Abstract
Migration to intestinal mucosa putatively depends on local activation because gastrointestinal lymphoid tissue induces expression of intestinal homing molecules, whereas skin-draining lymph nodes do not. This paradigm is difficult to reconcile with reports of intestinal T cell responses after alternative routes of immunization. We reconcile this discrepancy by demonstrating that activation within spleen results in intermediate induction of homing potential to the intestinal mucosa. We further demonstrate that memory T cells within small intestine epithelium do not routinely recirculate with memory T cells in other tissues, and we provide evidence that homing is similarly dynamic in humans after subcutaneous live yellow fever vaccine immunization. These data explain why systemic immunization routes induce local cell-mediated immunity within the intestine and indicate that this tissue must be seeded with memory T cell precursors shortly after activation.
Naive T cell recirculation is primarily restricted to secondary lymphoid tissues, blood, and lymph (Butcher and Picker, 1996; von Andrian and Mackay, 2000). However, most infections are initiated at body surfaces, including the gut (Neutra and Kozlowski, 2006). Before CD8 T cells can participate in the eradication of local infections, microbial antigen must get from the site of infection to secondary lymphoid tissue, cognate antigen-specific T cells must undergo activation and expansion, and then CD8 T cells must migrate to the site of infection to effect pathogen clearance. Because these events consume valuable time, it is perhaps not surprising that a portion of memory T cells remain distributed at body surfaces after primary infections, where they may be better positioned to respond immediately in the event of reinfection (Hogan et al., 2001; Masopust et al., 2001b; Reinhardt et al., 2001). In support of this hypothesis, memory CD8 T cells within the gut epithelium (intraepithelial lymphocytes [IELs]) are functionally distinct from those in lymphoid tissue and exhibit immediate cytotoxicity upon reinfection (Kim et al., 1999; Masopust et al., 2001a). The vulnerability of the intestinal mucosa to infection because of its proximity with the microbial world, large surface area, and identity as a major reservoir of HIV infection, and the putative role that in situ memory T cells play in protection, have provided substantial motivation to understand how memory is established and maintained at such body surfaces (Hayday et al., 2001; Nagler-Anderson, 2001; Belyakov and Berzofsky, 2004; Cheroutre, 2005; Haase, 2005; Liu et al., 2006; Gebhardt et al., 2009).
T cells use α4β7 integrin and CCR9 to migrate to small intestine epithelium, and they use different homing receptors, such as cutaneous lymphocyte-associated antigen (CLA), to migrate to skin (von Andrian and Mackay, 2000; Johansson-Lindbom and Agace, 2007). Gut and skin homing receptor expression is coupled to the location of T cell activation (Campbell and Butcher, 2002; Stagg et al., 2002; Mora et al., 2003, 2005). Priming within mesenteric LN (mLN) induces α4β7 expression. In contrast, priming within inguinal LN (iLN) induces expression of skin homing molecules. These observations support the hypothesis that local infection and subsequent priming within gastrointestinal-associated lymphoid tissue (GALT) is required for dissemination of CD8 T cells into the small intestine. Although numerous observations are consistent with this hypothesis (Gallichan and Rosenthal, 1996; Belyakov et al., 1998), there are several examples in which alternative routes of immunization result in effector T cell responses within the intestinal mucosa (Stevceva et al., 2002; Masopust et al., 2004; Liu et al., 2006). Reconciling these seemingly disparate observations is essential for understanding the relationship between immunization route and the establishment of local protective cellular immunity.
Another issue of relevance to protective immunity is whether memory CD8 T cells continuously recirculate between blood, lymphoid tissue, and mucosal epithelium or whether IELs are long-term sessile residents. Numerous studies support the general concept that effector memory CD8 T cells recirculate between nonlymphoid tissues and blood (Butcher and Picker, 1996). For instance, if the vasculature of two mice is conjoined via parabiosis, preexisting memory CD8 T cells originating from both mice can be isolated from a single lung, liver, or peritoneal cavity (Jungi and Jungi, 1981; Klonowski et al., 2004), demonstrating that memory cells enter certain tissues via the blood. The memory T cell population in lung airways is thought to be short lived but maintained by the continuous recruitment of the systemic population (Ely et al., 2006). Memory CD8 T cells also exit tissues using afferent lymphatic vessels, which drain interstitial fluid from peripheral tissues (Olszewski et al., 1995; Lehmann et al., 2001; Bromley et al., 2005; Debes et al., 2005). Indeed, Mackay et al. (1990) isolated antigen-experienced lymphocytes directly from the hind limb afferent lymphatics of sheep. Memory T cells putatively egress with the afferent lymph as it filters through proximal LNs, pools within the thoracic duct, and then rejoins the blood supply. Thus, memory CD8 T cells in most peripheral tissues may be transient visitors, constantly patrolling peripheral organs in the event of reinfection.
However, it is unclear whether the recirculation paradigm applies to virus-specific memory CD8αβ IEL. Unlike most tissues, including the subjacent lamina propria, this avascular compartment consists of T cells residing among a monolayer of columnar epithelial cells that lies above a basement membrane and lacks blood vessels and afferent lymphatics. Although antigen specificity was not determined, parabiosis studies showed limited mixing of IEL between conjoined mice (Klonowski et al., 2004). Lastly, memory IELs are phenotypically and functionally distinct from CD8 T cells in blood (Masopust et al., 2006). As these issues impact our understanding of how epithelial memory CD8 T cell populations are established, instructed to express unique properties, and maintained, we investigated whether priming within GALT is required for CD8 T cell migration to small intestine and also whether IELs routinely recirculate or are long-term residents.
RESULTS
Dynamic T cell migration program in spleen
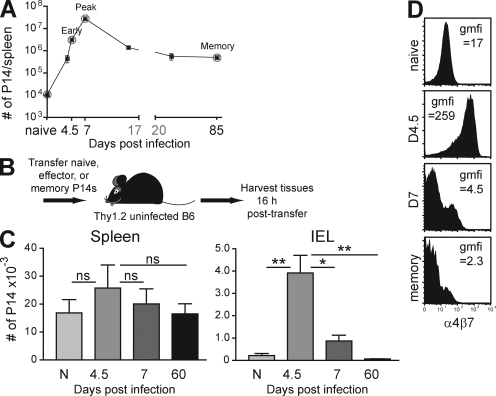
To examine the dynamics of CD8 T cell homing, we used the lymphocytic choriomeningitis virus (LCMV) and P14 transgenic CD8 T cell system, which provides access to large numbers of naive, effector, and memory CD8 T cells of known specificity. To generate effector and memory CD8 T cells, naive Thy1.1+ P14 were transferred to naive mice, which were then challenged i.p. with the Armstrong strain of LCMV. In response to infection, P14 underwent clonal expansion, which peaked 7 d after challenge, followed by contraction of the population and formation of stable memory by ∼1 mo later (Fig. 1 A). We first examined the trafficking potential of cells isolated from spleen. We transferred 2 × 106 naive P14 splenocytes or 2 × 106 P14 splenocytes isolated 4.5, 7, or 60 d after LCMV Armstrong infection into naive Thy1.2+ recipients. It should be noted that day-4.5 CD8 T cells represent early effectors that are still undergoing clonal expansion. The next day, lymphocytes were harvested from recipient tissues (Fig. 1 B).
Figure 1.
Only early effector CD8 T cells migrate to intestinal epithelium and express α4β7. (A) Dynamics of P14 response to LCMV. The day 37 time point represents 12 mice analyzed from days 31 to 50 after infection. (B) Experimental design consists of transferring P14 at different stages of differentiation and harvesting tissues the next day. (C) Numbers of naive (N), effector (isolated 4.5 or 7 d after infection), and memory (isolated 60 d after infection) cells isolated from recipient spleen and intestinal epithelium (IEL). ns, not significant, *, P < 0.05; **, P < 0.01, unpaired Student’s t test. Error bars indicate SEM. (D) Virus-specific P14 CD8 T cells were analyzed for expression of α4β7. gmfi, geometric mean fluorescence intensity of α4β7 staining. All plots are gated on Thy1.1+ CD8+ lymphocytes and are representative of at least three independent experiments totaling >10 mice/time point.
As expected, naive CD8 T cells populated blood and secondary lymphoid tissues but were excluded from the IEL compartment of the small intestine (Fig. 1 C and Fig. S1). When early effector P14 splenocytes isolated from spleen (or blood; Fig. S2) were transferred, they were recovered from spleen and the epithelium of the intestinal mucosa. These data demonstrated that early effector CD8 T cells were capable of homing from spleen to gut. Pertussis toxin treatment of donor cells inhibited effector cell homing to intestinal epithelium, demonstrating that this migration was chemokine dependent (Fig. S1).
Remarkably, CD8 T cells lost the ability to migrate to the IEL compartment by 7 d after infection (Fig. 1 C). Memory CD8 T cells were entirely excluded from migrating to the intestinal epithelium. Thus, CD8 T cells were endowed with the ability to migrate to intestinal epithelium only early during the response.
We then tested whether effector cells were contaminated with a cofactor that permitted T cells, regardless of differentiation state, to migrate to the intestinal epithelium. When day-4.5 and memory P14 splenocytes were mixed and transferred to the same naive recipient, both populations of cells were equivalently recovered from spleen. However, memory CD8 T cells were ∼20-fold more efficient at trafficking to LN and early effectors were >50-fold more efficient at migrating to the small intestinal epithelium (Fig. S3). This indicated that effector and memory CD8 T cells exhibit intrinsically different homing properties.
To test this conclusion further, we examined the dynamics of α4β7 expression, which is required for homing to the small intestine. Fig. 1 D shows that α4β7 expression is transiently up-regulated on all early effector LCMV-specific CD8 T splenocytes and is then rapidly down-regulated. Thus, the timing of α4β7 expression reflected the transient ability of T cells to traffic to the gut epithelium.
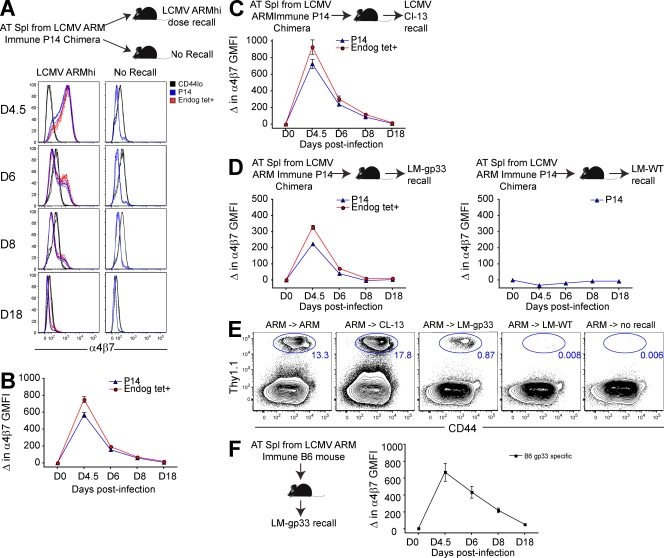
We then tested whether reactivation of memory CD8 T cells would result in transient reexpression of α4β7. Splenocytes isolated from LCMV-immune Thy1.1+ P14 chimeras (Fig. 1) were transferred to naive mice. Recipients were boosted the next day with pathogens that contained cognate gp33 antigen, including LCMV Armstrong strain, LCMV clone 13 strain, or recombinant Listera monocytogenes (LM-gp33). In each case, secondary donor CD8 T cell responses were coupled with transient reexpression of α4β7 and dissemination into the small intestinal epithelium (Fig. 2). Endogenous H-2Db/gp33-specific reactivated memory CD8 T cells also transiently up-regulated α4β7 upon boosting (Fig. 2), so this phenomenon was not unique to transgenic CD8 T cells. In contrast, challenge with LM that lacked recombinant cognate gp33 antigen (LM-WT) failed to induce α4β7 expression among memory P14, suggesting that the infectious milieu alone was insufficient and that cognate antigen was required.
Figure 2.
Antigen-dependent reexpression of α4β7 by spleen-derived transgenic and endogenous memory CD8 T cells upon infection with virus or bacteria. (A–D) Splenocytes isolated from P14 immune chimeras (>30 d after LCMV Arm infection) were transferred to naive recipients. (A and B) The next day, recipients were challenged with high-dose LCMV Arm or left unchallenged. (A) α4β7 expression was monitored in blood among donor P14 (Thy1.1+/gp33 tetramer+), nontransgenic gp33-specific cells (Thy1.1−/gp33 tetramer+), and CD44lo (naive) CD8 T cells. Representative flow cytometry data are shown. (B) Change in GMFI of α4β7 expression relative to α4β7 GMFI of memory P14 transferred to unchallenged mice that were analyzed on the same day. (C and D) As in A and B, except mice were challenged with LCMV Cl−13 (C) or LM-gp33 (D), or the noncognate antigen bearing inflammation control LM-WT. (E) 19 d after infection, small intestine IEL of these mice were examined for the presence of donor P14. (F) Splenocytes from LCMV Arm–immune C57BL/6J mice (which did not contain P14) were transferred to naive CD45.1 recipients. Recipients were challenged the next day with LM-gp33, and α4β7 expression among CD45.1− gp33-tetramer+ CD8 T cells was monitored in blood. At least three mice were analyzed at each time point in each experiment. Error bars indicate SEM. One of two experiments with similar results is shown.
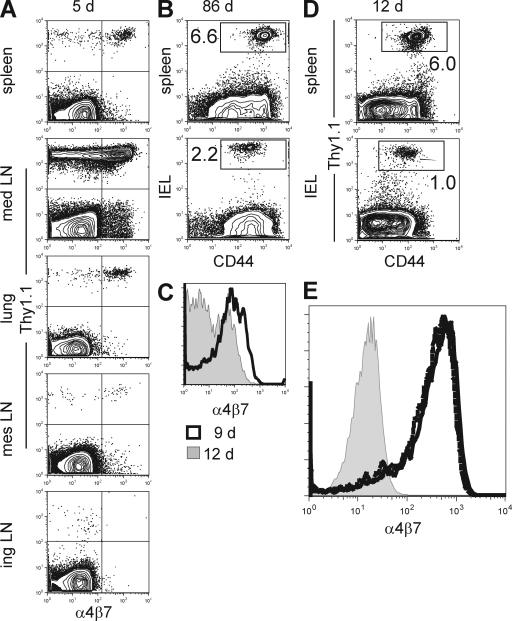
It should be noted that LCMV infects the spleen, almost 100% of early effector splenocytes expressed α4β7, and there are ∼20-fold more early effectors in spleen than GALT (7.2 × 106 early effector P14 in spleen versus 3.4 × 105 in mLNs). Thus, it is unlikely that activation in GALT was required for α4β7 expression. To examine this issue more stringently, mice were infected intranasally with influenza virus. 5 d later, almost no activated virus-specific CD8 T cells could be detected within GALT, yet flu-specific CD8 T cells in spleen- and lung-associated tissues expressed α4β7 and memory CD8 T cells were established within small intestinal epithelium (Fig. 3, A and B). Intramuscular injection of a DNA vaccine into the anterior tibialis (located within lower hind limb) also led to transient α4β7 expression and the establishment of CD8 T cells within intestinal epithelium (Fig. 3, C and D). Moreover, disruption of lymphocyte egress from GALT via treatment of mice with FTY720 did not affect α4β7 expression among effector CD8 T cells in spleen (Fig. 3 E). Collectively, these data demonstrate that activation in GALT is not required for α4β7 expression among CD8 T cells.
Figure 3.
Memory CD8 T cells do not retain α4β7 expression regardless of anatomical location or immunization route. Naive Thy1.1+ P14 were transferred to naive mice. (A and B) The next day, mice were infected intranasally with 500 pfu of recombinant influenza virus that expresses gp33. 5 (A) or 86 (B) d later, lymphocytes were isolated from the indicated tissues and stained with α-Thy1.1, CD8, and α4β7 or CD44. Plots are gated on CD8+ lymphocytes. (C and D) Alternatively, 200 µg DNA that expresses the glycoprotein of LCMV under control of the CMVie promoter was administered intramuscularly into both anterior tibialis muscles. (C) 9 and 12 d later, Thy1.1+ cells were examined for expression of α4β7 (gated on CD8+ Thy1.1+ lymphocytes). (D) 12 d after immunization, lymphocytes were isolated from spleen and IEL and stained with α-CD8α, Thy1.1, and CD44 antibodies. Plots are gated on CD8α+ lymphocytes. (E) Naive Thy1.1+ P14 were transferred to naive C57BL/6J mice. Control mice received normal drinking water, whereas treated mice were exposed to 2 µg/ml FTY720 in the drinking water ad libitum for the duration of the experiment. The next day, both groups of mice were immunized with LCMV, and α4β7 expression among Thy1.1+ P14 in spleen was compared among control mice (black line), FTY720-treated mice (dashed line), and CD44lo CD8 T cells isolated from spleens of naive mice (gray histogram). Plots are gated on Thy1.1+ CD8+ lymphocytes. All data are representative of two experiments with at least three mice per group in each experiment.
Homing potential among distinct lymphoid tissues
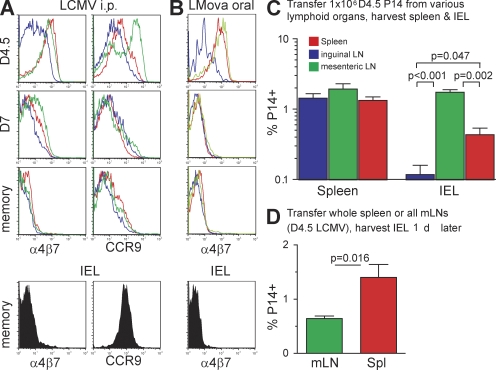
We next tested whether memory CD8 T cells in mucosal tissues also lacked α4β7, and we determined the dynamics of CCR9 expression, which is also involved in migration to gut (Zabel et al., 1999; Kunkel et al., 2000; Johansson-Lindbom and Agace, 2007). Fig. 4 A demonstrates that neither α4β7 nor CCR9 expression is maintained on memory CD8 T cells isolated from any lymphoid tissue. Although IEL memory CD8 T cells expressed CCR9, α4β7 was down-regulated by day 7 after infection (Fig. 4 and Fig. S4). Thus, LCMV-specific memory CD8 T cells, regardless of location, did not coexpress the essential homing molecules required for entry into the intestinal mucosa.
Figure 4.
Memory CD8 T cells do not retain α4β7 expression regardless of anatomical location or immunization route. (A and B) Expression of α4β7 and/or CCR9 by P14 isolated from various tissues 4.5, 7, or 60 d after i.p. LCMV infection (A) or by OT-I after oral LMova infection (B). All plots are gated on Thy1.1+ CD8+ lymphocytes. (C) 106 Thy1.1+ P14 isolated from spleen (red), iLN (blue), or mLN (green) 4.5 d after LCMV infection was transferred to naive mice. The next day, lymphocytes were harvested from recipient spleen and small intestinal epithelium, and the proportion of CD8+ lymphocytes that were Thy1.1+ was determined. Only p-values of <0.05 are shown. (D) Recipient mice received the entire single cell suspension derived from either one spleen or the complete cluster of mLN derived from one mouse isolated 4.5 d after LCMV infection. The next day, the proportion of CD8+ lymphocytes that were Thy1.1+ was determined. Error bars indicate SEM. All data are representative of at least two experiments with at least three mice per group in each experiment.
We then determined whether α4β7 expression was maintained on memory CD8 T cells after oral infection. OT-I transgenic CD8 T cells specific for ovalbumin were transferred to mice before oral infection with recombinant LM expressing ovalbumin (LM-ova; Masopust et al., 2001a; Pope et al., 2001). As shown in Fig. 4 B, memory CD8 T cells did not retain α4β7 after oral infection in any tissue analyzed, including the IEL compartment.
Expression of gut homing molecules on early effector CD8 T cells varied by location (Fig. 4, A and B). Those isolated from skin-draining iLN up-regulated less α4β7 than cells in spleen and mLN. And early effectors in mLN expressed higher levels of CCR9 than those isolated from spleen or iLN. We compared the homing potential of each population on a per cell basis by transferring 106 P14 isolated from each lymphoid tissue 4.5 d after LCMV infection to separate recipients (Fig. 4 C). With regard to homing to intestinal epithelium, P14 cells derived from mLN were the most efficient, and iLN-derived cells migrated poorly. P14 derived from spleen exhibited an intermediate ability to traffic to mucosal epithelium. When one considers the fact that there are ∼20-fold more early effector T cells in spleen than mLN, it is likely that more IEL effectors are derived from spleen than from mLN after an LCMV infection. To test this hypothesis, all lymphocytes derived from one spleen versus all mLNs derived from one mouse were transferred into separate recipients. Although recovery varied among different experiments, transfer of spleen consistently resulted in 2.5–4-fold more P14 within the gut epithelium than the transfer of mLN on a per-organ basis (Fig. 4 D, n = 5 per group for the experiment shown).
Memory IELs are resident
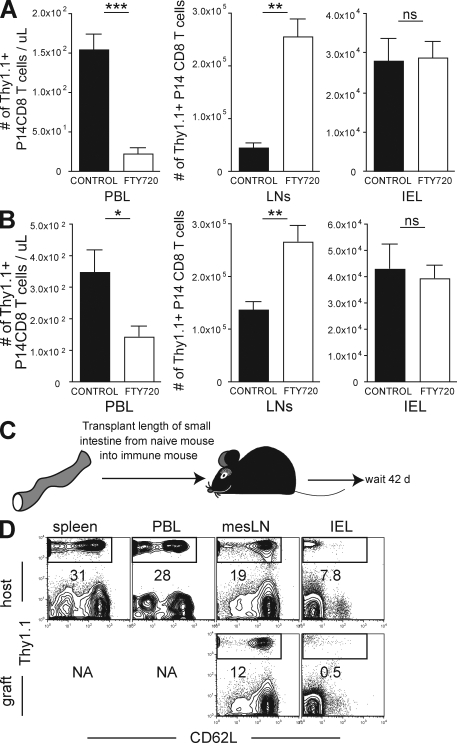
FTY720 treatment causes an accumulation of recirculating lymphocytes within lymphoid tissue and a corresponding decrease within other tissues that contain recirculating lymphocytes (Schwab and Cyster, 2007). To further test whether IELs recirculate, we treated LCMV-immune mice with FTY720 and examined whether treatment induced a reduction in memory CD8 T cells within the intestinal epithelium. LCMV-immune P14 chimeras (generated as described in Fig. 1 A) were treated with FTY720 for 2 or 30 d in drinking water. As shown in Fig. 5 A, treatment led to a rapid depletion of memory CD8 T cells in blood within 2 d, and this reduction was maintained for 30 d with continuous FTY720 treatment (Fig. 5 B). As expected, a reciprocal increase in P14 was observed in iLN, suggesting that lymphocyte recirculation through the iLN was interrupted. However, there was no reduction in the number of P14 IEL, even after 30 d of treatment (Fig. 5, A and B). These data support the hypothesis that IELs do not recirculate via afferent lymphatics.
Figure 5.
Memory CD8 T cells in intestinal epithelium do not recirculate. Naive P14 cells were transferred to naive C57BL/6J mice, and recipients were infected with LCMV. 90 d later, 2 µg/ml FTY720 was dissolved in drinking water (white bars) or mice were maintained on normal drinking water (black bars). (A and B) 2 (A) or 30 (B) d after FTY720 treatment, the number of LCMV-specific P14 memory CD8 T cells was determined in blood (PBL), iLNs, lung, or small intestinal epithelium (IEL). Data shown is one of five experiments with three mice per group with similar results. Immune mice were generated by transferring naive Thy1.1+ P14 into naive C57BL/6J mice and infecting recipients with LCMV. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001, unpaired Student’s t test. (C) 2 mo later, 7 cm of small intestine, along with associated mesentery and mLN, were transplanted from naive mice into immune mice. (D) 42 d after transplantation, lymphocytes were isolated from host spleen, blood (PBL), mLN, and intestinal epithelium (IEL), as well as donor mLN and IEL. The presence of host memory P14 was determined in each tissue by Thy1.1 staining and flow cytometry. All plots are gated on CD8+ lymphocytes and are representative of one of a total of three mice examined in two independent experiments. NA, not applicable.
We wished to independently test whether memory CD8 T cells recirculated through gut epithelium over a period of several weeks. To this end, we removed 7 cm of small intestine from naive C57BL/6J mice and transplanted this tissue into C57BL/6J mice that had been immunized with LCMV 2 mo previously and contained large numbers of Thy1.1+ memory P14 CD8 T cells (Fig. 5 C). Blood vessels were ligated upon transplantation and donor gut remained viable and appeared healthy by visual inspection upon cessation of the experiment. This method allowed us to test whether memory CD8 T cells, which are present in all tissues of the recipient mouse, would equilibrate with donor gut epithelium. Association of donor small intestine with donor mLN remained intact and served as a positive control for recirculation between graft and host. 42 d after transplantation, host and donor tissues were removed and assessed for the presence of host memory P14. As shown in Fig. 5 D, donor mLNs have similar frequencies of memory P14 CD8 T cells compared with host mLNs, as expected. Within the donors’ IEL compartment, there were far fewer host memory P14 CD8 T cells compared with host epithelium, indicating minimal recirculation.
Dynamic α4β7 and CLA expression after human s.c. yellow fever vaccination
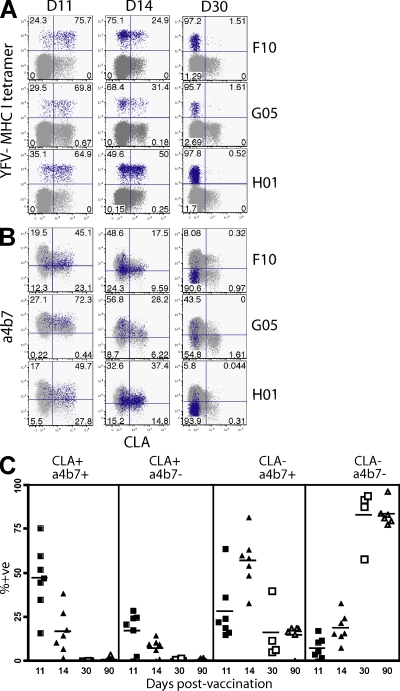
Our observations in mice revealed that α4β7 was expressed by early effector CD8 T cells but not memory CD8 T cells. Expression of the putative skin homing molecule, functional P-selectin ligand, was similarly dynamic and was coexpressed with α4β7 on effector splenocytes shortly after LCMV infection (Fig. S5). We wished to examine whether expression of peripheral homing molecules was also dynamic after a primary immunization of humans. To follow a primary CD8 T cell response in humans, we examined the response to the live attenuated yellow fever virus (YFV)–17D vaccine (Miller et al., 2008). We identified an HLA-A2–restricted immunodominant epitope that we used to construct MHC class I tetramers. Volunteers were vaccinated s.c. and a tetramer+ population in PBMC could be visualized by day 11. Because immunization was delivered s.c., we examined expression of CLA, which is involved in skin homing. CLA expression on YFV-specific T cells was remarkably transient, peaking on day 11, decreasing substantially by day 14, and was not detectable by day 30 after immunization (Fig. 6 A). When CD8 T cells were costained for both CLA and α4β7 molecules, early effectors expressed detectable levels of both homing molecules (Fig. 6 B). However, by day 30, YFV-specific effector CD8 T cells had predominantly down-regulated CLA and α4β7, and this phenotype persisted among memory CD8 T cells (90 d after infection; Fig. 6 C). Thus, human virus-specific CD8 T cells also express a very dynamic pattern of homing molecule expression and support the hypothesis that CD8 T cell homing to body surfaces occurs predominantly upon recent activation.
Figure 6.
Primary human CD8 T cell response to s.c. yellow fever vaccine results in only short-term expression of α4β7 and CLA. Blood was isolated from HLA-A2–positive volunteers 11, 14, 30, and 90 d after s.c. vaccination with YFV-17D. (A) Expression of CLA versus staining with MHC class I tetramers that recognize YFV-specific CD8 T cells from three representative patients. (B) CLA versus α4β7 expression. HLA2-YFV tetramer+ cells are blue and HLA2-YFV tetramer− cells are gray. Numbers indicate percentage of tetramer+ cells in each quadrant. All plots are gated on CD3+ CD8+ lymphocytes. (C) Summary of CLA and α4β7 expression among YFV tetramer+ cells. Longitudinal analysis is shown, although some patients were not examined on days 30 and 90. Horizontal bars show the mean. Day 11, n = 7; day 14, n = 7; day 30, n = 4; day 90, n = 6.
DISCUSSION
Our data support a model by which early effector CD8 T cells migrate into the intestinal mucosa and then down-regulate α4β7, differentiate into long-lived memory CD8 T cells in situ, and remain resident without recirculating. Analysis of a primary human immune response to s.c. immunization suggests that these migration dynamics reflect what occurs at other body surfaces, such as skin, which may also harbor resident memory CD8 T cells (Clark et al., 2006). This observation has several implications. Obviously, analysis of blood-borne lymphocytes will not reflect the quantity and quality of nonrecirculating T cells in tissues. Defining correlates of cell-mediated protection after vaccination will require sampling T cells directly from portals of pathogen entry. The fact that memory CD8 T cells may remain sessile within intestinal epithelium supports the hypothesis that microenvironmental cues instruct their unique differentiation profile (Masopust et al., 2006). Most importantly, these data indicate that body surfaces must be seeded with memory T cell precursors shortly after priming or boosting, as memory T cells in other compartments will not equilibrate into this tissue. Consequently, in the absence of cognate antigen restimulation, it is unlikely that peripheral memory is maintained by a stem cell–like population of central memory CD8 T cells located in other tissues (Sallusto et al., 2004). Rather, the population is maintained in situ, which may have implications for the longevity of in situ protective immunity.
Local lymphoid tissue preferentially induces expression of homing molecules that help target activated T cells to associated nonlymphoid tissues (Butcher and Picker, 1996; von Andrian and Mackay, 2000). This has been best characterized in response to immunization with nonreplicating agents or in vitro. Our data extend these findings by demonstrating that T cells activated in vivo in response to a viral or bacterial infection also exhibit distinct homing properties that are dependent on the lymphoid environment within which they are primed (Fig. 4). However, our data demonstrates that priming within local lymphoid tissue may not be required for homing into the gut epithelium (Fig. 1–4). Rather, priming in spleen, a central lymphoid organ which contains the majority of antigen-experienced CD8 T cells even after local infections (Haanen et al., 1999; Marshall et al., 2001; Masopust et al., 2004), results in a promiscuous homing program that drives migration to the intestinal mucosa with intermediate efficiency (Fig. 4 and Fig. S5). This observation may help reconcile the many studies demonstrating the distinctive homing properties of T cells primed in intestinal versus skin draining lymphoid tissue (Campbell and Butcher, 2002; Mora et al., 2003, 2005) and those studies that have demonstrated promiscuous T cell distribution after local infections (Stevceva et al., 2002; Masopust et al., 2004; Liu et al., 2006). Our results do not exclude other possible mechanisms that could contribute to the dissemination of effector T cell responses, including recirculation of dendritic cells or early effector T cells through different LNs, where they may access a variety of homing signals (Belyakov et al., 2004; Liu et al., 2006). Defining what conditions regulate the establishment of intestinal CD8 T cell memory after nonoral routes of immunization will have obvious ramifications for vaccination.
In contrast to LNs, spleen does not drain tissues via the lymphatic system, nor is it directly associated with any particular body surface. Therefore, induction of a promiscuous homing program after activation within this tissue makes sense both teleologically and mechanistically and may enhance host immunity by ensuring that adaptive T cell responses examine all tissues for infection. Of course it remains quite possible that repeated infections, which may limit T cell reactivation to local tissues, could enhance the preferential accumulation of memory T cells specifically at the original site of infection. It is more difficult to speculate on the advantage of restricting recirculation through intestinal epithelium (Fig. 5), as this presumably limits the immediate availability of memory CD8 T cells in the event of local reinfection. It may be related to the fact that the function and differentiation state of small intestine IELs differs markedly from memory CD8 T cells in blood (Masopust et al., 2006). Regardless, the observation that the potential to home to the gut epithelium dissipates shortly after pathogen control highlights how quickly CD8 T cell homing properties change throughout the response (Figs. 1–4). These rapid changes in homing potential and α4β7 expression were defined in mouse studies that used various immunogens (LCMV, L. monocytogenes, influenza virus, and DNA vaccination) and routes of immunization (i.p., oral, intranasal, and intramuscular). When this principle was tested in humans in the context of a live attenuated vaccine, we found that homing molecule expression was similarly dynamic (Fig. 6). It remains important to determine whether long-term tissue residence will define the paradigm for memory T cells at other body surfaces, and how this relates to both control and pathogenesis of mucosal infections, such as HIV.
MATERIALS AND METHODS
Generation of virus-specific naive, effector, and memory donor CD8+ T cells and infections.
Animal protocols were approved by the Institutional Animal Care and Use Committees at the University of Minnesota and/or Emory University. Naive donor antigen-specific CD8 T cells were isolated directly from spleens of naive Thy1.1+ transgenic P14 mice. For effector and memory cell generation, 2 × 105 naive Thy1.1+ transgenic P14 CD8 T cells isolated from spleen were transferred i.v. into C57BL/6 recipients. The next day, recipients were infected with 2 × 105 pfu LCMV (Armstrong strain). Single cell suspensions of lymphocytes isolated from challenged mice were prepared 4.5, 7, and 30–60 d after infection. For cotransfer experiments (Fig. S3), Ly5.1+ P14 were used to generate memory CD8 T cells. For analyzing influenza specific responses, 2 × 105 naive Thy1.1+ transgenic P14 CD8 T cells isolated from spleen were transferred i.v. into C57BL/6 recipients. The next day, mice were infected intranasally with 500 pfu of recombinant influenza virus that expresses gp33 in a total volume of 30 µl. Alternatively, 200 µg DNA was administered intramuscularly into both anterior tibialis muscles in a total volume of 100 µl of sterile phosphate-buffered saline. The DNA plasmid used in these experiments expresses the glycoprotein of LCMV under control of the CMVie promoter (pCMV-GPm; gift from J.L. Whitton [Scripps research Institute, La Jolla, CA] and H. Ertl [Wistar Institute, Philadelphia, Pennsylvania]). DNA stocks were prepared using the endotoxin-free gigaprep kit (QIAGEN).
For secondary infections, splenocytes from LCMV-immune C57BL/6J mice were transferred to CD45.1 mice (Fig. 2 F) or splenocytes from LCMV P14 immune chimeras (generated as described in the previous paragraph) were transferred to naive C57BL/6J mice (Fig. 2, A–E). Recipients were challenged the next day with 2 × 106 pfu LCMV Arm i.v., 2 × 106 pfu LCMV Cl−13 i.v., 2 × 103 cfu LM-gp33 i.v., or 2 × 103 LM-WT i.v. LM-gp33 was provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA).
In vivo migration assays.
Before transfer, donor cells were positively selected for CD8 expression by magnetic separation (>95% purity), and total cells transferred (into C57BL/6J mice by tail vein injection) were normalized between groups via addition of splenocytes isolated from naive nontransgenic mice (Figs. 1 and 4). The next day (12–18 h later, depending on the experiment) recipient mice were perfused and lymphocytes were harvested from a variety of tissues (Masopust et al., 2004). In Fig. 4, donor lymphocytes were derived from spleen, iLN, or mLN as indicated. IEL were isolated as follows: small intestine was removed, Peyer’s patches were dissected, and the intestines were cut longitudinally and then into 1-cm pieces. Gut pieces were incubated with 15.4 mg/ml DTE in 10% HBSS/Hepes Bicarbonate (30 min at 37°C, shaking at 200 rpm). Lymphocytes were purified on a 44/67% percoll gradient (800 g at 20°C for 20 min). For assessing the role of chemokine signaling on migration, after CD8 enrichment, lymphocytes were treated with 25 ng/ml pertussis toxin (R&D Systems) at a concentration of 15 × 106 cells/ml for 1 h at 37°C. Control cells were treated equivalently except for the addition of pertussis toxin.
Identification and phenotyping of mouse donor CD8 T cells.
Recovered mouse cells were stained with fluorochrome-conjugated anti-CD8, -Thy1.1, -CD62L, and -α4β7 (BD) or with anti-CCR9 (Pabst et al., 2004), followed by secondary staining with mouse anti–rat IgG (Jackson ImmunoResearch Laboratories, Inc.). Anti-CCR9 antibody was provided by R. Forster (Institute for Immunology, Hannover Medical School, Hannover, Germany). Functional P-selectin binding was determined by staining with purified P-selectin–IgG fusion protein (BD) followed by secondary staining with allophycocyanin-conjugated AffiniPure goat anti–human IgG (Fcγ fragment specific; Jackson ImmunoResearch Laboratories, Inc.). Cells were analyzed on a FACSCalibur or LSR II (BD).
FTY720 treatment.
Mice were exposed ad libitum to drinking water containing dissolved FTY720 at a concentration of 2 µg/ml.
Intestinal transplantation.
Heterotopic small intestinal transplantation was performed as previously described (Newell et al., 1997; Wang et al., 2006). In brief, intestinal grafts were transplanted by anastomosing the graft portal vein and superior mesenteric artery to the recipient inferior vena cava and infrarenal aorta, respectively. A stoma was formed from the proximal graft and the distal graft was anastomosed to the recipient jejunum.
Study subjects and blood samples.
Blood samples were obtained from healthy volunteers (18–40 yr old) recruited after informed consent in a double-blind controlled trial. The live-attenuated YFV-17D vaccine (YF-Vax) was administered alone or in combination with pooled immune serum globulin. Approval for all procedures was obtained from the Emory University Institutional Review Board. Exclusion criteria for subjects were a previous history of vaccination for or exposure to flaviviruses as indicated by serology, travel to endemic areas, and those recommendations established by the Advisory Committee on Immunization Practices and Vaccination. Samples were analyzed before and at various times after vaccination as indicated in the text. PBMCs were purified from CPT (BD) containers
Phenotyping of human YFV-specific CD8 T cells.
HLA2-YFV–specific tetramers conjugated to streptavidin APC were prepared in house and used to stain 200–300 µl of whole blood. A 10-min incubation at room temperature was followed by addition of appropriate antibodies and a further 30-min incubation. The antibodies used were anti-CLA, -CD3, -CD8 (BD), or -α4β7 (clone ACT-1). Red blood cells were removed by lysing with FACS lysis buffer (BD) before washing cells with PBS. Samples were acquired on an LSRII flow cytometer (BD) and analyzed with FlowJo software (Tree Star, Inc.).
Online supplemental material.
Fig. S1 shows that migration of early effector CD8 T cells to small intestinal epithelium is chemokine dependent. Fig. S2 shows that early effector CD8 T cells migrate from blood to small intestinal epithelium. Fig. S3 shows that effectors do not elicit bystander migration of memory cells to small intestinal epithelium. Fig. S4 shows that CD8 T cells gradually lose α4β7 expression after entry into small intestinal epithelium. Fig. S5 shows transient coexpression of functional P-selectin binding ligand and α4β7 among effector splenocytes. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090858/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Reinhold Forster for kindly providing anti-CCR9 antibody and Dr. Hao Shen for providing LM-gp33.
This work was supported by grants from the National Institutes of Health and the Arnold and Mabel Beckman Foundation.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CLA
- cutaneous lymphocyte-associated antigen
- GALT
- gastrointestinal-associated lymphoid tissue
- IEL
- intraepithelial lymphocyte
- iLN
- inguinal LN
- LCMV
- lymphocytic choriomeningitis virus
- mLN
- mesenteric LN
- YFV
- yellow fever virus
References
- Belyakov I.M., Berzofsky J.A. 2004. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 20:247–253 10.1016/S1074-7613(04)00053-6 [DOI] [PubMed] [Google Scholar]
- Belyakov I.M., Derby M.A., Ahlers J.D., Kelsall B.L., Earl P., Moss B., Strober W., Berzofsky J.A. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA. 95:1709–1714 10.1073/pnas.95.4.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyakov I.M., Hammond S.A., Ahlers J.D., Glenn G.M., Berzofsky J.A. 2004. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J. Clin. Invest. 113:998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley S.K., Thomas S.Y., Luster A.D. 2005. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6:895–901 10.1038/ni1240 [DOI] [PubMed] [Google Scholar]
- Butcher E.C., Picker L.J. 1996. Lymphocyte homing and homeostasis. Science. 272:60–66 10.1126/science.272.5258.60 [DOI] [PubMed] [Google Scholar]
- Campbell D.J., Butcher E.C. 2002. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195:135–141 10.1084/jem.20011502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H. 2005. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol. Rev. 206:114–131 10.1111/j.0105-2896.2005.00284.x [DOI] [PubMed] [Google Scholar]
- Clark R.A., Chong B., Mirchandani N., Brinster N.K., Yamanaka K., Dowgiert R.K., Kupper T.S. 2006. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176:4431–4439 [DOI] [PubMed] [Google Scholar]
- Debes G.F., Arnold C.N., Young A.J., Krautwald S., Lipp M., Hay J.B., Butcher E.C. 2005. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6:889–894 10.1038/ni1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely K.H., Cookenham T., Roberts A.D., Woodland D.L. 2006. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 176:537–543 [DOI] [PubMed] [Google Scholar]
- Gallichan W.S., Rosenthal K.L. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879–1890 10.1084/jem.184.5.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T., Wakim L.M., Eidsmo L., Reading P.C., Heath W.R., Carbone F.R. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10:524–530 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- Haanen J.B., Toebes M., Cordaro T.A., Wolkers M.C., Kruisbeek A.M., Schumacher T.N. 1999. Systemic T cell expansion during localized viral infection. Eur. J. Immunol. 29:1168–1174 [DOI] [PubMed] [Google Scholar]
- Haase A.T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783–792 [DOI] [PubMed] [Google Scholar]
- Hayday A., Theodoridis E., Ramsburg E., Shires J. 2001. Intraepithelial lymphocytes: exploring the third way in immunology. Nat. Immunol. 2:997–1003 10.1038/ni1101-997 [DOI] [PubMed] [Google Scholar]
- Hogan R.J., Zhong W., Usherwood E.J., Cookenham T., Roberts A.D., Woodland D.L. 2001. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J. Exp. Med. 193:981–986 10.1084/jem.193.8.981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B., Agace W.W. 2007. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215:226–242 10.1111/j.1600-065X.2006.00482.x [DOI] [PubMed] [Google Scholar]
- Jungi T.W., Jungi R. 1981. Immunological memory to Listeria monocytogenes in rodents. IV. Studies on origin and fate of tissue-positioned T memory cells. Immunology. 44:789–798 [PMC free article] [PubMed] [Google Scholar]
- Kim S.K., Schluns K.S., Lefrançois L. 1999. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163:4125–4132 [PubMed] [Google Scholar]
- Klonowski K.D., Williams K.J., Marzo A.L., Blair D.A., Lingenheld E.G., Lefrançois L. 2004. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 20:551–562 10.1016/S1074-7613(04)00103-7 [DOI] [PubMed] [Google Scholar]
- Kunkel E.J., Campbell J.J., Haraldsen G., Pan J., Boisvert J., Roberts A.I., Ebert E.C., Vierra M.A., Goodman S.B., Genovese M.C., et al. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761–768 10.1084/jem.192.5.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C., Wilkening A., Leiber D., Markus A., Krug N., Pabst R., Tschernig T. 2001. Lymphocytes in the bronchoalveolar space reenter the lung tissue by means of the alveolar epithelium, migrate to regional lymph nodes, and subsequently rejoin the systemic immune system. Anat. Rec. 264:229–236 10.1002/ar.1163 [DOI] [PubMed] [Google Scholar]
- Liu L., Fuhlbrigge R.C., Karibian K., Tian T., Kupper T.S. 2006. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 25:511–520 10.1016/j.immuni.2006.06.019 [DOI] [PubMed] [Google Scholar]
- Mackay C.R., Marston W.L., Dudler L. 1990. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171:801–817 10.1084/jem.171.3.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D.R., Turner S.J., Belz G.T., Wingo S., Andreansky S., Sangster M.Y., Riberdy J.M., Liu T., Tan M., Doherty P.C. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA. 98:6313–6318 10.1073/pnas.101132698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Jiang J., Shen H., Lefrançois L. 2001a. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348–2356 [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Marzo A.L., Lefrançois L. 2001b. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Usherwood E.J., Cauley L.S., Olson S., Marzo A.L., Ward R.L., Woodland D.L., Lefrançois L. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875–4882 [DOI] [PubMed] [Google Scholar]
- Masopust D., Vezys V., Wherry E.J., Barber D.L., Ahmed R. 2006. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176:2079–2083 [DOI] [PubMed] [Google Scholar]
- Miller J.D., van der Most R.G., Akondy R.S., Glidewell J.T., Albott S., Masopust D., Murali-Krishna K., Mahar P.L., Edupuganti S., Lalor S., et al. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 28:710–722 10.1016/j.immuni.2008.02.020 [DOI] [PubMed] [Google Scholar]
- Mora J.R., Bono M.R., Manjunath N., Weninger W., Cavanagh L.L., Rosemblatt M., Von Andrian U.H. 2003. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 424:88–93 10.1038/nature01726 [DOI] [PubMed] [Google Scholar]
- Mora J.R., Cheng G., Picarella D., Briskin M., Buchanan N., von Andrian U.H. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316 10.1084/jem.20041645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler-Anderson C. 2001. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 1:59–67 10.1038/35095573 [DOI] [PubMed] [Google Scholar]
- Neutra M.R., Kozlowski P.A. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148–158 10.1038/nri1777 [DOI] [PubMed] [Google Scholar]
- Newell K.A., He G., Hart J., Thistlethwaite J.R., Jr 1997. Treatment with either anti-CD4 or anti-CD8 monoclonal antibodies blocks alphabeta T cell-mediated rejection of intestinal allografts in mice. Transplantation. 64:959–965 10.1097/00007890-199710150-00004 [DOI] [PubMed] [Google Scholar]
- Olszewski W.L., Grzelak I., Ziolkowska A., Engeset A. 1995. Immune cell traffic from blood through the normal human skin to lymphatics. Clin. Dermatol. 13:473–483 10.1016/0738-081X(95)00087-V [DOI] [PubMed] [Google Scholar]
- Pabst O., Ohl L., Wendland M., Wurbel M.A., Kremmer E., Malissen B., Förster R. 2004. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J. Exp. Med. 199:411–416 10.1084/jem.20030996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C., Kim S.K., Marzo A., Masopust D., Williams K., Jiang J., Shen H., Lefrançois L. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409 [DOI] [PubMed] [Google Scholar]
- Reinhardt R.L., Khoruts A., Merica R., Zell T., Jenkins M.K. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 410:101–105 10.1038/35065111 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Geginat J., Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745–763 10.1146/annurev.immunol.22.012703.104702 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Cyster J.G. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8:1295–1301 10.1038/ni1545 [DOI] [PubMed] [Google Scholar]
- Stagg A.J., Kamm M.A., Knight S.C. 2002. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur. J. Immunol. 32:1445–1454 [DOI] [PubMed] [Google Scholar]
- Stevceva L., Alvarez X., Lackner A.A., Tryniszewska E., Kelsall B., Nacsa J., Tartaglia J., Strober W., Franchini G. 2002. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques. J. Virol. 76:11659–11676 10.1128/JVI.76.22.11659-11676.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U.H., Mackay C.R. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020–1034 10.1056/NEJM200010053431407 [DOI] [PubMed] [Google Scholar]
- Wang J., Dong Y., Sun J.Z., Taylor R.T., Guo C., Alegre M.L., Williams I.R., Newell K.A. 2006. Donor lymphoid organs are a major site of alloreactive T-cell priming following intestinal transplantation. Am. J. Transplant. 6:2563–2571 10.1111/j.1600-6143.2006.01516.x [DOI] [PubMed] [Google Scholar]
- Zabel B.A., Agace W.W., Campbell J.J., Heath H.M., Parent D., Roberts A.I., Ebert E.C., Kassam N., Qin S., Zovko M., et al. 1999. Human G protein–coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256 10.1084/jem.190.9.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.