Abstract
The discovery that IGF-I mRNAs encoding isoforms of the pro-IGF-I molecule are differentially regulated in response to mechanical stress in skeletal muscle has been the impetus for a number of studies designed to demonstrate that alternative splicing of IGF-I pre-mRNA involving exons 4, 5, and 6 gives rise to a unique peptide derived from pro-IGF-I that plays a novel role in myoblast proliferation. Research suggests that after injury to skeletal muscle, the IGF-IEb mRNA splice variant is up-regulated initially, followed by up-regulation of the IGF-IEa splice variant at later time points. Up-regulation of IGF-IEb mRNA correlates with markers of satellite cell and myoblast proliferation, whereas up-regulation of IGF-IEa mRNA is correlated with differentiation to mature myofibers. Due to the apparent role of IGF-IEb up-regulation in muscle remodeling, IGF-IEb mRNA was also named mechano-growth factor (MGF). A synthetically manufactured peptide (also termed MGF) corresponding to the 24 most C-terminal residues of IGF-IEb has been shown to promote cellular proliferation and survival. However, no analogous peptide product of the Igf1 gene has been identified in or isolated from cultured cells, their conditioned medium, or in vivo animal tissues or biological fluids. This review will discuss the relationship of the Igf1 gene to MGF and will differentiate actions of synthetic MGF from any known product of Igf1. Additionally, the role of MGF in satellite cell activation, aging, neuroprotection, and signaling will be discussed. A survey of outstanding questions relating to MGF will also be provided.
There is inadequate evidence to support the hypothesis that mechano-growth factor is a product of Igf1gene expression in vivo, or that it plays a definitive role not fulfilled by full-length IGF-I in the expansion of precursor cell populations during muscle or CNS repair/remodeling.
Over the past 10 yr, literature has arisen asserting that an alternative splicing event in the IGF-I gene occurs very early in skeletal muscle in response to mechanical stimuli such as tenectomy and electrical stimulation. This splicing event is posited to result in production of an IGF-I mRNA species that is translated into a precursor and subsequently processed to generate a peptide distinct from the canonical 70-amino-acid mature IGF-I peptide. The unique peptide derived from the pro-IGF-I precursor has been named mechano-growth factor, or MGF (1). Although data have been generated indicating that a synthetic version of this peptide may increase precursor cell proliferation, we propose to show that critical evidence is lacking that this peptide is indeed generated from expression of the endogenous Igf1 gene. Moreover, we propose that there is insufficient data providing proof of principle that this peptide is generated in vivo or to possess a novel role in activation and proliferation of progenitor cells during the initial phases of repair after tissue injury. Because the existence and action of MGF depends on certain features of the Igf1 gene and transcription unit, we will begin by reviewing the structure of the Igf1 gene and its expression products.
IGF-I Gene Structure and Expression
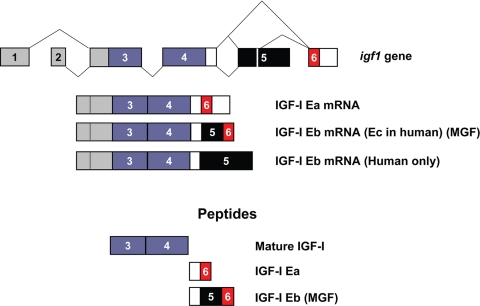
IGF-I promotes cell proliferation, differentiation, and survival (2,3). The synthesis of IGF-I is highly regulated at the level of mRNA abundance in a manner consistent with its roles in promoting cellular and tissue growth; furthermore, the complex structure of the IGF-I gene and transcription unit has for many years been appreciated as a major platform for regulation of IGF-I expression (4,5). The Igf1 gene spans more than 90 kb of chromosomal DNA, and alternative splicing of IGF-I pre-mRNA can produce multiple mRNA species, depending on the inclusion of alternative leader and C-terminal exons (6,7,8). All mRNA splice variants contain exons 3 and 4, which encode the mature 70-amino-acid IGF-I peptide consisting of the B, C, A, and D domains. mRNAs containing exon 4 spliced to exon 6 are designated as IGF-IEa (9), whereas those containing exon 4 spliced to exon 5 and exon 6 are designated IGF-IEb in rodents and IGF-IEc in humans (Fig. 1) (10,11,12). These mRNA splice variants encode C-terminal extensions termed E-domains to denote their positions relative to the BCAD domains of mature IGF-I. Translation of these alternatively spliced mRNAs is predicted to result in generation of pre-pro-IGF-Is, which are processed to yield the mature IGF-I molecule and the protein products of the E-domains, the E-peptides.
Figure 1.
Splicing and peptide products of the Igf1 gene. The Igf1 gene contains six exons; exons 1 and 2 (gray) serve as alternative leader exons at the 5′-end of the mRNA, whereas exons 3 and 4 (purple) are common to all splice variants. Several transcripts can be derived by alternative splicing at the C terminus, including exon 4 spliced directly to exon 6 (Ea) or exon 4 spliced to exon 5 spliced to exon 6. IGF-I mRNA containing exon 5 is referred to as Eb in rodents (Ec in humans) and has also been referred to as MGF (33). In humans, but not rodents, mRNAs containing exon 5 spliced to exon 4 have been identified (9) and are designated as Eb. Peptide products, derived from pro-IGF-I and referred to in the text, are shown.
Seminal work examining the translation and stability of Igf1 gene products demonstrated that human fibroblasts secrete an approximately 21.5-kDa peptide (13); additionally, in vitro translation of human IGF-IEa and IGF-IEb mRNAs revealed major bands of approximately 17.5 kDa for IGF-IEa and approximately 22 kDa for IGF-IEb (14). The existence of high relative molecular mass (Mr) IGF-I molecules was also shown using in vitro translation with rat IGF-IEa and IGF-IEb mRNA sequences (15). Collectively, these data suggest that the E-peptides are indeed translated and exist as a part of pro-IGF-I. Indeed, antiserum directed against a 13-amino-acid peptide with sequence identical to a portion of the E-domain of human IGF-IEa recognized a protein of approximately 19 kDa as determined by SDS-PAGE (16), and antiserum developed against a 23-amino-acid peptide with a sequence identical to a portion of the human Eb peptide was immunoreactive with molecules of various sizes that were presumed to represent precursor forms of IGF-I (17). Although these observations suggest that the E-peptides are stable as part of pro-IGF-I, whether they are stable or functional apart from the pro-IGF-I molecule is unknown. An approximately 2-kDa band (consistent with the Mr of the Ea peptide) was recognized by an IGF-IEa monoclonal antibody in FLAG-pro-IGF-IEa-transfected HEK293 cells, suggesting that a stable Ea peptide is generated under these conditions; however, this band was not evident in nontransfected IM9 lymphocytes (18). Investigation into the stability, secretion, and functions of the various E-peptides is currently ongoing, and recent work indicates a possible role for these peptides in mediating cellular uptake of IGF-I (19).
The MGF Hypothesis
The role of E-peptides in muscle repair and neuronal protection has also been the focus of intense study. Early work indicated that muscles exposed to stretch and (or) electrical stimulation responded with increased levels of the IGF-IEb mRNA splice variant (20,21,22). Because increases in IGF-IEb mRNA were seen after mechanical damage, and owing to its expression in a mechano-sensitive manner, IGF-IEb mRNA was named mechano-growth factor (MGF). Although the term MGF signifies its responsiveness after mechanical stress, it is nonetheless the exact same mRNA species as IGF-IEb and does not differ in sequence from IGF-IEb mRNA found in multiple tissues (23). The idea that the IGF-IEb transcript required a second name, i.e. MGF, resulted not only from findings that this splice variant is increased after mechanical stress but also from the opinion that species-specific designations for IGF-I splice variants could be simplified (24). However, the unconventional addition of MGF to the IGF-I nomenclature itself caused significant confusion; indeed, in 2003 (25), it finally became necessary for the authors themselves to provide clarification in an effort to discriminate MGF from the original consensus terminology established in 1991 (26). Despite this clarification, there still remains ambiguity in the relatedness of MGF to authentic IGF-I within the literature.
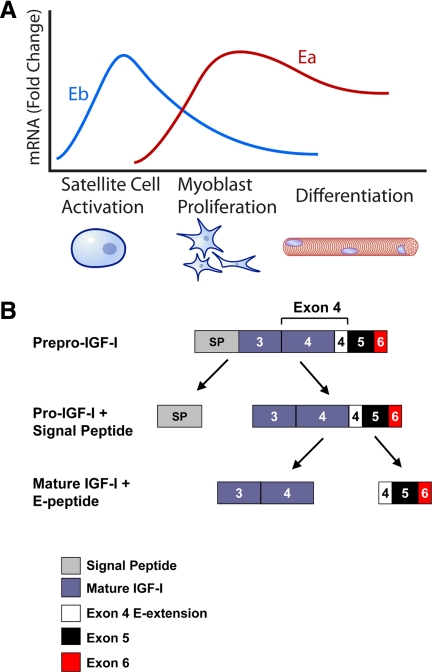
Although the nomenclature surrounding MGF is problematic, a number of studies have been published that attempt to define the role of IGF-IEb/MGF in muscle repair and survival. In aggregate, results from these studies have led to the formation of what we term the MGF hypothesis (Fig. 2). The MGF hypothesis asserts that during acute skeletal muscle repair after exercise or injury, levels of a specific splice variant that contains exon 5 (IGF-IEb in rodents, Ec in humans; Fig. 1) increase above preinjury levels. After several days, levels of this transcript decrease, and levels of the IGF-IEa splice variant increase above their preinjury levels concomitant with the decline in levels of IGF-IEb mRNA (27,28); these molecular events correlate with the mitogenic and myogenic differentiation events, respectively, that lead to muscle repair (Fig. 2A). The initial wave of IGF-IEb mRNA is posited to be translated and then processed to form two independent peptides: mature IGF-I and an E-peptide (or portion of the E-peptide), which is now referred to as MGF (Fig. 2B). MGF is then responsible for activating quiescent satellite cells (27), which proliferate to provide a pool of myogenic precursor cells for muscle repair. MGF itself, however, despite promoting proliferation, opposes differentiation of precursor cells (28). At this point, the levels of IGF-IEb/MGF decline, whereas levels of IGF-IEa increase (Fig. 2A). Like IGF-IEb, IGF-IEa propeptide is processed to generate mature IGF-I as well as an E-peptide, and it is argued that this increase of IGF-IEa is responsible for myoblast differentiation, presumably through the actions of mature IGF-I (28).
Figure 2.
The MGF hypothesis. A, The MGF hypothesis suggests that after muscle injury such as that caused by exercise, the Igf1 gene is first spliced toward the Eb (MGF) splice variant. Presumably, IGF-IEb mRNA will then be translated and processed to yield mature IGF-I as well as the autonomous C-terminal MGF peptide. The MGF peptide is then responsible for activating quiescent satellite cells to enter the cell cycle and develop into mononucleated myoblasts; MGF then promotes myoblast proliferation. However, MGF also inhibits differentiation; thus, levels of MGF must decrease for differentiation to occur. During the myoblast proliferative stage, splicing of the Igf1 gene is increasingly shifted toward the Ea splice variant, which promotes further myoblast proliferation and potentiates myoblast differentiation into multinucleated myotubes. Thus, the MGF hypothesis suggests that MGF is responsible for satellite cell activation and proliferation, whereas IGF-IEa is responsible for differentiation. The events outlined above describe the MGF hypothesis, but little has been shown with respect to endogenous proteins acting in the manner described. Thus, although the graph depicts cellular repair events that coincide with IGF-I splice variant mRNA levels, extreme caution should be employed when extrapolating mRNA data to actions at the protein level, as the MGF hypothesis does. In particular, it is essential to realize that levels of IGF-IEb mRNA are far lower than levels of IGF-IEa mRNA. Panel A does not reflect this, but rather illustrates the fold changes in the respective splice variants compared to unstimulated levels. B, Proposed generation of MGF. Processing of pre-pro-IGF-I contains at least two general steps; the first step involves removal of the signal peptide, and a second step liberates the E-peptide from mature IGF-I.
At present, definitive evidence supporting the events outlined in the MGF hypothesis is lacking, and there exists significant confusion surrounding Igf1 gene splicing, specifically with respect to MGF. MGF has been variously referred to as an mRNA splice variant of IGF-I (i.e. IGF-IEb mRNA) (21,29,30); a peptide product of IGF-I gene expression, i.e. the Eb peptide encoded by the 3′ end of the IGF-IEb mRNA (31,32); and a synthetic peptide that corresponds to the C-terminal 24 amino acids of the IGF-IEb propeptide (28,33). Obviously, these definitions are incongruent with each other and generate confusion. More importantly, the levels of IGF-IEb mRNA are normally vastly lower than IGF-IEa mRNA in skeletal muscle and in other extrahepatic tissues (23,30,34). IGF-I gene expression increases after injury (35,36,37,38), and there is no evidence that splicing of IGF-I pre-mRNA to yield IGF-IEa mRNA is inhibited or that IGF-IEa mRNA is degraded during the early phase of repair. Accordingly, the abundance of IGF-IEb mRNA in skeletal muscle in vivo has never been shown to exceed or even approach that of IGF-IEa mRNA, including during the early proliferative period of muscle repair when IGF-IEb mRNA levels undoubtedly show a transient increase above their preinjury levels (27,34,39). Moreover, IGF-IEb mRNA is predicted to encode canonical full-length IGF-I as well as MGF (10,12). Together, these facts create a major confound to the MGF hypothesis: under conditions where the MGF peptide, whatever it may be, are predicted to be elevated, there is no basis for positing that mature IGF-I and/or pro-IGF-IEa are not also present and indeed in elevated amounts. Because MGF and mature IGF-I are proposed under the MGF hypothesis to have distinct and, indeed, countervailing functions (MGF as an activator of satellite cells and mitogen and IGF-I as a differentiation factor), the presence of levels of IGF-I similar to or greater than MGF even during the early phase of muscle repair/remodeling creates an obvious conundrum. This conflict could only be resolved by demonstrating that IGF-IEa mRNA is not translated during the proliferative phase or that pro-IGF-IEa or mature IGF-I are degraded or sequestered from signaling during the early proliferative stage of repair/remodeling. We are unaware of any evidence to support either of these possibilities. It is also important to keep in mind that previous cell culture studies have provided abundant evidence that the full-length mature IGF-I molecule sequentially stimulates both myoblast proliferation and myogenic differentiation by activating the ERK and phosphoinositide 3-kinase (PI3K)/Akt pathways, respectively (40,41,42,43,44,45,46,47). Thus, experimental results have never been consistent with the need to invoke distinct pro-IGF-I-derived extracellular signals to explain the switch from proliferation to differentiation. Finally, there is no evidence that the IGF-IEb (Ec in humans)/MGF transcript generates a stable 24-amino-acid E-peptide with residues corresponding to the synthetically derived 24-amino-acid MGF peptide (Fig. 3), nor is the evidence convincing that stably increased amounts of the Eb peptide are generated under conditions accompanying repair and regeneration of muscle, brain, or other tissues in which there is an undoubted increase in the levels of IGF-IEb mRNA.
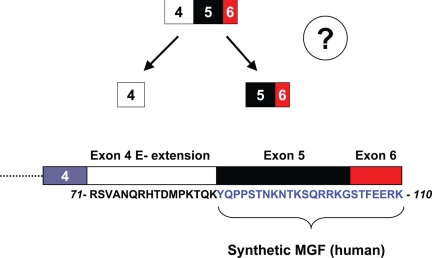
Figure 3.
Required step for derivation of MGF from the rest of the E-peptide. For MGF to exist as an endogenous 25-amino-acid peptide (24 amino acids in humans due to absence of one codon within exon 5; human sequence is shown), a processing step must occur that removes the 16-amino-acid E-extension from the peptide generated from exon 5 and exon 6 (see also Table 2); this step has not been observed in any system to date. Additionally, there is no identified sequence or motif within the intact 41-amino-acid E-peptide that would suggest this step occurs. For an endogenous peptide equivalent to the synthetic MGF to exist, proteolytic cleavage must occur that liberates those residues encoded by exon 5–6 (blue text) from those encoded by the exon 4 E-extension (black text). No furin or furin-like convertase, which are known to process pro-IGF-I, cleave scissile bonds on the carboxyl end of lysine or on the amino end of tyrosine.
Does MGF Exist in Vivo?
Lack of availability of specific antibodies had previously complicated detection of IGF-IEb/MGF at the protein level. Recently however, several independent groups have reported generation of antibodies raised against C-terminal amino acids corresponding to MGF (Table 1) (32,33,48). These antibodies are immunoreactive with endogenous proteins possessing Mr higher than 15 kDa in gerbil, rat, and human tissues, suggesting that the antibody is recognizing a sequence within a pro-IGF-IEb peptide, rather than an approximately 3-kDa endogenous 24-amino-acid (human) or 25-amino-acid (rodent) peptide corresponding to synthetic MGF (Table 2 and Fig. 3).
Table 1.
Peptide sequences used for MGF antibody generation
Table 2.
Estimated Mrof components of mouse pre-pro-IGF-I
| No. of residues | Estimated mass (kDa) | |
|---|---|---|
| Pre-pro-IGF-IEb | 159 | 17.92 |
| Pro-IGF-IEb | 111 | 12.57 |
| Mature IGF-I | 70 | 7.68 |
| Eb peptide | 41 | 4.89 |
| Exon 4 extension | 16 | 1.95 |
| MGF | 25 | 2.94 |
Number of residues corresponding to each component of pre-pro-IGF-I were determined from NCBI Reference Sequence NP_034642.2 [IGF-I isoform 1 pre-proprotein (Mus musculus)] (11). Estimated average molecular masses were determined by computing mass of peptides with hydrogen N-terminal and free acid C-terminal groups. Although the Eb peptide represents 41 residues of the pre-pro-IGF-IEb sequence, its cleavage from mature IGF-I likely produces a 40-amino-acid peptide due to cleavage at Arg71. Cleavage of pro-IGF-I yields a 71-amino-acid peptide that contains the 70-residue mature IGF-I as well as Arg71 as the most C-terminal residue. Arg71 is subsequently removed, presumably by carboxypeptidase activity, thus yielding mature IGF-I and the Eb peptide.
These findings raise the question as to whether an endogenous 24-amino-acid peptide product equivalent to the MGF peptide exists. Processing of pro-IGF-I, like other hormones, is mediated through the actions of members of the subtilisin-like proprotein convertase (SPC) family (49). Members of this family, including furin, rely on a multiple-basic-residue motif to direct cleavage at the scissile bond, with preference for Arg at the P1 position. Furin itself also relies heavily on the presence of Arg at the P4 position, whereas the requirement for Arg at P4 is less stringent for other proprotein convertases such as Kex2, which is able to recognize basic or alipathic residues at that position (50). Research investigating the role of SPCs in pro-IGF-IEa processing revealed that furin, as well as several other SPC family members, regulated cleavage at Arg residues that leads to removal of the E-domain from mature IGF-I (51,52). However, these studies detected no cleavage at the exon 4–6 boundary; suggesting that neither SPCs nor other endogenous proteases in these cells cleave the most C-terminal residue in exon 4 (Lys86). This is important because both pro-IGF-IEa and pro-IGF-IEb possess the same 16-amino-acid exon 4 E-extension, thus strongly suggesting that Lys86 is not acted upon by any endogenous protease in a wide range of cells (52). The first residue of exon 5 within human pro-IGF-IEb is Tyr87; for an endogenous 24-amino-acid MGF to exist, amino-terminal cleavage at Tyr87 must occur (Fig. 3), and no SPCs with this function have been identified. Thus, no known furin or furin-like protease can cleave at the amino acid required to generate MGF, and if an endogenous peptide product equivalent to the synthetic MGF peptide exists, it must be generated by a novel mechanism. A nontrypsin serine protease-like enzyme was purified from rat synaptosomes that cleave pro-cholecystokinin C-terminal to a single lysine residue to generate cholecystokinin-8 (53). In theory, such an activity could cleave pro-IGF-I between Lys86 and Tyr87 to generate MGF. However, experimental evidence for this possibility is currently lacking.
Is MGF an Activator of and Mitogen for Satellite Cells?
Satellite cells are skeletal muscle stem cells that provide a source of nuclei to postmitotic muscle tissue and were first described by Mauro almost 50 yr ago (54). Subsequent research revealed that satellite cells reside between the basal lamina and the sarcolemma of the myofiber and that they are quiescent during adulthood but active during the postnatal growth period (55,56). Upon stimulus, satellite cells are activated to enter the cell cycle whereupon they express muscle-specific transcription factors such as MyoD (57). Progenitor cells then proliferate and become fusion-competent myoblasts and finally differentiate and fuse to form new myofibers or fuse with existing damaged myofibers (58,59). Additionally, these cells can self-renew, thus replenishing the satellite cell pool (60). Activation of satellite cells can be induced by several factors including epidermal growth factor (61), hepatocyte growth factor (62), and nitric oxide (63,64), whereas proliferation and differentiation can be promoted by other factors, such as IGF-I (65).
Studies to define a potential direct involvement of IGF-IEb in satellite cell activation were first reported in 2003 (39). In this study, rat tibialis anterior (TA) muscles were damaged by stretch and electrical stimulation, or by myotoxin injection, and regenerating muscles were analyzed at different time points up to 24 d after damage. It was found that levels of IGF-IEb mRNA were elevated at earlier time points during recovery than IGF-IEa mRNA; furthermore, the earlier increases in levels of IGF-IEb generally correlated with increased MyoD and M-cadherin mRNA, which were used as markers of satellite cell activation. Thus, the levels of IGF-IEb mRNA increased above control at early time points (∼1–4 d) but then declined, followed by increased levels of IGF-IEa transcripts at d 5–24. It should be noted, however, that the absolute number of transcripts for the Ea mRNA was always about 10-fold greater than the number of Eb transcripts, even when the latter were elevated, though fold changes of Eb and Ea were similar. In a follow-up study (27), these investigators found that levels of IGF-IEb (MGF) mRNA peaked before maximal M-cadherin immunoreactivity and mRNA in regenerating TA muscle but that levels of IGF-IEa mRNA reached peak elevation after maximal M-cadherin expression. Taken together with earlier results demonstrating that IGF-IEb mRNA is increased in concert with MyoD mRNA in a surgical overload model, the authors speculated that because maximal IGF-IEb mRNA levels were seen before the appearance of markers of satellite cell activation, the protein products generated from the IGF-IEb mRNA transcript were responsible for satellite cell activation.
In a more recent study, addition of a synthetic MGF peptide (originally described in Ref. 33) (see sequence in Table 1) increased the number of desmin-positive myogenic precursor cells derived from healthy and diseased muscles (66), suggesting that synthetic MGF can influence committed skeletal muscle progenitor cell proliferation. However, because desmin may be expressed in quiescent and activated satellite cells (67), a direct role of synthetic MGF on satellite cell activation per se, remains inconclusive.
In contrast to the above, work from the Barton laboratory (68) suggests that IGF-IEb may not activate satellite cells. When anterior hind-limb muscle was injected with recombinant adenovirus with a vector encoding murine IGF-IEb in either 2-wk- or 6-month-old mice, hypertrophy was detected in 2-wk, but not 6-month, TA muscles 4 months after injection (68). This finding was important because it suggested that the actions of IGF-IEb were effective in developing muscle (presumably possessing an active satellite cell pool) but not in mature muscle (which are absent a significantly active satellite cell pool). Thus, the ability of IGF-IEb mRNA products to activate quiescent satellite cells was not conclusively demonstrated in this study.
In summary, several studies have investigated the role of IGF-IEb mRNA expression during muscle recovery from injury. Speculation is widespread that an independent Eb peptide derived from pro-IGF-I (i.e. MGF) mediates satellite cell activation; indeed, it has generally come to be taken as having been established fact. However, there is a lack of definitive evidence at present for a direct action of MGF on satellite cell activation. Although administration of synthetic MGF to C2C12 myoblasts (28) or committed myoblast precursors (66) has been shown to increase their proliferation, whether MGF is an effector of, or whether increases in IGF-IEb mRNA simply correlate with, activation of satellite cells has yet to be established. Therefore, until conclusive evidence demonstrates a direct role for MGF in satellite cell activation, correlations between markers of satellite cell activation and increased MGF should only be interpreted as such.
MGF and Aging
Depending on skeletal muscle type, approximately 1.5–7% of total nuclei are satellite cells (69). Satellite cell number declines with age, which may result in overall decreased recruitment or activation (70,71,72); indeed, decreased activation of aged satellite cells is associated with loss of Notch (73) and possibly other factors that are present in, or are responsive to, circulating factors in young animals (74). The effect of aging on the expression of IGF-IEb mRNA (Ec in humans) has been the topic of several research studies, particularly in skeletal muscle. It has been proposed that age-related sarcopenia results from lowered expression of MGF (presumably derived from IGF-IEb propeptide, according to the MGF hypothesis); hence, MGF represents a target for therapeutic intervention in preventing age-related muscle loss due to its supposed ability to activate satellite cells.
Research addressing the involvement of MGF in muscle repair/regeneration in young vs. old animals was pioneered by Goldspink’s laboratory. In an early study, it was shown in rats that there was no significant difference in IGF-IEb mRNA levels in unperturbed muscle of young (3 months old), middle-aged (12 months), or old (24 months) animals (34). However, increased IGF-IEb mRNA was noted in all age groups 5 d after surgical ablation of the distal gastrocnemius tendon, with the young rats showing the greatest IGF-IEb mRNA induction and old rats showing the least induction. These results suggested that in rodents, levels of IGF-IEb mRNA are elevated in muscles that compensate for overload induced by tenectomy but that the magnitude of elevation decreases as a function of age. These findings are in contrast to a study that evaluated levels of IGF-IEb after stimulated isometric contraction (simulating resistance exercise) in young (6 months old) and old (30 months) rats (75). Twenty-four and 48 h after the resistance exercise bout, levels of IGF-IEb mRNA were elevated to the same degree in both young and old rat medial gastrocnemius, demonstrating that there was no age-related decline in IGF-IEb mRNA responsiveness to isometric contraction.
In human skeletal muscle, there were no differences in IGF-IEc (corresponding to rodent IGF-IEb) mRNA levels in muscle between young (∼29.5 yr) and old (∼74.4 yr) men. However, levels of IGF-IEc in vastus lateralus were increased 2.5 h after knee extension resistance exercise in young but not old men, suggesting the possibility that IGF-IEc mRNA responses may be sensitive to age (25).
In a longitudinal study performed in elderly men (∼74 yr old), increases in IGF-IEc mRNA were seen after 5 and 12 wk GH administration or resistance training compared with the beginning of the study. Furthermore, when elderly subjects receiving GH also performed resistance exercise, there was an even greater elevation of IGF-IEc mRNA than with resistance training or GH alone (76). In contrast, young men receiving either GH or placebo for 14 d before an acute exercise bout showed no increased IGF-IEc mRNA levels 2.5 h after exercise (30), a finding in contrast with the earlier observations in young men (mentioned above) that used similar exercise protocols, sampling techniques, and experimental methodologies (25).
Thus, in humans, whether IGF-IEc mRNA is increased after mechanical stress is not entirely clear. In elderly men, IGF-IEc (MGF) mRNA is increased after long-term exercise and GH administration but not after an acute bout of exercise. Evidence for elevation of IGF-IEc mRNA in young men 2.5 h after an acute bout of resistance exercise is conflicting; therefore, it is difficult to draw conclusions with respect to IGF-IEc mRNA up-regulation after exercise in young vs. old humans. Increased sample sizes and a greater number of sampling points during an extended recovery period may clarify the magnitude of difference in IGF-IEc levels after exercise in young and old individuals. In rodents, however, there is evidence IGF-IEb (MGF) mRNA is up-regulated in response to compensatory overload in some models, and these increases are attenuated in older animals. Given these observations, if aging reduces levels of IGF-IEb, is this due to altered mRNA splicing or decreased IGF-IEb mRNA stability? What factors are absent or present in aged muscle that mediate these splicing events? Does impaired satellite cell activation and muscle mass result, in some part, from deficiency of a locally produced Eb peptide? Would provision of Eb peptide prevent age-related sarcopenia or improve regeneration/repair after injury in aged skeletal muscle, and if so, what are the biological processes that are mediated?
MGF as a Neuroprotective Agent
Early evidence for a putative neuroprotective effect of MGF was published in 2004 (77). In this study, rabbit IGF-IEb (MGF) cDNAs encoding mature IGF-I as well as the C-terminal Eb region (referred to as MGF in this study) were injected into rat facial muscles before avulsion of the facial nerve. Those muscles that had received the MGF plasmid injection were almost completely protected from motoneuron loss 1 month after nerve avulsion. In a more recent study (78), im injection of similar plasmids prevented the loss of muscle force and weight as well as prevented the loss of spinal cord motoneurons of 120-d-old SOD1G93A mice, a model of amyotrophic lateral sclerosis. Thus, it was concluded that MGF could contribute to preservation of muscular phenotypes during onset of motoneuron-targeted disease. However, immunoreactive bands detected by Western blot with a polyclonal anti-MGF antibody were evident in the 25- to 37-kDa range and were presented as evidence that plasmid injection induced MGF, despite the fact that the actual protein products of the plasmid expression were not characterized. Characterization of these immunoreactive bands is important because there is no known Igf1 gene product that contains the C-terminal 24 amino acids of IGF-IEb greater than approximately 17.8 kDa, which corresponds to pre-pro-IGF-IEb (Table 2). Until effects of an endogenous peptide equivalent to MGF are differentiated from those of mature IGF-I in this model, it is premature to assign any role to a putative in vivo MGF, especially in the case of a protein possessing Mr an order of magnitude greater than the putative MGF peptide.
In a study performed in gerbils, brains underwent transient ischemia and levels of MGF were assessed at different time points during recovery (33). An antibody raised against a peptide sequence derived from the 24 most C-terminal amino acids of pro-IGF-IEb was used to determine endogenous MGF protein levels. Immunoreactive bands (between 15 and 25 kDa, possibly consistent with a pro-form of IGF-I) were observed in ischemia-resistant portions of the hippocampus, prompting the authors to suggest a possible link between endogenous MGF production and resistance to ischemic brain injury, despite the high Mr protein detected. Administration of a synthetic MGF peptide immediately after reperfusion was associated with increased survival of neurons, suggesting that the synthetic MGF peptide was protective against neuronal degradation.
Synthetic MGF has also been shown to promote survival in response to neurotoxins through a mechanism that may involve heme-oxygenase-1 (79). Addition of synthetic MGF to SH-SY5Y cultured neurons protected against neurotoxin-induced mitochondrial stress and apoptosis concordant with MGF-stimulated increases in heme-oxygenase-1, which was found to be required for neuroprotective effects of MGF. Additionally, MGF was protective in vivo in rat substantia nigra dopamine neurons; rats receiving MGF infusion were protected from motor behavior deficits as well as neuronal cell death. Overall, the authors suggested that synthetic MGF peptide administration may represent a new strategy to protect neurons from oxidative stress-induced neurotoxicity seen in Parkinson’s disease.
These studies provide evidence that im injection of mammalian expression vectors encoding IGF-IEb cDNA, or administration of synthetic MGF peptide, is neuroprotective; indeed, both the synthetic peptide and injections of vectors encoding IGF-IEb cDNA promoted survival. However, a stable protein product of the Igf1 gene corresponding exactly to those amino acids contained in the synthetic MGF peptide has not yet been identified. Furthermore, the protein products resulting from overexpression were not shown to represent pro-IGF-I, mature IGF-I, the Eb peptide, or the 24 most C-terminal residues of pro-IGF-I (MGF); hence, the identity of the immunoreactive bands remain unknown. Due to their size, these bands most likely represent pro-IGF-IEb or perhaps even nonspecific antibody binding. Additionally, until the 24-amino-acid MGF peptide is shown to exist in vivo, neuroprotective actions of the synthetic MGF peptide should be considered independent from expression of the endogenous IGF-I Eb peptide and, indeed, any product of the Igf1 gene.
Putative MGF Signaling Pathways
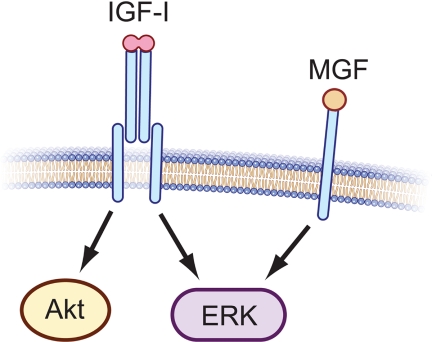
Although many studies had previously examined end-point outcomes of IGF-IEb overexpression or synthetic MGF administration, only recently have the potential signaling mechanisms involved in effecting these outcomes been investigated. Two recent studies provide evidence that synthetic MGF may act through ERK in C2C12 myoblasts and H9c2 cardiomyocytes. Addition of synthetic MGF peptide to C2C12 (31) or Hc92 (80) cells stimulated phosphorylation of ERK, but not Akt, even in the presence of a monoclonal IGF-I receptor (IGF-IR) neutralizing antibody, suggesting that synthetic MGF-mediated ERK activation is independent of IGF-IR and that synthetic MGF does not activate Akt (Fig. 4). These findings have, collectively, led to speculation that MGF is responsible for muscle progenitor cell proliferation through ERK activation, whereas mature IGF-I promotes differentiation and protein synthesis through actions of Akt. However, because no evidence to date suggests that pro-IGF-I is processed to yield a peptide product equivalent to synthetic MGF, the effects of synthetic MGF peptide administration on myoblast proliferation and ERK activation cannot, at present, be correlated with effects of any natural Igf1 gene product. Nevertheless, these data illuminate larger questions. For example, does a stable Eb peptide exist endogenously, and if so, through what mechanism is it generated? If this peptide exists, does it bind to a receptor or exert biological action similar to that of synthetic MGF? Alternatively, if the effects ascribed to MGF actually represent effects of proIGF-IEb, then through what receptor does this proIGF-I isoform signal? How does the MGF hypothesis incorporate a preponderance of cell culture data showing that full-length IGF-I increases myoblast proliferation through activation of ERK but later promotes myogenic differentiation and hypertrophy by activating the PI3K/Akt pathway (40,41,42,43,44,45,46)? Do the data describing synthetic MGF-mediated survival and ERK signaling suggest its potential use as a therapeutic agent, despite a current lack of evidence for the existence of the Eb peptide in vivo, and because IGF-I itself can act as a mitogen, what advantage is gained by endogenous generation or exogenous administration of MGF?
Figure 4.
IGF-IR and MGF signaling are distinct. Signaling through the IGF-IR activates a number of intracellular signaling pathways including PI3K/Akt and MAPK kinase/ERK. Conversely, MGF synthetic peptide has been proposed to signal through an IGF-IR-independent mechanism to activate ERK, but not Akt (31,80). A specific MGF receptor has not yet been identified; thus, that monomeric MGF binds to a single-chain transmembrane receptor as illustrated, is speculative.
Summary and Future Directions
The majority of studies evaluating the role of MGF in muscle repair have focused on increases in IGF-IEb (Ec in humans) mRNA after damage induced by myotoxin, stretch/stimulation, or exercise. In an effort to correlate changes in IGF-IEb mRNA levels with assumed increased levels of MGF protein, synthetic peptides have been generated whose amino acid sequences are identical to that contained in the C-terminal-most 24 amino acids in pro-IGF-IEb. The choice of this sequence to represent a putative Eb peptide has been described as “somewhat mysterious” (6); indeed, there is no evidence for such a peptide existing in biological systems, and pro-IGF-IEb contains no identified regulatory or proteolytic sites from which this peptide could be liberated from either pro-IGF-IEb or the E peptide extension encoded by exon 4 of the Igf1 gene (Fig. 3). Thus, until it can be confirmed that a protein product derived from pro-IGF-IEb that is identical to the synthetic MGF peptide exists, interpretation of findings resulting from use of this peptide should not be considered secondary to increased IGF-IEb mRNA or pro-IGF-IEb but rather autonomous effects of the synthetic MGF peptide itself. Indeed, work using an MGF-specific antibody demonstrated the presence of immunoreactive bands at molecular weights that correspond to pro-IGF-I rather than MGF (31,32,33,78,80), suggesting that MGF sequence is stable only when a part of pro-IGF-IEb. Finally, the role of MGF (whether as a synthetic peptide or putative protein product of the Igf1 gene) in satellite cell activation is unclear. Whether IGF-IEb/MGF activates satellite cells or whether increased levels of IGF-IEb mRNA simply correlate with satellite cell activation requires further investigation.
To more adequately address the proposed function and relevance of MGF in tissue proliferation, growth, survival, and satellite cell activation, several concepts should be addressed.
1) Demonstration that MGF exists in vivo
An effort should be made to determine the existence and stability of a peptide product identical to synthetic MGF. The identification of preptin, a 34-amino-acid peptide derived from the E-peptide of pro-IGF-II, provides an illustration of a process through which identification of MGF could be made in vivo (81). By using matrix-assisted laser desorption/ionization-time of flight mass spectrometry and reverse-phase HPLC, the investigators detected a 3.95-kDa peptide among purified peptides from secretory granules isolated from cultured murine βTC6-F7 β-cells. After N-terminal protein sequencing, this peptide was identified as a 34-amino-acid fragment of pro-IGF-II. These investigators then characterized preptin by preptin-specific RIA in βTC6-F7 islet β-cell granules and pancreatic tissue sections stained with preptin antisera. Finally, the investigators identified that preptin is cosecreted with insulin and functions to increase glucose-stimulated insulin secretion. Thus, identification, sequencing, characterization, and functional outcome determinations in this manner would go far in demonstrating the existence of MGF in vivo.
2) Relevance
In addition to the numerous MGF overexpression models described above, it is necessary to determine whether loss of IGF-IEb/MGF prevents growth, proliferation, survival, or satellite cell activation. Generation of knockout mouse models incapable of producing IGF-IEb may benefit evaluation of these processes in vivo.
3) Identification of a receptor
Synthetic MGF peptide may exert its effects through an IGF-IR-independent mechanism; this is not surprising because presumably, the tertiary conformation of synthetic MGF differs from mature IGF-I due to considerable differences in primary structure. Identification of a receptor and characterization of its actions will facilitate understanding of synthetic MGF-mediated biological outcomes. With respect to endogenous Eb peptide or MGF in vivo, however, it will be necessary to confirm their existence as outlined in point 1 before attempting to identify a bona fide cognate receptor. Alternatively, if effects ascribed to MGF are due actually to proIGF-IEb, then it will be necessary to determine whether this molecule signals through the canonical IGF-I receptor, or a novel receptor.
4) Defining signaling pathways
Two studies to date have shown that synthetic MGF activates ERK but not Akt. Defining the signaling pathways both upstream and downstream of MGF-mediated ERK activation, as well as discovering other signaling molecules activated by MGF, is essential to define the mechanisms through which MGF exerts its effects.
5) Nomenclature
The term MGF has been used to describe IGF-IEb mRNA, pro-IGF-IEb, and the synthetic MGF peptide corresponding to the C-terminal end of pro-IGF-IEb. Because discrepancy exists with respect to this naming schema (e.g. no evidence that a stable 24-amino-acid protein derived from Igf1 gene exists), we submit that the term MGF be used only when referring to synthetic MGF. Additionally, because MGF was named as a result of observing increased IGF-IEb mRNA levels in response to mechanical stimuli, and because there currently exists no link between IGF-IEb mRNA and synthetic MGF, we submit that the term MGF be avoided when referring to an IGF-I mRNA splice variant. When examining levels of IGF-IEb mRNA or protein, they should be referenced as IGF-IEb (or Ec in the case of human study) to avoid confusion.
In summary, numerous studies report that levels of the IGF-IEb splice variant increase after tissue damage. However, relating this phenomenon to the overall MGF hypothesis, which is strongly based on evidence from synthetic MGF peptide administration, is difficult due to lack of detection of an in vivo MGF, uncertainty about whether effects ascribed to MGF in actuality reflect actions of endogenous IGF-IEb, as well as a lack of evidence that mature IGF-I or pro-IGF-IEa is not also present at high levels during this period of satellite cell activation and proliferation. There is, however, support for mitogenic effects of synthetic MGF, but whether an equivalent peptide is derived from endogenous pro-IGF-I remains to be established.
Footnotes
This work was supported by National Institute on Aging Grant R01AG026012 to M.L.A. and an appointment to the Postgraduate Research Participation Program at the U.S. Army Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. Army Medical Research and Materiel Command (R.W.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 3, 2010
Abbreviations: IGF-IR, IGF-I receptor; MGF, mechano-growth factor; Mr, relative molecular mass; PI3K, phosphoinositide 3-kinase; SPC, subtilisin-like proprotein convertase; TA, tibialis anterior.
References
- Goldspink G 2005 Research on mechano growth factor: its potential for optimising physical training as well as misuse in doping. Br J Sports Med 39:787–788; discussion 787–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman R 2006 Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev 17:305–323 [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR 1995 Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- Adamo ML, Ben-Hur H, LeRoith D, Roberts Jr CT 1991 Transcription initiation in the two leader exons of the rat IGF-I gene occurs from disperse versus localized sites. Biochem Biophys Res Commun 176:887–893 [DOI] [PubMed] [Google Scholar]
- Rotwein P, Bichell DP, Kikuchi K 1993 Multifactorial regulation of IGF-I gene expression. Mol Reprod Dev 35:358–363; discussion 363–354 [DOI] [PubMed] [Google Scholar]
- Barton ER 2006 The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab 31:791–797 [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Winn N, Rosenthal N, Grounds MD 2005 Reconciling data from transgenic mice that overexpress IGF-I specifically in skeletal muscle. Growth Horm IGF Res 15:4–18 [DOI] [PubMed] [Google Scholar]
- Yang H, Adamo ML, Koval AP, McGuinness MC, Ben-Hur H, Yang Y, LeRoith D, Roberts Jr CT 1995 Alternative leader sequences in insulin-like growth factor I mRNAs modulate translational efficiency and encode multiple signal peptides. Mol Endocrinol 9:1380–1395 [DOI] [PubMed] [Google Scholar]
- Rotwein P, Pollock KM, Didier DK, Krivi GG 1986 Organization and sequence of the human insulin-like growth factor I gene. Alternative RNA processing produces two insulin-like growth factor I precursor peptides. J Biol Chem 261:4828–4832 [PubMed] [Google Scholar]
- Chew SL, Lavender P, Clark AJ, Ross RJ 1995 An alternatively spliced human insulin-like growth factor-I transcript with hepatic tissue expression that diverts away from the mitogenic IBE1 peptide. Endocrinology 136:1939–1944 [DOI] [PubMed] [Google Scholar]
- Bell GI, Stempien MM, Fong NM, Rall LB 1986 Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res 14:7873–7882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Jr CT, Lasky SR, Lowe Jr WL, Seaman WT, LeRoith D 1987 Molecular cloning of rat insulin-like growth factor I complementary deoxyribonucleic acids: differential messenger ribonucleic acid processing and regulation by growth hormone in extrahepatic tissues. Mol Endocrinol 1:243–248 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Shaw DS 1986 Purification and biologic properties of fibroblast somatomedin. J Biol Chem 261:10293–10298 [PubMed] [Google Scholar]
- Rotwein P, Folz RJ, Gordon JI 1987 Biosynthesis of human insulin-like growth factor I (IGF-I). The primary translation product of IGF-I mRNA contains an unusual 48-amino acid signal peptide. J Biol Chem 262:11807–11812 [PubMed] [Google Scholar]
- Bach MA, Roberts Jr CT, Smith EP, LeRoith D 1990 Alternative splicing produces messenger RNAs encoding insulin-like growth factor-I prohormones that are differentially glycosylated in vitro. Mol Endocrinol 4:899–904 [DOI] [PubMed] [Google Scholar]
- Powell DR, Lee PD, Chang D, Liu F, Hintz RL 1987 Antiserum developed for the E peptide region of insulin-like growth factor IA prohormone recognizes a serum protein by both immunoblot and radioimmunoassay. J Clin Endocrinol Metab 65:868–875 [DOI] [PubMed] [Google Scholar]
- Siegfried JM, Kasprzyk PG, Treston AM, Mulshine JL, Quinn KA, Cuttitta F 1992 A mitogenic peptide amide encoded within the E peptide domain of the insulin-like growth factor IB prohormone. Proc Natl Acad Sci USA 89:8107–8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HE, Westwood M, White A, Clayton PE 2001 Monoclonal antibodies to the carboxy-terminal Ea sequence of pro-insulin-like growth factor-IA (proIGF-IA) recognize proIGF-IA secreted by IM9 B-lymphocytes. Growth Horm IGF Res 11:10–17 [DOI] [PubMed] [Google Scholar]
- Pfeffer LA, Brisson BK, Lei H, Barton ER 2009 The insulin-like growth factor (IGF)-I E-peptides modulate cell entry of the mature IGF-I protein. Mol Biol Cell 20:3810–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Alnaqeeb M, Simpson H, Goldspink G 1996 Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil 17:487–495 [DOI] [PubMed] [Google Scholar]
- McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G 1999 Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516(Pt 2):583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Alnaqeeb M, Simpson H, Goldspink G 1997 Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat 190(Pt 4):613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe Jr WL, Lasky SR, LeRoith D, Roberts Jr CT 1988 Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol 2:528–535 [DOI] [PubMed] [Google Scholar]
- Goldspink G 2005 Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology (Bethesda) 20:232–238 [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD 2003 Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer EM, Modern concepts of insulin-like growth factors: Proc Second International Symposium on Insulin-Like Growth Factors/Somatomedins, San Francisco, CA, 1991 [Google Scholar]
- Hill M, Wernig A, Goldspink G 2003 Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Goldspink G 2002 Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett [Erratum (2006) 580:2530] 522:156–160 [DOI] [PubMed] [Google Scholar]
- Evans RM, Harridge SD, Velloso CP, Yang SY, Goldspink G, Orrell RW July 2009 Investigation of MGF mRNA expression in patients with amyotrophic lateral sclerosis using parallel in vivo and in vitro approaches. Amyotroph Lateral Scler 10.1080/17482960903089775 [DOI] [PubMed] [Google Scholar]
- Aperghis M, Velloso CP, Hameed M, Brothwood T, Bradley L, Bouloux PM, Harridge SD, Goldspink G 2009 Serum IGF-I levels and IGF-I gene splicing in muscle of healthy young males receiving rhGH. Growth Horm IGF Res 19:61–67 [DOI] [PubMed] [Google Scholar]
- Philippou A, Papageorgiou E, Bogdanis G, Halapas A, Sourla A, Maridaki M, Pissimissis N, Koutsilieris M 2009 Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro. In Vivo 23:567–575 [PubMed] [Google Scholar]
- Philippou A, Stavropoulou A, Sourla A, Pissimissis N, Halapas A, Maridaki M, Koutsilieris M 2008 Characterization of a rabbit antihuman mechano growth factor (MGF) polyclonal antibody against the last 24 amino acids of the E domain. In Vivo 22:27–35 [PubMed] [Google Scholar]
- Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Górecki DC, Zabłocka B 2005 A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J 19:1896–1898 [DOI] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G 2001 Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett 505:259–263 [DOI] [PubMed] [Google Scholar]
- Jennische E, Skottner A, Hansson HA 1987 Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand 129:9–15 [DOI] [PubMed] [Google Scholar]
- Edwall D, Schalling M, Jennische E, Norstedt G 1989 Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology 124:820–825 [DOI] [PubMed] [Google Scholar]
- Adams GR, Haddad F 1996 The relationships among IGF-1, DNA content, and protein accumulation during skeletal muscle hypertrophy. J Appl Physiol 81:2509–2516 [DOI] [PubMed] [Google Scholar]
- DeVol DL, Rotwein P, Sadow JL, Novakofski J, Bechtel PJ 1990 Activation of insulin-like growth factor gene expression during work-induced skeletal muscle growth. Am J Physiol 259:E89–E95 [DOI] [PubMed] [Google Scholar]
- Hill M, Goldspink G 2003 Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549:409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adi S, Bin-Abbas B, Wu NY, Rosenthal SM 2002 Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143:511–516 [DOI] [PubMed] [Google Scholar]
- Clemmons DR 2009 Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab 20:349–356 [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR 1997 The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272:6653–6662 [DOI] [PubMed] [Google Scholar]
- Engert JC, Berglund EB, Rosenthal N 1996 Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135:431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SE, Allen RE 1990 The effects of bFGF, IGF-I, and TGF-β on RMo skeletal muscle cell proliferation and differentiation. Exp Cell Res 187:250–254 [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ 2001 Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3:1009–1013 [DOI] [PubMed] [Google Scholar]
- Tureckova J, Wilson EM, Cappalonga JL, Rotwein P 2001 Insulin-like growth factor-mediated muscle differentiation. J Biol Chem 276:39264–39270 [DOI] [PubMed] [Google Scholar]
- Koyama T, Nakaoka Y, Fujio Y, Hirota H, Nishida K, Sugiyama S, Okamoto K, Yamauchi-Takihara K, Yoshimura M, Mochizuki S, Hori M, Hirano T, Mochizuki N 2008 Interaction of scaffolding adaptor protein Gab1 with tyrosine phosphatase SHP2 negatively regulates IGF-I-dependent myogenic differentiation via the ERK1/2 signaling pathway. J Biol Chem 283:24234–24244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko IV, Furalyov VA, Khotchenkov VP, Popov VO 2006 Monoclonal antibodies to mechano-growth factor. Hybridoma (Larchmt) 25:300–305 [DOI] [PubMed] [Google Scholar]
- Rouillé Y, Duguay SJ, Lund K, Furuta M, Gong Q, Lipkind G, Oliva Jr AA, Chan SJ, Steiner DF 1995 Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases. Front Neuroendocrinol 16:322–361 [DOI] [PubMed] [Google Scholar]
- Rockwell NC, Thorner JW 2004 The kindest cuts of all: crystal structures of Kex2 and furin reveal secrets of precursor processing. Trends Biochem Sci 29:80–87 [DOI] [PubMed] [Google Scholar]
- Duguay SJ, Lai-Zhang J, Steiner DF 1995 Mutational analysis of the insulin-like growth factor I prohormone processing site. J Biol Chem 270:17566–17574 [DOI] [PubMed] [Google Scholar]
- Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF 1997 Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem 272:6663–6670 [DOI] [PubMed] [Google Scholar]
- Viereck JC, Beinfeld MC 1992 Characterization of a cholecystokinin 8-generating endoprotease purified from rat brain synaptosomes. J Biol Chem 267:19475–19481 [PubMed] [Google Scholar]
- Mauro A 1961 Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E, Gibson MC, Champion T 1978 Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool 206:451–456 [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP 1971 Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170:421–435 [DOI] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA 2008 The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14:82–91 [DOI] [PubMed] [Google Scholar]
- Adams GR 2006 Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab 31:782–790 [DOI] [PubMed] [Google Scholar]
- Chargé SB, Rudnicki MA 2004 Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238 [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA 2008 Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2:22–31 [DOI] [PubMed] [Google Scholar]
- Golding JP, Calderbank E, Partridge TA, Beauchamp JR 2007 Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp Cell Res 313:341–356 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE 1998 HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194:114–128 [DOI] [PubMed] [Google Scholar]
- Wozniak AC, Anderson JE 2007 Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 236:240–250 [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE 2006 Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 290:C1487–C1494 [DOI] [PubMed] [Google Scholar]
- Machida S, Booth FW 2004 Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc 63:337–340 [DOI] [PubMed] [Google Scholar]
- Ates K, Yang SY, Orrell RW, Sinanan AC, Simons P, Solomon A, Beech S, Goldspink G, Lewis MP 2007 The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett 581:2727–2732 [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z 2007 Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ER 2006 Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol 100:1778–1784 [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S, Kjaer M 2005 The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451:319–327 [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J 2004 Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 29:120–127 [DOI] [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM 2005 Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci 118:4813–4821 [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z 2006 Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294:50–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA 2003 Notch-mediated restoration of regenerative potential to aged muscle. Science 302:1575–1577 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA 2005 Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764 [DOI] [PubMed] [Google Scholar]
- Haddad F, Adams GR 2006 Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol 100:1188–1203 [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G 2004 The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol 555:231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aperghis M, Johnson IP, Cannon J, Yang SY, Goldspink G 2004 Different levels of neuroprotection by two insulin-like growth factor-I splice variants. Brain Res 1009:213–218 [DOI] [PubMed] [Google Scholar]
- Riddoch-Contreras J, Yang SY, Dick JR, Goldspink G, Orrell RW, Greensmith L 2009 Mechano-growth factor, an IGF-I splice variant, rescues motoneurons and improves muscle function in SOD1(G93A) mice. Exp Neurol 215:281–289 [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych P, Handforth A 2009 C-terminal mechano growth factor protects dopamine neurons: a novel peptide that induces heme oxygenase-1. Exp Neurol 220:255–266 [DOI] [PubMed] [Google Scholar]
- Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M 2009 IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med 15:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Phillips AR, Cooper GJ 2001 Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet β-cells and enhances insulin secretion. Biochem J 360:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]