Abstract
Context
The rate of adoption of new therapies for cardiovascular diseases following the publication of favorable clinical trial results has been studied; however, less is known about the rates of de-adoption of a drug when negative studies are published.
Objective
To evaluate the use of nesiritide before and after March and April 2005 publications in 2 high-impact journals that suggested an increased risk of renal failure and mortality with intravenous nesiritide for acute decompensated heart failure.
Design, Setting, and Patients
Analysis of a large prospective hospital database, developed for quality and utilization benchmarking, of 491 acute care US hospitals at which 385 627 inpatient admissions occurred with a primary International Classification of Diseases, Ninth Revision (ICD-9) code for heart failure between January and August 2001 (prior to nesiritide release) and January 2004 to December 2005 (before and after publication periods). In addition, any patient admitted who received nesiritide in the absence of a primary or secondary heart failure code was evaluated for potential off-label use of the drug.
Main Outcome Measure
Use of nesiritide and other intravenous vasoactive therapy among patients admitted with heart failure.
Results
Nesiritide use decreased from a peak of 16.6% (2351 of 14 167 admissions) in March 2005 to 5.6% (611 of 10 822 admissions) in December 2005 (P<.001). Among those patients treated with nesiritide, the mean duration of treatment changed minimally, from 2.3 to 2.1 days. Although the use of inotropes also decreased during the period under study, the changes were more modest; furthermore, of those patients who were prescribed intravenous vasoactive therapy, a higher percentage were prescribed inotropes after publication (3272 [21.5%] of 15 193 patients from January-April 2005 vs 5750 [29.6%] of 19 445 patients from May-December 2005, P<.001). The use of nesiritide, in the absence of an ICD-9 heart failure code, was small.
Conclusions
Rapid de-adoption of nesiritide occurred following 2 publications suggesting risk with the drug. Further analyses are required to evaluate the consequences of these changes on patient outcomes and to anticipate how publications of adverse findings can influence practice.
THE ADOPTION OF EVIDENCE-based therapies for the treatment of cardiovascular diseases has been extensively studied in the clinical trials setting, registries, and claims data.1-6 The rapidity with which medications are accepted into clinical practice following the publication of positive trials data varies. For example, the uptake of some heart failure medications, such as angiotensin-converting enzyme (ACE) inhibitors was slow7-11 despite the publication of landmark studies12,13 and incorporation of the therapy into clinical practice guidelines.14 Conversely, the use of spironolactone increased significantly after release of the Randomized Aldactone Evaluation Study (RALES) trial data,15 with unexpected consequences.4,16
The factors that lead to a decrease in the use of a drug or other intervention have not been as extensively studied. The observation has been made that drugs may fall out of favor as other therapeutic options evolve.5,17-19 However, less is known about the impact on practice of published studies suggesting adverse effects or possible safety concerns following regulatory approval. A few anecdotal examples do exist, however. In the 1970s, the use of lipid-lowering drugs such as clofibrate decreased in the years following the publication of a series of adverse articles emanating from the Coronary Drug Project.20 Prescriptions for α-adrenergic blockers fell, albeit modestly, after data suggested a possible adverse effect in hypertension relative to thiazide diuretics.21 The publication of an outcomes analysis from the Women's Health Initiative22 had an impact on prescriptions written for hormone replacement therapy in women aged 55 years and older.23 The data, derived from the records of a pharmacy benefits management company, suggested rapid decreases in both new and repeat prescriptions. However, 2 prior clinical trials that failed to demonstrate prevention of coronary heart or cerebrovascular events with hormone replacement therapy did not change prescribing practice.
In the area of acutely decompensated heart failure, the approval of nesiritide in August 2001 for the relief of dyspnea and acute lowering of pulmonary capillary wedge pressure on the basis of a series of trials24-26 provided a potential new avenue for pharmacologic treatment. A survival indication was not included for this or any other drug in use for acutely decompensated heart failure. However, 2 publications in prominent medical journals in the spring of 2005 reported associations between nesiritide use and adverse effects, specifically, worsening renal function27 and death.28 Subsequently, a commentary-type publication appeared in which a prominent author called for the withdrawal of nesiritide from the market.29
Box. Timeline of Events Related to Nesiritide Use.
1998
April—New Drug Application filed with the US Food and Drug Administration (FDA)
1999
April—FDA action letter requesting more data37
2000
July—Publication of article outlining symptom relief with nesiritide 24
2001
August—FDA approval of nesiritide
September—Launch of nesiritide
2002
March—Publication of Vasodilation in the Management of Acute Congestive Heart Failure study results25
December—Publication of PRECEDENT safety study results26
2005
March—Publication of analysis suggesting worsening renal function27
April—Publication of analysis suggesting increased mortality28
June-July—Dissemination of Braunwald Panel summary statement on appropriate indications for nesiritide administration, including a Dear Health Care Provider letter by Scios38
July—Publication of a critical commentary29
2006
January—Publication of Wall Street Journal article showing decrease in nesiritide sales39
We hypothesized that there would be a rapid decrease in the use of nesiritide. Furthermore, we anticipated an increase in alternative therapies for advanced heart failure with a focus on therapies (dobutamine and milrinone) that have had a well-known potential association with increased mortality.30-36 This trade-off raised the possibility that the publication and subsequent dissemination of the nesiritide data had an unexpected impact on practice and patient care. Because no other drugs had been approved for the treatment of acutely decompensated heart failure since the launch of nesiritide in September 2001 (Box), we also were able to compare recent trends in the use of intravenous vasoactive therapy with practice before the medication's approval.
Methods
We used data from Premier's Perspective Comparative Database, a large US hospital clinical and economic database developed for quality and utilization benchmarking. This database includes patient-level data on each admission from approximately 800 acute care hospitals across the United States, providing nationally representative information on nearly 5 million annual hospital discharges at both rural and urban hospitals. All data are organized by discharge month. We use the term patient to refer to a discrete admission; an individual patient may be included in the database more than once.
To avoid any possible issues regarding the use of protected health information in the analyses, dates of admission and discharge were reported by month and year; day-of-service detail was provided using chronological days; and the age of patients older than 89 years were assigned an age of 89 years. The Saint Louis University Institutional Review Board approved the study and waived the requirement for patient informed consent.
Data were obtained from several periods to allow for examination of changes in prescribing patterns following the release of the 2 sentinel articles challenging the safety of nesiritide (March 29 and April 20, 2005). The specific periods were selected to allow for before-and-after publication comparisons and a reference point before nesiritide was introduced. Specifically, we examined the period January to April 2005, which includes the 4 months just prior to the publication of the mortality article28 and the subsequent 8 months in the same calendar year (May-December). To compare on a year-over-year basis, we divided the previous year into the same periods (January-April 2004 and May-December 2004). In addition, we obtained data for the time frame of January to August 2001, representing the period prior to the approval and introduction of nesiritide to the market (September 2001) to examine secular trends in the utilization of vasoactive therapies. The number of hospitals (n=491) varied slightly over time, contributing to differences in the number of patients during each period.
Variables in the PREMIER database include patient demographic information (eg, age, sex, and race based on UB92 coding), admission and discharge dates by month and year, concurrent background heart failure therapy (ACE inhibitor, angiotensin-receptor antagonist, β adrenergic antagonist, digoxin, diuretic, aldosterone antagonist), type of admission, length of stay (including days in intensive care), intravenous drug used (including day of initiation and discontinuation of therapy), patient discharge status, primary and secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (limited to 1 secondary code in the 2001 sample), hospital characteristics (size, geographic location, and teaching status), payer type, and the specialty of both the admitting and attending physician.
Inclusion criteria included (1) a primary ICD-9 diagnosis of heart failure (a detailed list is available on request), (2) age older than 18 years, and (3) acute care inpatient status. Standardized charge codes were used to identify drugs administered during the hospitalization. We defined intravenous vasoactive therapy as any one of the following: nesiritide, nitroglycerin, sodium nitroprus-side, dobutamine, dopamine, or milrinone. We also recorded the use of all 3 available intravenous loop diuretics (furosemide, bumetanide, and torsemide). We defined cardiology care if either the admitting or attending physician was coded as a cardiologist.
Secondary analyses were performed in which all admissions with a secondary ICD-9 code for heart failure were considered. Additionally, we were interested in the use of nesiritide for patients who did not have a primary or secondary ICD-9 code for heart failure to understand the scope of use that might fall outside the approved labeling for the drug and the associated underlying primary diagnoses in this cohort. We also separately analyzed the use of intravenous drugs in patients treated in hospitals that contributed patient data throughout 2004 and 2005; the number decreased modestly from 341 in the January-April 2004 period to 320 from March to December 2005.
Statistical Analyses
A χ2 analysis was used to compare frequency of drug use across the periods of observation. One-way analysis of variance was used to test for overall differences in total length of stay, accumulative days receiving therapy, and day of initiation of drug across the 4 periods examined in 2004 and 2005. Tukey post hoc tests were then conducted to determine exactly which periods differed significantly from one another.
We used logistic regression to identify characteristics associated with physician use of nesiritide among patients with heart failure. The following characteristics were included: age (19-64, 65-74, 75-84, ≥85), race (black, other), sex, use of background heart failure medications as defined above, hospital location (urban or rural) and geographical region, hospital teaching status, hospital size (defined by 0-100, 101-400, 401-600, >601 beds), physician (cardiologist, other), and payer type. Odds ratios (ORs) were calculated to evaluate risk. Time interaction variables for before and after publication of the survival article were used to evaluate risks based on patient and hospital characteristics for both periods, as well as change in risk between periods. Data management and analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC). Differences were considered statistically significant at a 2-sided P<.05 level.
Results
Baseline Characteristics and Demographics
The total number of hospitalizations across all periods with a primary ICD-9 diagnosis code for heart failure was 385 627. Patients were predominantly women, white, and elderly (mean [SD] age, 71.9 [13.7] years; median age, 75.0 years). The majority, 314 431 (81.5%), had a primary ICD-9 diagnosis code of 428.0 for unspecified heart failure with an additional 23 877 patients (6.2%) as codes 428.1 to 428.9. Other prevalent diagnoses included hypertensive heart disease with heart failure, code 402.91, in 19 612 patients (5.1%) and rheumatic heart failure, code 398.91, in 10 937 patients (2.8%). The median length of stay was 4.0 days. Additional variables are shown in Table 1.
Table 1.
Patient and Hospital Characteristics, Heart Failure Population for January-August 2001 and January 2004–December 2005 (N = 385 627)*
| No. (%) of Admissions | ||||||
|---|---|---|---|---|---|---|
| Variables | Jan-Aug 2001 (n = 89 726) |
Jan-Apr 2004 (n = 54 378) |
May-Dec 2004 (n = 97 823) |
Jan-Apr 2005 (n = 54 257) |
May-Dec 2005 (n = 89 443) |
All Periods (n = 385 627) |
| Age, y | ||||||
| 0-64 | 22 710 (25.3) | 14 430 (26.5) | 26 555 (27.2) | 14 484 (26.7) | 25 044 (28.0) | 103 223 (26.8) |
| 65-74 | 21 513 (24.0) | 11 947 (22.0) | 21 378 (21.9) | 11 764 (21.7) | 19 200 (21.5) | 85 802 (22.3) |
| 75-84 | 28 537 (31.8) | 17 148 (31.5) | 30 589 (31.3) | 16 963 (31.3) | 27 313 (30.5) | 120 550 (31.3) |
| ≥85 | 16 966 (18.9) | 10 853 (20.0) | 19 301 (19.7) | 11 046 (20.4) | 17 886 (20.0) | 76 052 (19.7) |
| Race | ||||||
| White | 59 875 (66.7) | 33 732 (62.0) | 60 022 (61.4) | 33 504 (61.8) | 53 922 (60.3) | 241 055 (62.5) |
| Black | 16 743 (18.7) | 11 334 (20.8) | 21 248 (21.7) | 11 417 (21.0) | 19 261 (21.5) | 80 003 (20.8) |
| Other | 13 108 (14.6) | 9312 (17.1) | 16 553 (16.9) | 9336 (17.2) | 16 260 (18.2) | 64 569 (16.7) |
| Male | 40 025 (44.6) | 25 709 (47.3) | 45 833 (46.9) | 26 222 (48.3) | 43 412 (48.5) | 181 201 (47.0) |
| 428.× heart failure diagnosis | 77 592 (86.5) | 48 055 (88.4) | 86 243 (88.2) | 47 857 (88.2) | 78 561 (87.8) | 338 308 (87.7) |
| Admission type | ||||||
| Emergency | 60 376 (67.3) | 38 825 (71.4) | 68 738 (70.3) | 38 051 (70.1) | 63 446 (70.9) | 269 436 (69.9) |
| Urgent | 17 174 (19.1) | 9524 (17.5) | 16 540 (16.9) | 9088 (16.8) | 13 988 (15.6) | 66 314 (17.2) |
| Elective | 8696 (9.7) | 5396 (9.9) | 11 302 (11.6) | 6517 (12.0) | 11 088 (12.4) | 42 999 (11.1) |
| Other/unknown | 3480 (3.9) | 633 (1.2) | 1243 (1.3) | 601 (1.1) | 921 (1.0) | 6878 (1.8) |
| Discharge status | ||||||
| Died | 4177 (4.7) | 2346 (4.3) | 3772 (3.9) | 2283 (4.2) | 3069 (3.4) | 15 647 (4.1) |
| Home | 68 219 (76.0) | 39 929 (73.4) | 72 742 (74.4) | 40 143 (74.0) | 67 405 (75.4) | 288 438 (74.8) |
| Continued care | 16 100 (17.9) | 11 504 (21.2) | 20 209 (20.7) | 11 266 (20.8) | 17 990 (20.1) | 77 069 (20.0) |
| Other/unknown | 1230 (1.4) | 599 (1.1) | 1100 (1.1) | 565 (1.0) | 979 (1.1) | 4473 (1.2) |
| Hospital bed count | ||||||
| 0-100 | 4471 (5.0) | 2245 (4.1) | 3886 (4.0) | 2488 (4.6) | 4109 (4.6) | 17 199 (4.5) |
| 101-400 | 44 661 (49.8) | 25 841 (47.5) | 45 618 (46.6) | 25 467 (46.9) | 42 077 (47.0) | 183 664 (47.6) |
| 401-600 | 23 900 (26.6) | 14 366 (26.4) | 26 297 (26.9) | 14 286 (26.3) | 22 630 (25.3) | 101 479 (26.3) |
| ≥601 | 16 694 (18.6) | 11 926 (21.9) | 22 022 (22.5) | 12 016 (22.2) | 20 627 (23.1) | 83 285 (21.6) |
| Oral therapy† | ||||||
| β-Blocker CHF | 31 582 (35.2) | 31 514 (58.0) | 60 202 (61.5) | 35 068 (64.6) | 60 118 (67.2) | 218 484 (56.7) |
| β-Blocker any | 38 869 (43.3) | 35 111 (64.6) | 66 233 (67.7) | 38 256 (70.5) | 65 003 (72.7) | 243 472 (63.1) |
| ACE inhibitor or ARB | 51 690 (57.6) | 34 837 (64.1) | 63 812 (65.2) | 35 810 (66.0) | 59 241 (66.2) | 245 390 (63.6) |
| Digoxin | 38 114 (42.5) | 17 991 (33.1) | 30 867 (31.6) | 16 473 (30.4) | 26 129 (29.2) | 129 574 (33.6) |
| Aldosterone antagonist | 15 816 (17.6) | 11 465 (21.1) | 21 615 (22.1) | 12 211 (22.5) | 19 897 (22.3) | 81 004 (21.0) |
| Cardiologist | 20 476 (22.8) | 12 873 (23.7) | 22 971 (23.5) | 12 663 (23.3) | 21 848 (24.4) | 90 831 (23.6) |
| Teaching hospital | 29 673 (33.1) | 19 241 (35.4) | 35 758 (36.6) | 19 859 (36.6) | 32 850 (36.7) | 137 381 (35.6) |
| Urban hospital | 79 297 (88.4) | 48 251 (88.7) | 87 314 (89.3) | 48 043 (88.6) | 79 538 (88.9) | 342 443 (88.8) |
| Payer type | ||||||
| Medicare | 65 255 (72.7) | 41 009 (75.4) | 73 958 (75.6) | 41 094 (75.7) | 67 238 (75.2) | 288 554 (74.8) |
| Medicaid | 5204 (5.8) | 3419 (6.3) | 6422 (6.6) | 3408 (6.3) | 5909 (6.6) | 24 362 (6.3) |
| Managed care | 15 308 (17.1) | 7529 (13.9) | 13 113 (13.4) | 7391 (13.6) | 12 028 (13.5) | 55 369 (14.4) |
| Self-pay | 1690 (1.9) | 1288 (2.4) | 2390 (2.4) | 1406 (2.6) | 2536 (2.8) | 9310 (2.4) |
| Other | 2269 (2.5) | 1133 (2.1) | 1940 (2.0) | 958 (1.8) | 1732 (1.9) | 8032 (2.1) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB: angiotensin receptor blocker; β-blocker any, use of any drug within the β-blocker class; β-blocker CHF, use of a β-blocker that is approved for heart failure; cardiologist, care by a cardiologist listed as either the admitting or attending physician; continued care, care at a chronic care facility, such as a skilled nursing facility.
Percentages may not sum to 100 due to rounding.
Patients received more than 1 type of therapy.
Use of Intravenous Medication
During hospitalization, 322 692 patients (83.7%) received loop diuretic; 33 068 (8.6%), nitroglycerin; 3513 (0.9%), sodium nitroprusside; 2709 (5.9%), dopamine; 24 018 (6.2%), dobutamine; and 7173 (1.9%), milrinone. For the period January 2004-December 2005, 37 354 (12.6%) of 295 901 admissions included nesiritide.
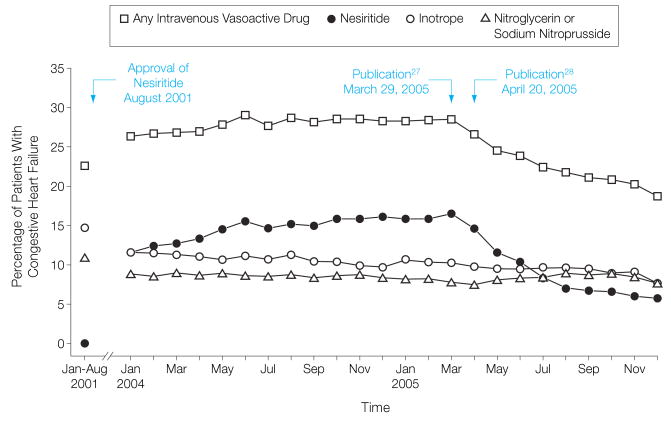
The use of nesiritide peaked in March 2005, administered during 2351 (16.6%) of 14 167 admissions and then declined significantly from April 2005 through December 2005 (Figure 1) from 1876 (14.6%) of 12 839 to 611 (5.6%) of 10 822 admissions (P<.001).
Figure 1.
Use of Intravenous Vasoactive Drugs Over Time
Trends in intravenous vasoactive therapies with composite baseline data for January-August 2001 (n=87 726) and monthly data for January 2004-December 2005 (n=295 901). The peak of nesiritide use occurred in March 2005 followed by a marked decline coinciding with the publication dates of pivotal safety articles. Overall use of vasoactive drugs also declined.
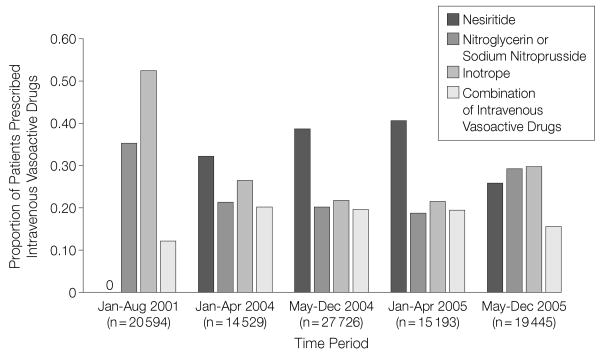
When analyzed by time frame, the use of inotropes and other vasodilators decreased until April 2005. The use of inotropes decreased further and the use of nitroglycerin or nitroprusside increased slightly after April 2005. Overall, the percentage of patients receiving intravenous vasoactive therapy declined from 28.0% (15 193 of 54 257) in January-April 2005 to 21.7% (19 445 of 89 443) in May-December 2005 (P<.001). However, of those patients receiving such therapy, the proportion receiving an inotrope increased from 21.5% (3272 of 15 193) in January-April 2005 to 29.6% (5750 of 19 445) in May-December 2005 (P<.001; Figure 2).
Figure 2.
Proportions of Drug Use Among Patients Receiving Intravenous Vasoactive Therapy
The use of individual agents in patients prescribed intravenous vasoactive therapy for the treatment of acute decompensated heart failure. Following increases in nesiritide use through April 2005, a greater proportion of patients were treated with inotropic drugs or vasodilators.
Furthermore, a separate analysis was limited to data supplied by hospitals that contributed patient information throughout all 4 periods in 2004 and 2005. This approach yielded a 15.6% decrease in the number of hospitals to 320 but only a 5.8% decrease in admissions to 278 874. With this narrowed population, the percentage of patients receiving nesiritide decreased significantly (P<.001) from 12.6% (6450 of 51 341) to 7.6% (6340 of 83 237), in parallel with the results for the entire sample from 12.5% (6813 of 54 378) to 7.8% (7006 of 89 443; P<.001). The trends in use of other intravenous vasoactive therapies was also similar.
Timing of Administration of Nesiritide
Comparison of mean values between January-April and May-December 2005 revealed a mean (SD) decrease in length of stay from 8.0 (7.9) to 7.6 (7.4) days, respectively (P<.005), and length of therapy from 2.3 (1.7) to 2.1 (1.4) days, respectively (P<.001). The day of initiation changed minimally from 1.9 (2.2) to 2.0 (2.5) days, respectively (P<.002).
Predictors of Nesiritide Use
Decreases in nesiritide use were found in all subgroups analyzed (Table 2). In a logistic regression analysis, a number of factors, including younger patient age, nonblack race, male sex, hospital location outside the Northeast region, care by a cardiologist, and background use of heart failure medications predicted use of nesiritide up to April 2005. Following the publication of the mortality article, the odds ratio for use among elderly patients (age ≥85 years) relative to a reference group (19-64 years) declined in a statistically significant manner, possibly reflecting heightened concerns about the potential risks of nesiritide in this population. Similar findings were observed for urban and nonteaching hospitals and the Northeast region.
Table 2.
Logistic Regression Results Modeling Nesiritide Use, Heart Failure Population for January 2004–December 2005 (n = 295 901)
| January 2004-April 2005 (n = 206 458) |
May 2005-December 2005 (n = 89 443) |
||||||
|---|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | Patients Taking Nesiritide, % | OR (95% CI) | P Value | Patients Taking Nesiritide, % | OR Change, P Value |
| Age, y | |||||||
| 19-64 | 1.00 | 15.0 | 1.00 | 8.2 | |||
| 65-74 | 1.17 (1.13-1.22) | <.001 | 16.5 | 1.07 (0.99-1.15) | .08 | 8.9 | .03 |
| 75-84 | 1.14 (1.10-1.19) | <.001 | 15.2 | 1.04 (0.97-1.12) | .30 | 8.1 | .02 |
| ≥85 | 0.96 (0.92-1.01) | .09 | 11.5 | 0.83 (0.76-0.91) | <.001 | 5.7 | .003 |
| Race | |||||||
| Black | 0.90 (0.87-0.93) | <.001 | 13.9 | 0.93 (0.87-0.99) | .02 | 7.9 | .35 |
| Other | 1.00 | 14.9 | 1.00 | 7.8 | |||
| Sex | |||||||
| Men | 1.24 (1.20-1.27) | <.001 | 16.9 | 1.16 (1.10-1.22) | <.001 | 8.9 | .02 |
| Women | 1.00 | 12.8 | 1.00 | 6.8 | |||
| Concurrent therapy* | |||||||
| ACE inhibitor | 0.93 (0.90-0.95) | <.001 | 14.9 | 0.88 (0.83-0.93) | <.001 | 7.9 | .08 |
| No ACE inhibitor | 1.00 | 14.5 | 1.00 | 7.7 | |||
| ARB | 1.07 (1.03-1.10) | <.001 | 15.9 | 0.99 (0.92-1.06) | .74 | 8.3 | .055 |
| No ARB | 1.00 | 14.5 | 1.00 | 7.8 | |||
| β-Blocker | 1.62 (1.57-1.66) | <.001 | 17.4 | 1.60 (1.51-1.70) | <.001 | 9.1 | .79 |
| No β-blocker | 1.00 | 10.4 | 1.00 | 5.2 | |||
| Digoxin | 1.26 (1.23-1.30) | <.001 | 18.2 | 1.27 (1.20-1.34) | <.001 | 9.9 | .87 |
| No digoxin | 1.00 | 13.1 | 1.00 | 7.0 | |||
| Aldosterone antagonist | 1.66 (1.61-1.71) | <.001 | 22.0 | 1.63 (1.54-1.72) | <.001 | 12.1 | .56 |
| No aldosterone antagonist | 1.00 | 12.6 | 1.00 | 6.6 | |||
| Hospital type | |||||||
| Urban | 1.01 (0.97-1.05) | .56 | 14.7 | 0.83 (0.77-0.89) | <.001 | 7.7 | <.001 |
| Rural | 1.00 | 14.8 | 1.00 | 9.0 | |||
| Nonteaching | 0.99 (0.96-1.02) | .55 | 14.4 | 0.78 (0.74-0.83) | <.001 | 7.6 | <.001 |
| Teaching | 1.00 | 15.2 | 1.00 | 8.3 | |||
| Hospital bed count | |||||||
| 0-100 | 0.69 (0.64-0.74) | <.001 | 9.4 | 0.77 (0.67-0.88) | <.001 | 5.6 | .17 |
| 101-400 | 1.00 | 15.1 | 1.00 | 8.5 | |||
| 401-600 | 0.95 (0.92-0.98) | <.001 | 14.6 | 0.76 (0.71-0.81) | <.001 | 7.0 | <.001 |
| >601 | 0.83 (0.81-0.86) | <.001 | 15.0 | 0.69 (0.64-0.74) | <.001 | 7.8 | <.001 |
| Physician | |||||||
| Cardiologist | 1.93 (1.88-1.99) | <.001 | 23.0 | 1.80 (1.71-1.90) | <.001 | 12.2 | .02 |
| Other | 1.00 | 12.2 | 1.00 | 6.4 | |||
| Region | |||||||
| Midwest | 1.67 (1.59-1.75) | <.001 | 17.4 | 1.38 (1.26-1.51) | <.001 | 9.1 | <.001 |
| Northeast | 0.81 (0.76-0.85) | <.001 | 9.4 | 0.58 (0.52-0.65) | <.001 | 4.0 | <.001 |
| South | 1.51 (1.44-1.58) | <.001 | 15.8 | 1.38 (1.28-1.49) | <.001 | 8.9 | .04 |
| West | 1.00 | 12.2 | 1.00 | 6.4 | |||
| Payer type | |||||||
| Medicare | 1.00 | 14.7 | 1.00 | 7.8 | |||
| Medicaid | 1.02 (0.97-1.09) | .44 | 14.0 | 1.09 (0.98-1.21) | .14 | 8.6 | .34 |
| Charity | 1.19 (0.98-1.44) | .08 | 15.8 | 0.93 (0.62-1.39) | .73 | 7.8 | .28 |
| Managed care | 1.06 (1.01-1.10) | .01 | 15.6 | 0.92 (0.85-1.00) | .04 | 7.8 | .003 |
| Self-pay | 0.90 (0.82-0.98) | .02 | 13.4 | 0.90 (0.77-1.06) | .20 | 7.9 | .96 |
| Workers comp | 1.97 (1.34-2.91) | <.001 | 28.2 | 1.36 (0.57-3.24) | .48 | 12.0 | .45 |
| Government payer† | 0.97 (0.82-1.14) | .70 | 15.1 | 0.76 (0.53-1.10) | .15 | 6.6 | .25 |
| Other or unknown | 0.68 (0.58-0.80) | <.001 | 9.9 | 0.65 (0.48-0.88) | .006 | 5.3 | .76 |
Abbreviations: ACE: angiotensin-converting enzyme; ARB, angiotensin receptor blocker; β-blocker: β-blocker approved for heart failure; CI, confidence interval; OR, odds ratio.
All reference groups for concurrent therapies are those individuals not receiving the specific drug.
Including the Veterans Health Administration, Tricare, and Indian Health Services.
Use of Nesiritide Among Patients With a Secondary ICD-9 Code for Heart Failure
During calendar years 2004 and 2005, 806 069 patients were hospitalized with secondary diagnoses of heart failure. The leading primary ICD-9 diagnoses among the 23 823 nesiritide users were acute myocardial infarction (25.6%, n=6104), respiratory illness or failure (19.5%, n=4645), and coronary atherosclerosis (7.8%, n=1866). We observed a significant decrease in nesiritide use that was similar to the decrease in the primary cohort.
Use of Nesiritide Among Patients Without an ICD-9 Heart Failure Code
A total of 3190 patients during the January 2004-December 2005 period received nesiritide without a primary or secondary diagnosis of heart failure (4.96% of all nesiritide use). A more conservative estimate that removes all patients with cardiovascular codes (with the exception of nonspecific chest pain, conduction disorders, cardiac dysrhythmias, and essential hypertension) yielded 569 patients (0.88%).
Comment
The adoption of new cardiovascular medications and the influences on physician decision making that affect prescribing practice have been extensively studied. Prescribing practices vary by provider characteristics and can be affected by a wide range of factors.40-43 For the condition of congestive heart failure, available data suggest that some medications, such as ACE inhibitors, were underused after publication of definitive randomized placebo-controlled clinical trials that demonstrated a survival benefit.7 Other medications, such as aldactone, may have been rapidly adopted albeit in inappropriate populations.4,16 However, most of the focus has been on the adoption rather than its opposite, when safety or efficacy of an established drug is brought into question.
Recently, several analyses were published that suggested the existence of a safety concern with the intravenous drug nesiritide, approved by the US Food and Drug Administration for the treatment of symptomatic, acutely decompensated heart failure. Using data from a representative cohort of patients with heart failure admitted to acute care hospitals in the United States during the periods immediately before and after the publication dates, we observed a highly significant decrease in the use of the drug. The decrease occurred in all subgroups. Use among elderly patients was initially lower than in other age groups and declined at a more rapid rate. At the same time, for those patients receiving nesiritide, the duration of therapy decreased and the time from admission to administration increased slightly.
These findings suggest that physicians may respond rapidly in the face of highly publicized negative postapproval data, perhaps at a greater speed and to a greater extent than when positive efficacy data for a new medication are published. Furthermore, we found that there were downstream consequences. For example, there was a lack of compensatory uptake of alternative intravenous vasoactive therapies, although inotrope use became more likely among patients offered this treatment option. The overall percentage of patients receiving nondiuretic intravenous therapy, which increased dramatically after nesiritide approval, has fallen to levels below those observed prior to nesiritide approval. Hence, the publication of articles that call into question safety (and potentially efficacy) of approved medications may have important and early effects on patient care.
Prior studies have shown that the use of cardiovascular drugs may also decrease in the absence of major new findings. In the cases of digoxin for congestive heart failure and lidocaine for routine administration following suspected acute myocardial infarction, changes in use appear to be related to background secular trends17,19 rather than to a particular publication or highly publicized new clinical findings. Indeed, when safety concerns have been described, the medication in question has often been removed from the market as in the cases of rofecoxib, mibefradil, and short-acting nifedipine. However, the way in which postmarketing data can affect practice remains an important area for investigation.44
In addition, in our study, off-label administration of nesiritide appears to have comprised a small proportion of overall use. With a conservative assumption, namely that any primary or secondary ICD-9 code for heart failure reflects on-label use, an estimate of approximately 5% was derived.
Further study is required to assess the degree to which current trends with nesiritide will continue and the long-term impact on the pharmacological treatment of patients with decompensated heart failure. Newer trial data and alternative drug therapies may influence practice and modify current approaches; nevertheless, the reassessment of nesiritide has had pronounced and rapid effects on practitioners, patients, and—by extension—industry.39
Limitations
It is possible that the change in nesiritide prescribing reflects a wide array of influences including the mass media and changes in marketing.45 A definitive causal relationship with any single factor cannot be made. However, the fact that a continual decrease was seen over a period of 10 months suggests, from an analytical perspective, that the publications27,28 had a pronounced influence on practice.
Additionally, we do not know details about antecedent care or prior use of intravenous vasoactive therapy in this patient cohort. The reasons for the admission (eg, noncompliance, new arrhythmia, etc) are not known. Furthermore, it is possible that a given patient may be admitted more than once during the period under study and therefore contributes to the database with each admission. We did not specifically look at dosing of intravenous therapy, because most doses are based on patient weight and may be frequently changed during the course of the hospitalization. However, it is possible that the average per-kilogram dose of nesiritide declined during the period under study and this would likely represent an important shift toward on-label use, for which a dose of 0.01 μg/kg per minute is standard.
We were unable to pinpoint the exact timing of the beginning of the decrease in nesiritide use, because the article suggesting a detrimental effect on mortality was published on the 20th day of the month (April 2005). Our data provides month and year of admission (as well as day of service detail) but does not include the precise calendar date. Nevertheless, the impact, if sudden, would have been limited to the last 10 days of the month. Furthermore, since the database is driven by month of discharge, the data we have for December 2005 does not include all hospitalizations in that month. Patients admitted in December 2005 and discharged in January 2006, are not captured in the database. Because these patients may have longer lengths of stay and hence be considered sicker and more likely to receive intravenous vasoactive therapy, we may have slightly underestimated the use of nesiritide in that month. Finally, we may have underestimated the participation of a cardiologist in the care of the patients because we were limited to the admitting physician of record and the attending physician during the hospitalization.
Conclusions
In conclusion, we have observed a rapid de-adoption of a drug prescribed for decompensated heart failure after a series of publications brought into question its clinical safety profile. Because intravenous vasoactive therapy use was increasingly driven by nesiritide, the overall use of these therapies also declined. However, among patients on intravenous vasoactive therapy, a higher proportion was prescribed intravenous inotropic drugs. The rate of de-adoption appears to be faster than what has been conventionally described for the adoption of new heart failure medications. Whether the magnitude of these changes can be anticipated or are reproducible in other therapeutic areas remains to be seen.
Acknowledgments
Funding/Support: This work was funded in part by National Institutes of Health grant RO1 AG021515 (Dr Hauptman).
Footnotes
Author Contributions: Dr Hauptman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hauptman, Schnitzler, Burroughs.
Acquisition of data: Hauptman, Schnitzler.
Analysis and interpretation of data: Hauptman, Schnitzler, Swindle, Burroughs.
Drafting of the manuscript: Hauptman.
Critical revision of the manuscript for important intellectual content: Hauptman, Schnitzler, Swindle, Burroughs.
Statistical analysis: Hauptman, Schnitzler, Swindle, Burroughs.
Obtained funding: Hauptman.
Administrative, technical, or material support: Burroughs.
Study supervision: Schnitzler, Burroughs.
Financial Disclosures: Dr Hauptman reports that in the area of decompensated heart failure he has received honoraria as a member of the speakers bureau from Scios and Johnson & Johnson before September 2005 and grant support from Abbott Laboratories. He currently reports that he receives or will receive grant support and consultancy fees from Orqis Medical Corp, Otsuka Maryland Research Institute Inc, and NovaCardia Inc. In the area of chronic heart failure, Dr Hauptman reports that he has served as an investigator, consultant, and speaker for GlaxoSmithKline and has been on the speaker's bureau for AstraZeneca and King Pharmaceuticals. No other authors reported relevant financial disclosures.
Role of the Data Source: Premier Healthcare Informatics served as the supplier and vendor of the database. The authors purchased this database using funds from the NIH grant. The company had no role whatsoever in the analysis of the data and no one at the company contributed to the drafting of the manuscript. One computer programmer helped clarify questions raised during the analysis of the database and several definitions in the data dictionary. This was the extent of the company's involvement in this study.
References
- 1.Lamas GA, Pfeffer MA, Hamm P, Wertheimer J, Rouleau JL, Braunwald E, The SAVE Investigators Do the results of randomized clinical trials of cardiovascular drugs influence medical practice? N Engl J Med. 1992;(327):241–247. doi: 10.1056/NEJM199207233270405. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar SR, McAlister FA, Soumerai SB. Synergy between publication and promotion: comparing adoption of new evidence in Canada and the United States. Am J Med. 2003;115:467–472. doi: 10.1016/s0002-9343(03)00422-4. [DOI] [PubMed] [Google Scholar]
- 3.Kizer JR, Cannon CP, McCabe CH, et al. Trends in the use of pharmacotherapies for acute myocardial infarction among physicians who design and/or implement randomized trials vs physicians in routine clinical practice: the MILIS-TIMI experience. Am Heart J. 1999;137:79–92. doi: 10.1016/s0002-8703(99)70462-x. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt B, Agoston I, Knowlton AA. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–214. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 5.Col NF, McLaughlin TJ, Soumerai SB, et al. The impact of clinical trials on the use of medications for acute myocardial infarction: results of a community based study. Arch Intern Med. 1996;156:54–60. [PubMed] [Google Scholar]
- 6.Tu K, Mamdani MM, Jacka RM, Forde NJ, Roth-well DM, Tu JV. The striking effect of the Heart Outcomes Prevention Evaluation (HOPE) on ramipril prescribing in Ontario. CMAJ. 2003;168:553–557. [PMC free article] [PubMed] [Google Scholar]
- 7.Masoudi FA, Rathore SS, Wang Y, et al. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724–731. doi: 10.1161/01.CIR.0000138934.28340.ED. [DOI] [PubMed] [Google Scholar]
- 8.Rich MW, Brooks K, Luther P. Temporal trends in pharmacotherapy for congestive heart failure at an academic medical center 1990-1995. Am Heart J. 1998;135:367–372. doi: 10.1016/s0002-8703(98)70309-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith NL, Chan JD, Rea TD, et al. Time trends in the use of beta blockers and other pharmacotherapies in older adults with congestive heart failure. Am Heart J. 2004;148:710–717. doi: 10.1016/j.ahj.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Feinglass J, Lee P, et al. Heart failure between 1986 and 1994: temporal trends in drug prescribing practices, hospital readmissions, and survival at an academic medical center. Am Heart J. 1997;134:901–909. doi: 10.1016/s0002-8703(97)80013-0. [DOI] [PubMed] [Google Scholar]
- 11.Gambassi G, Forman DE, Lapane KL, et al. Management of heart failure among very old persons living in long-term care: has the voice of trials spread? Am Heart J. 2000;139:85–93. doi: 10.1016/s0002-8703(00)90313-2. [DOI] [PubMed] [Google Scholar]
- 12.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 13.SOLVD Investigators. Effects of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 14. [January 31, 2006];ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. doi: 10.1016/j.jacc.2005.08.022. American College of Cardiology Web site. http://www.acc.org/qualityandscience/clinical/guidelines/failure/update/index.pdf. [DOI] [PubMed]
- 15.Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 16.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 17.Hussain Z, Swindle J, Hauptman PJ. Digoxin use and digoxin toxicity in the post-DIG Trial era. J Card Fail. 2006;12:343–346. doi: 10.1016/j.cardfail.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Young JB. Whither Withering's legacy: digoxin's role in our contemporary pharmacopeia for heart failure. J Am Coll Cardiol. 2005;46:505–507. doi: 10.1016/j.jacc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Soumerai SB, McLaughlin TJ, Gurwitz JH, et al. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279:1358–1363. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 20.Friedman L, Wenger NK, Knatterud GL. Impact of the Coronary Drug Project findings on clinical practice. Control Clin Trials. 1983;4:513–522. doi: 10.1016/0197-2456(83)90032-6. [DOI] [PubMed] [Google Scholar]
- 21.Stafford RS, Furberg CD, Finkelstein SN, Cock-burn IM, Alehegn T, Ma J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996-2002. JAMA. 2004;291:54–62. doi: 10.1001/jama.291.1.54. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Hsia J, Johnson JE, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 23.Kim N, Gross C, Jeptha C, et al. The impact of clinical trials on the use of hormone replacement therapy: a population based study. J Gen Intern Med. 2005;20:1026–1031. doi: 10.1111/j.1525-1497.2005.0221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 25.Publication Committee for the VMAC Investigators. Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;(287):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 26.Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002;144:1102–1108. doi: 10.1067/mhj.2002.125620. [DOI] [PubMed] [Google Scholar]
- 27.Sackner Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 28.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 29.Topol EJ. Nesiritide—not verified. N Engl J Med. 2005;353:113–116. doi: 10.1056/NEJMp058139. [DOI] [PubMed] [Google Scholar]
- 30.Bayram M, De Luca L, Massie BM, Gheorghiade M. Reassessment of dobutamine, dopamine and milrinone in the management of acute heart failure syndromes. Am J Cardiol. 2005;96(suppl):47G–58G. doi: 10.1016/j.amjcard.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Ewy GA. Inotropic infusions for chronic congestive heart failure: medical miracles or misguided medicinals? J Am Coll Cardiol. 1999;33:572–575. doi: 10.1016/s0735-1097(98)00596-8. [DOI] [PubMed] [Google Scholar]
- 32.Packer M. The development of positive inotropic agents for chronic heart failure: how have we gone astray? J Am Coll Cardiol. 1993;22(suppl A):119A–126A. doi: 10.1016/0735-1097(93)90474-f. [DOI] [PubMed] [Google Scholar]
- 33.Felker GM, O'Connor CM. Between Scylla and Charybdis: the choice of inotropic agent for decompensated heart failure. Am Heart J. 2001;142:932–933. doi: 10.1067/mhj.2001.119611. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138(1 pt 1):78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 35.Oliva F, Latini R, Politi A, et al. Intermittent 6-month low dose dobutamine infusion in severe heart failure: DICE multicenter trial. Am Heart J. 1999;138(1 pt 2):247–253. doi: 10.1016/s0002-8703(99)70108-0. [DOI] [PubMed] [Google Scholar]
- 36.Cuffe MS, Califf RM, Adams KF, Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 37.Bethesda, Md: US Dept of Health and Human Services, Food and Drug Administration Center for Drug Evaluation; May 25, 2001. [September 7, 2006]. Cardiovascular and Renal Drugs Advisory Committee 92nd meeting. transcript. http://www.fda.gov/ohrms/dockets/ac/01/transcripts/3749t2.rtf. [Google Scholar]
- 38.Horton DP. Fremont, Calif: Scios Inc; Jul 13, 2005. [September 6, 2006]. Dear Health Care Provider. letter. http://www.fda.gov/medWatch/safety/2005/natrecor2_DHCP.htm. [Google Scholar]
- 39.Hensley S. J & J net rises 79% on lower taxes: revenue slips on soft drug sales. Wall Street Journal. 2006 January 25;:A3. [Google Scholar]
- 40.Roumie CL, Halasa NB, Edwards KM, Zhu Y, Dittus RS, Griffin MR. Differences in antibiotic prescribing among physicians, residents, and nonphysician clinicians. Am J Med. 2005;118:641–648. doi: 10.1016/j.amjmed.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Kozyrskyj AL, Dahl ME, Chateau DG, Mazowita GB, Klassen TP, Law BJ. Evidence-based prescribing of antibiotics for children: role of socioeconomic status and physician characteristics. CMAJ. 2004;171:139–145. doi: 10.1503/cmaj.1031629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamann J, Langer B, Leucht S, Busch R, Kissling W. Medical decision making in antipsychotic drug choice for schizophrenia. Am J Psychiatry. 2004;161:1301–1304. doi: 10.1176/appi.ajp.161.7.1301. [DOI] [PubMed] [Google Scholar]
- 43.Watkins C, Harvey I, Carthy P, Moore L, Robinson E, Brawn R. Attitudes and behaviour of general practitioners and their prescribing costs: a national cross sectional survey. Qual Saf Health Care. 2003;12:29–34. doi: 10.1136/qhc.12.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaya FT, Gu A. Assessing potential risks and evaluating expected benefits. Manag Care Interface. 2005;18:27–30. [PubMed] [Google Scholar]
- 45.Kesselheim AS, Fischer MA, Avorn J. The rise and fall of Natrecor for congestive heart failure: implications for drug policy. Health Aff (Millwood) 2006;25:1095–1102. doi: 10.1377/hlthaff.25.4.1095. [DOI] [PubMed] [Google Scholar]