A wide spectrum of amyloid structures is revealed in drusen. The data further support a commonality between age-related macular degeneration and other neurodegenerative diseases characterized by amyloid deposits.
Abstract
Purpose.
Drusen are a hallmark of eyes affected by age-related macular degeneration. In previous study, a conformational-specific antibody showed drusen to contain nonfibrillar amyloid structures. The current study was undertaken to assess the presence of additional amyloid structures in drusen.
Methods.
Sections from human donor eyes were reacted with M204, a monoclonal antibody that recognizes nonfibrillar oligomers; OC, a polyclonal antibody that recognizes amyloid fibrils of various molecular weights; and WO1 and WO2, monoclonal antibodies that are specifically reactive to mature amyloid fibrils. Electron microscopy was used as an independent means of investigating the presence of amyloid fibrils in drusen.
Results.
The presence of nonfibrillar oligomers was verified using the M204 antibody. OC and WO antibodies stained a wide spectrum of vesicular structures. OC reactivity showed extensive overlap with Aβ immunoreactivity, whereas a partial overlap was seen between Aβ reactivity and that of the WO antibodies. The presence of amyloid fibrils was also visualized by electron microscopy.
Conclusions.
These data reveal the presence of a wide spectrum of amyloid structures in drusen. The results are significant, given that specific conformational forms of amyloid are known to be pathogenic in a variety of neurodegenerative diseases. Deposition of these structures may lead to local toxicity of the retinal pigmented epithelium or induction of local inflammatory events that contribute to drusen biogenesis and the pathogenesis of AMD.
Age-related macular degeneration (AMD) is characterized by the presence of drusen, which are extracellular deposits that accumulate beneath the retinal pigmented epithelium. Many protein and lipid constituents of drusen are similar to those found in deposits characteristic of other age-related degenerative disorders such as Alzheimer disease (AD) and other amyloid diseases.1,2 These include amyloid β (Aβ), vitronectin, amyloid P, apolipoprotein E, and inflammatory mediators such as acute phase reactants and complement components. The finding that C5, C5b9, and C3 fragments, which are components of the complement cascade, are often present in drusen support a role for local inflammation in drusen biogenesis.3–5 This notion is bolstered by the discovery that a polymorphism in complement factor H, a regulator of the alternative complement pathway, significantly increases the risk factor for AMD.6–8 Despite its potential importance in the pathogenesis of AMD and AD, the initiating events leading to the inflammatory response are largely unknown.
The commonalities between AMD and AD can also be seen in a transgenic mouse model that expressed human apoE4,9 an allelic variant that shows a strong positive association with AD.10 Aged mice of this strain exhibit a retinal phenotype that replicates many features of AMD when the animals are fed a high-fat diet. Of interest, the pathologic features of this retinal model are attenuated by anti-Aβ antibody,11 supporting a role for Aβ toxicity in the retina. Retinal phenotypes of existing transgenic mouse models of AD that overexpress Aβ in neuronal cells have also been examined,12–14 and retinal disease, as well as a decrease in retinal function, as assessed by ERG, have been observed. Because the different promoters used for these mouse models were chosen based on their known activity in cortical neurons, the nature of Aβ-induced retinal disease in these AD mouse models varied, inasmuch as these promoters show various degrees of activity in different retinal cell types. It is likely that Aβ-induced toxicity in the retina, as in the brain, is due to formation of toxic amyloid structures, inasmuch as Aβ oligomers exert cellular toxicity, whereas soluble Aβ monomers do not.15,16
One distinguishing characteristic of amyloid diseases is the presence of abundant fibrils that are 6 to 15 nm in diameter, of various lengths, and often twisted.17 Fibrils are an end product of a stepwise misfolding of the proteins or peptides that accumulate in the deposits of many age-related degenerative disorders.18,19 For example, amyloid fibrils of AD plaques and Lewy bodies of Parkinson disease consist primarily of Aβ peptide and α-synuclein, respectively. Potentially amyloidogenic proteins share neither sequence homology nor structural similarity as soluble monomeric proteins. Remarkably, however, they display common structural features at specific stages in a misfolding process that leads to the formation of spherical and protofibrillar oligomers, as well as fibrillar forms.16,20 For example, soluble nonfibrillar oligomers formed by several amyloidogenic peptides and proteins are recognized by the conformation-specific A11 antibody.21 Given that a growing body of evidence points to a pathogenic role for soluble nonfibrillar oligomers in amyloid diseases,22 the A11 antibody has greatly facilitated the identification of such toxic species in diseased tissues.21,23–27 Antibodies that specifically recognize structural determinants of amyloid fibrils not present in monomeric or nonfibrillar oligomeric forms, also have been described. These include the OC28 antibody, which recognizes a wide molecular weight range of amyloid fibrils, and the WO1 and WO229 antibodies, which show a preference for large, insoluble, mature fibrils.
Despite the presence of several potentially amyloidogenic proteins in drusen and other similarities between drusen and amyloid deposits, amyloid fibrils have not been reported in drusen. Recently, the A11 antibody demonstrated the presence of nonfibrillar oligomers in drusen,26 suggesting that amyloid oligomers may be involved in drusen biogenesis and/or participate directly in local RPE toxicity. In this study, we verified the presence of amyloid oligomers using the monoclonal antibody M204, which binds amyloid oligomers but not monomers or fibrils (Supplementary Fig. S1, http://www.iovs.org/cgi/content/full/51/3/1304/DC1). We also used the WO1, WO2, and OC antibodies to investigate the presence of amyloid fibrils in drusen. These latter antibodies stained a wide spectrum of vesicular elements in drusen with reactivity predominantly localized to the outer shells of these structures. Such structures have been shown to contain Aβ,30,31 and colocalization showed that WO antibodies and OC staining overlapped that of Aβ-specific antibody. These observations indicate that drusen-associated vesicles may in fact contain fibrillar amyloid composed, at least in part, by Aβ. In addition, fibrillar structures consistent with amyloid fibrils were detected in sub-RPE deposits by staining with WO antibodies and electron microscopy (EM). These structures were immunopositive for vitronectin but not for Aβ. These data reveal the presence of a wide spectrum of amyloid structures in drusen and support the idea that similar protein misfolding events, which are characteristic of amyloid diseases, are involved in the pathogenesis of AMD.
Methods
Preparation of Oligomers and Fibrils for Dot Blot Assay
Aβ oligomers and fibrils as well as Q36, α-synuclein, and prion fibrils were prepared from respective synthetic peptides or recombinant proteins, as previously described.21,32 The fibrils were confirmed by EM and thioflavin S binding. An equal amount (40 nanomoles) of proteins or peptides was spotted onto nitrocellulose membrane, dried, and incubated with the primary antibody. Signals were detected with enhanced chemiluminescence (GE Life Sciences, Piscataway, NJ).
Human Tissue
Human donor eyes were obtained from the Oregon Lions Sight and Hearing Foundation and were managed according to the guidelines of the Declaration of Helsinki for research involving human tissue. Eyes and brain samples were also obtained from the Alzheimer Disease Research Center (ADRC) of the University of Southern California. This project was approved by the institutional review board of the University of Southern California School of Medicine. Eyes of nine individuals that contained drusen were analyzed, six of which had clinically documented atrophic AMD. Their ages ranged from 75 to 91 years. Two control eyes, ages 74 and 90, were also examined. Eyes were stored at 4°C and processed 24 to 48 hours postmortem. The neural retina was removed from the posterior pole; the remaining eye cup was cut into 1 × 0.5-cm rectangles, immediately embedded in OCT (Tissue-Tek, Sakura Finetec, Torrance, CA) and frozen. Both macular and peripheral areas that contained drusen were used. The tissue was not fixed to avoid interference with antigen detection by nonfibrillar oligomer antibodies. Eight-micrometer-thick frozen sections were cut on a cryostat (CM3050 S; Leica Microsystems, Bannockburn, IL) at −18°C.
Confocal Immunofluorescence Microscopy
Sections were blocked overnight at 4°C or for 1 hour at room temperature in blocking solution (phosphate-buffered saline containing 2% BSA and 2% goat serum) and incubated for 2 hours with the primary antibody at 2 to 10 μg/mL in blocking solution (rabbit monoclonal M204, mouse monoclonal antibodies WO1 and WO2,29 mouse monoclonal antibody 6E10 [Covance Research Products, Inc., Dedham, MA], and rabbit polyclonal antibody OC28) followed by incubation for 1 hour with FITC-conjugated or Texas red–conjugated secondary antibodies (Vector Laboratories, Inc., Burlingame, CA) at a dilution of 1:100 in blocking solution. For double labeling using antibodies raised in the same species (rabbit) an intervening formaldehyde treatment was used between the first and second primary antibodies.33 DAPI was used to visualize nuclei. Images were acquired with an inverted laser scanning confocal microscope (Zeiss LSM-510; Carl Zeiss Meditec, Thornwood, NY) or a spinning-disc confocal microscope (UltraVIEW VoX; PerkinElmer, Waltham, MA). Specimens were scanned under the same conditions for laser intensity, magnification, brightness, gain, and pinhole size.
Immunogold Electron Microscopy
Ocular tissue was fixed in paraformaldehyde, rinsed in 0.1 M phosphate buffer and dehydrated through a graded ethanol series. The specimen was embedded in LR White resin (Electron Microscopy Sciences, Hatfield, PA), sectioned, and stained for Aβ with the 6E10 antibody followed by a secondary antibody conjugated to 25-nm gold particles (Ted Pella, Redding, CA), as previously described.30 Staining for vitronectin was performed similarly using a rabbit antibody against human vitronectin (Quidel Corp., San Diego, CA) and a secondary goat anti-rabbit antibody conjugated to 15 nm colloidal gold (Ted Pella).
Results
Nonfibrillar Amyloid Oligomer Core Structures in Drusen
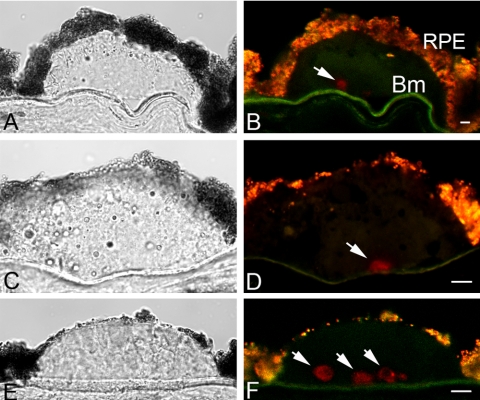
Previously, the presence of core structures in drusen that contain nonfibrillar amyloid was revealed by a rabbit polyclonal antibody, A11.26 This antibody was raised against a molecular mimic of nonfibrillar oligomer made from the Alzheimer Aβ peptide, but was found to detect toxic oligomers made from a variety of other amyloidogenic proteins.21 Thus, the A11 antibody is able to recognize a common structural conformation displayed by nonfibrillar oligomeric forms of diverse proteins and peptides. Because A11 is a polyclonal antibody preparation, it is likely that a heterogenous variety of antigenic epitopes is recognized. To validate the results obtained using A11, we took advantage of a rabbit monoclonal antibody that was recently generated by using the same immunogen as for A11 and binds specifically to amyloid oligomers, but not monomers or fibrils (Supplementary Fig. S1, http://www.iovs.org/cgi/content/full/51/3/1304/DC1). This antibody, mAb M204, bound to drusen-associated structures similar in appearance to those identified by the polyclonal A11 antibody (Fig. 1). These cores within drusen were 10 to 20 μm in size and were located in close proximity to the Bruch membrane. Many drusen contained a single core (Figs. 1B, 1D), whereas a few large drusen were observed to contain multiple cores (Figs. 1E, 1F). Sections from eight donor eyes with drusen were all positive for M204-immunoreactive drusen cores, whereas control eyes without drusen displayed no immunoreactivity (data not shown). Not all drusen sections displayed M204-immunoreactive cores, perhaps because they were out of the plane of section or simply were not present in some drusen. These results are consistent with those obtained with the polyclonal A11 antibody and confirm the presence of nonfibrillar amyloid oligomers in core elements of drusen.
Figure 1.
Immunolocalization of nonfibrillar amyloid oligomers in drusen by the rabbit M204 monoclonal antibody. Sections containing drusen from three individuals are shown. One-micrometer-thick optical images were acquired on an inverted laser scanning confocal microscope. (A, C, E) Differential interference contrast images; (B, D, F) confocal fluorescence images of amyloid oligomer cores (red, Cy3 channel). Arrows: M204-positive cores. Lipofuscin autofluorescence in RPE appeared as an orange-yellow fluorescence. RPE, retinal pigmented epithelium; Bm, Bruch membrane. Scale bar, 10 μm.
Fibrillar Amyloid Structures in Drusen
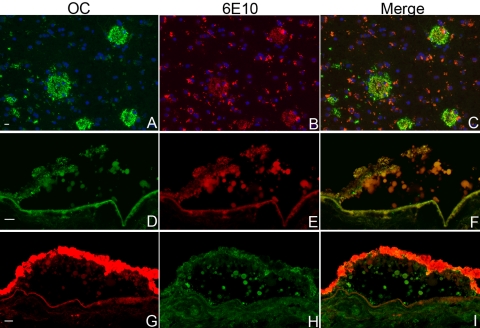
We first investigated the presence of amyloid fibrils in drusen using the OC antibody, which is a rabbit polyclonal antibody that was obtained by immunization with a morphologically homogeneous population of sonicated Aβ42 fibrils.28 The OC antibody binds fibrils made from a variety of amyloidogenic proteins and peptides, but not soluble monomers or nonfibrillar oligomers. It binds Aβ fibrils with molecular masses between 8 and 200 kDa based on Western blot and size-exclusion chromatography, suggesting interaction with a population of soluble small fibrils or fibril nuclei, as well as with larger insoluble mature fibrils.28 Thus, the amyloid species recognized by OC and M204/A11 are structurally distinct. As expected, OC showed extensive reactivity with AD plaques (Figs. 2A–C), due to their known concentration of Aβ fibrils. Whether the Aβ immunoreactivity associated with vesicular structures in drusen30,31 is due to the presence of monomeric or fibrillar amyloid structures has not been investigated. In this study, similar to AD brain, Aβ reactivity in drusen showed extensive, but incomplete, overlap with that of OC (Figs. 2D–I). Of note, we observed that the 6E10 and OC antibodies appeared to compete for the same epitope. In double-labeling experiments when 6E10 was applied first, the OC signal was obviously weaker and vice versa. This observation and the large extent of overlap in immunoreactivity pattern between these antibodies suggest that the amyloid fibrils recognized by OC may, in part, be formed by Aβ.
Figure 2.
Co-localization of amyloid fibril structures with Aβ in AD brain and in drusen. Amyloid fibrils were visualized by the OC rabbit polyclonal antibody, whereas Aβ was visualized by the 6E10 mouse monoclonal antibody. Extensive overlap was observed for both senile plaques from AD brain (A–C) and drusen (D–I). The AD brain section was reacted first with OC and then 6E10, whereas the drusen sections were reacted first with 6E10, then OC. The specific staining of the senile plaques was seen clearly in the green channel. These structures were also present in the red channel, which represents specific 6E10 reactivity. Lipofuscin autofluorescence was strong in the red channel, and appeared as clusters of small granules. In the retinal sections, lipofuscin autofluorescence was present within the RPE in both the green and red channels, which made the autofluorescence immunoreactivity within RPE difficult to discern. In the AD brain, section nuclei were stained by DAPI (blue). Images were acquired on an inverted laser scanning confocal microscope. Scale bars, 10 μm.
Recently, we discovered that vitronectin, an abundant protein component of drusen, readily forms amyloid oligomers and fibrils in vitro,34 raising the possibility that it also forms amyloid structures in vivo. Double staining with a vitronectin antibody and the A11 anti-amyloid oligomer antibody showed that they do not colocalize in drusen.26 To test the possibility that vitronectin is localized to the site of amyloid fibrils, we performed double staining with a vitronectin antibody and the OC antibody. Overall colocalization of vitronectin and OC was poor (data not shown), despite the fact that this vitronectin antibody is capable of recognizing amyloid fibrils made of vitronectin in vitro.34 These results suggest that vitronectin does not contribute in a significant way to amyloid fibril formation at the shell of vesicular structures in drusen.
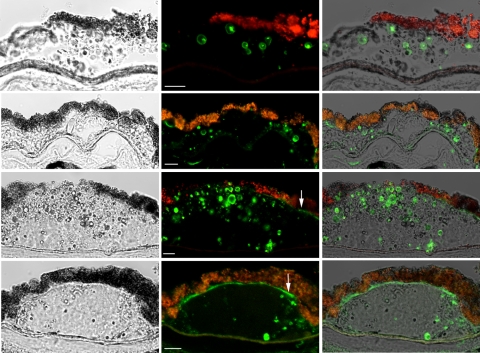
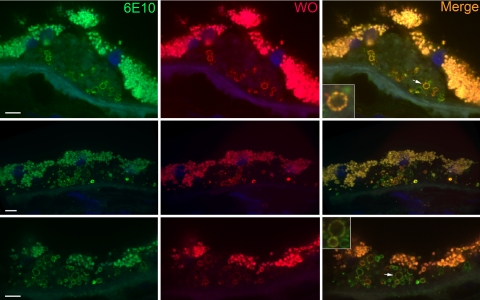
We next investigated whether drusen contains amyloid fibrils by using the monoclonal antibodies WO1 and WO2, which were made against Aβ fibrils29 (Fig. 3). In addition to Aβ (1-40) amyloid fibrils, these antibodies also show reactivity with fibrils formed by a variety of amyloidogenic proteins and peptides including IAPP (islet amyloid polypeptide), poly Gln (Q42), and transthyretin, but not other biological fibrils such as collagen, elastin, or nonnative globular protein aggregates.29,35 WO1 and WO2 antibodies yielded similar patterns of reactivity; hence, no distinction is made between the two in the data presented. In contrast to OC that recognizes fibrils of a wide range of molecular masses, the preferred epitope for the WO1 antibody is mature amyloid fibrils rather than protofibrils.36 Figure 3 shows that WO identified vesicular structures within drusen similar to those bound by the 6E10 and OC antibodies. Thin optical sections showed that these fibrils were deposited predominantly at the periphery of vesicular structures (Fig. 3), but not all vesicles stained with WO. The morphology of drusen is quite heterogeneous37,38; not all drusen displayed vesicular structures, and those that did not contain vesicular structures showed little or no reactivity with WO antibodies (data not shown). In certain instances, immunoreactivity was also seen in sub-RPE deposits, suggesting the presence of mature amyloid fibrils at these sites (Fig. 3, bottom, arrows). Like OC, the pattern of WO reactivity in drusen resembled that of Aβ.30,31 To determine whether the fibrils recognized by WO contain Aβ, we performed double staining with 6E10 and WO antibodies. Figure 4 shows thin optical sections obtained with a spinning-disc confocal microscope. Both antibodies stained the periphery of vesicular structures, as expected. The immunoreactivity for both antibodies resembled beads on a string that decorated the edge of the vesicles. In some drusen, extensive overlap was observed for certain vesicles even to the individual beads that lined the edge of these vesicles (Fig. 4, insets), whereas in others, WO appeared to stain a subset of Aβ-positive vesicles. The remarkable degree of overlap in immunoreactivity of some vesicles again supports the notion that the mature amyloid fibrils recognized by WO may be formed by Aβ.
Figure 3.
Mature amyloid fibrils recognized by the WO antibody were localized to the shell of vesicular structures. Shown are representative sections containing drusen from four individuals. Left: differential interference contrast images; middle: WO reactivity (green) reflecting the presence of mature amyloid fibrils; right: overlay of the differential interference contrast and fluorescent images. WO reactivity was seen largely at the surface of vesicular structures. Not all vesicles were stained. Sub-RPE deposit appeared to stain strongly for WO (arrows, bottom). Red-orange granules were due to RPE lipofuscin autofluorescence. Scale bar, 10 μm.
Figure 4.
Mature amyloid fibrils are present in a subset of Aβ-positive vesicles. In general, the pattern of WO reactivity appeared to represent a subset of Aβ containing vesicles. The images are thin optical sections obtained from a spinning-disc confocal microscope, which allowed for the resolution of punctate staining at the shell of the vesicles. In some vesicles costaining was visible at the level of these puncta, suggesting that Aβ forms amyloid fibrils at these sites. Inset, arrows: magnified images of these vesicles. Nuclei are stained by DAPI (blue). Scale bar, 10 μm.
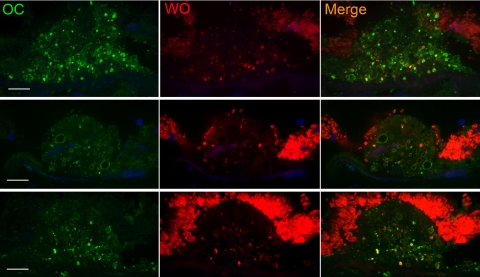
The staining patterns of Aβ and WO antibodies prompted us to compare the distribution pattern of the structures stained by OC and WO antibodies (Fig. 5). Again reactivity of both antibodies was restricted to the shell of vesicles in the drusen. Similar to those observed for Aβ and WO, vesicles that were immunopositive for WO represented a subset of OC-positive vesicles in many drusen, indicating that their structural composition in terms of amyloid fibrils is not identical. The OC antibody recognized a broader range of vesicles than WO, a result consistent with their antigenic specificities. WO preferentially binds mature amyloid fibrils,36 whereas OC reacts with a spectrum of small protofibrils to large fibrils.28 These results show that amyloid fibrils, ranging from low-molecular-mass fibrils to mature fibrils, are present in drusen and may be formed by Aβ.
Figure 5.
Vesicles that stained positively for mature amyloid fibrils (WO, red) represented a subset of OC-positive vesicles (green). Nuclei were stained with DAPI (blue). Images were thin optical sections obtained by spinning-disc confocal microscope. Scale bar, 10 μm.
Amyloid Fibrils in Drusen Visualized by Electron Microscopy
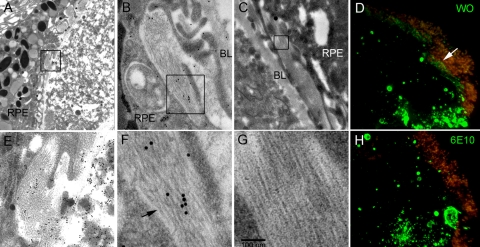
Electron microscopy was performed as an independent approach to investigate the presence of amyloid fibrils in drusen. Figure 6 shows three different drusen (Figs. 6A–C, with higher magnification of the boxed area shown in Figs. 6E–G, respectively) and associated sub-RPE deposits. The section shown in Figures 6A and 6E was reacted with an antibody against vitronectin and visualized with a secondary antibody conjugated to 15-nm gold particles. Vitronectin reactivity appeared throughout the drusen and in adjacent sub-RPE deposits inside the RPE basal lamina. Of note, long strands of ∼10-nm diameter fibrils are abundant in these sub-RPE deposits (Fig. 6E). Such fibril dimension and morphology is typical of amyloid fibrils.17 Figures 6B and 6F show a section of a druse with sub-RPE fibrils reacted with the 6E10 anti-Aβ antibody followed by a secondary antibody conjugated to 25-nm gold particles. The 6E10 staining pattern appeared to be nonspecific inasmuch as the gold particles were seen distributed randomly throughout. Nevertheless, the diameter of the fibrils can be measured against the 25-nm gold particles. The fibril diameter also appeared to be ∼10 nm, consistent with that of amyloid fibrils. Some strands appeared to be twisted (Fig. 6F, arrow), a morphologic characteristic often seen in amyloid fibrils. Figures 6C and 6G are micrographs from a different druse with a similar pattern of amyloid fibrils in the sub-RPE deposits. To determine whether the sub-RPE fibrils may be formed by Aβ, we stained adjacent sections from a drusen specimen that contained sub-RPE deposits with WO (Fig. 6D) and 6E10 (Fig. 6H) antibodies. Both showed strong staining of vesicles that are more concentrated at the bottom right corner of the figures. Similar to that shown in Figure 3, WO stained sub-RPE deposits in addition to vesicles (Fig. 6D). Although 6E10 has the ability to bind to Aβ fibrils in senile plaques of AD brain (Fig. 2), no reactivity was seen in the sub-RPE deposits (Fig. 6H). These data suggest that amyloid fibrils in these sub-RPE deposits are not derived from Aβ and are likely to be formed by different proteins or peptides. Amyloid fibrils at the edge of vesicular structures were not identified by EM. A previous EM study of Aβ-stained drusen vesicles did not reveal obvious structures consistent with amyloid fibrils,30 perhaps because the few vesicles examined by EM contained OC-reactive small fibril seeds rather than WO-reactive mature fibrils. Further studies of more of these vesicular structures are needed to address whether some vesicles are lined with EM-visible amyloid fibrils. Nevertheless, the current data obtained from immunofluorescence and EM clearly demonstrate the presence of bona fide amyloid fibrils in substructural elements of drusen and in sub-RPE deposits.
Figure 6.
Abundant amyloid fibrils of ∼10 nm diameter were present in sub-RPE deposits. (A) Electron micrograph of drusen section with sub-RPE deposit stained for vitronectin and visualized with a secondary antibody conjugated to 15-nm gold particles. (B) A section from another drusen with sub-RPE deposit incubated with 6E10 followed by a secondary anti-mouse antibody conjugated to 25-nm gold particles. (C) Drusen section with sub-RPE deposit containing fine fibrils. (D) Frozen section of drusen with sub-RPE deposits visualized with WO antibodies. In this large druse the vesicular structures were concentrated to the bottom right of the image. Sub-RPE deposit also stained positively (white arrow). (E–G) Magnified images of boxed areas shown in (A–C), respectively. (F) Fibrils with twisted morphology (black arrow) are indicated. The diameter of the fibrils and the fibril morphology were consistent with that of amyloid fibrils. (H) Adjacent section of the same druse shown in (D) reacted with 6E10 antibody. Vesicular structures were again concentrated to the bottom right of the image as in (D). However, no staining was observed beneath the RPE. BL: basal lamina.
Discussion
The similarities between drusen and deposits of amyloid diseases with regard to protein and lipid content have been noted previously.2,31 These include proteins in the complement cascade, pointing to involvement of local inflammation in AMD disease pathogenesis. Although amyloidogenic proteins such as Aβ and transthyretin have been found in drusen,30,31 abundant amyloid fibrils, such as those characteristic of AD plaques, have not been noted in EM studies of drusen. For this reason, drusen have not been considered amyloid deposits. We used conformation-dependent, sequence-independent antibodies to show that a variety of amyloid structures are indeed present in drusen. These antibodies, WO and OC, do not recognize natively folded monomers; they specifically recognize fibrillar epitopes of amyloid fibrils that are distinct from those present in nonfibrillar oligomers. Moreover, they do not react with other fibrillar biomolecules such as collagen or elastin.28,29 Of note, their reactivity was seen predominantly at the shell of vesicular structures previously shown to contain Aβ.30,31 Indeed, we demonstrate a high degree of colocalization between Aβ and OC immunofluorescence in these vesicles (Fig. 2). Given the tendency of Aβ to form fibrillar structures, our evidence strongly suggests that the OC-positive fibrils are formed, at least in part, by Aβ. The reason that Aβ and fibrillar structures preferentially form at the edges of vesicles is not clear. Perhaps molecular components that are present at these sites facilitate seeding for the growth of amyloid fibrils. Vesicles labeled by the WO antibodies represent a subset of OC-positive vesicles (Fig. 5); this pattern was mirrored by colocalization of Aβ and WO (Fig. 4). These results can be explained by in vitro evidence demonstrating that OC recognizes fibrillar structures of a broad range of molecular weight,28 whereas WO preferentially binds large insoluble amyloid fibrils.36 Thus, vesicles that harbor WO-positive fibrils appear to comprise a subpopulation of amyloid-containing vesicles, which may explain why they have not been observed by EM. Also, the prevalence of soluble protofibrils over large fibrils in these vesicles may explain a tendency of drusen to stain positively for thioflavin T and Congo red, but not to emit green birefringence.2,30 Notably, nonfibrillar oligomers in diffuse AD plaques also stain poorly with fibril specific dyes such as Congo red and thioflavin S.21
The staining of WO antibodies in sub-RPE deposits (Fig. 3) indicated the presence of amyloid fibrils in this compartment, which was confirmed by EM, where abundant amyloid fibrils of ∼10 nm diameter were observed in drusen-associated sub-RPE deposits; amyloid fibrils appeared to be the main constituent of these deposits. Whether abundant amyloid fibrils are common constituents of sub-RPE deposits awaits future investigation.
Vitronectin is an abundant protein present in drusen1,2,4,39 that readily forms amyloid fibrils in vitro.34 To see whether vitronectin forms amyloid fibrils in vivo, double staining using an antibody against human vitronectin and OC antibody was performed, whereupon little co-localization was observed (data not shown). On the other hand, the immunogold result shown in Figure 6B suggests that the amyloid fibrils in sub-RPE deposits may be composed of vitronectin. However, the density of gold particles was lower when compared to that in adjacent drusen material. Future biochemical analyses are needed to unambiguously identify the protein/peptide that makes up the amyloid fibrils in sub-RPE deposits.
In sum, we demonstrated in this study that a wide range of amyloid structures are present in vesicular elements of drusen. These include nonfibrillar oligomers as well as protofibrils and mature amyloid fibrils. Of these structures the nonfibrillar oligomers appeared to be most abundant. They were observed in all drusen-containing tissue, although, as noted previously, not all drusen demonstrated reactivity. This finding was followed by OC- and WO-positive drusen. Reactivity to these antibodies, as well as that for Aβ, was observed only in drusen that contain vesicular structures. Of the nine drusen-containing eyes, four were positive. This frequency is the same as has been reported for Aβ.40 The observation that amyloid structures are present in drusen is significant. In other neurodegenerative diseases these structures have been demonstrated to participate in disease pathogenesis. Together, our data add to the list of similarities shared between drusen and amyloid deposits of other age-related neurodegenerative diseases.
Supplementary Material
Acknowledgments
The authors thank Ernesto Barron for his contribution to the EM experiments; the Oregon Lions Sight and Hearing Foundation for providing eyes for the study; and the ADRC Neuropathology Core, University of Southern California, for providing the eye and brain specimens.
Footnotes
Supported by the National Eye Institute in the form of a Vision Core Grant to Doheny Eye Institute (EY03040) and National Institutes of Health Grants R01 NS46356 (RW), NIH R01 EY11527 (LVJ), and NIH R24 EY017404 (LVJ); a grant from the Arnold and Mabel Beckman Foundation (RL, JC); and a Network Grant from the Larry L. Hillblom Foundation (CGG, RL, JC).
Disclosure: J.M. Isas, None; V. Luibl, None; L.V. Johnson, Pfizer, Inc. (C); R. Wetzel, None; C.G. Glabe, Kinexis, Inc. (C); R. Langen, None; J. Chen, None
References
- 1.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A 2002;99(23):14682–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins RF, Russell SR, Anderson DH, et al. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J 2000;14(7):835–846 [PubMed] [Google Scholar]
- 3.Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 2001;20(6):705–732 [DOI] [PubMed] [Google Scholar]
- 4.Johnson LV, Leitner WP, Staples MK, et al. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res 2001;73(6):887–896 [DOI] [PubMed] [Google Scholar]
- 5.Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res 2000;70(4):441–449 [DOI] [PubMed] [Google Scholar]
- 6.Edwards AO, Ritter R, 3rd, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308(5720):421–424 [DOI] [PubMed] [Google Scholar]
- 7.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 2005;102(20):7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005;308(5720):419–421 [DOI] [PubMed] [Google Scholar]
- 9.Malek G, Johnson LV, Mace BE, et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc Natl Acad Sci U S A 2005;102(33):11900–11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalaria RN. Arteriosclerosis, apolipoprotein E, and Alzheimer's disease. Lancet 1997;349(9059):1174 [DOI] [PubMed] [Google Scholar]
- 11.Ding JD, Lin J, Mace BE, et al. Targeting age-related macular degeneration with Alzheimer's disease based immunotherapies: anti-amyloid-beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vision Res 2008;48(3):339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning A, Cui J, To E, et al. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci 2008;49(11):5136–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez SE, Lumayag S, Kovacs B, et al. Beta-amyloid deposition and functional impairment in the retina of the APPswe/PS1DeltaE9 transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis Sci 2009;50(2):793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazawa M, Inokuchi Y, Okuno T, et al. Reduced retinal function in amyloid precursor protein-over-expressing transgenic mice via attenuating glutamate-N-methyl-d-aspartate receptor signaling. J Neurochem 2107(1):279–290 [DOI] [PubMed] [Google Scholar]
- 15.Glabe CG. Conformation-dependent antibodies target diseases of protein misfolding. Trends Biochem Sci 2004;(10):542–547 [DOI] [PubMed] [Google Scholar]
- 16.Glabe CG. Structural classification of toxic amyloid oligomers. J Biol Chem 2008;283(44):29639–29643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunde M, Blake C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv Protein Chem 1997;50:123–159 [DOI] [PubMed] [Google Scholar]
- 18.Margittai M, Langen R. Fibrils with parallel in-register structure constitute a major class of amyloid fibrils: molecular insights from electron paramagnetic resonance spectroscopy. Q Rev Biophys 2008;41(3–4):265–297 [DOI] [PubMed] [Google Scholar]
- 19.Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol 2006;1:151–170 [DOI] [PubMed] [Google Scholar]
- 20.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci 1999;24(9):329–332 [DOI] [PubMed] [Google Scholar]
- 21.Kayed R, Head E, Thompson JL, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003;300(5618):486–489 [DOI] [PubMed] [Google Scholar]
- 22.Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life 2007;59(4–5):332–345 [DOI] [PubMed] [Google Scholar]
- 23.Kokubo H, Kayed R, Glabe CG, et al. Oligomeric proteins ultrastructurally localize to cell processes, especially to axon terminals with higher density, but not to lipid rafts in Tg2576 mouse brain. Brain Res 2005;31(1–2):224–228 [DOI] [PubMed] [Google Scholar]
- 24.Kokubo H, Kayed R, Glabe CG, et al. Soluble Abeta oligomers ultrastructurally localize to cell processes and might be related to synaptic dysfunction in Alzheimer's disease brain. Brain Res 2005;1031(2):222–228 [DOI] [PubMed] [Google Scholar]
- 25.Lesne S, Koh MT, Kotilinek L, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006;440(7082):352–357 [DOI] [PubMed] [Google Scholar]
- 26.Luibl V, Isas JM, Kayed R, et al. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J Clin Invest 2006;116(2):378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanbe A, Osinska H, Saffitz JE, et al. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A 2004;101(27):10132–10136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayed R, Head E, Sarsoza F, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener 2007;2:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci U S A 2002;99(3):1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson DH, Talaga KC, Rivest AJ, et al. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res 2004;78(2):243–256 [DOI] [PubMed] [Google Scholar]
- 31.Johnson LV, Leitner WP, Rivest AJ, et al. The Alzheimer's A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A 2002;99(18):11830–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayed R, Glabe CG. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol 2006;413:326–344 [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Larson L. Simultaneous demonstration of multiple antigens by indirect immunofluorescence or immunogold staining. Histochemistry 1985;83:47–56 [DOI] [PubMed] [Google Scholar]
- 34.Shin T, Isas JM, Hsieh CL, et al. Formation of soluble amyloid oligomers and amyloid fibrils by the multifunctional protein vitronectin. Mol Neurodegener 2008;3(1):16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen P, Nielsen JL, Dueholm MS, et al. Amyloid adhesins are abundant in natural biofilms. Environ Microbiol 2007;9(12):3077–3090 [DOI] [PubMed] [Google Scholar]
- 36.Williams AD, Sega M, Chen M, et al. Structural properties of Abeta protofibrils stabilized by a small molecule. Proc Natl Acad Sci U S A 2005;102(20):7115–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis 1999;5:28 [PubMed] [Google Scholar]
- 38.Rudolf M, Clark ME, Chimento MF, et al. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci 2008;49(3):1200–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hageman GS, Mullins RF, Russell SR, et al. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J 1999;13(3):477–484 [DOI] [PubMed] [Google Scholar]
- 40.Dentchev T, Milam AH, Lee VM, et al. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis 2003;9:184–190 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.