Abstract
Vectors derived from adeno-associated virus (AAV) are promising candidates for neural cell transduction in vivo because they are nonpathogenic and achieve long-term transduction in the central nervous system. AAV serotype 2 (AAV2) is the most widely used AAV vector in clinical trials based largely on its ability to transduce neural cells in the rodent and primate brain. Prior work in rodents suggests that other serotypes might be more efficient; however, a systematic evaluation of vector transduction efficiency has not yet been performed in the primate brain. In this study, AAV viral vectors of serotypes 1–6 with an enhanced green-fluorescent protein (GFP) reporter gene were generated at comparable titers, and injected in equal amounts into the brains of Chlorocebus sabaeus. Vector injections were placed in the substantia nigra (SN) and the caudate nucleus (CD). One month after injection, immunohistochemistry for GFP was performed and the total number of GFP+ cells was calculated using unbiased stereology. AAV5 was the most efficient vector, not only transducing significantly more cells than any other serotype, but also transducing both NeuN+ and glial-fibrillary-acidic protein positive (GFAP+) cells. These results suggest that AAV5 is a more effective vector than AAV2 at delivering potentially therapeutic transgenes to the nigrostriatal system of the primate brain.
Introduction
Viral vectors can be useful tools for expressing desired genes in brain cells to achieve therapeutic benefit. Adeno-associated virus (AAV), a nonpathogenic parvovirus, has become the most widely used vector in gene therapy applications in human. Twelve vector serotypes have been identified, and are of particular interest, because of capsid-associated tissue tropisms identified in some of them.1,2,3 These tropisms have been described in rodents, but work in nonhuman primate and human clinical trials has been more circumscribed. Over 40 clinical trials have been approved using AAV vectors, all of them are serotype 2, or using AAV serotype 2 (AAV2) vector genomes and AAV1 capsids.4 There has been no systematic exploration of tissue tropism or vector transduction efficiency using AAV vectors of multiple serotypes in the nonhuman primate, which has led to limitations in the vectors available for clinical use.
The goal in human neurosurgical procedures delivering viral vectors to the brain is to make as little perturbation of brain tissue as is possible while delivering the needed gene for the appropriate duration. Viral vectors that can generate a large overall area of transduction and have a tropism for the desired cell type may allow for the delivery of the smallest possible amount of vector, resulting in maximal gene delivery while minimizing tissue damage, inappropriate spread, or the possibility of incorporation into the host genome. In this study, we have generated high-titer vectors from AAV serotypes 1–6 with a green-fluorescent protein (GFP) reporter gene, and injected those vectors into the substantia nigra (SN) and caudate nucleus (CD) of old world nonhuman primates, Chlorocebus sabaeus, the St Kitts subspecies of African green monkey. Tissue sections throughout the extent of the injection site were analyzed for GFP expression by immunohistochemistry, and GFP+ cells were counted using unbiased stereology. We labeled and counted the numbers of vector-transduced cells by serotype, determined the vector with greatest transduction efficiency for these two regions of the nonhuman primate brain, and identified the most promising of these vector subtypes for use in human clinical trials targeting the nigrostriatal system.
Results
Viral vectors of AAV serotypes 1–6 with the gene for GFP were injected into the brains of St Kitts green monkeys. The animals survived for 30 days in good health with postoperative clinical evaluation showing no evidence of behavioral abnormalities, loss of appetite, weight loss, or other abnormalities. Brain tissue processed for immunohistochemistry using the antibody to GFP and a 3,3′-diamino-benzidine (DAB) secondary showed numerous cells around the injection site that contained DAB chromogen. Vector injections of 1 × 1012 viral genomes/ml were placed into SN (Figure 1) and CD (Figure 2), and transduced cells were detected in those regions and along the injection tract, with no evidence of spillage into the ventricles.
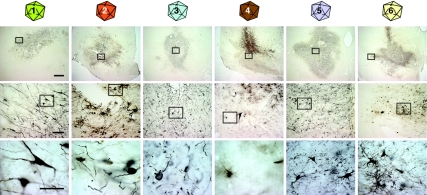
Figure 1.
Bright-field microscopy of immunohistochemistry for GFP on transduced substantia nigra tissue. Tissue that has been transduced with vectors of each serotype (by column) is shown in progressive magnifications (by row) with boxes in low power images showing the area magnified in subsequent rows. All vectors were able to transduce neural cells to produce the gene product GFP. Bars are 1 mm in the top row, 100 µm in the middle row, and 10 µm in the bottom row. GFP, green-fluorescent protein.
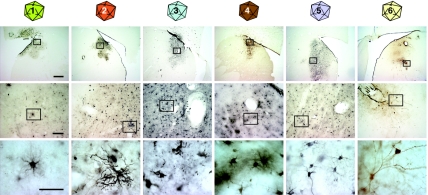
Figure 2.
Bright-field microscopy of immunohistochemistry for GFP on transduced caudate tissue. Tissue that has been transduced with vectors of each serotype (by column) is shown in progressive magnifications (by row) with boxes in low power images showing the area magnified in subsequent rows. All vectors were able to transduce neural cells to produce the gene product GFP. Bars are 1 mm in the top row, 100 µm in the middle row, and 10 µm in the bottom row. GFP, green-fluorescent protein.
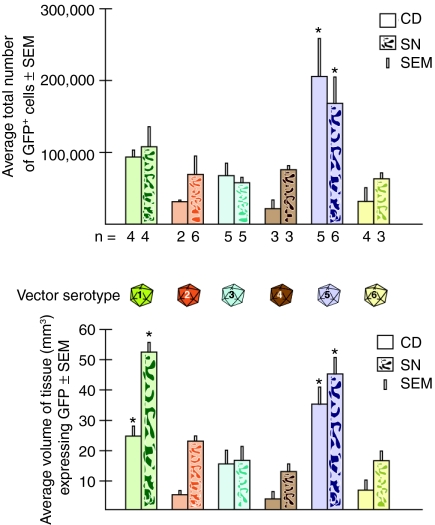
Transduction efficiency was measured by determining both the number of GFP+ cells and the volume of tissue containing GFP+ cells. The correlation between these parameters was strongly positive in the CD (Pearson correlation coefficient 0.88, P < 0.0001, n = 23) and less strongly positive in the SN (Pearson correlation coefficient 0.67, P < 0.0001, n = 27). AAV serotype 5 transduced 1.85 × 105 cells in CD and SN, a significantly higher number of cells than any other serotype (analysis of variance, degrees of freedom (5.23), F = 7.88, P < 0.0002; post hoc Student–Newman–Keuls test P < 0.05) (Figure 3a).
Figure 3.
Quantification of vector transduction. (a) The numbers of transduced cells expressing GFP, determined by unbiased stereology, are shown for each vector serotype in the CD and the SN. The number (n) of monkeys studied was as follows: for AAV1, n = 4 CD, n = 4 SN; AAV2, n = 2 CD, n = 6 SN; AAV3, n = 5 CD, n = 5 SN; AAV4, n = 3 CD, n = 3 SN; AAV5, n = 5 CD, n = 6 SN; AAV6, n = 4 CD, n = 3 SN. AAV5 transduced significantly (*) more cells than any other vector type in both CD and SN. (b) The volume of tissue (mm3) containing transduced cells expressing GFP is shown by serotype in CD and SN. AAV1 and AAV5 transduced significantly (*) more tissue volume than other vectors studied. AAV, adeno-associated virus; CD, caudate nucleus; GFP, green-fluorescent protein; SN, substantia nigra.
Both AAV5 and AAV1 resulted in significantly higher transduction volume than other serotypes (analysis of variance, degrees of freedom (5.23), F = 21.51, P < 0.0001; post hoc Student–Newman–Keuls test P < 0.05) (Figure 3b) generating 40.87 and 38.61 mm3 of transduced tissue, respectively. These volumes were significantly higher than those generated by any other serotype (8.22–18.22 mm3) (Figure 3b). AAV1 and AAV5 were not different from each other in volume of transduction. There were significant differences in volume of transduction between CD and the SN when all vectors were analyzed together (analysis of variance, degrees of freedom (1.15), F = 20.1, P < 0.0004), but this was not due to any specific serotype. Cell counts were not significantly different between the two targets for all serotypes. Density (count/volume) was also significantly different between CD and SN for all serotypes together (analysis of variance, degrees of freedom (1.15), F = 11.8, P < 0.004), but this was not due to any serotype alone. There were no significant differences in density between any of the serotypes.
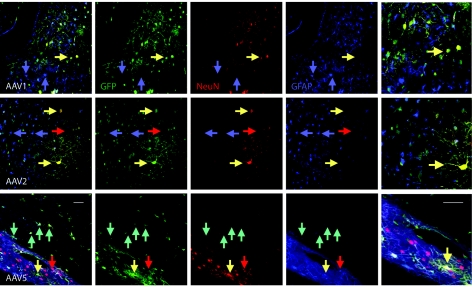
In order to determine what types of cells were being transduced by AAV5, we performed fluorescence immunohistochemistry for multiple labels on transduced tissue that was evaluated using confocal microscopy (Figure 4). We also evaluated the type of cells transduced by AAV1, because it has the largest effects next to AAV5, and by AAV2, because it is the most often used vector for gene therapy applications to date. The antibody for GFP was visualized with a fluorescein isothiocyanate secondary (green), the antibody for glial-fibrillary-acidic protein (GFAP) with CY5 (blue), and the antibody for NeuN with Rhodamine Red-X (red). All vectors transduced neurons and glial cells, but to a different extent. Although AAV2 transduced more neurons than glial cells (~65% of transduced cells that could be assigned a phenotype were NeuN+ neurons), AAV5 transduced neurons and glial cells with apparently equal efficiency (~47% were GFAP+, 53% NeuN+).
Figure 4.
Confocal microscopy of fluorescence immunohistochemistry for neural phenotypes in tissue transduced by AAV1, AAV2, and AAV5. Tissue was labeled with the antibody to GFP (green), the neuronal marker NeuN (red), and the glial marker GFAP (blue). Merged images are shown in the left column (and again at higher power in the far right column), followed by separated images, and show several yellow cells at yellow arrows indicating NeuN+ cells (red) that are also GFP+ (green). Blue arrows point to GFAP+ cells (blue) that are not GFP+, and red arrows indicate NeuN+ (red) cells that are not GFP+. In the case of AAV5, there are also aqua arrows that indicate GFAP+ (blue) cells that are also GFP+ (green). Bar = 100 µm. AAV, adeno-associated virus; GFP, green-fluorescent protein; GFAP, glial-fibrillary-acidic protein.
Discussion
This study systematically compares the transduction efficiency of AAV vector serotypes 1–6 in the nonhuman primate brain. All six serotypes of AAV were able to transduce endogenous cells to generate the reporter gene GFP. AAV3 was the least reliable of the vectors, with several injections resulting in no detectable transduced cells at 1 month; total transduced cell numbers were the lowest with this serotype. AAV5 was the most efficient of the vectors studied (Figure 3), labeling the most cells over the largest volume, by a very large margin, regardless of injection location in the brain. AAV1 also labeled cells over a larger volume than any of the other serotypes except AAV5. Furthermore, AAV5 effectively transduced glial cells as well as neurons, whereas AAV2 is generally considered to have a greater tropism for neurons.5,6,7,8,9 This finding agrees with the results of previous studies in rodents: in mice and rats AAV5 led to a higher number of transduced cells and demonstrated the ability to infect glial cells stably and effectively, thus being superior to serotypes 1, 2, 6, 7, 8, and 9 (ref. 10,11,12,13,14). These findings indicate that using AAV5 as a vector for gene delivery instead of AAV2 could improve gene delivery in the human central nervous system considerably, especially when the transgene produces a protein normally manufactured in glial cells.
The present study was carried out in a large number of monkeys, all evaluated at 30 days after vector injection. It is possible that some serotypes might lead to more gene expression over longer periods of time. Most AAV serotype capsids share >80% amino acid similarity, yet these differences can lead to large differences in terms of tropism. AAV5 was the most evolutionarily distinct of over 100 AAV variants recovered from human and nonhuman primates, with <62% amino acid similarity to AAV1–4 and AAV6 (ref. 15). Moreover, AAV5 demonstrates a distinct combination of characteristics that are likely responsible for the efficiency of this serotype;5,6 it binds to the N-linked form of α(2,3) sialic acid,7 uses platelet-derived growth factor receptor8 and has the potential for retrograde transport.9 Although we did see what might have been retrograde transport in monkeys that were injected into CD in one hemisphere, and the SN in the other, we did not evaluate retrograde transport systematically because we did not have enough animals injected in this way across serotypes for meaningful comparisons.
Even though it has proven to be an effective vector in the nonhuman primate, AAV5 may not be the most efficient vector at transducing neural cells across mammalian species. In their study on feline brain Vite et al.16 found AAV5 to be so inefficient that direct injection into the brain failed to transduce any cells.16 In the canine brain AAV2 and AAV8 have been found to be effective at neural transduction17,18 although, just as in human and nonhuman primate, due to the lack of studies of the other AAV serotypes in this species, AAV2 is the most widely used vector system in canine brain.
Both old and new world nonhuman primates have received direct injection of AAV2 vector into the brain, including macaque,19,20,21 cynomolgus,17 and rhesus monkeys22 and transduction of neural cells has been described in all of them. Although other serotypes of AAV vectors are rarely used, AAV1 has been used successfully to transduce neural cells in macaque monkey20 and pseudotyped vectors AAV2/1 and AAV2/5 have been used successfully for transduction of neural cells in macaque brain.19 Interestingly, pseudotyped vectors AAV2/1 and AAV2/5 when injected into the macaque striatum showed greater vector copy numbers than AAV2. This finding correlates well with our observation that AAV2 performed unimpressively in the caudate and was surpassed by both serotypes 1 and 5 in numbers of cells transduced.
Of the seven active clinical trials using AAV vectors in human brain, five are directed at Parkinson's disease therapeutics and all have used AAV2. Our study and that of others19 indicate that AAV2 is inefficient at transducing neural cells in the striatum. In our study, AAV2 was surpassed in transduction efficiency by every other serotype injected into the CD except for AAV3. AAV5 transduces more cells, and therefore more glial cells than any other vector studied, making it a superior vector for use in the delivery of glial-derived neurotrophic factor family ligands to the SN and CD of primates. Additionally, using AAV5 may actually be safer than using AAV2, having a decreased risk of crossreactions with pre-existing antibodies. Antibodies against AAV, predominantly against serotype 2, are present in 50–80% of the population23,24 and have caused problems in previous clinical studies.25,26
AAV5 and AAV1 transduce cells over a greater volume of tissue than AAV2, AAV3, AAV4, and AAV6 (Student–Newman–Keuls test at P < 0.05), and AAV5 transduces more cells than any of the other serotypes (P < 0.05 by Student–Newman–Keuls test). Combined with possible retrograde transport to the SN (which may depend on the extent of the destruction of the nigrostriatal pathway), these greater effects could allow the reduction of viral protein load for achieving an equivalent effect.
In summary, the AAV5 vector is more efficient than AAV2 in the nonhuman primate brain, resulting in a far greater number of cells transduced for a given viral load. Using a viral vector with greater efficiency at transducing the desired cell type could provide an advance for delivery of therapeutic genes to the nigrostriatal system.
Materials and Methods
All animal work complied with National Research Council guidelines and was approved by the Axion Research Foundation's animal care and use committee.
Vector construction. The vector used in this study was the TRUF-eGFP vector. This vector contains the GFP gene driven by the cytomegalovirus promoter. The TRUF-eGFP vector was used for all viral preps in conjunction with the appropriate AAV helper plasmid for each respective AAV serotypes 1–6 used in the experiments.
Vector production. The pTRUF-eGFP vector was packaged into vector particles with AAV serotypes of 1–6 at the University of North Carolina Vector Laboratories. Vector particles were manufactured as described in detail in ref. 27. Briefly, human embryonic kidney-293 cells grown to 80–90% confluent in Dulbecco's modified Eagle's medium + 10% fetal bovine serum, 37 °C, 5% CO2 are transfected with TRUF-eGFP, the XX680 Adenoviral helper plasmid and an AAV helper plasmid (pAAV1, pAAV2, pAAV3, pAAV4, pAAV5, or pAAV6, depending on which serotype is being prepared) using polyethylenimine as the transfection reagent.
Cells are harvested 48 hours later, lysed in a hypotonic buffer (10 mmol/l HEPES, 1.5 mmol/l MgCl2, 10 mmol/l KCL, and 350 mg of spermine/l), and then homogenized in a Kontes glass homogenizer (Kimball Chase, Vineland, NJ). The resulting nuclei suspension is then clarified by centrifugation, sonicated for 25 pulses with a Branson sonifier (cycle 50, output 5) and then incubated at 37 °C with 10 mg/ml of benzonase for 30–60 minutes.
Nuclear lysates were centrifuged through a CsCl gradient, and peak vector fractions were subjected to a second CsCl centrifugation step. The pooled peak fractions are then dialyzed in sterile Pierce Slide-A-Lyser cassettes twice against phosphate-buffered saline for 2 hours each (total dilution factor of 1:1 × 106). Following purification the individual batches of vector are stored at −80 °C until used. Titers of vector genomes in the viral preparations were determined using the dot-blot method.27 Viral titers between the different preps ranged from 2–4 × 1012 vg/ml which is within the margin of error for the assay. Using these manufacturing conditions, vectors are consistently produced with minimal contamination of cellular proteins (see representative preparations in Supplementary Figure S1). Viral preps tested for residual CsCl by atomic absorption has shown that these methods yield stocks of virus with ≤2 ng of residual CsCl per 1 × 1012 vector genomes (data not shown).
Stereotactic surgeries. Fully mature adult St Kitts (African) green monkeys (C. sabaeus), with no stigmata of advanced age, were anesthetized with ketamine (10 mg/kg intramuscularly) and sodium pentobarbital (15–25 mg/kg intravenously), intubated and the scalp was prepped, shaved, and mounted into a stereotactic frame using sterile technique. The animal was monitored for vital signs, electrocardiography, pO2, temperature and given a 30–50 ml/hour intravenous infusion of Lactated Ringer's solution. Each animal received 300,000 Units of Flocillin before surgery. After the animal was anesthetized to a level that showed no deep pain responses, the scalp was incised. Small 1–2 mm holes in the cranium were drilled at the desired coordinates (David Kopf, Tujunga, CA). A 22-gauge needle connected to a Hamilton syringe (Hamilton, Reno, NV) was lowered through drilled holes to the desired depth, and left in situ for 2 minutes before and after injection. The vector-loaded 100-µl Hamilton syringe was driven by a perfusion pump delivering vector at 1 µl/minute (Stoelting Instruments, Wood Dale, IL) for 10 minutes, and a total of 10 µl of vector was delivered to each target. Targets were the left or right CD and left or right SN. A sorbitol control injection was made on the same structure on the opposite side in some animals. The needle was slowly withdrawn (1 mm/min) over a period of 5 minutes. Animals survived for 30 days before killing after induction with ketamine (10 mg/kg) followed by an overdose of pentobarbital solution given until loss of deep corneal reflexes (usually 50–100 mg/kg intravenously).
DAB immunohistochemistry. Animals were perfused with heparinized saline, followed by 4% paraformaldehyde, and brains were removed. Blocks of tissue surrounding the injection target were isolated, postfixed, and immersed in 30% sucrose. Coronal sections of blocks of tissue for DAB immunohistochemistry were cut at 50 µm at room temperature on a vibratome. Free-floating sections comprising every fourth section were stored in buffer at 4 °C. One series, or every fourth section, was immunolabeled for GFP using a mouse monoclonal antibody against GFP (Invitrogen # A11120 at 1:250; R&D Systems, Minneapolis, MN). GFP immunoreactivity was visualized using biotinylated horse anti-goat immunoglobin G (BA-9500 at 1:250; Vector Laboratories, Burlingame, CA), and the ABC technique with nickel-intensified DAB as chromagen. Sections were examined under a light microscope, and brains in which the injection tract was found to be well placed within the target region were analyzed.
Fluorescence immunohistochemistry. Triple label fluorescence immunohistochemistry was performed on frozen sections of free-floating caudate tissue that were cut on a sliding microtome at 40 µm thickness. Sections were permeabilized and blocked with 0.3% triton and normal donkey serum in potassium phosphate-buffered saline buffer. Primary antibodies generated in rabbit, mouse, and guinea pig were diluted in triton buffer and applied together onto tissue sections for 72 hours at 4 °C. Sections were then rinsed, and incubated in secondary antibodies for 1 hour at room temperature. Sections were rinsed, mounted onto gel coated slides, and coverslipped using a PVA DABCO mountant.28 Antibodies used were: rabbit anti-GFP (1:100, AB3080; Millipore, Temecula, CA); guinea pig anti-GFAP (1:250, #031223; Advanced Immunochemicals, Long Beach, CA); mouse anti-NeuN (1:100, MAB377; Millipore); secondary antibodies used were: donkey-anti-guinea pig CY5, donkey anti-mouse-Rhodamine Red-X, and donkey anti-rabbit-fluorescein isothiocyanate (Jackson ImmunoResearch, West Grove, PA). Tissue sections were examined under a fluorescence microscope equipped with confocal lasers (Zeiss LSM510; Zeiss, Thornwood, NY), and images captured using LSM 510 software (Zeiss). Images were arranged using Adobe Photoshop (Adobe Systems, San Jose, CA).
Stereology: cell counts, volume, and transduction efficiency (density). We quantified the number of GFP+ cells and volume of tissue occupied by GFP immunoreactivity using Stereo Investigator 7 software (MicroBrightField, Williston, VT). Counts were made from at least three animals per serotype/target area. Every fourth section was analyzed using the optical fractionator probe. The area containing concentrated GFP immunoreactivity was outlined at ×2.5 magnification and GFP+ cells were counted at ×20. The volume of spread of concentrated GFP immunoreactivity was determined by multiplying the area of the outline by the section thickness and the sampling interval, using the Cavalieri method.29 Density was determined by dividing the cell count by the volume.
Phenotype. To determine the identity of GFP+-transduced cells in tissue, we performed triple-labeling immunofluorescence with the antibodies to GFP, NeuN, and GFAP. We took confocal photomicrographs of nonadjacent sections and counted at least 33 cells from each of three animals per serotype for a total of at least 100 cells. The cells were scored as NeuN+, GFAP+, or undetermined. Percentages of neurons and glial cells were then calculated.
SUPPLEMENTARY MATERIALFigure S1. Silver stained polyacrylamide gel electrophoresis (PAGE) of AAV GAP vectors of different serotypes.
Supplementary Material
Silver stained polyacrylamide gel electrophoresis (PAGE) of AAV GAP vectors of different serotypes.
Acknowledgments
We thank Heidi Tucker for excellent technical assistance and the staff of St Kitts Biomedical Research Foundation for their assistance with surgeries, sample collections, and care of the monkeys during the experiment, especially Clive Wilson, Ernell Nisbett, Steve Whittaker, Junior Swanston, Shervin Liddie, and Xavier Morton. Supported by NINDS 5U01NS046028, the Michael J. Fox Foundation for Parkinson's Research, and the Axion Research Foundation. The authors declared no conflict of interest regarding the vectors in this study.
REFERENCES
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Smith A, Collaco R., and , Trempe JP. Methods in Molecular Biology: AAV Vector Delivery to Cells in Culture. Vol. 246. Springer Verlag: Clifton, NJ; 2004. pp. 167–177. [DOI] [PubMed] [Google Scholar]
- Virella-Lowell I, Zusman B, Foust K, Loiler S, Conlon T, Song S, et al. Enhancing rAAV vector expression in the lung. J Gene Med. 2005;7:842–850. doi: 10.1002/jgm.759. [DOI] [PubMed] [Google Scholar]
- Mueller C., and , Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Chiorini JA, Afione S., and , Kotin RM. Adeno-associated virus (AAV) type 5 Rep protein cleaves a unique terminal resolution site compared with other AAV serotypes. J Virol. 1999;73:4293–4298. doi: 10.1128/jvi.73.5.4293-4298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J., and , Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Dass B, Gasmi M, Bakay R, Stansell JE, Tuszynski M, et al. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol Ther. 2008;16:1737–1744. doi: 10.1038/mt.2008.170. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, et al. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, et al. Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Vandenberghe LH, Parente MK, Carnish ER, Wilson JM., and , Wolfe JH. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite CH, Passini MA, Haskins ME., and , Wolfe JH. Adeno-associated virus vector-mediated transduction in the cat brain. Gene Ther. 2003;10:1874–1881. doi: 10.1038/sj.gt.3302087. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G, et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther. 2008;16:916–923. doi: 10.1038/mt.2008.41. [DOI] [PubMed] [Google Scholar]
- Ciron C, Cressant A, Roux F, Raoul S, Cherel Y, Hantraye P, et al. AAV1-, AAV2- and AAV5-mediated human alpha-iduronidase gene transfer in the brain of nonhuman primate: vector diffusion and bio distribution. Hum Gene Ther. 2009;20:350–360. doi: 10.1089/hum.2008.155. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Miyachi S, Kitagawa R, Wada K, Nihira T, Ren YR, et al. Neuronal specificity of alpha-synuclein toxicity and effect of Parkin co-expression in primates. Neuroscience. 2007;144:743–753. doi: 10.1016/j.neuroscience.2006.09.052. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci USA. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Sereno MS, Brandt CD, Kim HW, Parrott RH, et al. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol. 1971;94:359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- Parks WP, Boucher DW, Melnick JL, Taber LH., and , Yow MD. Seroepidemiological and Ecological Studies of the Adenovirus-Associated Satellite Viruses. Infect Immun. 1970;2:716–722. doi: 10.1128/iai.2.6.716-722.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Peden CS, Burger C, Muzyczka N., and , Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW., and , Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Markakis EA., and , Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Silver stained polyacrylamide gel electrophoresis (PAGE) of AAV GAP vectors of different serotypes.