Abstract
Intrinsic abnormalities in transplanted eutopic endometrium are believed to contribute to the pathogenesis of pelvic endometriosis. Herein we investigated transcriptomic differences in human endometrial stromal fibroblasts (hESFs) from women with (hESFendo) vs. without (hESFnonendo) endometriosis, in response to activation of the protein kinase A (PKA) pathway with 8-bromoadenosine-cAMP (8-Br-cAMP). hESFnonendo (n = 4) and hESFendo (n = 4) were isolated from eutopic endometrium and treated ± 0.5 mm 8-Br-cAMP for 96 h. Purified total RNA was subjected to microarray analysis using the whole-genome Gene 1.0 ST Affymetrix platform. A total of 691 genes were regulated in cAMP-treated hESFnonendo vs. 158 genes in hESFendo, suggesting a blunted response to cAMP/PKA pathway activation in women with disease. Real-time PCR and ELISA validated the decreased expression of decidualization markers in hESFendo compared with hESFnonendo. In the absence of disease, 8-Br-cAMP down-regulated progression through the cell cycle via a decrease in cyclin D1, cyclin-dependent kinase 6, and cell division cycle 2 and an increase in cyclin-dependent kinase inhibitor 1A. However, cell cycle components in hESFendo were not responsive to 8-Br-cAMP, resulting in persistence of a proliferative phenotype. hESFendo treated with 8-Br-cAMP exhibited altered expression of immune response, extracellular matrix, cytoskeleton, and apoptosis genes. Changes in phosphodiesterase expression and activity were not different among experimental groups. These data support that eutopic hESFendo with increased proliferative potential can seed the pelvic cavity via retrograde menstruation and promote establishment, survival, and proliferation of endometriosis lesions, independent of hydrolysis of cAMP and likely due to an inherent abnormality in the PKA pathway.
Endometrial stroma from subjects with endometriosis has increased proliferation potential and a blunted response to cAMP, which is independent of hydrolysis of cAMP by phosphodiesterase.
In endometriosis, endometrium (uterine lining)-like tissue is found primarily on the pelvic peritoneum and ovaries (1). Peritoneal disease is the most common form, occurring in 6–10% of women of reproductive age and is believed to derive mainly from retrograde menstruation and transplantation of eutopic (within the uterus) endometrial tissue fragments and cells to the peritoneum (2). Resulting lesions undergo neoangeogenesis; proliferation; invasion of surrounding structures; infiltration by sensory and sympathetic nerves (3); and elicit a local inflammatory response, adhesion formation, and fibrosis (1,4). Intrinsic abnormalities of the transplanted eutopic endometrium are believed to enable establishing disease in the pelvis (4). Thirty-five to 50% of women with pelvic pain and/or infertility have endometriosis (5), with infertility due to ovulatory dysfunction, poor oocyte quality in response to the inflammatory environment, and abnormal eutopic endometrium with compromised embryonic implantation (1).
Transcriptomic analyses investigating differences in eutopic endometrium from women with and without endometriosis (6,7) reveal abnormalities in the proliferative (estrogen-dominant)-to-secretory [progesterone (P4) dominant] phase transition (7), normally observed in the menstrual cycle (8). Several studies support resistance to P4 action in this tissue (9,10). P4 signaling and the protein kinase A (PKA) pathway intersect and are key to decidualization (differentiation) of human endometrial stromal fibroblasts (hESFs), which are central to successful embryonic implantation, placental function, and pregnancy progression (11,12). Impaired decidualization in vivo has been suggested as an underlying mechanism for infertility in women with endometriosis (10). In vitro, decidualization of hESFs can be induced by P4 with estradiol priming (13,14) and/or activation of the PKA pathway by relaxin, prostaglandins, and cAMP analogs (13,15,16,17,18). We (8) and others (16) have demonstrated a blunted expression of specific decidual markers in response to activation of the cAMP/PKA pathway in eutopic hESFs from women with endometriosis (hESFendo) vs. without disease (hESFnonendo). The molecular bases of these differences are not well understood.
Herein we investigated the transcriptome resulting from activation of the PKA pathway by the cAMP analog, 8-bromoadenosine-cAMP (8-Br-cAMP), in hESFs from women without and with endometriosis. The data demonstrate that activation of the PKA pathway in hESFnonendo results in down-regulation of genes involved in cell cycle progression and up-regulation of their inhibitors as well as regulation of genes involved in cytoskeletal changes important in hESF differentiation and key signaling pathways and markers of the decidualization process. In contrast, there is dysregulation of the actions of cAMP, in the absence of changes in phosphodiesterases, differentiation of hESFendo, and persistence of a proliferative phenotype involving key regulators of the cell cycle, including cyclins and cyclin inhibitors in the presence of disease.
Materials and Methods
Subjects
Endometrial tissue biopsies were obtained from 14 reproductive-age women. Six subjects had endometriosis (n = 1 minimal, n = 2 mild, n = 1 moderate-severe, n = 2 severe), based on visualization of lesions during laparoscopy and their histologic evaluation (Table 1). Staging of endometriosis was according to the revised American Fertility Society classification system (19). Participating subjects with endometriosis were 22–46 yr old (mean 33.5 ± 3.0). Controls were eight regularly cycling premenopausal subjects (37–49 yr old, mean 44.4 ± 1.7) undergoing endometrial biopsy or hysterectomy for benign reasons (Table 1), had no history and no evidence of endometriosis at laparoscopy, were documented not to be pregnant, and had not been on hormonal therapies for at least 3 months before tissue sampling.
Table 1.
Characteristics of patients donated endometrial biopsy samples for the study
| Patient | Cycle phase | hESF used in experiments | Diagnosis at laparoscopy | Age (yr) | Ethnicity |
|---|---|---|---|---|---|
| Endometriosis | |||||
| 233 | MSE | Microarray, ELISA, validation | Mild endometriosis, pelvic pain | 31 | Caucasian |
| 243 | ESE | Microarray, ELISA, validation | Minimal endometriosis, bilateral ovarian cyst, intramural myoma | 46 | Caucasian |
| 267 | LSE | Microarray, ELISA, validation | Mild endometriosis, pelvic pain | 32 | Caucasian |
| 288 | PE | Microarray, ELISA, validation, PDE assays | Severe endometriosis | 22 | Caucasian |
| 279 | PE | PDE assays | Moderate-severe endometriosis, pelvic pain | 30 | Unspecified |
| 381 | MSE | PDE assays | Severe endometriosis, pelvic pain, myoma | 40 | Asian |
| No endometriosis | |||||
| 229 | LSE | Microarray, ELISA, validation | Pelvic pain (no endometriosis at laparoscopy) | 47 | Caucasian |
| 236 | PE | Microarray, ELISA, validation | Symptomatic pelvic prolapse | 47 | Caucasian |
| 237 | ESE | Microarray, ELISA, validation | Intramural myoma, left paratubal cyst | 39 | Caucasian |
| 238 | ESE | Microarray, ELISA, validation | Endometrial polyp | 41 | Black |
| 285 | PE | PDE assays | Intramural myoma, pelvic adhesions | 37 | Caucasian |
| 293 | PE | PDE assays | Intramural myoma | 49 | Caucasian |
| 298 | ESE | PDE assays | Endometrial polyp | 46 | Asian |
| 326 | PE | PDE assays | Intramural myoma | 49 | Asian |
PE, Proliferative endometrium, MSE, midsecretory endometrium; LSE, late secretory endometrium.
The University of California, San Francisco, Committee on Human Research and the Stanford University Committee on the Use of Human Subjects in Research approved the study. Written informed consent was obtained from subjects. Samples were also obtained through the University of California, San Francisco, National Institutes of Health Human Endometrial Tissue and DNA Bank with appropriate institutional review, approvals, and informed consent from all subjects.
Isolation, culture, and decidualization of endometrial stromal cells
All samples were collected, transported, and processed as described (13,20). Decidualization was performed by treating hESFs cultured in low-serum medium with 0.5 mm 8-Br-cAMP for 96 h and confirmed by measuring decidual markers, IGF binding protein (IGFBP)-1, and prolactin (PRL), in conditioned media by ELISA (13). Time zero (t-0) control samples were collected before initiating treatment. Cells cultured for the corresponding time periods without treatment served as additional controls. hESF cell morphology before and after decidualization was documented. Cells lysates in RLT lysis buffer (QIAGEN, Valencia, CA) containing β-mercaptoethanol and conditioned media were collected after 96 h of incubation.
Total RNA isolation, microarray hybridization, and real-time RT-PCR
Total RNA was isolated from hESFs from subjects without (n = 4) and with (n = 4) endometriosis and purified using RNeasy Plus minikit (QIAGEN). Samples were quantified by spectroscopy, and purity was analyzed by the 260:280 absorbance ratio. RNA quality and integrity were assessed using Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) with all samples having high-quality RNA [RNA integrity number (RIN) = 9.7–10]. Hybridization was performed with Human Gene 1.0 ST arrays (Affymetrix, Inc, Santa Clara, CA). Briefly, 100 ng of total RNA from each sample were reverse transcribed to cDNA, followed by overnight in vitro transcription to generate cRNA, which was reverse transcribed, and the 5.5 μg of sense cDNA were fragmented and labeled. The quality of cDNA and fragmented cDNA was assessed in the Agilent bioanalyzer. Microarrays were hybridized, washed, stained, and scanned according to the protocol described in the WT sense target labeling assay manual from Affymetrix (version 4; FS450_0007).
For quantitative (Q) RT-PCR analysis, 1 μg of RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). The real-time RT-PCR reaction was carried out for 40 cycles with the primers listed in Table 2.
Table 2.
Primer sequences used in real-time RT-PCR experiments
| Gene | Sense primer 5′–3′ | Antisense primer 5′–3′ |
|---|---|---|
| IGFBP1 | CTATGATGGCTCGAAGGCTC | TTCTTGTTGCAGTTTGGCAG |
| PRL | CATCAACAGCTGCCACACTT | CGTTTGGTTTGCTCCTCAAT |
| FOXO1A | AAGAGCGTGCCCTACTTCAA | CTGTTGTTGTCCATGGATGC |
| CXCR4 | GGGCCTGAGTGCTCCAGTAG | GGGTAGAAGCGGTCACAGAT |
| SST | CCCAGACTCCGTCAGTTTCT | ATCATTCTCCGTCTGGTTGG |
| IL13AR2 | CCTTGAAAACAACAAATGAAACC | TAGTTATATTTGTAACCGGTCTGCTTT |
| DKK1 | CATCAGACTGTGCCTCAGGA | CCACAGTAACAACGCTGGAA |
| DKK3 | ATGAGTATGAAGTTGGCAGC | TATTGCACATCTACCCACAG |
| CCND1 | GTGGGTGTGCAAGCCAGGT | TTCCTGTCCTACTACCGCCT |
| CDC2 | GCTTATGCAGGATTCCAGGTT | CAATCCCCTGTAGGATTTGGT |
| CDKN1A (p21) | GTCCGTCAGAACCCATGC | GCTTCCTCTTGGAGAAGATCA |
| CDK6 | GCCTCTTTTTCGTGGAAGTT | AATTGGTTGGGCAGATTTTG |
| RPL19 | GCAGATAATGGGAGGAGCC | GCCCATCTTTGATGAGCTTC |
Microarray gene expression data analysis and statistical analysis
To minimize technical (nonbiological) variability among arrays, densitometry values between arrays were normalized using the Robust Multichip Average function and further transformed to the logarithmic scale (log2). Probes with a known GenBank accession ID correspondence were selected for functional analysis. Statistically significant differences between groups were determined using statistical analysis of microarrays (21) and RankProd (22) methods, using the Bioconductor (http://www.bioconductor.org/) packages Siggene and RankProd, respectively, both run under R software (http://www.r-project.org/). We used two different statistical tests to identify the most robustly differentially expressed genes. Functional annotations were carried out using the Ingenuity Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA, http://www.ingenuity.com/), in which gene symbols and fold changes of the up- and down-regulated genes were imported.
Hierarchical clustering
Hierarchical clustering is an unsupervised way of grouping samples based only on their gene expression similarities (23). Herein we conducted hierarchical cluster analysis of differentially expressed genes from all samples (the combined gene list) using the smooth correlation for distance measure algorithm (GeneSpring 7.3) to identify samples with similar patterns of gene expression. The output data are also displayed graphically as a dendrogram of hESFnonendo and hESFendo samples. The raw data files are stored at the National Center for Biotechnology Information gene Expression Omnibus database, number GSE17504.
Microarray validation by real-time PCR
The most highly up- or down-regulated genes, as well as some genes of interest, based on previous transcriptomic analyses (4), were chosen for validation by QRT-PCR. QRT-PCR was performed in duplicate according to the manufacturer’s instructions and as described above, with the above primers.
ELISA
Conditioned media from cultured hESFs were subjected to ELISA to determine IGFBP1 and PRL concentrations (Alpha Diagnostic International, San Antonio, TX, and Diagnostic Systems Labs, Webster, TX, respectively). Levels of IGFBP1 and PRL for each sample were normalized to total RNA.
Phosphodiesterase (PDE) activity
Reagents were purchased from Sigma (St. Louis, MO) unless otherwise specified. Protease and phosphatase inhibitor were from Roche Diagnostics (Indianapolis, IN). Isolated hESF cells from subjects (n = 4 with and n = 3 without endometriosis) were cultured as described above with or without subsequent treatment for 96 h with 0.5 mm 8-Br-cAMP. Cell pellets were lysed in 500 μl lysis buffer [50 mm Tris-HCl, 1% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, and protease and phosphatase inhibitors (pH 7.4)] and then gently rotated at 4 C for 30 min. Lysates were then spun for 20 min at 15,000 × g at 4 C. The supernatant was removed and protein concentration determined by the BCA protein assay. The supernatant was then diluted 5-fold in 10 mm potassium phosphate (pH 6.8), 25 mm β-mercaptoethanol, and 1 mg/ml BSA, and 10 μl were assayed for cAMP PDE activity in a final volume of 100 μl, as described (24), with 100 nm [3H]cAMP as substrate and 2 mm EGTA. [3H]cAMP was from Amersham Healthcare (Piscataway, NJ). The activity of the PDE4 family was determined in the presence of 1 μm rolipram. The cAMP PDE assay was also performed in the presence of 100 μm isobutylmethylxanthine (IBMX) to determine the apparent contribution of the PDE8 family, which is insensitive to IBMX (25,26).
Statistical evaluation
Statistical analysis of data generated in the ELISA was performed using a two-tailed type 3 Student’s t test. For QRT-PCR and PDE assays, the nonparametric Mann-Whitney test was used. Significance was determined at P ≤ 0.05.
Results
Samples cluster by treatment (not disease)
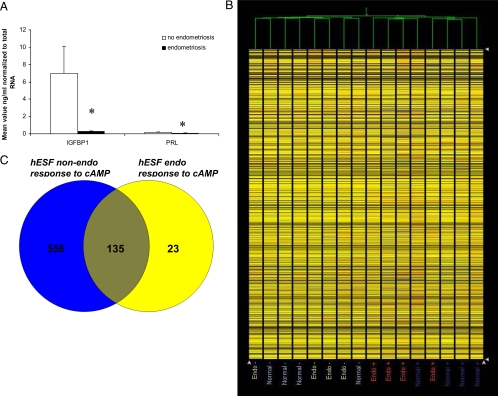
hESFs from women with and without endometriosis have different responses to 8-Br-cAMP with regard to secretion of the decidualization markers, IGFBP1 and PRL (Fig. 1A). To determine hESF sample clustering patterns, hierarchical clustering analysis of the expression profiles of hESFendo and hESFnonendo was conducted, using all gene sets (Fig. 1B). As demonstrated in Fig. 1B, samples clustered based on treatment, and not on disease, subject age, or how the tissue was obtained, consistent with our previous observations on whole endometrial tissue (7). Figure 1C demonstrates the Venn diagram of common and unique genes regulated in hESFendo and hESFnonendo by 8-Br-cAMP treatment (supplemental Tables S1 and S2, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Interestingly, the total number of genes regulated by cAMP was much lower in hESFendo, only 135 genes being common between the two groups.
Figure 1.
A, IGFBP1 and PRL protein secretion in conditioned medium from hESFs treated with 0.5 mm cAMP for 96 h, normalized to total RNA, n = 4. *, Significance accepted at P ≤ 0.05. Error bars, ±sem. B, Hierarchical clustering analysis of hESFnonendo (no endometriosis) and hESFendo (endometriosis) samples treated with (+) or without (−) 0.5 mm cAMP for 96 h. C, Venn diagram of shared and unique genes between hESFendo and hESFnonendo response to cAMP treatment (gene lists in supplemental Tables S1 and S2).
The PKA pathway-regulated transcriptomes of hESFnonendo and hESFendo
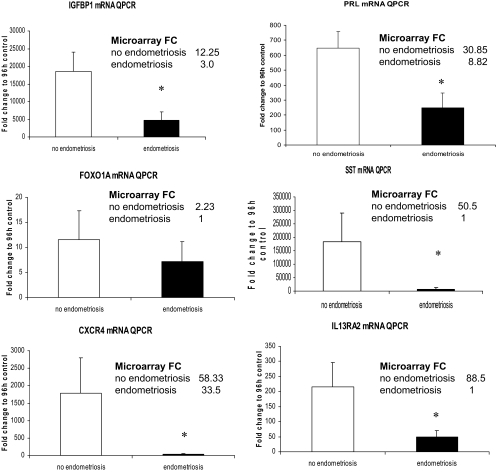
Gene expression profiles were derived from hESFnonendo and hESFendo in response to 8-Br-cAMP or vehicle for 96 h, and four comparison groups were generated: A) hESFnonendo treated with cAMP vs. vehicle control (i.e. no cAMP); B) hESFendo treated with cAMP vs. vehicle control; C) vehicle control hESFendo vs. vehicle control hESFnonendo; and D) cAMP-treated hESFendo vs. cAMP-treated hESFnonendo (supplemental Tables S1–S3 and Table 3). Group A presented with the highest number of regulated genes (319 up- and 414 down-regulated) after 1.5-fold cutoff. Remarkably, only 158 genes were regulated in hESFendo by cAMP, with 77 up- and 95 down-regulated genes (supplemental Tables S1 and S2). Analysis of microarray data demonstrated that mRNA levels of IGFBP1 and PRL in response to 8-Br-cAMP were significantly higher in hESFnonendo in response to cAMP (group A) (12.25- and 30.8-fold, respectively), compared with hESFendo (group B) (3.0- and 8.8-fold, respectively) (supplemental Tables S1 and S2). Moreover, analysis of group D showed that IGFBP1 and PRL levels were lower in cAMP-treated hESFendo (4.2- and 4-fold, respectively) (Table 3) but not in vehicle controls (supplemental Table S3). There was a general trend of diminished response in hESFs from women with endometriosis to cAMP (as seen from lower expression of the cAMP-exclusively regulated somatostatin gene, SST) and overall a more limited response of cAMP/P4 coregulated genes characteristic of decidualization (i.e. PRL, IGFBP1, FOXO1A, SFRP1, TNFAIP5, MMPs, IL-8, IL-6, IL13RA2, IL-1B) (supplemental Table S2). IGFBP1, PRL, SST, and FOXO1A gene expression was further validated by QPCR (Fig. 2).
Table 3.
List of up- and down-regulated genes in hESFendo treated with 0.5 mm cAMP for 96 h (hESFendo cAMP) vs. hESFnonendo cAMP, expressed as fold change (FC)
| Gene symbol | FC (hESFendo cAMP/hESFnonendo cAMP) | Description | |
|---|---|---|---|
| CST1 | 3.53 | Cystatin SN | |
| ANGPT2 | 3.28 | Angiopoietin 2 | |
| AK5 | 2.78 | Adenylate kinase 5 | |
| DSC3 | 2.66 | Desmocollin 3 | |
| ATP1B1 | 2.58 | ATPase, Na+ | |
| ITGA4 | 2.56 | Integrin, α4 (antigen CD49D, α4 subunit of VLA-4 receptor) | |
| ARHGDIB | 2.50 | ρ-GDP dissociation inhibitor (GDI)-β | |
| MYCN | 2.45 | v-myc Myelocytomatosis viral related oncogene, neuroblastoma derived (avian) | |
| PDE8B | 2.41 | Phosphodiesterase 8B | |
| HSD17B2 | 2.40 | Hydroxysteroid (17-β) dehydrogenase 2 | |
| DHRS3 | 2.23 | Dehydrogenase | |
| HEY1 | 2.21 | Hairy | |
| CHRM2 | 2.20 | Cholinergic receptor, muscarinic 2 | |
| CCND2 | 2.19 | Cyclin D2 | |
| KCNMB4 | 2.18 | Potassium large conductance calcium-activated channel, subfamily M, β-member 4 | |
| PSG4 | 2.08 | Pregnancy specific β-1-glycoprotein 4 | |
| DOCK9 | 2.06 | Dedicator of cytokinesis 9 | |
| TNFSF15 | 2.05 | TNF (ligand) superfamily, member 15 | |
| MXRA5 | 2.05 | Matrix-remodeling associated 5 | |
| FAM46C | 1.93 | Family with sequence similarity 46, member C | |
| IL1B | −1.45 | IL-1, β | |
| CHI3L1 | −1.69 | Chitinase 3-like 1 (cartilage glycoprotein-39) | |
| TNFAIP3 | −1.84 | tumor necrosis factor, α-induced protein 3 | |
| KLHL23 | −1.85 | Kelch-like 23 (Drosophila) | |
| SLIT2 | −1.86 | Slit homolog 2 (Drosophila) | |
| POSTN | −1.88 | Periostin, osteoblast specific factor | |
| REN | −1.89 | Renin | |
| CLIC2 | −1.90 | Chloride intracellular channel 2 | |
| PDGFRL | −1.91 | Platelet-derived growth factor receptor-like | |
| C13orf33 | −1.92 | Chromosome 13 open reading frame 33 | |
| IL33 | −1.94 | IL-33 | |
| TMEM154 | −1.94 | Transmembrane protein 154 | |
| MYC | −1.95 | v-myc Myelocytomatosis viral oncogene homolog (avian) | |
| NOG | −1.95 | Noggin | |
| TLR4 | −1.96 | Toll-like receptor 4 | |
| OLFML1 | −1.98 | Olfactomedin-like 1 | |
| CGA | −1.98 | Glycoprotein hormones, α polypeptide | |
| RXFP1 | −1.98 | Relaxin | |
| BDKRB1 | −1.99 | Bradykinin receptor B1 | |
| DKK1 | −1.99 | Dickkopf homolog 1 (Xenopus laevis) | |
| RDH10 | −1.99 | Retinol dehydrogenase 10 (all-trans) | |
| KYNU | −2.01 | Kynureninase (l-kynurenine hydrolase) | |
| PEG10 | −2.05 | Paternally expressed 10 | |
| CXCL12 | −2.05 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) | |
| SLC7A8 | −2.06 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 8 | |
| IL1R1 | −2.07 | IL-1 receptor, type I | |
| HSD11B1 | −2.08 | Hydroxysteroid (11-β) dehydrogenase 1 | |
| BMP3 | −2.09 | Bone morphogenetic protein 3 (osteogenic) | |
| IGFBP2 | −2.09 | insulin-like growth factor binding protein 2, 36 kDa | |
| TWIST1 | −2.11 | Twist homolog 1 (acrocephalosyndactyly 3 | |
| ABCC9 | −2.11 | ATP-binding cassette, sub-family C (CFTR | |
| EFNB2 | −2.13 | Ephrin-B2 | |
| EMILIN2 | −2.16 | Elastin microfibril interfacer 2 | |
| LIF | −2.16 | Leukemia inhibitory factor (cholinergic differentiation factor) | |
| MFAP4 | −2.16 | Microfibrillar-associated protein 4 | |
| CDH13 | −2.16 | Cadherin 13, H-cadherin (heart) | |
| GNG11 | −2.16 | Guanine nucleotide binding protein (G protein), γ 11 | |
| PTGS2 | −2.17 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G | |
| MOBKL2B | −2.18 | MOB1, Mps one binder kinase activator-like 2B (yeast) | |
| (Continued) | |||
Table 3A.
Continued
| Gene symbol | FC (hESFendo cAMP/hESFnonendo cAMP) | Description |
|---|---|---|
| IL13RA2 | −2.19 | IL-13 receptor, α 2 |
| CD68 | −2.19 | CD68 molecule |
| IL6 | −2.20 | IL-6 (interferon, β 2) |
| NOX4 | −2.22 | NADPH oxidase 4 |
| IL24 | −2.29 | IL-24 |
| SNCA | −2.31 | Synuclein, α (non A4 component of amyloid precursor) |
| ERAP2 | −2.34 | Endoplasmic reticulum aminopeptidase 2 |
| PTGES | −2.37 | Prostaglandin E synthase |
| ABCA9 | −2.38 | ATP-binding cassette, sub-family A (ABC1), member 9 |
| IL8 | −2.43 | IL-8 |
| EDNRA | −2.47 | Endothelin receptor type A |
| GADD45G | −2.53 | Growth arrest and DNA-damage-inducible, γ |
| ROR2 | −2.55 | Receptor tyrosine kinase-like orphan receptor 2 |
| ABCA8 | −2.56 | ATP-binding cassette, sub-family A (ABC1), member 8 |
| IL11 | −2.57 | IL-11 |
| HGF | −2.57 | Hepatocyte growth factor (hepapoietin A |
| ALDH1A1 | −2.59 | Aldehyde dehydrogenase 1 family, member A1 |
| ENPP1 | −2.60 | Ectonucleotide pyrophosphatase |
| ABCA6 | −2.62 | ATP-binding cassette, sub-family A (ABC1), member 6 |
| NOV | −2.63 | Nephroblastoma overexpressed gene |
| ALDH1A2 | −2.64 | Aldehyde dehydrogenase 1 family, member A2 (ALDH1A2), transcript variant 3, mRNA |
| IGFBP3 | −2.75 | IGF binding protein 3 |
| NR4A2 | −2.78 | Nuclear receptor subfamily 4, group A, member 2 |
| THSD7A | −2.84 | Thrombospondin, type I, domain containing 7A |
| ENPEP | −2.87 | Glutamyl aminopeptidase (aminopeptidase A) |
| C4orf31 | −2.89 | Chromosome 4 open reading frame 31 |
| EDG2 | −3.06 | Endothelial differentiation, lysophosphatidic acid G-protein-coupled receptor, 2 |
| SFRP1 | −3.28 | Secreted frizzled-related protein 1 |
| PTHLH | −3.32 | PTH-like hormone |
| EDNRB | −3.42 | Endothelin receptor type B (EDNRB), transcript variant 2, mRNA |
| FST | −3.57 | Follistatin |
| KGFLP1 | −3.69 | Keratinocyte growth factor-like protein 1 |
| KGFLP1 | −3.69 | Keratinocyte growth factor-like protein 1 |
| KGFLP1 | −3.69 | Keratinocyte growth factor-like protein 1 |
| CCL8 | −3.88 | Chemokine (C-C motif) ligand 8 |
| FGF7 | −3.91 | Fibroblast growth factor 7 (keratinocyte growth factor) |
| TNFAIP6 | −3.91 | TNF, α-induced protein 6 |
| ENPP2 | −3.95 | Ectonucleotide pyrophosphatase |
| PRL | −3.99 | Prolactin |
| LTBP1 | −4.08 | Latent TGF-β binding protein 1 |
| COL15A1 | −4.13 | Collagen, type XV, α1 |
| CCL2 | −4.16 | Chemokine (C-C motif) ligand 2 |
| IGFBP1 | −4.17 | IGFBP1 |
| MMP8 | −5.35 | Matrix metallopeptidase 8 (neutrophil collagenase) |
| SST | −5.36 | Somatostatin |
| TFPI2 | −7.12 | Tissue factor pathway inhibitor 2 |
Figure 2.
QPCR validation of microarray data of gene expression in hESFnonendo and hESFendo decidualized in vitro with 0.5 mm with 8Br-cAMP for 96 h, expressed as fold change to the expression in 96 h vehicle controls. *, Significance accepted at P ≤ 0.05 (Mann-Whitney test). Error bars, ±sem. FC, Fold change; SST, somatostatin; CXCR4, C-X-C chemokine receptor type 4.
Functional network analysis
The four study groups (A–D) were subjected to canonical pathway analysis and generation of networks. Genes were selected based on the fold change cutoff (1.5-fold) and P < 0.05. In group A, 691 genes were identified as regulated after applying both parametric statistical analysis of microarrays and nonparametric (RankProd) tests, from which 351 molecules were eligible for generating networks and pathways. In group B, 158 genes were regulated, with 103 molecules eligible for generating networks and pathways. In group C, of 245 molecules identified as different between control hESFendo vs. control hESFnonendo, 202 were eligible for generating networks. In group D, 105 genes were different between cAMP-treated hESFendo vs. hESFnonendo, with 96 eligible for generating networks and pathways. Although numerous pathways were identified as regulated, many canonical pathways are connected with each other because many genes participate in multiple pathways.
In group A, the five leading pathways with a high ratio of regulated molecules per total number of molecules in the pathway were (ratio; P value): hepatic fibrosis (12 of 135; 0.089), cell cycle G2/M DNA damage checkpoint regulation (five of 43; 0.116), caveolar-mediated endocytosis (seven of 81; 0.086), cAMP mediated signaling (10 of 159, 0.063), and G protein-coupled signaling (11 of 202, 0.054) (supplemental Table S4). The activation of other pathways in this group is worth mentioning, including vascular endothelial growth factor, tight junction, notch, bone morphogenetic protein, TGF-β, integrin, and sonic hedgehog signaling pathways and others known to be actively involved in endometrial physiology.
There is a blunted response to cAMP in hESFendo vs. hESFnonendo [Fig. 1A, (13)], and therefore, few genes were anticipated to be regulated per total number of molecules in each pathway. The top five pathways in group B (hESFendo+cAMP vs. vehicle control) were (ratio; P value): cell cycle G2/M DNA damage checkpoint regulation (three of 43; 0.07), lipopolysaccharide/IL-1-mediated inhibition of retinoid X receptor function (five of 198; 0.025), caveolar-mediated endocytosis (three of 81; 0.037), tight junction signaling (four of 164; 0.024), calcium signaling (four of 202; 0.02) (supplemental Table S5). The complete lists of regulated genes and canonical pathways are presented as supplemental data (supplemental Tables S1–S6) and Table 3. Of special interest is the list of canonical pathways differentially regulated in cAMP treatment of hESFendo vs. hESFnonendo. Pathways, uniquely activated in hESFendo response to cAMP (group B) and in hESFnonendo response to cAMP (group A) are presented in supplemental Table S6.
Cell cycle G1/S and G2/M DNA damage checkpoint regulation pathways
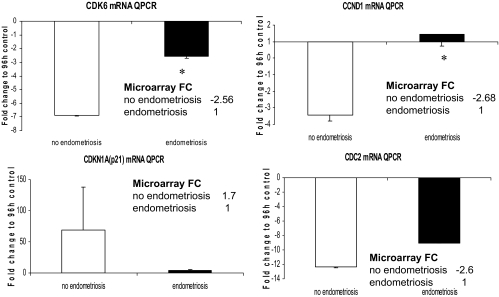
Microarray analysis of the hESFnonendo treated with vs. without cAMP (group A) demonstrated down-regulation of cell cycle pathways due to significant down-regulation of cyclin (CCN)-D1 (−2.68-fold) and cyclin-dependent kinase (CDK)-6 (−2.56-fold), up-regulation of cyclin-dependent kinase inhibitor (CDKN)-1A (p21) (+1.7-fold), decrease in CDKN3 (−1.9-fold) and cell division cycle 2 (−2.6). These data, indicating a decrease in cell cycling in normal hESF on decidualization, support previous reports (18,27). However, in contrast, microarray analysis revealed that in hESFendo down-regulation of cell cycle pathway members in response to cAMP (group B) was not observed, and there was no change in CCND1, CDK6, CDKN1A, and cell division cycle 2, with only CDKN3 decreased 2.87-fold. In groups C and D, CCND2 was up-regulated 1.68- and 2.19-fold, respectively, indicating that even in vehicle-treated hESFendo, the basal level of this cell cycle regulator is higher than in hESFnonendo (supplemental Tables S1–S4). Expression of mRNA for some of the cell cycle genes was validated by QRT-PCR and comparison with the microarray data are presented in Fig. 3. Very recently, we demonstrated that 5-bromo-2′-deoxyuridine incorporation is significantly decreased in cAMP-treated hESFnonendo vs. hESFendo (27). These data suggest that hESFendo exhibits higher proliferation potential under the decidualizing stimulus (cAMP) and are consistent with the transcriptomic analyses herein.
Figure 3.
All hESFs were treated with or without 0.5 mm cAMP for 96 h. Validation by real-time QPCR expression of cell cycle genes’ mRNA. *, Significance accepted as P ≤ 0.05. Error bars, ±sem. FC, Fold change.
Differentiation
Several canonical signaling pathways were regulated in hESFs after cAMP treatment, as determined by IPA analysis, and some of them exhibited differences between hESFendo vs. hESFnonendo.
Immune genes
The most highly regulated gene (88.5-fold) on hESFnonendo decidualization with cAMP was IL13RA2, high-affinity IL-13 receptor-α2, which was not regulated in response to cAMP in hESFendo (supplemental Tables S1 and S2), indicating marked dysregulation in the setting of endometriosis. CXCR4 (C-X-C chemokine receptor type 4), the receptor for CXCL12 (stromal cell derived factor 1), was significantly up-regulated 58.3- and 33.5-fold in hESFnonendo and hESFendo, respectively, in response to cAMP treatment. QRT-PCR validation of IL13RA2 and CXCR4 expression in hESFs before and after cAMP treatment confirmed the microarray data and underscored diminished responsiveness of hESFendo to cAMP (Fig. 2).
Wnt signaling pathway
The Wnt inhibitor Dickkopf-1, exclusively regulated by P4 (20), was significantly lower in hESFendo compared with hESFnonendo, and treatment with or without cAMP had no effect on its expression. SFRP1 (an inhibitor of the Wnt receptor Frizzled) was 3.27-fold decreased in hESFendo. Furthermore, increased expression of nemo-like kinase protein (a negative regulator of Wnt signaling that translocates from the cytoplasm to nucleus and decreases transcription of CCND1) was found in hESFnonendo in response to cAMP but not in hESFendo. Overall, IPA suggests activation of the Wnt canonical and noncanonical pathways in endometriosis.
Forkhead box O (FOXO) signaling pathway
FOXO1A is important in the decidualization process (28,29), and, interestingly, the FOXO1A transcript was increased in hESFnonendo, but not hESFendo, in response to cAMP. FOXO transcription factors up-regulated Bcl-2-interacting mediator of cell death (BIM) expression, as reported in other cell types (30), which is an initiator of apoptosis (31,32).
ERK/MAPK signaling pathway
We and others recently found that the MAPK pathway is constitutively active in hESFendo vs. hESFnonendo (27,33). Herein dual-specificity phosphatases 1 and 4, which are known inhibitors of ERK1/2, were up-regulated 4.4- and 3.26-fold, respectively, in hESFnonendo treated with cAMP. This can lead to increased E-twenty six (ETS) transcription factors, such as ETS2 [which was up-regulated 2.7-fold (supplemental Table S1)] that are involved in stem cell development, cell senescence and death, and tumorogenesis (34). ERK/MAPK signaling did not change significantly in hESFendo on cAMP treatment.
Cytoskeleton and extracellular matrix (ECM) genes
Changes in the cytoskeleton and cell shape are hallmarks of hESF decidualization. Several cytoskeletal structural genes and some ECM genes were down-regulated in hESFnonendo in response to cAMP. α2-Smooth muscle actin and γ2-smooth muscle actin were significantly down-regulated during the normal decidualization process (6.7- and 6.0-fold, respectively) (35), whereas in cAMP-treated hESFendo, only α2-smooth muscle actin was decreased, consistent with increased migration/motility of stromal cells in endometriosis. Filamins A, B, and C, which are involved in remodeling the cytoskeleton and participate in anchoring membrane proteins for the actin cytoskeleton, were greater than 2-fold decreased in response to cAMP in hESFnonendo, whereas only filamin B was (down)regulated in hESFendo (supplemental Tables S1 and S2). Myosin light-chain kinase, myosin heavy-chain 10, and myosin light-chain 9 regulatory unit were all greater than 2-fold down-regulated in decidualized hESFnonendo but not in cAMP-treated hESFendo (except for myosin light-chain 9 regulatory unit). Fibronectin 1, anilin, laminin-α4, matrix-remodeling associated 5, and some kinesin family members were significantly down-regulated in hESFnonendo, but not hESFendo, after treatment with cAMP (supplemental Tables S1 and S2).
Transgelin (TAGLN, SM22), a smooth muscle actin-binding protein specific for fibroblasts and smooth muscle cells and a repressor of matrix metalloproteinase (MMP)-9 (36), was down-regulated (15.5- and 11.3-fold) after decidualization with cAMP in hESFnonendo and hESFendo, respectively. MMP10 and MMP8 were markedly (15.6- and 15.8-fold, respectively) up-regulated in hESFnonendo in response to cAMP, supporting earlier reports (15,37,38) and their role in matrix remodeling during the normal decidualization process and preparation for implantation. Of note is that the MMP8 transcript was 5.35-fold lower in hESFendo, whereas MMP1, MMP3, MMP10, and MMP12 were greater than 2-fold up-regulated in hESFendo vs. hESFnonendo. Collagen type VIα1, collagen type VIα3, and collagen α-1(XIV) chain (COL14A1; undulin) transcripts were significantly decreased in hESFnonendo response to cAMP and were not regulated in hESFendo in the present study.
Tenascin C (hexabrachion), an extracellular matrix protein that binds collagen, fibronectin, and integrins, which can act as an inhibitor of cell adhesion to fibronectin (39), was the only gene in our study that was 8.15-fold down-regulated in cAMP-treated hESFendo but not regulated by cAMP in hESFnonendo. Levels of the tenascin C transcript were slightly (1.6-fold) lower in untreated hESFendo vs. hESFnonendo.
Validation and functional analysis of PDE regulation
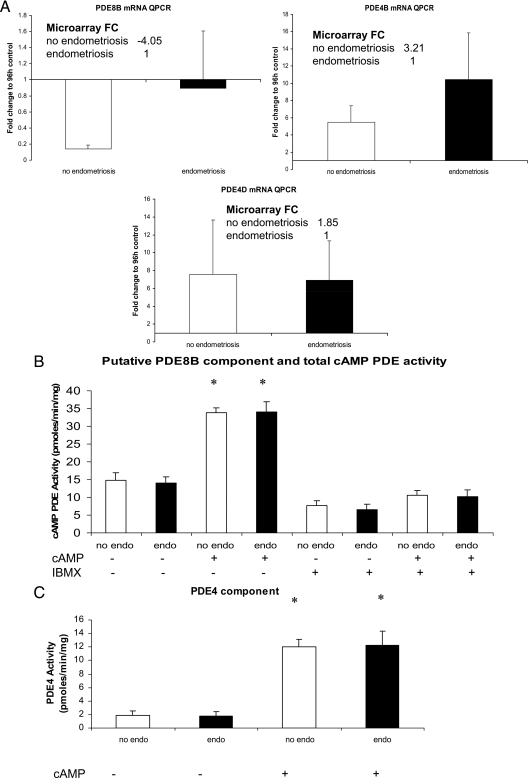
Because of a blunted response of hESFendo vs. hESFnonendo to 8-Br-cAMP, we investigated whether the resistance to activation of the PKA pathway is due to hydrolysis of the cAMP analog 8-Br-cAMP by specific PDEs. The PDE4 family is the largest PDE family in the mammalian PDE superfamily and encompasses four genes, PDE4A–D (40). In the current microarray study, PDE4B and PDE4D were up-regulated 3.2- and 1.85-fold, respectively, by cAMP in hESFnonendo but not hESFendo (supplemental Tables S1 and S2), although QRT-PCR validation did not confirm these differences (Fig. 4A). Our microarray analysis also identified PDE8B, a cAMP-specific PDE, whose transcript decreased significantly (−4.0-fold) in hESFnonendo, but not hESFendo, after treatment with cAMP. Moreover, levels of PDE8B by microarray analysis were significantly higher in hESFendo compared with hESFnonendo, both untreated and treated with cAMP (2.2- and 2.41-fold, respectively, groups C and D). The analysis of PDE8B transcripts levels by QRT-PCR between cAMP-treated hESFnonendo and hESFendo did not detect a statistically significant difference but did confirm the trend observed by microarray analysis (Fig. 4A).
Figure 4.
Analysis of PDE4 and PDE8 in hESFnonendo and hESFendo. A, Validation by real-time QPCR expression of PDE8B, PDE4B, and PDE4D mRNAs. All hESFs were treated with or without 0.5 mm cAMP for 96 h. B, Total and IBMX-insensitive cAMP PDE activity in hESFs was determined as described in Materials and Methods. Shown is the cAMP PDE activity of hESF lysates (n = 4 with and n = 3 without endometriosis) using 100 nm [3H] cAMP as substrate in the absence of added inhibitors (Total) or in the presence of 100 μm IBMX to measure the apparent PDE8 component (+IBMX). cAMP-PDE activity is expressed as picomoles of cAMP hydrolyzed per minute per milligram of total cell protein. Error bars, ±sem. C, PDE4 activity in hESF lysates. The PDE4 cAMP PDE activity in hESFs was determined as described in Materials and Methods by calculating the activity that was sensitive to 1 μm of the PDE4 selective inhibitor rolipram. The data represent n = 3 hESFnonendo or hESFendo assayed in duplicate. Error bars, ±sem. No endo, hESFnonendo; endo, hESFendo. *, Significant difference (P ≤ 0.05) compared with respective control. FC, Fold change.
To investigate whether the observed microarray analysis changes in the PDE4 and PDE8 families, translated into significant functional effects, we investigated the cAMP PDE activity of cultured hESFnonendo and hESFendo. The contribution of PDE4 to the cAMP hydrolytic activity in hESFnonendo and hESFendo was delineated by the use of rolipram, a potent and selective inhibitor of all PDE4 isoenzymes. Because there is no available specific PDE8 inhibitor available, the PDE8 component was measured in the presence of IBMX. IBMX is a nonselective PDE inhibitor, which inhibits all families of cAMP-hydrolyzing PDEs except PDE8 (25,26); therefore, the cAMP hydrolytic activity that remained in the presence of IBMX was taken as the putative PDE8 component. There were no observed changes in the putative PDE8 component identified as the cAMP hydrolytic activity resistant to IBMX when comparing nondecidualized hESFendo vs. hESFnonendo or when comparing cAMP-decidualized hESFs from the same subjects (Fig. 4B). Also, no changes between total cAMP hydrolytic activities were observed between nondecidualized hESFnonendo and hESFendo as well as between cAMP-decidualized hESFnonendo and hESFendo (Fig. 4B). Furthermore, no significant difference in PDE4 activity in lysates of hESFendo vs. hESFnonendo was observed (Fig. 4C). Decidualization with 8-Br-cAMP produced a robust 6.7-fold increase in PDE4 activity in both hESFnonendo and hESFendo, with no difference between them (Fig. 4C).
Discussion
General comments
The current study presents two important concepts. The first focuses on genes, pathways, and biological processes activated or repressed in response to the decidualizing stimulus/activation of the PKA pathway in hESFnonendo. The second is the difference in response to the decidualizing stimulus/activation of the PKA pathway of hESFendo compared with hESFnonendo.
In general, our data support evidence reported by us and others regarding decreased expression of cell cycle-regulating genes on decidualization in vivo and in vitro in hESFs from women without endometriosis (8,15,17,41,42,43,44). This is further supported by early studies on histology and mitotic figures and rates of DNA synthesis in secretory endometrium (45). The cell cytoskeleton and extracellular matrix genes, such as actins, fibulins, kinesins, myosins, and MMPs, were significantly regulated in cAMP-treated hESFnonendo, consistent with the characteristic changes in endometrial stromal cell morphology during the decidualization process (46). Characterization of global gene expression in response to cAMP confirmed earlier findings of our group using a more limited microarray platform (15). Importantly, in the current study, we identified new genes and pathways that are important for the process of decidualization, including vascular endothelial growth factor, Notch, tight junction, G protein-coupled receptor signaling, axonal guidance signaling, and others (supplemental Table S4).
Differentiation vs. proliferation in hESFnonendo vs. hESFendo
Overall, our study demonstrated that hESFendo displays a blunted response to cAMP/PKA pathway activation with a decrease in the total number of genes regulated, compared with hESFnonendo. Subsequently hESFendo demonstrated a diminished differentiation capacity, reflected in decreased expression of decidualization markers, cytoskeletal components, matrix-degrading enzymes, immune genes, and key signaling pathways involved in the differentiation process, known and revealed herein.
In a comprehensive microarray analysis of endometrial tissue biopsies from women with and without endometriosis, we demonstrated that there is a delayed transition from the proliferative to secretory phase, with persistent expression of genes involved in mitosis and proliferation in early secretory endometrium (ESE) of women with endometriosis but not in those without disease (7). Even though ESE samples clustered according to their cycle phase, genes involved in cell proliferation maintained a fingerprint consistent with the proliferative phase (7). Johnson et al. (47) reported that eutopic endometrium from women with endometriosis demonstrated increased proliferation and decreased apoptosis, compared with endometrial tissue from women without disease (47). These observations are consistent with the present microarray analysis and validated by QRT-PCR (Fig. 3 and supplemental tables). Taken together, the data strongly support an abnormal transition from the estrogen-dominant proliferative phase to the progesterone (and PKA)-dominant secretory phase in endometrium in the setting of endometriosis (47,48,49).
Because cell cycle progression is inhibited in the hESFnonendo in response to cAMP, with differentiation/decidualization biomarkers being up-regulated, the data support decreased proliferation and increased differentiation as hallmarks of the endometrial stromal fibroblast in the secretory phase of menstrual cycle. In addition, the observed blunted response of hESFendo to cAMP inhibition of proliferation and induction of decidualization biomarkers may account for the increased survival of endometrial cells and the persistent proliferative phenotype of endometrial tissue during the proliferative-secretory transition in women with vs. without disease (7). Interestingly, the cell cycle pathway in human endometrium may be regulated in part by microRNAs (miRNAs), and recently we demonstrated that of seven miRNAs significantly down-regulated in women with vs. without endometriosis, five are involved in regulation of cell cycle (50). These observations support increased transitioning through the cell cycle in endometrium from women with endometriosis and that miRNAs are potential mediators in the delayed proliferative to secretory transition of endometrium from women with endometriosis (7).
cAMP hydrolysis
Because 8-Br-cAMP was used to induce decidualization, a G protein-coupled-receptor/G protein/cyclase defect in hESFendo has been excluded (40). Thus, hydrolysis of 8-Br-cAMP by PDEs (40,51) was considered as a possibility for the blunted response to cAMP treatment in hESFendo vs. hESFnonendo. Of the 11 mammalian PDE families (PDEs1–11), PDE4, PDE7, and PDE8 are the cAMP-specific PDEs (26). Hydrolysis of cyclic nucleotides by the PDEs provides the major cellular mechanism for the dampening of cellular cyclic nucleotide signaling (26,52). Our results demonstrate that cAMP treatment of hESFnonendo and hESFendo leads to a robust induction of PDE4 activity, confirming that intracellular cAMP is involved in regulating its own synthesis and degradation (53,54). Despite changes in PDE4 and PDE8 mRNA transcripts detected in our microarray studies of hESFendo vs. hESFnonendo, no apparent changes were observed in functional characterization of these cAMP-PDE activities in these cells under the conditions tested. It should be noted that the PDE8 family is poorly understood and no specific inhibitor exists for this enzyme family. Therefore, our functional evidence for assessing PDE8 activity is based on the noted insensitivity of this PDE to the PDE inhibitor IBMX (25,26). Because the cAMP PDE activities in hESFnonendo and hESFendo were not significantly different, we conclude that hESFnonendo and hESFendo do not have dysregulated cAMP homeostasis. However, with dysregulation of the cAMP/PKA pathway in hESFendo not being attributed to altered hydrolysis of cAMP, there may still be a difference in the expression of downstream mediators, such as cAMP response element modulator (supplemental Tables S1 and S2). Because PDEs are regulated by receptors coupled to cAMP (55,56), the fact that the observed abnormality in the PKA signaling pathway in women with vs. without disease did not affect PDE4 induction suggests that there are differences in the sensitivity of different genes to cAMP/PKA activation or that there are specific coregulators that alter transcription of a subgroup of genes (e.g. PDE4D vs. IGFBP1 and CCND1). This characterization represents the first comparison of cAMP hydrolysis/PDE activity in eutopic endometrium from women with and without endometriosis.
Other pathway involvement in the pathogenesis of endometriosis
Of note is the involvement of the Wnt pathway in the pathogenesis of endometriosis (44,57). The data herein further support this and provide important insight into these observations. For example, the Wnt inhibitor secreted frizzled related protein (SFRP)-1 is highly expressed in endometrial stroma in the proliferative phase, compared with the secretory phase, in normal endometrium (57,58) as well as in endometriotic tissue compared with eutopic endometrium, and direct regulation of SFRP1 by estrogen has been suggested (57). Transcripts for both Wnt inhibitors, Dickkopf-1 and SFRP1, were decreased in untreated hESFendo in the present study, suggesting a potential contribution of the Wnt pathway to development and/or maintenance of endometriosis with its persistent proliferative signature that is amplified in response to steroid hormones and activation of the PKA pathway.
Dysregulation of immune genes in the setting of endometriosis is well documented (7,58). In the present study, IL13RA2 was the most highly up-regulated gene in hESFnonendo but not hESFendo in response to cAMP. The function of IL13RA2 in endometrial physiology or pathology is largely unknown. Chen et al. (59) demonstrated that IL13RA2 is cleaved and solubilized in vitro by MMP8, which was increased in our array data 15.8-fold on decidualization of hESFnonendo, suggesting a contribution of that mechanism to the normal decidualization process in human endometrium.
Many genes involved in cell shape and cytoskeleton functioning were decreased in hESFnonendo on decidualization; whereas these changes were blunted in hESFendo. Collagens VI are present in endometrial stroma in proliferative and ESE as a component of the intercellular collagenous network, with a decrease in expression during the midsecretory phase (Refs. 60 and 61 and herein). No alteration in collagen, smooth muscle actin, fibronectin, kinesins, and other ECM molecule expression in hESFendo on cAMP treatment is consistent with impaired decidualization of hESF from women with endometriosis, which is accompanied by impaired morphological transformation to the decidualized phenotype. Earlier studies demonstrated down-regulation of tenascin C by P4 in the stroma of normal endometrium and dysregulation of it in endometriosis (62). Interestingly, tenascin C was one of the most highly down-regulated genes (0.16-fold) in the proliferative-to-early secretory transition in women without endometriosis (8), and whether that is due to progesterone and/or activation of the PKA pathway in hESFs is uncertain. Studies of tenascin C up-regulation in tumor ECM by epithelial growth factor and down-regulation by steroid hormones (63) underscore the complexity of regulation of this ECM glycoprotein.
Summary and conclusions
The results presented herein demonstrate that eutopic endometrial stromal fibroblasts from women with endometriosis differ from those obtained from women without disease. These differences lie in processes that are related to cell survival, cell proliferation, neoangiogenesis, immune function, cell shape, and ECM modification that may account for why a subgroup of women develops endometriosis, despite most women experiencing retrograde menstruation (64,65). The data support the postulate that in endometriosis, eutopic endometrial cells with increased proliferative potential, as well as decreased immunogenicity, when seeded into the pelvic cavity after retrograde menstruation, are endowed with properties to establish endometriotic lesions. Altered immune response, impaired ECM degradation, cell cytoskeleton changes, and establishment of a blood supply and accompanying innervation likely promote further survival and growth of transplanted/implanted stromal cells from the eutopic endometrium.
From a clinical perspective, in view of the role of decidual cells in tissue hemostasis (66,67), an impaired decidual response of hESFs may also lead to premenstrual uterine spotting in patients with endometriosis, although this remains to be determined.
The current and earlier studies (13,16,68) are convincing in that whereas the endometrium from women with endometriosis exhibits a molecular signature of P4 resistance, there is an apparent impairment of the PKA pathway in hESFs. The observations that the decidualization process involves activation of the PKA- and progesterone receptor-dependent signaling pathways that interface with and are influenced by a multitude of other pathways are important in understanding basic mechanisms underlying differentiation of this tissue in response to steroid hormones. Likewise, investigation into the effects of progesterone on hESFendo vs. hESFnonendo is currently underway in our laboratory. Furthermore and of equal importance, abnormal signaling pathways in endometrium (described herein and Refs. 27,33 and 68) and endometriosis lesions (9,69) of women with disease offer new opportunities to develop novel, targeted therapies to treat endometriosis-related pain and/or infertility.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreement 1U54HD055764-03 (to L.C.G.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure summary: The authors have nothing to disclose.
First Published Online January 12, 2010
Abbreviations: 8-Br-cAMP, 8-Bromoadenosine-cAMP; CCN, cyclin; CDK, cyclin-dependent kinase; CDKN, cyclin-dependent kinase inhibitor; ECM, extracellular matrix; ESE, early secretory endometrium; FOXO, Forkhead box O; hESF, human endometrial stromal fibroblast; IBMX, isobutylmethylxanthine; IGFBP, IGF binding protein; IPA, ingenuity pathway analysis; miRNA, microRNA MMP, matrix metalloproteinase; P4, progesterone; PDE, phosphodiesterase; PKA, protein kinase A; PRL, prolactin; Q, quantitative; SFRP, secreted frizzled related protein.
References
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Sampson JA 1927 Peritoneal endometriosis due to the menstrual dissemination of endometrial tissues into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- Berkley KJ, Rapkin AJ, Papka RE 2005 The pains of endometriosis. Science 308:1587–1589 [DOI] [PubMed] [Google Scholar]
- Burney RO, Giudice LC 2008 The pathogenesis of endometriosis. In: Nezhat CN, Nezhat FR, Nezhat C, eds. Nezhat’s operative gynecologic laparoscopy and hysteroscopy. New York: Cambridge University Press; 251–257 [Google Scholar]
- Eskenazi B, Warner ML 1997 Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24:235–258 [DOI] [PubMed] [Google Scholar]
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Osteen K, Lessey BA, Giudice LC 2003 Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 144:2870–2881 [DOI] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC 2007 Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC 2006 Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- Bulun SE 2009 Endometriosis. N Engl J Med 360:268–279. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC, 2010 Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med, 28:51–58 [DOI] [PubMed] [Google Scholar]
- Giudice LC, Telles TL, Lobo S, Kao L 2002 The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann NY Acad Sci 955:252–264; discussion 293–295, 396–406 [DOI] [PubMed] [Google Scholar]
- Giudice LC 1999 Genes associated with embryonic attachment and implantation and the role of progesterone. J Reprod Med 44:165–171 [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC 2009 Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod 80:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Irwin JC 1999 Roles of the insulinlike growth factor family in nonpregnant human endometrium and at the decidual: trophoblast interface. Semin Reprod Endocrinol 17:13–21 [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC 2003 Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 16:47–66 [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ 2006 Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril 85:564–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ 2001 Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7:135–148 [DOI] [PubMed] [Google Scholar]
- Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW, Unterman TG, Brosens JJ, Kim JJ 2007 Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 21:2334–2349 [DOI] [PubMed] [Google Scholar]
- The American Fertility Society 1985 Revised American Fertility Society classification of endometriosis. Fertil Steril 43:351–352 [DOI] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC 2006 Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91:1453–1461 [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G 2001 Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling R, Armengaud P, Amtmann A, Herzyk P 2004 Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573:83–92 [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D 1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks 2nd JL, , Zoraghi R, Francis SH, Corbin JD 2007 N-terminal domain of phosphodiesterase-11A4 (PDE11A4) decreases affinity of the catalytic site for substrates and tadalafil, and is involved in oligomerization. Biochemistry 46:10353–10364 [DOI] [PubMed] [Google Scholar]
- Hambartsoumian E, Srivastava RK, Seibel MM 2001 Differential expression and regulation of inducible nitric oxide synthase (iNOS) mRNA in human trophoblasts in vitro. Am J Reprod Immunol 45:78–85 [DOI] [PubMed] [Google Scholar]
- Francis SH, Turko IV, Corbin JD 2001 Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acids Res Mol Biol 65:1–52 [DOI] [PubMed] [Google Scholar]
- Velarde MC, Aghajanova L, Nezhat CR, Giudice LC 2009 Increased MEK/ERK activity in human endometrial stromal fibroblasts of women with endometriosis reduces cAMP inhibition of cyclin D1. Endocrinology 150:4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinius L, Kessler C, Schroeder J, Handwerger S 2006 Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol 189:179–187 [DOI] [PubMed] [Google Scholar]
- Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ 2006 FOXO1A differentially regulates genes of decidualization. Endocrinology 147:3870–3876 [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J 2003 FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol 162:613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K, Nakazawa S, Hirai H, Ozawa K, Inaba T 2004 Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitors. Mol Cell Biol 24:6172–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ 2006 Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol 20:35–44 [DOI] [PubMed] [Google Scholar]
- Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA, Arici A 2008 Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3532–3540 [DOI] [PubMed] [Google Scholar]
- Seth A, Watson DK 2005 ETS transcription factors and their emerging roles in human cancer. Eur J Cancer 41:2462–2478 [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT 1999 Blastocyst invasion and the stromal response in primates. Hum Reprod 14(Suppl 2):45–55 [DOI] [PubMed] [Google Scholar]
- Nair RR, Solway J, Boyd DD 2006 Expression cloning identifies transgelin (SM22) as a novel repressor of 92-kDa type IV collagenase (MMP-9) expression. J Biol Chem 281:26424–26436 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Groth P, Haendler B, Hess-Stumpp H, Kratzschmar J, Seidel H, Thaele M, Weiss B 2005 Gene expression during the implantation window: microarray analysis of human endometrial samples. Ernst Schering Res Found Workshop 139–157 [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, Gorstein F, Osteen KG 1994 Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest 94:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE 2005 Meet the tenascins: multifunctional and mysterious. J Biol Chem 280:26641–26644 [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo J 2007 Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC 2000 Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 141:3510–3513 [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G 2005 DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril 84(Suppl 2):1180–1190 [DOI] [PubMed] [Google Scholar]
- Lu Z, Hardt J, Kim JJ 2008 Global analysis of genes regulated by HOXA10 in decidualization reveals a role in cell proliferation. Mol Hum Reprod 14:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YY, Lin CS, Sun YL, Chang CC, Tsai HD, Wu JC 2002 In vivo gene transfer of leukemia inhibitory factor (LIF) into mouse endometrium. J Assist Reprod Genet 19:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC, Ferenczy A 1995 The endometrial cycle: morphologic and biochemical events. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive endocrinology, surgery, and technology. New York: Raven Press; 171–194 [Google Scholar]
- Gellersen B, Brosens J 2003 Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 178:357–372 [DOI] [PubMed] [Google Scholar]
- Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, Vega M, Boric MA 2005 Augmented cell survival in eutopic endometrium from women with endometriosis: expression of c-myc, TGF-β1 and bax genes. Reprod Biol Endocrinol 3:45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresman GF, Vighi S, Buquet RA, Contreras-Ortiz O, Tesone M, Rumi LS 2000 Apoptosis and expression of Bcl-2 and Bax in eutopic endometrium from women with endometriosis. Fertil Steril 74:760–766 [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee HW, Kim YH, Koo YH, Chae HD, Kim CH, Lee PR, Kang BM 2009 Down-regulation of p21-activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Hum Reprod 24:1133–1141 [DOI] [PubMed] [Google Scholar]
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC 2009 MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod 15:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M, Butt E, Nolte C, Fischer L, Halbrugge M, Beltman J, Jahnsen T, Genieser HG, Jastorff B, Walter U 1991 Characterization of Sp-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole-3′,5′-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J 279(Pt 2):521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD 2005 High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res 17:5–9 [DOI] [PubMed] [Google Scholar]
- Conti M, Kasson BG, Hsueh AJ 1984 Hormonal regulation of 3′,5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology 114:2361–2368 [DOI] [PubMed] [Google Scholar]
- Erdogan S, Houslay MD 1997 Challenge of human Jurkat T-cells with the adenylate cyclase activator forskolin elicits major changes in cAMP phosphodiesterase (PDE) expression by up-regulating PDE3 and inducing PDE4D1 and PDE4D2 splice variants as well as down-regulating a novel PDE4A splice variant. Biochem J 321(Pt 1):165–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen JV, Joseph DR, Conti M 1989 The mRNA encoding a high-affinity cAMP phosphodiesterase is regulated by hormones and cAMP. Proc Natl Acad Sci USA 86:8197–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Nemoz G, Sette C, Vicini E 1995 Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev 16:370–389 [DOI] [PubMed] [Google Scholar]
- Cheng CW, Smith SK, Charnock-Jones DS 2008 Transcript profile and localization of Wnt signaling-related molecules in human endometrium. Fertil Steril 90:201–204 [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC 2003 Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 88:3860–3866 [DOI] [PubMed] [Google Scholar]
- Chen W, Tabata Y, Gibson AM, Daines MO, Warrier MR, Wills-Karp M, Hershey GK 2008 Matrix metalloproteinase 8 contributes to solubilization of IL-13 receptor α2 in vivo. J Allergy Clin Immunol 122:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylona P, Kielty CM, Hoyland JA, Aplin JD 1995 Expression of type VI collagen mRNAs in human endometrium during the menstrual cycle and first trimester of pregnancy. J Reprod Fertil 103:159–167 [DOI] [PubMed] [Google Scholar]
- Lessey BA, Gui Y, Apparao KB, Young SL, Mulholland J 2002 Regulated expression of heparin-binding EGF-like growth factor (HB-EGF) in the human endometrium: a potential paracrine role during implantation. Mol Reprod Dev 62:446–455 [DOI] [PubMed] [Google Scholar]
- Harrington DJ, Lessey BA, Rai V, Bergqvist A, Kennedy S, Manek S, Barlow DH, Mardon HJ 1999 Tenascin is differentially expressed in endometrium and endometriosis. J Pathol 187:242–248 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kawakatsu H, Furukawa Y, Saito M 1995 Regulation of EGF-induced tenascin-C by steroids in tenascin-C-non-producing human carcinoma cells. Int J Cancer 63:720–725 [DOI] [PubMed] [Google Scholar]
- Cramer DW, Missmer SA 2002 The epidemiology of endometriosis. Ann NY Acad Sci 955:11–22; discussion 34–36, 396–406 [DOI] [PubMed] [Google Scholar]
- Missmer SA, Cramer DW 2003 The epidemiology of endometriosis. Obstet Gynecol Clin North Am 30:1–19, vii [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Hickey M, Huang SJ, Schatz F 2009 Decidualized human endometrial stromal cells mediate hemostasis, angiogenesis, and abnormal uterine bleeding. Reprod Sci 16:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood CJ, Krikun G, Rahman M, Caze R, Buchwalder L, Schatz F 2007 The role of decidualization in regulating endometrial hemostasis during the menstrual cycle, gestation, and in pathological states. Semin Thromb Hemost 33:111–117 [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Velarde MC, Giudice LC 2009 The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology 150:3863–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu SK, Lee J, Speights Jr VO, Starzinski-Powitz A, Arosh JA 2009 Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFκB and β-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol 23:1291–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.