Abstract
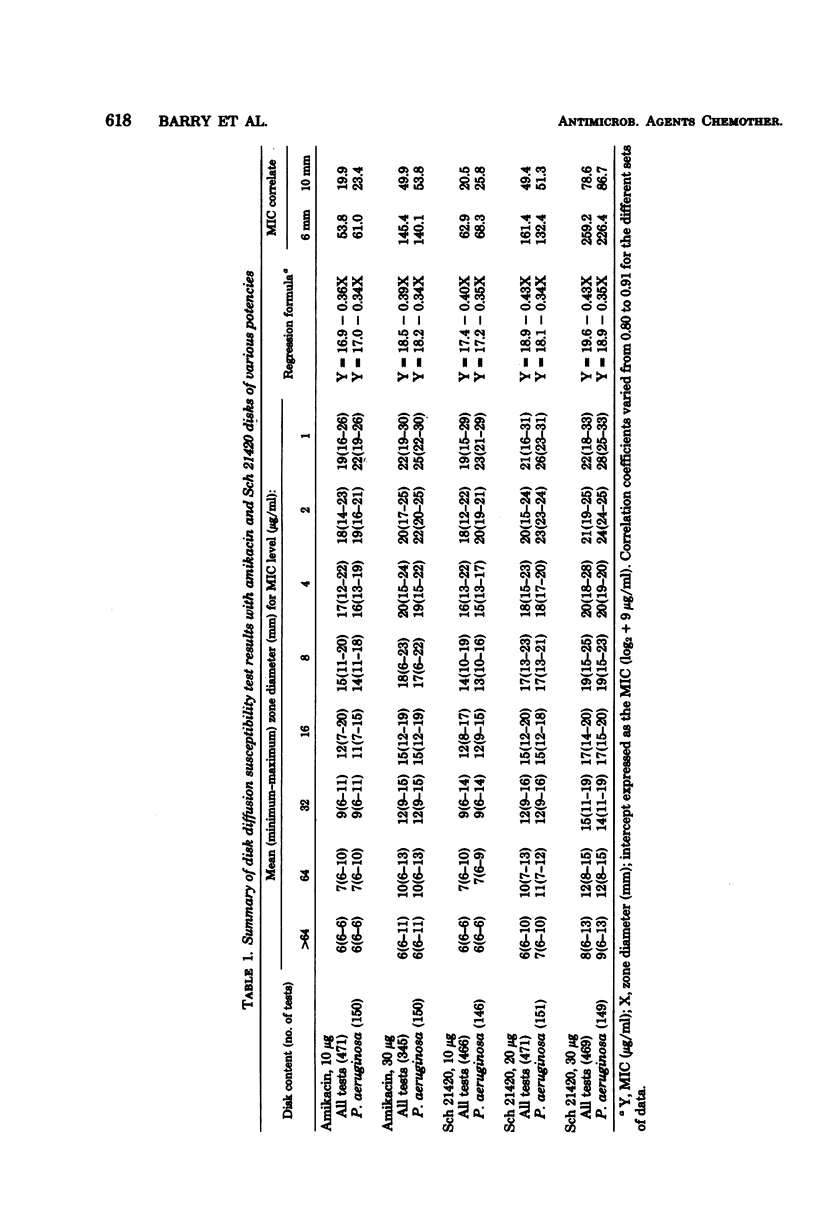
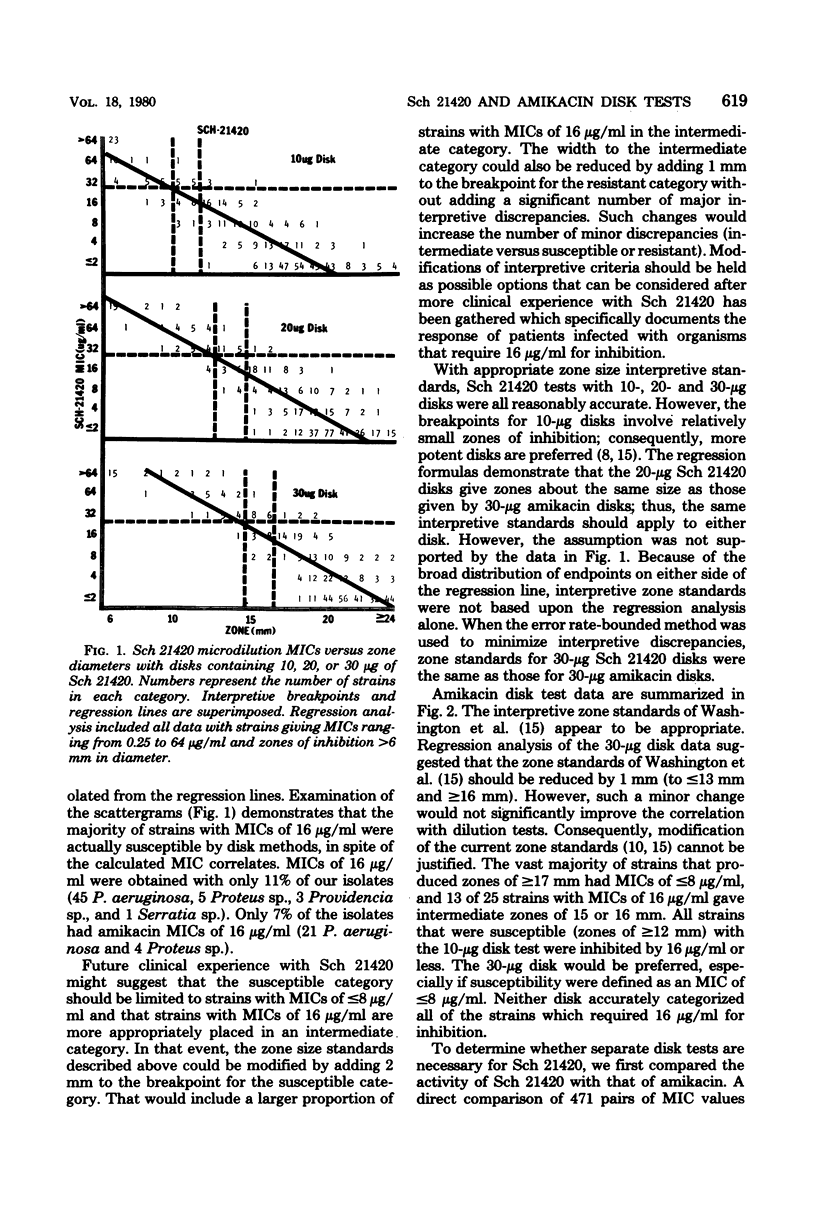
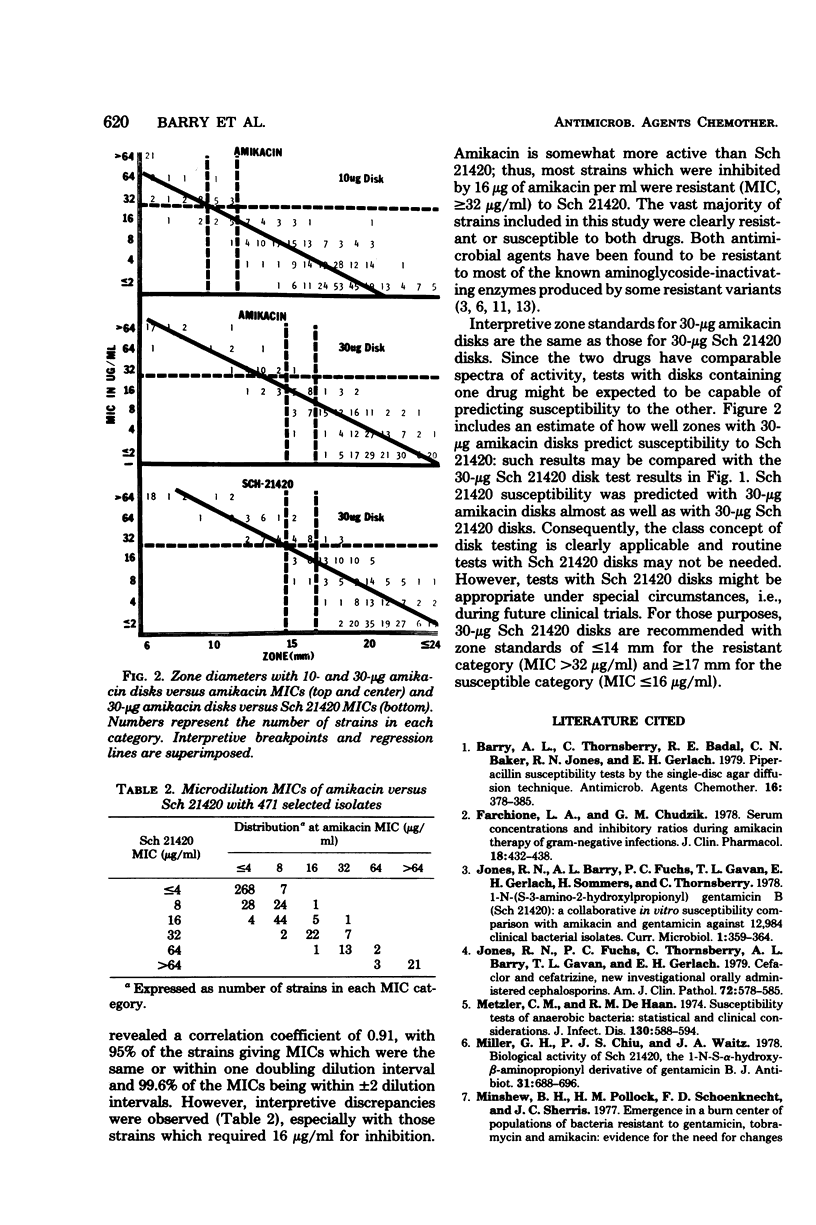
Disk susceptibility tests with two structurally related aminoglycosides (amikacin and Sch 21420) were evaluated. Tests with 10- and 30-micrograms amikacin disks confirmed previous recommendations for interpretive zone standards; 30-micrograms disks are preferred. Tests with 10-, 20-, and 30-micrograms Sch 21420 disks led to similar conclusions. The 30-micrograms Sch 21420 disks are recommended, with zone standards of less than or equal to 14 mm for the resistant category (minimal inhibitory concentration, greater than or equal to 32 micrograms/ml) and greater than or equal to 17 mm for the susceptible category (minimal inhibitory concentration, less than or equal to 16 micrograms/ml). If a minimal inhibitory concentration breakpoint of less than or equal to 8 micrograms/ml is preferred for defining the susceptible category, somewhat different zone standards may be used (less than or equal to 15 mm and greater than or equal to 19mm). Further evaluation documented the fact that tests with 30-micrograms amikacin disks predicted resistance or susceptibility to Sch 21420 almost as well as did a 30-micrograms Sch 21420 disk. Thus, the class concept of disk testing was judged to applicable, and routine testing with Sch 21420 may not be required.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Thornsberry C., Badal R. E., Baker C. N., Jones R. N., Gerlach E. H. Piperacillin susceptibility tests by the single-disk agar diffusion technique. Antimicrob Agents Chemother. 1979 Sep;16(3):378–385. doi: 10.1128/aac.16.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchione L. A., Chudzik G. M. Serum concentrations and inhibitory ratios during amikacin therapy of gram-negative infections. J Clin Pharmacol. 1978 Aug-Sep;18(8-9):432–438. doi: 10.1002/j.1552-4604.1978.tb02460.x. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Fuchs P. C., Thornsberry C., Barry A. L., Gavan T. L., Gerlach E. H. Cefaclor and cefatrizine, new investigational orally administered cephalosporins. In-vitro collaborative evaluation against clinical bacterial isolates and comparison with related antimicrobics. Am J Clin Pathol. 1979 Oct;72(4):578–585. doi: 10.1093/ajcp/72.4.578. [DOI] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Miller G. H., Chiu P. J., Waitz J. A. Biological activity of SCH 21420, the 1-N-S-alpha-hydroxy-beta-aminopropionyl derivative of gentamicin B. J Antibiot (Tokyo) 1978 Jul;31(7):688–696. doi: 10.7164/antibiotics.31.688. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Kunz L. J., Poitras J. W. Resistance to gentamicin, tobramycin and amikacin among clinical isolates of bacteria. Am J Med. 1977 Jun;62(6):873–881. doi: 10.1016/0002-9343(77)90655-6. [DOI] [PubMed] [Google Scholar]
- Nagabhushan T. L., Cooper A. B., Tsai H., Daniels P. J., Miller G. H. The syntheses and biological properties of 1-N-(S-4-amino-2-hydroxybutyryl)-gentamicin B and 1-N-(S-3-amino-2-hydroxypropionyl)-gentamicin B. J Antibiot (Tokyo) 1978 Jul;31(7):681–687. doi: 10.7164/antibiotics.31.681. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Fu K. P. 1-N HAPA gentamicin B, a new aminoglycoside active against gentamicin resistant isolates--activity compared to other aminoglycosides. J Antibiot (Tokyo) 1978 May;31(5):385–393. doi: 10.7164/antibiotics.31.385. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. In vitro studies with Sch 21420 and Sch 22591: activity in comparison with six other aminoglycosides and synergy with penicillin against enterococci. Antimicrob Agents Chemother. 1978 Aug;14(2):178–184. doi: 10.1128/aac.14.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Barry A. L., Jones R. N., Baker C. N., Badal R. E., Packer R. R. Comparison of in vitro activity of Sch 21420, a gentamicin B derivative, with those of amikacin, gentamicin, netilmicin, sisomicin, and tobramycin. Antimicrob Agents Chemother. 1980 Aug;18(2):338–345. doi: 10.1128/aac.18.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Yu P. K., Gavan T. L., Schoenknecht F. D., Thornsberry C. Interpretation of the disk diffusion susceptibility test for amikacin: report of a collaborative study. Antimicrob Agents Chemother. 1979 Mar;15(3):400–407. doi: 10.1128/aac.15.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Comparative in vitro activity of a semisynthetic derivative of gentamicin B (SCH 21420) and five other aminoglycosides. J Antibiot (Tokyo) 1978 Oct;31(10):1063–1064. doi: 10.7164/antibiotics.31.1063. [DOI] [PubMed] [Google Scholar]


