Abstract
Context: A reduction in maximal mitochondrial ATP production rate (MAPR) and mitochondrial DNA (mtDNA) abundance occurs with age in association with muscle weakness and reduced endurance in elderly people. Branched chain amino acids (BCAA) have been extensively used to improve physical performance.
Objective: The objective was to determine whether an 8-h infusion of BCAA enhances MAPR equally in healthy young and elderly adults.
Methods: Using a crossover study design, we compared the effect BCAA vs. saline infusion in 12 young (23.0 ± 0.8 yr) and 12 elderly (70.7 ± 1.1 yr) participants matched for sex and body mass index. Skeletal muscle MAPR and mtDNA abundance were measured in muscle biopsy samples obtained before and at the end of the 8-h infusion.
Results: In young participants, MAPR with the substrates glutamate plus malate (supplying electrons to complex I) and succinate plus rotenone (complex II) increased in response to BCAA infusion, relative to a decline in MAPR in response to the saline infusion. In contrast, MAPR was unaffected by BCAA infusion in the elderly participants. Moreover, mtDNA abundance was lower in the elderly compared with the young participants but was unaffected by the BCAA infusion. Insulin and C-peptide concentrations declined over time during the saline infusion, but these declines were prevented by the BCAA infusion.
Conclusions: BCAA increased skeletal muscle MAPR in the young participants in comparison with saline, but this effect was not seen in the elderly participants indicating, that unlike in the young, BCAA does not increase muscle mitochondrial function in the elderly.
Branched chain amino acids increased skeletal muscle mitochondrial ATP production rate in young people in comparison with saline, but this effect was not seen in the elderly participants.
Sarcopenia contributes to many of the chronic pathologies associated with aging, including frailty, insulin resistance, and type 2 diabetes (1,2). As the world’s population undergoes a relatively rapid advancement in age, the socioeconomic impact of sarcopenia and its related comorbidities will be overwhelming if allowed to go unchecked. In addition to sarcopenia, there is increasing evidence of age-related declines in skeletal muscle mitochondrial function (3,4) in association with reduced peak oxygen uptake. In particular, prior studies indicate that maximal mitochondrial ATP production rates (MAPR) and activity of mitochondrial oxidative enzymes decline with age. The age-related decline in mitochondrial function is associated with reductions in mitochondrial DNA (mtDNA) abundance (5,6).
Amino acids, particularly branch chain amino acids (BCAAs), provide an attractive nonpharmacological approach for the potential prevention and treatment of sarcopenia and its related comorbidities. Many athletes and bodybuilders use BCAA supplements with the belief that they act as an ergogenic aid by enhancing their physical performance and skeletal muscle accretion (7,8). In addition, amino acids, particularly BCAAs, may be used clinically to attenuate diet-induced muscle atrophy (9), to facilitate wound healing (10,11), and prevent sarcopenia (12,13,14). It was recently reported that 3 months of essential amino acid (15 g/d) supplementation increases lean body mass, basal muscle protein synthesis, and IGF-I expression in elderly women (15).
We previously reported that maximal MAPR, activity of mitochondrial enzymes, and abundance of mRNA gene transcripts encoding mitochondrial proteins were stimulated by an 8-h infusion of insulin plus a mixture of amino acids in healthy young participants (16). The above study suggests a unique role for amino acids in regulating both muscle mitochondrial function and protein synthesis and an intriguing interaction between amino acids and mitochondrial biogenesis. Mechanistically, BCAAs enhance cell signaling pathways [e.g. Akt-mammalian target of rapamycin (mTOR)] that regulate skeletal muscle protein synthesis (17), which in turn may also facilitate an enhancement in mitochondrial ATP production. BCAAs may also have important effects on intermediary metabolism, which also facilitate an enhancement mitochondrial function. For example, leucine provides carbon skeletons to the citric acid cycle at the level of acetyl-CoA that may acutely enhance both citric acid cycle flux and mitochondrial ATP production.
To our knowledge, no data exist that examines the efficacy of amino acid supplementation for improving skeletal muscle mitochondrial function in the elderly. This study was designed to examine the effects of a single 8-h infusion of BCAAs on skeletal muscle mitochondrial function in young and elderly adults. We hypothesized that: 1) the BCAAs would stimulate skeletal muscle MAPR and 2) the stimulatory effect of BCAA would be lower in elderly compared with young adults. Secondary measurements (e.g. mtDNA abundance, citrate synthase activity, and hormones and substrates) were performed to further the understanding of the underlying mechanism of enhanced skeletal muscle MAPR and the basic mechanisms of the regulation of mitochondrial biogenesis in humans.
Subjects and Methods
Subjects
Twelve healthy, sedentary elderly (65–80 yr) and 12 healthy, sedentary young (18–30 yr) participants matched for body mass index (BMI) and sex were studied in this randomized, placebo-controlled, crossover study (Table 1). Participants completed two separate inpatient admissions to the Mayo Clinic’s Center for Translational Science Activities’ Clinical Research Unit (CRU). Informed written and verbal consent was obtained from each participant with the Mayo Foundation Institutional Review Board’s approval.
Table 1.
Subject characteristics
| Young (n = 12) | Old (n = 12) | P | |
|---|---|---|---|
| Age (yr) | 23.4 (0.8) | 70.7 (1.1) | <0.001 |
| Weight (kg) | 74.5 (4.2) | 72.7 (3.3) | 0.53 |
| BMI (kg/m2) | 24.4 (1.0) | 24.5 (0.7) | 0.90 |
| Fat-free mass (kg) | 47.6 (3.5) | 42.2 (3.1) | 0.15 |
| Body fat (%) | 33 (3.1) | 40 (2.6) | 0.10 |
Values are shown as mean (sem). Male to female ratio is 50:50.
Participants underwent an initial screening that included a medical history; physical examination; resting electrocardiogram; and biochemical tests of renal, hepatic, hematological and metabolic function. Participants with evidence of diseases such as diabetes, cardiovascular disease, and thyroid dysfunction or a history of alcohol or substance abuse were excluded due to the potential effects these diseases may have on the outcome measures. Participants who reported using β-blockers were also excluded. Supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org presents prescription medication use by study group. Body composition was measured using dual x-ray absorptiometry (Lunar DPX-L; Lunar Radiation, Madison, WI) (18).
Experimental design
Within 1 month of the screening examination, participants were admitted to the CRU on the evening before their inpatient study day. Premenopausal participants were studied in the luteal phase of their menstrual cycle. Participants were provided a weight-maintaining diet (energy composition of carbohydrate, fat, and protein at the ratio of 50:30:20) for the 3 d before their inpatient study from the CRU metabolic kitchen (19). Upon CRU admission, on d 3 of the diet, participants received dinner at 1800 h and a standardized snack (5.5 kcal/kg) at 2200 h and then remained fasting until the completion of the study. The second study day was performed between 2 and 12 wk after the initial inpatient study.
At approximately 0600 h the following morning, an iv catheter was inserted in the dorsal hand vein and kept in a hot box to collect arterialized venous blood samples (20). The order of the infusions was randomized. The BCAA (Branchamin 4%; Baxter, Clintec Nutrition Division, Deerfield, IL) infusion was administered at 0.13 ml/kg fat-free mass per hour for 30 min and then reduced to 0.043 ml/kg fat-free mass per hour from 30 to 480 min. The volume of saline (0.9% normal saline) infused was equal to the BCAA solution. The BCAA infusion was administered for 8 h because we previously reported that a combined infusion of insulin and amino acids did not significantly affect gene expression or MAPR after only 4 h of infusion, whereas significant effects on both gene expression and MAPR were observed after 8 h of infusion (16). Preliminary studies were performed to determine the infusion rate of the BCAA mixture, which included an equimolar mixture of valine, leucine, and isoleucine, and that would achieve plasma concentrations of these BCAA greater than 3–6 times their baseline concentrations and would keep the leucine concentration 800 μmol/liter or greater.
Percutaneous muscle biopsies of the vastus lateralis (∼200–300 mg each) were performed under local anesthesia (lidocaine, 2%) at baseline (0 h) and 8 h (21). A portion of the muscle (∼50 mg) was kept on ice in saline-soaked gauze for measurement of MAPR with the remainder frozen in liquid nitrogen and stored at −80 C for later analyses.
Analytical procedures
Amino acid concentrations and protein dynamics
HPLC with precolumn o-phthalaldehyde derivatization was used for to determine plasma amino acid concentrations (HP 1090, 1046 fluorescence detector and cooling system; Hewlett Packard, Palo Alto, CA).
Hormones and substrates
Blood glucose concentration was measured during each test with the glucose oxidase method (Beckman Instruments, Fullerton, CA). Additional samples obtained at baseline and every 2 h were collected in EDTA-containing tubes and stored at −80 C for later batch analysis with hormone assays as previously described (19). Automated immunoenzymatic assay systems were used to measure plasma insulin and GH (Beckman Coulter Unicel DXI 800; Beckman) and C-peptide (Bayer-Centaur ACS: 180 Automated chemiluminescence system; Bayer Industries, Indianapolis, IN). Glucagon was measured by direct double-antibody RIA (Linco Research, St. Louis, MO). Nonesterified free fatty acids were measured with an enzymatic colorimetric assay (NEFA C; Wako Chemicals, Richmond, VA).
Mitochondrial analysis
Maximal MAPRs were assessed in isolated mitochondria using a bioluminescent technique, as previously described (3,22). Briefly, fresh muscle tissue (∼50 mg) was minced on a child glass plate and homogenized in buffer A (100 mm KCl, 50 mm Tris, 5 mm MgCl2, 1.8 mm ATP, 1 mm EDTA) (22) and spun at 1020 × g at 4 C. The supernatant was removed and spun at 10,000 g. The new pellet was resuspended in buffer A, recentrifuged at 9000 × g and supernatant removed. The resulting pellet was resuspended in buffer B (180 mm sucrose, 35 mm KH2PO4, 10 mg acetate, 5 mm EDTA) (22) and kept on ice. The reaction mixture included a luciferin-luciferase ATP monitoring reagent (BioThema, Haninge, Sweden), 35 μm ADP, and substrates. Substrates added (in millimole final concentration) were either 10 glutamate plus 1 malate (GM) or 20 succinate plus 0.1 rotenone (SR) with additional blank tubes used for measuring background. ATP production reactions were simultaneously monitored at 25 C for 20–25 min with an automated luminometer (BioOrbit 1251; Oy, Turku, Finland). The GM substrate combination was used to measure ATP production as index of primary electron transfer to complex I of the electron transport chain (ETC). The SR substrate provides electrons to complex II of the ETC. Mitochondrial and tissue homogenate citrate synthase activity was used to calculate mitochondrial ATP production in whole tissue as previously described (23).
mtDNA quantification
Real-time PCR (7900 HT Sequence Detection System; Applied Biosystems, Foster City, CA) was used to quantify mtDNA abundance from frozen muscle samples in duplicate as previously described (16). The mtDNA abundance was determined using two independent primers/probe sets to mtDNA-encoded reduced nicotinamide adenine dinucleotide dehydrogenase-1 and -4 genes and was normalized to the signal for 28S rDNA, which was coamplified within the same reaction well (16).
Statistical analysis
Data are presented as means ± sem. Three-way mixed-effects ANOVA was used to test the effects of treatment (BCAA vs. saline), age (young vs. elderly), time, and their interactions on the dependent outcome measures using SAS 9.0 (SAS Institute, Cary, NC). The data were examined for departures from normality and log transformations were used for the parameters when the variables were not normally distributed. Fisher’s least significant differences test was used for post hoc analysis. Using the data from a previous study (16) and assuming the correlation between the two studies is equal to 0.5, a sample size of 12 participants per group was estimated to provide a power greater than 95% to test the effect of BCAA and 90% to test the effect of age and their interaction. For single measurements between age groups and treatment groups, paired two-tailed t tests were used. Statistical significance was set at P < 0.05.
Results
Amino acid concentrations
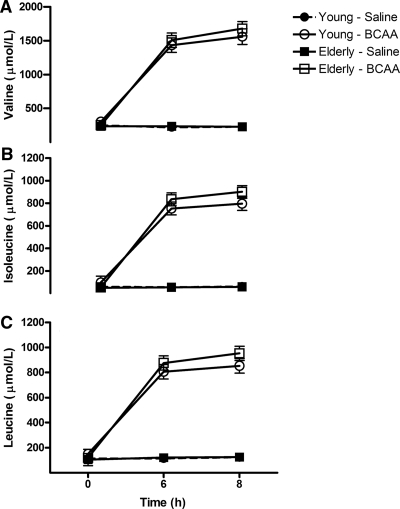
Figure 1 demonstrates that the plasma concentrations of valine, isoleucine, and leucine increased 3- to 5-fold in response to the BCAA infusion compared with saline (P < 0.0001 for all treatmenttime interaction) in both young and elderly participants (P > 0.05 for age and treatmenttimeage interaction). In contrast, the BCAA infusion resulted in reductions (21–48%, P < 0.05) in seven of the 12 non-BCAA amino acids measured in the young group (13–62%, P < 0.05) and 10 of 12 non-BCAA amino acids in the elderly group (Supplemental Table 2).
Figure 1.
Branched chain amino acid concentrations. A–C, Plasma concentrations of valine, isoleucine, and leucine, respectively, during the 8-h infusion of either BCAA or saline. Data presented as mean ± sem. Mixed-effects ANOVA revealed significant treatmenttime (P < 0.001) for all three amino acids.
Hormones and substrates
Insulin
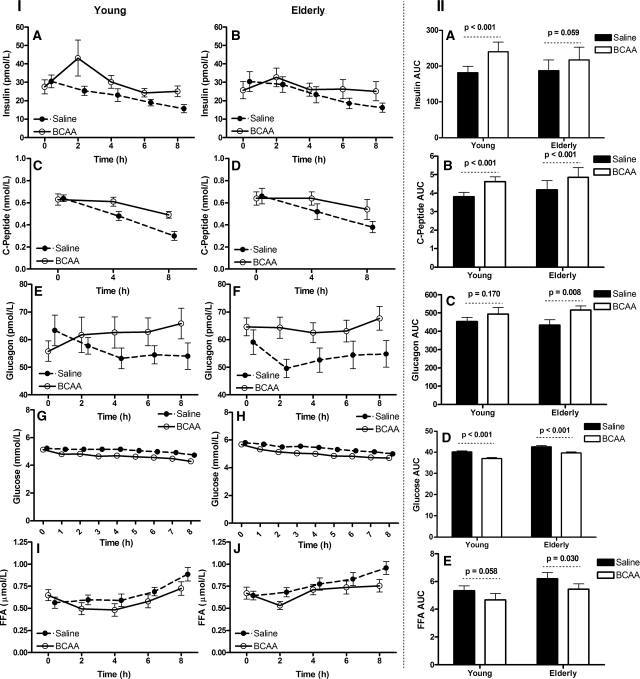
Baseline insulin concentration did not differ between age groups or by treatment (all P > 0.05) (Fig. 2IA and IB). As depicted in Fig. 2, IA-IB, plasma insulin concentrations initially increased in response to the BCAA infusion, followed by a gradual decline toward baseline in both the young and elderly participants. In contrast, insulin declined from baseline in both age groups during the saline infusion. ANOVA revealed a significant treatmenttime effect (P < 0.01), with both age groups responding similarly (P = 0.26 for treatmenttimeage interaction). Moreover, the BCAA infusion increased the insulin area under the curve (AUC) in the young (Fig. 2IIA). The BCAA infusion increased the insulin AUC in the elderly, but this change did not reach the level of statistical significance (Fig. 2IIA).
Figure 2.
Hormones and substrates. Panels IA and IB present the plasma insulin concentrations during the 8 h infusion of either BCAA or saline. Panels IC and ID present the plasma C-peptide concentrations during the 8-h infusion of either BCAA or saline. Panels IE and IF present the plasma glucagons concentrations during the 8-h infusion of either BCAA or saline. Panels IG and IH present the plasma glucose concentrations during the 8-h infusion of either BCAA or saline. Panels II and IJ present the plasma FFA concentrations during the 8 h infusion of either BCAA or saline. Panel IIA–IIE present the insulin, C-peptide, glucagons, glucose, and FFA area under the curve (AUC) during the 8-h infusion of either BCAA or saline, respectively. Data presented as mean ± sem. Mixed-effects ANOVA was used to test the main effects of age and treatment and their interaction.
C-peptide
Baseline C-peptide concentrations did not differ between treatments or age groups (all P > 0.05) (Fig. 2IC and 2ID). The BCAA infusion maintained the C-peptide concentrations at higher concentrations at 4 and 8 h compared with the saline trial (Fig. 2IC and 2ID). ANOVA revealed a significant treatmenttime (P < 0.01), with both age groups responding similarly (P > 0.05 for treatmenttimeage interaction). Subsequently, the C-peptide AUCs were significantly higher under the BCAA condition compared with saline in both the young and elderly participants (Fig. 2IIB).
Glucagon
Baseline glucagon concentrations did not differ between treatments or age groups (all P > 0.05) (Fig. 2IE and 2IF). Glucagon concentrations declined from baseline in both age groups during the saline infusion (Fig. 2IE and 2IF). ANOVA revealed a significant treatmenttime effect (P < 0.0001), with both age groups responding similarly (P = 0.47 for treatmenttimeage interaction). Moreover, the infusion of BCAA increased the glucagon AUC among the elderly participants (Fig. 2IIB).
Glucose
Baseline glucose concentrations did not differ between treatments or age groups (all P > 0.05) (Fig. 2IG and 2IH). The BCAA infusion resulted in significant decline in glucose from baseline compared with the saline infusion (P < 0.01 for the treatmenttime) in both the young and elderly participants (P > 0.05 for the treatmenttimeage) (Fig. 2IG and 2IH). Glucose AUCs were lower on the BCAA trial in both young and elderly participants (Fig. 2IID).
Free fatty acids (FFAs)
Baseline FFA concentrations did not differ between treatments or age groups (all P > 0.05) (Fig. 2II-IJ). FFA levels gradually increased, in both age groups, during saline infusion (Fig. 2II-IJ), but initially declined during BCAA infusion followed by a gradual increase in both young and elderly participants. ANOVA revealed significant timetreatment (P < 0.001) and timeage (P = 0.043) interactions, with no agetreatment (P = 0.884) or timetreatmentage (P = 0.751) interaction. The BCAA infusion resulted in similar reductions in the FFA AUC in young and elderly participants (Fig. 2IIE).
Serum testosterone
As expected, total serum testosterone was lower in elderly males than young males (330 ± 53 vs. 537 ± 58 ng/dl, P = 0.025).
MAPR
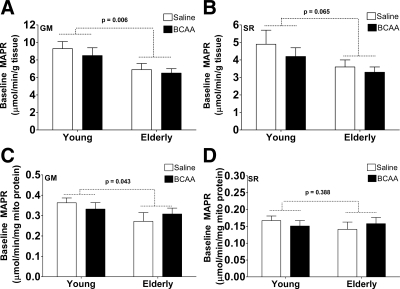
Baseline MAPR with the GM and SR substrates (Fig. 3, A and B) were lower in the elderly than the young (P < 0.05 for age) when normalized to tissue weight. Similarly, baseline MAPR with the GM substrate (Fig. 3C) was lower in the elderly than the young when normalized to mitochondrial protein content.
Figure 3.
MAPR. A and B, Baseline mitochondrial MAPR expressed per gram of tissue during the saline (open bars) and branched chain amino acid (filled bars) infusions by age group. C and D, Baseline mitochondrial MAPR expressed per milligram of mitochondrial protein during the saline (open bars) and branched chain amino acid (filled bars) infusions by age group. MAPR was significantly lower in the elderly participants than the young when GM (complex I) was used and a trend when SR (complex II) was used. Data presented as mean ± sem. Mixed-effects ANOVA was used to test the main effects of age and treatment and their interaction.
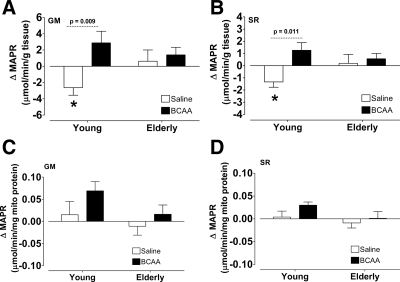
Within the young group (Fig. 4, A and B), MAPR declined from baseline during the saline infusion (P < 0.05) when normalized to tissue weight, and relative to this control trial, MAPR was elevated with the infusion of BCAA for both substrate combinations used (P < 0.05 for treatment). Similar trends were also observed when the MAPR data were expressed relative to mitochondrial protein content (Fig. 4, C and D). There were no significant changes in MAPR in the elderly participants whether normalized to tissue weight or mitochondrial protein content. Baseline citrate synthase activity (units per gram tissue per minute) levels were lower in the elderly than the young under both the saline (13.2 ± 1.1 vs. 16.2 ± 1.2, respectively) and BCAA (12.8 ± 0.9 vs. 15.6 ± 0.8, respectively) conditions, although these differences did not reach the level of statistical significance (P = 0.10 for age). There were no changes in citrate synthase activity in response to either BCAA or saline in either age group.
Figure 4.
MAPR. A and B, Change in MAPR in response to saline (open bars) or BCAA (filled bars) normalized to tissue weight. C and D, Change in MAPR in response to saline (open bars) or BCAA (filled bars) normalized to mitochondrial protein. MAPR was significantly lower in the elderly participants than the young when GM (complex I) was used and a trend when SR (complex II) was used. C and D, Change in MAPR in response to saline (open bars) or BCAA (filled bars). Data presented as mean ± sem. Mixed-effects ANOVA was used to test the main effects of age and treatment and their interaction.
MtDNA quantification
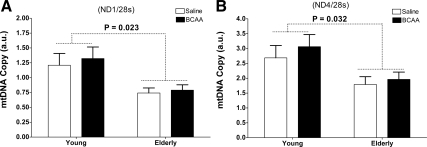
mtDNA abundance was significantly lower in the elderly compared with the young during BCAA administration using both genome markers studied (all P < 0.05 for age) (Fig. 5). There were no significant changes in mtDNA abundance within or between treatments, irrespective of age.
Figure 5.
mtDNA copy number. A and B, mtDNA abundance after either an 8-h infusion of saline (open bars) or BCAA (filled bars) infusions assessed using primers and probes directed to mitochondrial-encoded genes nicotinamide adenine dinucleotide hydroxide dehydrogenase-1 (ND1, A) and 4 (ND4, B) normalized to 28 sec. Data presented as mean ± sem. Mixed-Effects ANOVA was used to test the main effects of age and treatment and their interaction.
Discussion
The current study examined the effects of an 8-h infusion of BCAA on skeletal muscle mitochondrial ATP production in sedentary young and elderly adults. The primary finding was that the infusion of BCAA increased MAPR in young participants when compared with saline when substrates that primarily provide electrons to complex I (GM) or complex II (SR) of the ETC were used. In contrast, there were no significant changes in MAPR in the elderly participants in response to BCAA. The infusion of BCAA also increased plasma concentrations of insulin, C-peptide, and glucagon in both groups relative to the time-related declines in these hormones that were observed during the saline condition. Moreover, BCAA although increasing plasma concentrations of leucine, isoleucine and valine reduced the concentrations of many other amino acids, consistent with the reduction in protein breakdown and endogenous amino acid influx (24,25).
This study aids in the understanding of the underlying physiological processes stimulated by BCAA, which are readily available as nutritional supplements. As is seen with other medications and supplements, there were variations in the physiological response that were associated with aging. Specifically, BCAA stimulated MAPR in the young but not the elderly. Although the underlying mechanisms for these responses remain unclear, it is possible that BCAA-induced stimulation of insulin secretion may play a role. In support of this contention, it has been previously reported that a physiological infusion of insulin, in conjunction with amino acids, enhances skeletal muscle MAPR, gene transcripts encoding mitochondrial proteins, and mitochondrial protein synthesis in young adults (16). Moreover, it was also recently demonstrated that insulin action is an important determinant of skeletal MAPR in people with type 1 diabetes (26). Indeed, the BCAA-induced increase in the insulin AUC was greater in young compared with the elderly, which may have contributed to the difference in MAPR outcomes. However, the present data suggest that the effects BCAA-induced enhancement in MAPR are probably driven more by the acute elevation of BCAA rather than insulin because the differences in insulin exposure between conditions was relatively small when compared with our previous (insulin and amino acid) infusion studies. It is also possible that age-related insulin resistance played a role in the blunting of the BCAA-induced enhancement in MAPR among the elderly. In a corollary, it has been shown that skeletal muscle protein synthesis is blunted in response to insulin (27), and therefore, it is conceivable that this mechanism may also be involved in the blunting of the response to BCAA.
The BCAA-induced enhancement of cell signaling pathways (e.g. Akt-mTOR) that regulate skeletal muscle protein synthesis (17) may also play a role in facilitating the acute enhancement in mitochondrial function. Indeed, it has recently been reported that leucine supplementation enhances postprandial mitochondrial protein synthesis in male Wistar rats (28). Moreover, BCAAs have been shown to activate the Akt-mTOR cell signaling pathway (29,30). In addition to the known effects of BCAAs on the Akt-mTOR signaling pathway, future studies are needed to examine the effects of BCAA on sirtuin1, peroxisomal proliferator-activated receptor-γ coactivator 1-α, mitochondrial transcription factor A signaling pathways, which directly regulate mitochondrial biogenesis and function. The timing of the muscle biopsies (0 and 8 h) precluded the evaluation of the role of these cell signaling pathways (and others) in the regulation of the effect of BCAA on mitochondrial function because it is well recognized that cell signaling pathways are rapidly turned on (<30 min) and off (<60 min) (31,32). Therefore, it is possible that these signaling pathways were involved in the BCAA-induced enhancement of MAPR that we observed in the young.
Consistent with our previous reports (3,33), the present data indicate that sedentary elderly adults have lower MAPRs than sedentary younger adults. It could be hypothesized that age-related declines in sex hormones (e.g. testosterone) may mediate in part the age-related decline in MAPRs. As expected, the elderly men had lower testosterone concentrations than young men. It could also be postulated that the lack of response to BCAA (particularly in elderly men) may be related to reduction in circulating sex hormones. However, the present study was not designed to properly address this issue. Future studies are warranted to examine the role of age-related declines in circulating sex hormones on mitochondrial function. Interestingly, we recently reported that MAPRs are lower in women than men, which may be related to differences in sex hormones (33). Although we did not observe a significant sex effect on MAPRs in the present study, it should be recognized that the present study was not powered to detect a main effect of sex on MAPRs.
It could be argued that the BCAA dose might have been insufficient to exert a stimulatory effect on mitochondrial function in the elderly. Indeed, healthy elderly adults have been shown to be resistant to the anabolic effects of amino acids (34); therefore, it may be possible that elderly adults are also resistant to the stimulatory effects of BCAAs on mitochondrial function. Further studies are warranted to examine whether higher doses of BCAAs result in improvements in mitochondrial function among the elderly. However, it should be noted that the leucine concentrations achieved in the present study were about 33% higher than the leucine concentrations reported previously (34) and were severalfold higher than saline.
Amino acids, including BCAAs, are well known to have pluripotent effects on the pancreas, including the promotion of insulin and glucagon secretion in humans and animals (35,36,37,38). Indeed, the present data clearly demonstrate that the infusion of BCAAs affects both insulin and glucagon secretion. Of the BCAAs, leucine has been reported to be the most potent stimulator of insulin secretion in animals (39) However, in lean healthy humans, it has been reported that the a 5-h iv infusion of leucine has only a limited effect on insulin secretion (40). Differences in the duration (8 vs. 5.5 h) of infusion and the achieved leucine concentration (∼800 vs. ∼300 μmol/liter) between our previous study (40) and the present study may account for the variation in insulin secretion between these two studies. The effect of BCAA infusion to prevent the time-related decline in glucagon may be due in part to the larger decline in plasma glucose compared with the saline trial. Administration of amino acids has been shown to directly stimulate glucagon secretion (38), although this effect is strongest with non-BCAAs (e.g. alanine, arginine, asparagine, and glycine) and the effects of BCAA appears to be minimal (35). Therefore, it is probable that the BCAA-induced elevation in the glucagon AUCs is a counterregulatory response to the greater decline in plasma glucose concentrations that were observed in the BCAA condition.
We recognize that a limitation of the present investigation is that we did not directly measure cardiorespiratory fitness (peak oxygen uptake) or physical activity, which likely has affects on mitochondrial density and function. However, we previously demonstrated that MAPRs are lower in sedentary elderly adults than sedentary younger adults matched for BMI, age, and physical activity (33).
In conclusion, the BCAA infusion enhanced skeletal muscle MAPR in the young but not the elderly. Whether this effect is a direct effect of BCAAs on muscle, or requires the action of insulin or other hormones and signaling molecules remains uncertain. Muscle mtDNA abundance was lower in the elderly than the younger participants and was unaffected by the infusion of BCAAs. Further studies are warranted to examine the exact mechanism by which BCAAs enhance MAPRs in the young but not the elderly and the potential effect of chronic dietary supplementation on changes in MAPRs to evaluate the clinical utility of these widely available nutritional supplements.
Supplementary Material
Acknowledgments
We gratefully acknowledge the skillful technical assistance of the Mayo Clinic Center for Translational Science Activities’ Clinical Research Unit. L.L.T. and K.S.N. was responsible for experimental design, collection and analysis of data, and manuscript preparation; B.A.I. was responsible for analysis of data and manuscript preparation; A.T. and M.L.B. was responsible for experimental design and collection of data; K.K. was responsible for analysis of data; and K.R.S. was responsible for experimental design and collection of data.
Footnotes
This work was supported in part by an unrestricted grant from Ajinomoto, the David Murdock Dole Professorship (to K.S.N.), and Public Service Grants RO1 AG09531 from the National Institute on Aging (NIA), 1 UL1 RR024150-01 and 1 KL2 RR024151-01 (to B.A.I.) from the National Center for Research Resources (NCRR), National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available (http://www.ncrr.nih.gov/). Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overviewtranslational.asp.
Disclosure Summary: The authors have no conflicts of interest.
First Published Online December 18, 2009
Abbreviations: AUC, Area under the curve; BCAA, branch chain amino acid; BMI, body mass index; CRU, Clinical Research Unit; ETC, electron transport chain; FFA, free fatty acid; GM, glutamate plus malate; MAPR, mitochondrial ATP production rate; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; SR, succinate plus rotenone.
References
- Dutta C, Hadley EC 1995 The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci 50A:1–4 [DOI] [PubMed] [Google Scholar]
- Melton 3rd LJ, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL 2000 Epidemiology of sarcopenia. J Am Geriatr Soc 48:625–630 [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS 2005 Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI 2003 Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS 2003 Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52:1888–1896 [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS 1996 Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromiak JA, Antonio J 2002 Use of amino acids as growth hormone-releasing agents by athletes. Nutrition 18:657–661 [DOI] [PubMed] [Google Scholar]
- Armsey Jr TD, Grime TE 2002 Protein and amino acid supplementation in athletes. Curr Sports Med Rep 1:253–256 [DOI] [PubMed] [Google Scholar]
- Layman DK 2003 The role of leucine in weight loss diets and glucose homeostasis. J Nutr 133:261S–267S [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Chinkes DL, Irtun O, Wolfe RR 2002 Anabolic action of insulin on skin wound protein is augmented by exogenous amino acids. Am J Physiol Endocrinol Metab 282:E1308–E1315 [DOI] [PubMed] [Google Scholar]
- Collins CE, Kershaw J, Brockington S 2005 Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutrition 21:147–155 [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR 1998 Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101:2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ 2006 Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr 84:623–632 [DOI] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR 2003 Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ 2009 Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. Journal of Clin Endocrinol Metab 94:1630–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS 2003 Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA 100:7996–8001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS 2001 Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care 4:39–43 [DOI] [PubMed] [Google Scholar]
- Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW 1993 Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 68:867–873 [DOI] [PubMed] [Google Scholar]
- Charlton MR, Adey DB, Nair KS 1996 Evidence for a catabolic role of glucagon during an amino acid load. J Clin Invest 98:90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland KC, Kenney FA, Nair KS 1992 Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol 263:E1010–E1014 [DOI] [PubMed] [Google Scholar]
- Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J 1995 Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. J Clin Invest 95:2926–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom R, Lundin A, Hultman E 1990 A sensitive method for measuring ATP-formation in rat muscle mitochondria. Scand J Clin Lab Invest 50:143–152 [DOI] [PubMed] [Google Scholar]
- Short KR, Nygren J, Barazzoni R, Levine J, Nair KS 2001 T(3) increases mitochondrial ATP production in oxidative muscle despite increased expression of UCP2 and -3. Am J Physiol Endocrinol Metab 280:E761–E769 [DOI] [PubMed] [Google Scholar]
- Louard RJ, Barrett EJ, Gelfand RA 1995 Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 44:424–429 [DOI] [PubMed] [Google Scholar]
- Louard RJ, Barrett EJ, Gelfand RA 1990 Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci (Lond) 79:457–466 [DOI] [PubMed] [Google Scholar]
- Karakelides H, Asmann YW, Bigelow ML, Short KR, Dhatariya K, Coenen-Schimke J, Kahl J, Mukhopadhyay D, Nair KS 2007 Effect of insulin deprivation on muscle mitochondrial ATP production and gene transcript levels in type 1 diabetic subjects. Diabetes 56:2683–2689 [DOI] [PubMed] [Google Scholar]
- Fujita S, Glynn EL, Timmerman KL, Rasmussen BB, Volpi E 2009 Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia 52:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet C, Zangarelli A, Mishellany A, Rousset P, Sornet C, Dardevet D, Boirie Y 2004 Mitochondrial and sarcoplasmic proteins, but not myosin heavy chain, are sensitive to leucine supplementation in old rat skeletal muscle. Exp Gerontol 39:745–751 [DOI] [PubMed] [Google Scholar]
- Proud CG 2004 mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun 313:429–436 [DOI] [PubMed] [Google Scholar]
- Jefferson LS, Kimball SR 2003 Amino acids as regulators of gene expression at the level of mRNA translation. J Nutr 133:2046S–2051S [DOI] [PubMed] [Google Scholar]
- Korf U, Derdak S, Tresch A, Henjes F, Schumacher S, Schmidt C, Hahn B, Lehmann WD, Poustka A, Beissbarth T, Klingmüller U 2008 Quantitative protein microarrays for time-resolved measurements of protein phosphorylation. Proteomics 8:4603–4612 [DOI] [PubMed] [Google Scholar]
- Blagoev B, Ong SE, Kratchmarova I, Mann M 2004 Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol 22:1139–1145 [DOI] [PubMed] [Google Scholar]
- Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS 2009 Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes doi: 10.2337/db09-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ 2005 Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424 [DOI] [PubMed] [Google Scholar]
- Rocha DM, Faloona GR, Unger RH 1972 Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest 51:2346–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaru A, Shirohara H, Moriyama A, Otsuki M 1998 Effects of branched-chain-enriched amino acid solution on insulin and glucagon secretion and blood glucose level in liver cirrhosis. Scand J Gastroenterol 33:853–859 [DOI] [PubMed] [Google Scholar]
- Floyd Jr JC, Fajans SS, Conn JW, Knopf RF, Rull J 1966 Stimulation of insulin secretion by amino acids. J Clin Invest 45:1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Welle SL, Tito J 1990 Effect of plasma amino acid replacement on glucagon and substrate responses to insulin-induced hypoglycemia in humans. Diabetes 39:376–382 [DOI] [PubMed] [Google Scholar]
- Kuhara T, Ikeda S, Ohneda A, Sasaki Y 1991 Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am J Physiol 260:E21–E26 [DOI] [PubMed] [Google Scholar]
- Nair KS, Matthews DE, Welle SL, Braiman T 1992 Effect of leucine on amino acid and glucose metabolism in humans. Metabolism 41:643–648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.