Abstract
Polyamine analogues have demonstrated significant activity against human breast cancer cell lines as single agents as well as in combination with other cytotoxic drugs. This study evaluates the ability of a polyamine analogue N1, N11-bis(ethyl)norspermine (BENSpm) to synergize with six standard chemotherapeutic agents, 5-fluorouracil (FU), fluorodeoxyuridine, cis- diaminechloroplatinum(II) (DDP), paclitaxel, docetaxel, and vinorelbine, in four human breast cancer cell lines and one immortalized, non-tumorigenic mammary epithelial cell line. BENSpm exhibited synergistic inhibitory effect on cell proliferation in combination with 5-FU or paclitaxel in human breast cancer cell lines (MDA-MB-231 and MCF-7) and either antagonistic or less effective in the non-tumorigenic MCF-10A cell line. Synergism was highest with 120 hour concomitant treatment or pre-treatment with BENSpm for 24 hours followed by concomitant treatment for 96 additional hours. Since the cytotoxic effects of many polyamine analogues and cytotoxic agents are believed to act, in part, through induction of the polyamine catabolic enzymes SSAT and SMO, the role of these enzymes on synergistic response was evaluated in MDA-MB-231- and MCF-7-treated with BENSpm and 5-FU or paclitaxel. Combination treatments of BENSpm with 5-FU or paclitaxel resulted in induction of SSAT mRNA and activity in both cell lines compared to either drug alone, while SMO mRNA and activity were increased only in MDA-MB-231 cells. Induction was greater with BENSpm/paclitaxel combination than BENSpm/5-FU. Further, RNAi studies demonstrated that both SSAT and SMO play a significant role in the response of MDA-MB-231 cells to treatment with BENSpm and 5-FU or paclitaxel. In MCF-7 cells, only SSAT appears to be involved in the response to these treatments. In an effort to translate combination studies from in vitro to in vivo, and to form a basis for clinical setting, the in vivo therapeutic efficacy of BENSpm alone and in combination with paclitaxel on tumor regression was evaluated in xenograft mice models generated with MDA-MB-231 cells. Intraperitoneal exposure to BENSpm or taxol singly and in combination for 4 weeks resulted in significant inhibition in tumor growth These findings help elucidate the mechanisms involved in synergistic drug response and support combinations of polyamine analogues with chemotherapeutic agents which could potentially be used in the treatment of breast cancer.
Keywords: BENSpm, polyamine analogue, combination study, breast cancer
INTRODUCTION
Metastatic breast cancer is the second leading cause of cancer death among women in the United States despite substantive advances in the field. One of the major obstacles in treating breast cancer is that tumors that are initially responsive to systemic treatments often progress into a more resistant form that does not respond well to current therapies [46]. Nearly 75% of patients with axillary-positive nodes and 30% of patients with axillary-negative nodes will eventually relapse with metastatic disease in the absence of systemic therapy; current adjuvant approaches provide moderate improvement in outcomes [21]. Therefore, alternative therapies are being investigated that may be effective against breast cancer regardless of its hormone dependency or stage [23].
Combination chemotherapy is now considered one of the mainstays of the treatment of breast cancer [45] [67]. Several novel agents that target specific pathways are being investigated for their efficacy in combination with standard chemotherapeutic agents [21] [35, 44, 45, 50]. Polyamine analogues are one such class of agents that have shown very promising anti-tumor activity in several in vitro and in vivo models [6, 15, 24, 40, 41, 52, 53, 58, 59, 62]. The natural polyamines are small, ubiquitous polycations that are essential for normal cell growth [14, 69]. Their metabolism is regulated at multiple levels including biosynthesis, catabolism, import, and export [71]. The key, rate-limiting biosynthetic enzymes are ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC). The polyamine catabolic enzymes act through two pathways: 1) a two-step process involving acetylation by spermidine/spermine N1-acetyltransferase (SSAT) followed by oxidation by N1-acetylpolyamine oxidase (APAO), and 2) the direct oxidation of polyamines by spermine oxidase (SMO) [31, 42, 71]. The natural polyamines have multiple feedback mechanisms to regulate their intracellular levels including activation of the catabolic enzymes, decreased polyamine import, and increased polyamine export. Polyamine analogues have been developed to mimic the natural polyamines and alter their normal function with the goal of inhibiting proliferation and/or inducing apoptosis [4, 39, 49, 55]
A polyamine analogue that has shown significant activity against human breast cancer cells in vitro and in vivo is N1, N11-bis(ethyl)norspermine, BENSpm (also known as DENSpm) [14, 23, 24, 41, 71] and it has been examined in Phase I and II clinical studies [34, 74]. Results from the Phase II study of BENSpm for women with advanced refractory breast cancer revealed that, although BENSpm was not active as a single agent in the dose and schedule chosen, this analogue could be safely administered [74].
The ability of natural polyamines to bind to and alter the conformation of DNA led to studies investigating the potential of polyamine analogues to interact with DNA. In fact, polyamine analogues were found to result in DNA strand breaks [42], inhibit nucleosome formation in cell-free systems and alter the structure of chromatin and influence gene expression in cellular systems [2, 3, 27, 65]. Based on the hypothesis that polyamine analogues can synergize with standard DNA-reactive cytotoxic drugs, several studies evaluated the combination of chemotherapeutic agents with either polyamine analogues or drugs targeting the polyamine metabolic pathway [16, 22, 25, 33, 43, 47, 61, 63, 64, 68, 77]. The findings from these studies overall were mixed with results being agent-, schedule-, and cell-line-dependent. Additionally, many of these studies were limited in the number of cell lines examined or the examination of their effects on normal cell lines or tissues. A more thorough examination of different treatment regimens in both tumorigenic and non-tumorigenic cell lines is required to focus in vivo clinical testing.
The cytotoxic effects of many polyamine analogues, including BENSpm, are believed to act, in part, through induction of SSAT and/or SMO [13, 24, 26, 58]. Several recent studies have also implicated SSAT in the response of cancer cell lines to chemotherapeutic agents. SSAT regulates cellular polyamine homeostasis and SSAT induction results in significantly altered polyamine metabolism. One study using DNA microarray technology identified SSAT as one of the most up-regulated genes in MCF-7 breast cancer cells treated with 5-FU [51]. A separate study reported that treatment of A2780 ovarian cancer cells with oxaliplatin or cisplatin synergistically induces SSAT mRNA expression [36, 37, 70].
The encouraging in vitro and in vivo studies with BENSpm combined with its safety in administration in Phase II clinical studies make this polyamine analogue appealing to examine in combination with traditional cytotoxic drugs. In this study, the ability of BENSpm to synergize with six standard chemotherapeutic drugs (5-fluorouracil (FU), fluorodeoxyuridine, cis- diaminechloroplatinum(II) (DDP), paclitaxel, docetaxel, and vinorelbine) using three treatment schedules was examined in four human breast cancer cell lines and one immortalized non-tumorigenic mammary epithelial cell line. The chemotherapeutic agents used in this study were chosen because they are all currently used in the treatment of breast cancer and they represent a wide spectrum of mechanisms of action. The in vitro results suggested combinations for examination in an in vivo model and translation of these synergistic combination treatments to a xenograft mouse model of MDA-MB-231 led to significant tumor regression. These findings help further elucidate the mechanism involved in the synergistic drug response and will aid in the design of more useful combinations for the treatment of breast cancer.
MATERIALS AND METHODS
Cell lines and culture conditions
The acquisition and maintenance of the breast cancer cell lines, MDA-MB-231, Hs578t, MCF-7, and T47D, have been previously described [33]. MCF-10A cells were cultured in DMEM/F-12 (Mediatech, Herndon, VA) supplemented with 5% heat-inactivated horse serum (Mediatech), 10 µg/mL insulin (Sigma, St Louis, MO), 100 ng/mL cholera toxin (Sigma), 0.5 µg/mL hydrocortisone (Sigma), and 20 ng/mL recombinant EGF (Sigma). The SMO stable siRNA clones were generated by annealing and inserting the following oligonucleotides (Invitrogen, Carlsbad, CA) into the pSilencer 2.1-U6 neo expression vector (Ambion, Austin, TX) according to the manufacturer’s instructions:
SMO Forward, 5’ GAT CCG CAC TTC TTG AGC AGG GTT TTC AAG AGA AAC CCT GCT CAA GAA GTG CTT TTT TGG AAA
SMO Reverse, 5’ AGC TTT TCC AAA AAA GCA CTT CTT GAG CAG GGT TTC TCT TGA AAA CCC TGC TCA AGA AGT GCG
The following oligonucleotides (Invitrogen) targeting the SSAT gene were annealed to form the hairpin siRNA template insert that was then ligated into the pSilencer 2.1-U6 hygro expression vector (Ambion) according to the manufacturer’s instructions:
SSAT Forward, 5’ GAT CCG TGA TCC TCC CAC CTC AGC TTC AAG AGA GCT GAG GTG GGA GGA TCA CTT TTT TGG AAA
SSAT Reverse, 5’ AGC TTT TCC AAA AAA GTG ATC CTC CCA CCT CAG CTC TCT TGA AGC TGA GGT GGG AGG ATC ACG
Lipofectamine was used to transfect four µg of the targeting plasmid or a nonsense control plasmid (Ambion) into MDA-MB-231 and MCF-7 cells. Single clones representing MDA-MB-231 nonsense control, MDA-MB-231 SMO, MDA-MB-231 SSAT, MDA-MB-231 SMO/SSAT, MCF-7 nonsense control, MCF-7 SMO, MCF-7 SSAT, and MCF-7 SMO/SSAT were chosen. Clones were selected and maintained in DMEM (Mediatech) supplemented with 5% FBS (Mediatech), 1% glutamine (Mediatech), and 500 µg/ml G418 (Sigma) or 500 µg/ml hygromycin (Roche) as required.
Reagents
BENSpm was synthesized as described previously [4]. A concentrated stock solution of 10 mM was prepared in water and was stored at −20°C. 5-FU was obtained from the Johns Hopkins Oncology Center pharmacy and was stored in water at −20°C. Paclitaxel (a gift from Bristol-Myers/Squibb) was stored at 4°C as a 10-mM solution in DMSO. All drugs were diluted in cell culture medium to the desired final concentration except for docetaxel, which was initially diluted in water and then in cell culture medium. 5-(and -6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate (CM-H2DCFDA), mixed isoforms, was purchased from Molecular Probes (Eugene, OR) and catalase was purchased from Sigma.
MTT and cell growth assays
MTT assays were performed as described previously [33]. Briefly, cells were plated in 96-well dishes and were treated as outlined below. All experiments were performed at least three times in quadruplicate. The results from the MTT assays were validated by direct comparison to a conventional cell growth assay. For the cell growth assay, cells were plated at a cell density of 5,000 cells/well in six-well tissue culture plates. After attachment overnight, the medium was replaced and cells were treated as described below. At the end of the treatment period, cells were detached by trypsinization and counted using a Coulter particle counter.
Treatment schedules
Three different treatment schedules were used to mimic potentially relevant clinical schedules that have been successfully examined in vivo [33]. The first treatment schedule evaluated simultaneous exposure to both the polyamine analogue and cytotoxic drug for 120 hours. In the second treatment schedule, the cells were exposed to 24 hour treatment with the cytotoxic drug alone followed by treatment with medium containing polyamine analogue for additional 96 hours. In both treatment schedules, medium was discarded after 24 hours and the cell monolayer was washed with drug-free medium, and fresh medium containing either cytotoxic drug and polyamine analogue or only the polyamine analogue was added for 96 hours. The third treatment schedule used 24 hour polyamine analogue treatment followed by removal of the medium and addition of medium containing both the polyamine analogue and cytotoxic drug for 96 hours. A lengthy treatment with the polyamine analogue was used for all treatment schedules because previous studies have demonstrated that sustained exposure is required for optimal polyamine analogue activity [4].
Median effect/combination index analysis
The median effect/combination index (CI) model was used to determine synergy, additivity, or antagonism of the combination treatments [19]. Cell cultures were treated with each agent individually at its IC50 concentration and at fixed multiples (two and three times) and fractions (0.75, 0.50, and 0.25) of the IC50 concentrations [33]. The agents (polyamine analogue and cytotoxic drug) were also combined in these same dose-fixed ratios to determine the CI. Synergy was defined as any CI value below 1, additivity as CI = 1, and antagonism as any CI above 1 ± SD. Experiments were done in quadruplicate, and each experiment yielded one CI value. The CI values shown represent the mean ± SD for at least three, independent experiments. CI values are shown only for fractional growth inhibition levels of 0.50, 0.75, and 0.90.
RNA isolation, RT, and real-time PCR
Total cellular RNA was isolated from cultured cell lines using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was synthesized from 3 µg of total RNA using MMLV reverse transcriptase (Invitrogen) and oligo-dT primers (Invitrogen). For Real Time PCR, cDNA was amplified using SYBR green (Sigma) using the following primers:
SMO forward 5’ CGCAGACTTACTTCCCCGGC
SMO reverse 5’ CGCTCAATTCCTCAACCACG
SSAT forward 5’ ATCTAAGCCAGGTTGCAATGA
SSAT reverse 5’ GCACTCCTCACTCCTCTGTTG
Real Time PCR data were acquired and analyzed using Sequence Detector v1.7 software (Perkin Elmer, Wellesley, MA) and were normalized to the GAPDH housekeeping gene. All experiments were performed four times in duplicate.
Analysis of SSAT and oxidase activity
SSAT activity was determined by using C14-labeled substrates and by scintillation counting of end products produced as previously described [11]. Protein concentrations were determined using the Bradford method [8]. SMO and APAO enzyme activity in cell lysates was assayed as described previously using either 250 µM spermine or N1-acetylspermine, respectively, as the substrate (Sigma, St Louis, MO) as a substrate [8]. All activity experiments were repeated at least three times in triplicate.
Xenograft mice model and treatment schedules
Four six-week old oophorectomized female Balb/c athymic nu/nu mice (Harlan Sprague Dawley, Madison, WI) were injected sub-cutaneously (s.c.) in the right flank with 1.5 × 106 MDA-MB-231 cells in a volume of 100 µl Hanks Balance Salt Solution (HBSS). As the tumors were established (30 mm3), the mice were randomized into four groups (n=10/group), control, BENSpm, paclitaxel and BENSpm/paclitaxel. While the control mice received intra-peritoneal (i.p.) injection of HBSS (5times/week) and HBSS with cremophor (once weekly), the three treatment groups received: group 1) 100 mg/kg BENSpm i.p. 5 days each week for 4 weeks, group 2) 5 mg/kg paclitaxel (cremophor used as excipient) i.p. once weekly for 4 weeks, and 3) BENSpm and paclitaxel. Tumors were measured with a caliper once weekly and tumor volume was calculated according to the formula, volume = length × width × height × 0.5236. Animals were monitored carefully for signs of toxicity and weighed once weekly. All mice were humanely euthanized at the end of experiment.
Statistics
Time to 6-fold tumor increase relative to baseline was evaluated to assess the effects of two drugs (BENSpm or paclitaxel) and the combination (BENSpm and paclitaxel) on tumor growth inhibition. Death prior to the target tumor increase was a competing risk. Since censoring deaths from competing causes means that fewer animals at risk and impact of time-to-manifold tumor progression is overestimated due to the decreased denominator, a cumulative incidence function was used to account for the presence of a competing risk and obtain more precise estimates. Animals that were still alive at the end of the study but not yet had reached the target tumor volume were censored at the time of study end. None of the animals had to be sacrificed before reaching the 6-fold tumor increase. Difference in cumulative incidence functions between the treatment groups was compared using Gray’s method [30] and hazard ratios were estimated using a proportional hazards model of a competing risk described by Fine and Gray [28]. All tests were two-sided and considered statistically significant at P<0.05. The competing risk analyses were implemented in R software package (v 2.4.1).
RESULTS
Effect of BENSpm and chemotherapeutic drugs on MDA-MB-231 and MCF-7 cell proliferation
The initial combination studies were performed using estrogen receptor (ER)-negative MDA-MB-231 and ER-positive MCF-7 cells as they are representative of both hormone-independent and hormone-dependent breast cancer. Six chemotherapeutic agents (FdURd, 5-FU, C-DDP, paclitaxel, docetaxel, and vinorelbine) were tested in combination with BENSpm in both cell lines. The chemotherapeutic drugs used were selected because they all have antitumor activity in breast cancer, are currently in use in the treatment of breast cancer, and represent a broad spectrum of mechanisms of action. The symmetrically substituted polyamine analogue BENSpm was chosen for study because it induces SSAT and SMO in breast cancer cell lines and has been evaluated in Phase I and II clinical trials [34, 57, 74]. Three different treatment schedules were examined that are potentially clinically relevant: simultaneous exposure to both polyamine analogue and cytotoxic drug for 120 hours; cytotoxic drug exposure for 24 hours followed by polyamine analogue exposure for 96 hours; and polyamine analogue alone for 24 hours followed by simultaneous exposure for 96 hours.
The CI values for the combination studies using BENSpm with chemotherapeutic drugs were determined for MDA-MB-231 (Table 1) and MCF-7 (Table 2) cells using the median effect/CI model [19]. The two treatment schedules that allowed for the greatest amount of synergy in both cell lines were 120 hour concomitant treatment and pre-treatment with BENSpm for 24 hours followed by simultaneous treatment for an additional 96 hours. Using a concomitant treatment schedule, BENSpm was synergistic with FdURd, 5-FU, C-DDP, and paclitaxel and was primarily additive with docetaxel and vinorelbine (Table 1). Similar results were obtained when MDA-MB-231 cells were pre-treated with BENSpm followed by simultaneous treatment, although the concomitant treatment schedule was slightly more synergistic. In MCF-7 cells, BENSpm was synergistic with 5-FU and paclitaxel using a concomitant treatment schedule (Table 2). In contrast to the MDA-MB-231 cells, pre-treatment of MCF-7 cells with BENSpm resulted in synergy with agents, including FdURd, 5-FU, paclitaxel, and docetaxel. Pre-treatment with the chemotherapeutic drug followed by BENSpm alone produced mostly additive or antagonistic effects overall in both cell lines (Table 1 and Table 2). In general, the greatest amount of synergy in both cell lines was observed with BENSpm in combination with 5-FU and paclitaxel using a 120-hour simultaneous treatment regimen.
Table 1. Effects of BENSpm and chemotherapeutic drugs on MDA-MB-231 cells.
Combination index (CI) values for fractional growth inhibitions of 0.50, 0.75, and 0.90 are shown for combination studies using BENSpm with chemotherapeutic drugs using three different treatment schedules. CI values were determined as described in Materials and Methods. Values are the means ± SD of three experiments. Synergy CI < 1.00; CI = 1.00; antagonism CI > 1.00.
| Concomitant BENSpm and drug | |||
|---|---|---|---|
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.536 ± 0.138 | 0.626 ± 0.093 | 0.547 ± 0.147 |
| 5-FU | 0.507 ± 0.147 | 0.645 ± 0.068 | 0.642 ± 0.103 |
| C-DDP | 0.897 ± 0.172 | 0.765 ± 0.183 | 0.653 ± 0.139 |
| Paclitaxel | 0.483 ± 0.132 | 0.486 ± 0.212 | 0.413 ± 0.089 |
| Docetaxel | 1.043 ± 0.193 | 1.002 ± 0.214 | 0.971 ± 0.058 |
| Vinorelbine | 1.461 ± 0.206 | 0.861 ± 0.164 | 1.001 ± 0.167 |
| Drug then BENSpm | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 1.549 ± 0.116 | 1.569 ± 0.116 | 1.602 ± 0.174 |
| 5-FU | 1.558 ± 0.173 | 1.334 ± 0.188 | 1.807 ± 0.201 |
| C-DDP | 0.356 ± 0.109 | 0.210 ± 0.026 | 0.234 ± 0.015 |
| Paclitaxel | 1.137 ± 0.027 | 1.075 ± 0.102 | 1.089 ± 0.085 |
| Docetaxel | 0.952 ± 0.178 | 1.025 ± 0.182 | 1.167 ± 0.071 |
| Vinorelbine | 1.605 ± 0.181 | 1.494 ± 0.167 | 1.521 ± 0.217 |
| BENSpm the BENSpm and drug | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.892 ± 0.102 | 0.769 ± 0.094 | 0.756 ± 0.084 |
| 5-FU | 0.511 ± 0.056 | 0.525 ± 0.077 | 0.506 ± 0.085 |
| C-DDP | 0.907 ± 0.114 | 1.024 ± 0.125 | 0.990 ± 0.132 |
| Paclitaxel | 0.660 ± 0.189 | 0.657 ± 0.154 | 0.674 ± 0.184 |
| Docetaxel | 0.915 ± 0.065 | 0.964 ± 0.121 | 1.026 ± 0.184 |
| Vinorelbine | 0.914 ± 0.174 | 1.089 ± 0.147 | 1.091 ± 0.118 |
Table 2. Effects of BENSpm and chemotherapeutic drugs on MCF-7 cells.
Combination index (CI) values for fractional growth inhibitions of 0.50, 0.75, and 0.90 are shown for combination studies using BENSpm with chemotherapeutic drugs using three different treatment schedules. CI values were determined as described in Materials and Methods. Values are the means ± SD of three experiments. Synergy CI < 1.00; additivity CI = 1.00; antagonism CI > 1.00.
| Concomitant BENSpm and drug | |||
|---|---|---|---|
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 1.527 ± 0.351 | 1.132 ± 0.165 | 1.718 ± 0.145 |
| 5-FU | 0.645 ± 0.122 | 0.887 ± 0.044 | 0.918 ± 0.018 |
| C-DDP | 1.240 ± 0.107 | 1.344 ± 0.128 | 1.354 ± 0.126 |
| Paclitaxel | 0.214 ± 0.128 | 0.204 ± 0.059 | 0.201 ± 0.109 |
| Docetaxel | 0.942 ± 0.167 | 0.936 ± 0.167 | 1.268 ± 0.154 |
| Vinorelbine | 1.209 ± 0.159 | 1.428 ± 0.202 | 1.498 ± 0.174 |
| Drug then BENSpm | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 1.149 ± 0.128 | 1.316 ± 0.174 | 1.447 ± 0.084 |
| 5-FU | 1.586 ± 0.196 | 1.346 ± 0.214 | 1.155 ± 0.115 |
| C-DDP | 1.177 ± 0.165 | 1.319 ± 0.164 | 1.561 ± 0.175 |
| Paclitaxel | 0.724 ± 0.123 | 0.672 ± 0.147 | 0.640 ± 0.045 |
| Docetaxel | 1.134 ± 0.189 | 1.375 ± 0.166 | 1.263 ± 0.153 |
| Vinorelbine | 1.106 ± 0.084 | 1.146 ± 0.035 | 1.019 ± 0.170 |
| BENSpm then BENSpm and drug | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.799 ± 0.121 | 0.786 ± 0.124 | 0.782 ± 0.133 |
| 5-FU | 0.756 ± 0.168 | 0.773 ± 0.106 | 0.713 ± 0.169 |
| C-DDP | 0.939 ± 0.084 | 0.912 ± 0.087 | 1.098 ± 0.108 |
| Paclitaxel | 0.709 ± 0.059 | 0.605 ± 0.049 | 0.535 ± 0.067 |
| Docetaxel | 0.687 ± 0.107 | 0.638 ± 0.049 | 0.617 ± 0.116 |
| Vinorelbine | 1.025 ± 0.187 | 0.973 ± 0.152 | 0.993 ± 0.127 |
Effects of BENSpm and chemotherapeutic drugs on Hs578t, T47D, and MCF-10A cells
The activity of BENSpm in combination with all six chemotherapeutic drugs was then examined in two other breast cancer cell lines, ER-negative Hs578t and ER-positive T47D, and an immortalized non-tumorigenic mammary epithelial cell line, MCF-10A. Using the 120 hour concomitant treatment schedule in Hs578t cells, BENSpm was synergistic with FdURd, 5-FU, and paclitaxel (Table 3). Further BENSpm was synergistic with FdURd, 5-FU, C-DDP, paclitaxel, and vinorelbine in T47D cells. In MCF-10A cells, all of the chemotherapeutic agents were antagonistic with BENSpm except for paclitaxel; this combination was synergistic although the CI value was greater than that of the cancer cell lines examined. In general, the greatest synergy was observed using BENSpm in combination with 5-FU and paclitaxel in Hs578t and T47D cells.
Table 3. Effect of concomitant treatment with BENSpm and chemotherapeutic drugs on Hs578t, T47D, and MCF-10A cells.
Combination index (CI) values for fractional growth inhibitions of 0.50, 0.75, and 0.90 are shown for combination studies using BENSpm with chemotherapeutic drugs for a 120 hour concomitant treatment. CI values were determined as described in Materials and Methods. Values are the means ± SD of three experiments. Synergy CI < 1.00; additivity CI = 1.00; antagonism CI > 1.00.
| Hs578t cells | |||
|---|---|---|---|
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.908 ± 0.188 | 0.867 ± 0.103 | 0.852 ± 0.098 |
| 5-FU | 0.875 ± 0.157 | 0.843 ± 0.147 | 0.678 ± 0.128 |
| C-DDP | 1.357 ± 0.216 | 1.906 ± 0.218 | 1.792 ± 0.145 |
| Paclitaxel | 0.306 ± 0.073 | 0.355 ± 0.069 | 0.453 ± 0.089 |
| Docetaxel | 1.009 ± 0.139 | 0.995 ± 0.184 | 0.984 ± 0.110 |
| Vinorelbine | 1.405 ± 0.156 | 1.579 ± 0.261 | 1.883 ± 0.117 |
| T47D cells | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.847 ± 0.142 | 0.871 ± 0.167 | 0.899 ± 0.127 |
| 5-FU | 0.865 ± 0.132 | 0.951 ± 0.049 | 0.811 ± 0.065 |
| C-DDP | 0.825 ± 0.059 | 0.949 ± 0.148 | 0.903 ± 0.054 |
| Paclitaxel | 0.473 ± 0.137 | 0.411 ± 0.111 | 0.366 ± 0.119 |
| Docetaxel | 0.882 ± 0.211 | 0.807 ± 0.249 | 0.792 ± 0.129 |
| Vinorelbine | 0.665 ± 0.101 | 0.591 ± 0.284 | 0.679 ± 0.179 |
| MCF-10A cells | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 1.907 ± 0.137 | 1.711 ± 0.121 | 1.937 ± 0.138 |
| 5-FU | 1.797 ± 0.219 | 1.526 ± 0.231 | 1.843 ± 0.122 |
| C-DDP | 1.606 ± 0.116 | 1.716 ± 0.165 | 1.835 ± 0.169 |
| Paclitaxel | 0.606 ± 0.117 | 0.635 ± 0.154 | 0.682 ± 0.216 |
| Docetaxel | 1.557 ± 0.125 | 1.712 ± 0.165 | 1.208 ± 0.237 |
| Vinorelbine | 1.221 ± 0.203 | 1.767 ± 0.165 | 1.527 ± 0.187 |
Effects of pre-treatment with BENSpm followed by concomitant treatment with FdURd, 5-FU, or paclitaxel on Hs578t, T47D, and MCF-10A cells
Next, the activity of BENSpm with FdURd, 5-FU, and paclitaxel was examined in Hs578t, T47D, and MCF-10A cells that were pre-treated with BENSpm followed by simultaneous treatment (Table 4). These agents were chosen because they have shown the greatest amount of synergy overall with BENSpm and this treatment schedule was used since it was highly synergistic in MDA-MB-231 and MCF-7 cells. BENSpm was synergistic with all three agents in Hs578t cells; this treatment schedule produced smaller CI values in this cell line as compared with concomitant treatment. In T47D cells, this treatment also produced smaller CI values than simultaneous treatment and BENSpm was synergistic with each agent. BENSpm was very synergistic with FdURd in MCF-10A cells using this treatment schedule with a CI <0.30. BENSpm was slightly more synergistic with paclitaxel in this cell line versus concomitant treatment, but was less synergistic with 5-FU (CI > 0.90) versus concomitant treatment in MCF-10A cells.
Table 4. Combinative effect of BENSpm pretreatment followed by treatment with FdURd, 5-FU, or paclitaxel in Hs578t, T47D, and MCF-10A cells.
Combination index (CI) values for fractional growth inhibitions of 0.50, 0.75, and 0.90 are shown for combination studies with pretreatment with BENSpm for 24 hours followed by 96 hours simultaneous treatment. CI values were determined as described in Materials and Methods. Values are the means ± SD of three experiments. Synergy CI < 1.00; additivity CI = 1.00; antagonism CI > 1.00.
| Hs578t cells | |||
|---|---|---|---|
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.302 ± 0.046 | 0.380 ± 0.063 | 0.370 ± 0.057 |
| 5-FU | 0.484 ± 0.074 | 0.454 ± 0.061 | 0.436 ± 0.044 |
| C-DDP | 0.927 ± 0.134 | 0.908 ± 0.123 | 0.902 ± 0.205 |
| Paclitaxel | 0.741 ± 0.121 | 0.803 ± 0.154 | 0.881 ± 0.210 |
| Docetaxel | 0.598 ± 0.088 | 0.558 ± 0.081 | 0.529 ± 0.091 |
| Vinorelbine | 1.158 ± 0.214 | 1.183 ± 0.134 | 1.272 ± 0.224 |
| T47D cells | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 1.407 ± 0.169 | 1.349 ± 0.227 | 1.695 ± 0.430 |
| 5-FU | 1.569 ± 0.170 | 1.648 ± 0.351 | 1.623 ± 0.341 |
| C-DDP | 1.299 ± 0.286 | 1.213 ± 0.345 | 1.510 ± 0.114 |
| Paclitaxel | 0.825 ± 0.156 | 0.886 ± 0.124 | 0.776 ± 0.046 |
| Docetaxel | 1.178 ± 0.245 | 1.381 ± 0.249 | 1.659 ± 0.126 |
| Vinorelbine | 1.125 ± 0.127 | 1.297 ± 0.164 | 1.137 ± 0.097 |
| MCF-10A cells | |||
| Fractional Growth Inhibition | |||
| 0.5 | 0.75 | 0.9 | |
| FdUrd | 0.217 ± 0.045 | 0.234 ± 0.037 | 0.293 ± 0.047 |
| 5-FU | 0.957 ± 0.103 | 0.927 ± 0.123 | 0.905 ± 0.147 |
| C-DDP | 0.416 ± 0.045 | 0.418 ± 0.058 | 0.453 ± 0.065 |
| Paclitaxel | 0.554 ± 0.074 | 0.534 ± 0.049 | 0.523 ± 0.087 |
| Docetaxel | 0.173 ± 0.033 | 0.361 ± 0.037 | 0.399 ± 0.035 |
| Vinorelbine | 0.388 ± 0.057 | 0.315 ± 0.056 | 0.273 ± 0.089 |
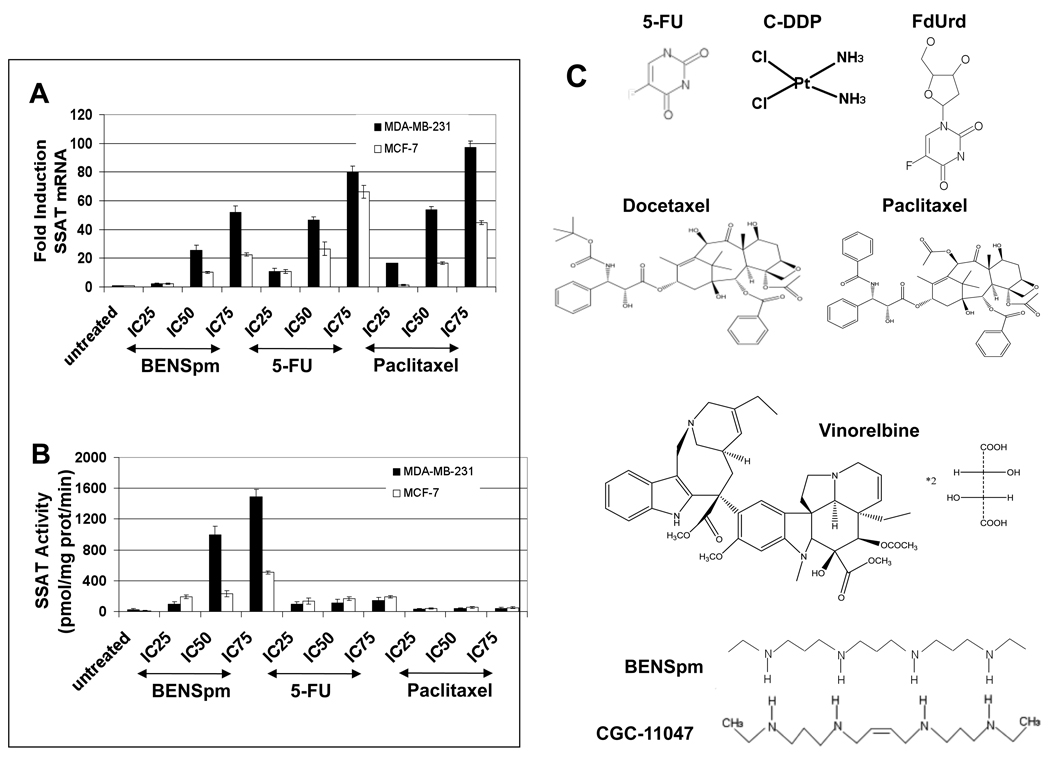
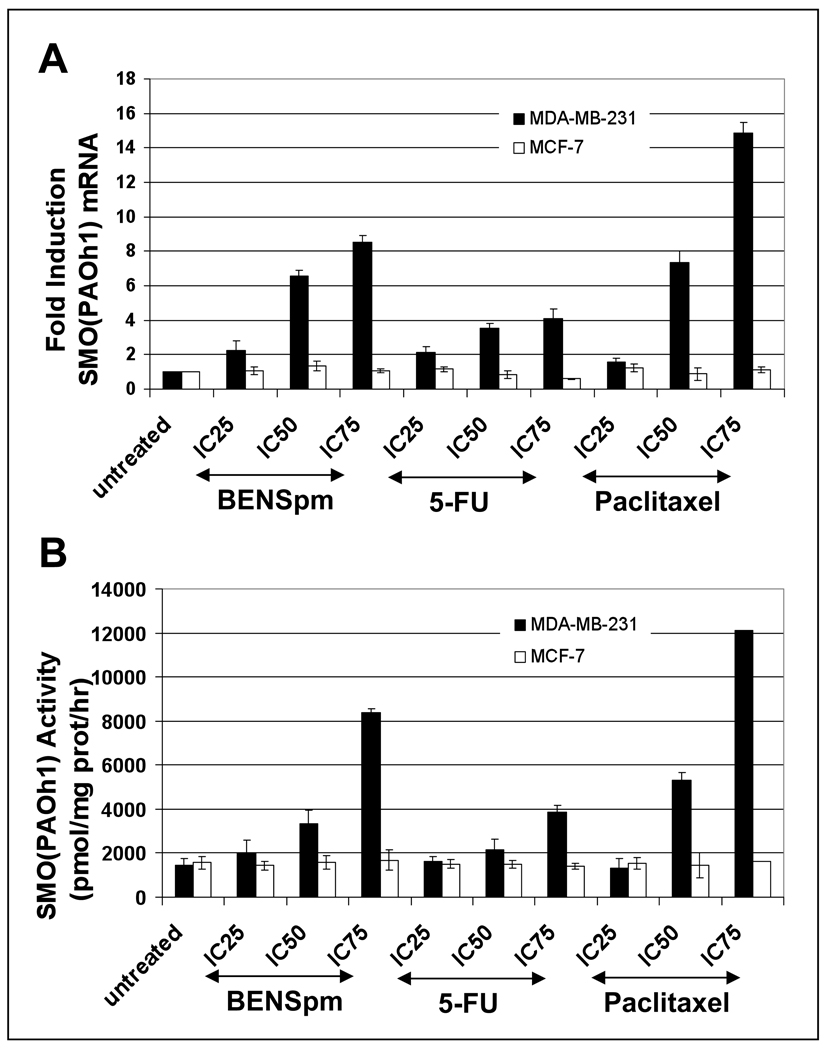
BENSpm, 5-FU, and paclitaxel induce SSAT and SMO mRNA and activity in MDA-MB-231 cells in a dose-dependent manner
Many polyamine analogues and chemotherapeutic agents act, in part, through the induction of the polyamine catabolic enzymes SSAT and SMO [11, 12, 17, 24, 26, 37, 51, 58]. We therefore examined the induction of both of these enzymes to determine if their increase was associated with the synergistic response of breast cancer cell lines to exposure to BENSpm with 5-FU and paclitaxel. MDA-MB-231 and MCF-7 cells were treated with a range of doses (IC25, IC50, and IC75) of BENSpm, 5-FU, and paclitaxel and real-time PCR and standard enzyme activity assays were performed to determine the effects on SSAT and SMO mRNA and activity. BENSpm treatment induced SSAT mRNA and enzyme activity in both MDA-MB-231 and MCF-7 cells with a greater induction in MDA-MB-231 cells (Figure 1). 5-FU and paclitaxel treatment also induced SSAT mRNA in both cell lines although there was no significant induction of SSAT activity. BENSpm treatment also induced SMO mRNA and activity in a dose-dependent manner in MDA-MB-231 cells (Figure 2). 5-FU treatment slightly induced SMO mRNA and activity whereas paclitaxel treatment induced SMO mRNA and activity more than BENSpm. No induction of SMO mRNA or activity was seen in MCF-7 cells. In addition, no APAO activity induction was seen in MDA-MB-231 or MCF-7 cells with any of these agents (data not shown). Treatment of MDA-MB-231 and MCF-7 cells with another chemotherapeutic agent, vinorelbine, did not significantly induce SSAT or SMO mRNA or activity, suggesting that the induction of these enzymes is not solely a stress-response (data not shown).
Figure 1. Dose response of SSAT mRNA and activity by BENSpm, 5-FU, or paclitaxel in MDA-MB-231 and MCF-7 cells.
MDA-MB-231 (black bars) and MCF-7 (white bars) cells were treated with IC25, IC50, and IC75 concentrations of BENSpm, 5-FU, and paclitaxel for 24 hours. A) Real-time PCR for SSAT mRNA was performed as described in Materials and Methods. All values were normalized to the GAPDH housekeeping gene. B) SSAT activity was assayed as described in Materials and Methods. SSAT activity is shown in pmol N1-acetylspermidine/mg protein/minute. All values are the means ± SD of three independent experiments performed in triplicate. C) Structures of six chemotherapeutic agents used in this study along with the structures of BENSpm and CGC-11047 is represented here.
Figure 2. Dose response of SMO mRNA and activity by BENSpm, 5-FU, or paclitaxel in MDA-MB-231 and MCF-7 cells.
MDA-MB-231 (black bars) and MCF-7 (white bars) cells were treated with IC25, IC50, and IC75 concentrations of BENSpm, 5-FU, and paclitaxel for 24 hours. A) Real-time PCR for SMO mRNA was performed as described in Materials and Methods. All values were normalized to the GAPDH housekeeping gene. B) SMO activity was assayed as described in Materials and Methods. All values are the means ± SD of three independent experiments performed in triplicate.
Synergistic SSAT and SMO mRNA and activity induction with BENSpm and 5-FU or paclitaxel
As most of the regulation of SSAT expression is post-transcriptional, a key question is whether the high induction of SSAT mRNA with 5-FU and paclitaxel treatment can be translated into greater SSAT activity induction by co-treatment with BENSpm. MDA-MB-231 and MCF-7 cells were treated with either BENSpm alone, paclitaxel alone, 5-FU alone, BENSpm and paclitaxel, or BENSpm and 5-FU for 48 hours. Real-time PCR and standard activity assays were performed to determine the effects on SSAT and SMO mRNA and activity (Figure 3A, B).
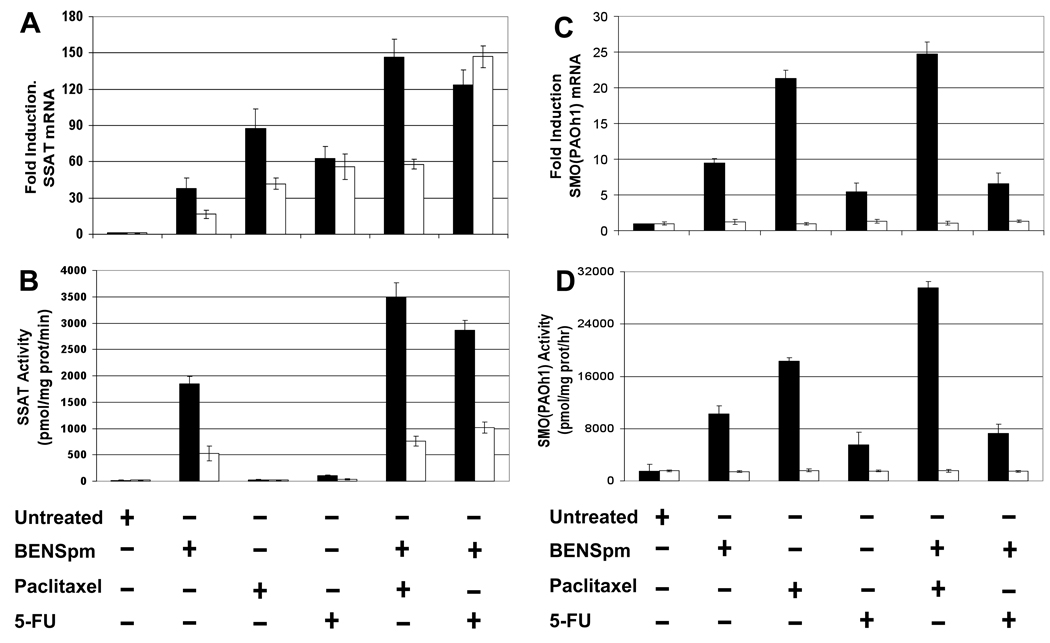
Figure 3. SSAT and SMO mRNA and activity induction by BENSpm with 5-FU or paclitaxel.
MDA-MB-231 (black bars) and MCF-7 (white bars) cells were treated for 48 hours with either BENSpm (IC50) alone, paclitaxel (IC50) alone, 5-FU (IC50) alone, BENSpm (IC50) and paclitaxel (IC50), or BENSpm (IC50) and 5-FU (IC50). A) Real-time PCR for SSAT mRNA was performed as described in Materials and Methods. B) SSAT activity was assayed as described in Materials and Methods. SSAT activity is shown in pmol N1-acetylspermidine/mg protein/minute. C) Real-time PCR for SMO mRNA was performed as described in Materials and Methods. D) SMO activity was assayed as described in Materials and Methods. Real time PCR for SSAT and SMO mRNA values were normalized to the GAPDH housekeeping gene. All values are the means ± SD of three independent experiments performed in triplicate.
In MDA-MB-231 cells, paclitaxel treatment induced the greatest levels of SSAT mRNA among the single treatment groups while the greatest amount of SSAT mRNA induction occurred with the combination of BENSpm and paclitaxel (Figure 3A). In MCF-7 cells, 5-FU treatment induced the greatest levels of SSAT mRNA while the combination of BENSpm and 5-FU induced the greatest levels of SSAT mRNA among all of the treatment groups. In both cell lines, the only single agent that induced SSAT activity was BENSpm while the combination of BENSpm with both paclitaxel and 5-FU induced greater activity in MDA-MB-231 cells as compared with BENSpm alone. The combination of BENSpm with 5-FU in MCF-7 cells resulted in approximately twice the amount of SSAT activity induction versus BENSpm alone.
In MDA-MB-231 cells, the greatest SMO mRNA and activity induction with a single cytotoxic agent was also seen with paclitaxel, which was approximately twice the induction seen with BENSpm alone (Figure 3C). The greatest induction of mRNA and activity with a combination treatment was observed with BENSpm and paclitaxel with SMO activity induced nearly 20-fold with BENSpm and paclitaxel treatment compared with untreated cells. No SMO mRNA or activity induction was seen in any of the treated MCF-7 cells (Figure 3C, D). In addition, no induction of APAO mRNA or activity was seen in either cell line using these treatment schedules (data not shown).
Knockdown of SSAT and SMO reduces the sensitivity of MDA-MB-231 cells to BENSpm and 5-FU or paclitaxel
A fundamental issue is whether SSAT and/or SMO play a role in the antiproliferative effects of BENSpm with 5-FU or paclitaxel. To address this question, MTT assays were performed using SSAT, SMO, and SSAT/SMO stable knockdown MDA-MB-231 and MCF-7 cells. Transfected MDA-MB-231 and MCF-7 cells were treated with dose-fixed ratios of BENSpm with paclitaxel or 5-FU for 120 hours and MTT assays were performed to examine the role that SSAT and SMO play alone and together on the cellular response to these combination treatments (Figure 4 and Figure 5).
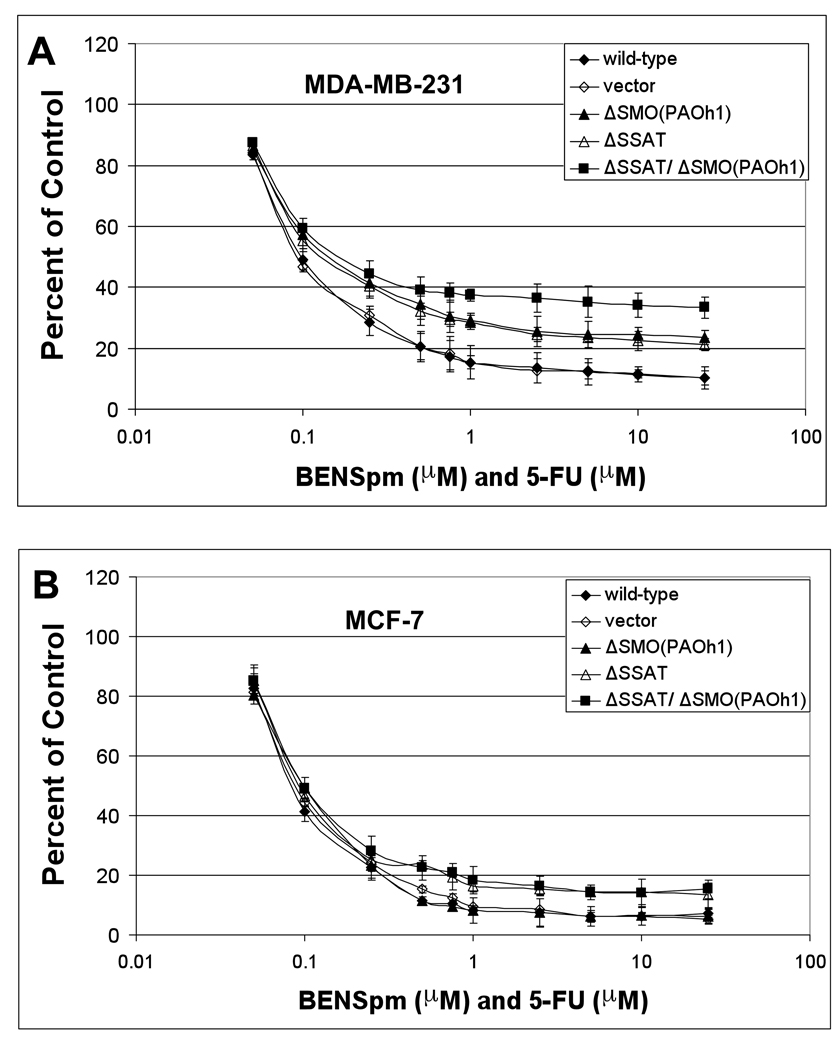
Figure 4. Knockdown of SSAT and SMO reduces the sensitivity of MDA-MB-231 cells to BENSpm and paclitaxel.
MDA-MB-231 (A) and MCF-7 (B) cells were treated with BENSpm and paclitaxel in a dose-fixed ratio for 120 hours as described in Materials and Methods. MTT assays were performed to determine the effects on cell growth. All values are the means ± SD of three independent experiments performed in quadruplicate.
Figure 5. Knockdown of SSAT and SMO reduces the sensitivity of MDA-MB-231 cells to BENSpm and 5-FU while the knockdown of SSAT reduces the sensitivity of MCF-7 cells to BENSpm and 5-FU.
MDA-MB-231 (A) and MCF-7 (B) cells were treated with BENSpm and 5-FU in a dose-fixed ratio for 120 hours as described in Materials and Methods. MTT assays were performed to determine the effects on cell growth. All values are the means ± SD of three independent experiments performed in quadruplicate.
When knockdown MDA-MB-231 cells were treated with BENSpm and paclitaxel simultaneously, the knockdown of either SSAT or SMO reduced their sensitivity while the double knockdown cells were even less sensitive to the combination treatment than either single knockdown cell line (Figure 4A). In MCF-7 cells, the knockdown of SSAT alone or in combination with SMO modestly reduced their sensitivity to BENSpm and paclitaxel treatment (Figure 4B). The sensitivity of MDA-MB-231 cells to the combination of BENSpm and 5-FU was reduced by the knockdown of each enzyme individually and was reduced even more so with their double knockdown (Figure 5A). As with BENSpm and paclitaxel, the sensitivity of MCF-7 cells to BENSpm and 5-FU was modestly reduced with the knockdown of SSAT but was not altered by the knockdown of SMO alone.
BENSpm and paclitaxel alone, and in combination, significantly inhibited tumor growth in xenograft mice model
From promising in vitro results it is evident that synergistic cytotoxic response of treatment with BENSpm and 5-FU or paclitaxel is mediated through activation of SSAT and SMO. In particular, MDA-MB-231 cells were more sensitive to concomitant combination treatment with BENSpm and paclitaxel, resulting in activation of both SSAT and SMO. Conversely knock down of either enzyme individually or in combination reduces the sensitivity of MDA-MB-231 cells to treatment.
Based on these results, the potential for synergy between BENSpm and paclitaxel was assessed in the MDA-MB-231 human breast cancer xenograft model. Although BENSpm has not been evaluated in in vivo mouse models of breast cancer, the maximum tolerated dose for BENSpm in MALME-3 melanoma xenografts has previously been established [5]. Based on these and other studies with different human cancer cell line xenograft models, and the dosing schedules in use for clinical trials, the treatment regimen for BENSpm used in an in vivo combination study was 100 mg/kg/day × 5, for 4 weeks.
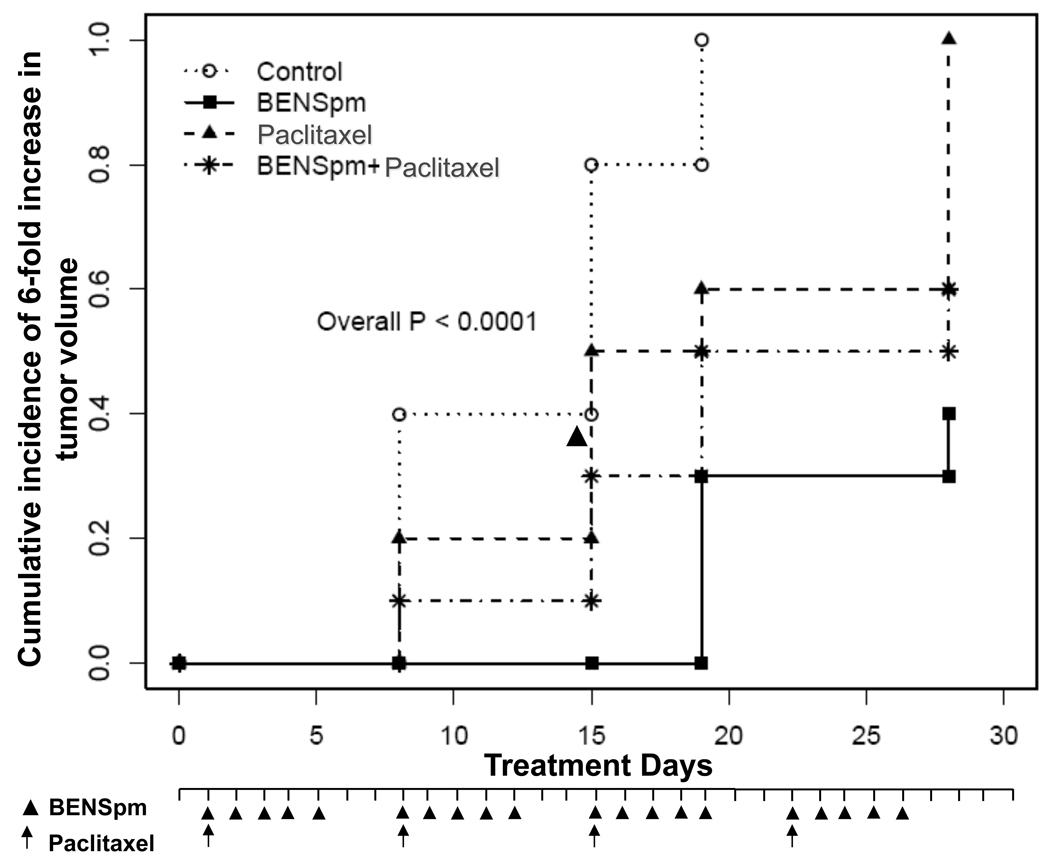
Tumor bearing mice were treated with either BENSpm or paclitaxel alone or in combination for a period of 4 weeks. Consistent with previous studies with other xenograft models, BENSpm significantly inhibits the growth of established breast xenograft tumors. At 100 mg/kg/day × 5, for 4 weeks, time to 6-fold increase in tumor volume was significantly longer than that in control (hazard ratio=7.9, 95% CI=3.1–20.5; P<0.0001). Similarly, significantly lower incidence rate of 6-fold tumor increase was observed in mice treated with 5 mg/kg paclitaxel alone (hazard ratio=2.1, 95% CI=1.0–4.1; P=0.04). In combination, BENSpm and paclitaxel significantly inhibited tumor growth in comparison to control (hazard ratio=4.1, 95% CI=1.7–10.3; P=0.002) (Fig. 6). However, no synergy was observed in the animals treated with the combination.
Figure 6. BENSpm alone and in combination with paclitaxel significantly inhibits tumor growth in MDA-MB-231 xenografts.
Mice bearing tumors generated by subcutaneous injection of MDA-MB-231 breast cancer cells were randomly divided into groups of 10 and treated intraperitoneally with either BENSpm alone (100mg/kg) for five days each week, paclitaxel alone (5 mg/kg weekly) or BENSpm/paclitaxel or control for a study period of 4 weeks. Treatment schedule is described in Materials and Methods. Time-to-6 fold tumor growth relative to baseline was evaluated using cumulative incidence function accounting for the presence of competing risks and represented graphically.
DISCUSSION
Much attention has been focused on developing therapies that combine the use of standard cytotoxic agents with more novel therapies to overcome disease relapse or resistance. One example of a novel therapeutic family is the polyamine analogues, which mimic the natural polyamines in self-regulating roles, but interfere with their normal cell-growth promoting functions [23, 41, 46]. Polyamine analogues have demonstrated significant activity as single agents in several tumor models [15, 24, 40, 52, 53, 58, 59, 62]. This study examined the activity of a leading polyamine analogue, BENSpm, in combination with multiple chemotherapeutic agents in four human breast cancer cell lines and an immortalized, non-tumorigenic human breast epithelial cell line. C-DDP is a DNA-reactive agent that results in the formation of inter-and intra-strand crosslinks [10]. Paclitaxel, docetaxel, and vinorelbine interfere with tubulin assembly or disassembly and induce G2/M blocks [9, 29]. 5-FU and FdURd are fluoropyrimidines with multiple mechanisms of action including inhibition of thymidylate synthase (TS) and misincorporation into DNA and/or RNA [48, 54]. 5-FU also induces SSAT expression in a range of colon cancer cell lines including HCT-116 and HT-29 [1, 7, 17, 76]. However, SSAT mRNA induction by 5-FU does not translate into protein unless BENSpm is added [1].
Several clinically relevant treatment schedules were examined in this study. Of the three treatment schedules tested, the two schedules that were most synergistic were simultaneous treatment with the polyamine analogue and cytotoxic drug for 120 hours and pre-treatment with the polyamine analogue for 24 hours followed by simultaneous treatment for 96 hours. The greatest synergy resulted using a concomitant treatment regimen. These findings are consistent with previous studies in the HCT -116 colon cancer cell line treated with BENSpm and oxaliplatin where the combination resulted in massive induction of SSAT activity and mRNA [37, 38]. Extension of this study to evaluate the effect of multiple drugs in combination on polyamine catabolism showed that a combination of 5-FU and oxaliplatin pre-treatment followed by treatment with BENSpm induced significantly higher levels of SSAT and SMO mRNA, protein and activity and resulted in greater depletion of spermine and spermidine pools than was observed when treated with oxaliplatin or 5-FU alone. It is noted that oxaliplatin/BENSpm combinations were more effective at SSAT induction than 5-FU/BENSpm [38]. However, these results are in contrast to a recent study that examined two asymmetrically substituted polyamine analogues, N1-ethyl-N11-[(cyclopropyl)methyl]-4,8-diazaundecane (CPENSpm) and N1-ethyl-N11-[(cycloheptyl)methyl]4,8-diazaundecane (CHENSpm), in combination with similar cytotoxic agents in breast cancer using the same treatment schedules [33]. In that study, the most synergy was seen when cells were treated with the cytotoxic drug alone for 24 hours followed by polyamine analogue treatment alone for 96 hours, while the concomitant treatment schedule was predominantly additive or antagonistic in the breast cancer cell lines examined. Importantly, several of the previously reported experiments that found synergy by pre-treating with the cytotoxic drug were repeated using CPENSpm and CHENSpm and similar CI values were obtained (data not shown). This suggests that the difference in results for various schedules between these two studies is primarily polyamine analogue-dependent. Although all of these analogues have anti-tumor activity, they have different mechanisms of action. CHENSpm and CPENSpm induce programmed cell death by altering tubulin polymerization or super-inducing the polyamine catabolic enzyme SSAT, respectively [12, 31, 73], while the treatment of breast cancer cell lines with BENSpm induces a G1 arrest that is associated with an induction of SSAT and SMO in a cell line-dependent manner [57]. The variable mechanisms of action of each of these analogues enable them to have a distinct ability to synergize with specific agents in a schedule-dependent manner.
BENSpm was not as effective when the cells were first treated with the cytotoxic agent alone. Treatment with the cytotoxic followed by BENSpm generally resulted in additivity or antagonism. This schedule-dependency is well documented in studies of other combinations. One study examining the combination of paclitaxel and vinorelbine using the human doxorubicin-resistant MCF-7 human breast carcinoma cell line found synergistic effects when the drugs were administered simultaneously [21]. However, when the drugs were administered sequentially, antagonistic effects resulted. Another study examined paclitaxel in combination with pemetrexed (folate antimetabolite) in MCF-7 cells and found an additive/antagonistic effect with simultaneous treatment, a synergistic effect for pemetrexed followed by paclitaxel, and an additive effect for paclitaxel followed by pemetrexed [44]. The mechanisms involved in the schedule-dependency of these agents are not yet known, although it is hypothesized that cell cycle accessibility is a major cause of additivity, antagonism and synergy. Exposure to the cytotoxic agent first may not only affect or alter the molecular targets of the polyamine analogue, but may prevent entry of the cell into the phase of the cell cycle required for activity of the polyamine analogue. The ability of BENSpm to synergize with cytotoxic agents using a concomitant treatment schedule is desirable since it is the most convenient combination treatment regimen in a clinical setting.
Of the cytotoxic agents evaluated, the two fluoropyrimidines, FdUrd and 5-FU, and paclitaxel were the most synergistic in the human breast cancer cell lines studied here. It is not known why these agents are so synergistic with BENSpm. The fluoropyrimidines were also found to be among the most synergistic agents with CPENSpm and CHENSpm in a previous study, but this is the first report of synergy between paclitaxel and a polyamine analogue [33]. It seems unlikely that stage of cell cycle is responsible for the synergy since FdUrd and 5-FU are reported to induce a G1 block whereas paclitaxel induces a G2/M block [29, 48, 54]. Also although paclitaxel and docetaxel are both tubulin-interfering agents, docetaxel did not synergize with BENSpm, suggesting that the G2/M block is not related to the synergy or antagonism of the combination. Both docetaxel and vinorelbine treatment result in a G2/M block and neither one of these agents synergized well with BENSpm in either cell line examined.
Two ER-negative breast cancer cell lines, MDA-MB-231 and Hs578t, two ER-positive breast cancer cell lines, MCF-7 and T47D, and one immortalized non-tumorigenic mammary epithelial cell line, MCF-10A, were utilized in this study. Findings were schedule- and agent-specific, but the patterns of synergy overall were not cell-line specific. FdURd, 5-FU, and paclitaxel were generally synergistic with BENSpm in all four cancer cell lines whereas C-DDP, docetaxel, and vinorelbine were commonly additive or antagonistic with BENSpm in each cancer cell line. The differences in synergy between the cancer cell lines and the non-tumorigenic cell line are noteworthy. The greatest synergy overall was seen with BENSpm in combination with 5-FU, FdUrd, and paclitaxel in the four cancer cell lines examined. When a concomitant treatment schedule was used, BENSpm was antagonistic with both 5-FU and FdURd in MCF-10A cells and was mildly synergistic with paclitaxel. However, the CI value for BENSpm and paclitaxel was higher in MCF-10A cells versus the cancer cell lines examined, suggesting that a differential sensitivity exists between the tumorigenic and non-tumorigenic cell lines.
Comparing the synergy seen with different breast cancer cell lines, BENSpm showed the most promise in combination with 5-FU and paclitaxel. The cytotoxic effects of several polyamine analogues are attributed to induction of the polyamine catabolic enzymes, SSAT and SMO [13, 24, 26, 58]. Recently, BENSpm was shown to induce SSAT and SMO activity in breast cancer cell lines in a cell-line dependent fashion [57]. DNA microarray technology was used to identify genes transcriptionally activated by 5-FU in MCF-7 cells, and SSAT was found to be among the most transcriptionally induced genes in 5-FU treated MCF-7 cells [51]. Additional studies have reported the ability of other chemotherapeutic agents to induce SSAT and SMO mRNA expression [17, 37]. For these reasons, we examined the role of the polyamine catabolic enzymes, SSAT and SMO, in the synergistic response of breast cancer cell lines to BENSpm treatment with 5-FU or paclitaxel.
BENSpm, 5-FU, and paclitaxel each induced SSAT mRNA in a dose- and time-dependent manner in both MDA-MB-231 and MCF-7 cells. Interestingly, both chemotherapeutic agents induced greater SSAT mRNA than BENSpm treatment alone in each cell line; however, neither of these agents alone induced significant SSAT activity (Figure 1). These results are consistent with a study that examined the ability of platinum drugs to induce polyamine catabolic enzymes in ovarian cancer cell lines [37]. This study in A2780 ovarian cancer cells demonstrated the ability of platinum drugs to induce SSAT mRNA, which was only translated into SSAT protein with co- or post-treatment with BENSpm. Based on these studies, it appears that several chemotherapeutic agents are able to induce SSAT mRNA although co-treatment with BENSpm appears to be required for its translation into protein. These results are consistent with the hypothesis that large increases in SSAT protein and activity are regulated at the post-transcriptional level [20]. It is reported that the transcriptional activation of SSAT is not due to enhanced BENSpm uptake but possibly through inactivation of IkB [18]. In contrast, SMO induction in MDA-MB-231 cells in response to BENSpm, 5-FU, or paclitaxel occurred at both the mRNA and activity level (Figure 2). Time course studies revealed that induction of SMO mRNA and activity began within six hours of treatment with each agent (data not shown). These results are consistent with the recent finding that most SMO regulation occurs at the level of mRNA [72].
Induction of SSAT is known to be a general stress response to a variety of factors and environmental conditions [56]. The possibility that the induction of SSAT, and perhaps other polyamine catabolic enzymes, represents a general response to environmental stress rather than a specific response to the chemotherapeutic agents selected was addressed by performing experiments using another chemotherapeutic agent, vinorelbine. Vinorelbine, was predominantly additive or antagonistic with BENSpm in several breast cancer cell lines (data not shown). Treatment of MDA-MB-231 or MCF-7 cells with vinorelbine over a similar dose-response and time-course did not significantly induce SSAT, APAO, or SMO mRNA or activity (data not shown). This finding suggests that the high induction of SSAT and SMO mRNA resulting from 5-FU or paclitaxel treatment is not solely a general stress response; rather the polyamine catabolic pathway may be a primary target of the chemotherapeutic agents that synergize with BENSpm.
Stable knock down of SSAT, SMO or both in MDA-MB-231 and MCF-7 cells by RNA interference was used to determine what role these enzymes play in the synergistic response of each cell line to treatment with BENSpm and 5-FU or paclitaxel. The knockdown of each enzyme alone partially reduced the sensitivity of MDA-MB-231 cells to BENSpm treatment with 5-FU or paclitaxel while MDA-MB-231 ΔSSAT/ΔSMO cells were the most resistant. While SSAT and SMO were found to play a major role in the response of MDA-MB-231 cells to treatment with BENSpm plus 5-FU or paclitaxel, these enzymes do not appear to play as great a role in the response of MCF-7 cells to these treatments. Neither SMO mRNA nor activity was induced by any of these agents in MCF-7 cells while SSAT played a modest role in response to BENSpm and paclitaxel and a greater role in the response to BENSpm with 5-FU. It will be important to examine the effects of these agents on the activity of ODC, one of the rate-limiting enzymes in polyamine biosynthesis, since BENSpm alone significantly reduces ODC activity in this cell line and because these agents often affect multiple polyamine metabolic enzymes. However, recent data demonstrated the ability of BENSpm and 5-FU to synergistically induce SSAT mRNA in human colon cancer cell lines; this induction was associated with a synergistic induction of apoptosis in wild-type and p53-null HCT116 colon carcinoma cells [17]. Transcriptome analysis from this study demonstrated that BENSpm and 5-FU treatment decreased the mitochondrial membrane potential, activated caspase 9, and led to the release of cytochrome c in both cell lines [17]. However, alteration in levels and metabolism of polyamines may not be completely responsible for cytotoxicity and induction of apoptosis. It was recently reported that BENSpm effectively induces apoptosis in chondrocytes independently of polyamine metabolism and levels [66].
BENSpm inhibits polyamine biosynthetic enzymes, induces polyamine catabolic enzymes like SSAT and SMO and inhibits cell growth in several different cancer cell lines in vitro [6]. Consistent antitumor activity has also been reported in several xenograft models. BENSpm given at 40 mg/kg 3 times/day for 6 days i.p. suppresses tumor growth of MALME-3 M human melanoma xenografts during treatment and up to 65 days after exposure [5]. Tumor regression was observed in other human solid tumor xenografts including A121 ovarian carcinoma, HT29 colon carcinoma, and SH-1 melanoma on i.p. treatment with 40–80 mg/kg BENSpm 3 times a day for 4 days [6] and in xenograft mouse models of pancreatic [15], bladder [62] and prostate cancer cell lines [60, 75].
In our study treatment of mice bearing MDA-MB-231 cell xenografts with BENSpm at 100 mg/kg/day 5 times a week for 4 weeks resulted in a significant decrease in tumor growth. Significant tumor regression was also evident with combination treatment with BENSpm and paclitaxel, although no synergism between the drugs was seen. Although 100 mg/kg BENSpm and weekly 5 mg/kg paclitaxel are both effective doses for tumor growth inhibition in breast carcinoma xenograft models, the treatment schedule chosen may not be the best to elicit a synergistic response. The lack of synergy observed may well be due to pharmacokinetic parameters that cannot be effectively modeled in vitro; this will necessitate additional experimentation to identify synergistic treatment schedules. Also, as treatment with 100 mg/kg/day of BENSpm in the schedule chosen was extremely effective, it may be possible to reduce the dose and/or frequency of BENSpm administration, thus reducing the possibility of potential toxicities and increasing the potential for synergy with paclitaxel. Finally, it is possible that sequential treatment may be more effective than the concurrent treatment used here. Thus, this study forms the basis for further exploration of optimal dose and schedule of combinative studies with cytotoxic drugs and BENSpm in vivo in preparation for human testing.
It is important to note that the data provided here may also be helpful in the development of combination treatment strategies with newly introduced polyamine analogues. One such agent, CGC-11047, that demonstrated effective antitumor properties in preclinical studies is currently in clinical trial [32]. The present study will form a platform for future studies of CGC-11047 in combination with chemotherapeutics that induce SSAT and/or SMO that could be translated clinically.
In conclusion, this study demonstrates that 5-FU or paclitaxel induces SSAT and SMO mRNA expression, and co-treatment with BENSpm leads to a large induction of SSAT and SMO activity in two representative human breast cancer cell lines. Knockdown studies suggest that induction of SSAT and SMO is correlated with the antiproliferative effects of BENSpm with 5-FU or paclitaxel in MDA-MB-231 cells. The induction of SSAT and SMO appears to play a specific role in the synergistic response of breast cancer cell lines to BENSpm with paclitaxel and 5-FU but not another chemotherapeutic, vinorelbine, suggesting specificity of effect. These results provide evidence that combining standard chemotherapeutic agents with polyamine analogues that induce SSAT and SMO is a rational approach to treating breast cancer. With further optimization of polyamine analogues and schedules, these results may lead to clinically relevant treatment strategies.
Acknowledgments
This work was supported by grants from the NIH, the Department of Defense and the Breast Cancer Research Foundation.
Abbreviations
- ODC
ornithine decarboxylase
- AdoMetDC
S-adenosylmethionine decarboxylase
- SSAT
spermidine/spermine N1-acetyltransferase
- APAO
N1-acetylpolyamine oxidase
- SMO
spermine oxidase
- BENSpm
N1, N11-Bis(ethyl)norspermine (also known as DENSpm, N1, N11- Diethylnorspermine)
- ER
estrogen receptor alpha
- TS
thymidylate synthase
- FdURd
fluorodeoxyuridine
- 5-FU
5-fluorouracil
- C-DDP
cis-diaminechloroplatinum(II)
- CPENSpm
N1-ethyl-N11-[(cyclopropyl)methyl]-4,8-diazaundecane
- CHENSpm
N1-ethyl-N11-[(cycloheptyl)methyl]4,8-diazaundecane
REFERENCES
- 1.Allen WL, McLean EG, Boyer J, McCulla A, Wilson PM, Coyle V, Longley DB, Casero RA, Jr, Johnston PG. The role of spermidine/spermine N1-acetyltransferase in determining response to chemotherapeutic agents in colorectal cancer cells. Mol Cancer Ther. 2007;6:128–137. doi: 10.1158/1535-7163.MCT-06-0303. [DOI] [PubMed] [Google Scholar]
- 2.Balasundaram D, Tyagi AK. Polyamine--DNA nexus: structural ramifications and biological implications. Mol Cell Biochem. 1991;100:129–140. doi: 10.1007/BF00234162. [DOI] [PubMed] [Google Scholar]
- 3.Basu HS, Pellarin M, Feuerstein BG, Shirahata A, Samejima K, Deen DF, Marton LJ. Interaction of a polyamine analogue, 1,19-bis-(ethylamino)-5,10,15-triazanonadecane (BE-4-4-4-4), with DNA and effect on growth, survival, and polyamine levels in seven human brain tumor cell lines. Cancer Res. 1993;53:3948–3955. [PubMed] [Google Scholar]
- 4.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1988;31:1183–1190. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 5.Bernacki RJ, Bergeron RJ, Porter CW. Antitumor activity of N,N'-bis(ethyl)spermine homologues against human MALME-3 melanoma xenografts. Cancer Res. 1992;52:2424–2430. [PubMed] [Google Scholar]
- 6.Bernacki RJ, Oberman EJ, Seweryniak KE, Atwood A, Bergeron RJ, Porter CW. Preclinical antitumor efficacy of the polyamine analogue N1, N11-diethylnorspermine administered by multiple injection or continuous infusion. Clin Cancer Res. 1995;1:847–857. [PubMed] [Google Scholar]
- 7.Boyer J, Allen WL, McLean EG, Wilson PM, McCulla A, Moore S, Longley DB, Caldas C, Johnston PG. Pharmacogenomic identification of novel determinants of response to chemotherapy in colon cancer. Cancer Res. 2006;66:2765–2777. doi: 10.1158/0008-5472.CAN-05-2693. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 9.Budman DR. Vinorelbine (Navelbine): a third-generation vinca alkaloid. Cancer Invest. 1997;15:475–490. doi: 10.3109/07357909709047587. [DOI] [PubMed] [Google Scholar]
- 10.Camargo SM, Francescato HD, Lavrador MA, Bianchi ML. Oral administration of sodium selenite minimizes cisplatin toxicity on proximal tubules of rats. Biol Trace Elem Res. 2001;83:251–262. doi: 10.1385/BTER:83:3:251. [DOI] [PubMed] [Google Scholar]
- 11.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 12.Casero RA, Jr, Celano P, Ervin SJ, Wiest L, Pegg AE. High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma. Biochem J. 1990;270:615–620. doi: 10.1042/bj2700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casero RA, Jr, Wang Y, Stewart TM, Devereux W, Hacker A, Smith R, Woster PM. The role of polyamine catabolism in anti-tumour drug response. Biochem Soc Trans. 2003;31:361–365. doi: 10.1042/bst0310361. [DOI] [PubMed] [Google Scholar]
- 14.Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 15.Chang BK, Bergeron RJ, Porter CW, Vinson JR, Liang Y, Libby PR. Regulatory and antiproliferative effects of N-alkylated polyamine analogues in human and hamster pancreatic adenocarcinoma cell lines. Cancer Chemother Pharmacol. 1992;30:183–188. doi: 10.1007/BF00686309. [DOI] [PubMed] [Google Scholar]
- 16.Chang BK, Gutman R, Black O., Jr Combined effects of alpha-difluoromethylornithine and doxorubicin against pancreatic cancer cell lines in culture. Pancreas. 1986;1:49–54. doi: 10.1097/00006676-198601000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Choi W, Gerner EW, Ramdas L, Dupart J, Carew J, Proctor L, Huang P, Zhang W, Hamilton SR. Combination of 5-fluorouracil and N1,N11-diethylnorspermine markedly activates spermidine/spermine N1-acetyltransferase expression, depletes polyamines, and synergistically induces apoptosis in colon carcinoma cells. J Biol Chem. 2005;280:3295–3304. doi: 10.1074/jbc.M409930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi W, Proctor L, Xia Q, Feng Y, Gerner EW, Chiao PJ, Hamilton SR, Zhang W. Inactivation of IkappaB contributes to transcriptional activation of spermidine/spermine N(1)-acetyltransferase. Mol Carcinog. 2006;45:685–693. doi: 10.1002/mc.20239. [DOI] [PubMed] [Google Scholar]
- 19.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Coleman CS, Pegg AE, Megosh LC, Guo Y, Sawicki JA, O'Brien TG. Targeted expression of spermidine/spermine N1-acetyltransferase increases susceptibility to chemically induced skin carcinogenesis. Carcinogenesis. 2002;23:359–364. doi: 10.1093/carcin/23.2.359. [DOI] [PubMed] [Google Scholar]
- 21.Culine S, Roch I, Pinguet F, Romieu G, Bressolle F. Combination paclitaxel and vinorelbine therapy: in vitro cytotoxic interactions and dose-escalation study in breast cancer patients previously exposed to anthracyclines. Int J Oncol. 1999;14:999–1006. doi: 10.3892/ijo.14.5.999. [DOI] [PubMed] [Google Scholar]
- 22.Das B, Rao AR, Madhubala R. Difluoromethylornithine antagonizes taxol cytotoxicity in MCF-7 human breast cancer cells. Oncol Res. 1997;9:565–572. [PubMed] [Google Scholar]
- 23.Davidson NE, Hahm HA, McCloskey DE, Woster PM, Casero RA., Jr Clinical aspects of cell death in breast cancer: the polyamine pathway as a new target for treatment. Endocr Relat Cancer. 1999;6:69–73. doi: 10.1677/erc.0.0060069. [DOI] [PubMed] [Google Scholar]
- 24.Davidson NE, Mank AR, Prestigiacomo LJ, Bergeron RJ, Casero RA., Jr Growth inhibition of hormone-responsive and -resistant human breast cancer cells in culture by N1, N12-bis(ethyl)spermine. Cancer Res. 1993;53:2071–2075. [PubMed] [Google Scholar]
- 25.Desiderio MA, Bergamaschi D, Mascellani E, De Feudis P, Erba E, D'Incalci M. Treatment with inhibitors of polyamine biosynthesis, which selectively lower intracellular spermine, does not affect the activity of alkylating agents but antagonizes the cytotoxicity of DNA topoisomerase II inhibitors. Br J Cancer. 1997;75:1028–1034. doi: 10.1038/bjc.1997.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 27.Feuerstein BG, Pattabiraman N, Marton LJ. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990;18:1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 29.Fitzpatrick FA, Wheeler R. The immunopharmacology of paclitaxel (Taxol), docetaxel (Taxotere), and related agents. Int Immunopharmacol. 2003;3:1699–1714. doi: 10.1016/j.intimp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 31.Ha HC, Woster PM, Yager JD, Casero RA., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci U S A. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacker A, Marton LJ, Sobolewski M, Casero RA., Jr In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;63:45–53. doi: 10.1007/s00280-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahm HA, Dunn VR, Butash KA, Deveraux WL, Woster PM, Casero RA, Jr, Davidson NE. Combination of standard cytotoxic agents with polyamine analogues in the treatment of breast cancer cell lines. Clin Cancer Res. 2001;7:391–399. [PubMed] [Google Scholar]
- 34.Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, Casero RA., Jr Phase I study of N(1),N(11)-diethylnorspermine in patients with non-small cell lung cancer. Clin Cancer Res. 2002;8:684–690. [PubMed] [Google Scholar]
- 35.Hawthorne TR, Austin JK., Jr Synergism of the polyamine analogue, N1,N11-bisethylnorspermine with cis-diaminedichloroplatinum (II) against murine neoplastic cell lines in vitro and in vivo. Cancer Lett. 1996;99:99–107. doi: 10.1016/0304-3835(95)04043-9. [DOI] [PubMed] [Google Scholar]
- 36.Hector S, Hawthorn L, Greco W, Pendyala L. Gene expression profiles after oxaliplatin treatment in A2780 ovarian carcinoma cells. American Association for Cancer Research. 2002:62. [Google Scholar]
- 37.Hector S, Porter CW, Kramer DL, Clark K, Prey J, Kisiel N, Diegelman P, Chen Y, Pendyala L. Polyamine catabolism in platinum drug action: Interactions between oxaliplatin and the polyamine analogue N1,N11-diethylnorspermine at the level of spermidine/spermine N1-acetyltransferase. Mol Cancer Ther. 2004;3:813–822. [PubMed] [Google Scholar]
- 38.Hector S, Tummala R, Kisiel ND, Diegelman P, Vujcic S, Clark K, Fakih M, Kramer DL, Porter CW, Pendyala L. Polyamine catabolism in colorectal cancer cells following treatment with oxaliplatin, 5-fluorouracil and N1, N11 diethylnorspermine. Cancer Chemother Pharmacol. 2008;62:517–527. doi: 10.1007/s00280-007-0633-2. [DOI] [PubMed] [Google Scholar]
- 39.Holst CM, Johansson VM, Alm K, Oredsson SM. Novel anti-apoptotic effect of Bcl-2: prevention of polyamine depletion-induced cell death. Cell Biol Int. 2008;32:66–74. doi: 10.1016/j.cellbi.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Jr, Davidson NE. A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res. 2003;9:2769–2777. [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Pledgie A, Casero RA, Jr, Davidson NE. Molecular mechanisms of polyamine analogs in cancer cells. Anticancer Drugs. 2005;16:229–241. doi: 10.1097/00001813-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Johansson VM, Oredsson SM, Alm K. Polyamine depletion with two different polyamine analogues causes DNA damage in human breast cancer cell lines. DNA Cell Biol. 2008;27:511–516. doi: 10.1089/dna.2008.0750. [DOI] [PubMed] [Google Scholar]
- 43.Johnson M, Shaw M, Rubenstein M, Guinan P. Effect of early and delayed difluoromethylornithine pretreatment upon cyclophosphamide chemotherapy. Clin Physiol Biochem. 1990;8:11–15. [PubMed] [Google Scholar]
- 44.Kano Y, Akutsu M, Tsunoda S, Izumi T, Mori K, Fujii H, Yazawa Y, Mano H, Furukawa Y. Schedule-dependent synergism and antagonism between pemetrexed and paclitaxel in human carcinoma cell lines in vitro. Cancer Chemother Pharmacol. 2004;54:505–513. doi: 10.1007/s00280-004-0839-5. [DOI] [PubMed] [Google Scholar]
- 45.Kano Y, Akutsu M, Tsunoda S, Mori K, Suzuki K, Adachi KI. In vitro schedule-dependent interaction between paclitaxel and SN-38 (the active metabolite of irinotecan) in human carcinoma cell lines. Cancer Chemother Pharmacol. 1998;42:91–98. doi: 10.1007/s002800050790. [DOI] [PubMed] [Google Scholar]
- 46.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97:825–833. doi: 10.1002/cncr.11126. [DOI] [PubMed] [Google Scholar]
- 47.Kingsnorth AN, Russell WE, McCann PP, Diekema KA, Malt RA. Effects of alpha-difluoromethylornithine and 5-fluorouracil on the proliferation of a human colon adenocarcinoma cell line. Cancer Res. 1983;43:4035–4038. [PubMed] [Google Scholar]
- 48.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 49.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 50.Marverti G, Piccinini G, Ghiaroni S, Barbieri D, Quaglino D, Moruzzi MS. N1,N12-bis(ethyl)spermine effect on growth of cis-diamminedichloroplatinum(II)-sensitive and -resistant human ovarian-carcinoma cell lines. Int J Cancer. 1998;78:33–40. doi: 10.1002/(sici)1097-0215(19980925)78:1<33::aid-ijc7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 51.Maxwell PJ, Longley DB, Latif T, Boyer J, Allen W, Lynch M, McDermott U, Harkin DP, Allegra CJ, Johnston PG. Identification of 5-fluorouracil-inducible target genes using cDNA microarray profiling. Cancer Res. 2003;63:4602–4606. [PubMed] [Google Scholar]
- 52.McCloskey DE, Casero RA, Jr, Woster PM, Davidson NE. Induction of programmed cell death in human breast cancer cells by an unsymmetrically alkylated polyamine analogue. Cancer Res. 1995;55:3233–3236. [PubMed] [Google Scholar]
- 53.McCloskey DE, Yang J, Woster PM, Davidson NE, Casero RA., Jr Polyamine analogue induction of programmed cell death in human lung tumor cells. Clin Cancer Res. 1996;2:441–446. [PubMed] [Google Scholar]
- 54.Nakagawa H, Yamada M, Fukushima M, Ikenaka K. Intrathecal 5-fluoro-2'-deoxyuridine (FdUrd) for the treatment of solid tumor neoplastic meningitis: an in vivo study. Cancer Chemother Pharmacol. 1999;43:247–256. doi: 10.1007/s002800050891. [DOI] [PubMed] [Google Scholar]
- 55.Oredsson SM, Alm K, Dahlberg E, Holst CM, Johansson VM, Myhre L, Soderstjerna E. Inhibition of cell proliferation and induction of apoptosis by N(1),N(11)-diethylnorspermine-induced polyamine pool reduction. Biochem Soc Trans. 2007;35:405–409. doi: 10.1042/BST0350405. [DOI] [PubMed] [Google Scholar]
- 56.Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 57.Pledgie A, Huang Y, Hacker A, Zhang Z, Woster PM, Davidson NE, Casero RA., Jr Spermine oxidase SMO(PAOh1), Not N1-acetylpolyamine oxidase PAO, is the primary source of cytotoxic H2O2 in polyamine analogue-treated human breast cancer cell lines. J Biol Chem. 2005;280:39843–39851. doi: 10.1074/jbc.M508177200. [DOI] [PubMed] [Google Scholar]
- 58.Porter CW, Ganis B, Libby PR, Bergeron RJ. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- 59.Porter CW, McManis J, Casero RA, Bergeron RJ. Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res. 1987;47:2821–2825. [PubMed] [Google Scholar]
- 60.Schipper RG, Deli G, Deloyer P, Lange WP, Schalken JA, Verhofstad AA. Antitumor activity of the polyamine analog N(1), N(11)-diethylnorspermine against human prostate carcinoma cells. Prostate. 2000;44:313–321. doi: 10.1002/1097-0045(20000901)44:4<313::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 61.Seidenfeld J, Barnes D, Block AL, Erickson LC. Comparison of DNA interstrand cross-linking and strand breakage by 1,3-bis(2-chloroethyl)-1-nitrosourea in polyamine-depleted and control human adenocarcinoma cells. Cancer Res. 1987;47:4538–4543. [PubMed] [Google Scholar]
- 62.Sharma A, Glaves D, Porter CW, Raghavan D, Bernacki RJ. Antitumor efficacy of N1,N11-diethylnorspermine on a human bladder tumor xenograft in nude athymic mice. Clin Cancer Res. 1997;3:1239–1244. [PubMed] [Google Scholar]
- 63.Shaw MW, Guinan PD, McKiel CF, Dubin A, Rubenstein M. Combination therapy using polyamine synthesis inhibitor alpha-difluoromethylornithine and adriamycin in treatment of rats carrying the Dunning R3327 MAT-LyLu prostatic adenocarcinoma. Prostate. 1987;11:87–93. doi: 10.1002/pros.2990110111. [DOI] [PubMed] [Google Scholar]
- 64.Shrestha RD, Fujimoto S, Okui K. Contradictory antitumor efficacies produced by the combination of DNA attacking drugs and polyamine antimetabolites. Jpn J Surg. 1987;17:263–268. doi: 10.1007/BF02470698. [DOI] [PubMed] [Google Scholar]
- 65.Snyder RD. Polyamine depletion is associated with altered chromatin structure in HeLa cells. Biochem J. 1989;260:697–704. doi: 10.1042/bj2600697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanic I, Cetrullo S, Facchini A, Stefanelli C, Borzi RM, Tantini B, Guarnieri C, Caldarera CM, Flamigni F. Effect of the polyamine analogue N1,N11-diethylnorspermine on cell survival and susceptibility to apoptosis of human chondrocytes. J Cell Physiol. 2008;216:153–161. doi: 10.1002/jcp.21387. [DOI] [PubMed] [Google Scholar]
- 67.Stearns V, Davidson NE, Flockhart DA. Pharmacogenetics in the treatment of breast cancer. Pharmacogenomics J. 2004;4:143–153. doi: 10.1038/sj.tpj.6500242. [DOI] [PubMed] [Google Scholar]
- 68.Thomas T, Kiang DT. Additive growth-inhibitory effects of DL-alpha-difluoromethylornithine and antiestrogens on MCF-7 breast cancer cell line. Biochem Biophys Res Commun. 1987;148:1338–1345. doi: 10.1016/s0006-291x(87)80279-6. [DOI] [PubMed] [Google Scholar]
- 69.Thomas T, Thomas TJ. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci. 2001;58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Varma R, Hector S, Greco WR, Clark K, Hawthorn L, Porter C, Pendyala L. Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother Pharmacol. 2007;59:711–723. doi: 10.1007/s00280-006-0325-3. [DOI] [PubMed] [Google Scholar]
- 71.Wallace HM, Fraser AV, Hughes A. A perspective of polyamine metabolism. Biochem J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Hacker A, Murray-Stewart T, Fleischer JG, Woster PM, Casero RA., Jr Induction of human spermine oxidase SMO(PAOh1) is regulated at the levels of new mRNA synthesis, mRNA stabilization and newly synthesized protein. Biochem J. 2005;386:543–547. doi: 10.1042/BJ20041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Webb HK, Wu Z, Sirisoma N, Ha HC, Casero RA, Jr, Woster PM. 1-(N-alkylamino)-11-(N-ethylamino)-4,8-diazaundecanes: simple synthetic polyamine analogues that differentially alter tubulin polymerization. J Med Chem. 1999;42:1415–1421. doi: 10.1021/jm980603+. [DOI] [PubMed] [Google Scholar]
- 74.Wolff AC, Armstrong DK, Fetting JH, Carducci MK, Riley CD, Bender JF, Casero RA, Jr, Davidson NE. A Phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2003;9:5922–5928. [PubMed] [Google Scholar]
- 75.Zagaja GP, Shrivastav M, Fleig MJ, Marton LJ, Rinker-Schaeffer CW, Dolan ME. Effects of polyamine analogues on prostatic adenocarcinoma cells in vitro and in vivo. Cancer Chemother Pharmacol. 1998;41:505–512. doi: 10.1007/s002800050774. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Ramdas L, Shen W, Song SW, Hu L, Hamilton SR. Apoptotic response to 5-fluorouracil treatment is mediated by reduced polyamines, non-autocrine Fas ligand and induced tumor necrosis factor receptor 2. Cancer Biol Ther. 2003;2:572–578. doi: 10.4161/cbt.2.5.532. [DOI] [PubMed] [Google Scholar]
- 77.Zirvi KA, Atabek U. In vitro response of a human colon tumor xenograft and a lung adenocarcinoma cell line to alpha-difluoromethylornithine alone and in combination with 5-fluorouracil and doxorubicin. J Surg Oncol. 1991;48:34–38. doi: 10.1002/jso.2930480107. [DOI] [PubMed] [Google Scholar]