Abstract
Cholera is a severe diarrheal disease caused by the motile Gram-negative rod Vibrio cholerae. Live-attenuated V. cholerae vaccines harboring deletions of the genes encoding cholera toxin have great promise for reducing the global burden of cholera. However, development of live vaccines has been hampered by the tendency of such strains to induce noncholeric reactogenic diarrhea in human subjects. The molecular bases of reactogenicity are unknown, but it has been speculated that reactogenic diarrhea is a response to V. cholerae’s flagellum and/or the motility that it enables. Here, we used an infant rabbit model of reactogenicity to determine what V. cholerae factors trigger this response. We found that V. cholerae ctx mutants that produced flagellins induced diarrhea, regardless of whether the proteins were assembled into a flagellum or whether the flagellum was functional. In contrast, ∼90% of rabbits infected with V. cholerae lacking all five flagellin-encoding genes did not develop diarrhea. Thus, flagellin production, independent of flagellum assembly or motility, is sufficient for reactogenicity. The intestinal colonization and intraintestinal localization of the nonreactogenic flagellin-deficient strain were indistinguishable from those of a flagellated motile strain; however, the flagellin-deficient strain stimulated fewer mRNA transcripts coding for proinflammatory cytokines in the intestine. Thus, reactogenic diarrhea may be a consequence of an innate host inflammatory response to V. cholerae flagellins. Our results suggest a simple genetic blueprint for engineering defined nonreactogenic live-attenuated V. cholerae vaccine strains.
Keywords: animal model, cholera, innate immunity, diarrhea
Cholera is a severe diarrheal disease that is caused by Vibrio cholerae, a motile curved Gram-negative rod. This disease remains a significant threat to health in many parts of the developing world, especially in Africa and Asia. It is estimated that there are several million cases of cholera in the world annually (1) and that more than 100,000 people die from this infection each year. Although rehydration therapy is effective and greatly reduces mortality when available, the continued burden of cholera, particularly in regions with socio-economic disruptions, has prompted the recommendation that vaccines to protect against infection with El Tor biotype Vibrio cholerae, the cause of the ongoing seventh pandemic, be developed (2).
V. cholerae is a noninvasive enteric pathogen. Humans contract cholera following ingestion of water or food that is contaminated with this organism. Bacteria that survive passage through the acidic gastric barrier colonize the small bowel, where they produce cholera toxin (CT), an A-B5–subunit type exotoxin. CT is thought to be the principal factor underlying the severe secretory diarrhea that is characteristic of cholera (3).
Since the initial cloning of ctxAB, the genes encoding the A and B subunits of CT (4), there have been several attempts to engineer live-attenuated V. cholerae vaccine strains via deletion of ctxA, which encodes the toxic moiety of CT (5). To date, ctxA live-attenuated oral V. cholerae vaccine strains have shown promise, but many of the candidate vaccine strains have led to side effects in volunteers. Such side effects, often referred to as vaccine “reactogenicity,” include noncholeric diarrhea and abdominal cramps (5). Comparative analyses of vaccine candidates suggests that reactogenicity may be linked to V. cholerae’s single polar flagellum and/or to bacterial motility. The vaccine strain Peru-3, a ctxA derivative of a Peruvian El Tor clinical isolate, caused diarrhea, whereas Peru-15, a spontaneously derived nonflagellated (nonmotile) derivative of Peru-3, did not (6, 7). Both strains engendered protection against challenge with wild-type V. cholerae in human trials, suggesting that the lack of reactogenicity does not simply result from a failure of Peru-15 to colonize. However, despite these findings, the precise cause of reactogenic diarrhea has not been identified, and the nature of the genetic changes that distinguish Peru-15 from Peru-3 remains unknown.
Several (not necessarily exclusive) hypotheses regarding the origins of reactogenicity have been proposed. One possibility is that flagellation and motility enable V. cholerae to penetrate the mucus layer covering the intestinal epithelial surface, and that the close proximity of the organism to the apical surface of epithelial cells elicits an inflammatory response that results in diarrhea (8). Additionally, it is possible that reactogenicity is induced by toxins still produced by the ctxA mutant, such as zonula occludens toxin, accessory cholera enterotoxin, hemolysin A, multifunctional autoprocessing repeats-in-toxin (MARTX) toxin, and/or hemagglutinin/protease, through direct enterotoxicity and/or through proinflammatory effects (9). The effect of these factors might be potentiated by close contact between the bacteria and the epithelium. Finally, recent work using tissue-culture models has led to the hypothesis that the five V. cholerae flagellins, which have been demonstrated to activate the Toll-like receptor 5 (TLR5) signaling pathway, could lead directly to reactogenic diarrhea by stimulating production of proinflammatory cytokines in the intestine (10, 11). Detection of lactoferrin and fecal leukocytes in the stools of volunteers with reactogenic diarrhea (12, 13) supports the idea that intestinal inflammation is associated with vaccine reactogenicity.
Investigation of the molecular basis of V. cholerae vaccine reactogenicity has been hampered by the lack of an animal model. Recently, however, we found that infant rabbits can serve as a model for severe cholera as well as for the reactogenic diarrhea caused by V. cholerae ctxA mutants. Oro-gastric inoculation of wild-type V. cholerae into infant rabbits that had been pretreated with cimetidine led to lethal, watery diarrhea in virtually all animals. Rabbits inoculated with wild-type V. cholerae usually died ∼24–30 h later; in contrast, rabbits inoculated with an isogenic V. cholerae ctxAB mutant exhibited no or minimal signs of disease during this time period. However, 36–60 h after inoculation of the ctxAB mutant, most of the animals developed noncholeric fecal diarrhea.
Here, we used infant rabbits to explore the genetic basis of reactogenic diarrhea. We found that the reactogenic vaccine strain Peru-3 caused diarrhea in most rabbits, whereas the nonreactogenic strain Peru-15 did not, thereby validating the relevance of the rabbit model for study of reactogenicity. Subsequently, we used an isogenic set of defined mutants to determine whether motility per se, production of a flagellum, or production of flagellin proteins underlies reactogenicity. These experiments revealed that neither motility nor a flagellum is required to induce reactogenic diarrhea; instead, production of flagellin proteins is sufficient cause. The intestinal colonization and the intraintestinal localization of the nonreactogenic flagellin-deficient strain were indistinguishable from those of a flagellated motile strain; however, the flagellin-deficient strain stimulated fewer mRNA transcripts coding for proinflammatory cytokines in the intestine. These data are consistent with the possibility that reactogenic diarrhea is linked to an innate host inflammatory response to V. cholerae flagellins and suggest a simple genetic blueprint for creating defined nonreactogenic live-attenuated V. cholerae vaccine strains.
Results
Nonreactogenic V. cholerae Vaccine Strains Do Not Elicit Diarrhea in an Infant Rabbit Model of Disease.
In our recent work developing an infant rabbit model of cholera, we noted that infant rabbits infected with V. cholerae lacking ctxAB developed noncholeric diarrhea, and we proposed that this animal model also might be useful for study of vaccine reactogenicity. Animals infected with a C6706 ctxAB mutant (here termed “Peru-NT”), a derivative of a 1991 El Tor Peruvian clinical isolate (14), were contaminated with loosely adherent fecal material on their perineums, hind legs, and tails by ∼36–60 h after inoculation (Fig. 1). At necropsy the large intestine contained soft, unformed fecal material; in contrast, the intestines of mock-infected rabbits contained hard, formed pellets. This fecal diarrhea appeared markedly different from the watery, mucin-rich fluid released from rabbits infected with wild-type V. cholerae, which closely approximates the “rice-water stool” produced by cholera patients. Fecal diarrhea was classified subjectively as severe (e.g., Fig. 1A) or mild (e.g., Fig. 1B), based on the amount of adherent feces. Almost all (13/18) rabbits infected with Peru-NT developed severe diarrhea, and only 1 remained clear of any fecal contamination. Diarrhea spontaneously remitted by ∼80 h after inoculation. In control experiments, we found that none of the 13 rabbits inoculated with buffer alone developed diarrhea.
Fig. 1.
Diarrhea in infant rabbits inoculated with Peru-NT (a ctxAB mutant) or one of its derivatives. Rabbits exhibiting severe, mild, and no diarrhea are shown in A, B, and C, respectively. The frequency with which rabbits infected with the indicated strains exhibited diarrhea and statistical analyses of these results are presented in D.
To confirm the relevance of this animal model for studies of vaccine reactogenicity, we compared the signs of disease exhibited by infant rabbits inoculated with either Peru-3 or Peru-15, two live-attenuated ctxA mutant vaccine strains that have been tested in humans. Volunteers inoculated with Peru-3 often developed self-limiting diarrhea, whereas diarrhea was not observed in volunteers who received Peru-15 (6, 7). Similarly, we found that most (12/18) rabbits inoculated with Peru-3 developed diarrhea, whereas only 2 of 13 rabbits inoculated with Peru-15 exhibited diarrhea. These observations suggest that infant rabbits are a valid model host for study of reactogenic diarrhea caused by ctx mutant live-attenuated V. cholerae vaccine strains.
Reactogenicity Depends on Flagellins but Not on Motility.
Peru-15 was isolated as a spontaneous nonmotile derivative of Peru-3 (7), and the mutation(s) that render Peru-15 nonflagellated (and hence nonmotile) are not known. In principle, the difference between the reactogenicity of Peru-3 and Peru-15 could result from Peru-15’s lack of a flagellum and/or flagellar proteins, from the strain’s lack of motility, or even from a mutation not linked to flagellation or motility. Furthermore, the differences between these strains might not have a direct connection to diarrheagenic pathways but instead might be coupled indirectly, for example, via differences in their capacities to colonize the rabbit intestinal tract. To begin to distinguish among these possibilities, we generated derivatives of Peru-NT containing a variety of mutations within genes needed for flagellar assembly and/or activity. Flagellar synthesis is a complex process that is coordinately regulated via several virulence-linked pathways (15); however, the mutations generated for this study disrupt only the synthesis of the individual flagellins and are not expected to influence other processes.
V. cholerae encodes five distinct flagellins, which are encoded within two operons, flaAC and flaDBE (16). All these flagellins are thought to be incorporated into V. cholerae’s single polar flagellum (visible in Fig. 2B); however, incorporation is dependent upon flaA. In the absence of flaA, the synthesis of other flagellins does not appear to be altered, but these flagellins are secreted, rather than incorporated into a filament (10). We generated derivatives of Peru-NT lacking one or more flagellin-encoding gene(s). Mutants lacking flagellins other than flaA were flagellated and motile, as expected, whereas strains lacking flaA were nonflagellated and nonmotile (Fig. 2 A–C and Fig. S1). The relative levels of flagellins produced by these strains are shown in Fig. 2D. As expected, Peru-NTΔflaABCDE, a strain in which all five V. cholerae flagellins were deleted, did not produce detectable flagellins (Fig. 2D). We also generated a strain lacking motB, which encodes a component of the flagellar motor. This mutant synthesizes wild-type levels of flagellins and assembles a flagellum (Fig. 2D and Fig. S1); however, this flagellum does not turn, and consequently the bacteria are nonmotile (Fig. 2A) (17). None of the mutants used in this study displayed any significant defects in growth in vitro.
Fig. 2.
Motility, flagellum, and flagellin production in Peru-NT or one of its derivatives. (A) The indicated strains were inoculated into 0.3% LB agar and photographed 12 h later. Peru-NT (B) or Peru-NTΔflaABCDE (C) were visualized using transmission electron microscopy. (Scale bars, 1 μm.) (D) A Western blot of whole-cell extracts from the indicated strains was probed with a 1:4000 dilution of antisera to V. parahaemolyticus polar flagellins (33).
We initially compared the diarrhea caused by Peru-NT, Peru-NTΔflaABCDE, and Peru-NT motB after their oro-gastric inoculation into infant rabbits. The majority (73%) of rabbits infected with the motB mutant developed diarrhea, a frequency that did not differ significantly from that observed with Peru-NT (94%). In marked contrast, PeruNTΔflaABCDE led to diarrhea in only 12% (4/32) of inoculated rabbits (Fig. 1D; P < 0.0001 relative to Peru-NT). Together, these data are consistent with the possibility that a functional flagellum (i.e., motility) is not required to induce reactogenicity, but that production of flagellin proteins and/or a flagellar structure are critical.
To assess whether the flagellum itself is required for diarrhea, and to begin to decipher which flagellin(s) promote reactogenic diarrhea, we infected rabbits with derivatives of Peru-NT that lacked a subset of V. cholerae’s flagellin-encoding genes. Two nonflagellated strains, Peru-NTΔflaA and Peru-NTΔflaACD (Fig. S1), also caused diarrhea (Fig. 1D), albeit at a significantly lower frequency than Peru-NT (P < 0.01 for both strains versus Peru-NT). Therefore, the flagellum filament is not essential for induction of diarrhea. Because flagellins are thought to be secreted in the absence of flagellum production, these observations suggest that extracellular flagellin monomers can induce reactogenic diarrhea. Furthermore, diarrhea does not appear to be linked to a particular flagellin monomer. As noted above, strains lacking flaA caused diarrhea; additionally, rabbits inoculated with Peru-NTΔflaBCDE and Peru-NTΔflaABCDE pSW-flaA (which expresses flaA from a low-copy vector) developed diarrhea. Thus, no individual flagellin is essential for induction of diarrhea, although flaA appears to be sufficient. Notably, all of the strains lacking at least one but not all flagellin-encoding gene(s) differed significantly from both Peru-NT and Peru-NTΔflaABCDE in their frequency of causing diarrhea. This result suggests that, although a subset of flagellins can be sufficient to induce reactogenic diarrhea, the full complement of flagellins is a more potent stimulus. There does not appear to be a precise correlation between reactogenicity and net production of flagellin monomers (compare Figs. 1D and 2D); however, it is quite possible that the abundance of cell-associated monomers does not accurately reflect the level of secreted monomers.
Differential Intestinal Colonization or Localization of Peru-NT Flagellin Mutants Does Not Account for Reactogenic Diarrhea.
It is possible that these results do not indicate a direct reactogenic role for flagellin proteins but instead reflect differences in the colonization capacities of the various strains. To explore this possibility, we determined the number of cfu recovered for each strain in intestinal tissue homogenates 3 days after their inoculation into infant rabbits. All strains robustly colonized the mid and distal portions of the small intestine (∼1010 cfu g−1) as well as the midcolon. The number of cfu recovered from animals infected with the flagellin mutants did not differ significantly from the number of Peru-NT cfu recovered at any site (Fig. 3). These findings argue strongly against the idea that the capacity of these strains to colonize the intestine, at least as assessed by cfu recovered in intestinal homogenates, correlates with their stimulation of diarrhea.
Fig. 3.
Intestinal colonization of Peru-NT or one of its derivatives. Graphs show the number of cfu in tissue homogenates of the proximal (A), mid (B), and distal (C) small intestine (SI) and midcolon (D) 3 days after inoculation. Bars show the geometric mean for each group.
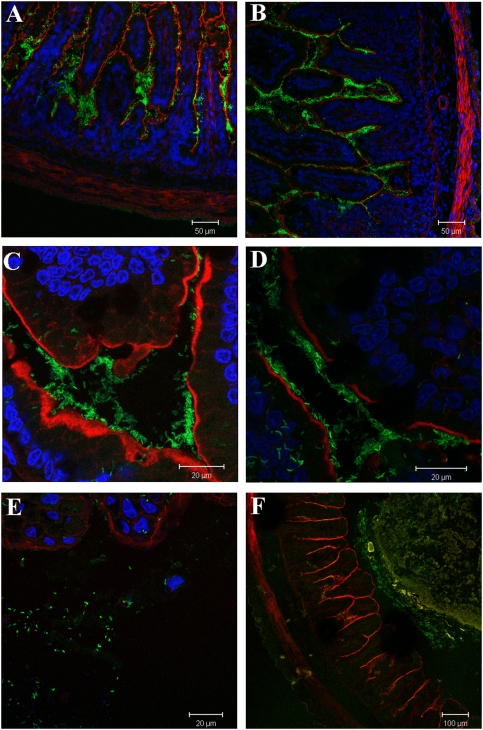
Although the numbers of Peru-NT and Peru-NTΔflaABCDE cfu recovered from the intestine were similar, the fine localization of these strains within the intestine could differ, given their dramatic differences in motility. To address this issue, we visualized Peru-NT and Peru-NTΔflaABCDE within infected tissues using confocal microscopy. Unexpectedly, we observed that these strains exhibited very similar patterns of localization in the small and large intestine. Like Peru-NT, the nonmotile Peru-NTΔflaABCDE could be found in close apposition to all parts of the villous surface as well as deep in the crypt-like structures of the infant rabbit intestine (compare Fig. 4 A and C with Fig. 4 B and D). This observation strongly suggests that flagellar-based motility is not required for V. cholerae to gain access to the intestinal crypts or to get close to the epithelial surface in this model host. In the large intestine, both strains usually were found in the lumen (Fig. 4E), frequently covering the surface of digesta (Fig. 4F), and occasionally in close proximity to the colonic epithelium.
Fig. 4.
Representative micrographs showing the localization of GFP-labeled (green) Peru-NT (A, C, and E) or Peru-NTΔflaABCDE (B, D, and F) in the distal small intestine (A–D) or midcolon (E and F) 3 days postinoculation of infant rabbits. Alexa Fluor 568-labeled phalloidin (red) was used to visualize the F-actin at the epithelial surface, and DAPI (blue) was used to detect cell nuclei. In F, Alexa Fluor 633-labeled wheat germ agglutinin (yellow) was used to detect mucin-covered digesta.
Peru-NT and Peru-NTΔflaABCDE Differ in Their Stimulation of Proinflammatory Cytokines.
Bacterial flagellin proteins, including all five V. cholerae flagellins, are known to stimulate production of proinflammatory cytokines by activation of TLR5 (10, 11, 18). We therefore compared the relative abundance of transcripts for several cytokines in tissue homogenates from rabbits infected with Peru-NT or Peru-NTΔflaABCDE and from mock-infected rabbits. We found that both strains led to elevations in transcripts for all cytokines measured compared with mock-infected rabbits (Fig. 5). However, IL-8 and IL-1β transcripts in tissue homogenates from the distal small intestines of rabbits infected with Peru-NTΔflaABCDE were ∼4-fold lower than in those from rabbits infected with Peru-NT (Fig. 5A). Similarly, there were significantly fewer TNFα and IL-1β transcripts in tissue samples from the midcolons of Peru-NTΔflaABCDE–infected rabbits than in Peru-NT–infected animals (Fig. 5B). Histologic examination of tissue sections from infected rabbits revealed that there were few to no heterophils (the rabbit equivalent of neutrophils) in samples from the small intestine; however, moderate numbers of heterophils were seen in the lamina propria, crossing the epithelium, and amid the digesta of the midcolon. There appeared to be more heterophils in colonic samples from Peru-NT–than in Peru-NTΔflaABCDE–infected rabbits, although this trend did not reach statistical significance.
Fig. 5.
Relative levels of transcripts for proinflammatory cytokines in homogenates from the small (A) and large (B) intestines of infant rabbits inoculated with Peru-NT (gray bars) or Peru-NTΔflaABCDE (black bars). Homogenates were obtained 3 days postinoculation. Transcript levels were determined by quantitative real-time PCR and normalized to GAPDH cDNA levels. The results are shown as log2 difference relative to the levels measured in samples from control rabbits inoculated with buffer. Stars indicate statistically significant (P < 0.05) different values in Peru-NT and Peru-NTΔflaABCDE samples.
Discussion
Live-attenuated V. cholerae vaccines have great promise for reducing the global burden of cholera, because a single oral dose often engenders long-lived protective immunity (5). However, development of such vaccines has been hampered by their reactogenicity. The molecular bases of reactogenicity are not known, but it has been speculated, based on the absence of reactogenic diarrhea associated with Peru-15, a nonflagellated V. cholerae ctxA mutant, that symptoms are a response to V. cholerae’s flagellum and/or the motility that it enables (7, 8). Here, we used an infant rabbit model of reactogenicity to define better the V. cholerae factors that contribute to this host response to earlier vaccine prototypes. We found that V. cholerae ctx mutants that produced flagellins induced diarrhea, regardless of whether the proteins were assembled into a flagellum or whether the flagellum was functional. In contrast, this response was absent in ∼90% of rabbits infected with V. cholerae lacking all five flagellin-encoding genes. Thus, our findings indicate that flagellin production, independent of motility or the presence of a flagellum, is sufficient for reactogenicity.
Previous studies have demonstrated that flagellins have proinflammatory effects that can contribute to diarrhea. Flagellins have been found to interact with TLR5 and to trigger MyD88- and NF-κB–dependent transcription of proinflammatory cytokines (19). Notably, all five V. cholerae flagellins, which can be secreted independently of flagellum filament assembly, contain the amino acid motif that stimulates TLR5, and purified V. cholerae flagellins were found to elicit TLR5-dependent IL-8 secretion from T84 cells (10). In addition, flagellins have been shown to activate the NLRC4 inflammasome, promoting IL-1β maturation and secretion (20–22). Release of IL-1β and other proinflammatory cytokines can induce diarrhea via several processes. For example, TNFα induces contraction of the actomyosin ring that controls tight junctions, resulting in diminished epithelial barrier function (23). In addition, IL-8 is chemotactic and promotes an influx of inflammatory cells. Neutrophil-derived 5′-AMP can lead to diarrhea by promoting chloride secretion (23). Collectively, these factors disrupt the equilibrium between the typical absorptive and secretory functions of the intestinal epithelium.
Consistent with the model outlined above, we observed flagellin-dependent increases in transcripts for several proinflammatory cytokines in tissue samples from infected rabbits. We found that transcripts for IL-8 and IL-1β were increased ∼8-fold in tissue samples from the small intestines of rabbits infected with Peru-NT, whereas their abundance in tissue from the small intestines of rabbits infected with Peru-NTΔflaABCDE differed by only ∼2-fold from those of mock-infected rabbits. We also detected statistically significant differences between the induction of TNFα and IL-1β in tissue samples from the large intestines of Peru-NT– and Peru-NTΔflaABCDE–infected rabbits. Our data are consistent with the hypothesis that flagellins are released in the rabbit intestine and induce synthesis of cytokines. However, if this process is dependent upon TLR5, it is unclear how the flagellins reach TLR5, which is found on the basolateral membrane of intestinal epithelial cells (24). It also is not currently known which site (small vs. large intestine) is the primary source of reactogenic diarrhea. Histologic analyses of the small intestine did not reveal an inflammatory response, whereas mild to moderate inflammation was detected within the large intestines both of Peru-NT– and Peru-NTΔflaABCDE–infected rabbits. The latter finding may reflect the elevation of IL-8 transcripts in this tissue in response to both bacterial strains. Collectively, these data suggest that an inflammatory infiltrate (i.e., heterophils) is not sufficient to induce diarrhea, although it may be a contributing factor. Perhaps the effects of such cells must be coupled with additional factors, such as the relative elevation of transcripts for cytokines that do not act as chemoattractants. Alternatively, it is possible that flagellins prompt reactogenicity through processes in addition to, or independent of, proinflammatory cytokines.
Our observations provide evidence against the idea that additional V. cholerae toxins, such as hemolysin A, MARTX toxin, or hemagglutinin/protease, are major contributors to reactogenicity. Fullner and colleagues (25) have proposed that these three toxins contribute to reactogenicity by promoting inflammation, primarily based on studies using a lung infection model. However, all these factors were intact in Peru-NTΔflaABCDE, which caused diarrhea in only 12% of rabbits. Furthermore, we found that 10 of 14 (71%) rabbits inoculated with a Peru-NT derivative in which hlyA, hap, and rtx were deleted still developed diarrhea, again suggesting that the toxins these genes encode are not the principal factors underlying reactogenicity. It is possible that these factors contribute to the diarrhea observed in the small minority of rabbits inoculated with Peru-NTΔflaABCDE.
Our findings also argue against the idea that intestinal colonization per se leads to diarrhea. No correlation was observed between the presence or absence of diarrhea in infected rabbits and the extent of colonization. In fact, there was no detectable difference between the number of cfu of Peru-NT and Peru-NTΔflaABCDE recovered from intestinal homogenates at 3 days postinfection. Furthermore, colonization of the large and small intestine by Peru-NT continued at a constant or even increasing level by day 6 postinoculation, even though diarrhea had ceased by this point. Future studies can explore the processes which account for the resolution of diarrhea despite continued bacterial presence within the intestine.
Our experiments unexpectedly revealed that the localizations of Peru-NT and Peru-NTΔflaABCDE within the intestine were indistinguishable. Both strains colonized throughout the small intestine, including deep within the crypts, and were found in close apposition to the intestinal epithelium. Thus, V. cholerae does not appear to depend on flagellar-based motility for spread within intestinal sites in this model. Flagella-independent motility has been observed for V. cholerae (26, 27), although the precise mechanism(s) underlying this process has not been identified.
Finally, our findings suggest a relatively straightforward approach to create genetically defined live-attenuated V. cholerae vaccine strains. Deletion of the two loci encoding the V. cholerae flagellins should render ctxA mutant strains nonreactogenic but not impair their ability to colonize the host. Furthermore, based on existing clinical data from trials of Peru-15, which does not produce flagellins, these potent activators of innate immunity are not required to generate protective immunity against V. cholerae (28). Nonetheless, the genetic plasticity of V. cholerae, as illustrated by the emergence of V. cholerae O139 in 1992 (29), will almost certainly require construction of new vaccine strains. We propose that deletion of the genes encoding flagellins be a standard part of the blueprint for creation of new live-attenuated V. cholerae vaccine strains and perhaps of live-attenuated vaccines protecting against other enteric pathogens as well.
Materials and Methods
Strains, Plasmids, and Culture Conditions.
The V. cholerae strains used in this study are all derivatives of the El Tor clinical isolate C6706 and are listed in Table S1. In addition to the genotypes noted within the text, all strains contained a chromosomal copy of gfp-mut3, under the control of the lac promoter, integrated within lacZ. Thus, these strains could be visualized with fluorescence microscopy. All bacterial strains were grown routinely in LB medium. For animal experiments, strains were grown overnight at 30 °C. Antibiotics were used at the following concentrations: streptomycin, 200 μg mL−1; spectinomycin, 50 μg mL−1; and carbenicillin, 50 μg mL−1.
The deletion mutants were constructed in C6706 ctxAB lacZ::gfp by allelic exchange using vectors based on pCVD442 as described (30). Details of the construction of the allele exchange vectors are available upon request. All mutants were confirmed by PCR analysis. Plasmid pJZ111 was used to introduce a gene encoding GFPmut3 into the V. cholerae lacZ locus. A derivative of the plasmid pSW25T, which is stably maintained in V. cholerae in vitro and in vivo without selection, was used to reintroduce the intact flaA gene under the control of its native promoter into Peru-NTΔflaABCDE.
Infant Rabbit Model.
Infant rabbit experiments were carried out as described in work to be published elsewhere. Briefly, 3-day-old infant rabbits were treated with cimetidine (50 mg kg−1 i.p.) 3 h before oro-gastric inoculation with V. cholerae strains. In all experiments, rabbits were inoculated with ∼1 × 109 cfu of V. cholerae suspended in sodium bicarbonate solution (2.5 g in 100 mL dH2O; pH, 9). The rabbits were monitored twice daily for signs of illness. Diarrhea was scored as follows: none, no fecal material evident on the perianal area, tail, or hind limbs; mild, light fecal staining of the perineum or hind legs or tail; severe, fecal material consisting of unformed or liquid stool staining large portions of the perineum, hind legs, and tail (Fig. 1 A and B). The rabbits usually were necropsied at 3 days postinoculation, and samples were collected for histologic and microscopic analyses and RNA extraction as well as for determining the numbers of V. cholerae cfu g−1 of tissue. Some rabbits were necropsied at 6 days postinoculation. The infant rabbits were housed together with the adult female. To limit litter-specific effects, at least two independent litters were used to test each mutant.
Confocal Microscopy.
Intestinal tissue was prepared for confocal microscopy as previously described. Briefly, tissue segments were fixed for 2 h in 4% paraformaldehyde (in PBS) on ice and then were transferred into 30% sucrose (in PBS) at 4 °C overnight. After washing in PBS, tissue segments were embedded in optimal cutting temperature (OCT) compound, quick-frozen, and sectioned. Slides were stained for 1 h with Alexa Fluor 568 phalloidin (1/50; A12380; Invitrogen) and/or Alexa Fluor 633 wheat germ agglutinin (1/200; W21404; Invitrogen) at room temperature in the dark. After washing, the slides were counterstained with DAPI, 1 μg mL−1, and examined using a Zeiss LSM 510 Meta upright confocal microscope.
RNA Isolation and Quantitative Real-Time PCR.
Quantitative real-time PCR (qPCR) assays were performed as previously described (31). Briefly, RNA was isolated from tissue sections homogenized in TRIzol (Invitrogen) and then treated with DNase I (Ambion) on RNeasy mini columns (QIAGEN). Specific DNA primers, which were designed using Primer Express 2 software (Applied Biosystems), were used for the reverse-transcription reactions (sequences are available on request). Each RT reaction mixture contained 5 μg RNA. SYBR Green PCR master mix and an ABI Prism 7000 (Applied Biosystems) were used to perform qPCR experiments. GAPDH was used as a control gene, and all gene transcript levels were normalized to GAPDH transcript levels using the ΔΔCT method as described (32).
Supplementary Material
Acknowledgments
We are grateful to Liza Shakhnovich, Jay Zhu, and Frederique LeRoux for strains and plasmids and to Linda McCarter for flagellin antisera. We thank Brigid Davis for critical review of the manuscript and Lay Hong Ang in the Harvard Digestive Disease Centre for help with confocal microscopy. Work in M.K.W.’s laboratory was supported with grants from the National Institute of Allergy and Infectious Diseases (NIAID) (R37-AI42347), the Howard Hughes Medical Institute, the Institute for One World Health, the Harvard Catalyst (National Institutes of Health Grant #UL1 RR 025758-02) and a fellowship from China Scholarship Council (to H.R). Work in J.J.M’s laboratory was supported by NIAID Grant R01-AI018045.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915164107/DCSupplemental.
References
- 1.World Health Organization Cholera Unveiled Global Task Force on Cholera Control. 2003. http://www.who.int/cholera/en.
- 2.World Health Organization Cholera vaccines. Wkly Epidemiol Rec. 2001;76:117–124. [PubMed] [Google Scholar]
- 3.Sánchez J, Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci. 2008;65:1347–1360. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekalanos JJ, et al. Cholera toxin genes: Nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 5.Ryan ET, Calderwood SB, Qadri F. Live attenuated oral cholera vaccines. Expert Rev Vaccines. 2006;5:483–494. doi: 10.1586/14760584.5.4.483. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DN, et al. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 7.Kenner JR, et al. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 8.Mekalanos JJ, Sadoff JC. Cholera vaccines: Fighting an ancient scourge. Science. 1994;265:1387–1389. doi: 10.1126/science.8073279. [DOI] [PubMed] [Google Scholar]
- 9.Sears CL, Kaper JB. Enteric bacterial toxins: Mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison LM, et al. Vibrio cholerae flagellins induce Toll-like receptor 5-mediated interleukin-8 production through mitogen-activated protein kinase and NF-kappaB activation. Infect Immun. 2008;76:5524–5534. doi: 10.1128/IAI.00843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xicohtencatl-Cortés J, et al. Identification of proinflammatory flagellin proteins in supernatants of Vibrio cholerae O1 by proteomics analysis. Mol Cell Proteomics. 2006;5:2374–2383. doi: 10.1074/mcp.M600228-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Silva TM, et al. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadri F, et al. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut. 2004;53:62–69. doi: 10.1136/gut.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziejman M, et al. Comparative genomic analysis of Vibrio cholerae: Genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci USA. 2002;99:1556–1561. doi: 10.1073/pnas.042667999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed KA, et al. The Vibrio cholerae flagellar regulatory hierarchy controls expression of virulence factors. J Bacteriol. 2009;191:6555–6570. doi: 10.1128/JB.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klose KE, Mekalanos JJ. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardel CL, Mekalanos JJ. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64:2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SS, Mekalanos JJ. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect Immun. 2008;76:1282–1288. doi: 10.1128/IAI.00736-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: Making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molofsky AB, et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: Dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan VK, Hodges K, Hecht G. Enteric infection meets intestinal function: How bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119. doi: 10.1038/nrmicro2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee SH, et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–13615. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fullner KJ, et al. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J Exp Med. 2002;195:1455–1462. doi: 10.1084/jem.20020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown II, Häse CC. Flagellum-independent surface migration of Vibrio cholerae and Escherichia coli. J Bacteriol. 2001;183:3784–3790. doi: 10.1128/JB.183.12.3784-3790.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, et al. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci USA. 2008;105:9769–9774. doi: 10.1073/pnas.0802241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury MI, Sheikh A, Qadri F. Development of Peru-15 (CholeraGarde), a live-attenuated oral cholera vaccine: 1991-2009. Expert Rev Vaccines. 2009;8:1643–1652. doi: 10.1586/erv.09.137. [DOI] [PubMed] [Google Scholar]
- 29.Ramamurthy T, et al. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 30.Davis BM, Waldor MK. RNase E-dependent processing stabilizes MicX, a Vibrio cholerae sRNA. Mol Microbiol. 2007;65:373–385. doi: 10.1111/j.1365-2958.2007.05796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinones M, Davis BM, Waldor MK. Activation of the Vibrio cholerae SOS response is not required for intestinal cholera toxin production or colonization. Infect Immun. 2006;74:927–930. doi: 10.1128/IAI.74.2.927-930.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Scott ME, Dossani ZY, Sandkvist M. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc Natl Acad Sci USA. 2001;98:13978–13983. doi: 10.1073/pnas.241411198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.