Abstract
Catumaxomab, a monoclonal bispecific trifunctional antibody, was approved in the european Union in April 2009 for the intraperitoneal treatment of patients with malignant ascites. The marketing authorization holder Fresenius Biotech GmbH developed catumaxomab (Removab®) together with its partner TRiOn Pharma GmbH, Germany. it is the first substance worldwide with a regulatory label for the treatment of malignant ascites due to epithelial carcinomas. Since the peritoneum is of mesothelial origin and therefore lacks epCAM expression, the intraperitoneal administration of catumaxomab is an attractive targeted immunotherapeutic approach. Catumaxomab is able to destroy epCAM positive tumor cells in the peritoneal cavity known as the main cause of malignant ascites. in addition, catumaxomab is a potential therapeutic option for several primary tumors since the epCAM molecule is expressed on the majority of epithelial carcinomas. This review focuses on the clinical development of catumaxomab and indicates future directions.
Key words: catumaxomab, Removab®, monoclonal antibody, trifunctional, EpCAM, malignant ascites, peritoneal carcinomatosis, immunotherapy
Introduction
Catumaxomab-targeted immunotherapy
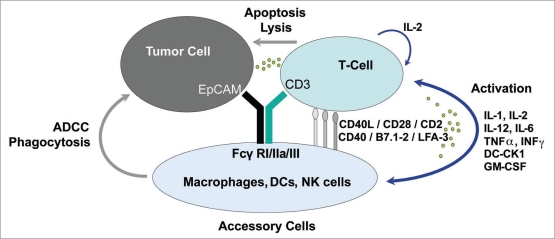
The bispecific (anti-EpCAM x anti-CD3) trifunctional antibody catumaxomab combines the characteristics of classical monoclonal antibodies and bispecific molecules. It is produced via quadroma technology and consists of mouse IgG2a and rat IgG2b.1 One specific antigenbinding site binds T cells via CD3, the other site binds tumor cells via the EpCAM antigen. The Fc region provides a third functional binding site that is able to selectively bind and activate Fcγ receptor I-, IIa- or III-positive accessory cells. Catumaxomab does not bind to inhibitory Fcγ receptor type IIb accessory cells, which may reduce the activation of certain classes of immune cells. The interaction of different immune effector cells at the tumor site results in a complex immune reaction leading to the elimination of tumor cells. In preclinical studies several killing mechanisms including T cell mediated lysis, cytotoxicity by released cytokines (e.g., IL1β, IL-2, IL-6, IL-12 or DC-CK1), phagocytosis or ADCC were identified. In addition, control tests with two parental antibodies simultaneously showed a much lower antitumor capacity compared with the bispecific antibodies.2,3 This result could be confirmed in a mouse tumor model with a surrogate bispecific trifunctional antibody. Furthermore, within the mouse model a long-lasting antitumor immunity was shown.4 Overall, catumaxomab enhances the activation of the patient’s own immune system against the tumor. The postulated mechanism of action is shown in Figure 1.
Figure 1.
The postulated mechanism of action of catumaxomab: The intact trifunctional antibody catumaxomab accelerates the recognition and destruction of tumor cells by different immune cells. ADCC, antibody-dependent cellular toxicity; DC-CK1, dendritic cell cytokine 1; iL, interleukin; IFNγ, interferon gamma; TnFα, tumor necrosis factor alpha; LFA, lymphocyte function antigen; nK, natural killer; GM-CSF, granulocyte monocyte colony stimulating factor.
The human epithelial cell adhesion molecule (EpCAM) is a type I transmembrane glycoprotein. It consists of two epidermal growth factor-like domains, one cysteine-poor region, one transmembrane domain and one short cytoplasmic tail. In normal tissues, EpCAM is only expressed baso-laterally and is shielded by tight junctions that limit its accessibility. In contrast, in tumor cells EpCAM is expressed on the whole cell surface, and therefore becomes more easily available for binding.5 EpCAM is one of the most frequently and most intensely expressed tumor-associated antigens, e.g., in ovarian, gastric, colon, pancreatic, prostate, lung and endometrial carcinoma.6,7 Thus EpCAM is an attractive target for antibody therapy of carcinomas of various origins, as evidenced also by the evaluation of several other EpCAM targeted antibodies in clinical development.8
EpCAM is expressed on the vast majority of the main epithelial cancers that cause malignant ascites, and is also expressed on tumor cells in the majority of malignant effusion due to these cancer types. Due to the fact that the inner layer of the peritoneal cavity is of mesothelial origin and thus lacking EpCAM expression, catumaxomab represents a targeted therapy in this indication and seemed to be a good model to prove the principle of the mechanism of action. In addition, all immune cells necessary for the mode of action are present in the peritoneal fluid.9
Malignant ascites is the accumulation of peritoneal fluid due to the spread of malignant cells in the peritoneal cavity. It is a typical late-stage manifestation of cancer associated with a poor prognosis. Common symptoms are increase of abdominal girth, abdominal pain, anorexia, nausea and vomiting.10 At present, there exist no evidence-based guidelines for the evaluation and treatment of malignant ascites. Repeated paracentesis often plays an important palliative role in patients in whom chemotherapy fails. Rapid re-accumulation of fluid leads to relatively short duration of symptom improvement and necessitates frequent drainage procedures. In April 2009 catumaxomab (Removab®) was approved in the European Union for the intraperitoneal treatment of malignant ascites in patients with EpCAM-positive carcinomas where standard therapy is not available or no longer feasible.
Clinical Studies
The clinical development of catumaxomab via intraperitoneal application comprises the indications malignant ascites, peritoneal carcinomatosis, ovarian cancer, and gastric cancer. Clinical studies with patients suffering from malignant ascites included one dose finding study,11 a PK/PD study12 as well as a pivotal Phase 2/3 trial.13 A Phase 1 study was conducted in patients with peritoneal carcinomatosis due to gastrointestinal cancers.14 Several Phase 2 trials were conducted in minimal residual disease setting with focus on gastric- and ovarian cancer. In addition, further routes of applications including intrapleural and intravenous were tested: Intrapleural infusion was clinically investigated in a Phase 1 study in patients with malignant pleural effusion.15 Catumaxomab was also administered intravenously during a Phase 1 study in patients with non-small cell lung cancer.16
An overview of the clinical development program (completed, ongoing and planned) is given in Table 1.
Table 1.
Clinical studies with catumaxomab
| Completed | |||
| Indication | Reference | Phase | No. of catumaxomab treated patients |
| Malignant ascites due to ovarian cancer | Ref. 11 (Burges et al. 2007) | 1/2 | 23 |
| Malignant ascites due to epithelial tumours (PK/PD study) | Ref. 12 (Ruf et al. 2008) | 2 | 13 |
| Malignant ascites due to epithelial tumours (pivotal study) | NCT00836654; Ref. 13 (Parsons et al. 2008) | 2/3 | 157 |
| Platinum refractory epithelial ovarian cancer (i.p. application) | AGO-Ovar 2.10, nCT00189345; Ref. 24 (Belau et al. 2007) | 2a | 45 |
| Peritoneal carcinomatosis due to epithelial gastrointestinal malignancies (i.p. application) | Ref. 14 (Ströhlein et al. 2009) | 1 | 24 |
| Intra-abdominal epithelial tumours (i.o. and i.p. application) | Ref. 27 (Ströhlein et al. 2008) | 1 | 12 |
| Malignant pleural effusion (i.pl. application) | Ref. 15 (Sebastian et al. 2009) | 1/2 | 24 |
| Non-small cell lung cancer (i.v. application) | Ref. 16 (Sebastian et al. 2007) | 1 | 21 |
| Ongoing | |||
| Gastric cancer (i.o. and i.p. application) | NCT00352833; Ref. 30 (Heiss et al. 2008) | 2 | 28 |
| Gastric cancer (i.o. and i.p. application after neoadjuvant CTx) | NCT00464893 | 2 | 54 |
| Ovarian cancer (consolidation, i.p. application) | NCT00377429 | 2 | 47 |
| Ovarian cancer (i.o. and i.p. application) | NCT00563836 | 2 | 41 |
| Malignant ascites due to ovarian cancer (i.p. application) | NCT00326885 | 2 | 35 (planned) |
| CASIMAS, malignant ascites due to epithelial carcinomas (i.p. application) | NCT00822809 | 3 | 156 (planned) |
| SECIMAS, malignant ascites due to epithelial carcinomas (i.p. application) | 2 | 30 (planned) | |
| Optimal interval between CTx and catumaxomab | in vitro | 30 (planned) | |
| Under discussion | |||
| Peritoneal carcinomatosis due to metastatic gastric adenocarcinoma | 3 | ||
Malignant ascites.
A first Phase 1/2 study was conducted in patients suffering from malignant ascites due to ovarian cancer.11 Twenty-three patients were treated with different dosages of catumaxomab after a premedication of 1,000 mg paracetamol. The maximum tolerated dose was determined for every single dose resulting in a final escalating scheme of 10, 20, 50, 200 and 200 µg catumaxomab. A total of 22 patients did not require paracentesis between the last infusion and the end of study at day 37. By the shift to an increase of leukocyte/tumor cell ratio in the ascites fluid it could be shown that the tumor cell elimination was caused by the proposed mechanism of action of catumaxomab and not simply by serial drainages of ascites.
The side effects with fever, nausea, vomiting and abdominal pain are due to cytokine release. The proportion of patients with TNFα and IL-6 values above the upper limit of normal increased to 60 and 80%, respectively. The increased levels could be measured on the days after catumaxomab infusion and decreased to almost baseline values before the next infusion.
Overall, the cytokine release is not only a side effect of trifunctional antibodies but rather a part of the mode of action. The cytokine profile indicates T helper cell type 1 activation.17
The promising results were the basis for the strategy of conducting a Phase 2/3 study with malignant ascites patients caused by different epithelial carcinomas in parallel with a PK/PD study.12,13
Patients with recurrent symptomatic malignant ascites were randomized 2:1 to catumaxomab plus paracentesis or paracentesis alone and according to underlying disease stratified for ovarian cancer or non-ovarian cancer group. Catumaxomab was administered as four 6-hour i.p. infusions on days 0, 3, 7 and 10 at doses of 10, 20, 50 and 150 µg, respectively. The primary endpoint, with focus on the patient, was puncture-free survival defined as time to next therapeutic puncture or time to death, whichever occurred first. Secondary endpoints included time to next therapeutic puncture, overall survival, as well as safety, tolerability and ascites signs and symptoms, and measurements of anti-drug antibodies. Patients enrolled in the study had symptomatic ascites with a volume of >1 liter, EpCAM-positive tumor cells in the ascites fluid, at least one previous puncture within 5 weeks before screening, and were either resistant to chemotherapy or chemotherapy was no longer feasible.
Overall, 258 patients were randomized. The demographics of the patients were equally matched in both study arms with regard to age, performance status, number of previous treatments and paracentesis, as well as time to last therapeutic puncture. For both strata these demographic characteristics were identical. In median, patients in the ovarian cancer stratum had three courses of chemotherapy prior to entering into this study. Patients in the non-ovarian cancer stratum in median had a single chemotherapy. The main underlying tumors in the non-ovarian cancer stratum were gastric carcinoma followed by breast carcinoma, pancreatic carcinoma, and colon carcinoma.
Data for the primary endpoint, median puncture-free survival, indicated clinically relevant prolongation for the pooled population of 46 days for catumaxomab versus 11 days for control (p < 0.0001). The benefit was confirmed in patients with malignant ascites independent of the primary tumor or other prognostic factors like metastasis or age (see Table 2).18 Median puncture free time was 77 versus 13 days (p < 0.0001). This suggests that catumaxomab-treated patients avoided approximately five therapeutic paracentesis procedures. Although the study was neither powered nor designed for overall survival, e.g., cross-over was allowed, a positive trend was seen.
Table 2.
Analysis of puncture-free survival in different subgroups (pivotal trial)
| Catumaxomab (n = 170) | Control (n = 88) | ||||
| n | Median PunFS (days) | n | Median PunFS (days) | ||
| Primary tumour | Ovarian-cancer | 85 | 52 | 44 | 11 |
| All non-ovarian cancer | 85 | 37 | 44 | 14 | |
| Gastric cancer | 46 | 44 | 20 | 15 | |
| Distant metastasis | No | 70 | 48 | 32 | 11 |
| Yes | 99 | 44 | 56 | 13 | |
| Liver metastasis | No | 133 | 49 | 68 | 14 |
| Yes | 36 | 27 | 20 | 9 | |
| Age range | >median | 84 | 44 | 44 | 11 |
| ≤median | 86 | 48 | 44 | 13 | |
| Total serum protein | >median | 81 | 55 | 43 | 13 |
| ≤median | 88 | 31 | 44 | 11 | |
Most of the patients (51%) in the non-ovarian cancer stratum were gastric cancer patients. For this reason, this subgroup was evaluated separately in a predefined way. Analysis of the primary endpoint, as well as main secondary endpoints like time to next puncture and overall survival, indicated a significant benefit to patients (see Table 3).19
Table 3.
Analysis of the gastric-cancer subgroup (pivotal trial)
| Gastric-cancer subgroup (n = 66) | Puncture free survival | Time to next therapeutic puncture | Overall survival |
| Catumaxomab group (n = 46) | 44 days | 118 days | 71 days |
| Control group (n = 20) | 15 days | 15 days | 44 days |
| p value | <0.0001 | <0.0001 | 0.0313 |
| Hazard ratio (95% CI) | 0.289 (0.151; 0.554) | 0.143 (0.057; 0.359) | 0.469 (0.232; 0.951) |
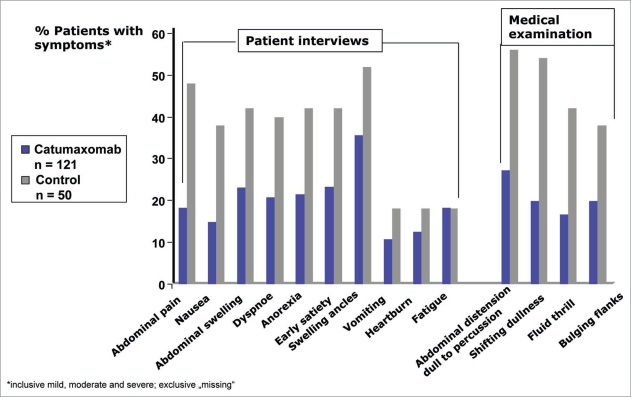
Another important secondary endpoint was the assessment of ascites symptoms through use of a patient questionnaire with a 4-point Likert scale of none, mild, moderate, and severe, for the following symptoms: anorexia, nausea, early satiety, vomiting, abdominal pain, abdominal swelling, dyspnea, fatigue, swelling of ankles and heartburn. Assessment of the following signs was performed after abdominal examination by the investigator: abdominal distension—dullness to percussion, shifting dullness, fluid thrill and bulging flanks. Analysis of ascites signs and symptoms 8 days after last catumaxomab infusion or after Day 0 (control) showed fewer patients with ascites signs and symptoms in the catumaxomab group compared to control. In 6 out of 10 symptom categories (anorexia, nausea, early satiety, abdominal pain, abdominal swelling, dyspnea) and in all 4 sign categories (abdominal distension-dull to percussion, shifting dullness, fluid thrill and bulging flanks) the differences were confirmed with p values <0.05 (see Fig. 2).
Figure 2.
Ascites symptoms: The benefit of the catumaxomab therapy was also confirmed in the assessment of ascites symptoms.
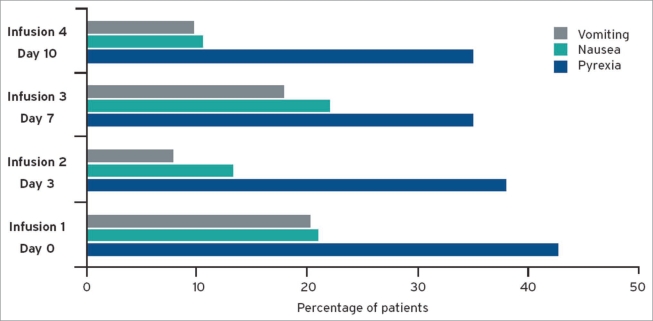
As the release of pro-inflammatory and cytotoxic cytokines is initiated by the binding of catumaxomab to immune and tumor cells, cytokine-release-related symptoms are one of the most common adverse events. The incidences were similar in the ovarian and non-ovarian cancer group. Most events like pyrexia, nausea and vomiting were manageable with standard symptomatic treatment; they were limited to the duration of catumaxomab treatment, with a median onset of one day after administration and a median duration of 1–2 days. A positive trend between cytokine-release-related symptoms and clinical outcome was seen. The majority of patients (83%) received all four i.p. infusions; the incidence of the symptoms did not increase despite up to 15 times ascending dosages of catumaxomab (see Fig. 3).20 The frequent occurrence of abdominal pain in the catumaxomab patients was considered at least partially as a symptom of peritoneal irritation following i.p. administration, which was in general manageable by standard pain medication. Transient increases in liver enzymes and bilirubin, as well as transient decreases of lymphocytes, were rarely considered clinically significant. Grade 4 adverse events were isolated cases and mostly related to progression of the underlying malignant disease, such as ileus. None of the deaths in the randomized part of the study were considered to be related to catumaxomab treatment.
Figure 3.
Incidence of pyrexia, nausea and vomiting: The expected and manageable cytokine release related symptoms did not increase during treatment.
Human anti-mouse antibody/human anti-rat antibody (HAMA/HARA) was observed in the minority of patients (<10%) before the 4th infusion and the majority of patients (>70%) after the last infusion. There were no changes in safety patterns reported for these HAMA/HARA positive patients in comparison to negative patients after receiving the last infusion. On the other hand, HAMA/HARA positive patients seemed to have an improved clinical outcome possibly due to their good physical condition and fit immune system.
Two additional analyses support the positive efficacy results. Besides the obstruction of lymphatic drainage via tumor cells, secretion of factors like vascular endothelial growth factor (VEGF) leads to the fluid accumulation in malignant ascites, e.g., the release of VEGF leads to tumor neovascularization associated with higher plasma efflux, as well as an increased permeability of the tumor capillaries. Therefore, the VEGF levels in 47 patients of the pivotal trial were analyzed. One day after the last catumaxomab infusion, 46 out of 47 patients showed a significantly reduced VEGF protein to total protein level compared to the measurement before catumaxomab treatment. The results can be correlated with tumor cell elimination in the malignant ascites fluid.21
In addition, EpCAM is known to be expressed very often on cancer stem cells.22 Therefore, further analysis were done regarding CD133+/EpCAM+ putative cancer stem cells in malignant ascites samples of 14 patients. After four i.p. catumaxomab infusions, the CD133+/EpCAM+ cells were completely eliminated from the peritoneal fluids of all 14 malignant ascites patients.23
In the corresponding pharmacokinetic trial with 13 patients, the mean elimination half life of catumaxomab was approximately 2 days. As expected for a locoregional application the highest concentration occurred in the ascites fluid, whereas the systemic exposure was low (<5%).12
Ovarian cancer.
A variety of indications in ovarian cancer were addressed in Phase 2 trials for catumaxomab, including peri-operative first line therapy, consolidation after first line standard therapy and platinum-refractory ovarian cancer.
Two different dose levels of catumaxomab were tested in a Phase 2 study (AGO-Ovar-2.10).24 A total of forty-five patients with platinum-refractory epithelial ovarian cancer were included.
Malignant ascites was not an inclusion criterion, but was permitted. The escalating dose scheme of catumaxomab (10, 20, 50, 100 µg) was safe and associated with a higher therapeutic index than the constant low dose scheme of four doses of 10 µg catumaxomab.
Safety data for these patients, as well as ovarian cancer patients who participated in other studies, will be available in the near future.
Peritoneal carcinomatosis and gastrointestinal malignancies.
A Phase 1 study of patients with peritoneal carcinomatosis (PC) due to epithelial gastrointestinal malignancies was conducted.14 PC, the preliminary stage of malignant ascites, is also associated with a poor outcome and deteriorating quality of life. Systemic chemotherapy has shown only unsatisfactory results.25 Twentyfour patients were treated with different dosages of catumaxomab resulted in an escalating dose scheme with maximum tolerated doses of 10, 20, 50, and 200 µg catumaxomab.
The trial showed that the i.p. application of catumaxomab in patients with PC is safe and technically feasible, and the results suggested that catumaxomab may represent a new concept for treatment of PC due to gastrointestinal malignancies. This conclusion can be supported by first efficacy hints such as
Eleven out of 24 patients were progression free one month after the start of treatment;
Seventeen patients were evaluable for RECIST: one CR, 3 PR and 7 SD;
Peritoneal lavage samples (baseline and at discharge) were obtained from ten patients: no EpCAM-positive tumor cells were detectable at either examination in one patient, the number decreased in six patients and increased slightly in three patients;
Median OS from the start of treatment was 273 days, the median OS from first diagnosis of PC was 502 days.
Peritoneal dissemination of tumor cells can also occur during surgical resection and lead to the development of peritoneal carcinomatosis.26 Therefore, another Phase 1 trial was conducted in the minimal residual disease situation involving treatment of patients with gastrointestinal tumors directly after abdominal surgery, with the objective of eliminating free peritoneal tumor cells.27 This was the first use of an intra-operative immunotherapy.
A total of 12 patients (8 gastric, 3 pancreatic, 1 colon cancer) were treated with one intra-operative (5, 10 or 20 µg) and four subsequent i.p. dosages of catumaxomab. No DLTs were observed up to the dose schedule of 20 µg intra-operative catumaxomab on day 0, followed by 10, 20, 50, 150 µg i.p. catumaxomab on day 7, 10, 13 and 16; this schedule can thus be regarded as safe and clinically feasible. The vast majority of observed side effects could be attributed to well-known cytokine release related symptoms and common postoperative reactions after major gastrointestinal surgery. No anastomotic leakage was diagnosed. The reported adverse events were generally mild to moderate in intensity and were mainly fully reversible. No patients had local intraperitoneal tumor recurrence or peritoneal carcinomatosis during follow up, so intraoperative catumaxomab may offer new opportunities for prevention of PC in gastrointestinal tract cancer.
Alternative Application Routes
Intrapleural application: malignant pleural effusion
The pleural cavity is another EpCAM negative region where spreading EpCAM positive tumor cells should be effectively destroyed by catumaxomab. Therefore, a Phase 1/2 trial was conducted in patients suffering from malignant pleural effusions due to lung or breast cancer. Overall, 24 patients were treated intrapleural with different dosages of catumaxomab. The maximum tolerated dose was defined by three escalating applications of 10, 20 and 50 µg, respectively and classified as feasible. Side effects were as expected by the mode of action.
The data obtained from pleural cells showing catumaxomab-induced proliferation of T cells and accessory cells and TH1-directed cytokine secretion. However, due to the high rate of screening failures based on rapid tumor progression and different drug tolerance (possibly due to heterogeneity of the patient population), further investigation is necessary.15
Intravenous administration: non-small cell lung cancer.
A Phase 1/2 study was conducted in patients suffering from nonsmall cell lung cancer where EpCAM is also overexpressed on the surface of tumor cells. This was the first attempt to administer catumaxomab intravenously. Overall, 21 patients in various stages of their disease were treated with different escalating doses of 2, 5 or 7.5 µg catumaxomab as a single i.v. infusion together with several doses of dexamethasone. The DLT was determined at 5 µg catumaxomab with 40 mg dexamethasone as premedication.16 Intravenous application of catumaxomab appears feasible, although further knowledge regarding PK/PD and safety profile is necessary. This trial is a basis for potential further evaluations in solid tumors. Further steps towards alternative administration routes are currently under consideration.
Ongoing Trials and Future Directions
Malignant ascites and peritoneal carcinomatosis.
An important step in the further development of i.p. catumaxomab is the reduction of the infusion time to 3 hours, the investigation of a repeated treatment cycle, as well as the management of side effects by corticosteroid co-medication. These questions will be addressed by the CASIMAS and SECIMAS studies.
The CASIMAS study (NCT00822809) is an on-going Phase 3b trial of catumaxomab in the approved indication malignant ascites. In order to make the treatment more convenient for patients, the tolerability of 3 hour infusions of catumaxomab instead of 6 hour is being evaluated. In addition, patients were randomized to receive a premedication of paracetamol and prednisolone versus paracetamol alone.
Prednisolone was chosen as additional premedication with the objective of reducing the cytokine release related symptoms without affecting the efficacy of catumaxomab as shown in vitro. The primary endpoint is a composite safety score calculated from the frequency and intensity of the main known AEs caused by catumaxomab, and secondary outcomes are efficacy measurements.
The feasibility of a repeated treatment cycle will be investigated in the SECIMAS study, which is designed as a follow up study of CASIMAS. This is a Phase 2 study in patients with malignant ascites requiring their first therapeutic puncture after treatment in the CASIMAS study. Patients will receive a second i.p. cycle of 10, 20, 50, 150 µg of catumaxomab. The development of antidrug-antibodies (ADAs) is typically observed 12 to 21 days after first exposure of a patient to trifunctional antibodies. Data from the pivotal study suggest that the safety of i.p. treatment with catumaxomab may not be compromised by the systemic occurrence of ADA. In addition, ADA responses were detected an average of 16 days earlier in plasma than in ascites fluid. Therefore, it is assumed that patients benefit from this extended treatment with catumaxomab.
Another open question is the effect of chemotherapy on catumaxomab. Chemotherapies are known for their influence on the quantity and function of peripheral T cells that are important for the mode of action of catumaxomab. Nevertheless, several recent studies have produced unexpected results indicating that there is a strong and developing case for combining chemotherapy and immunotherapy in cancer treatment.27 One proposed explanation is that tumor cell death due to chemotherapy yields tumor-antigen cross-presentation and enforces the enhancement of immunotherapy response.28 First experiments with co-cultures in vitro, e.g., catumaxomab + 5-FU or catumaxomab + cisplatin, have shown synergistic reactions on human gastric carcinoma cells when applied in a sequential combination. Therefore, an in vitro analysis will be conducted with peripheral blood cells of patients suffering from advanced gastric- or colon cancer. Blood samples will be analyzed during and after chemotherapy treatment. Leukocytes will be cultivated in presence of tumor cells and different concentrations of catumaxomab. The survival of tumor cells and T cells will be defined for analysis. Further studies investigating the combinability of chemotherapy and catumaxomab are currently under consideration.
Based on the promising Phase 1 trial in peritoneal carcinomatosis patients and the positive results of the gastric cancer subgroup in the pivotal malignant ascites trial, further development in PC is considered.
Gastric cancer.
Two Phase 2 studies were conducted with operable patients suffering from gastric cancer. During the first study, 55 patients were randomized to receive directly after surgery intra-operative 10 µg catumaxomab, followed by four ascending i.p. dosages or surgery alone.29 A total of 78% of the patients treated with catumaxomab received all five infusions. There were no clinically relevant differences in the incidence of surgically relevant complications between the surgery alone and the surgery plus catumaxomab group (see Table 4).
Table 4.
Surgically relevant complication in the gastric cancer study (minimal residual disease)
| Complication | Surgery alone n (%) | Surgery + catumaxomab n (%) |
| Anastomosis insufficiency | 2 (7.4) | 1 (3.7) |
| Wound healing disorders | 2 (7.4) | 3 (10.7) |
| Infections | 3 (11.1) | 4 (14.3) |
| Abdominal abscess | 1 (3.7) | 0 |
In the second study, 54 patients were treated intra-operatively and i.p. with catumaxomab after they received neoadjuvant chemotherapy. Final results are pending.
Ovarian cancer.
The efficacy of catumaxomab was investigated in two Phase 2 studies of ovarian cancer patients.
Analogously to the gastric cancer study, 41 ovarian cancer patients were treated peri-operatively with catumaxomab in a Phase 2 trial. The second study included 47 patients in the consolidation setting. After a complete response to platinum and taxane-based standard chemotherapy after optimal or suboptimal cytoreductive surgery, patients received four doses i.p. catumaxomab (10, 20, 50, 150 µg, respectively). Final study results of both studies are in preparation.
Conclusion
Catumaxomab is the first bispecific trifunctional antibody to receive marketing approval. The pivotal trial in patients with malignant ascites showed promising results that have to be transferred in daily patient care. Further trial concepts such as CASIMAS or SECIMAS will help to further optimize the use of catumaxomab in malignant ascites. Other subsequent development options are linked to earlier use of catumaxomab within the treatment algorithm for solid tumors to exploit the full potential of its mechanism of action. For further development, studies in peritoneal carcinomatosis, combination with chemotherapeutic regimens and alternative administration routes are under discussion.
Declaration of Interest
The authors are employees of Fresenius, which markets catumaxomab.
Abbreviations
- ADA
anti-drug antibody
- ADCC
antibody dependent cellular cytotoxicity
- AE
adverse event
- CR
complete response
- CTx
chemotherapy
- DLT
dose limited toxicity
- EpCAM
epithelial cell adhesion molecule
- Fc
fragment crystallisable
- Fcγ
Fc gamma-receptor
- HAMA
human anti-mouse antibody
- HARA
human anti-rat antibody
- IgG
immunglobulin G
- i.o.
intraoperative
- i.p.
intraperitoneal
- i.pl.
intrapleural
- n
number of patients
- OS
overall survival
- PK/PD
pharmacokinetic/pharmacodynamic
- PR
partial response
- PunFS
puncture-free survival
- RECIST
response evaluation criteria in solid tumors
- SD
stable disease
- VEGF
vascular endothelial growth factor
- 5-FU
5-fluorouracil
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/11221
References
- 1.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J Immunol. 1995;155:215–219. [PubMed] [Google Scholar]
- 2.Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, et al. Simultaneous Activation of T Cells and Accessory Cells by a New Class of Intact Bispecific Antibody Results in Efficient Tumor Cell Killing. J Immunol. 1999;163:1246–1252. [PubMed] [Google Scholar]
- 3.Riesenberg R, Buchner A, Pohla H, Lindhofer H. Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (αEpCAM × αCD3) J Histochem Cytochem. 2001;49:911–917. doi: 10.1177/002215540104900711. [DOI] [PubMed] [Google Scholar]
- 4.Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–2534. doi: 10.1182/blood.v98.8.2526. [DOI] [PubMed] [Google Scholar]
- 5.Litvinov SV, Bakker HAM, Gourevitch MM, Velders M, Warnaar SO. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epthelial cell-cell adhesion. Cell Adhes Commun. 1994;2:417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- 6.Went P, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCAM protein expression in human carcinomas. Hum Pathol. 2004;35:122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, et al. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128–135. doi: 10.1038/sj.bjc.6602924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichert JM, Valge-Archer V. Development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6:349–356. doi: 10.1038/nrd2241. [DOI] [PubMed] [Google Scholar]
- 9.Kubicka U, Olszewski WL, Tarnowski W, Bielecki K, Ziolkowska A, Wierzbicki Z. Normal human immune peritoneal cells: subpopulations and functional characteristics. Scand J Immunol. 1996;44:157–163. doi: 10.1046/j.1365-3083.1996.d01-297.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. doi: 10.1093/annonc/mdl499. [DOI] [PubMed] [Google Scholar]
- 11.Burges A, Wimberger P, Kümper C, Gorbounova V, Sommer H, Schmalfeldt B, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a Phase I/II Study. Clin Cancer Res. 2007;13:3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 12.Ruf P, Jäger M, Volovat C, Burges A, Heiss MM, Wimberger P, et al. Pharmacokinetics and in vivo stability of intraperitoneally administered therapeutic antibody catumaxomab. J Clin Oncol. 2008:26. doi: 10.1111/j.1365-2125.2010.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons S, Murawa PX, Koralewski P, Kutarska E, Kolesnik OO, Ströhlein MA, et al. Intraperitoneal treatment of malignant ascites due to epithelial tumors with catumaxomab: A phase II/III study. J Clin Oncol. 2008:26. [Google Scholar]
- 14.Ströhlein M, Lordick F, Rüttinger D, Schemanski OC, Jäger M, Lindhofer H, et al. Peritoneal carcinomatosis immunotherapy with the trifunctional anti-EpCAM x anti-CD3 antibody catumaxomab in patients with colon-, gastric- or pancreatic cancer: long-term results after a 2-year follow-up. J Clin Oncol. 2009;27:15. [Google Scholar]
- 15.Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, et al. Treatment of malignant pleural effusion with the trifuctional antibody catumaxomab (Removab) (anti-EpCAM x anti-CD3) J Immunother. 2009;32:195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 16.Sebastian M, Passlick B, Friccius-Quecke H, Jäger M, Lindhofer H, Kanniess F, et al. Treatment of non-small cell lung cancer patiets with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study. Cancer Immunol Immunother. 2007;56:1637–1644. doi: 10.1007/s00262-007-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiewe P, Hasmüller S, Kahlert S, Heinrigs M, Rack B, Marmé A, et al. Phase I Trial of the trifunctional anti-HER 2 x anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res. 2006;12:3085–3091. doi: 10.1158/1078-0432.CCR-05-2436. [DOI] [PubMed] [Google Scholar]
- 18.Heiss MM, Linke R, Schulze E, Friccius-Quecke H, Gamperl H, Lindhofer H, et al. Catumaxomab—a new treatment option for patients with malignant ascites. Ann Oncol. 2009:20. [Google Scholar]
- 19.Heiss MM, Linke R, Friccius-Quecke H, Klein A, Hennig M, Lindhofer H, et al. Catumaxomab treatment in gastric-cancer patients with malignant ascites—subgroup-analysis of a pivotal trial. Eur J Canc Suppl. 2009;7:363. [Google Scholar]
- 20.Bokemeyer C, Heiss MM, Gamperl H-J, Linke R, Schulze E, Friccius-Quecke H, et al. Safety of catumaxomab: cytokine-release-related symptoms as a possible predictive factor for efficacy in a pivotal phase II/III trial in malignant ascites. J Clin Oncol. 2009;27:15. [Google Scholar]
- 21.Jäger M, Schoberth A, Theissen B, Hess J, Friccius H, Lindhofer H. Decrease of VEGF within malignant ascites during catumaxomab treatment: results from a pivotal phase II/III study. J Clin Oncol. 2009;27:15. [Google Scholar]
- 22.Munz M, Baeuerle PA, Gires O. The Emerging Role of EpCAM in Cancer and Stem Cell Signaling. Cancer Res. 2009;69:5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 23.Lindhofer H, Schoberth A, Pelster D, Hess J, Herold J, Jäger M. Elimination of cancer stem cells (CD133+/EpCAM+) from malignant ascites by the trifunctional antibody catumaxomab: results from a pivotal Phase II/ III study. J Clin Oncol. 2009:15. [Google Scholar]
- 24.Belau A, Pfisterer J, Wimberger P, Kurzeder C, Du Bois A, Sehouli J, et al. Randomized, multicenter, two-dose level, open-label, phase Iia study with the intraperitoneally infused trifunctional bispecific antibody catumaxomb (anti-EpCAM x anti-CD3) to select the better dose level in platinum refractory epithelial ovarian cancer patients. J Clin Oncol. 2007;25:18. [Google Scholar]
- 25.Sadeghi B, Arvieux C, Glehen O, Rivoire M, Baulieux J, Fontaumard E, et al. Peritoneal Carcinomatosis from Non-Gynecologic Malignancies. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Koppe MJ, Boerman OC, Oyen WJG, Bleichrodt RP. Peritoneal Carcinomatosis of Colorectal Origin. Ann Surg. 2006;243:212–222. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ströhlein MA, Schemanski O, Jäger M, Schoberth A, Bartelheim K, Bokemeyer C, et al. Intraoperative immunotherapy with the trifunctional antibody catumaxomab in patients with advanced gastric-, colon- and pancreatic cancer: a pilot phase I study. Proc Gastrointest Cancer Symp. 2008:120. [Google Scholar]
- 28.Lake RA, Robinson BWS. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 29.Baxevanis CN, Perez SA, Papamichail M. Combinatiorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiss M, Berdov BA, Roman LD, Luft AV, Lampe P, Lindhofer H, et al. Intraoperative, adjuvant treatment of gastric cancer with the trifunctional antibody catumaxomab compared to surgery alone: a phase II study. Annals Oncol. 2008:19. [Google Scholar]