138.3.657-662.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M. Plasmid-mediated beta-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979 Jul;5(4):349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- Nugent M. E., Hedges R. W. The nature of the genetic determinant for the SHV-1 beta-lactamase. Mol Gen Genet. 1979 Oct 1;175(3):239–243. doi: 10.1007/BF00397222. [DOI] [PubMed] [Google Scholar]
- Petrocheilou V., Sykes R. B., Richmond M. H. Novel R-plasmid-mediated beta-lactamase from Klebsiella aerogenes. Antimicrob Agents Chemother. 1977 Jul;12(1):126–128. doi: 10.1128/aac.12.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitton J. S. Mechanisms of bacterial resistance to antibiotics. Ergeb Physiol. 1972;65:15–93. doi: 10.1007/3-540-05814-1_2. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Curtis N. A. The interplay of beta-lactamases and intrinsic factors in the resistance of gram-negative bacteria to penicillins and cephalosporins. Ann N Y Acad Sci. 1974 May 10;235(0):553–568. doi: 10.1111/j.1749-6632.1974.tb43290.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The chromosomal integration of a -lactamase gene derived from the P-type R-factor RP1 in Escherichia coli. Genet Res. 1972 Oct;20(2):231–237. doi: 10.1017/s0016672300013732. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Infectious drug resistance. Sci Am. 1967 Dec;217(6):19–28. doi: 10.1038/scientificamerican1267-19. [DOI] [PubMed] [Google Scholar]


