Abstract
Animal models for Duchenne muscular dystrophy (DMD) have species limitations related to assessing function, immune response, and distribution of micro- or mini-dystrophins. Nonhuman primates (NHPs) provide the ideal model to optimize vector delivery across a vascular barrier and provide accurate dose estimates for widespread transduction. To address vascular delivery and dosing in rhesus macaques, we have generated a fusion construct that encodes an eight amino-acid FLAG epitope at the C-terminus of micro-dystrophin to facilitate translational studies targeting DMD. Intramuscular (IM) injection of AAV8.MCK.micro-dys.FLAG in the tibialis anterior (TA) of macaques demonstrated robust gene expression, with muscle transduction (50–79%) persisting for up to 5 months. Success by IM injection was followed by targeted vascular delivery studies using a fluoroscopy-guided catheter threaded through the femoral artery. Three months after gene transfer, >80% of muscle fibers showed gene expression in the targeted muscle. No cellular immune response to AAV8 capsid, micro-dystrophin, or the FLAG tag was detected by interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) at any time point with either route. In summary, an epitope-tagged micro-dystrophin cassette enhances the ability to evaluate site-specific localization and distribution of gene expression in the NHP in preparation for vascular delivery clinical trials.
Introduction
Duchenne muscular dystrophy (DMD), inherited as an X-linked recessive disorder with monogenic mutations, is the most common devastating muscle disease of childhood. Potential gene replacement strategies are under investigation. Progress toward clinical gene therapy with mini- and micro-dystrophin constructs delivered by adeno-associated virus (AAV) has gained momentum.1,2 Proof-of-principle studies in the mdx mouse with micro-dystrophin have demonstrated reversal of the dystrophic process with reduced central nucleation, improvement in tetanic force measures and increased resistance to eccentric contractions.3,4,5 Gene replacement studies in mdx mice have also shown promise in heart6,7,8 and diaphragm muscle.9,10,11 In order to produce clinically meaningful outcomes of gene transfer, multiple muscle groups will require transduction. Both arterial and venous approaches have demonstrated success using rAAV6, rAAV8, and rAAV9 in preclinical studies in mdx mice and canine dystrophy.5,11,12 These approaches, although important, fail to replicate some potential obstacles related particularly to dosing and anatomical distribution of blood vessels that dominate the clinical environment. These requirements can be addressed, in part, in the rhesus macaque where the arteriovenous circulation to muscle presents anatomical similarities with DMD boys. In addition, the endothelial junctions of capillaries, the interior wall pressures, and size ratio of capillaries to muscle fibers more closely simulate the clinical setting.
Studies in the nonhuman primate (NHP) and the rhesus macaque, in particular, offer the opportunity to evaluate a naturally occurring AAV infection by humoral and T-cell immunity to this virus. Such conditions potentially more closely simulate clinical gene transfer. In addition, sophisticated tools are evolving to characterize the immune response in rhesus macaques through the use of major histocompatibility complex haplotyping and tetramer analysis to directly visualize antigen-specific T cells.13
One limitation in the NHP is a biomarker that permits precise assessment of vector-mediated gene expression and distribution. In this report, we describe the use of a FLAG tag for tracking micro-dystrophin distribution delivered by AAV8 under control of the muscle-specific creatine kinase promoter, MCK. The eight amino-acid FLAG epitope was originally designed for antibody-mediated identification and purification of recombinant proteins.14 FLAG is commonly used to tag proteins for in vitro cell culture assays and more recently in vivo when antibodies to a particular antigen are not available or are unreliable.14,15,16 This study represents the first time a FLAG epitope tag has been used as a transgene marker to differentiate between endogenous and vector-derived protein to assess transgene distribution in a NHP. Robust micro-dystrophin.FLAG expression was documented for at least 5 months with no apparent immunogenicity, supporting possible applicability for other preclinical gene replacement studies relevant to other forms of muscular dystrophy.
Results
Micro-dys.FLAG gene is highly expressed in mouse muscle tissue
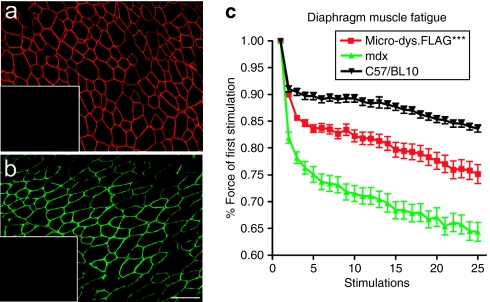
The presence of an endogenous dystrophin gene in rhesus macaques precludes studying distribution of a traditional micro-dystrophin transgene by immunohistochemistry. We generated a human micro-dystrophin cassette with an eight amino-acid FLAG protein tag (micro-dys.FLAG) fused in-frame at the C-terminus. The micro-dys.FLAG transgene was driven by the MCK promoter and packaged into a rAAV8 vector. A biopotency assay was conducted using 4-week-old mdx mice (n = 12) by injecting the tibialis anterior (TA) muscle with 1011 vector genomes (vg) of rAAV8.MCK.micro-dys.FLAG. Four weeks after gene delivery, 73.2 ± 10.4% of muscle fibers in the TA expressed micro-dys.FLAG as determined using a FLAG-specific antibody (Figure 1a) and confirmed with the N-terminal dystrophin (Dys3) antibody (Figure 1b). Micro-dystrophin does not stain for C-terminal dystrophin (Dys2).
Figure 1.
Micro-dystrophin.FLAG improves muscle function. (a) Immunostaining with anti-FLAG antibody reveals robust micro-dystrophin.FLAG expression in mdx mice. Inlay: contralateral limb muscle reveals no FLAG staining. (b) Immunostaining with N-terminal dystrophin antibody Dys3 confirms the presence of micro-dystrophin. Note: Dys3 antibody will only recognize human N-terminal dystrophin and does not crossreact with mouse dystrophin. Inlay: contralateral limb muscle reveals no dystrophin staining. Bar = 100 µm. (c) mdx diaphragms treated with AAV8.MCK.micro-dystrophin.FLAG are significantly protected from muscle fatigue compared to untreated mdx controls (***two-way analysis of variance, P < 0.001), but did not fully restore normal function compared to C57/BL10 strain controls.
Micro-dys.FLAG is functional
The value of the micro-dys.FLAG cassette is increased by demonstrating a capacity to reverse physiologic deficits in dystrophin-deficient muscle. We chose to do these studies in the diaphragm muscle of the mdx mouse because the severity of the dystrophic process closely simulates that seen in patients with DMD.17,18 We delivered 2 × 1011 vg (30 µl) of rAAV8.MCK.micro-dys.FLAG by direct injection to the left ventral quadrant of the diaphragm in 8-week-old mdx mice (n = 6). Eight weeks after transfer, the diaphragm was isolated, and two muscle strips from each mouse (1 mm width) were tested from the targeted area of gene transfer for resistance to fatigue (stimulation every second for 90 seconds—1 Hz amplitude, 80 ms duration, and 130 ms frequency) and compared to aged-matched mdx and C57/BL10 controls. Treatment with micro-dys.FLAG significantly protected the mdx diaphragm from fatigue (Figure 1c).
Micro-dys.FLAG gene transfer in NHPs
AAV8.MCK.micro-dystrophin.FLAG was delivered to the TA muscle of three rhesus macaques negative for AAV8 capsid–binding antibodies by enzyme-linked immunosorbent assay (ELISA) (subjects: RQ6661, RQ6698, and RQ6706). The left TA was injected with 5 × 1012 vg of rAAV8.MCK.micro-dys.FLAG (three injections ~333 µl each, 0.5 cm apart, and 0.5 cm beneath the fascia). The contralateral TA was injected with vector diluent in the same manner. These three macaques were prescreened on two occasions prior to gene transfer for antigen-specific enzyme-linked immunosorbent spot (ELISpot) interferon-γ (IFN-γ) responses directed against AAV8 capsid or micro-dys.FLAG peptide pools. Antibody and T-cell responses to the vector and transgene were analyzed every 2 weeks following gene transfer for 5 months.
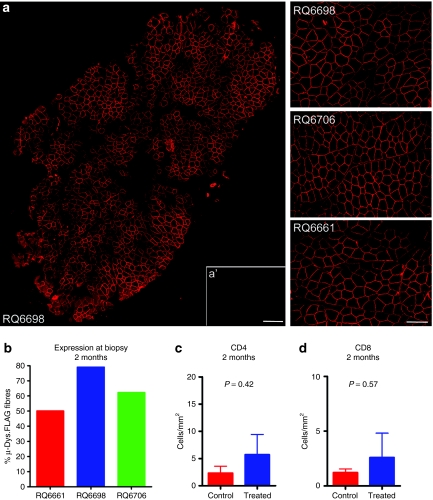
The efficiency of gene transfer was assessed 2 months after gene transfer by muscle biopsy from the site of injection via an open incision. This time point was chosen because it was well beyond the 4-week peak time for rAAV expression in muscle.19,20 Muscle tissue was sectioned and stained for micro-dys.FLAG and examined for immune cell infiltration. Robust gene expression (percentage of total muscle fibers expressing micro-dys.FLAG) was demonstrated in all three subjects: RQ6661, 50.1%; RQ6698, 79.1%; RQ6706, 62.3% (Figure 2a,b). Micro-dys.FLAG vg copy number was also assessed in intramuscular (IM)-treated subjects and found to be 2.5 × 106 ± 2.4 × 106 vg/µg genomic DNA in treated muscle compared to 0.009 ± 0.009 vg/µg in the contralateral limb (Supplementary Figure S2). Assessment of mononuclear cells by counts of CD4+ and CD8+ cells showed no difference in the number of cells in the vector versus sham-injected muscle (Figure 2c,d).
Figure 2.
Two-month muscle biopsy reveals widespread micro-dys.FLAG expression. (a) A biopsy of all three primates from the site of gene transfer revealed robust staining of micro-dystrophin FLAG localized to the sarcolemmal membrane compared to no staining in contralateral control (a′). Bar = 400 µm for montage and 200 µm for ×20 images. (b) Percentage of micro-dys.FLAG transduced muscle fibers from each biopsy. (c,d) Quantitative comparison of CD4 and CD8 T cells present in the biopsy of treated muscle versus the contralateral limb muscle. Error bars, standard deviation. Student's t-test was used to determine significance between sides (P > 0.05).
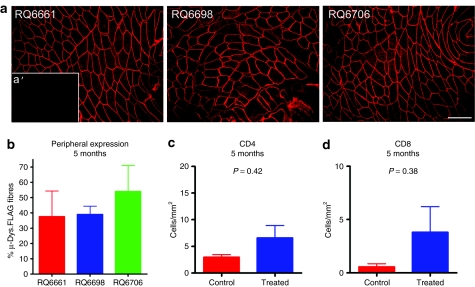
The three macaques were then killed and necropsied 5 months after gene transfer to assess long-term gene expression of micro-dys.FLAG. Muscle was removed corresponding to the peri-injection site of IM gene injections (the precise target site of gene transfer was removed at 2 months). The tissue was blocked, sectioned, and stained for micro-dys.FLAG expression. Robust gene expression was demonstrated in all three animals: RQ6661, 37.6 ± 16.7%; RQ6698, 39 ± 5.4%; RQ6706, 54 ± 17.1% (Figure 3a,b). Reduced expression compared to the muscle biopsy at 2 months was not unexpected, as this reflects the limited spread of vector from the target site of gene transfer. Mononuclear cell infiltration using specific markers for CD4+ and CD8+ cells showed no difference between the vector versus sham-injected muscle (Figure 3c,d; Supplementary Figure S1) as was observed in the 2-month biopsies.
Figure 3.
Persistent expression micro-dys.FLAG at 5 months. (a) Excision of the entire gene transfer site revealed sustained expression in all three primates. Bar = 200 µm. (a′) Contralateral control shows no FLAG staining. (b) Mean percentage of micro-dys.FLAG transduced muscle fibers from tissue blocks (six blocks each, ~0.5 × 0.5 × 0.5 cm) harvested from injection site for each subject (error bars ± SD). (c,d) Quantitative comparison of CD4 and CD8 T cells present in the biopsy tissue versus the contralateral muscle (error bars ± SD). Student's t-test was used to determine significance between sides (P > 0.05).
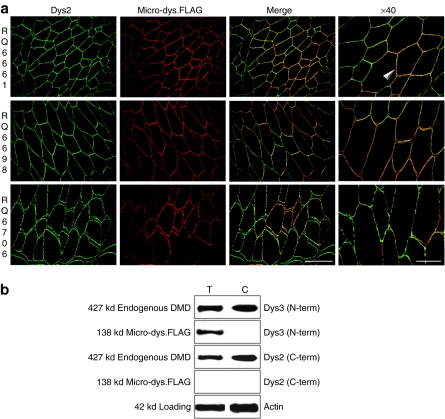
Consistent with functional activity, the micro-dys.FLAG protein was correctly localized to the sarcolemmal membrane and colocalized with full-length endogenous dystrophin. Immunofluorescent C-terminal dystrophin and anti-FLAG antibodies showed overlapping domains of expression (Figure 4a). Western blot analysis confirmed these findings with the N-terminal Dys3 dystrophin antibody detecting both the full-length endogenous 427 kd dystrophin band and the 138 kd micro-dys.FLAG band in treated (T) samples. The micro-dys.FLAG band was absent in the contralateral control (C) sample confirmed by the absence of C-terminal reactivity (Dys2). C-terminal Dys2 reactivity of the same immunoblot confirmed the endogenous dystrophin was full-length (Figure 4b).
Figure 4.
Localization of micro-dys.FLAG protein to the sarcolemma. (a) Immunofluorescence labeling for dystrophin C-terminus (Dys2) and micro-dys.FLAG (anti-FLAG) revealed colocalization to the sarcolemma (arrow). Intensity of micro-dys.FLAG expression was variable in some muscle fibers as demonstrated in merged images. Bar: ×20 = 200 µm, ×40 = 100 µm. (b) Western blot analysis using N-terminal (Dys3) and C-terminal (Dys2) antibodies to dystrophin indicate both full-length dystrophin (427 kd) and micro-dystrophin.FLAG (138 kd) in treated (T) samples. A Dys3 positive band at 138 kd is absent in contralateral control (C) tissue, whereas the endogenous full-length 427 kd band is intact. Dys2 positive band at 427 kd present in all samples is detecting full-length dystrophin. Actin is shown as loading control (42 kd). DMD, Duchenne muscular dystrophy.
Immune responses following direct IM injection
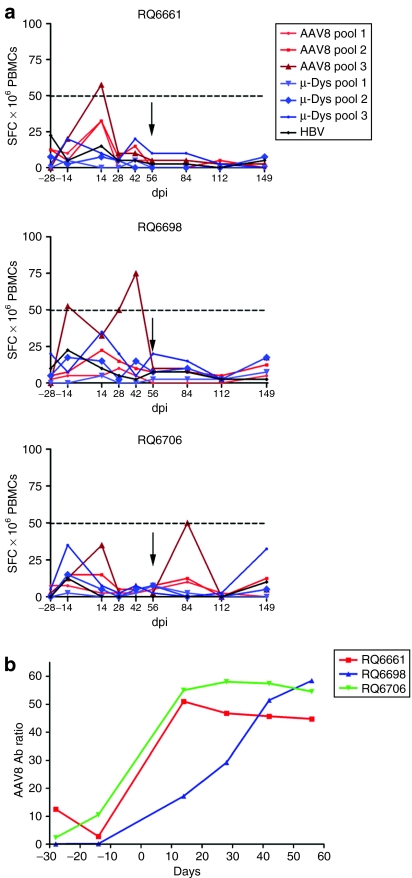
Antigen-specific T cells directed against AAV8 capsid and micro-dys.FLAG proteins were evaluated using overlapping peptide pools encompassing the full-length protein (18-mers overlapping by 11). Peptides were divided into three pools representing N-terminal, mid, and C-terminal regions of the protein for high-throughput analyses. At 2-week intervals, peripheral blood mononuclear cells (PBMCs) were isolated from each animal and assayed for IFN-γ ELISpot responses to transgene and capsid. A positive response is defined as ≥50 spot-forming colonies per 106 PBMCs.
No micro-dys.FLAG T-cell response was detected above background in any animal at any time point. In similar fashion, there was virtually no detectable capsid-specific T-cell response, except for two transient, marginally positive responses to the AAV8 capsid pool 3 at 14 days after gene transfer in animal RQ6661 and at day 42 in animal RQ6698 (82 and 77 spot-forming colonies/million PBMCs, respectively) (Figure 5).
Figure 5.
Cellular immune response. (a) Enzyme-linked immunosorbent spot assay for detection of transgene and/or capsid-specific T cells in PBMCs by interferon-γ secretion. Fifty reactive spots per million cells (dotted line) represent the threshold for a significant response. Black arrow indicates the time of biopsy. The only marginally positive responses to be noted were at day 14 for RQ6661 and day 42 for RQ6698. These were not sustained and had no influence on gene expression. (b) AAV8 binding Ab titers. Antibodies to AAV8 capsid in serum were quantified by enzyme-linked immunosorbent assay and monitored every 2 weeks. AAV8 Ab ratio = ratio OD with AAV8 coat/without AAV8 coat. All three animals demonstrated rapid seroconversion in AAV8 Abs after immunization with rAAV8.MCK.micro-dys.FLAG. Ab, antibody; HBV, hepatitis B virus; OD, optical density; PBMC, peripheral blood mononuclear cell; SFC, spot-forming colonies.
Anti-AAV-binding antibodies to the AAV8 capsid were detected in all animals as early as 2 weeks after gene transfer (Figure 5b) confirming delivery of an immunogenic capsid dose to all three animals.
Targeted vascular delivery of micro-dystrophin to gastrocnemius muscle
The NHP provides a testing paradigm for vascular delivery translational studies due to anatomic parallels with humans. Mirroring a potential clinical protocol, anti-AAV8 serostatus was determined for four rhesus macaques. Four animals were identified: two were negative (subjects: 01D299 and RQ6539) and two were positive (subjects: 03E101 and 03E062) for pre-existing AAV8 antibodies. Two baseline bleeds were performed to collect PBMCs for serial IFN-γ ELISpot analysis of capsid and transgene along with serum for AAV8 antibody–binding ELISAs. These analyses were repeated at 2-week intervals throughout the study.
The left hindlimb of four rhesus macaques (4–8 kg animals) was perfused with 2 × 1012 vg/kg rAAV8.MCK.micro-dys.FLAG in 2.5 ml/kg saline, through a catheter advanced to the sural artery, which is the arterial supply for the gastrocnemius muscle. This muscle was chosen as a proof of principle that vector could be specifically targeted by vascular delivery. Specific needs will dictate the muscle groups to be targeted in a clinical trial. Two tourniquets were used to compartmentalize delivery, one above the knee ~0.5 inches above the catheter tip and a second above the ankle. A preflush (2.5 ml/kg over 60 seconds) of sterile saline was given with the proximal tourniquet occluding flow to minimize arterial blood supplying the gastrocnemius. After occluding flow completely with both tourniquets, the vector was delivered over 60 seconds followed by a 10-minute dwell time. A postflush (2.5 ml/kg over 60 seconds) was delivered immediately before removing the tourniquet. None of the animals suffered noticeable edema or adverse effects from the procedure.
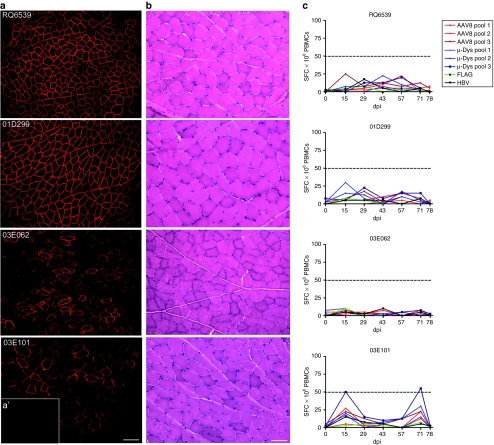
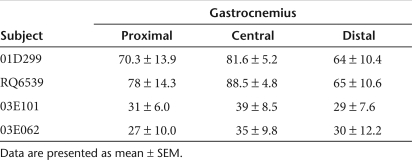
Three months after transfer, the macaques were euthanized, and the gastrocnemius muscle was removed, cut into blocks (~1.0 × 0.75 cm), and snap-frozen. Micro-dystrophin expression was visualized by immunofluorescence using an anti-FLAG antibody. All four monkeys had efficient transduction of the gastrocnemius muscle (Figure 6a; Table 1), with subjects 01D299 and RQ6539 (those without pre-existing AAV8 antibodies) demonstrating 82 and 89% transduction, respectively in the central portion of the muscle, and subjects 03E101 and 03E062 (those with pre-existing AAV8 antibodies) demonstrating 39 and 35% transduction, respectively. Expression levels were also confirmed with quantitative PCR in subjects treated by isolated limb perfusion. Micro-dys.FLAG vg copy number was assessed and found to be 2.2 × 105 ± 1.7 × 105 vg/µg genomic DNA in treated muscle from subjects 01D299 and RQ6539 (without pre-existing AAV8 antibodies) compared to 2.8 × 103 ± 1.3 × 103 vg/µg in subjects 03E101 and 03E062 (those with pre-existing AAV8 antibodies) (Supplementary Figure S2). Histological assessment in all samples with gene expression revealed no inflammation, necrosis, or change in morphology (Figure 6b).
Figure 6.
Targeted vascular delivery of micro-dystrophin.FLAG in nonhuman primates. (a) The targeted gastrocnemius muscle from all four primates revealed robust staining of micro-dystrophin.FLAG localized to the sarcolemmal membrane at 3 months after gene transfer. Bar = 200 µm, ×10 images. (a′) Contralateral control shows no FLAG staining. (b) Hematoxylin and eosin staining from isolated muscle tissue at the site of gene transfer revealed no evidence of tissue damage or cellular infiltration. (c) Enzyme-linked immunosorbent spot assay for detection of transgene and/or capsid-specific T cells in PBMCs. Pool 1 is composed of aa14-240 (actin-binding domain), aa253-327 (hinge 1), and aa337-427 (first part of spectrin repeat 1). Pool 2 is composed of aa428-447 (remainder of spectrin repeat 1), aa448-556 (spectrin repeat 2), aa557-667 (spectrin repeat 3), aa668-717 (hinge 2), and aa2932-3040 (spectrin repeat 24). Pool 3 is composed of aa3041-3112 (hinge 4), aa3080-3360 (cysteine repeat region), plus the sequence of the FLAG tag. Two marginally positive responses against micro-dys pool 3 in animal 03E101 were noted at days 15 and 71. These responses were not sustained and had no influence on long-term gene expression. HBV, hepatitis B virus; PBMC, peripheral blood mononuclear cell; SFC, spot-forming colonies.
Table 1.
Micro-dystrophin percent transduction following vascular delivery
Immune responses following vascular delivery
Antigen-specific T-cell responses were monitored every 2 weeks over the course of the study using PBMCs. There were no significant IFN-γ responses to capsid or transgene peptide pools up to and including the day of necropsy 3 months after gene transfer (Figure 6c). To eliminate the possibility that a T-cell response could be occurring in the muscle at a level below the threshold of detection in the peripheral blood, T cells were directly isolated from necropsied muscle tissue. No IFN-γ ELISpot response to capsid or micro-dys.FLAG was observed using these muscle-derived T cells. Moreover, clonal expansion and restimulation with micro-dys.FLAG and capsid peptide pools also failed to elicit a positive response, further confirming our findings that AAV8.micro-dys.FLAG was not immunogenic when delivered by a vascular route. These data demonstrate that rAAV8 is efficient at transducing the underlying musculature of the primate host in the presence of anti-AAV antibodies and without eliciting a T-cell immune response to vector or transgene that could preclude expression. FLAG-tagged micro-dystrophin was also shown to correctly localize to the muscle sarcolemma and persist for at least 3–5 months without apparent diminution.
Discussion
Demonstration of persistent FLAG-tagged human micro-dystrophin expression in a NHP has significant advantages in facilitating the goal of intravascular delivery for human gene therapy trials in DMD. In the NHP studies, the intravascular sites of delivery provide anatomical thoroughfares for muscle-specific targeting and offer information on viral dosing to achieve robust levels of muscle transduction that are well within those expected to represent a therapeutic range.21 We demonstrated sustained micro-dystrophin.FLAG expression for at least 3–5 months by either IM or intravascular routes without overt T-cell immune responses to vector capsid or the micro-dys.FLAG transgene. The vascular delivery method detailed herein is clinically applicable for targeted delivery in terms of vector dose, volume of administration, intra-arterial pressure, and catheterization technique. The studies therefore represent a useful preclinical paradigm to continue to test vascular delivery to specific muscle groups with implications for whole limb delivery. Micro-dystrophin.FLAG correctly localized to the sarcolemmal membrane in the presence of endogenous dystrophin allowing accurate assessment of the delivery technique and percentage of muscle fibers transduced.
As a nonhuman serotype, AAV8 may have particular advantages for clinical gene therapy by permitting evasion of pre-existing AAV2 natural immunity and reduced antigen-presenting cell transduction in the human host.22,23,24 All animals (whether naive or AAV antibody positive) rapidly seroconverted following vector administration by either route, which may preclude vector administration. However, transgene expression was observed following vascular delivery to two AAV8 positive animals, which suggests that gene transfer is possible in seropositive animals. Although these data are encouraging, naive animals without pre-existing AAV8 antibody demonstrated approximately twofold higher expression than animals with pre-existing antibodies, implying that such antibodies partially block transduction as observed in other models.23
The use of an eight amino-acid FLAG epitope tag fused to the C-terminus of micro-dystrophin allowed for assessment of transgene distribution in the presence of endogenous macaque dystrophin. Detection of the FLAG epitope was critical for the success of the study to accurately assess vascular distribution. FLAG did not elicit a T-cell immune response as measured by IFN-γ ELISpot induction and thus represents an ideal strategy to tag proteins also expressed endogenously.
The findings in this NHP study have implications for other muscle diseases as well. The recently reported success in type 2D limb-girdle muscular dystrophy IM α-sarcoglycan (SGCA) clinical gene transfer by an AAV vector bodes well for vascular delivery in this form of dystrophy.25 Labeling SGCA with a FLAG-tag and performing vascular delivery studies such as the one described herein should expedite translation to the clinic.
Materials and Methods
Micro-dystrophin gene construction. The human micro-dystrophin cassette contained the (R4–R23/Δ71–78) domains as previously described, and the FLAG epitope (DYKDDDDK) was added to the C-terminus as a translational fusion by PCR.3 The complementary DNA was codon optimized for human usage and synthesized by GenScript (Piscataway, NJ). It includes a consensus Kozak sequence, an SV40 intron, and synthetic polyadenylation site (53 base pairs). A muscle creatine kinase promoter/enhancer was used to drive muscle-specific gene expression. The MCK micro-dystrophin.FLAG expression cassette was cloned between AAV2 inverted terminal repeats using flanking XbaI restriction enzyme sites in a plasmid derived from pCMVβ (Clontech, Mountain View, CA). MscI/SmaI restriction enzyme digestions were used to confirm ITR integrity.
rAAV vector production. rAAV vectors were produced by a modified cross-packaging approach whereby the AAV type 2 ITRs can be packaged into multiple AAV capsid serotypes.26 Production was accomplished using a standard 3 plasmid DNA CaPO4 precipitation method using HEK293 cells. Two hundred ninety-three cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin. The production plasmids were as follows: (i) pAAV.MCK.microdys.FLAG, (ii) rep2-cap8 modified AAV helper plasmids encoding cap serotype 8-like isolate rh.74, and (iii) an adenovirus type 5 helper plasmid (pAdhelper) expressing adenovirus E2A, E4 ORF6, and VA I/II RNA genes. A quantitative PCR-based titration method was used to determine an encapsidated vg titer utilizing a Prism 7500 TaqMan detector system (PE Applied Biosystems, Foster City, CA).27 The primer and fluorescent probe targeted the MCK promoter and were as follows: MCK forward primer, 5-CCCGAGATGCCTGGTTATAATT-3; MCK reverse primer, 5-GCTCAGGCA CAGGTGTTG-3; and MCK probe, 5-FAM-CCAGACATGTGGCTGCTCCCCC-TAMRA-3.
Animals and treatments
IM injection of the TA of mouse: All procedures were approved by the Research Institute at Nationwide Children's Hospital Institutional Animal Care and Use Committee. Three to four-week-old mdx mice and normal age-matched C57/BL10 were used for IM injection. Mice were anesthetized and maintained on 1–4% Isoflurane (in oxygen). Both hindlimbs were shaved, and the TA muscle was injected with 3 × 1010 vg of rAAV8.micro-dystrophin.FLAG or normal saline (30 µl volume) using a 30-gauge insulin syringe.
IM injection of the diaphragm of mouse: Briefly, mice were weighed and anesthetized with a combination of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively). A single abdominal incision was made from the base of the sternum to just above the pelvis (~1 cm incision). The diaphragm was identified, and 30 µl of the vector preparation in sterile saline was delivered using a 32-gauge needle. The abdominal wall was closed with 4.0 Vicryl Plus continuous sutures, and skin wound was closed with sterile surgical staples. Mice were treated with a postoperative dose of buprenorphine 0.01 mg/kg subcutaneously for pain. The animals are allowed to recover on a 37 °C warmer.
Diaphragm fatigue protocol in mouse: Mice were euthanized, and the diaphragm dissected with rib attachments and central tendon intact, and placed in K-H buffer (5 mmol/l KCl, 137 mmol/l NaCl, 1.2 mmol/l NaH2PO4-H2O, and 1.2 mmol/l MgSO4). A 1 mm wide section (from rib to tendon) of diaphragm was isolated and attached to a force transducer. The diaphragm strip was looped around a basket assembly attached to the transducer (the rib cartilage serves as the anchor), and the tendon was pierced by a pin. The muscle was stretched to optimal length (length at maximum twitch force) for measurement of twitch contractions and rested for 5 minutes before starting the muscle fatigue protocol. The muscle fatigue protocol measured the force exerted by the muscle when stimulated every second for 90 seconds (1 Hz amplitude, 80 ms duration, and 130 ms frequency). Following the muscle fatigue protocol, the muscle strip was removed from the apparatus, the rib cartilage removed and weighed.
NHP IM injection: Three 7-year-old Chinese rhesus macaques that did not possess AAV8-binding antibodies above background were studied (ELISA performed using 1:50 serum dilution). All NHPs were housed in pairs to promote socialization. The macaques were anesthetized using Telazol (5 mg/kg IM) and treated with buprenorphine (0.01 mg/kg) IM preoperatively. The TA of both hindlimbs was shaved and prepared with 95% EtOH and povidone solution, and the animal secured to a warming blanket (37 °C) that overlies the surgery table. The TA was visualized by blunt dissection, and 5 × 1012 vg of rAAV8.micro-dystrophin.FLAG or normal saline (1 ml volume total) was injected in three sites 0.5 cm apart. The fascial layer and skin incision were closed with 3–4 interrupted Vicryl sutures and skin bond, and the injection site was marked with tattoo ink. For the muscle biopsy at 8 weeks, an incision was made to visually expose the TA muscle, using sterile drapes and scalpels. A small muscle biopsy was obtained (block 0.75–1.0 × 0.5 cm), with bleeding controlled by direct pressure. The wound was closed with interrupted Vicryl sutures.
Isolated limb perfusion in rhesus macaques: Rhesus macaques were sedated with IM Telazol (3–6 mg/kg), intubated and secured to a heated procedure table at 37 °C. General anesthesia was administered with Isoflurane (in oxygen) 1–4% during the procedure. The left groin was shaved with extension to the mid thigh, and prepped with povidone–iodine solution followed by 95% ethanol. A groin incision was made over the femoral bundle, and the femoral artery was isolated. The femoral artery was catheterized using fluoroscopy-guided 3-0 f catheter (Cook) that was advanced to the sural branch of the popliteal artery. Prior to vector administration, a prevector flush of saline (2.5 ml/kg) was given over 1 minute. This was immediately followed by occluding blood flow to the extremity using a standard phlebotomy tourniquet placed proximal to the tip of the catheter that was typically right above the knee. A second tourniquet was placed at the base of the gastrocnemius. rAAV8.MCK.micro-dys.FLAG was infused over 60 seconds at a dose of 2 × 1012 vg/kg in 2.5 ml/kg of Tris buffered saline. The extremity remained isolated from the circulation for 10 minutes. A postvector flush (2.5 ml/kg) was infused over 1 minute and then the tourniquets were released. Direct pressure was applied for 10 minutes to control bleeding, and the wound was closed with 4-0 Vicryl suture.
Gene expression analyses. TA and gastrocnemius skeletal muscles were collected from treated and contralateral control limbs at 8 weeks and 5 months after treatment, respectively, for subjects treated by IM injection. A single 1.0 × 0.5 cm block was removed at biopsy at the 8-week time point, and the remainder of the TA was removed and blocked at necropsy at 5 months. Subjects treated by isolated limb perfusion were killed at 3 months at which time the entire gastrocnemius muscle was removed and blocked (0.75–1.0 × 0.5 cm blocks). Muscles were embedded in 7% gum tragacanth and flash-frozen in isopentane cooled in liquid nitrogen. Cryostat sections (12 µm) for FLAG immunofluorescence were incubated with anti-FLAG polyclonal primary antibody (F7425; Sigma, St Louis, MO) at a dilution of 1:175 in blocking buffer [phosphate-buffered saline (PBS), 10% goat serum, 0.1% Triton X-100] for 1 hour at 25 °C in a wet chamber. For dystrophin-specific staining, Dys2 (NCL-Dys2; Novocastra Laboratories, Newcastle, UK) primary antibody was used (1:3 in blocking buffer). Sections were then washed with PBS three times, each for 20 minutes and reblocked. Visualization was achieved by incubation for 45 minutes at 25 °C with an Alexa 568 goat anti-rabbit or Alexa 488-conjugated IgG1 isotype-specific goat anti-mouse antibody at a 1:300 dilution (Molecular Probes, Carlsbad, CA). Sections were washed with PBS three times for 20 minutes and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Fluorescence staining was visualized using a Zeiss Axioskop 2 Plus Microscope (Zeiss, Thornwood, NY), and images were captured with a Zeiss AxioCam MRC5 camera (Zeiss). The number of fibers with sarcolemmal staining were expressed as percentage of all fibers. Means for IM necropsy studies were obtained from the mean of six blocks surrounding the original injection site. Means for isolated limb perfusion studies were obtained by counting four ×10 fields from six muscle blocks in proximal, central, and distal regions for each muscle.
Western blot analysis. Tissue sections (10–20 µm thick) from micro-dys.FLAG treated and untreated muscle were collected into a microcentrifuge tube and homogenized with 100 µl homogenization buffer (125 mmol/l Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate, 4 mol/l urea, 5% βME and protease inhibitor cocktail). Protein levels were quantified using RC/DC method (BioRad Laboratories, Hercules, CA). Protein samples (50 µg per lane) were electrophoresed on a 3–8% polyacrylamide Tris-Acetate gel (NuPAGE; Invitrogen, Carlsbad, CA) and then transferred to a polyvinylidene fluoride membrane (Amersham Biosciences, Pittsburgh, PA). After blocking for 1 hour in 5% nonfat dry milk in TBST (100 mmol/l Tris-HCl, pH 8.0, 167 mmol/l NaCl, 0.1% Tween), the western blot was incubated overnight with dystrophin monoclonal antibody NCL-DYS2 or DYS3 (Novocastra Laboratories) at a dilution of 1:100, followed by horseradish peroxidase–labeled goat anti-mouse IgG (GE Healthcare, Piscataway, NJ) at a dilution of 1:2,000 for 1 hour. Immunoreactive bands were visualized with the use of the ECL Plus western blotting detection system (GE Healthcare) and Hyperfilm ECL (Amersham Biosciences). Signal intensities were measured with ImageQuant software (GE Healthcare).
Quantitative PCR to detect genome copy number. TaqMan quantitative PCR was used to quantify the number of vg copies compared to contralateral control tissue as described.27 A vector-specific primer probe set amplified a portion of the unique sequence of the MCK promoter within the micro-dystrophin.FLAG cassette. The rhesus erythropoietin gene was used as an internal control to normalize for genomic input and confirm the absence of PCR inhibitors in the sample DNA. Copy number is reported as vg per microgram of genomic DNA.
Mononuclear cell analysis. Immunohistochemistry was performed to identify immune cells. Tissue sections were incubated with monoclonal antibodies to CD4 (L200; BD Biosciences, San Jose, CA), CD8 (RPA-T8; BD Biosciences) and were diluted 1:50 with PBS. Visualization was achieved with DAB substrate using the Super Sensitive Polymer-HRP IHC Detection System for Automation (QD410-YAX; BioGenex, San Ramon, CA). The entire muscle section was analyzed, and the number of mononuclear cells were counted and expressed as total number per mm2.
IFN-γ ELISpot analysis. ELISpot assays were performed on fresh PBMCs, which were added at a concentration of 2 × 105/well in duplicate wells of a 96-well flat-bottom membrane plate (Millipore, Billerica, MA). Three peptide pools were used for the AAV8 capsid protein (Genemed Synthesis, San Antonio, TX), containing 34–36 peptides, each 18 amino-acid long and overlapping by 11 residues. Three peptide pools encompassed the micro-dystrophin.FLAG protein (Genemed Synthesis) and contained 36–42 peptides, each 20 amino-acid long and overlapping by 10 residues. The final two peptides of the micro-dystrophin.FLAG pool 3 were specific for the FLAG-tag epitope. Pool 1 is composed of aa14-240 (actin-binding domain), aa253-327 (hinge 1), and aa337-427 (first part of spectrin repeat 1). Pool 2 is composed of aa428-447 (remainder of spectrin repeat 1), aa448-556 (spectrin repeat 2), aa557-667 (spectrin repeat 3), aa668-717 (hinge 2), and aa2932-3040 (spectrin repeat 24). Pool 3 is composed of aa3041-3112 (hinge 4), aa3080-3360 (cysteine repeat region), plus the sequence of the FLAG-tag. Concanavalin A (Sigma) served as a positive control and a hepatitis B virus peptide pool as a negative control. Peptides were added directly to the wells at a final concentration of 1 µg/ml in 200 µl of AIM-HS [Aim-V lymphocyte media (Invitrogen) supplemented with 2% human AB serum (Gemini-BioScience, Basel, Switzerland)]. Monkey IFN-γ ELISpot kits were purchased from U-CyTech (Utrecht, the Netherlands). After the addition of PBMCs and peptides, the plates were incubated at 37 °C for 48 hours and then developed according to the manufacturer's protocol. IFN-γ spot formation was counted using a Cellular Technologies systems analyzer (Cellular Technologies, Cleveland, OH).
ELISA analysis. An ELISA was performed to measure the level of circulating AAV8 capsid–binding antibody in serum. Immulon-4 96-well plates (ISC BioExpress, Kaysville, UT) were coated with 100 µl of 2 × 1010 vg/ml AAV8 viral stock in carbonate buffer (pH 9.4; Pierce, Rockford, IL) per well. Plates were sealed overnight at 4 °C. Plates were blocked with 280 µl per well of a 5% nonfat dry milk and 1% normal goat serum (Invitrogen) in PBS for 3 hours at 25 °C. Rhesus plasma was diluted at a 1:50 ratio in solution identical to the blocking solution, and 100 µl added in duplicate to both wells coated with AAV8 particles in carbonate buffer and wells coated with carbonate buffer alone. Plates were incubated at 25 °C for 1 hour before being washed five times with 280 µl of PBS-T (0.05% Tween). Blocking solution was used again to dilute the secondary antibody, goat anti-monkey IgG-HRP (Sigma) at a 1:10,000 dilution. Wells received 250 µl of the secondary antibody and were incubated at 25 °C for 30 minutes before being washed five times and blotted dry. Tetramethylbenzidine (100 µl/well; Pierce) was added and incubated at 25 °C for 10 minutes in the dark, before the addition of 100 µl of 1N H2SO4 (Acros Organics, Geel, Belgium) to stop the reaction. The OD450 was measured using a Wallace 1420-050 Multilabel Counter (PerkinElmer, Waltham, MA). Samples were considered positive if the OD450 average of the antigen-coated wells was three times greater than wells coated with carbonate buffer alone.
SUPPLEMENTARY MATERIALFigure S1. Micro-dystrophin.FLAG gene transfer does not induce histopathological changes. (a) Hematoxylin and eosin staining from isolated muscle tissue at the site of gene transfer revealed no evidence of tissue damage or cellular infiltration in all three subjects. (b,c) CD4+ and CD8+ mononuclear cell stains show sparsely, scattered positive cells (arrows) following gene transfer in perivascular and endomysial sites. No mononuclear cell invasion of muscle fibers was detected. Scale bar = 100 μm, Magnified image in panel c, scale bar = 50 μm.Figure S2. Vector genome copy number. Histogram demonstrating micro-dystrophin.FLAG copy number determined by quantitative Taqman qPCR. Subjects treated by intramuscular injected (IM) are compared with those treated by isolated limb perfusion without (ILP AAV-) and with (ILP AAV+) pre-existing immunity to AAV8.
Supplementary Material
Micro-dystrophin.FLAG gene transfer does not induce histopathological changes. (a) Hematoxylin and eosin staining from isolated muscle tissue at the site of gene transfer revealed no evidence of tissue damage or cellular infiltration in all three subjects. (b,c) CD4+ and CD8+ mononuclear cell stains show sparsely, scattered positive cells (arrows) following gene transfer in perivascular and endomysial sites. No mononuclear cell invasion of muscle fibers was detected. Scale bar = 100 μm, Magnified image in panel c, scale bar = 50 μm.
Vector genome copy number. Histogram demonstrating micro-dystrophin.FLAG copy number determined by quantitative Taqman qPCR. Subjects treated by intramuscular injected (IM) are compared with those treated by isolated limb perfusion without (ILP AAV-) and with (ILP AAV+) pre-existing immunity to AAV8.
Acknowledgments
We thank the Viral Vector Core at Nationwide Children's Hospital for vector production. Research supported by the Children's Hospital Foundation (J.R.M.); National Institutes of Health U54 (1U54NS055958-01A1) to J.R.M., L.G.C., K.R.C., Z.S., and C.M.W.; Muscular Dystrophy Association to J.R.M.; Jesse's Journey Foundation for Gene and Cell Therapy, Ruth L. Kirschstein National Research Service Award postdoctoral fellowship (1F32AR055008) to L.R.R.-K.
REFERENCES
- Rodino-Klapac LR, Chicoine LG, Kaspar BK., and , Mendell JR. Gene therapy for duchenne muscular dystrophy: expectations and challenges. Arch Neurol. 2007;64:1236–1241. doi: 10.1001/archneur.64.9.1236. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chamberlain JS, Tapscott SJ., and , Storb R. Gene therapy in large animal models of muscular dystrophy. ILAR J. 2009;50:187–198. doi: 10.1093/ilar.50.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS., and , Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, et al. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med. 2007;5:45. doi: 10.1186/1479-5876-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Lai Y, Long C, Li D., and , Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008;19:851–856. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, et al. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS., and , Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Mah C, Fraites TJ Jr, Cresawn KO, Zolotukhin I, Lewis MA., and , Byrne BJ. A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol Ther. 2004;9:458–463. doi: 10.1016/j.ymthe.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Ghosh A, Long C, Bostick B, Smith BF, Kornegay JN, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN., and , Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Cerretti DP, et al. A short polypeptide marker sequence useful for recombinant protein identification and purification. Biotechnology (N Y) 1988;6:1204–1210. [Google Scholar]
- Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele E., and , Strayer DS. Strategies for CNS-directed gene delivery: in vivo gene transfer to the brain using SV40-derived vectors. Gene Ther. 2007;14:939–949. doi: 10.1038/sj.gt.3302939. [DOI] [PubMed] [Google Scholar]
- Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y, et al. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord. 2008;18:342–348. doi: 10.1016/j.nmd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado K, Rafael JA, Mills PL, Cole NM, Faulkner JA, Wang K, et al. Transgenic mdx mice expressing dystrophin with a deletion in the actin-binding domain display a “mild Becker” phenotype. J Cell Biol. 1996;134:873–884. doi: 10.1083/jcb.134.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin SW, Giles-Davis W, Li Y, Zhou D, Xiang ZQ, et al. A preclinical animal model to assess the effect of pre-existing immunity on AAV-mediated gene transfer. Mol Ther. 2009;17:1215–1224. doi: 10.1038/mt.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Murphy SL, Giles-Davis W, Edmonson S, Xiang Z, Li Y, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther. 2007;15:792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KR, Liu X, McGrath JP., and , Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Micro-dystrophin.FLAG gene transfer does not induce histopathological changes. (a) Hematoxylin and eosin staining from isolated muscle tissue at the site of gene transfer revealed no evidence of tissue damage or cellular infiltration in all three subjects. (b,c) CD4+ and CD8+ mononuclear cell stains show sparsely, scattered positive cells (arrows) following gene transfer in perivascular and endomysial sites. No mononuclear cell invasion of muscle fibers was detected. Scale bar = 100 μm, Magnified image in panel c, scale bar = 50 μm.
Vector genome copy number. Histogram demonstrating micro-dystrophin.FLAG copy number determined by quantitative Taqman qPCR. Subjects treated by intramuscular injected (IM) are compared with those treated by isolated limb perfusion without (ILP AAV-) and with (ILP AAV+) pre-existing immunity to AAV8.