Abstract
GH promotes longitudinal growth and regulates multiple cellular functions in humans and animals. GH signals by binding to GH receptor (GHR) to activate the tyrosine kinase, Janus kinase 2 (JAK2), and downstream pathways including signal transducer and activator of transcription 5 (STAT5), thereby regulating expression of genes including IGF-I. GH exerts effects both directly and via IGF-I, which signals by activating the IGF-I receptor (IGF-IR). IGF-IR is a cell surface receptor that contains intrinsic tyrosine kinase activity within its intracellular domain. In this study, we examined the potential role of IGF-IR in facilitating GH-induced signal transduction, using mouse primary calvarial osteoblasts with Lox-P sites flanking both IGF-IR alleles. These cells respond to both GH and IGF-I and in vitro infection with an adenovirus that drives expression of Cre recombinase (Ad-Cre) dramatically reduces IGF-IR abundance without affecting the abundance of GHR, JAK2, STAT5, or ERK. Notably, infection with Ad-Cre, but not a control adenovirus, markedly inhibited acute GH-induced STAT5 activity (more than doubling the ED50 and reducing the maximum activity by nearly 50%), while sparing GH-induced ERK activity, and markedly inhibited GH-induced transactivation of a STAT5-dependent luciferase reporter. The effect of Ad-Cre on GH signaling was specific, as platelet-derived growth factor-induced signaling was unaffected by Ad-Cre-mediated reduction of IGF-IR. Ad-Cre-mediated inhibition of GH signaling was reversed by adenoviral reexpression of IGF-IR, but not by infection with an adenovirus that drives expression of a hemagglutination-tagged somatostatin receptor, which drives expression of the unrelated somatostatin receptor, and Ad-Cre infection of nonfloxed osteoblasts did not affect GH signaling. Notably, infection with an adenovirus encoding a C-terminally truncated IGF-IR that lacks the tyrosine kinase domain partially rescued both acute GH-induced STAT5 activity and GH-induced IGF-I gene expression in cells in which endogenous IGF-IR was reduced. These data, in concert with our earlier findings that GH induces a GHR-JAK2-IGF-IR complex, suggest a novel function for IGF-IR. In addition to its role as a key IGF-I signal transducer, this receptor may directly facilitate acute GH signaling. The implications of these findings are discussed.
In vitro deletion of IGF-1R in mouse primary osteoblasts using the Cre-lox system results in desensitization to acute GH-induced STAT5 signaling and IGF-1 gene expression.
GH is a 22-kDa protein produced largely in the anterior pituitary. By interacting with GH receptor (GHR), GH signals powerful growth-promoting and metabolic effects, including longitudinal bone growth, enhanced bone mass, lipolysis, muscle growth, amino acid transport, and modulation of insulin sensitivity (1,2). GHR is a widely expressed approximately 620-residue transmembrane glycoprotein found in many species which binds GH in its extracellular domain (ECD) and signals by regulated interaction of its approximately 350-residue intracellular domain (ICD) with signaling molecules (3,4). Conserved ECD structural features make GHR a member of the cytokine receptor superfamily that includes prolactin and erythropoietin receptors (5). Crystallographic, kinetic, functional, and biochemical evidence indicates that active GHR is a homodimer that reflects GH-induced conformational changes (6,7,8). Although GHR has no enzymatic activity, GH causes its enhanced association with and activation of Janus kinase 2 (JAK2), the cytoplasmic tyrosine kinase required for nearly all GH functions (9,10). JAK2, like other Janus kinases, has a C-terminal tyrosine kinase domain and a nonfunctional kinase-like domain (11). GHR-JAK2 association relies on the proline-rich Box1 GHR ICD element and the N-terminal region of JAK2, particularly its approximately 450 residue FERM domain (12,13,14,15,16,17).
GH engagement of GHR triggers several signaling pathways including signal transducer and activator of transcription (STAT)5b and ERKs (3,4). GH-induced STAT5b activation requires GHR tyrosine phosphorylation and causes gene expression (e.g., IGF-I, acid-labile subunit of the IGF binding protein complex, suppressor of cytokine signaling proteins, P450 enzymes, and serine protease inhibitor 2.1; Refs. 18,19,20,21,22,23,24,25,26,27,28). Animal models and human mutations indicate that disrupted STAT5 signaling is associated with growth deficiency and other impaired GH actions and STAT5 is required for overgrowth seen in suppressor of cytokine signaling-2-deficient mice (26,29,30,31,32). Skeletal muscle-specific STAT5 knockout reduces postnatal growth, skeletal size, and skeletal muscle IGF-I content, suggesting local (vs. systemic) IGF-I generated in a STAT5 (presumably GH)-dependent fashion regulates growth (33,34). Unlike STAT5b, GH-induced ERK and phosphoinositide-3 kinase activation do not require the entire GHR ICD, but only JAK2 coupling (13,14,15,35,36,37). Some have observed GH-induced ERK activity even in the absence of JAK2, but this is not universally seen (38,39).
IGF-I is a peptide synthesized in liver and other tissues that signals via type I IGF-I receptor (IGF-IR), a widely expressed cell surface heterotetramer with intrinsic kinase activity in its β-subunit cytoplasmic domains (40). Activated IGF-IR enlists intracellular signaling pathways to cause proliferation, antiapoptosis, and other actions (41,42). IGF-IR is encoded by a single mRNA including both the α- and β-chains. Cleavage of this α-β-precursor and disulfide linkage between the two chains occurs posttranslationally such that the mature α-chain is completely extracellular and the β-chain spans the membrane. Disulfide linkages between α-chains allow formation of the mature α2β2 heterotetrameric assemblage that is the mature receptor.
Whereas IGF-I can be an effector of GH action, intriguing data suggest that GH and IGF-I may act collaboratively. In particular, synergy in signal transduction by the two ligands has been observed in several cell systems (43,44,45) and GH-induced formation of a complex that includes GHR, JAK2, and IGF-IR has been observed (45,46), suggesting that IGF-IR may be a proximal component in GH signaling. We recently initiated studies of GH action in primary osteoblasts, cells in which IGF-I signaling exerts important effects on bone anabolism (47). In the course of those studies, we observed that genetic deletion of IGF-IR impacted GH-dependent STAT5 activation. The current studies test the effects of Cre recombinase expression in IGF-IR-floxed primary osteoblasts on GH signaling and GH-mediated gene expression. We find that IGF-IR deletion markedly desensitizes osteoblasts in terms of GH-induced STAT5 activation and IGF-I gene expression. This effect is specific in that another growth factor [platelet-derived growth factor (PDGF)] signaling system is unaffected by IGF-IR deletion. Furthermore, GH-dependent STAT5 signaling is rescued by reexpression of IGF-IR and, interestingly, reconstitution with a truncated IGF-IR devoid of the majority of its ICD partially restores GH-dependent STAT5 activity and IGF-I gene expression. These data suggest a novel role for IGF-IR in acute GH signaling that does not depend on IGF-I or IGF-IR tyrosine kinase activity.
Results
Deletion of IGF-IR in osteoblasts selectively reduces GH-induced STAT5 tyrosine phosphorylation
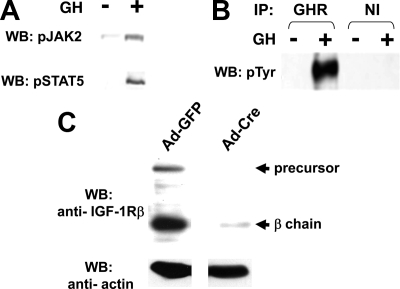
To study the impact of IGF-IR on acute GH signaling, we harvested calvarial osteoblasts from newborn mice bearing lox-P sites flanking both IGF-IR alleles (47,48). We first verified the GH responsiveness of these primary osteoblasts. Cells were serum-starved and treated with vehicle or GH (500 ng/ml) for 15 min, after which detergent extracts were resolved by SDS-PAGE and immunoblotted with anti-pTyr JAK2 and anti-pTyr STAT5 (Fig. 1A). This revealed GH-dependent JAK2 and STAT5 phosphorylation. Consistent with this, immunoprecipitation of these extracts with anti-GHR vs. nonimmune serum followed by antiphosphotyrosine blotting demonstrated substantial GH-dependent tyrosine phosphorylation of GHR and/or coprecipitated comigrating JAK2 (Fig. 1B). These responses verified that primary osteoblasts constitute an excellent in vitro model system for assessing GH signaling. A unique feature of IGF-IR-floxed osteoblasts is the potential for in vitro Cre-mediated deletion of IGF-IR to test its role in GH signaling (47). For this, we infected cells with Ad-Cre, a nonreplicative adenovirus that drives Cre expression. Notably, cells infected with Ad-Cre, but not the control virus adenovirus encoding green fluorescent protein (Ad-GFP), exhibited dramatic specific reduction of both the IGF-IR precursor and the β-chain (Fig. 1C). As previously reported (47), Ad-Cre infection did not affect GHR abundance (data not shown).
Figure 1.
Characterization of IGF-IR-floxed primary osteoblasts. A, GH-stimulated JAK2 and STAT5 phosphorylation. Primary osteoblasts were harvested from newborn mice bearing lox-P sites flanking both IGF-IR alleles. Serum-starved cells were treated with vehicle or GH (500 ng/ml) for 15 min. Detergent extracts were resolved by SDS-PAGE and immunoblotted with anti-pTyr JAK2 and anti-pTyr STAT5, respectively. B, Osteoblasts harvested and serum starved as in A were treated with vehicle or GH (500 ng/ml) for 15 min. Detergent extracts were immunoprecipitated with either anti-GHR or nonimmune (NI) serum. Precipitated proteins were resolved by SDS-PAGE and blotted with antiphosphotyrosine. The blots shown are representative of two independent experiments. C, IGF-IR-floxed osteoblasts were infected with either 800 MOI Ad-GFP or 800 MOI Ad-Cre for 48 h. After overnight serum starvation, detergent extracts were resolved by SDS-PAGE and immunoblotted with anti-IGF-IRβ and anti-β-actin, respectively.
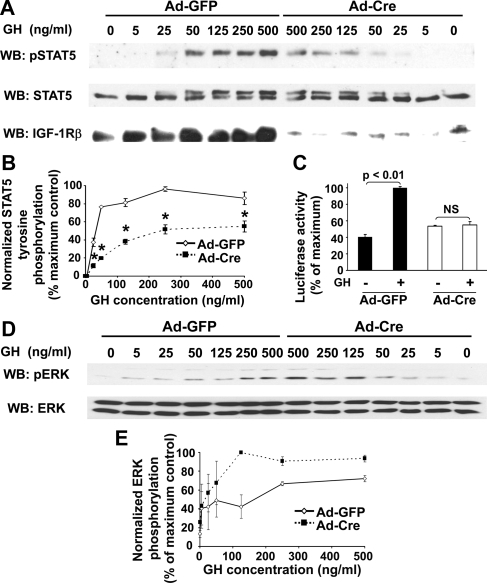
We next evaluated GH-dependent signaling in the setting of IGF-IR deletion (Fig. 2). Osteoblasts infected with Ad-GFP vs. Ad-Cre were treated with varying GH concentrations (0–500 ng/ml) for 10 min. In Ad-GFP- infected cells, STAT5 tyrosine phosphorylation was detected with as little as 25 ng/ml GH and nearly peaked with 50 ng/ml (Fig. 2A). In cells infected with Ad-Cre to delete IGF-IR, GH-induced STAT5 phosphorylation was quite reduced at each concentration compared with control cells, confirming our previous results with 500 ng/ml (47). This reduced phospho-STAT5 signal was not explained by differences in the STAT protein present in the samples from Ad-GFP- vs. Ad-Cre-infected cells, as detected by anti-STAT5 blotting of the same samples. Notably, the anti-STAT5 blotting revealed the expected GH-induced retardation of migration of phosphorylated STAT5, and comparison of Ad-GFP- vs. Ad-Cre-infected cells confirmed the marked reduction in the ability of GH to cause this shift in the Ad-Cre-infected cells. Blotting also revealed the effectiveness of IGF-IR reduction in the Cre-expressing cells. Densitometric evaluation (Fig. 2B) of these GH concentration-dependence experiments indicated that the ED50 for GH-induced STAT5 phosphorylation (normalized for STAT5 abundance) in Ad-GFP-infected cells was approximately 35 vs. approximately 85 ng/ml in those cells infected with Ad-Cre. Furthermore, the maximum signal achieved over the concentration range tested was approximately 44% reduced in the Ad-Cre-infected cells. Time course experiments showed similar timing of STAT5 activation, but with markedly reduced intensity in Ad-Cre cells (data not shown). Thus, IGF-IR deletion led to marked desensitization to GH-induced STAT5 phosphorylation and also greatly blunted the maximal response. Extracts from GH concentration dependence experiments were also assessed by anti-phospho-ERK blotting (Fig. 2, D and E). In contrast to STAT5, GH-induced ERK activity was not impaired (and, in fact, was increased at some GH concentrations) by IGF-IR deletion. This result suggests that the inhibitory effect on STAT5 activation was relatively specific in terms of GH-induced signaling pathways.
Figure 2.
Effect of IGF-IR deletion on GH signaling in IGF-IR-floxed osteoblasts. A and B, GH-induced STAT5 activity. Primary osteoblasts were infected with Ad-Cre vs. Ad-GFP, as indicated, as in Fig. 1C. Serum-starved cells were treated with the indicated concentrations of GH for 10 min, after which detergent extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-IRβ. A, Representative immunoblots. B, Densitometric quantitation of pSTAT5/STAT5 signals from two (5, 25, 50, and 125 ng/ml) or six (250 and 500 ng/ml) independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± se. Asterisk indicates P < 0.02 for comparison of Ad-GFP- vs. Ad-Cre-infected cells at each GH concentration. C, GH-induced STAT5-dependent reporter gene activity. Floxed IGF-IR cells treated with Ad-GFP or Ad-Cre were infected with Ad-GHRE-luc and then stimulated with GH (50 ng/ml; 6 h). Luciferase activity was measured in cell extracts. Mean ± se for triplicates is shown, normalized to maximum response. D and E, GH-induced ERK activity. D, Blots in A were reprobed with anti-pERK and anti-ERK. E, Densitometric quantitation of pERK/ERK signal from the same experiments as in B above.
We also examined consequences of STAT5 activity in terms of STAT5-dependent reporter gene expression (Fig. 2C). Primary floxed IGF-IR osteoblasts were infected with either Ad-GFP or Ad-Cre, as well as Ad-GH response element (GHRE)-luc (49,50), in which the firefly luciferase gene is driven by a basal promoter with eight repeats of the STAT5-dependent GHRE from the serine protease inhibitor 2.1 gene cloned into the 5′-untranslated region. Based on our dose-response data, we stimulated with 50 ng/ml GH for 6 h. As expected, GH promoted approximately 2.4-fold increase in luciferase activity in Ad-GFP-infected cells. However, GH did not change luminescence in Ad-Cre-infected cells. These data indicate that IGF-IR deletion by Ad-Cre treatment renders osteoblasts much less sensitive to GH for STAT5 activation and STAT5-dependent reporter gene expression.
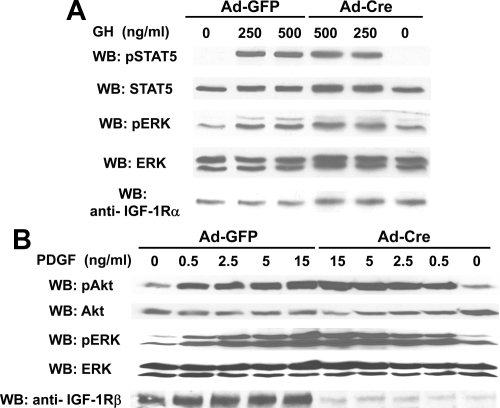
We further tested the specificity of our findings in two ways. First, primary calvarial osteoblasts were isolated from wild-type (nonfloxed) mice and infected with Ad-GFP vs. Ad-Cre (Fig. 3A). As expected, Ad-Cre infection did not alter IGF-IR levels. Nor did Ad-Cre infection alter GH- induced STAT5 or ERK activation. Thus, the effects of Ad-Cre on IGF-IR-floxed osteoblasts are not due to Ad-Cre infection per se, suggesting they are instead related to IGF-IR deletion. Second, we asked whether IGF-IR deletion globally affected signaling. IGF-IR-floxed osteoblasts were infected with Ad-GFP or Ad-Cre and exposed to varying concentrations of PDGF for 10 min (Fig. 3B). Notably, Ad-Cre-mediated IGF-IR deletion did not affect PDGF-induced Akt or ERK phosphorylation. Thus, the effect of IGF-IR deletion on GH signaling is not attributable to nonspecific global signaling effects engendered by either Ad-Cre infection or IGF-IR deletion.
Figure 3.
Impact of Ad-Cre infection on GH signaling does not reflect global effects on cell integrity or other pathways. A, Adenoviral infection of nonfloxed primary osteoblasts. Primary osteoblasts from mice without floxed IGF-IR genes were serum starved and treated with GH at the indicated concentrations for 10 min, after which detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted with the indicated antibodies. Note the lack of effect of Ad-Cre vs. Ad-GFP on GH-induced signaling. The blots shown are representative of two independent experiments. B, Ad-Cre-mediated IGF-IR deletion did not affect PDGF signaling. IGF-IR-floxed primary osteoblasts were infected with Ad-GFP vs. Ad-Cre, as in Figs. 1 and 2. Serum-starved cells were treated with PDGF at the indicated concentrations for 10 min. Detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pAkt, anti-pERK, and anti-IGF-IRβ. The blots shown are representative of two independent experiments.
Reconstitution with IGF-IR rescues GH-induced STAT5 activation in Ad-Cre-infected IGF-IR-floxed osteoblasts
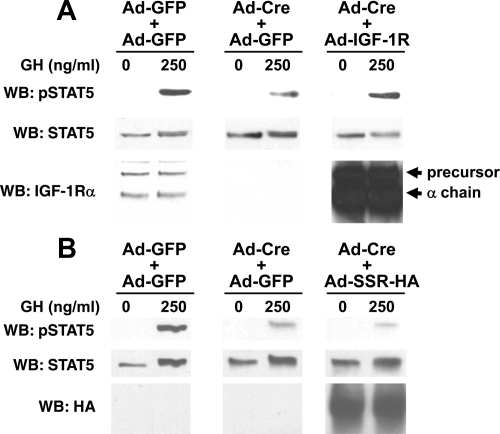
If Cre-mediated endogenous IGF-IR deletion blunts GH signaling, we reasoned this signaling defect should be rescued by exogenous IGF-IR reexpression. To test this, we infected primary IGF-IR-floxed osteoblasts with Ad-GFP, Ad-Cre, or Ad-Cre and Ad-IGF-IR (Ad-Cre plus Ad-IGF-IR) (Fig. 4A). Ad-IGF-IR drives expression of the wild-type human IGF-IR. To control for the dual infection of Ad-Cre and Ad-IGF-IR, the Ad-GFP and Ad-Cre infections were actually carried out as two doses (Ad-GFP plus Ad-GFP and Ad-Cre plus Ad-GFP, respectively). As expected, endogenous IGF-IR expression was greatly reduced in Ad-Cre-infected cells and these cells exhibited markedly reduced GH-induced STAT5 activation compared with Ad-GFP-infected cells. Coinfection with Ad-IGF-IR yielded high IGF-IR expression (precursor and mature forms), as detected by immunoblotting. Notably, GH-induced STAT5 activation was restored in IGF-IR-expressing Ad-Cre-infected cells.
Figure 4.
Reconstitution with IGF-IR rescues GH-induced STAT5 activation in Ad-Cre-infected IGF-IR-floxed osteoblasts. A, Floxed-IGF-IR primary osteoblasts were infected with either Ad-GFP only (1600 MOI), Ad-GFP plus Ad-Cre (800 MOI each simultaneously), or Ad-IGF-IR plus Ad-Cre (800 MOI each simultaneously), as in Figs. 1–3. Serum-starved cells were treated with vehicle or GH (250 ng/ml) for 10 min, after which detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-IRα. Positions of IGF-IR precursor and IGF-IRα chain are indicated. The blots shown are representative of two separate experiments. Note the rescue of GH-induced STAT5 activity by IGF-IR expression. B, The experiment in A was repeated, except that Ad-SSR-HA was used instead of Ad-IGF-IR for infection. Immunoblotting was with anti-pSTAT5, anti-STAT5, and anti-HA. The blots shown are representative of three separate experiments. Note the lack of rescue of GH-induced STAT5 activity by SSR-HA expression.
As a control, we performed the same experiment, but instead of Ad-IGF-IR, we coinfected with Ad-SSR-HA, an adenovirus that drives expression of a hemagglutination-tagged somatostatin receptor (Fig. 4B). Unlike Ad-IGF-IR, coinfection of Ad-SSR-HA with Ad-Cre did not rescue GH-induced STAT5 signaling. Thus, adenoviral expression of an irrelevant surface receptor did not suffice. These findings indicate that Ad-IGF-IR expression specifically rescues the decreased GH-induced STAT5 activation in IGF-IR-floxed osteoblasts infected with Ad-Cre.
IGF-IR tyrosine kinase inhibitor does not prevent GH-induced STAT5 activation
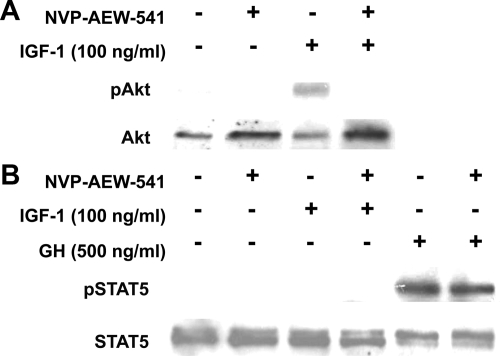
The rescue of GH-induced STAT5 activation by IGF-IR reexpression could, in principle, be explained by the ability of this receptor to act as a tyrosine kinase, although we note that exogenous IGF-I was not needed to allow GH signaling. We tested whether IGF-IR kinase activity was necessary for GH-induced STAT5 activation by employing a specific IGF-I kinase inhibitor, NVP-AEW-541 (51). As expected, pretreatment of IGF-IR-floxed osteoblasts with NVP-AEW-541 prevented IGF-I-induced IGF-IR signaling, assessed by anti-phospho-Akt blotting (Fig. 5A), verifying that this drug inhibits IGF-IR kinase activity in primary osteoblasts. However, NVP-AEW-541 treatment did not affect GH-induced STAT5 activation in these osteoblasts (Fig. 5B), suggesting the tyrosine kinase activity of IGF-IR is not needed to impact GH signaling.
Figure 5.
IGF-IR tyrosine kinase inhibitor does not prevent GH-induced STAT5 activation. A and B, IGF-IR-floxed primary osteoblasts were serum starved and pretreated with vehicle or NVP-AEW-541 (5 mm, as indicated) for 16 h before stimulation with IGF-I (A, 100 ng/ml) or GH (B, 500 ng/ml) for 10 min. Detergent extracts were resolved by SDS-PAGE and immunoblotted with anti-pAkt and anti-Akt (A) or anti-pSTAT5 and anti-STAT5 (B). The blots shown are representative of two separate experiments. Note inhibition of IGF-I, but not GH, signaling by NVP-AEW-541.
Partial rescue of GH-induced STAT5 signaling by an IGF-IR lacking the intracellular domain of its β-chain
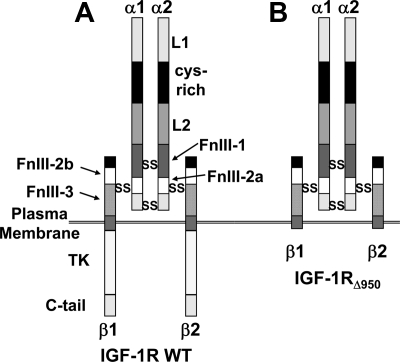
The data in Figs. 2 and 4 collectively indicate that IGF-IR contributes to GH-dependent STAT5 activation, but that blockade of IGF-IR kinase activity has no effect on GH signaling. This suggests that an IGF-IR component(s) other than the kinase domain impacts GH action. IGF-IR is a disulfide-linked heterotetramer comprised by two extracellular α-chains and two transmembrane β-chains, as depicted in Fig. 6A. To probe the issue of IGF-IR involvement in GH action further, we prepared an adenovirus to direct expression of a mutant IGF-IR that is truncated at residue 950 (Y-950 is replaced by a stop codon). IGF-IRΔ950 includes the entire α-chain and a β-chain ICD truncated just proximal to its tyrosine kinase domain (52) (Fig. 6B). This mutant thus includes only the ICD juxtamembrane 30 residues and lacks the kinase domain.
Figure 6.
IGF-IR structure. A and B, Diagram of the full-length IGF-IR (A) and C-terminally truncated IGF-IRΔ950 mutant (B). IGF-IR is a disulfide-linked heterotetramer consisting of an α2β2 assemblage in which the α-chain is entirely extracellular and the β-chain is a transmembrane protein that harbors a tyrosine kinase in its intracellular domain. L, leucine-rich repeat domain; Cys-rich, cysteine-rich; FnIII, fibronectin III; TK, tyrosine kinase domain.
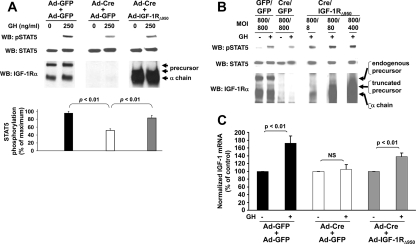
We coinfected IGF-IR-floxed osteoblasts with Ad-Cre plus Ad-GFP vs. Ad-Cre plus Ad-IGF-IRΔ950 and assessed GH-induced STAT5 phosphorylation (Fig. 6A). Notably, infection with Ad-IGF-IRΔ950, like Ad-IGF-IR, substantially restored (by ∼76%) Ad-Cre-mediated loss of GH-induced STAT5 phosphorylation (densitometric quantitation in Fig. 7A, lower panel), strongly suggesting that the majority of the ICD, including the kinase domain, is not necessary for the rescue effect on GH-induced STAT5 signaling. We note that the adenoviral reconstitution achieved very high levels of expression of the truncated IGF-IR in Fig. 7A (and of the wild-type IGF-IR in Fig. 4A). We sought to verify our findings by using conditions that yielded more moderate expression and thus performed a rescue experiment with lower levels of Ad-IGF-IRΔ950 infection (Fig. 7B). Notably, infection with Ad-Cre plus Ad-IGF-IRΔ950 using Ad-IGF-IRΔ950 at multiplicity of infection (MOI) as low as eight enabled reconstitution of truncated IGF-IR to levels slightly less in abundance than endogenous IGF-IR; still, the diminished GH-induced STAT5 activation was increased under these circumstances and even further still with MOIs of 80 and 400. (Indeed, in this particular experiment, the level of GH-induced STAT5 phosphorylation under these latter two conditions exceeds that observed with Ad-GFP-only infection; reasons for this finding are unclear.)
Figure 7.
Partial rescue of GH-induced STAT5 signaling by an IGF-IR lacking the intracellular domain of its β-chain. A, Upper panel, Floxed-IGF-IR primary osteoblasts were infected with either Ad-GFP only (1600 MOI), Ad-GFP plus Ad-Cre (800 MOI each simultaneously), or Ad-IGF-IRΔ950 plus Ad-Cre (800 MOI each simultaneously). Serum-starved cells were treated with vehicle or GH (250 ng/ml) for 10 min, after which detergent extracts were resolved by SDS-PAGE and sequentially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-IRα. Lower panel, Densitometric quantitation of pSTAT5 signals from three independent experiments. In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± se. P values are indicated. B, The experiment in A was repeated, except that Ad-IGF-IRΔ950 was used at 8, 80, or 400 MOI. Note that rescue of GH-induced STAT5 activity was afforded by Ad-IGF-IRΔ950, despite the reduced level of expression of the truncation mutant achieved with 8–400 MOI compared with 800 MOI used in A. C, Floxed IGF-IR primary osteoblasts were infected as in A. Serum-starved cells were treated with vehicle or GH (50 ng/ml) for 6 h. Total RNA was extracted, and quantitative real-time PCR for IGF-I (normalized for β-actin) was performed. Pooled data from two separate experiments (one in triplicate and one in duplicate) are represented as mean ± se. In each case, the value of the vehicle-treated samples is considered 100%. P values are indicated.
GH-dependent IGF-I gene expression is believed to be mediated largely by STAT5 activation via enhancer elements that bind STAT5 (25,26,29,31). We sought to determine whether the effects of IGF-IR deletion and reconstitution on GH-induced STAT5 signaling in primary osteoblasts would be reflected in the levels of IGF-I mRNA expressed in response to GH (Fig. 7C). Primary calvarial IGF-IR-floxed osteoblasts were infected with Ad-GFP plus Ad-GFP vs. Ad-Cre plus Ad-GFP vs. Ad-Cre plus Ad-IGF-IRΔ950, and serum-starved cells were treated for 6 h with GH (50 ng/ml) or vehicle. IGF-I mRNA abundance was measured by reverse transcription and real-time PCR. As we showed with 500 ng/ml previously (47), GH treatment with 50 ng/ml significantly increased IGF-I mRNA (∼1.8-fold increase) in Ad-GFP-infected cells. However, 50 ng/ml GH had no effect on IGF-I mRNA abundance in Ad-Cre- infected cells. These data are consistent with the findings in Fig. 2, A and B, that IGF-IR deletion by Ad-Cre infection renders osteoblasts much less sensitive to GH for STAT5 activation and thus consequent IGF-I gene expression. Notably, reconstitution with Ad-IGF-IRΔ950 in the setting of deletion of endogenous IGF-IR resulted in a partial rescue (∼1.4-fold) of GH-induced IGF-I mRNA abundance. This degree of restoration is akin to the partial rescue of GH-induced STAT5 activation described in Fig. 7A. These data strongly suggest that the use of IGF-IR in acute GH signaling leading to IGF-I gene expression does not depend on the intact IGF-IR ICD. In other data (not shown), Ad-IGF-IRΔ932, which encodes another truncation mutant lacking all but three ICD residues (stop after R-932), also partially rescued GH-induced STAT5 activation, furthering the conclusions drawn with Ad-IGF-IRΔ950.
Discussion
GH is among the most intensely studied endocrine hormones. Its somatogenic effects have been intimately related to those of IGF-I. The complicated physiological relationship between GH and IGF-I has become better understood in the past decade. IGF-I is an effector of GH; GH induces IGF-I expression and secretion in various tissues. The original somatomedin hypothesis held that GH stimulated hepatic secretion of IGF-I, which interacted with IGF-IR in tissues that responded with growth (53,54). Studies of genetically modified mice with global and liver-specific IGF-I knockout, as well as acid-labile subunit knockout, suggested circulating IGF-I is mainly liver derived, but that circulating (in contrast to autocrine/paracrine-derived) IGF-I does not contribute to normal postnatal growth unless a threshold reduction is achieved (55,56,57,58,59). However, recent studies in which IGF-I knockin was engineered in the context of liver-specific IGF-I knockout indicated that endocrine IGF-I contributes approximately 30% of adult body size and supports postnatal development (60). A study in which mice with combined generalized knockout of both GHR and IGF-I genes were compared with mice with individual knockout of each further illustrates the complicated relationship between GH and IGF-I (61). Combined knockout yielded severe (>80%) growth retardation, significantly greater than with either GHR or IGF-I knockout alone, suggesting the GH and IGF-I signaling pathways serve both independent and overlapping growth functions.
In the current work, we used an osteoblast model to examine the relationship between the GHR and IGF-IR signaling systems. Such a model is particularly appealing in that GH and IGF-I strongly affect bone formation and remodeling in vertebrates (reviewed in Ref. 62). Loss of GH action results in inadequate long bone growth and short stature, and hypersecretion of GH in acromegaly leads to connective tissue and bony overgrowth or, if before growth plate closure, gigantism (2,62). As above, it has been difficult to decipher in vivo the degree to which GH affects bone directly vs. indirectly through systemic or local IGF-I. Similarly, some IGF-I effects on bone may be GH independent. Chondrocytes and osteoblasts express GHR (47,63,64,65,66,67,68,69), and GH-induced JAK2 and/or STAT5 activation are detected in several osteoblast cell lines (47,70,71,72). Furthermore, GH promotes proliferation of osteoblastic cells in vitro (47,66,73,74) and trabecular bone formation in IGF-I-deficient mice is enhanced by GH (75), suggesting IGF-I-independent effects. Likewise, GH exerts direct (IGF-I independent) antiapoptotic effects in primary osteoblasts in vitro (47). Others have observed that decreased bone length in global STAT5 knockout mice, unlike GHR knockout mice, is unaccompanied by defective trabecular bone remodeling or growth plate width, suggesting STAT5 alone may not mediate all GH effects on bone homeostasis or that other pathways may compensate for lack of STAT5 (76). Thus, it remains unclear the degree to which GH action in bone cells is direct (vs. IGF-I dependent) and mediated by STAT5. Osteoblasts and chondrocytes produce IGF-I, at least partially in response to GH; however, other factors, such as PTH, estrogens, and glucocorticoids powerfully regulate IGF-I expression, particularly in osteoblasts (47,62). Osteoblasts also express IGF-IR and can thus respond to IGF-I and -II (47). IGF-I stimulates osteoblast proliferation, collagen stability, and bone mineralization (77,78,79). Importantly, osteoblast-specific IGF-IR deletion in vivo results in reduced bone formation and mineralization of osteoid, decreased cancellous bone volume, and altered trabecular structure (48). Whether this implies a role for IGF signaling via IGF-IR vs. the presence of IGF-IR per se is not yet certain.
We found that deletion of IGF-IR rendered primary osteoblasts markedly less responsive to GH in terms of net STAT5 phosphorylation. (Whether this decrease in net STAT5 phosphorylation reflects diminished GH-induced phosphorylation vs. increased GH-induced dephosphorylation has yet to be determined.) This was manifested by significant reductions in GH-induced transactivation of a STAT5-dependent luciferase reporter gene and in IGF-I gene expression in the IGF-IR-deleted cells. This effect was not attributable to decreased expression of GHR, JAK2, or STAT5 in the IGF-IR-deleted cells, and it was specific in that acute PDGF signaling was not altered. Notably, reconstitution of IGF-IR-deleted cells with wild-type IGF-IR by adenoviral infection substantially rescued GH-induced signaling. Furthermore, reexpression of truncated IGF-IRs that lack the majority of the intracellular domain (including the kinase domain) also partially rescued GH-induced STAT5 activation and IGF-I gene expression, a finding consistent with the lack of effect of IGF-IR tyrosine kinase inhibition on GH signaling.
We emphasize that IGF-I gene expression is a notable and relevant GH-dependent effect that is believed to be substantially related to GH-induced STAT5 signaling, although other GH-dependent pathways may also affect IGF-I expression levels. Notably, our findings show that at the same GH concentration (50 ng/ml), deletion of IGF-IR resulted in a roughly 75% reduction in GH-induced STAT5 activation and nearly complete inhibition of both GH-induced STAT5-dependent reporter activation and GH-induced accumulation of IGF-I mRNA. In concert with the similar partial rescue of GH-induced STAT5 activation and GH-induced IGF-I mRNA accumulation by reexpression of the truncated IGF-IR, these data suggest the novel hypothesis that the presence of IGF-IR, or more specifically its ECD and/or transmembrane domain, contributes to the ability of GHR to augment net GH-induced STAT5 phosphorylation and downstream signaling, even in the absence of added IGF-I or a competent IGF-IR kinase response. Whereas our data clearly fit such a hypothesis, we note that rescue by the truncated IGF-IR was less than complete. This may suggest that a deleted region(s) in such a receptor mutant might also contribute to GH signaling. Furthermore, the method of IGF-IR reconstitution of Cre-expressing cells (adenoviral expression) likely introduces aberrant trafficking of the reexpressed receptor (wild-type or mutant) that may alter its ability to function normally. Thus, it is difficult at this point to draw definitive conclusions as to meaning of the partial (rather than complete) rescue achieved by expression of the IGF-IR truncation mutant. Rather, the strong conclusion is that significant rescue was seen with this mutant, whereas adenoviral expression of another cell surface receptor under the same conditions yielded no such rescue.
How could IGF-IR influence early aspects of GH signaling? We view this question in the context of our previous investigation of GH-IGF-I signaling cross talk in mouse preadipocyte fibroblasts (45). 3T3-F442A and 3T3-L1 cells express GHR, JAK2, and IGF-IR, and are highly responsive to GH and IGF-I (19,37,39,43,80,81,82,83,84,85,86). By GHR or IGF-IR immunoprecipitation, we found that GH treatment caused GHR tyrosine phosphorylation and IGF-I treatment caused IGF-IR tyrosine phosphorylation, as expected (45). In addition, we made the unanticipated observation that acute GH treatment resulted in specific coimmunoprecipitation of a complex including tyrosine phosphorylated GHR and JAK2, as well as nontyrosine-phosphorylated IGF-IR (45). This GH-dependent GHR-JAK2-IGF-IR complex formation occurred in the absence of IGF-I and did not require tyrosine phosphorylation of any of the members of the complex. However, we also observed in preadipocytes that cotreatment with GH plus IGF-I significantly augmented GH-dependent signals including STAT5-dependent gene transactivation, even though IGF-I itself had no effect (45). Furthermore, cotreatment of 3T3-L1 cells with IGF-I enabled enhanced acute GH-induced GHR tyrosine phosphorylation and GHR disulfide linkage, the latter of which corresponds to acquisition of the activated receptor conformation (45).
We do not yet know exactly how our findings of GH-induced GHR-JAK2-IGF-IR complex formation and GH-IGF-I signaling synergy in preadipocytes relate to our current findings in osteoblasts that genetic deletion of IGF-IR blunts GH-induced STAT5 signaling and IGF-I gene expression. In preliminary experiments in osteoblasts, we have detected GH-induced coimmunoprecipitation of tyrosine phosphoproteins whose migration in SDS-PAGE are consistent with being JAK2 and GHR (data not shown). We hypothesize that in GH-responsive tissues, GHR and IGF-IR reside in close proximity and that GH promotes functionally relevant enhancement in this association such that GH signaling is augmented in comparison to what it would be in the absence of IGF-IR. We note that the ability to detect GH-inducible complex formation may vary between cell and tissue types, depending on the relative expression levels of the components and the ability of the complex to withstand detergent solubilization that is a prerequisite to coimmunoprecipitation. Furthermore, the ability of IGF-I engagement of the GHR-associated IGF-IR to augment GH-dependent signaling may also vary between cells.
We note that cross talk of several sorts between IGF-IR and other signaling systems have been reported. For example, Ozbay and Nahta (87) detected coimmunoprecipitation of IGF-IR and leptin receptor (ObR) in several human breast cancer cell lines and showed that IGF-I treatment caused activation of ObR downstream signaling molecules. Additionally, short hairpin RNA-mediated silencing of IGF-IR in human breast cancer cells led to enhanced epidermal growth factor (EGF)-induced EGFR and ERK tyrosine phosphorylation, suggesting functionally relevant cross talk between EGFR and IGF-IR (88). We note similarities and differences between these observations and those reported herein and in our previous studies for GHR and IGF-IR. In our work, GHR-JAK2-IGF-IR complex formation was strongly augmented by GH, but not by IGF-I; we do not know the ligand dependencies for the IGF-IR-ObR interactions reported. Furthermore, we have yet to observe an effect of IGF-I treatment alone on GHR tyrosine phosphorylation. Conversely, the effect of IGF-IR deletion on EGF-induced ERK activation is reminiscent of our findings in Fig. 2, D and E, for GH-induced ERK activation in osteoblasts lacking IGF-IR. Clearly, further studies are required to determine the degree to which our findings of GHR-IGF-IR cross talk may relate to findings in other systems.
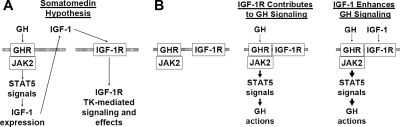
Our current findings further exemplify the complex relationships between GH and IGF-I, which are schematically summarized in Fig. 8. According to the classical somatomedin hypothesis and amended versions that have more recently emerged (Fig. 8A), GH interacts with GHR, causing JAK2/STAT5 activation and IGF-I production. IGF-I then acts in an endocrine (or paracrine/autocrine) fashion by stimulating IGF-IR signaling. In addition to this mode of GH action, we see our recent and current findings (Fig. 8B) as collectively indicating that GH promotes GHR-JAK2-IGF-IR association, allowing IGF-IR to act as a facilitator of GH signaling. Furthermore, in the presence of IGF-I, GH action can be even further augmented. As GH action is important in many aspects of physiology and pathophysiology, a better understanding of the determinants underlying GH-induced complex formation and mechanisms by which IGF-IR can augment GH signaling may have substantial biological and therapeutic significance. Future in vivo experiments to test these hypotheses will be facilitated by our finding that a kinase-deficient IGF-IR allows substantial rescue of the GH signaling defect found with endogenous IGF-IR deletion. Thus, tissue-specific in vivo reexpression of a kinase-deficient IGF-IR in the context of, for example, an osteoblast-specific IGF-IR knockout may allow understanding of such IGF-IR-augmented GH signaling effects.
Figure 8.
GH, IGF-I, and IGF-IR interrelationships (see text for details). A, Schematized somatomedin hypothesis of GH action. GH interacts with GHR, causing JAK2/STAT5 activation and IGF-I production. IGF-I then acts in an endocrine fashion by stimulating IGF-IR signaling. B, Schematization of our findings. GH promotes GHR-JAK2-IGF-IR association. IGF-IR acts as a component of GH signaling. In the presence of IGF-I, GH action is augmented.
Materials and Methods
Materials
Recombinant human GH was kindly provided by Eli Lilly & Co. (Indianapolis, IN). Recombinant human IGF-I was purchased from Novozymes GroPep Ltd. (Thebarton, South Australia, Australia). NVP-AEW541 was from Novartis Pharma AG (Basel, Switzerland). Other routine reagents were from Sigma-Aldrich. (St. Louis, MO), unless otherwise noted. Cell culture medium, α-MEM, was obtained from Cellgro-Mediatech (Herndon, VA), and fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA).
Antibodies
Polyclonal anti-STAT5, anti-IGF-IRβ, and anti-IGF-IRα antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal anti-phospho-STAT5 was purchased from Zymed Laboratories, Inc. (South San Francisco, CA). Monoclonal anti-phosphotyrosine antibody 4G10 (pTyr) and affinity-purified polyclonal anti-mitogen-activated protein kinase antibody (anti-ERK, recognizing both ERK1 and ERK2) were obtained from Upstate Biotechnology (Lake Placid, NY). Affinity-purified polyclonal antiactive mitogen-activated protein kinase antibody (anti-active ERK, recognizing the dually phosphorylated Thr-183 and Tyr-185 residues corresponding to the active forms of ERK1 and ERK2) was from Promega (Madison, WI). Polyclonal anti-phospho-JAK2, anti-phospho-AKT, and anti-AKT antibodies were from Cell Signaling Technology (Beverly, MA). Rat anti-HA monoclonal antibody (clone 3F10) was from Roche Applied Science (Indianapolis, IN). Monoclonal anti-β-actin antibody was from Sigma-Aldrich.
Cells and cell culture
Osteoblasts were isolated from calvaria of newborn Igf1rflox/flox mice by serial digestion in 1.6 mg/ml collagenase type I (Worthington, Lakewood, NJ) solution (47). Calvaria were digested in 10 ml of digestion solution for 15 min at 37 C with constant agitation. The digestion solution was collected, and digestion was repeated with fresh digestion solution an additional four times. Digestions three to five (containing the osteoblasts) were pooled together, centrifuged, washed with α-MEM containing 10% FBS and 1% penicillin/streptomycin, and plated overnight at 37 C in a humidified incubator supplied with 5% CO2. HEK-293 cells were maintained in DMEM (low glucose) (Cellgro, Inc.) supplemented with 7% fetal bovine serum (Biofluids, Rockville, MD) and 50 μg/ml gentamicin sulfate, 100 U/ml penicillin, and 100 μg/ml streptomycin (all Biofluids). In general, a single newborn mouse calvaria prep produced a yield of primary osteoblasts sufficient for roughly ten samples of 1 × 106 cells each in experiments outlined below.
Generation of recombinant adenoviruses
To generate an adenovirus encoding hIGF-IR-FLAG, the pCDNA-hIGF-IR cDNA (a gift of Dr. C. Roberts, Oregon Health and Sciences University, Portland, OR) served as a template to amplify full length hIGF-IR; using that incorporated a NotI 5′ restriction site, a C-terminal FLAG tag, and an XbaI 3′ restriction site. This fragment was subcloned into the pAdTrack shuttle vector. pAdTrack-hIGF-IRFLAG was linearized with PacI and cotransformed with pAdEASY (a helper plasmid) into Escherichia coli BJ5138 cells. Colonies harboring recombinants were selected by virtue of kanamycin resistance. Linearized recombinant plasmid was transfected into HEK-293 cells and high titer viral stock was obtained. Adenovirus expressing hIGF-IR1-932-FLAG was constructed similarly.
Adenovirus preparation and infection
Adenoviruses were amplified by infecting HEK-293 cells (49,50). Cells were harvested when cytopathic effects became apparent. After lysis by five freeze/thaw cycles, cell debris was pelleted by centrifugation and subject to further cesium chloride purification procedures. Concentration of purified virus was calculated by measuring the value of OD260. For deletion of the IGF-IR, osteoblasts containing floxed IGF-IR alleles were cultured to 70% confluence and then, in the absence of serum, were infected with Ad-Cre or, as a control, an Ad-GFP, at 800 MOI, unless otherwise noted (47). After 1 h, culture medium containing 10% FBS was added, and the cells were allowed to recover for 48 h before stimulation.
Cell starvation, cell stimulation, and protein extraction
Serum starvation of primary osteoblasts (∼1 × 106 cells/6 cm2 dish/sample) was accomplished by substitution of 0.5% (wt/vol) BSA (fraction V, Roche Molecular Biochemicals, Indianapolis, IN) for serum in their respective culture media for 16–20 h before experiments. Stimulations were performed at 37 C. The cells were stimulated in starvation medium and stimulations were terminated by washing the cells once with and then harvesting by scraping in ice-cold PBS in the presence of 0.4 mm sodium orthovanadate (PBS-vanadate). Pelleted cells were collected by brief centrifugation and solubilized for 30 min at 4 C in fusion lysis buffer (1% (vol/vol) Triton X-100, 150 mm NaCl, 10% (vol/vol) glycerol, 50 mm Tris-HCl (pH 8.0), 100 mm NaF, 2 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 10 mm benzamidine, and 10 μg/ml aprotinin). After centrifugation at 15,000 × g for 15 min at 4 C, the detergent extracts were electrophoresed under reducing conditions or subjected to immunoprecipitations, as indicated.
Immunoprecipitation, electrophoresis, and immunoblotting
For immunoprecipitation, cell extracts (500–1000 μg protein; ∼6–12 × 106 cells) were mixed with either 2 μl of AL47 or 5 μl of polyclonal anti-IGF-IRβ antibody and incubated at 4 C overnight with continuous agitation. Protein A-Sepharose beads (Amersham Biosciences, Piscataway, NJ) were added and incubated at 4 C for an additional hour. The beads were washed four times with lysis buffer adjusted to 0.5% (vol/vol) Triton X-100. Laemmli sample buffer eluates were resolved by SDS-PAGE and immunoblotted as indicated.
Proteins resolved by SDS-PAGE were transferred to Hybond ECL nitrocellulose membranes (Amersham Biosciences). The membranes were blocked with TBST buffer [20 mm Tris-HCl (pH 7.6), 150 mm NaCl, and 0.1% (vol/vol) Tween 20] containing 2% (wt/vol) BSA and incubated with primary antibodies (0.5–1 μg/ml) as specified in each experiment. After three washes with TBST, the membranes were incubated with appropriate secondary antibodies (1:7500 dilution) and washed. The bound antibodies were detected with SuperSignal chemiluminescent substrate (Pierce Chemical Co., Rockford, IL). Membrane stripping was performed according to the manufacturer’s suggestions (Amersham Biosciences).
Transactivation assay
Floxed IGF-IR cells treated with Ad-GFP or Ad-Cre, as above, were infected with Ad-GHRE-luc, as previously described (49,50), in triplicate for each condition. Cells were stimulated with vehicle vs. GH (50 ng/ml) for 6 h. Luciferase activity was measured in cell extracts, as previously described (15,17,89). Data are displayed as the mean ± SE normalized for the maximum signal within the assay.
Figure presentation and densitometric analysis
Immunoblots shown are in all instances representative of at least two experiments, as indicated in figure legends. In some figures, irrelevant intervening lanes from original immunoblots have been cropped for clarity of presentation; spaces between lanes clearly indicate this cropping. In all cases, only data from the same original blots are incorporated in figures with consistent brightness/contrast adjustment made across each blot. For densitometric analysis, only data from within each blot are compared with each other. Densitometric quantitation of ECL immunoblots was performed using a high-resolution scanner and the ImageJ 1.30 program (developed by W. S. Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD). Pooled data from several experiments are displayed as mean ± se. The significance of differences (P value) of pooled results was estimated using paired t tests.
RNA preparation and real-time PCR analysis
Total RNA was isolated from primary osteoblasts using the RNeasy RNA isolation kit as recommended by the manufacturer (Qiagen, Valencia, CA). cDNA was synthesized using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). The cDNA was amplified in the LightCycler 480 Real-Time PCR System (Roche) using SYBR Green reagent (Roche) and sequence-specific primers. PCR were performed in triplicate for each cDNA, averaged, and normalized to endogenous β-actin reference transcripts. Primer sequences used were as follows: IGF-I, F5′-GTGTGGACCGAGGGGCTTTTACTTC-3′; R5′-GCTTCAGTGGGGCACAGTACATCTC-3′; β-actin, F5′-TGCGTGACATCAAAGAGAAG-3′; R F5′-GATGCCACAGGATTCCATAC-3′.
Acknowledgments
We appreciate helpful conversations with Drs. J. Messina, L. Deng, L. Liu, J. Xu, and X. Li and the generous provision of reagents by those named in the text. This work was supported by National Institute of Health Grants DK46395 (to S.J.F.) and AR052746 (to T.L.C.) and a Veteran’s Affairs Merit Review Award (to T.L.C.). Parts of this work were presented at the 89th and 90th Annual Meetings of The Endocrine Society in Toronto, Canada, and San Francisco, CA, in 2007 and 2008, respectively.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 4, 2010
Abbreviations: Ad-Cre, Nonreplicative adenovirus that drives Cre expression; Ad-GFP, adenovirus encoding green fluorescent protein; Ad-SSR-HA, adenovirus that drives expression of a hemagglutination-tagged somatostatin receptor; ECD, extracellular domain; EGF, epidermal growth factor; FBS, fetal bovine serum; GHRE, GH response element; GHR, GH receptor; ICD, intracellular domain; IGF-IR, IGF-I receptor; JAK2, Janus kinase 2; MOI, multiplicity of infection; ObR, leptin receptor; PDGF, platelet-derived growth factor; STAT, signal transducer and activator of transcription.
References
- Isaksson OG, Eden S, Jansson JO 1985 Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol 47:483–499 [DOI] [PubMed] [Google Scholar]
- Kaplan S 1999 Hormonal regulation of growth and metabolic effects of growth hormone. In: Kostyo J, Goodman HM, eds. Handbook of physiology. New York: Oxford University Press; 129–143 [Google Scholar]
- Frank SJ, Messina JL 2002 Growth hormone receptor. In: Oppenheim JJ, Feldman M, eds. Cytokine reference on-line. London, UK: Academic Press, Harcourt; 1–21 [Google Scholar]
- Carter Su C, Schwartz J, Smit LS 1996 Molecular mechanism of growth hormone action. Annu Rev Physiol 58:187–207 [DOI] [PubMed] [Google Scholar]
- Bazan JF 1990 Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 87:6934–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA 1992 Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA 1991 Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 254:821–825 [DOI] [PubMed] [Google Scholar]
- Frank SJ 2002 Receptor dimerization in GH and erythropoietin action—it takes two to tango, but how? Endocrinology 143:2–10 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang J, Kopchick JJ, Frank SJ 1999 Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem 274:33072–33084 [DOI] [PubMed] [Google Scholar]
- Silvennoinen O, Witthuhn BA, Quelle FW, Cleveland JL, Yi T, Ihle JN 1993 Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci USA 90:8429–8433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SJ, Gilliland G, Kraft AS, Arnold CS 1994 Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 135:2228–2239 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay MC, Finidori J, Kelly PA 1994 Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology 135:1292–1298 [DOI] [PubMed] [Google Scholar]
- VanderKuur JA, Wang X, Zhang L, Campbell GS, Allevato G, Billestrup N, Norstedt G, Carter-Su C 1994 Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem 269:21709–21717 [PubMed] [Google Scholar]
- Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiang J, Sakai I, Kraft AS 1995 Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem 270:14776–14785 [DOI] [PubMed] [Google Scholar]
- Tanner JW, Chen W, Young RL, Longmore GD, Shaw AS 1995 The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J Biol Chem 270:6523–6530 [DOI] [PubMed] [Google Scholar]
- He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ 2003 Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol 17:2211–2227 [DOI] [PubMed] [Google Scholar]
- Hansen LH, Wang X, Kopchick JJ, Bouchelouche P, Nielsen JH, Galsgaard ED, Billestrup N 1996 Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J Biol Chem 271:12669–12673 [DOI] [PubMed] [Google Scholar]
- Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C 1996 The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol 10:519–533 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos A, Moutoussamy S, Renaudie F, Clauss M, Kayser C, Gouilleux F, Kelly PA, Finidori J 1996 Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor. Mol Endocrinol 10:998–1009 [DOI] [PubMed] [Google Scholar]
- Wang X, Darus CJ, Xu BC, Kopchick JJ 1996 Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol Endocrinol 10:1249–1260 [DOI] [PubMed] [Google Scholar]
- Yi W, Kim SO, Jiang J, Park SH, Kraft AS, Waxman DJ, Frank SJ 1996 Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol 10:1425–1443 [DOI] [PubMed] [Google Scholar]
- Bergad PL, Shih HM, Towle HC, Schwarzenberg SJ, Berry SA 1995 Growth hormone induction of hepatic serine protease inhibitor 2.1 transcription is mediated by a Stat5-related factor binding synergistically to two γ-activated sites. J Biol Chem 270:24903–24910 [DOI] [PubMed] [Google Scholar]
- Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE 1999 STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol 158:111–116 [DOI] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR 2001 STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142:3836–3841 [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW 1997 Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94:7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR 1998 Binding of STAT5a and STAT5b to a single element resembling a γ-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol Endocrinol 12:675–687 [DOI] [PubMed] [Google Scholar]
- Woelfle J, Billiard J, Rotwein P 2003 Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem 278:22696–22702 [DOI] [PubMed] [Google Scholar]
- Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG 2003 Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349:1139–1147 [DOI] [PubMed] [Google Scholar]
- Chia DJ, Subbian E, Buck TM, Hwa V, Rosenfeld RG, Skach WR, Shinde U, Rotwein P 2006 Aberrant folding of a mutant Stat5b causes growth hormone insensitivity and proteasomal dysfunction. J Biol Chem 281:6552–6558 [DOI] [PubMed] [Google Scholar]
- Fang P, Kofoed EM, Little BM, Wang X, Ross RJ, Frank SJ, Hwa V, Rosenfeld RG 2006 A mutant signal transducer and activator of transcription 5b, associated with growth hormone insensitivity and insulin-like growth factor-I deficiency, cannot function as a signal transducer or transcription factor. J Clin Endocrinol Metab 91:1526–1534 [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS 2002 Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b). Mol Endocrinol 16:1394–1406 [DOI] [PubMed] [Google Scholar]
- Klover P, Hennighausen L 2007 Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology 148:1489–1497 [DOI] [PubMed] [Google Scholar]
- Frank SJ 2007 Growth hormone, insulin-like growth factor I, and growth: local knowledge. Endocrinology 148:1486–1488 [DOI] [PubMed] [Google Scholar]
- Möller C, Hansson A, Enberg B, Lobie PE, Norstedt G 1992 Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem 267: 23403–23408 [PubMed] [Google Scholar]
- Argetsinger LS, Hsu GW, Myers Jr MG, Billestrup N, White MF, Carter-Su C 1995 Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem 270:14685–14692 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C 1996 Growth hormone, interferon-γ, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J Biol Chem 271:29415–29421 [DOI] [PubMed] [Google Scholar]
- Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, Millard K, Palethorpe K, Nielsen K, Clyde-Smith J, Hancock JF, Waters MJ 2008 An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat Cell Biol 10:740–747 [DOI] [PubMed] [Google Scholar]
- Jin H, Lanning NJ, Carter-Su C 2008 JAK2, but not Src family kinases, is required for STAT, ERK, and Akt signaling in response to growth hormone in preadipocytes and hepatoma cells. Mol Endocrinol 22:1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, Jacobs S, Francke U, Ramachandran J, Fujita-Yamaguchi Y 1986 Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J 5:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D 2000 Insulin-like growth factor I receptor signaling—overlapping or redundant pathways? Endocrinology 141:1287– 1288 [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D 2001 Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22:818–835 [DOI] [PubMed] [Google Scholar]
- Ashcom G, Gurland G, Schwartz J 1992 Growth hormone synergizes with serum growth factors in inducing c-fos transcription in 3T3-F442A cells. Endocrinology 131:1915–1921 [DOI] [PubMed] [Google Scholar]
- Edmondson SR, Russo VC, McFarlane AC, Wraight CJ, Werther GA 1999 Interactions between growth hormone, insulin-like growth factor I, and basic fibroblast growth factor in melanocyte growth. J Clin Endocrinol Metab 84:1638–1644 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim SO, Yang N, Jiang J, Frank SJ 2004 Physical and functional interaction of growth hormone and IGF-1 signaling elements. Mol Endocrinol 18:1471–1485 [DOI] [PubMed] [Google Scholar]
- Xu J, Keeton AB, Franklin JL, Li X, Venable DY, Frank SJ, Messina JL 2006 Insulin enhances growth hormone induction of the MEK/ERK signaling pathway. J Biol Chem 281:982–992 [DOI] [PubMed] [Google Scholar]
- DiGirolamo DJ, Mukherjee A, Fulzele K, Gan Y, Cao X, Frank SJ, Clemens TL 2007 Mode of growth hormone action in osteoblasts. J Biol Chem 282:31666–31674 [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL 2002 Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem 277:44005–44012 [DOI] [PubMed] [Google Scholar]
- Frank SJ, Wang X, He K, Yang N, Fang P, Rosenfeld RG, Hwa V, Chaudhuri TR, Deng L, Zinn KR 2006 In vivo imaging of hepatic growth hormone signaling. Mol Endocrinol 20:2819–2830 [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang J, Warram J, Baumann G, Gan Y, Menon RK, Denson LA, Zinn KR, Frank SJ 2008 Endotoxin-induced proteolytic reduction in hepatic growth hormone receptor: a novel mechanism for GH insensitivity. Mol Endocrinol 22:1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Echeverría C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F 2004 In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell 5:231–239 [DOI] [PubMed] [Google Scholar]
- Lee CT, Park KH, Adachi Y, Seol JY, Yoo CG, Kim YW, Han SK, Shim YS, Coffee K, Dikov MM, Carbone DP 2003 Recombinant adenoviruses expressing dominant negative insulin-like growth factor-I receptor demonstrate antitumor effects on lung cancer. Cancer Gene Ther 10:57–63 [DOI] [PubMed] [Google Scholar]
- Salmon Jr WD, Daughaday WH 1957 A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 49:825–836 [PubMed] [Google Scholar]
- Daughaday WH 2000 Growth hormone axis overview–somatomedin hypothesis. Pediatr Nephrol 14:537–540 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Powell-Braxton L, Bondy C 1999 Effects of Igf1 gene deletion on postnatal growth patterns. Endocrinology 140:3391–3394 [DOI] [PubMed] [Google Scholar]
- Liu JL, LeRoith D 1999 Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology 140:5178–5184 [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D 1999 Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA 96:7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO, Ohlsson C 1999 Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci USA 96:7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D 2002 Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratikopoulos E, Szabolcs M, Dragatsis I, Klinakis A, Efstratiadis A 2008 The hormonal action of IGF1 in postnatal mouse growth. Proc Natl Acad Sci USA 105:19378–19383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A 2001 Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol 229:141–162 [DOI] [PubMed] [Google Scholar]
- Giustina A, Mazziotti G, Canalis E 2008 Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 29:535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R, Haynes KM, Werther GA, Waters MJ 1988 The ontogeny of growth hormone receptors in the rabbit tibia. Endocrinology 122:2562–2569 [DOI] [PubMed] [Google Scholar]
- Barnard R, Ng KW, Martin TJ, Waters MJ 1991 Growth hormone (GH) receptors in clonal osteoblast-like cells mediate a mitogenic response to GH. Endocrinology 128:1459–1464 [DOI] [PubMed] [Google Scholar]
- Kassem M, Mosekilde L, Eriksen EF 1994 Growth hormone stimulates proliferation of normal human bone marrow stromal osteoblast precursor cells in vitro. Growth Regul 4:131–135 [PubMed] [Google Scholar]
- Nilsson A, Swolin D, Enerback S, Ohlsson C 1995 Expression of functional growth hormone receptors in cultured human osteoblast-like cells. J Clin Endocrinol Metab 80:3483–3488 [DOI] [PubMed] [Google Scholar]
- Werther GA, Haynes K, Edmonson S, Oakes S, Buchanan CJ, Herington AC, Waters MJ 1993 Identification of growth hormone receptors on human growth plate chondrocytes. Acta Paediatr Suppl 82(Suppl 391):50–53 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, Salles JP, Ohlsson C, de Vries CP, Engelbregt MJ, Netelenbos JC 1996 Growth hormone binds to a single high affinity receptor site on mouse osteoblasts: modulation by retinoic acid and cell differentiation. J Endocrinol 150:465–472 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, Swolin D, Netelenbos JC, Isaksson OG, Ohlsson C 1997 Estrogen enhances growth hormone receptor expression and growth hormone action in rat osteosarcoma cells and human osteoblast-like cells. J Endocrinol 155:159–164 [DOI] [PubMed] [Google Scholar]
- Takahashi MO, Takahashi Y, Iida K, Okimura Y, Kaji H, Abe H, Chihara K 1999 Growth hormone stimulates tyrosine phosphorylation of focal adhesion kinase (p125(FAK)) and actin stress fiber formation in human osteoblast-like cells, Saos2. Biochem Biophys Res Commun 263:100–106 [DOI] [PubMed] [Google Scholar]
- Gerland K, Bataillé-Simoneau N, Baslé M, Fourcin M, Gascan H, Mercier L 2000 Activation of the Jak/Stat signal transduction pathway in GH-treated rat osteoblast-like cells in culture. Mol Cell Endocrinol 168:1–9 [DOI] [PubMed] [Google Scholar]
- Morales O, Lindgren U, Haldosén LA 2000 Growth hormone-regulated intracellular signaling in UMR 106 osteosarcoma cells. J Bone Miner Res 15:2284–2290 [DOI] [PubMed] [Google Scholar]
- Slootweg MC, van Buul-Offers SC, Herrmann-Erlee MP, van der Meer JM, Duursma SA 1988 Growth hormone is mitogenic for fetal mouse osteoblasts but not for undifferentiated bone cells. J Endocrinol 116:R11–R13 [DOI] [PubMed] [Google Scholar]
- Kassem M, Blum W, Ristelli J, Mosekilde L, Eriksen EF 1993 Growth hormone stimulates proliferation and differentiation of normal human osteoblast-like cells in vitro. Calcif Tissue Int 52:222–226 [DOI] [PubMed] [Google Scholar]
- Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B 2001 The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res 16:2320–2329 [DOI] [PubMed] [Google Scholar]
- Sims NA, Clément-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, Baron R, Kelly PA 2000 Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J Clin Invest 106:1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman HL, La Tour D, Linkhart TA, Mohan S, Baylink DJ, Strong DD 1990 Insulin-like growth factor-I and insulin-like growth factor-II induce c-fos in mouse osteoblastic cells. Calcif Tissue Int 46:258–262 [DOI] [PubMed] [Google Scholar]
- Rydziel S, Delany AM, Canalis E 1997 Insulin-like growth factor I inhibits the transcription of collagenase 3 in osteoblast cultures. J Cell Biochem 67:176–183 [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL 2000 Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674–2682 [DOI] [PubMed] [Google Scholar]
- Liang L, Zhou T, Jiang J, Pierce JH, Gustafson TA, Frank SJ 1999 Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology 140:1972–1983 [DOI] [PubMed] [Google Scholar]
- Kim SO, Houtman JC, Jiang J, Ruppert JM, Bertics PJ, Frank SJ 1999 Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3–F442A fibroblasts. J Biol Chem 274:36015–36024 [DOI] [PubMed] [Google Scholar]
- Huang Y, Kim SO, Jiang J, Frank SJ 2003 Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. Modulation of EGF-induced trafficking and signaling. J Biol Chem 278:18902–18913 [DOI] [PubMed] [Google Scholar]
- Huang Y, Chang Y, Wang X, Jiang J, Frank SJ 2004 Growth hormone alters epidermal growth factor receptor binding affinity via activation of ERKs in 3T3-F442A cells. Endocrinology 145:3297–3306 [DOI] [PubMed] [Google Scholar]
- Carter-Su C, Stubbart JR, Wang XY, Stred SE, Argetsinger LS, Shafer JA 1989 Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase. J Biol Chem 264:18654–18661 [PubMed] [Google Scholar]
- Christoffersen CT, Tornqvist H, Vlahos CJ, Bucchini D, Jami J, De Meyts P, Joshi RL 1998 Insulin and insulin-like growth factor-I receptor mediated differentiation of 3T3-F442A cells into adipocytes: effect of PI3-kinase inhibition. Biochem Biophys Res Commun 246:426–430 [DOI] [PubMed] [Google Scholar]
- Boney CM, Gruppuso PA, Faris RA, Frackelton Jr AR 2000 The critical role of Shc in insulin-like growth factor-I-mediated mitogenesis and differentiation in 3T3-L1 preadipocytes. Mol Endocrinol 14:805–813 [DOI] [PubMed] [Google Scholar]
- Ozbay T, Nahta R 2008 A novel unidirectional cross-talk from the insulin-like growth factor-I receptor to leptin receptor in human breast cancer cells. Mol Cancer Res 6:1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedemann J, Sohail M, Macaulay VM 2007 Dual silencing of the EGF and type 1 IGF receptors suggests dominance of IGF signaling in human breast cancer cells. Biochem Biophys Res Commun 355:700–706 [DOI] [PubMed] [Google Scholar]
- Kim SO, Jiang J, Yi W, Feng GS, Frank SJ 1998 Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem 273:2344–2354 [DOI] [PubMed] [Google Scholar]