Abstract
The solicitation behaviours performed by dependent young are under selection from the environment created by their parents, as well as wider ecological conditions. Here we show how mechanisms acting before hatching enable canary offspring to adapt their begging behaviour to a variable post-hatching world. Cross-fostering experiments revealed that canary nestling begging intensity is positively correlated with the provisioning level of their own parents (to foster chicks). When we experimentally increased food quality before and during egg laying, mothers showed higher faecal androgen levels and so did their nestlings, even when they were cross-fostered before hatching to be reared by foster mothers that had been exposed to a standard regime of food quality. Higher parental androgen levels were correlated with greater levels of post-hatching parental provisioning and (we have previously shown) increased faecal androgens in chicks were associated with greater begging intensity. We conclude that androgens mediate environmentally induced plasticity in the expression of both parental and offspring traits, which remain correlated as a result of prenatal effects, probably acting within the egg. Offspring can thus adapt their begging intensity to variable family and ecological environments.
Keywords: parental care, maternal effects, parental effects, indirect genetic effects, parent–offspring conflict, egg hormones
1. Introduction
Animal families have become model systems for understanding how the social environment influences the course of evolution (see Trivers 1974; Godfray 1995a; Mock & Parker 1997; Moore et al. 1997; Wolf & Brodie 1998; Wolf et al. 1998; Kölliker 2005; Uller 2008; Zeh & Zeh 2008). Traits of particular interest are the solicitation behaviours performed by dependent young as they demand resources and the provisioning behaviour shown by parents in response. Each trait has a genetic basis (reviewed by Kölliker & Richner 2001; Kölliker 2005), but the phenotype expressed also depends on the social environment created by the other family members, which contains genes, and therefore the environment can itself evolve (Kirkpatrick & Lande 1989; Cheverud & Moore 1994; Moore et al. 1997; Wolf & Brodie 1998; Wolf et al. 1998; Kölliker 2005; Smiseth et al. 2008). For example, the development of offspring solicitation behaviour may be modulated by so-called parental effects: aspects of the parents' phenotype, such as prenatal nourishment (reviewed by Mousseau & Fox 1998; Groothuis et al. 2005) or their inclination to supply care after birth (Wolf & Brodie 1998; Kedar et al. 2000; Kölliker 2005; Grodzinski & Lotem 2007), that are independent of direct genetic effects or environmental effects, or the interaction between the two. Likewise, the development of parental provisioning behaviour may be influenced by offspring effects: aspects of the offspring's phenotype, such as the intensity with which they demand food (Kölliker 2005).
Given this interplay of parental and offspring traits, how should their evolution proceed? Theoretical work predicts the coadaptation of genes determining offspring solicitation behaviour and genes influencing the action of parental effects (such as provisioning behaviour) ultimately leading to a genetic correlation between the two (see Wolf & Brodie 1998; Kölliker 2005). Experimental work has yielded results that are consistent with this prediction (e.g. Kölliker et al. 2000; Agrawal et al. 2001). For example, the more demanding are great tit Parus major nestlings, burying beetle Nicrophorus vespilloides larvae or mouse embryos, the more generous are their parents (Kölliker et al. 2000; Hager & Johnstone 2003; Curley et al. 2004; Lock et al. 2004, 2007). Furthermore, cross-fostering experiments involving distinct genetic strains show that these correlations are at least in part due to a genetic correlation (Hager & Johnstone 2003; Curley et al. 2004).
Nevertheless, three key questions remain unanswered. First, who gains most from this arrangement: parents or offspring (Kölliker et al. 2005; Muller et al. 2007; Uller 2008)? The coadaptation of parent and offspring traits is complicated by the potential for an evolutionary conflict of interest between parents and their young over the supply of parental investment (Trivers 1974; Parker & Macnair 1979; Godfray 1995a; Mock & Parker 1997; Müller et al. 2007; Uller 2008). Thus, the optimal solicitation behaviour for offspring may be suboptimal for parents, while the optimal provisioning behaviour for parents may be suboptimal for offspring. If parental and offspring behaviours become correlated, it is not obvious which party (if either) ends up expressing their optimal behaviour. We address this question experimentally elsewhere.
In this paper, we focus on two further unanswered questions. First, what proximate mechanisms mediate the observed phenotypic correlations between parent and offspring traits (Kölliker 2005)? Second, how do they explain observed variation from family to family within species in the expression of these characters? Family differences in parent and offspring behaviours (see Goodship & Buchanan 2006; Buchanan et al. 2007) may be entirely due to genetic variation, or they may reflect phenotypic plasticity, especially in variable environments (West-Eberhard 2003; Nussey et al. 2005). Hormones such as testosterone are prime candidates for inducing such plasticity (Mousseau & Fox 1998; Groothuis & Schwabl 2008).
To address these questions, we ran a series of experiments on domesticated canaries (Serinus canaria). Canaries breed readily in captivity, in experimentally controllable environments, enabling us to partition sources of variation in offspring and parental behaviours. In previous experimental work, we found considerable variation in faecal androgen levels and brood begging intensity from family to family, even after controlling for brood age and hunger (Buchanan et al. 2007). Here we show that some of this variation is due to a correlation between nestling begging behaviour and parental provisioning behaviour. We further demonstrate plasticity in this correlation, which is generated by hormonal mechanisms acting before hatching, and which is dependent on the quantity and quality of food available during nesting.
2. Material and methods
(a). Study species
Both Fife and Gloster type canaries were bred at the Sub-Department of Animal Behaviour at Madingley, Cambridgeshire, UK, in 1993–1995 and 1997–2002, and Fife type only during 2004–2006. Every year, birds were moved from aviaries in February or March, randomly assigned a partner and allowed to breed until 31 July at the latest. Each pair occupied a double breeding cage, furnished with a nest at one end and ad libitum quantities of food and water at the other (for further husbandry details, see Kilner 2002). The modal brood size was 3 (n=146 broods).
(i). Phenotypic correlation between nestling begging and parental provisioning
We used a cross-fostering experiment to measure offspring begging behaviour and parental provisioning after hatching independently, so as to detect evidence of a phenotypic correlation between the two traits. During 1997–2000, we created 13 dyads from 26 nests, and cross-fostered young within each dyad. Before hatching, 11 days after the onset of incubation, entire clutches were reciprocally cross-fostered between nests. To see whether offspring begging was related to parental provisioning independent of experience, we compared focal chick begging levels in their foster nest with focal parental provisioning levels to their foster chicks. Provisioning levels were inferred by the level of parental provisioning by examining the growth rate of the foster brood in their care (see below).
Measuring nestling begging intensity
Five days after hatching, nestlings were temporarily transferred from their nest in the breeding room to an identical, heated nest in the testing room. After being fed a standard meal, each chick was induced to beg alone under standardized conditions (further details in Kilner & Davies 1998; see the electronic supplementary material), and their behaviour was filmed after 40 and 80 min. Owing to a power cut we could not record begging from one family at 40 min, so the sample size at 40 min is 25 nests and at 80 min is 26 nests. From the videotapes, we measured postural begging intensity, an estimate of begging vigour that increases with nestling hunger (Kilner 1995), predicts parental provisioning behaviour (Kilner 1995, 2002) and has associated costs (Kilner 2001).
Measuring nestling growth rate
At hatching, chicks were marked for individual recognition. Chicks were weighed daily (or as frequently as time permitted) at roughly the same time each day between hatch day (day 0) and day 13. The rate of growth during this time was derived from the slope of a simple linear regression of chick mass on age (for all chicks: 0.924<R2<0.99).
Measuring whether chick growth rate is a good measure of foster parental provisioning
To test whether foster brood growth rates really do predict parental provisioning levels, in 1994 we observed the rate at which each nestling in broods of three was fed by parents for 1 hour either 11 (n=5 broods) or 12 (n=5 broods) days after hatching, and related that score to their mass gain that day. Nests were filmed from 10.00±15 min and the number of feeds each chick received was later quantified. Chicks were weighed in the same order each day, beginning at 16.00.
We used a linear mixed effect model in SPSS v. 15.0 to see whether foster chick daily mass gain was a good predictor of foster parental provisioning rate. The number of feeds per hour each chick received was the dependent variable, and each chick's daily mass gain and chick age were covariates. Brood identity was controlled for as a random factor. We checked for an interaction between the covariates.
We found that chick mass gain (independent variable) did predict the number of feeds each chick received in an hour (dependent variable) on that day (F1,22.7=14.98, p=0.001). There was no effect of chick age (F1,7.9=0.04, p=0.85) and no significant interaction between the number of feeds each chick received and chick age. This result confirms that foster offspring growth rates can be used to measure parental provisioning.
(ii). Plasticity in the phenotypic correlation between nestling and parental behaviour
Experimental manipulation of the environment during nesting
We manipulated the quantity and quality of food available to pairs from 39 days before pairing until 2 days before their chicks hatched. On 28 February 2006, each pair was assigned a double breeding cage (n=35), with the male and female separated by a metal barrier. Pairs were alternatively assigned to the ‘high’ or ‘low’ food quality treatment, each receiving ad libitum quantities of Haith's condition canary seed. Pairs in the high treatment also received one tablespoon of moistened Haith's rearing and condition soft food mixed with hard-boiled egg yolk and a small piece of broccoli every morning, as well as ad libitum quantities of dry Haith's rearing and condition soft food and Nutribird C15 pellets. After 39.5±0.40 days, birds were paired by removing the metal barrier between them. The food quality treatments ceased 11 days after the onset of incubation. At this point, clutches were cross-fostered for the last 2 days of incubation.
Our original intention was to cross-foster between and within food quality treatment groups evenly, creating four experimental treatments: original low-foster low (LL); original low-foster high (LH); original high-foster low; and original high-foster high (HH). By chance, however, we had insufficient breeding synchrony between treatments to achieve this design. This, together with brood failure, left us with no broods in the LL treatment. To keep the design balanced, we therefore selected only broods fostered into the high food quality treatment for analysis, so that we could compare the effect of original feeding treatment under uniform foster conditions. (Note that the results of our statistical analyses were qualitatively unchanged by restricting the dataset in this way.) In first clutches, there were ten broods in the HH treatment and six in the LH treatment. In second clutches, there were nine broods in the HH treatment and five in the LH treatment.
After cross-fostering, all nests received ad libitum quantities of Haith's condition canary seed and Haith's rearing and condition soft food mixed with egg yolk (as in the high food quality treatment), each refreshed twice per day. Five days after hatching, chick begging levels were measured as described in ‘Measuring nestling begging intensity’.
Nestling faecal samples were collected during the begging trial to assess androgen levels because we have previously shown in this species that chick faecal androgen levels are related to begging intensity (Buchanan et al. 2007). (Faecal samples were assessed instead of plasma because measuring plasma testosterone requires at least 80 μl of plasma, which is too much to take from a 5-day-old chick.) Here we were interested to know whether faecal androgen levels (and in turn nestling begging intensity) were affected by their mother's diet during egg laying. Finally, we inferred parental provisioning levels by measuring nestling growth rate, as described above in ‘Measuring nestling growth rate’.
Females were allowed to lay a second clutch immediately after fledging the first brood and it was not possible to resume the food quality treatments between clutches. Second broods were cross-fostered to a different pair from the first brood. Five days after hatching, we measured begging behaviour of nestlings in the second brood, and collected faecal samples for faecal androgen analysis, exactly as we had done for chicks from the first brood. We included ‘brood number’ as a fixed factor in analyses of chick faecal androgens and growth, and checked for interactions.
Hormonal correlates of plasticity
We investigated whether parental provisioning levels after hatching (inferred from nestling growth rates) were correlated with the concentration of maternal and paternal androgen levels. Male and female plasma testosterone levels were measured on pairing. Approximately 100 μl blood was taken from each adult, spun in a centrifuge, the plasma collected and then frozen for radioimmunoassay.
In addition, female testosterone levels during the laying of both first and second clutches were inferred from faecal androgen levels in samples collected daily for the 3 days preceding each egg (as in Schwabl 1996a; see the electronic supplementary material). A mean faecal androgen level was calculated per female for use in the statistical analysis. Faecal samples were used instead of plasma because we wanted to assess female hormone levels over the whole period of egg formation when circulating testosterone varies dramatically (as in Schwabl 1996a). If taken daily, repeated blood samples from females over this period would cause stress, decrease blood volume and affect their welfare adversely.
Faecal androgen and plasma analyses. Plasma samples were assayed for testosterone with direct radioimmunoassay using anti-testosterone serum (see Buchanan et al. 2007). The protocol for faecal analysis was based upon previously published protocols for faecal analysis of steroids, and subject to a full biological and biochemical validation for this species (Buchanan & Goldsmith 2004; Goodship 2006; Buchanan et al. 2007). The antiserum not only detects testosterone, but also cross-reacts with other androgen metabolites. For this reason, we refer to faecal androgens throughout.
Molecular sex determination. A blood sample (80 μl) was taken from each chick on day 8. Molecular sex determination methodology was based on Griffiths et al. (1998). We included chick sex as a fixed factor in analyses of chick faecal androgens and growth.
(b). Statistical analyses
(i). Phenotypic correlation between nestling begging and parental provisioning
Ideally, we would have used individual chick measures of growth and begging intensity in each analysis. However, the structure of the model we describe in this section was too complex for this to be possible. Consequently, mean values of begging and growth rates per brood are used instead.
To test whether mean brood begging intensity was correlated with the natural parent's provisioning levels, we investigated whether focal parental provisioning predicted the begging level of their brood, raised by foster parents, at both 40 and 80 min food deprivation. We used a linear mixed effect model (LME) in SPSS v. 15.0, with mean brood posture (in a foster nest) as the dependent variable and parental provisioning (measured as the growth rate of the foster brood in the focal nest) as a covariate. To rule out effects of the foster family on mean brood begging intensity, we controlled for the growth rate of the brood in the foster nest. Growth measures were log transformed to meet the assumptions of the model. Dyad was a random factor and hunger was a repeated measure (at 40 and 80 min). We checked the robustness of this result using a Spearman rank correlation on the relationship between mean brood begging intensity (mean of brood begging intensity at 40 and 80 min) and parental provisioning.
(ii). Plasticity in the phenotypic correlation between nestling and parental behaviour
A linear mixed effect model in SPSS v. 15.0 was used to investigate the effect of the food quality treatments on female faecal androgen levels. Original food quality treatment was a fixed factor. Clutch number nested within original female identity was controlled for as a random factor. However, there was no effect of clutch number as a fixed effect, nor was the interaction between clutch number and treatment significant. Mean female faecal androgen levels were log transformed to fit the assumptions of the model. A general linear model (GLM) was used to investigate the effect of food treatment on male plasma testosterone levels upon pairing, and also to investigate the correlation between male and female plasma testosterone levels on pairing. Plasma testosterone levels were log transformed to fit the assumptions of the model.
A linear mixed effect model in SPSS v. 15.0 was used to investigate the effect of food quality on individual chick faecal androgen levels. Original food quality treatment was a fixed factor. In this analysis, clutch number nested within original female identity was a random factor to control for multiple measures of both clutches and chicks within each female. There was no effect of clutch number, nor was the interaction between clutch number and treatment significant. Clutch number was therefore dropped from the model. Chick faecal androgen levels were log transformed to fit the assumptions of the model.
A linear mixed effect model was then used to investigate whether parental provisioning, as measured by individual foster chick growth rate, was predicted by the levels of maternal faecal androgens and paternal plasma androgens (which were not correlated; GLM: F1,24=0.41, p=0.53). Clutch number nested within original female identity was a random factor to control for multiple measures of both clutches and chicks within each female. There was no effect of clutch number, nor was the interaction between clutch number and treatment significant. Measurements of faecal androgens and plasma testosterone were log transformed to fit the assumptions of the model.
In each model, non-significant terms were sequentially deleted to yield the minimal model. All statistical tests reported are type III.
3. Results
(a). Phenotypic correlation between nestling begging and parental provisioning
We found that mean brood begging intensity and parental provisioning were positively correlated, even after cross-fostering. A linear mixed effects model showed that parents that provisioned at a higher rate (measured as the growth rate of the foster brood in the focal nest) produced chicks that begged more during controlled laboratory trials, even though these chicks were raised by foster parents (table 1; figure 1). A more robust measure of this relationship is a Spearman's rank correlation (although it cannot incorporate repeated measures, nor control for dyad). This also shows a significant positive relationship between parental effort and the mean begging level of their chicks (mean of brood begging at 40 and 80 min; n=26, Spearman's ρ=0.46, p=0.022).
Table 1.
Linear mixed effects model examining the prenatal link between provisioning levels (measured as mean growth rate of the foster brood parents reared) and mean focal brood begging intensity when reared by foster parents. (Focal brood growth rate was left in the model to control for the effect of foster parental provisioning rates on focal brood begging levels; removing this term makes little qualitative difference to the results. n=26 dyads, from 52 broods. Time (40 or 80 min after food deprivation) was a repeated measure and dyad was a random factor. The mean of both begging and growth was used for each brood. The p values are type III and are italicized when p<0.05.)
| dependent=mean focal brood begging | B | d.f. | F | p-value |
|---|---|---|---|---|
| (a) minimal model | ||||
| log growth rate of foster brood in focal nest (focal parental provisioning levels) | 13.23 | 1,32.89 | 4.62 | 0.039 |
| log growth rate of focal brood in foster nest (control for foster parental provisioning levels) | −0.13 | 1,32.89 | <0.001 | 0.98 |
Figure 1.
The relationship between offspring begging intensity (when raised by foster parents) and their true parents' provisioning effort (inferred from the mean growth rate of foster young in the focal nest) at (a) 40 min and (b) 80 min after feeding. Each data point represents a mean across chicks in the brood. The logarithmic regression line is shown.
(b). Plasticity in the phenotypic correlation between nestling and parental behaviour
(i). Effect of environmental manipulation on parent and offspring androgen levels
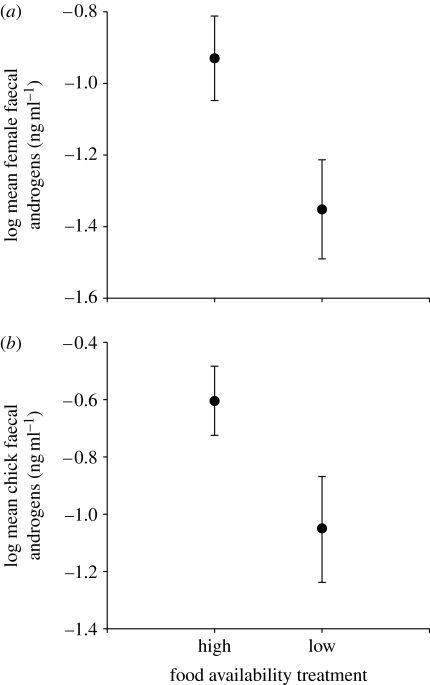
Females in the high food quality treatment had greater levels of faecal androgens during egg laying compared with those in the low food quality treatment (GLM: F1,28=4.56, p=0.042; figure 2a). There was no significant difference between male testosterone levels at pairing in the high and low quality food treatments (GLM: F1,25=0.99, p=0.33). There was no correlation between male and female plasma testosterone levels at pairing (GLM: F1,19=0.003, p=0.96).
Figure 2.
(a) The effect of the food quality treatment on maternal faecal androgen levels during egg laying (linear mixed model; clutch number nested within female identity was a random factor; n=19 in the high food quality treatment group, 10 in the low food quality treatment group). (b) The downstream effect of the maternal food quality treatment during egg laying on nestling faecal androgen levels. Offspring were cross-fostered before hatching and reared under uniform conditions of food quality (linear mixed model; clutch number nested within female identity was a random factor; n=95 chicks from the 18 successful nests in the high food quality treatment group and 6 successful nests in the low food quality treatment group). Predicted means and standard errors after controlling for random factors are shown.
Experimentally manipulating environmental conditions not only changed the levels of faecal androgens in mothers (figure 2a), but had similar downstream effects on their young. Nestlings hatching from eggs laid by mothers that experienced the high food quality treatment before egg-laying had greater levels of faecal androgens than those whose mothers had experienced the low food quality treatment (LME: F1,20.16=5.98, p=0.024; figure 2b). Note that these nestlings were neither exposed directly to the food quality manipulations experienced before and during laying by their mothers, nor reared in the natal nest after hatching. The change in their levels of faecal androgens with food quality must therefore be attributable to a prenatal maternal influence, probably transmitted through environmental conditions in the egg (Schwabl 1993, 1996a).
(ii). Effect of environmental manipulation on parental provisioning
We know from our previous work that canary nestling faecal androgen levels and begging intensity are positively correlated (Buchanan et al. 2007). The results of our food quality experiment additionally show that the extent of parental provisioning is positively correlated with maternal and paternal androgen levels (table 2). The growth rate of chicks was positively related to the plasma testosterone levels of their foster fathers and the faecal androgen levels of their foster mothers (table 2). There was also a positive effect on growth of the size of the egg the chick hatched from (table 2).
Table 2.
Linear mixed effects model examining hormonal correlates of provisioning effort, measured as the growth rate of the chicks that these parents rear. (n=90 chicks from 24 clutches reared by 18 foster females from 20 original females. Clutch number nested within female was included as a random factor. The p values are type III and are italicized when p<0.05.)
| term in model | B | d.f. | F | p-value |
|---|---|---|---|---|
| (a) minimal model | ||||
| log foster male plasma testosterone | 0.11 | 1,21.10 | 11.69 | 0.003 |
| log foster female faecal androgens | 0.31 | 1,23.13 | 9.63 | 0.005 |
| egg mass | 0.55 | 1,68.33 | 7.79 | 0.007 |
| (b) terms dropped from model | ||||
| original treatment | ||||
| high | 0.11 | 1,19.93 | 1.02 | 0.33 |
| low | 0 | |||
| original clutch size | −0.06 | 1,20.488 | 0.78 | 0.39 |
| foster brood size | 0.03 | 1,19.55 | 0.18 | 0.68 |
| chick sex | ||||
| female | −0.017 | 1,46.10 | 0.13 | 0.72 |
| male | 0 | |||
Taken together, our results suggest that prenatal cues match nestling demands to the extent of parental provisioning. Furthermore, there is environmentally induced plasticity in both parental provisioning and offspring begging intensity, which is mediated by androgens.
4. Discussion
In many species, offspring vary from family to family in the intensity of their demands, even after controlling for factors such as age and hunger (e.g. Kedar et al. 2000; Kölliker et al. 2000; Agrawal et al. 2001; Hager & Johnstone 2003; Curley et al. 2004; Lock et al. 2004, 2007; Goodship & Buchanan 2007). One theoretical explanation for this variation is that offspring characters are selected by the family environment in which they develop, with the result that genes for offspring traits become linked to parental genes influencing the developmental environment (Wolf & Brodie 1998; Kölliker & Richner 2001; Kölliker 2003, 2005; Royle et al. 2004; Kölliker et al. 2005; Räsänen & Kruuk 2007). Our experimental results are consistent with this suggestion, and in this regard are similar to those obtained previously for burying beetles (Lock et al. 2004, 2007), mice (Hager & Johnstone 2003; Curley et al. 2004) and great tits (Kölliker et al. 2000).
However, our work differs from previous studies in two key ways. First, we have shown that the positive correlation between the strength of the offspring's demands and the provisioning level of their parents when provisioning after hatching is maintained by a prenatal maternal effect on nestling begging intensity (figures 1 and 2). Second, our experiments reveal plasticity both in the extent of parental provisioning and in the intensity of offspring demands, because they covary with wider environmental conditions. In response to superior food quality, mothers showed elevated levels of faecal androgens during the egg-laying period (figure 2a), and these levels of faecal androgens were positively correlated with the extent of offspring provisioning after hatching (table 2). Remarkably, their offspring also showed higher levels of faecal androgens (figure 2b) even though they were not directly exposed to either the food quality manipulations or the post-hatching behaviour of their own mothers or fathers. Androgens thus apparently mediate environmentally induced plasticity in the expression of both parental and offspring traits (table 2), which remain correlated as a result of prenatal effects, probably acting within the egg (Schwabl 1993; Groothuis et al. 2005; Groothuis & Schwabl 2008). These results complement a study of great tits (P. major), which showed that females supplemented with dietary carotenoids provisioned at a higher level and produced chicks that begged more (Helfenstein et al. 2008). Carotenoids could therefore also mediate the covariation between parental and offspring behaviour.
Our experiments suggest that variation among families in offspring solicitation intensity, and covariation within families between parent and offspring behaviour, is not solely the result of genetic variation (see Kölliker 2005; Dor & Lotem 2009). Our feeding experiment suggests that it partly results from the wider environmental conditions experienced by mothers (and fathers) during nesting and egg laying (see also Kölliker et al. 2000; Moreno et al. 2008). Highly demanding offspring are the progeny of parents that experienced ready access to high-quality resources during this critical period, whereas more weakly begging young are produced by parents that were more food-deprived. Depending on their particular environmental circumstances, the same pair might therefore produce offspring of different behavioural types, although the extent to which the environment induces plasticity might vary among individuals (Eising et al. 2008). Our measure of parental provisioning combines maternal and paternal care, so it is not possible for us to infer whether the correlation between parental behaviour and offspring begging differs between parents (as in the great tit: see Kölliker et al. 2000).
What is the mechanistic basis of the prenatal effect driving the post-natal correlation between parental and offspring behaviour? We speculate that this maternal effect might involve the action of maternal steroids deposited in the egg yolk. In his ground-breaking work on canaries, Schwabl (1993) found variation among freshly laid clutches in the concentration of maternally derived testosterone in the egg yolk and subsequently showed that this variation could be explained by levels of maternal faecal androgens during egg laying, which themselves varied with environmental conditions (photoperiod, in this case; Schwabl 1996a). He also found that canary nestlings emerging from eggs that had been injected with testosterone soon after laying were more inclined to gape in the 24 hours after hatching (Schwabl 1996b). Our results show that the prenatal environment affected both maternal androgen levels, which were related to parental provisioning, and the androgen levels of chicks, which were related to begging intensity (Buchanan et al. 2007). Schwabl's results, together with our own, therefore suggest that maternally derived yolk testosterone is a prenatal cue that conveys information about the wider environment from the canary mother to her unborn young, which then guides the development of nestling begging and parental behaviour expressed after hatching (Müller et al. 2007; Groothuis & Schwabl 2008; Moore & Johnston 2008).
This study, together with previous work on other species, shows that the development of nestling begging behaviour is influenced by both prenatal and post-hatching cues (Kedar et al. 2000; Kölliker et al. 2000; Madden & Davies 2006; Grodzinski & Lotem 2007; Langmore et al. 2008). What influences the relative importance of these two factors? It is possible that canaries bias begging development to be more greatly influenced by prenatal cues because they are a better source of environmental information than any cues available from parents after hatching. Variation in nest visit rate might be a good indicator of food quality for an insectivorous species, but for a seed-regurgitator such as the canary, visiting the nest just two or three times an hour, such variation may be too low to be informative. In addition, canary nestlings may experience perceptual constraints as a consequence of their relatively slow development, which is again perhaps the result of their seed-eating habit (Newton 1972), and these may limit their ability to gather information about the wider environment. Thus embryonic birds developing within the egg might be better able to detect environmental information conveyed hormonally than newly hatched nestlings with relatively poorly developed sensory systems (Khayutin 1985).
The existence of prenatal maternal effects on nestling begging intensity is hypothesized to be the result of an evolutionary conflict of interest between parents and their young over the provision of parental investment (Groothuis et al. 2005; Müller et al. 2007; Smiseth et al. 2008; Uller 2008). Parents are selected to balance investment in their current brood against their future reproduction, and this might explain why the extent of their provisioning is carefully regulated by androgens to match their current supply of food. At the same time, mothers and fathers must defend their residual reproductive value against the demands of the current brood, which is under selection to be more demanding than is optimal from the parents' perspective (Trivers 1974; Godfray 1995b; Mock & Parker 1997). Maternal effects may have evolved initially to limit nestling begging intensity, but they may persist because they now serve the evolutionary interests of offspring too, as a valuable source of information about the wider environment (Wolf et al. 1999; Groothuis et al. 2005; Müller et al. 2007; Moore & Johnston 2008; Uller 2008). In addition, by generating covariance between parental provisioning behaviour and offspring begging intensity, maternal effects enable offspring to show a rapid evolutionary response to changes in provisioning behaviour, counteracting any evolutionary advantage that parents collectively might temporarily have gained (Moore et al. 1997; Wolf & Brodie 1998; Wolf et al. 1999; Stamps 2003; West-Eberhard 2003; Kölliker et al. 2005; Moore & Pizzari 2005). The covariance of parental and offspring phenotypes thus promotes social (co)evolution fuelled by parent–offspring conflict (Moore et al. 1997; Wolf & Brodie 1998; Wolf et al. 1999; Kölliker et al. 2005). However, whether the maternal effects we have identified here currently serve the evolutionary interests of parents, offspring or both parties remains to be determined.
Acknowledgments
R.M.K. was supported by a NERC studentship, then a Junior Research Fellowship at Magdalene College Cambridge, a Royal Society Dorothy Hodgkin Research Fellowship (sponsored by the Wolfson Foundation) and finally a Royal Society University Research Fellowship. The experiments were funded by two NERC small grants, a NERC standard research grant NER/A/S/2002/00776, which also supported C.A.H., and a Royal Society equipment grant. Additional funding for C.A.H. was provided by the Isaac Newton Trust and a Junior Research Fellowship at Newnham College Cambridge. K.L.B. was supported by BBSRC grant S18938. We are grateful to L. Barden, N. Bates, C. Donovan, P. Heavens, P. Hynes, I. Millar, J. Nightingale and S. Shelton for their help in maintaining the birds and constructing equipment; T. Roberts, M. Wade and J. Theaker for supplying the birds; J. Theaker for his invaluable advice on husbandry; and L. Fleming and N. Evans at the University of Glasgow for performing the molecular analyses. We thank N. Davies, M. Clinchy, R. Johnstone, M. Kölliker, C. Lessells, D. Nussey and L. Zanette for their invaluable comments on the manuscript, and C. Lessells and K. Moyes for statistical advice. Author contributions: C.A.H. co-designed and ran the food quality experiment, analysed the behavioural and hormonal data and co-wrote the paper; K.L.B. oversaw the hormonal analyses by C.A.H. in K.L.B.'s laboratory; R.M.K. conceived and designed the experiments, ran the cross-fostering experiment and co-wrote the paper. Work described in this paper was carried out under Home Office Licence PPL 80/1838.
References
- Agrawal A.F., Brodie E.D., III, Brown J.2001Parent–offspring coadaptation and the dual genetic control of maternal care. Science 292, 1710–1712 (doi:10.1126/science.1059910) [DOI] [PubMed] [Google Scholar]
- Buchanan K.L., Goldsmith A.R.2004Non-invasive endocrine data for behavioural studies: the importance of validation. Anim. Behav 67, 183–185 (doi:10.1016/j.anbehav.2003.09.002) [Google Scholar]
- Buchanan K.L., Goldsmith A.R., Hinde C.A., Griffith S.C., Kilner R.M.2007Does testosterone mediate the trade-off between nestling begging and growth in the canary (Serinus canaria)?. Horm. Behav 52, 664–671 (doi:10.1016/j.yhbeh.2007.08.009) [DOI] [PubMed] [Google Scholar]
- Cheverud J., Moore A.J.1994Quantitative genetics and the role of the environment provided by relatives in behavioral evolution. In Quantitative genetic studies of behavioral evolution (ed Boake C.) pp. 67–100 Chicago, IL: University of Chicago Press [Google Scholar]
- Curley J.P., Barton S., Surani A., Keverne E.B.2004Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc. R. Soc. Lond. B 271, 1303–1309 (doi:10.1098/rspb.2004.2725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor R., Lotem A.2009Heritability of nestling begging intensity in the house sparrow (Passer domesticus). Evolution 63, 738–748 (doi:10.1111/j.1558-5646.2008.00598.x) [DOI] [PubMed] [Google Scholar]
- Eising C.M., Pavlova D., Groothuis T.G.G., Eens M., Pinxten R.2008Maternal yolk androgens in European starlings: affected by social environment or individual traits of the mother?. Behaviour 145, 51–72 (doi:10.1163/156853908782687232) [Google Scholar]
- Godfray H.C.J.Evolutionary theory of parent–offspring conflict. Nature 376, 1995a1133–1138 (doi:10.1038/376133a0) [DOI] [PubMed] [Google Scholar]
- Godfray H.C.J.Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat 146, 1995b1–24 (doi:10.1086/285784) [Google Scholar]
- Goodship N. M.2006Endocrine control of nestling behaviour in the pied flycatcher Ficedula hypoleuca. PhD thesis, Cardiff University; [DOI] [PubMed] [Google Scholar]
- Goodship N.M., Buchanan K.L.2006Nestling testosterone is associated with begging behaviour and fledging success in the pied flycatcher, Ficedula hypoleuca. Proc. R. Soc. B 273, 71–76 (doi:10.1098/rspb.2005.3289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship N.M., Buchanan K.L.2007Nestling testosterone controls begging behaviour in the pied flycatcher, Ficedula hypoleuca. Horm. Behav 52, 454–460 (doi:10.1016/j.yhbeh.2007.06.008) [DOI] [PubMed] [Google Scholar]
- Griffiths R., Double M.C., Orr K., Dawson R.J.G.1998A DNA test to sex most birds. Mol. Ecol 7, 1071–1075 (doi:10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- Grodzinski U., Lotem A.2007The adaptive value of parental responsiveness to nestling begging. Proc. R. Soc. B 274, 2449–2456 (doi:10.1098/rspb.2007.0658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G., Schwabl H.2008Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them?. Phil. Trans. R. Soc. B 363, 1647–1661 (doi:10.1098/rstb.2007.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis T.G.G., Muller W., von Engelhardt N., Carere C., Eising C.2005Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev 29, 329–352 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- Hager R., Johnstone R.A.2003The genetic basis of family conflict resolution in mice. Nature 421, 533–535 (doi:10.1038/nature01239) [DOI] [PubMed] [Google Scholar]
- Helfenstein F., Berthouly A., Tanner M., Karadas F., Richner H.2008Nestling begging intensity and parental effort in relation to prelaying carotenoid availability. Behav. Ecol 19, 108–115 (doi:10.1093/beheco/arm103) [Google Scholar]
- Kedar H., Rodriguez-Girones M.A., Yedvab S., Winkler D.W., Lotem A.2000Experimental evidence for offspring learning in parent–offspring communication. Proc. R. Soc. Lond. B 267, 1723–1727 (doi:10.1098/rspb.2000.1201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayutin S.N.1985Sensory factors in the behavioral ontogeny of altricial birds. Adv. Study Behav 15, 105–152 [Google Scholar]
- Kilner R.1995When do canary parents respond to nestling signals of need?. Proc. R. Soc. Lond. B 260, 343–348 (doi:10.1098/rspb.1995.0102) [Google Scholar]
- Kilner R.M.2001A growth cost of begging in captive canary chicks. Proc. Natl Acad. Sci. USA 98, 11 394–11 398 (doi:10.1073/pnas.191221798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R.M.2002Sex differences in canary (Serinus canaria) provisioning rules. Behav. Ecol. Sociobiol 52, 400–407 (doi:10.1007/s00265-002-0533-8) [Google Scholar]
- Kilner R.M., Davies N.B.1998Nestling mouth colour: ecological correlates of a begging signal. Anim. Behav 56, 705–712 (doi:10.1006/anbe.1998.0785) [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Lande R.1989The evolution of maternal characters. Evolution 43, 485–503 (doi:10.2307/2409054) [DOI] [PubMed] [Google Scholar]
- Kölliker M.2003Estimating mechanisms and equilibria for offspring begging and parental provisioning. Proc. R. Soc. Lond. B 270, S110–S113 (doi:10.1098/rsbl.2003.0032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M.2005Ontogeny in the family. Behav. Genet 35, 7–18 (doi:10.1007/s10519-004-0852-9) [DOI] [PubMed] [Google Scholar]
- Kölliker M., Richner H.2001Parent–offspring conflict and the genetics of offspring solicitation and parental response. Anim. Behav 62, 395–407 (doi:10.1006/anbe.2001.1792) [Google Scholar]
- Kölliker M., Brinkhof M., Heeb P., Fitze P., Richner H.2000The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. Lond. B 267, 2127–2132 (doi:10.1098/rspb.2000.1259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker M., Brodie E.D., Moore A.J.2005The coadaptation of parental supply and offspring demand. Am. Nat 166, 506–516 (doi:10.1086/491687) [DOI] [PubMed] [Google Scholar]
- Langmore N.E., Maurer G., Adcock G.J., Kilner R.M.2008Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 62, 1689–1699 (doi:10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]
- Lock J.E., Smiseth P.T., Moore A.J.2004Selection, inheritance, and the evolution of parent–offspring interactions. Am. Nat 164, 13–24 (doi:10.1086/421444) [DOI] [PubMed] [Google Scholar]
- Lock J.E., Smiseth P.T., Moore P.J., Moore A.J.2007Coadaptation of prenatal and postnatal maternal effects. Am. Nat 170, 709–718 (doi:10.1086/521963) [DOI] [PubMed] [Google Scholar]
- Madden J.R., Davies N.B.2006A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. R. Soc. B 273, 2343–2351 (doi:10.1098/rspb.2006.3585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock D.W., Parker G.A.1997The evolution of sibling rivalry Oxford, UK: Oxford University Press [Google Scholar]
- Moore A.J., Pizzari T.2005Quantitative genetic models of sexual conflict based on interacting phenotypes. Am. Nat 165, S88–S97 (doi:10.1086/429354) [DOI] [PubMed] [Google Scholar]
- Moore A.J., Brodie E.D., Wolf J.B.1997Interacting phenotypes and the evolutionary process. 1. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 (doi:10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- Moore M.C., Johnston G.I.H.2008Toward a dynamic model of deposition and utilization of yolk steriods. Integr. Comp. Biol 48, 411–418 (doi:10.1093/icb/icn079) [DOI] [PubMed] [Google Scholar]
- Moreno J., Lobato E., Morales J., Merino S., Martinez-De La Puente J., Tomas G.2008Pre-laying nutrition mediates maternal effects on offspring immune capacity and growth in the pied flycatcher. Oecologia 156, 727–735 (doi:10.1007/s00442-008-1029-7) [DOI] [PubMed] [Google Scholar]
- Mousseau T.A., Fox C.W.1998Maternal effects as adaptations Oxford, UK: Oxford University Press [Google Scholar]
- Müller W., Lessells M., Korsten P., von Engelhardt N.2007Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat 169, E84–E96 (doi:10.1086/511962) [DOI] [PubMed] [Google Scholar]
- Newton I.1972The finches London, UK: Collins [Google Scholar]
- Nussey D.H., Postma E., Gienapp P., Visser M.E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- Parker G.A., Macnair M.R.1979Models of parent–offspring conflict IV suppression: evolutionary retaliation by the parent. Anim. Behav 27, 1210–1235 (doi:10.1016/0003-3472(79)90068-X) [Google Scholar]
- Räsänen K., Kruuk L.E.B.2007Maternal effects and evolution at ecological time-scales. Funct. Ecol 21, 408–421 (doi:10.1111/j.1365-2435.2007.01246.x) [Google Scholar]
- Royle N.J., Hartley I.R., Parker G.A.2004Parental investment and family dynamics: interactions between theory and empirical tests. Popul. Ecol 46, 231–241 (doi:10.1007/s10144-004-0196-6) [Google Scholar]
- Schwabl H.1993Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA 90, 11 446–11 450 (doi:10.1073/pnas.90.24.11446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H.Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool 276, 1996a157–163 (doi:10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- Schwabl H.Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol 114, 1996b271–276 (doi:10.1016/0300-9629(96)00009-6) [DOI] [PubMed] [Google Scholar]
- Smiseth P.T., Wright J., Kölliker M.2008Parent–offspring conflict and co-adaptation: behavioural ecology meets quantitative genetics. Proc. R. Soc. B 275, 1823–1830 (doi:10.1098/rspb.2008.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J.2003Behavioural processes affecting development: Tinbergen's fourth question comes of age. Anim. Behav 66, 1–13 (doi:10.1006/anbe.2003.2180) [Google Scholar]
- Trivers R.L.1974Parent–offspring conflict. Am. Zool 14, 249–264 (doi:10.1093/icb/14.1.249) [Google Scholar]
- Uller T.2008Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol 23, 432–438 (doi:10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J.2003Developmental plasticity and evolution Oxford, UK: Oxford University Press [Google Scholar]
- Wolf J.B., Brodie E.D.1998The coadaptation of parental and offspring characters. Evolution 52, 299–308 (doi:10.2307/2411068) [DOI] [PubMed] [Google Scholar]
- Wolf J.B., Brodie E.D., Cheverud J.M., Moore A.J., Wade M.J.1998Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol 13, 64–69 (doi:10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- Wolf J.B., Brodie E.D., Moore A.J.1999The role of maternal and paternal effects in the evolution of parental quality by sexual selection. J. Evol. Biol 12, 1157–1167 (doi:10.1046/j.1420-9101.1999.00138.x) [Google Scholar]
- Zeh J.A., Zeh D.W.2008Viviparity-driven conflict: more to speciation than meets the fly. Ann. NY Acad. Sci 1133, 126–148 (doi:10.1196/annals.1438.006) [DOI] [PubMed] [Google Scholar]