Abstract
Background
We report that elevated connexin-43 (Cx-43) in stem cells preconditioned with insulin like growth factor-1 (IGF-1) is cytoprotective and reprograms the cells for cardiomyogenic differentiation.
Methods and Results
Sca-1+ cells were preconditioned with 100nM IGF-1 for 30-minutes followed by 8-hours (h) of oxygen glucose deprivation (OGD) to assess the cytoprotective effects of preconditioning. LDH release assay, cytochrome-c release and mitochondrial membrane potential assay showed improved survival of preconditioned Sca-1+ cells (PCSca-1+) under OGD as compared to non-preconditioned Sca-1+ cells (non-PCSca-1+) via PI3K/Akt dependent caspase-3 downregulation. We observed PI3K/Akt dependent upregulation of cardiac specific markers including MEF-2c (2.5-fold), GATA4 (3.1-fold) and Cx-43 (3.5-fold). Cx-43 inhibition with specific RNAi reduced the cell survival under OGD and post-transplantation. In vivo studies were performed in a female rat model of acute myocardial infarction (n=78). Animals were grouped to receive intramyocardially 70μl DMEM without cells (group-1), or containing male 1×106 non-PCSca-1+ (group-2) or PCSca-1+ (group-3) cells labeled with PKH26. Survival of the PCSca-1+ was 5.5-fold higher in group-3 compared to group-2 on 7-days post-transplantation. Confocal imaging after actinin and Cx-43 specific immunostaining showed extensive engraftment and myogenic differentiation of PCSca-1+. As compared to group-2, group-3 showed increased blood vessel density (22.3±1.7/microscopic field, p<0.0001) and attenuated infarction size (23.3±3.6%; p=0.002). Heart function indices including ejection fraction (56.2±3.5; p=0.029) and fractional shortening (24.3±2.1; p=0.03) were improved in group-3 compared to group-2.
Conclusions
Preconditioning with IGF-1 reprograms Sca-1+ for pro-survival signaling and cardiomyogenic differentiation with an important role for Cx-43 in this process.
Keywords: Apoptosis, differentiation, preconditioning, connexin-43
Introduction
The electrical and mechanical integrity of the heart is compromised after myocardial infarction due to massive loss of functioning myocytes. The heart cell therapy provides a non-conventional corrective measure to compensate for myocyte loss in the infarcted heart.1–4 Nevertheless, poor survival of donor cells is one of the major concerns which hampers a better prognosis.5 Additionally, poor engraftment and lack of functional coupling of donor cells with the viable host tissue greatly impede cell-to-cell signaling and electrical communication. Most previous strategies have addressed the issue of cell survival alone albeit with limited success.6–8 As functional improvement of the heart is proportional to the number of injected cells; we therefore proposed that a strategy which concurrently addresses both these issues would increase the effectiveness of the procedure. Connexin-43 (Cx-43), with its dual role as an anti-apoptotic and as a gap junctional protein can effectively resolve both these issues.9–11
The connexin (Cx) family of genes encodes for more than 20 proteins of which Cx-30, Cx-37, Cx-40, Cx-43 and Cx-45 have been extensively studied for their role in the heart.12 Cx-43 is predominantly synthesized in the plasma membrane of cardiomyocytes and forms intercellular channels to link cytoplasmic compartments of the adjacent myocytes. As an alternative to paracrine mechanism of intercellular communication, Cx-43 ensures a direct transfer of ions and signaling molecules that regulates intracellular calcium and cell survival via releasing ATP, NAD+ or glutamate and propagation of electrical impulses.13,14 Gap junctional intercellular communication is also important for cellular proliferation and differentiation.15 Under physiological conditions, Cx-43 participates in cellular response to ischemia.16,17 Moreover, localization of Cx-43 in intracellular structures such as the mitochondria seems to be cardioprotective.18,19 Any reduction in Cx-43 renders the heart more susceptible to electrical instability. Cell-based delivery of Cx-43 transgene prevented ventricular arrhythmia post-infarction.19 The functional versatility of Cx-43 supports our study rationale that pharmacological targeting of Cx-43 in stem cells may improve their survival and integration in the infarcted heart. Different growth factors potentiate Cx-43 expression.20–22 Insulin like growth factor-1 (IGF-1) increases intracellular Cx-43.22 Our results emphasize the importance of IGF-1/IGF-1R interaction to initiate downstream survival signaling involving Cx-43 which primarily curtailed stem cell apoptosis and promoted their survival; the factors crucial for subsequent engraftment of donor cells in the infarcted heart. These are novel findings which underscore the need to exploit a dual role for the preconditioning induced Cx-43 to promote stem cell survival and their electromechanical coupling post-transplantation for better prognosis.
Materials and Methods
The experimental protocols are described in the expanded Supplemental Materials and Methods. The lists of antibodies and primers sequence used are given in Supplemental Table-I and Online Table-II.
Statistical Analysis
All experiments were performed at least three times to assess reproducibility of the results. The data were described as mean±SEM. Student’s t-tests or one-way or two-way analyses of variance (ANOVA) were performed to analyze statistical differences in each response variable. Pre-specified comparisons between groups were made and Bonferroni or Tukey adjustment for multiple comparisons was done when appropriate. A value of p≤0.05 was considered statistically significant.
The authors had full access to the data and took responsibility for its integrity. All authors read and agreed to the manuscript as written.
Results
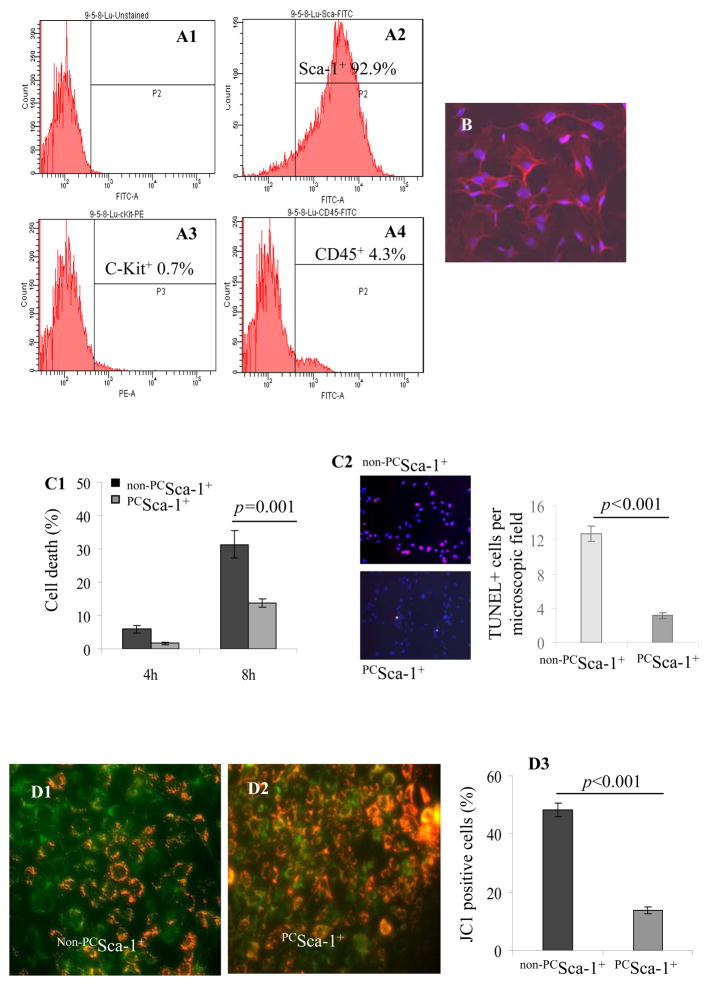
Flowcytometry showed that 92.9% cells expressed Sca-1 and were very low in ckit (0.7%) and CD45 (4.3%) expression (Figure 1A1–A4). The homogeneity of the purified cells was confirmed by fluorescent immunostaining for Sca-1 antigen (Figure 1B). These cells were later used in all the in vitro and in vivo experiments.
Figure 1.
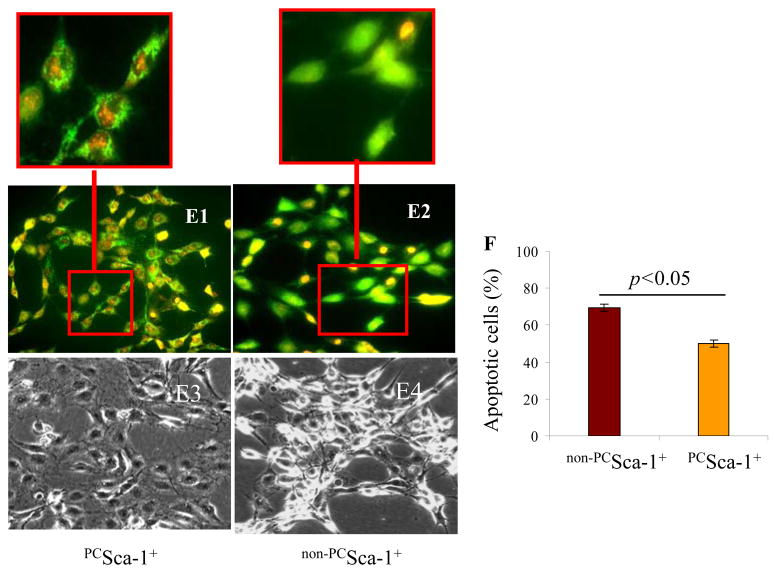
Flowcytometry of purified mice Sca-1+ cells for surface markers (A1) Unlabelled Sca-1 cells (as a control) and (A2) labeled cells showing 92.9% Sca-1+ (A3) 0.7% ckit+ and (A4) 4.3% CD45 cell population. (B) Immunostaining of cells for Sca-1 antigen (red=Sca-1 antigen; blue=DAPI; magnification x200). (C1) LDH release assay showed 31.4% cell death in BSA treated non-PCSca-1+ as compared to 13.7% in PCSca-1+. (C2) TUNEL staining showed that 8-h OGD caused higher TUNEL positivity in non-PCSca-1+ as compared to PCSca-1+. JC-1 staining of (D1) non-PCSca-1+ and (D2) PCSca-1+ for mitochondrial membrane potential after OGD. (D3) Significantly lower PCSca-1+ (13.8%) showed depolarized mitochondrial membrane potential as compared to non-PCSca-1+ (47.6%). Cytochrome-c specific immunostaining of (E1) PCSca-1+ and (E2) non-PCSca-1+ after OGD treatment. A typical punctuate distribution of cytochrome-c (green) was observed in PCSca-1+ as compared to the diffused appearance in non-PCSca-1+ (red boxed areas in E1 and E2 have been enlarged for clarity). Cell nuclei were observed by propidium iodide staining (red). Phase contrast microscopy showed better preserved cell morphology in (E3) PCSca-1+ as compared to (E4) non-PCSca-1+. (F) Annexin-V-FITC/PI staining showed reduced apoptosis in PCSca-1+ (50.5%) as compared to non-PCSca-1+ (69.7%).
Cytoprotection by IGF-1 pretreatment
The percentage cell viability under OGD was significantly higher in PCSca-1+ as compared to non-PCSca-1+. Preconditioning reduced the non-viable cells from 5.9% to 1.7% under 4-h OGD and from 31.4% to 13.7% under 8-h OGD (p=0.001 by two-way ANOVA; Figure 1C). These results were confirmed by TUNEL which showed that 8-h OGD caused higher TUNEL positivity in non-PCSca-1+ as compared to PCSca-1+ (Figure 1C2). JC-1, a cationic dye which exhibits membrane potential-dependent accumulation in the mitochondria, was used to detect early stage of apoptosis (Figure 1D1–D3). IGF-1 pretreatment significantly reduced the percentage of early apoptotic cells (green) under OGD (Figure 1D3). Moreover, cytochrome-c specific immunostaining revealed a typical punctuate distribution of fluorescence in the mitochondria of PCSca-1+ (Figure 1E1) whereas release of cytochrome-c from the mitochondria after OGD was indicated by diffused fluorescence in the cytoplasm (Figure 1E2). Phase contrast microscopy revealed well-preserved cell morphology in PCSca-1+ (Figure 1E3) as compared to non-PCSca-1+ (Figure 1E4). Annexin V-FITC/PI staining coupled with flowcytometry showed that preconditioning reduced apoptotic cells from 69.7% in non-PCSca-1+ to 50.5% in PCSca-1+ (Figure 1F). Taken together, IGF-1 preconditioning offered cytoprotection against OGD in vitro.
Cell signaling in IGF-1 initiated cytoprotection
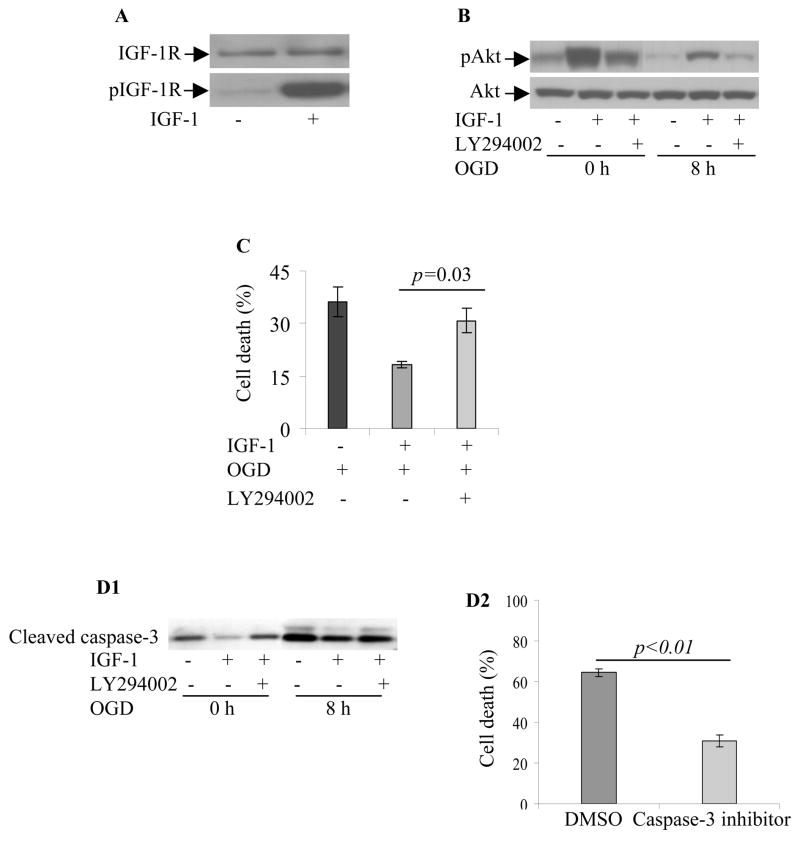
Sca-1+ cells expressed IGF-1 receptors which were phosphorylated by 30-min pretreatment with 100nM IGF-1 (Figure 2A). PI3K/Akt signaling plays a significant role downstream of IGF-1/IGF-1R ligand/receptor interaction 23. We observed higher pAkt in PCSca-1+ as compared to non-PCSca-1+ (Figure 2B). OGD for 8-h significantly reduced pAkt in non-PCSca-1+ while still remaining high in PCSca-1+ (Figure 2B). Pretreatment with PI3K inhibitor (LY294002; 40μM) abolished pAkt in PCSca-1+. The effect of LY294002 was more pronounced in the cells subjected to 8-h OGD as compared to normoxia. LDH-release assay showed higher PCSca-1+ survival under 8-h OGD as compared to non-PCSca-1+ (Figure 2C). However, cytoprotection was abolished in PCSca-1+ pretreated with LY294002 (17.9% in PCSca-1+ vs 31.2% in IGF-1+LY294002 treated cells). Western blotting showed that 8-h OGD increased caspase-3 cleavage (activation of caspase-3) in non-PCSca-1+ as compared to PCSca-1+. Contrarily, pretreatment with LY294002 showed a higher level caspase-3 cleavage in PCSca-1+ thus showing an inverse relation between dynamics of caspase-3 cleavage and Akt phosphorylation (Figure 2D1). Treatment with caspase-3 inhibitor and subsequent exposure to 8-h OGD showed enhanced cell survival under OGD (31.1%) as compared to inhibitor non-treated cells (64.9%) (Figure 2D2).
Figure 2.
Representative blots showing higher phosphorylation of (A) IGF-1R and (B) Akt in PCSca-1+ as compared to non-PCSca-1+. Treatment of the cells with PI-3K inhibitor (LY294002; 40μM) prior to preconditioning abolished pAkt under normoxia and after 8-h OGD. Total Akt remained unchanged. (C) LDH release assay showed that cell death was attenuated in PCSca-1+ as compared to non-PCSca-1+ under 8-h OGD. Pretreatment of cells with LY294002 abolished the cytoprotective effects of preconditioning under OGD. (D1) Downstream of pAkt, caspase-3 cleavage (active form) was significantly prevented in PCSca-1+ which otherwise was increased in non-PCSca-1+ under 8-h OGD. Pre-treatment of cells with LY294002 increased caspase-3 cleavage. (D2) Cells treated with caspase-3 inhibitor (Z-DEVD-FMK; 20μM) showed lower cell death as compared to the cells treated with DMSO (vehicle of caspase inhibitor).
Preconditioning with IGF-1 favored cardiomyogenesis
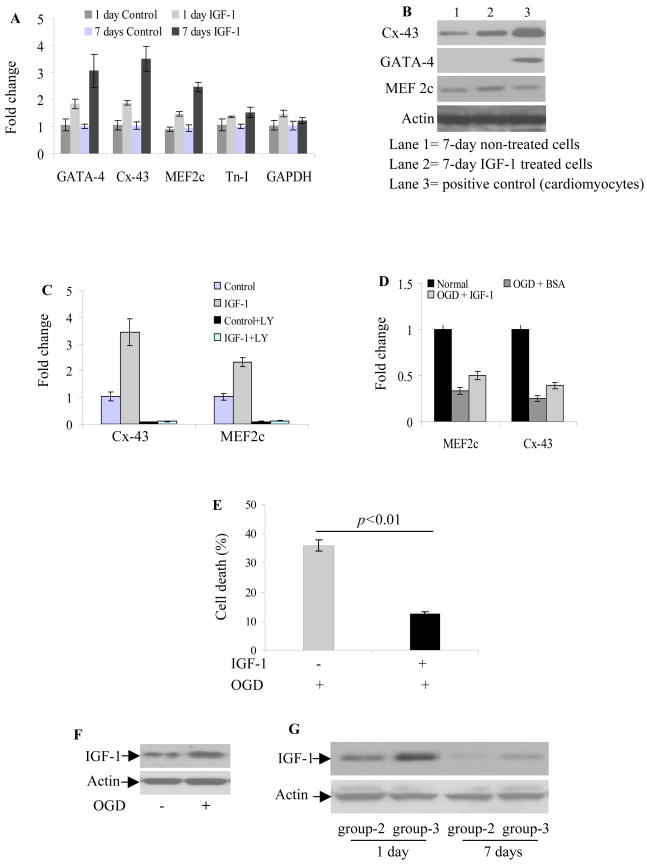
Although IGF-1 treatment for 1-day and 7-days significantly induced mRNA expression of cardiac marker proteins, 7-days IGF-1 treatment was more effective in upregulating GATA-4 (3.1-fold), Cx-43 (3.5-fold) and MEF-2c (2.5-fold) whereas troponin-I (Tn-I) upregulation remained insignificant (Figure 3A). Western blotting confirmed these findings except for GATA-4 which remained undetectable (Figure 3B). LY294002 pretreatment reversed the effect of IGF-1 on cells grown in normoxia (Figure 3C). We observed that cardiac-specific gene expression was sensitive to anoxia. Cells cultured with 0.5% BSA for 12-h under OGD showed abrogation of Cx-43 and MEF2c whereas presence of IGF-1 during OGD significantly enhanced their gene expression (Figure 3D). The presence of IGF-1 during OGD also offered cytoprotection besides promoting cardiac-specific proteins (Figure 3E). These data suggested that OGD not only imposed a survival challenge for cells but also impeded their differentiation. Even though downregulation of cardiac markers under OGD was not seen fully reversed from our 12-h study, the presence of IGF-1 supported Sca-1+ cells towards cardiac differentiation. Hence, it is reasonable to project a more pronounced effect of IGF-1 in vivo considering the kinetics of its expression in the infarcted heart which significantly affects the transplanted cells. In accordance with Western blotting results (Figure 3F), PCSca-1+ transplanted hearts showed higher IGF-1 on day-1 and day-7 (Figure 3G).
Figure 3.
(A) Real-time PCR of Sca-1+ after 1-day and 7-days IGF-1 treatment in culture. The cells cultured for 1-day and 7-days without IGF-1 were used as controls. IGF-1 treatment for 7-days induced higher mRNA expression of GATA-4 (3.1-fold), Cx-43 (3.5-fold) and MEF2c (2.5-fold) whereas troponin-I (Tn-I) upregulation was statistically insignificant. (B) Western blot on cell lysate from these treatment groups confirmed these findings. Whereas GATA-4 protein expression remained undetectable, both Cx-43 and MEF-2c protein level were significantly increased after 7-days IGF-1 treatment under normoxia. (C) Cx-43 and MEF-2c mRNA induction by IGF-1 treatment was abolished by LY294002 (IGF-1 without LY; MEF2C p=0.0003 and Cx-43 p=0.0021; IGF-1 with LY; MEF2C p=0.99 and Cx-43 p=1; two-way ANOVA,). (D) The expression of Cx-43 and MEF-2c mRNA was sensitive to anoxia and was abolished in Sca-1+ after 12-h OGD. However, effect of OGD was less pronounced in Sca-1+ with continuous IGF-1 presence during 12-h OGD treatment. (E) LDH release assay showed that continuous treatment with IGF-1 during OGD greatly reduced cell death from 35.8% to 12.4%. (F) Representative blots of Sca-1+ after 8-h OGD showing enhanced IGF-1 protein expression as compared to Sca-1+ grown under normoxia. (G) Representative blots of protein samples from rat heart on 1-day and 7-days after cell engraftment. PCSca-1+ showed higher IGF-1 expression on 1-day and 7-days as compared to non-PCSca-1+.
Cx-43 confers cytoprotection
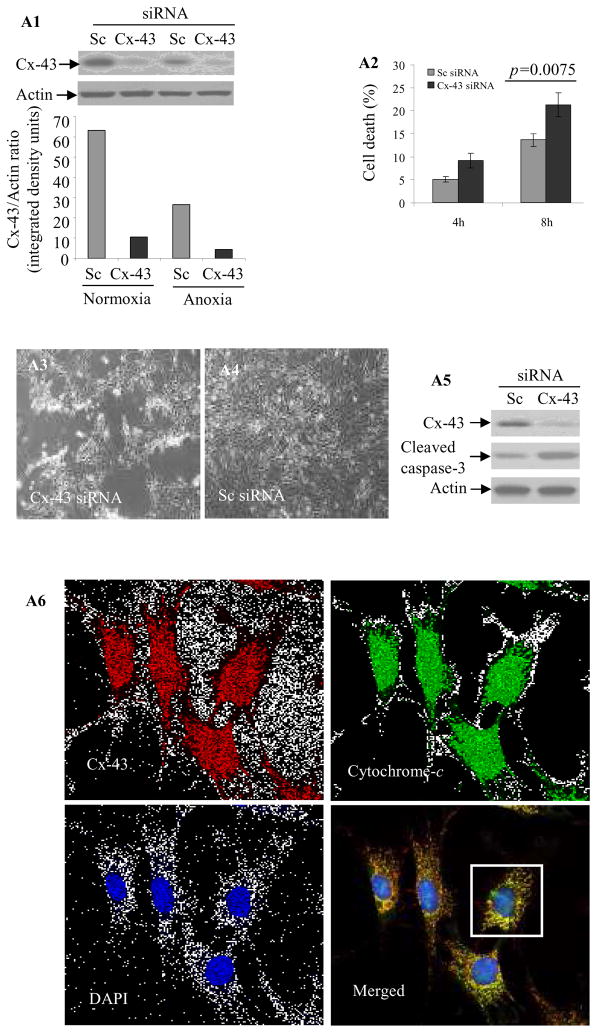
Since we observed that IGF-1 preconditioning increased Cx-43 in Sca-1+, we established its role in cytoprotection. Cx-43 was abolished in Cx-43 siRNA transfected cells grown under normoxia or OGD as compared to Sc siRNA transfected cells (Supplemental Figure I). These results were confirmed by Western blotting (Figure 4A1). LDH assay showed higher cell death in Cx-43 siRNA transfected cells under 4-h and 8-h OGD as compared to Sc siRNA transfected cells (p=0.0075 by two way ANOVA; Figure 4A2). Additionally, Sca-1+ transfected with Cx-43 siRNA showed shrunk and rounded morphology (Figure 4A3) as compared to Sc siRNA transfected cells (Figure 4A4). Elucidating the mechanism for increased death in Cx-43 siRNA transfected cells under 8-h OGD, we observed that caspase-3 cleavage was significantly increased (Figure 4A5). Double fluorescence immunostaining revealed punctate and co-localized distribution of Cx-43 (red) and cytochrome-c (green) in the mitochondria (Figure 4A6). Figure 4A7 shows magnified image of a Sca-1+ cell from Figure 4A6 (white box) to show co-localization of Cx-43 (red) and cytochrome-c (green). Western blotting on sub-cellular fractions confirmed a higher presence of Cx-43 in the mitochondrial fraction in PcSca-1+ (Figure 4B). VDAC was used as an indicator of purity of the mitochondrial fraction. Real-time PCR based gene-array showed that 10 genes from the apoptotic cascade had more than 2-fold higher expression in Cx-43 siRNA transfected cells (Supplemental Figure II). The expression of caspase recruitment domain family member-10 (Card-10) which increased 6.02-fold in Cx-43 siRNA transfected cells under normoxia led to higher sensitivity to anoxia and increased to more than 10-fold (Supplemental Figure III). We observed that elevation of Cx-43 in response to IGF-1 treatment abrogated Card-10 with concomitant increase in cell survival under OGD (Figure 4C).
Figure 4.
(A1) Western blot of Sc siRNA (control) or Cx-43 siRNA transfected Sca-1+ cells grown under normoxia or OGD. Cx-43 siRNA successfully abolished Cx-43 expression. (A2) LDH release assay showing higher cell death in Cx-43 siRNA transfected cells as compared to Sc siRNA transfected cells under 4-h and 8-h OGD. Similarly, Cx-43 siRNA transfected cells (A3) showed more obvious rounded and shrunk morphology under 8-h OGD as compared to (A4) Sc siRNA transfected cells. (A5) Western blot showing elevated caspase-3 cleavage in Cx-43 siRNA transfected cells as compared to Sc siRNA cells. (A6) Double fluorescence immunostaining showing distribution of co-localized Cx-43 (red) and cytochrome-c (green) in mitochondria as punctate bodies in Sca-1+. (A7) The magnified image of a Sca-1+ cell from A6 (white box; original magnification=63x) showing clear apposition of red (Cx-43) and cytochrome-c (green). (B) Western blot showing higher level Cx-43 in the mitochondrial fraction of Sca-1+ after IGF-1 treatment as compared with cells without IGF-1 treatment. (C) IGF-1 treatment significantly increased Cx-43 level with concomitant suppression of Card-10 as compared to BSA treated cells. (D) Real-time PCR for mice sry-gene in female rat hearts (n= 4/group) on day-7 post-transplantation of male Sca-1+. The cells were transfected with Sc siRNA or Cx-43 siRNA. Cx-43 siRNA significantly reduced the survival of Sca-1+.
Sca-1+ from male mice transfected with Cx-43 siRNA (n=4) or Sc siRNA cells (n=4) were transplanted in a female rat heart model of acute myocardial infarction. Real-time PCR for sry-gene on day-7 myocardial samples showed that survival of Cx-43 siRNA transfected cells was significantly lower as compared to Sc siRNA transfected cells (Figure 4D).
In vivo proliferation and survival of PCSca-1+
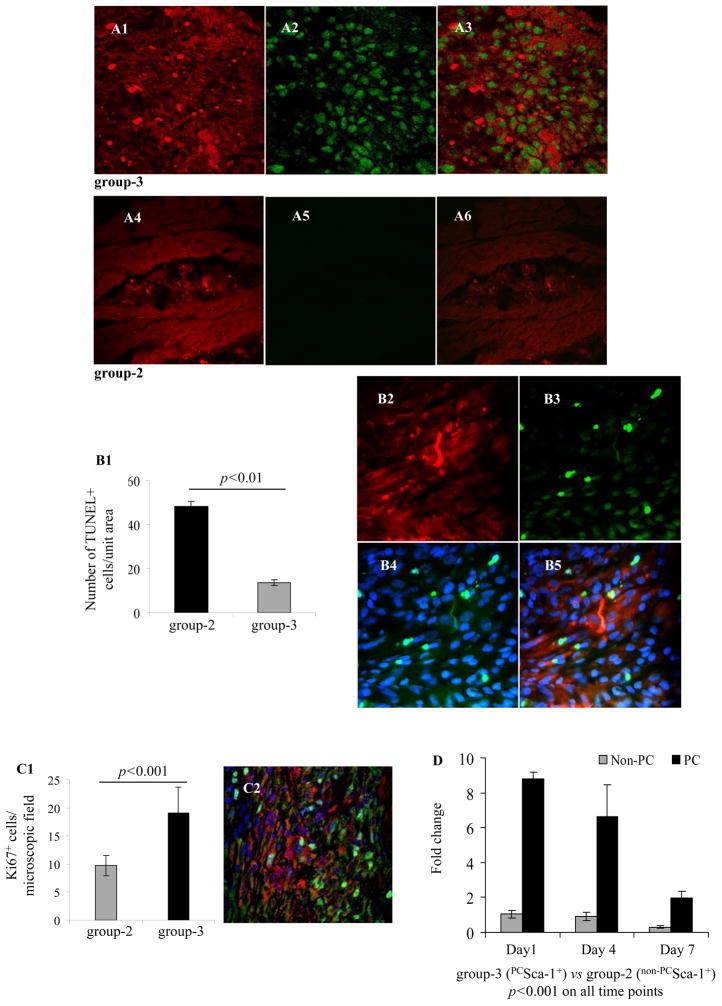
Immunostaining showed pronounced pAkt at the site of cell graft in PCSca-1+ transplanted animal (group-3) (Figure 5A1–A3) as compared to non-PCSca-1+ transplanted animals (group-2) (Figure 5A4–A6). The number of TUNEL+ cells was significantly higher in group-2 as compared to group-3 on day-7 after engraftment (Figure 5B1–B5). We also observed higher presence of Ki67+ cells in group-3 (p<0.001 vs group-2) especially in the cell engrafted regions (Figure 5C1–C2). For an overall estimation of donor cell survival, entire LV was used for mice sry-gene estimation in female recipient rat heart on 1-day, 4-days and 7-days after engraftment of male donor cells. Significantly higher cell survival was observed in group-3 as compared to group-2 on all the studied time-points (Supplemental Table-III and Figure 5D). DMEM injected female animal hearts (group-1) were used as a control.
Figure 5.
Confocal microscopy images of rat heart tissue sections on 1-day after transplantation of PKH 26 labeled (A1-A3) PCSca-1+ and (A4-A6) non-PCSca-1+(red fluorescence). (A3) Merged image showing higher pAkt levels (green) in group-3 animal hart as compared to (A6) group-2. (B1–B5) TUNEL staining on histological sections from various treatment groups on day-7 showed that the number of TUNEL+ cells was lower in group-3 as compared to group-2. B2–B5 are representative Figures of the TUNEL stained histological sections on day-7 after cell engraftment in group-3 (B2 red= PKH26; B3 green=TUNEL staining; B4 merged image of TUNEL staining (green) with DAPI (blue) to visualize all the cell nuclei; B5= B2–B4 merged image. (C1) Graph showing higher number of Ki67+ cells in group-3 as compared to group-2 on 7-day after cell engraftment as determined by Ki67 specific immunostaining. (C2) Representative confocal images of Ki67+ cells (green) in infarction area 7-days after PKH26 labeled PCSca-1+ engraftment (red). DAPI was used for visualization of the nuclei (blue). (D) Real-time PCR for mouce sry-gene to determine donor cell survival in the female recipient heart. PCSca-1+ survival was significantly higher as compared to non-PCSca-1+ on all time-points of engraftment.
Angiomyogenic fate of PCSca-1+
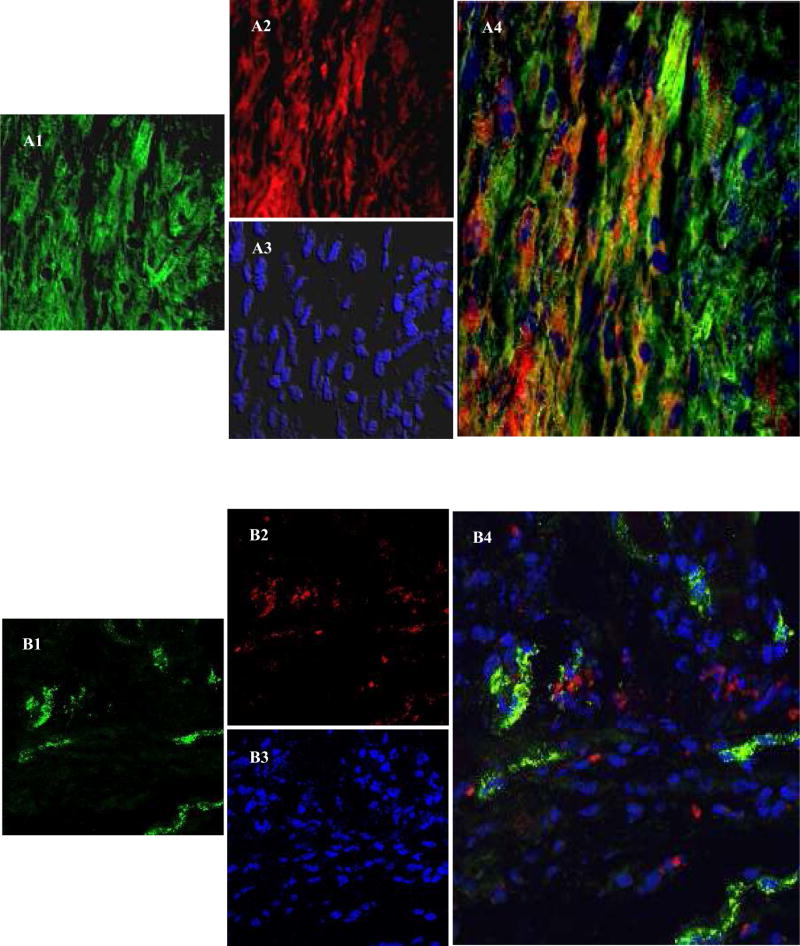
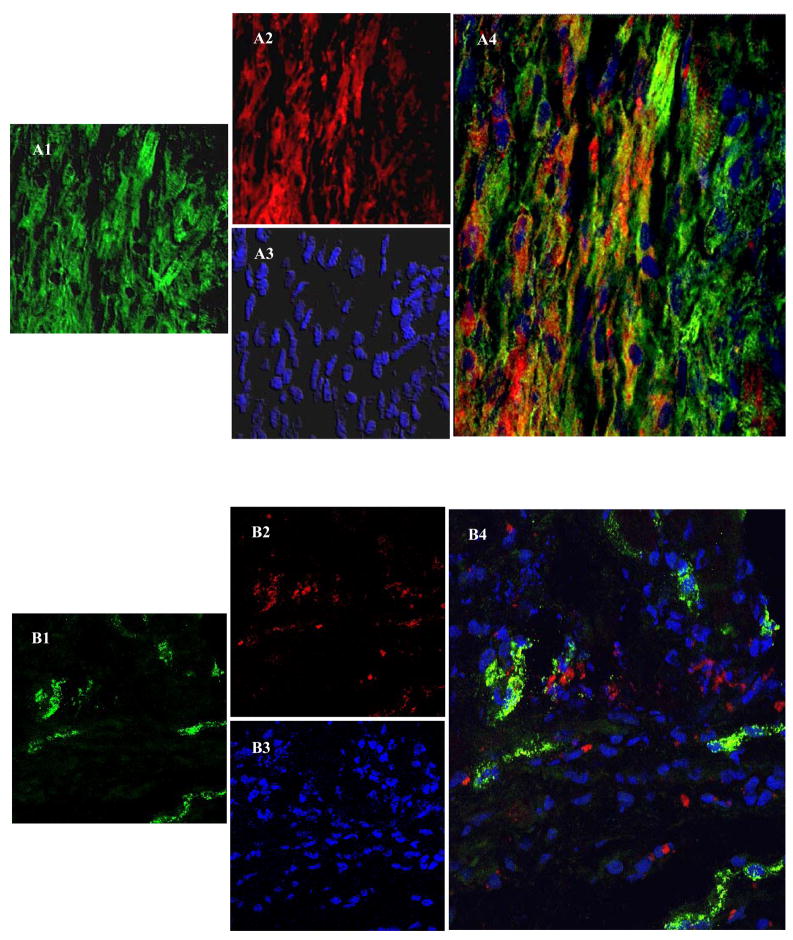
Confocal imaging after immunostaining for α-sarcomeric actinin 7-days after cell engraftment depicted extensive neomyogenesis in PCSca-1+ transplanted area (Figure 6A1–A4). Co-localization of PKH26 labeled cells (red) with cardiac actinin (green) was observed in the infarct and peri-infarct regions thus indicating myogenic differentiation of the engrafted cells. However, propensity of differentiating non-PCSca-1+ was obviously low as compared to PCSca-1+ (Figure 6B1–B4). These results suggest that Sca-1+ has inherent differentiation potential which gets accentuated by preconditioning. Immunostaining of histological sections from group-3 for myosin heavy chain (slow-isoform) and Cx-43 showed that neofibers also expressed Cx-43 and were well-engrafted in the host tissue (Figure 6C1–C4). Electron microscopy confirmed the presence of tight junctions and myofilaments in the newly differentiating Sca-1+ (Figure 6D1–D4).
Figure 6.
Confocal images of histological sections immunostained for α-sarcomeric actinin on 7-day after cell engraftment; (Figure A1–A4) group-3 and (Figure B1–B4) group-2. C1–C4 shows immunostaining of the histological sections for myosin heavy chain (MHC) at 6-weeks after PCSca-1+ transplantation. Primary antigen-antibody reaction in all the panels was detected by secondary antibody conjugated with Alexa Flour-488 (green A1, B1 & C1). The cells were labeled with PKH-26 (red; A2, B2 & C2). The nuclei were visualized with DAPI (blue; A3, B3 & C3). C3 is a merged image of MHC (green) counterstained for Cx-43 expression (cyan) and DAPI (blue). Merged images (A4 & C3–C4) showed extensive myogenesis in group-3 animal hearts as compared to (B4) group-2 in the center of the infarct region. Figure C4 shows co-localization of MHC (green) and Cx-43 (cyan) in the newly formed myofibers in the cell engrafted region (red). Large yellow and red boxes are magnified in Figure C4 to show co-localization of Cx-43 (cyan), MHC (green) and PKH26 (red). (D1–D4) Ultra-structure studies of the rat heart tissue at 6-weeks in group-3. D1 shows tight junctions between two adjacent differentiating cells (Yellow box). D3 shows typical newly differentiating myofibers with visible filaments (red box). D2 and D4 represent magnified images of D1 and D3 respectively.
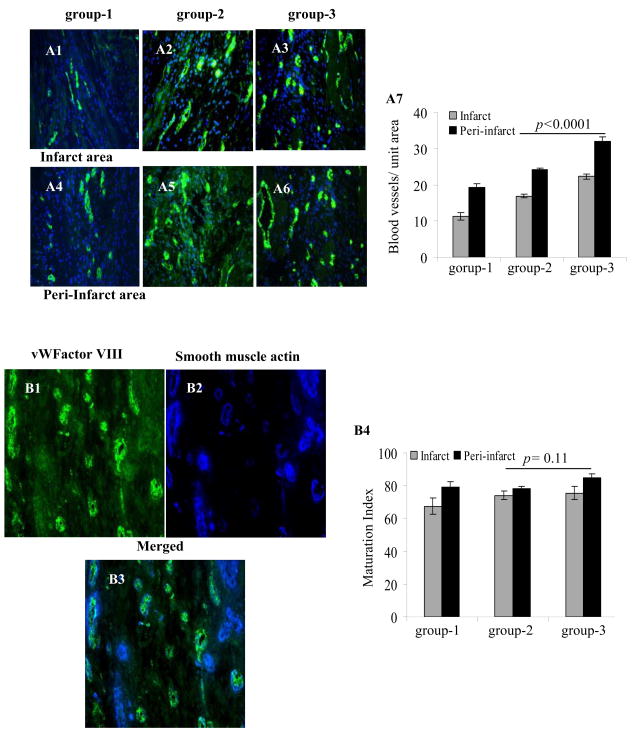
To evaluate angiogenesis and maturation index at 6-weeks time-point, histological sections were immunostained for vWFactor-VIII alone (Figure 7A1–A7) or with SMA (Figure 7B1–B4) respectively. Highest angiogenic activity was observed in group-3 (Figure 7A7). By two-way ANOVA, blood vessel density in infarct and peri-infarct regions (22.3±1.7; 32 ±2.2 respectively) was higher as compared to group-2 (16.9±1.5, p<0.0001; 24.3±1.4, p<0.0001) and group-1 (11.3±1.6 p<0.0001; 19.3±2.1 p<0.0001). There was no significant difference in maturation index amongst the three groups in the infarct region but group-3 showed higher maturation index in peri-infarct region as compared with group-2 (p=0.11 by two-way ANOVA; Figure 7B1–B4). However, total number of mature blood vessels was higher in group-3 as compared to the other groups.
Figure 7.
Immunostaining of rat heart tissue sections for vWFactor-VIII (green) in the infarct (A1–A3) and peri-infarct (A4–A6) areas 6-weeks in group-1 (A1 & A4), group-2 (A2 & A5) or group-3 (A3 & A6). The nuclei were visualized with DAPI (blue). (A7) Blood vessel density was highest in group-3 in the infarct and peri-infarct areas. (B1–B4) Double immunostaining of rat heart tissue samples for vWFactor-VIII (green) and smooth muscle actin (SMA, blue) for determination of maturation index of the newly formed blood vessels in group-3. Blood vessel maturation index changed insignificantly between the three treatment groups. (Magnification=x400).
Infarction size and the heart function
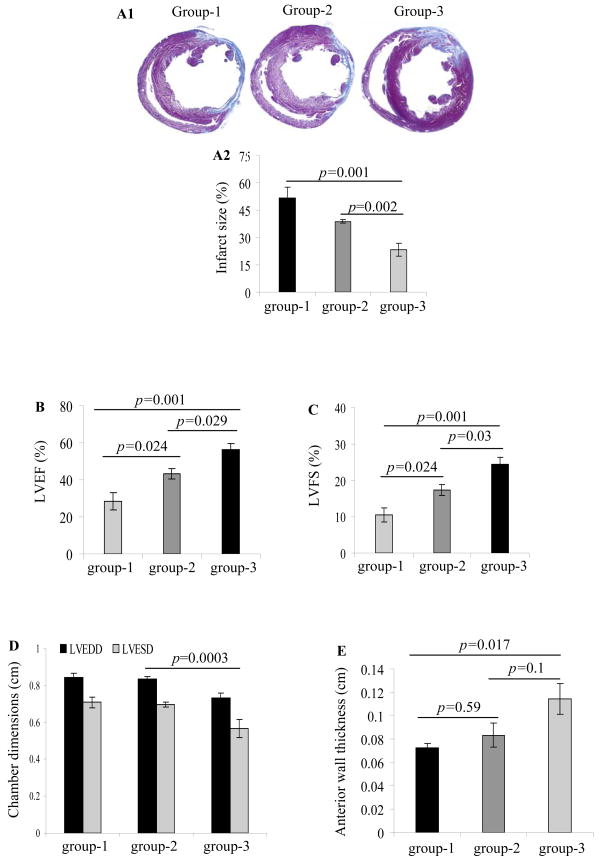
Cross-sections at mid-papillary muscle level showed transmural infarction in all the animals. Marked LV-wall thinning was observed at 6-weeks in group-1 with 51.9±5.6% infarction of the LV (Figure 8A1). Comparatively, infarction size at 6-weeks was attenuated in group-2 (38.8±1.2%) and group-3 (23.3±3.6%) (p=0.002 vs group-2) (Figure 8A2).
Figure 8.
(A1–A2) Measurement of the infarction size by Masson’s trichome staining on formalin fixed paraffin embedded histological sections. Infarction size was significantly attenuated in group-3 as compared to group-1 and group-2. (B–E) Indices of heart function including ejection fraction, fractional shortening, LV chamber dimensions, and wall thickness improved significantly in the cell transplanted groups-2 and 3 as compared to group-1 at 6-weeks after their respective treatment. However, the highest improvement was evident in group-3.
The indices of systolic function including LVEF and LVFS were higher in group-2 (43.3±2.8%; p=0.024 and 17.3±1.4%; p=0.024) and group-3 (56.2±3.5%; p=0.001 and 24.3±2.1%; p=0.001) as compared to group-1 (28.3±4.7%; 10.5±2%) (Figure 8B & 8C). LVEF (p=0.029) and LVFS (p=0.03) between group-3 and group-2 also showed significant difference. LVESD (cm) was smaller in group-3 (0.57±0.05; p=0.0003 vs all other groups by two-way ANOVA) as compared to group-1 (0.71±0.03) and group-2 (0.7±0.01) (Figure 8D). LV-wall thickness improved from 0.07cm in group-1 to 0.08±0.01cm in group-2 (group-2 vs group-1 p=0.59) and 0.11±0.01cm in group-3 (group-3 vs group-1 p=0.02) (Figure 8E).
Discussion
IGF-1 acts through IGF-1/IGF-1R ligand/receptor system and significantly affects LV remodeling.24 The IGF-1 autocrine and paracrine systems in the viable cardiomyocytes are altered transiently in response to myocardial infarction.25 Therefore, strategies were designed to manipulate intrinsic IGF-1 for its continuous participation in myocardial repair. To this end, IGF-1 protein and transgene delivery either by direct DNA injection or by ex-vivo cell based delivery has been studied.26,27 We have recently shown that IGF-1 transgene overexpression in the mesenchymal stem cells promoted their survival post-transplantation.28 Similar beneficial effects were achieved by preconditioning to activate IGF-1 mediated signaling in the stem cells. Our preconditioning approach with IGF-1 protein is simpler and much safer to follow in the clinical perspective as compared with IGF-1 transgene overexpression. The results of preconditioning also implied that IGF-1 was not required to be carried by stem cells. Preconditioning improved cell survival through classical survival signaling involving Akt activation which preserved mitochondrial integrity and prevented cytochrome-c release thus preventing caspase-3 cleavage with a possible role for Cx-43. Multiple cellular and physiological functions of IGF-1 have been reported through activation of PI3K–Akt.27,29,30 Interestingly, 30-min preconditioning in this study generated pAkt which remained elevated after 8-h OGD in vitro and for 24-h after transplantation. We infer that better engraftment of PcSca-1+ after transplantation in this study was due to improved cell survival, reduced cell apoptosis together with increased cell proliferation.
Another interesting observation in this study was elevation of IGF-1 in the infarcted heart after PCSca-1+ engraftment. Besides adoption of cardiac phenotype, the beneficial effect of the heart cell therapy is attributed to release of paracrine factors which gets accentuated by their genetic and pharmacological manipulation.6,31 Western blotting showed copious expression of IGF-1 from Sca-1+ under OGD in vitro and in the infarcted heart until 7-days of observation. Additionally, we observed that short-term IGF-1 treatment for preconditioning and continuous presence of IGF-1 during OGD had distinct effects on stem cells in terms of cardiomyogenic gene expression. Firstly, the timing of IGF-1 paracrine release was optimal to provide a second window of preconditioning when the effects of initial in vitro preconditioning vanished. Secondly, in agreement with our in vitro results from supplementation of recombinant IGF-1 for 7-days in culture under normoxia as well as anoxia, duration of IGF-1 paracrine release in vivo spanning over 7-days prompted expression of cardiac specific marker proteins including Cx-43. Therefore, PCSca-1+ manifested enhanced cardiomyogenesis as compared to their non-PCSca-1+ counterparts. This was evident from immunohistological studies where α-sarcomeric actinin and myosin heavy chain (slow isoform) positive structures were co-localized with Cx-43 expression thus signifying stem cells engraftment and coupling with existing fibers. We therefore inferred that cardiomyogenesis observed in this study was due to extensive transdifferentiation of PCSca-1+. Although the neofibers were connected by gap junctions, their real contribution to contractility was not determined. Additional studies are therefore needed to demonstrate that neofibers were functionally competent and contributed to improved heart function. Based on the current data, it is safe to say that improvement in global heart function was due to implantation of MSCs forming neofibers and release of paracrine factors.1,2
We also discovered that Cx-43 expression indeed played an important role in cytoprotective effects of preconditioning. As discussed earlier, Cx-43 is responsible for electrical coupling and maintenance of homeostasis between the adjacent cardiomyocytes. In other cells such as astrocytes, gap junctions composed of Cx-43 reduced apoptotic neuronal damage in cerebral ischemia.32 Moreover, Cx-43 hemichannels are capable of responding to extracellular cues and induce survival signals via extracellular signal-regulated kinase (ERK) activation.33 Cx-43 is also located in the mitochondrial membrane of cardiomyocytes and is up-regulated in response to ischemic preconditioning.34 Additionally, Cx-43 translocates into the mitochondrial membrane in response to ischemic stress.35 In both the cases, Cx-43 becomes modulator of mitochondrial functions during cell apoptosis. We observed that abrogation of Cx-43 in Sca-1+ caused poor cell survival under OGD and in the infarcted heart. Although mechanism of Cx-43 in conferring cytoprotection after preconditioning requires more and in-depth studies, our data showing punctate and co-localized distribution of Cx-43 and cytochrome-c in Sca-1+ cells confirms its mitochondrial distribution. Supporting an anti-apoptotic role for Cx-43, further studies will be required to delineate the mechanism by which Cx-43 prevents cytochrome-c translocation from the mitochondria into the cytoplasm. We are currently working on our hypothesis that Cx-43 either integrates into the membrane transition pores or develop a complex with cytochrome-c through hydrophobic interaction thus preventing its release into the cytoplasm. Furthermore, expression of Card-10, with its well-documented role in caspase activation in the context of apoptosis, showed sensitivity to Cx-43 overexpression as a result of IGF-1 treatment.36 Taken together, preconditioning with IGF-1 concurrently incurred cytoprotection leading to cardiomyogenesis which were not mutually exclusive molecular events, but instead, intertwined with each other.
These are important findings albeit with limitations which warrant additional studies. One limitation is the lack of functional evidence for electrical coupling of the newly formed myofibers. Selection of optimal cell type remains a major challenge for transplantation in the heart. Bone marrow derived cells in this regard were the focus of interest2 and have been extensively studied for their ability to adopt cardiac phenotype in the infarcted heart.6,7 More recent studies have shown that they share similar electrical characteristics as native cardiomyocytes.37 We were particularly interested in Sca-1+ cell population due to association of Sca-1 antigen with cell growth activity and differentiation potential.38 Sca-1+ have wide distribution in the body tissues including skeletal muscle, heart and bone marrow and have been manipulated to adopt cardiac phenotype.39 High level Sca-1 expressing mesenchymal multipotent stem cells differentiate into cardiomyocytes.40 Further studies should focus on defining Sca-1+ with multiple surface markers to select sub-lineages with greater cardiomyogenic capacity and assessing functional coupling. The cytoprotective role of IGF-1 induced Cx-43 could implicate Bcl10 and NFκB signaling. Despite these study limitations, overwhelmingly beneficial effects of IGF-1 preconditioning, simplicity, reproducibility, and easy adoptability together with lack of safety issues make this approach highly appealing for clinical applications.
Supplementary Material
Acknowledgments
Funding Sources. This work was supported by National Institutes of Health grants# R37-HL074272; HL-080686; HL087246 (M.A) and HL087288; HL089535 (Kh.H.H).
Footnotes
Potential Clinical Impact of Findings
The hostile microenvironment of ischemic myocardium causes extensive cell death. Preconditioning of stem cells by short-term treatment with growth factor is shown here as a simple, safe, effective and potential therapeutic intervention in the clinical perspective to promote their survival post-engraftment. IGF-1 preconditioning also promoted Cx-43 translocation to the mitochondria. Studies are being carried out to portray a possible role for the mitochondrial Cx-43 in cytoprotection. The potential benefits of preconditioning the stem cells before transplantation would be enormous and will revolutionalize our thinking on the strategies for cell based therapies.
Disclosures. None
References
- 1.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–9. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 2.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–37. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 3.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 4.Couzin J. Clinical trials. A shot of bone marrow can help the heart. Science. 2006;313:1715–6. doi: 10.1126/science.313.5794.1715a. [DOI] [PubMed] [Google Scholar]
- 5.Pouzet B, Vilquin JT, Hagege AA, Scorsin M, Messas E, Fiszman M, Schwartz K, Menasche P. Factors affecting functional outcome after autologous skeletal myoblast transplantation. Ann Thorac Surg. 2001;71:844–50. doi: 10.1016/s0003-4975(00)01785-9. [DOI] [PubMed] [Google Scholar]
- 6.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 7.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–50. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–21. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 9.Giardina SF, Mikami M, Goubaeva F, Yang J. Connexin-43 confers resistance to hydrogen peroxide-mediated apoptosis. Biochem Biophys Res Commun. 2007;362:747–52. doi: 10.1016/j.bbrc.2007.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krysko DV, Mussche S, Leybaert L, D’Herde K. Gap junctional communication and connexin-43 expression in relation to apoptotic cell death and survival of granulosa cells. J Histochem Cytochem. 2004;52:1199–207. doi: 10.1369/jhc.3A6227.2004. [DOI] [PubMed] [Google Scholar]
- 11.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–24. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 12.Duffy HS, Fort AG, Spray DC. Cardiac connexins: genes to nexus. Adv Cardiol. 2006;42:1–17. doi: 10.1159/000092550. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin-43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retamal MA, Schalper KA, Shoji KF, Orellana JA, Bennett MV, Saez JC. Possible involvement of different connexin-43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J Membr Biol. 2007;218:49–63. doi: 10.1007/s00232-007-9043-y. [DOI] [PubMed] [Google Scholar]
- 15.Worsdorfer P, Maxeiner S, Markopoulos C, Kirfel G, Wulf V, Auth T, Urschel S, von Maltzahn J, Willecke K. Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells. Stem Cells. 2008;26:431–9. doi: 10.1634/stemcells.2007-0482. [DOI] [PubMed] [Google Scholar]
- 16.Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin-43 in hypoxic preconditioning. J Neurosci. 2008;28:681–95. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutnik CM, Pocrnich CE, Liu H, Laird DW, Shao Q. The protective effect of functional connexin-43 channels on a human epithelial cell line exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:800–6. doi: 10.1167/iovs.07-0717. [DOI] [PubMed] [Google Scholar]
- 18.Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin-43 regulates apoptosis. Biochem Biophys Res Commun. 2007;352:97–103. doi: 10.1016/j.bbrc.2006.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–24. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 20.Srisakuldee W, Nickel BE, Fandrich RR, Jiang ZS, Kardami E. Administration of FGF-2 to the heart stimulates connexin-43 phosphorylation at protein kinase C target sites. Cell Commun Adhes. 2006;13:13–9. doi: 10.1080/15419060600631326. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S. G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med. 2006;203:87–97. doi: 10.1084/jem.20051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia G, Mitra AK, Cheng G, Gangahar DM, Agrawal DK. Angiotensin II and IGF-1 regulate connexin-43 expression via ERK and p38 signaling pathways in vascular smooth muscle cells of coronary artery bypass conduits. J Surg Res. 2007;142:137–42. doi: 10.1016/j.jss.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Kulik G, Weber MJ. Akt-dependent and -independent survival signaling pathways utilized by IGF-I. Mol Cell Biol. 1998;18:6711–8. doi: 10.1128/mcb.18.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desnoyers L, Simonette RA, Vandlen RL, Fendly BM. Novel non-isotopic method for the localization of receptors in tissue sections. J Histochem Cytochem. 2001;49:1509–18. doi: 10.1177/002215540104901204. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KG, Devlin GP, Conaglen JV, Stuart SP, Mervyn Aitken W, Bass JJ. Changes in IGFs in cardiac tissue following myocardial infarction. J Endocrinol. 1999;163:433–45. doi: 10.1677/joe.0.1630433. [DOI] [PubMed] [Google Scholar]
- 26.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial IGF-1 delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanemitsu N, Tambara K, Premaratne GU, Kimura Y, Tomita S, Kawamura T, Hasegawa K, Tabata Y, Komeda M. IGF-1 enhances the efficacy of myoblast transplantation with its multiple functions in the chronic myocardial infarction rat model. J Heart Lung Transplant. 2006;25:1253–62. doi: 10.1016/j.healun.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Haider HKh, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 29.Liu TB, Fedak PW, Weisel RD, Yasuda T, Kiani G, Mickle DA, Jia ZQ, Li RK. Enhanced IGF-1 expression improves smooth muscle cell engraftment after cell transplantation. Am J Physiol Heart Circ Physiol. 2004;287:H2840–9. doi: 10.1152/ajpheart.00439.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. IGF promotes engraftment, differentiation, and functional improvement after transfer of ESCs for myocardial restoration. Stem Cells. 2004;22:1239–45. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 31.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 32.Nakase T, Fushiki S, Sohl G, Theis M, Willecke K, Naus CC. Neuroprotective role of astrocytic gap junctions in ischemic stroke. Cell Commun Adhes. 2003;10:413–7. doi: 10.1080/cac.10.4-6.413.417. [DOI] [PubMed] [Google Scholar]
- 33.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–57. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 34.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana R, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin-43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin-43 to the inner mitochondrial membrane of cardiomyocytes through HSP-90 dependent TOM pathway and its importance for cardioprotection. Circ Res. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 36.Bouchier-Hayes L, Martin SJ. CARD games in apoptosis and immunity. EMBO reports. 2002;3:616–621. doi: 10.1093/embo-reports/kvf139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pijnappels DA, Schalij MJ, Ramkisoensing AA, van TJ, de Vries AA, van Der LA, Ypey DL, Atsma DE. Forced alignment of MSCs undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 38.Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–43. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 40.Gojo S, Gojo N, Takeda Y, Mori T, Abe H, Kyo S, Hata J, Umezawa A. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res. 2003;288:51–9. doi: 10.1016/s0014-4827(03)00132-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.