Abstract
The adrenal capsule is postulated to harbor stem/progenitor cells, the progenies of which contribute to the growth of adrenocortex. We discovered that cells in the adrenal capsule are positive for Ptch1 and Gli1, genes indicative of responsiveness to the stimulation of Hedgehog (Hh) ligands. On the other hand, Sonic hedgehog (Shh), one of the mammalian Hh ligands, is expressed in the adrenocortex underneath the adrenal capsule, possibly acting upon the Hh-Responsive capsule. To investigate the functional significance of Shh in adrenal growth, we ablated Shh in an adrenocortex-specific manner using the Steroidogenic factor 1-Cre mouse. Loss of Shh in the adrenocortex led to reduced proliferation of capsular cells and a 50–75% reduction in adrenocortex thickness and adrenal size. The remaining adrenocortex underwent proper zonation and was able to synthesize steroids, indicating that Shh is dispensable for differentiation of adrenocortex. When these animals reached adulthood, their adrenocortex did not undergo compensatory growth in response to a high level of plasma ACTH, and the size of the adrenal remained significantly smaller than the control adrenal. Using a genetic lineage-tracing model, we further demonstrated that the Hh-responding cells in the adrenal capsule migrated centripetally into the adrenocortex. Our results not only provide the genetic evidence to support that the adrenal capsule contributes to the growth of adrenocortex in both fetal and adult life but also identify a novel role of Shh in this process.
Growth of the fetal adrenal cortex depends upon expansion of progenitor cells in the adrenal capsule, which is regulated by a local factor, Sonic hedgehog.
Organogenesis requires a balance between cell proliferation and differentiation. Progenitor cells of the organ primordium undergo self-renewal and expansion while simultaneously differentiating into organ-specific cell types. In developing mouse adrenal, Steroidogenic factor 1 (SF1)-expressing cells from the adrenogonadal primordium serve as the founders for the future cortex (1). The SF1-expressing primordium is later encapsulated by the mesenchymal capsule, which consists of a single coelomic epithelium and underlying layers of fibroblast-like cells (2). Cells in the adrenal capsule and subcapsular cortical region have a higher proliferation index (3). It is proposed that the progenies of these proliferating cells migrate centripetally and differentiate into adrenocortical cells (2,4,5). When the adrenal parenchyma is removed (a process called enucleation), the remaining capsule and subcapsular cells undergo regeneration, eventually leading to restoration of a functional cortex (6,7). These observations together point to the possible existence of stem/progenitor cells in the adrenal capsule and subcapsular region.
Establishment of the adrenal primordium and its growth are regulated by a network of transcription regulators. SF1, a putative orphan nuclear receptor, is expressed at as early as 9 days post coitum (dpc) in mouse adrenal cortex, and the disruption of Sf1 causes adrenal aplasia (1). Dax1, which is also a member of the nuclear hormone receptor superfamily, represses SF1-mediated transcription and is specifically expressed in the highly proliferating cortical area (8,9,10). Loss of functions of the Dax1 gene causes congenital adrenal hypoplasia in humans (11,12). Other transcription factors such as transcription factor 21 (Tcf21, also known as Pod1), pre-B cell leukemia transcription factor 1 (Pbx1), Wilms tumor 1 (Wt1), and CBP/p300-interacting transactivator with ED-rich tail 2 (Cited2) are also involved in adrenal development (13,14,15,16). The finding that Wnt4 and β-catenin, key components of the Wnt canonical signaling pathway, are essential for adrenal development (17,18), suggests that the adrenal development is also under the influence of secreted signaling molecules.
Sonic hedgehog (SHH), a paracrine/autocrine morphogen, elicits its function by binding to the membrane-bound complex consisting of receptor Patched1 (PTCH1) and Smoothened (SMO). Binding of SHH to PTCH1 releases the inhibitory effect of PTCH1 on SMO, therefore allowing SMO to activate the downstream signaling pathway that involves transcriptional regulation via Gli1, Gli2, and Gli3 transcription factors (19). SHH is known to regulate expression of genes involved in cell proliferation (20) and plays critical roles in progenitor cell renewal, cell lineage specification, and tissue regeneration in the organs of various species. For example, self-renewal of neurosphere-forming stem cells in adult mouse forebrain requires the presence of Shh (21). In mouse retina, SHH stimulates progenitor cell proliferation and diversification (22). SHH is also involved in differentiation of mouse and human embryonic stem cells (23,24). Expression of Shh was found in the developing mouse adrenal (25) and Gli3 mutant mice developed adrenal agenesis (26), suggesting a critical role of the Hh signaling in adrenal development.
In this study, we tested the hypothesis that the adrenal capsule contributes to the growth and expansion of the adrenal gland. Using a loss-of-function genetic model and a lineage-tracing experiment, we demonstrated that Shh is involved in the expansion of the progenitor pool in the adrenal capsule. SF1-positive adrenocortical cells control the expansion of the adrenocortex by producing SHH that acts upon the adrenal capsule.
Materials and Methods
Animals and experimental protocols
The Ptch1-LacZ reporter line and Shh floxed mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and Gli1-LacZ mice were provided by Dr. Alexandra Joyner (27,28). We first generated Shh+/− mice by crossing Shhf/f male mice to EIIa-Cre female mice, which express the Cre recombinase ubiquitously since the one-cell stage (29). Then the EIIa-Cre was removed by backcrossing to inbred strain (C57BL/6) female. The resulting Shh+/− mice were mated with Sf1/Cre transgenic mice (30) to obtain the Shh+/−;Sf1/Cre mouse line. Conditional Shh knockout (KO) mice were then generated by mating Shh+/−;Sf-1/Cre with Shhf/f mice. Female and male mice were paired together and checked for the presence of a vaginal plug the next morning. The day when the vaginal plug was detected was considered 0.5 d of gestation, or 0.5 dpc. All procedures described were reviewed and approved by the Institutional Animal Care and Use Committee at University of Illinois and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals. All experiments were performed on at least three animals for each genotype.
Histological analysis, LacZ staining, and TUNEL
The specimens were fixed with 4% paraformaldehyde/PBS overnight, and embedded in paraffin following standard sectioning and stain in hematoxylin/eosin. For LacZ staining, fresh tissues were prefixed in 4% paraformaldehyde/PBS for 30 min, followed by three washes in PBS. The tissues were stained in LacZ staining solution as described elsewhere (31). For further immunohistochemical staining, tissues after LacZ staining were fixed and embedded in paraffin following standard sectioning procedure. Terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay was performed on paraffin sections with Roche’s TUNEL assay kit (Roche Co., Ltd., Indianapolis, IN) according to the manufacturer’s instructions.
Immunohistochemistry
For immunohistochemical analysis, paraffin-embedded sections (regular samples or samples after lacZ staining) were dewaxed and rehydrated in a series of alcohol to PBS. The endogenous peroxidase activity was blocked by 3% H2O2 in methanol for 8 min and rinsed with PBS three times for 5 min each. Slides were pretreated in 0.1 mm citric acid for 20 min in the microwave. After preincubating with 1.5% normal donkey serum in PBS for 30 min, sections were incubated with either anti-SF1, anti-cytochrome P450 (CYP)21 (1:1000, kindly provided by Dr. B-c Chung, Academia Sinica, Taipei, Taiwan), anti-CYP11b2 (1:1, kindly provided by Dr. C. Gomez-Sanchez, University of Mississippi Medical Center, Jackson, MS), anti-phenylethanolamine N-methyltransferase (PNMT), anti-tyrosine hydroxylase (TH) (1:1000, Millipore Corp., Billerica, MA), anti-3β-hydroxysteroid dehydrogenase (HSD) (1:1000, kindly provided by Dr. K. Morohashi, National Institutes of Natural Sciences, Okazaki, Japan) or anti-Ki-67 antibody (1:1000, BD Biosciences, San Jose, CA) in PBS-Tween 20 containing 1.5% normal donkey serum at 4 C overnight. After rinsing with PBS-Tween 20, sections were incubated with secondary antibody for 30 min and processed for signal detection according to the manufacturer’s protocol [TSA kit, PerkinElmer (Waltham, MA) or ABC kit, Vector, Burlingame, CA)]. For double-fluorescent staining, two different primary antibodies produced by different species were mixed and applied, followed by appropriate secondary antibodies. The total cell numbers in the capsule were obtained by counting the numbers of nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI). The numbers of proliferating cells were obtained by counting nuclei positive for Ki-67 and DAPI. For each genotype, three sections that were 5 μm apart were counted, and at least three animals were counted for each genotype.
Hormone assays
Plasma was collected from 18.5 dpc or postpartum d 5 (P5) mice that were killed by decapitation. Mice at the age of P21 were exposed to carbon dioxide until they stopped breathing (∼2 min), followed by cervical dislocation. Blood was collected by cardiac puncture. For measurement of basal ACTH level at the age of 1 yr, mice were single caged for at least 1 month and blood was collected from the submandibular pouch (Animal bleeding lancet, MEDIpoint, Mineola, NY) within 30 sec when the cage was touched. Plasma was collected in ice-cold EDTA-rinsed tubes. Hormonal analyses were performed using EIA kits for corticosterone (Assay Designs, Ann Arbor, MI; sensitivity 77.89 pmol/ml), ELISA kits for ACTH (Calbiotech, Spring Valley, CA; sensitivity 0.01 pmol/ml) according to the manufacturer’s instructions.
Whole mount in situ hybridization
Tissues were fixed in 4% paraformaldehyde in PBS at 4 C overnight and processed according to standard nonradioisotopic procedures using digoxigenin-labeled RNA probes. The hybridization temperature of the Shh (kindly provided by Dr. Gen Yamada Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan) and the Gli1 (kindly provided by Dr. Alexandra Joyner Department of Physiology and Neuroscience, New York University School of Medicine, New York, NY) probes was 65 C.
Quantitative real-time RT-PCR (Q-PCR)
Total RNA was isolated from P5 mice pituitary using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The reverse transcription was performed with total RNA and Omniscript reverse transcription kits (QIAGEN, Valencia, CA) with random primers (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA (5 ng) was used as the template together with 250 nm of each primer in a MJ Research PTC-200 (Bio-Rad Laboratories, Hercules, CA) with DyNAmo SYBR green qPCR kits (Finnzymes, Valencia, CA) to follow the progress of DNA synthesis. Primers used were GTGTTTCCTGGCAACGGAGATG (forward) and CATGAAGCCACCGTAACGCTTG (reverse) for Pomc. RNA amounts were calculated with relative standard curves and normalized by 36b4.
Fate-mapping analysis of Gli1-positive cells in fetal adrenal
Fate-mapping analyses were conducted by analyzing Gli1-CreERT2;R26R mice (32,33). The Gli1-CreERT2 mice were crossed with R26R-LacZ reporter mice (34) to generate Gli1-CreERT2/+;R26R/R26R males, which were subsequently used to impregnate ICR (Imprinting Control Region) inbred females. A 20 mg/ml stock solution of tamoxifen (T-5648, Sigma-Aldrich, St. Louis, MO) was prepared in corn oil. Tamoxifen (1 mg/10 g body weight) was administered to the pregnant ICR mouse females via oral gavage at 14.5 and 15.5 dpc. The embryos were collected at 18.5 dpc and P5 and were processed for whole-mount X-gal staining. No overt teratological effects were observed after tamoxifen administration under these conditions (35).
Statistical analysis
Student’s t test was used for statistical analysis in hormone measurement and cell counting.
Results
Expression of Shh and its downstream signaling components in fetal adrenal
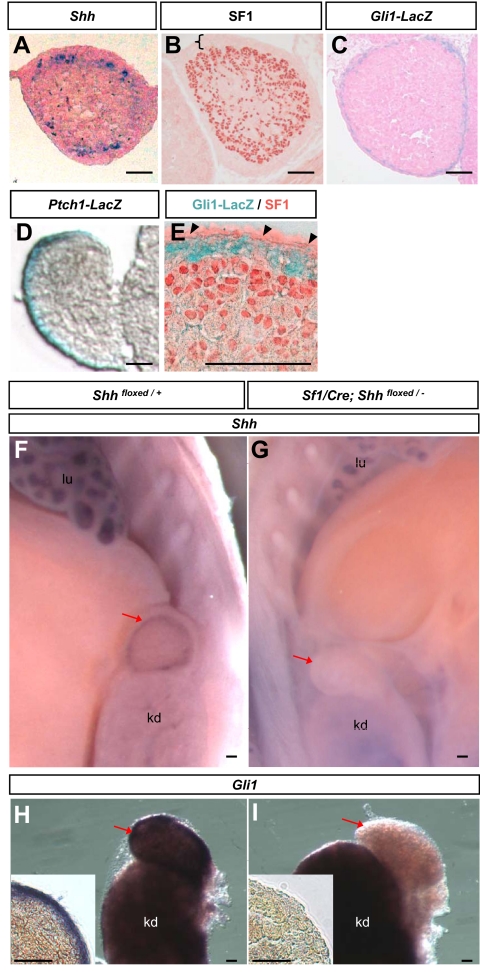
To investigate whether Shh is involved in organogenesis of mouse adrenal, we first characterized the expression of Shh and its downstream signaling component Gli1 and Ptch1 using in situ hybridization (Shh) and LacZ reporter lines (Gli1-LacZ and Ptch1-LacZ). In most tissues, binding of SHH to its receptor PTCH1 results in the activation of Smo, which leads to the activation/inhibition of the Gli family of transcription factors (19). The immediate molecular action in response to SHH activation is the up-regulation of Gli1 and Ptch1 (19). Thus presence of Gli1 and Ptch1 is used as an indicator of an active Hh pathway. At 14.5 dpc, when the adrenal primordium is encapsulated and has separated completely from the gonad, Shh mRNA was expressed in cell clusters in the adrenocortex immediately underneath the capsule (Fig. 1A) consistent with previous findings (25). At this stage, most adrenocortical cells were positive for SF1 whereas the adrenal capsule was devoid of SF1 expression (Fig. 1B). The Hh downstream components Gli1 and Ptch1, on the other hand, were expressed exclusively in the adrenal capsule (Fig. 1, C and D). Using double staining for Gli1-LacZ and SF1, we confirmed that the adrenal capsule, with the exception of the coelomic epithelium, was positive for Gli1-LacZ and negative for SF1 (Fig. 1E).
Figure 1.
Expression of Shh and its downstream signaling components in 14.5 dpc fetal adrenal. In situ hybridization for Shh (A) and immunohistochemistry for SF1 (B) were performed on wild-type adrenal. Positive signals were blue in A and red in B. The black bracket demarcates the adrenal capsule, which is negative for SF1. C, Adrenal sections from Gli1-LacZ reporter embryos after LacZ stain. D, Adrenal sections from Ptch1-LacZ reporter embryos after LacZ stain. E, Double staining for SF1 (red) and LacZ (blue) were conducted on adrenal sections from Gli1-LacZ embryos. Whole-mount in situ hybridization for Shh (F and G) and Gli1 (H and I) were performed on control (Shhfloxed/+) and Shh conditional KO (Sf1-Cre; Shhfloxed/−) embryos, respectively. Images in the inlets were sections of whole-mount adrenal samples in panels G and H. Black arrowheads, coelomic epithelium; red arrows, adrenal; lu, lung; kd, kidney. Scale bars, 100 μm.
Effects of Shh ablation in SF1-positive adrenocortical cells
To identify the source of Shh and its functional roles in adrenal development, we ablated Shh specifically in the SF1-positive cells using Sf1/Cre transgenic mice (30). Expression of Shh in the adrenal was lost in the Shh conditional KO embryos (or Sf1/Cre; Shhfloxed/−; Fig. 1F) in comparison with the presence of Shh in the control genotypes (Sf1/Cre, or Shhfloxed/+, or Sf1/Cre; Shhfloxed/+; only Shhfloxed/+ is shown in Fig. 1G), confirming that we have generated a complete KO in the adrenal. Expression of Gli1 was also lost in the Shh conditional KO adrenal (Fig. 1, H and I), further demonstrating that Shh is responsible for the activation of the Hh pathway in the adrenal capsule.
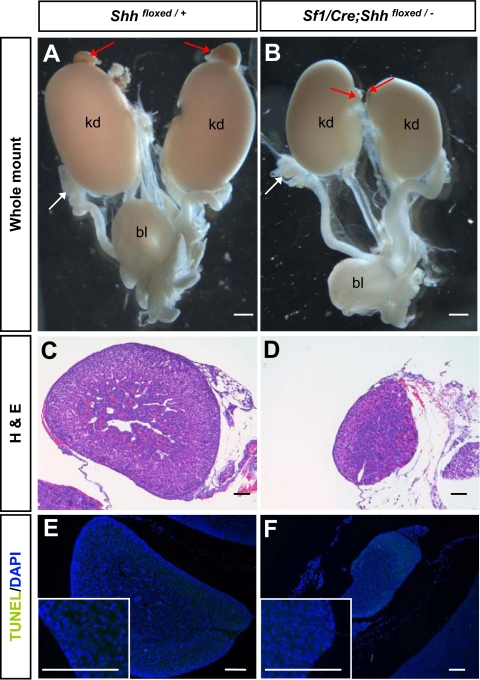
The effects of loss of Shh became evident at P5 when the size of adrenals in the Shh conditional KO animals was reduced to 25–50% of the size of the control littermates (Fig. 2, A–D). The body weights of control and KO animals were not different (control, 3.62 ± 0.6 g, n = 24; KO, 3.65 ± 0.8 g, n = 9; P = 0.9). However, the thickness of the adrenocortex in the Shh KO animals was significantly reduced (Fig. 2, C and D). The reduced adrenal size could result from increased apoptosis, decreased cell proliferation, or both. TUNEL assay revealed that few TUNEL-positive cells were present in the adrenal cortex or medulla, and no differences were found between the control and Shh KO mice at any stages analyzed (Fig. 2, E and F). Fetal ovaries were used as a positive control for TUNEL staining (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Online Journals web site at http://endo.endojournals.org). This result excluded apoptosis as a cause for reduced adrenal size in the absence of Shh.
Figure 2.
Effects of Shh ablation in adrenal morphology and apoptosis. A and B, Whole-mount light-field microscopic images of the urogenital system from control and Shh conditional KO animals at P5. White and red arrows indicate gonad and adrenal, respectively. C and D, Hematoxylin and eosin (H&E) staining of P5 adrenal gland for histology. E and F, TUNEL assay was performed on adrenal sections for apoptosis. Green nuclear staining represented positive signals for fragmented DNA, and blue staining was the DAPI nuclear counterstain. Images in the inlets were higher magnification of the outer layer (capsule and part of the cortex) of adrenals in E and F. bl, Bladder; kd, kidney. Scale bars, 100 μm. Fetal ovaries, the positive control tissue for apoptosis, were shown in supplemental Fig. 1.
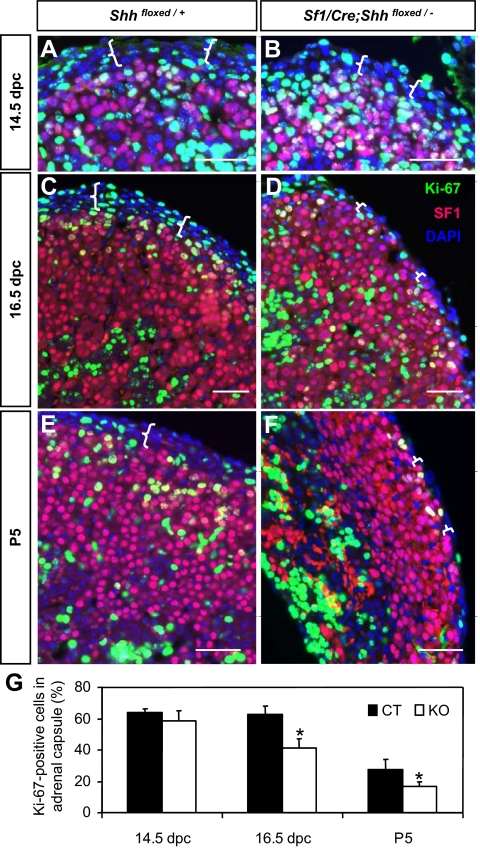
We then assessed adrenal growth by triple immunofluorescent staining for the proliferation marker Ki-67, SF1, and DAPI nuclear dye (Fig. 3). At 14.5 dpc, numbers of Ki-67-positive cells in the adrenal were not affected by the loss of Shh (Fig. 3, A, B, and G). However, at 16.5 dpc and P5, a significant decrease in Ki-67-positive cell numbers was observed in the SF1-negative capsule in the Shh KO adrenal (Fig. 3, C–F, and supplemental Fig. 2). The thickness of SF1-negative capsule was reduced from four to five cell layers in the controls to one to two cell layers in the KO (demarcated by white brackets in Fig. 3). We obtained the percentage of Ki-67-positive cells in the capsule based on the total cell numbers (nuclear DAPI staining) and numbers of Ki-67-positive cells. SF1 staining was used to distinguish capsular cells (SF1-negative) and adrenocortical cells (SF1-positive). The percentage of proliferating cells in the SF1-negative capsule was significantly reduced in the Shh KO adrenal at 16.5 dpc and P5 (Fig. 3G). These results demonstrate that Shh from the SF1-positive adrenocortical cells controls expansion of the adrenal capsule. Loss of Shh and consequent decreased proliferation in the capsule leads to stunted growth of adrenal cortex, probably due to a diminishing supply of stem/precursor cells.
Figure 3.
Effects of Shh ablation on adrenal cell proliferation at 14.5 dpc (A and B), 16.5 dpc (C and D), and P5 (E and F). Triple immunofluorescence for Ki-67 (green), SF1 (magenta), and DAPI (blue) was performed on adrenal sections. White brackets outline the adrenal capsule, which was negative for SF1. G, Percentage of Ki-67-positive proliferating cells in SF1-negative adrenal capsule at different stages. Black and white bars represent control (CT) and Shh conditional KO, respectively (P < 0.05 vs. control littermates). Scale bars, 50 μm.
Effects of Shh ablation in adrenal differentiation and steroidogenesis
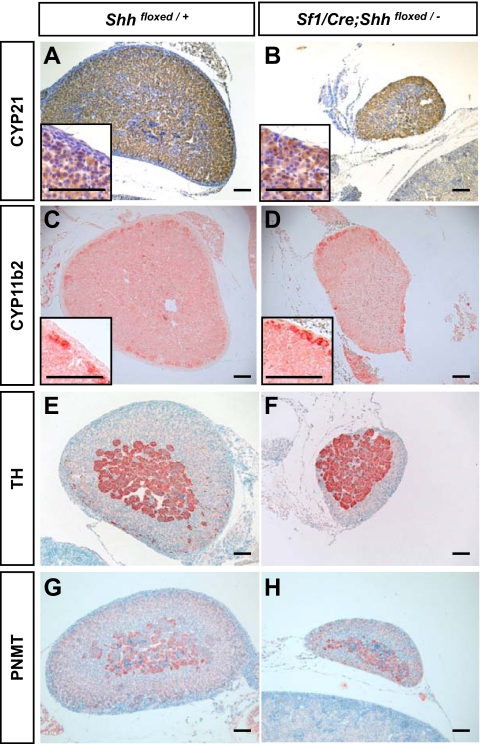
The decrease in size and proliferation in the Shh conditional KO adrenal prompted us to examine whether the hypoplastic adrenal differentiates properly. Despite a reduction in size, the Shh conditional KO adrenal expressed the cortex-specific enzymes 21-hydroxylase (CYP21) and aldosterone synthase (CYP11b2) in a pattern similar to that of the control (Fig. 4, A–D). In addition, the adrenal medulla differentiated properly based on the expression of medullar marker TH (Fig. 4, E and F) and PNMT (Fig. 4, G and H). It is apparent that histological differentiation and zonation of the adrenal cortex and medulla do not require the presence of Shh.
Figure 4.
Effects of Shh ablation on adrenocortex differentiation and zonation. Immunohistochemistry for CYP21 (brown staining in A and B), CYP11B2 (red staining in C and D), TH (red staining in E and F), and PNMT (light red staining in G and H) were performed on adrenal sections from control and Shh conditional KO at P5. Scale bars, 100 μm.
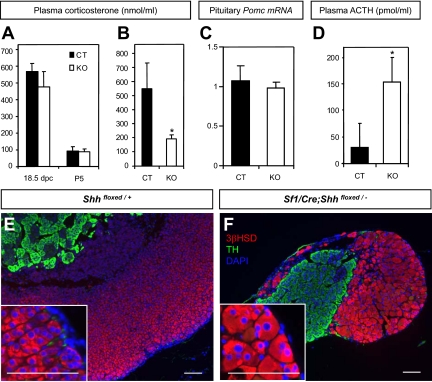
To further examine the steroidogenic ability of the Shh KO adrenal, we measured the level of plasma corticosterone and found that it was similar in the control and Shh conditional KO animals at 18.5 dpc and P5 (Fig. 5A). The level of proopiomelancortin (POMC) mRNA in the pituitary was also similar in the control and Shh conditional KO at P5 (Fig. 5C). In contrast, a significant decrease in plasma corticosterone was observed in Shh conditional KO animals at P21 immediately after carbon dioxide euthanasia (Fig. 5B, P < 0.01). Despite this defect, the Shh conditional KO animals survived to adulthood without apparent systemic problems.
Figure 5.
Effects of Shh ablation on adrenal physiology perinatally and in adulthood. A and B, Corticosterone levels in plasma (ng/ml) was measured by EIA at 18.5 dpc and P5 (A) and P21(B). Black and white bars represent control (CT) and Shh conditional KO, respectively (P < 0.01 vs. control littermates; 18.5 dpc: CT, n = 3, KO, n = 2; P5: CT, n = 19; KO, n = 7; P21: CT, n = 10; KO, n = 4). C, Pituitary Pomc mRNA level was measured by Q-PCR at P5 (CT, n = 5; KO, n = 3). D, Basal ACTH levels in plasma (pg/ml) in 1-yr-old mice was measured by ELISA (P < 0.01 vs. control littermates; CT, n = 8; KO, n = 4). E and F, Double-fluorescent immunohistochemistry for 3βHSD (magenta) and TH (green) was performed on adrenal sections from 1-yr-old control and Shh KO mice with DAPI nuclear counterstain (blue). Higher magnification of the adrenocortex was shown in the inlets. Scale bars, 100 μm.
Effects of Shh ablation in long-term adrenal growth
Insufficient corticosterone causes increased ACTH, leading to adrenal hypertrophy and hyperplasia in adult animals. Mice with blunted stress response could have long-term ACTH overstimulation, leading to progressively larger adrenal upon aging (36). Shh conditional KO mice had a higher level of ACTH at the age of 1 yr (Fig. 5D). However, this elevated ACTH did not result in an increase in adrenal size in the Shh KO animals, in which the adrenal size was 20–30% of that of the control littermates. Cortical cells in the Shh conditional KO adrenal underwent hypertrophy but their numbers remain significantly lower than the control (Fig. 5, E and F). The adrenocortical cells in the Shh KO adrenal appeared to respond to ACTH. However, loss of Shh abolished the proliferation in the capsule, leading to stunted growth of adrenal cortex even in adulthood.
Contribution of Hh-responding cells in the adrenal capsule to the adrenal cortex
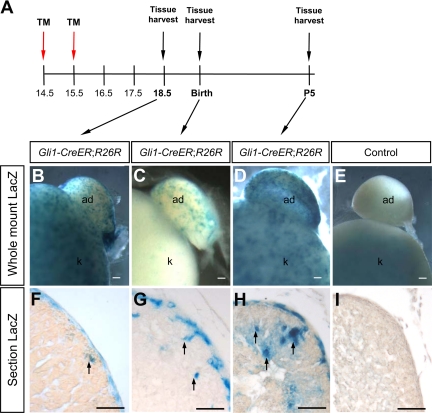
It is interesting to note that in the absence of Shh, the adrenocortex was underdeveloped (Fig. 2), although defects in proliferation were found in the capsule (Fig. 3). This observation led to the possibility that the progenies of the proliferating capsular cells eventually become a part of the adrenocortex. To test this possibility, we established a genetic fate-mapping system taking advantage of the exclusive expression of Gli1 in the adrenal capsule (Fig. 1D). We used the Gli1-CreERT2 model that expresses a tamoxifen-inducible Cre recombinase (CreERT2) in cells responding to Hh (32,33). Temporal addition of tamoxifen defines the time point when Gli1-expressing cells are marked, and all their progenies can then be traced by the presence of permanent LacZ reporter (35). We applied tamoxifen treatments to pregnant female carrying embryos with Gli1-CreERT2 and R26R LacZ reporter genes at 14.5 and 15.5 dpc (Fig. 6A). Adrenals were harvested and stained for LacZ at 18.5 dpc, birth, and P5. At all three time points, LacZ-positive cells were found in clusters in the capsule (Fig. 6, B–D) rather than the entire capsule as found in the Gli1-LacZ adrenal (Fig. 1C). At E18.5, or 3 d after tamoxifen treatment, most LacZ staining was concentrated in patches in the capsule, and only a few LacZ-positive cells were found in the cortex immediately underneath the capsule (Fig. 6F). At birth, clusters of LacZ-positive cells were found in the deeper part of the cortex. At P5, or approximately 9 d after tamoxifen treatment, LacZ-positive cells moved further inward into the cortex (Fig. 6F). The capsule remained LacZ-positive at P5, as was the case at 18.5 dpc and birth. No LacZ-positive cells were found in the adrenal of R26R reporter and Gli1-CreERT2-negative animals after tamoxifen treatments (Fig. 6, E and I). This finding demonstrates conclusively that Gli1-positive Hh-responding capsular cells migrate from the capsule and become parts of the adrenal cortex.
Figure 6.
Fate-mapping experiment for Gli1-positive capsular cells. A, Tamoxifen (TM) treatments were given to the pregnant female carrying Gli1-CreERT2;R26R or control R26R embryos at 14.5 and 15.5 dpc. Adrenals were harvested and stained for LacZ (blue) at either 18.5 dpc (B and F), birth (C and G), or P5 (D, E, H, and I). Black arrows indicate Lac-Z-positive cells that migrate into the adrenocortex. Scale bars, 100 μm. ad, Adrenal; k, kidney.
Discussion
Shh derived from SF1-positive cortical cells stimulates cell proliferation in the adrenal capsule
We identified the mechanism of how Shh signaling regulates adrenal development. Acting as a paracrine factor, SHH derived from the SF1-positive adrenocortex stimulates the proliferation of the Hh-responding cells in the adrenal capsule. In the absence of Shh, cells in the adrenal capsule fail to expand and therefore produce fewer progenies that eventually differentiate into the adrenocortical cells. The generation of Sf1/Cre;Shh conditional KO embryos enables us not only to identify the specific cell type that produces functional Shh, but also avoid the complications due to growth retardation and general failure of organ formation in the global Shh KO. However, in addition to the adrenocortex, SF1/Cre also targets cells in the anterior pituitary and other parts of the brain (30), raising the possibility that the impaired adrenal growth in the Shh conditional KO animals could be secondary to pituitary defects. We examined the anterior pituitary of the conditional KO animals and observed no differences in their development compared with the controls (data not shown). POMC, the precursor of ACTH, which is produced by the anterior pituitary, is known to stimulate proliferation of adrenocortical cells (37,38). We detected no differences in expression of Pomc at P5. In addition, mice lacking POMC production did not develop adrenal abnormalities until P7 (39), indicating that POMC or ACTH is not essential for fetal adrenal development. Based on the fact that the Shh conditional KO animals had normal neonatal Pomc expression and showed reduced proliferation and stunted adrenal growth as early as at 16.5 dpc, we conclude that the primary cause of hypoplastic adrenal is the loss of adrenal Shh, rather than a result secondary to pituitary problems. Using a similar model, Ching and Vilain (40) found that loss of Shh led to underdevelopment of adrenals. Our study provides further in-depth analysis of the involvement of Shh in adrenal growth and a novel interaction between adrenocortex and adrenal capsule. In the study of Ching and Vilain (40), the right adrenal was reported missing. We observed the same left and right asymmetrical development of the adrenal in the Shh conditional KO adults. However, in all KO animals analyzed, the right adrenals were present (Supplemental Fig. 3).
Proliferation defects were observed in fetal adrenal lacking either Shh (this study) or β-catenin in the SF1-postive cortical cells (18). However, the cellular domains affected were different: in the Shh KO model, proliferation was decreased mainly in the capsule whereas in the β-catenin KO model, decreased proliferation was found in the cortex. This difference implies that Shh and β-catenin belong to two separate pathways (Hh and canonical WNT), which target distinct cell populations during adrenal development.
Gli1-positive Hh-responding cells in the adrenal capsule are potential progenitor cells for the adrenocortex
Gottschau (4) in 1883 first described the histogenesis of adrenal, which laid the foundation for the “cell migration” theory that adrenocortical cells move centripetally from the capsule toward the medulla. Lineage-tracing experiments using trypan blue or bromodeoxyuridine pulse chase further expanded the notion that the adrenal capsule and its neighboring cells migrate inward and contribute to adrenocortex (5,41). However, these experiments provided only circumstantial observations, and alternative explanations such as incomplete penetration of the dye and bromodeoxyuridine were proposed. By using a genetic fate-mapping approach, we provided the evidence demonstrating that adrenal capsular cells migrate centripetally and become a part of adrenocortex. Moreover, we identified that the migration occurs at least in one specific cell population (Gli1 positive) that responds to SHH. Although our results strongly support that the Gli1-positive cell in the capsule are potential progenitors of the adrenocortex, these results do not exclude other possible sources of progenitor cells in the adrenal (42,43). In addition to the adrenal capsule, others have proposed that each cortical zone (zona glomerulosa, zona fasciculata, and zona reticularis) contains its own progenitor/stem cell population that are able to differentiate into cells unique for the particular zone. An undifferentiated zone between the zona glomerulosa and zona fasciculata was also proposed to be the stem cell zone (43). The stunted adrenocortex and decreased adrenal size in the Shh conditional KO animals argue that adrenal capsular cells are a major source of progenitor cells. These progenitor/stem cells constantly replenish the adrenocortical cell populations. If other stem cells are present in the adrenocortex, these cells are not sufficient to compensate for the loss of stem cells in the adrenal capsule in the Shh conditional KO adrenal. Indeed, in the adult Shh conditional KO mice, the adrenal gland remains small despite elevated plasma ACTH. The adrenocortical cells underwent significant hypertrophy in response to elevated ACTH; however, their numbers were not increased. This is consistent with the idea that expansion of progenitor/stem cells in the adrenal capsule is the major mechanism for adrenal growth in both fetal and adult life.
In our fate-mapping results, LacZ-positive cells marked by Gli1-CreERT2 were observed in clusters in the capsule rather than the entire capsule as found in the Gli1-LacZ adrenal. This observation indicated that the Gli1-CreERT2 was not sufficient to induce recombination in every Gli1-positive cell, which was also reported in other systems (35). However, this insufficient activity provided us a unique opportunity to trace the fate of the clustering cells, which presumably derive from a single progenitor (44,45).
Shh is not required for differentiation of adrenal cortex and medulla
Although Shh is essential for expansion of adrenal capsule and cortex, it is not required for the zonation and differentiation of the adrenal. Expression of markers for the cortex and medulla was not affected by the loss of Shh. Expression of PNMT in the medulla requires a high level of glucocorticoids (46). Decreased PNMT expression was observed in mice deficient in glucocorticoid production or signaling, such as the Cyp21 KO, Crhr1 KO, Sf1 heterozygous, and glucocorticoids receptor KO mice (47,48,49,50). The presence of PNMT in the Shh conditional KO adrenal suggests that the hypoplastic cortex in the Shh KO animals is still able to synthesize sufficient glucocorticoids to maintain the differentiation of the adrenal medulla. This notion is further supported by the observation that the level of plasma corticosterone was similar in control and Shh conditional KO animals at 18.5 dpc and P5. This was not unexpected due to the fact that at 18.5 dpc, maternal-derived corticosterone is the major source of glucocorticoids in fetal blood (51). At P5, corticosterone is maintained at a basal level, and the hypothalamic-pituitary-adrenal axis is hyposensitive to most stresses (52,53). At P21, however, the reduced corticosterone level in the Shh conditional KO animals indicates that the hypoplastic cortex does not have the same ability to produce corticosterone in response to carbon dioxide euthanasia. The well-defined zona glomerulosa and zona fasciculata indicate that Shh is not involved in zonation of the adrenocortex. However, fewer cells in the Shh KO cortex were unable to produce adequate corticosterone in response to an acute demand such as stress.
The presence of functional adrenal cortex in the absence of Shh also argues that a founder population of adrenocortical cells must be present and maintained. We believe that the original SF1-positive cells from the adrenogonadal primordium are the founder population (54). These SF1-positive cells apparently are the source of SHH although their survival and differentiation do not require SHH. SHH derived from these SF1-positive cells then acts upon the second progenitor population in the adrenal capsule, which is essential for fetal adrenal growth as well as expansion of the cortex in adulthood in response to ACTH.
In summary, we provided genetic evidence demonstrating the significance of the adrenal capsule in adrenocortex expansion via the signaling of SHH. Through a unique interaction between the SF1-positive cortical cells (source of SHH) and SF1-negative capsular cells (targets of SHH), progenitor cells in the adrenal capsule expand. The Hh-responding capsular cells then migrate inward and contribute to the adrenocortex. Findings of this research provide not only an understanding of how a precursor cell population is regulated during organogenesis but also potential applications for development of stem cell therapy for treatment of adrenal insufficiency.
Supplementary Material
Acknowledgments
We thank Dr. Bon-chu Chung (Academia Sinica, Taipei, Taiwan) for the SF1 antibody and the CYP21 antibody, Dr. Celso Gomez-Sanchez (University of Mississippi Medical Center, Jackson, MS) for the CYP11B2 antibody, Dr. Ken Morohashi (National Institutes of Natural Sciences, Okazaki, Japan) for the 3βHSD antibody, and Dr. Gen Yamada (Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan) for his comments and help. We also thank Dr. Ana Vieira for maintaining the mouse colony and all the Yao laboratory members for their assistance and support.
This study is dedicated to our co-author Dr. Keith Parker, who passed away on December 13, 2008.
Footnotes
This work was supported by National Institute of Health Grants [HD46861 (to H.H.Y.) and HD046743 and DK54480 to (K.L.P.)]; a Grant-in-Aid for Scientific Research on Priority Areas, Mechanisms of Sex Differentiation (to S.M. and D.M.); and the Global Center of Excellence Cell Fate Regulation Research and Education Unit (to S.M. and D.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 29, 2010
Abbreviations: CYP, Cytochrome P450; DAPI, 4′,6-diamidino-2-phenylindole; dpc, days post coitum; Hh, hedgehog; HSD, hydroxysteroid dehydrogenase; KO, knockout; P5, postpartum d 5; PNMT, phenylethanolamine N-methyltransferase; POMC, proopiomelancortin; PTCH1, Patched1; Q-PCR, quantitative real-time RT-PCR; SF1, steroidogenic factor 1; SHH, sonic hedgehog; SMO, smoothened; TH, tyrosine hydroxylase; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling.
References
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Zwemer RL, Wotton RM, Norkus MG 1938 A study of corticoadrenal cells. Anat Rec 72:249–263 [Google Scholar]
- Mesiano S, Jaffe RB 1997 Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18:378–403 [DOI] [PubMed] [Google Scholar]
- Gottschau M 1883 Structur und embryonale Entwickelung der Neben-nieren bei Saugethieren. Arch Anat Phys (S):412–458 [Google Scholar]
- Salmon TN, Zwemer RL 1941 A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anat Rec 80:421–429 [Google Scholar]
- Skelton FR 1959 Adrenal regeneration and adrenal-regeneration hypertension. Physiol Rev 39:162–182 [DOI] [PubMed] [Google Scholar]
- Perrone RD, Bengele HH, Alexander EA 1986 Sodium retention after adrenal enucleation. Am J Physiol 250:E1–E12 [DOI] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL 1997 DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AK, McCabe ER 2004 Molecular mechanisms of DAX1 action. Mol Genet Metab 83:60–73 [DOI] [PubMed] [Google Scholar]
- Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K 2002 Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7:717–729 [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwarz H, Kaplan JC, Camerino G, Meitinger T, Monaco A 1994 Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672–676 [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, Meitinger T, Monaco A, Sassone-Corsi P, Camerino G 1994 An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R 1993 WT-1 is required for early kidney development. Cell 74:679–691 [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Vanden Heuvel GB, Igarashi P 1998 Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev 71:37–48 [DOI] [PubMed] [Google Scholar]
- Schnabel CA, Selleri L, Cleary ML 2003 Pbx1 is essential for adrenal development and urogenital differentiation. Genesis 37:123–130 [DOI] [PubMed] [Google Scholar]
- Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S 2001 Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet 29:469–474 [DOI] [PubMed] [Google Scholar]
- Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S 2002 Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 143:4358–4365 [DOI] [PubMed] [Google Scholar]
- Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD 2008 Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135:2593–2602 [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP 2001 Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15:3059–3087 [DOI] [PubMed] [Google Scholar]
- Chari NS, McDonnell TJ 2007 The sonic hedgehog signaling network in development and neoplasia. Adv Anat Pathol 14:344–352 [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sánchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A 2005 Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, Kageyama R, Wallace VA 2009 Progenitor cell proliferation in the retina is dependent on notch-independent sonic hedgehog/Hes1 activity. J Cell Biol 184:101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC 2005 Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23:781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Peljto M 2008 Differentiation of mouse embryonic stem cells to spinal motor neurons. Curr Protoc Stem Cell Biol 5:1H.1.1-1H.1.9 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP 1995 Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172:126–138 [DOI] [PubMed] [Google Scholar]
- Bose J, Grotewold L, Ruther U 2002 Pallister-Hall Syndrome Phenotype in mice mutant for Gli3. Hum Mol Genet 11:1129–1135 [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL 2001 Gli1 can rescue the in vivo function of Gli2. Development 128:5161–5172 [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL 2002 Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129:4753–4761 [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H 1996 Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA 93:5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL 2006 Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44:419–424 [DOI] [PubMed] [Google Scholar]
- Nagy A 2003 Manipulating the mouse embryo: a laboratory manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Ahn S, Joyner AL 2004 Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118:505–516 [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL 2005 In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature 437:894–897 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G 2007 Molecular analysis of coordinated bladder and urogenital organ formation by hedgehog signaling. Development 134:525–533 [DOI] [PubMed] [Google Scholar]
- Shih MC, Hsu NC, Huang CC, Wu TS, Lai PY, Chung BC 2008 Mutation of mouse Cyp11a1 promoter caused tissue-specific reduction of gene expression and blunted stress response without affecting reproduction. Mol Endocrinol 22:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivariz FE, Iturriza F, McLean C, Hope J, Lowry PJ 1982 Stimulation of adrenal mitogenesis by N-terminal proopiomelanocortin peptides. Nature 297:419–422 [DOI] [PubMed] [Google Scholar]
- Pepper DJ, Bicknell AB 2009 The stimulation of mitogenic signaling pathways by N-POMC peptides. Mol Cell Endocrinol 300:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U 2005 Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 146:2555–2562 [DOI] [PubMed] [Google Scholar]
- Ching S, Vilain E 2009 Targeted disruption of sonic hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis 47:628–637 [DOI] [PubMed] [Google Scholar]
- Zajicek G, Ariel I, Arber N 1986 The streaming adrenal-cortex—direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol 111:477–482 [DOI] [PubMed] [Google Scholar]
- Jones IC 1948 Variation in the mouse adrenal cortex with special reference to the zona reticularis and to brown degeneration, together with a discussion of the cell migration theory. Q J Microsc Sci 89:53–74 [PubMed] [Google Scholar]
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y 1994 A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology 135:431–438 [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, Weinberg WC 1987 The histogenesis of the rat adrenal cortex: a study based on histologic analysis of mosaic pattern in chimeras. J Exp Zool 243:217–223 [DOI] [PubMed] [Google Scholar]
- Iannaccone P, Morley S, Skimina T, Mullins J, Landini G 2003 Cord-like mosaic patches in the adrenal cortex are fractal: implications for growth and development. FASEB J 17:41–43 [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, Axelrod J 1965 Adrenaline synthesis: control by the pituitary gland and adrenal glucocorticoids. Science 150: 1464–1465 [DOI] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schütz G 1995 Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621 [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Tajima T, Eisenhofer G, Haidan A, Aguilera G 1999 Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J 13:1185–1194 [DOI] [PubMed] [Google Scholar]
- Yoshida-Hiroi M, Bradbury MJ, Eisenhofer G, Hiroi N, Vale WW, Novotny GE, Hartwig HG, Scherbaum WA, Bornstein SR 2002 Chromaffin cell function and structure is impaired in corticotropin-releasing hormone receptor type 1-null mice. Mol Psychiatry 7:967–974 [DOI] [PubMed] [Google Scholar]
- Bland ML, Fowkes RC, Ingraham HA 2004 Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18:941–952 [DOI] [PubMed] [Google Scholar]
- Hu MC, Hsu NC, El Hadj NB, Pai CI, Chu HP, Wang CK, Chung BC 2002 Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol Endocrinol 16:1943–1950 [DOI] [PubMed] [Google Scholar]
- Schapiro S, Geller E, Eiduson S 1962 Neonatal adrenal cortical response to stress and vasopressin. Proc Soc Exp Biol Med 109:937–941 [DOI] [PubMed] [Google Scholar]
- Levine S 1994 The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann NY Acad Sci 746:275–288; discussion 289–293 [DOI] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi KI 2008 Developmental links between the fetal and adult adrenal cortex revealed by lineage tracing. Mol Cell Biol 28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.