Abstract
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein highly expressed in many prostate cancers, and can be targeted with radiolabeled antibodies for diagnosis and treatment of this disease. To serve as a radioimmunotherapeutic agent, a kinetically inert conjugate is desired to maximize tumor uptake and tumor radiation dose with minimal nonspecific exposure to bone marrow and other major organs. In this study, we assessed the pharmacokinetics and biodistribution of the 7E11 monoclonal antibody (MAb) radiolabeled with the lutetium-177 (177Lu) - tetraazacyclododecanetetraacetic acid (DOTA) conjugate system (177Lu-7E11) versus those of the 7E11 MAb radiolabeled with the indium-111 (111In) – glycyl-tyrosyl-(N,-diethylenetriaminepentaacetic acid)-lysine hydrochloride (DTPA) conjugate system (111In-7E11, also known as ProstaScint®), to determine the feasibility of using 111In-7E11 as a pretherapeutic agent for 177Lu-7E11 radioimmunotherapy. Pharmacokinetic and biodistribution studies of 177Lu-7E11 in LNCaP xenograft mice were performed at 2, 8, 12, 24, 72, and 168 hours after radiopharmaceutical administration. For 111In-7E11, pharmacokinetic and biodistribution studies were performed at 8, 24, and 72 hours. Parallel studies of 177Lu-7E11 in nontumor bearing mice at 8, 24, and 72 hours postinjection served as controls. Gamma scintigraphy was performed, followed by autoradiography and tissue counting to demonstrate and quantify the distributions of radioconjugated MAb in the tumor and normal tissues. Both 177Lu- and 111In- 7E11 conjugates demonstrated an early blood pool phase in which uptake was dominated by the blood, lung, spleen and liver, followed by uptake and retention of the radiolabeled antibody in the tumor which was most prominent at 24 h. Total accumulation of radioconjugated MAb in tumor at 24 h was greater in the case of 177Lu-7E11 in comparison to that of 111In-7E11. Continued accumulation in tumor was observed for the entire time course studied for both 177Lu-7E11 and 111In-7E11. The liver was the only major organ demonstrating a significant difference in accumulation between the two conjugates. In conclusion, pharmacokinetic and biodistribution studies of 177Lu-7E11 in LNCaP xenograft mouse models support its potential application as a radioimmunotherapeutic agent targeting prostate cancer, and the distribution and tumor uptake of 111In-7E11 appear to be similar to those of 177Lu-7E11, supporting its use as a pretherapeutic tool to assess the potential accumulation of 177Lu-7E11 radioimmunotherapeutic at sites of prostate cancer. However, the different accumulation patterns of the 111In and 177 Lu immunoconjugates in liver will likely prevent the use of 111In-7E11 as a true dosimetry tool for 177Lu-7E11 radioimmunotherapy.
Keywords: prostate cancer, immunoscintigraphy, lutetium-177, indium-111, radioimmunotherapy, prostate-specific membrane antigen, bioluminescence imaging
Introduction
Metastatic prostate cancer may be treated using androgen ablation, surgery, or radiation therapy [1, 2]. Currently, there is no single treatment that appears superior to the others for metastatic disease, and none of them significantly prolongs survival [3]. There has been continued interest in utilizing antibodies to deliver radionuclides and other therapeutics to prostate cancer metastases with the goal of decreasing toxicity to normal tissues and increasing therapeutic efficacy by carrying the therapeutic directly to the tumor target [4–6]. A similar strategy previously has been used successfully to target prostate cancer for scintigraphic imaging using radiolabeled antibodies to prostate-specific membrane antigen (PSMA). PSMA is a 100 kD transmembrane glycoprotein that is overexpressed in poorly differentiated and metastatic prostate carcinoma, and appears to be upregulated following androgen-ablation therapy [7, 8]. The specific role of PSMA in the evolution of prostate cancer itself is not fully understood, but PSMA is recognized to affect tumor angiogenesis in general [9]. The murine monoclonal antibody (MAb) 7E11 recognizes a PSMA epitope located on the cytoplasmic side of the prostate cancer cell membrane; therefore it is able to bind to PSMA after prostate cancer cell apoptosis or necrosis [10–12]. The 7E11.C5.3 antibody was developed using lymph node cancer of the prostate (LNCaP), a cell line that was developed from a heavily pretreated patient with hormone refractory prostate carcinoma. The cell line has been used to characterize 7E11 uptake in many studies [8, 13].
A radiolabeled form of 7E11 MAb (7E11/CYT356), 111In-capromab pendetide (ProstaScint®, Cytogen Corporation, Princeton NJ), has been utilized for several years as a single photon emission computed tomography (SPECT) radionuclide imaging agent to identify sites of residual disease, local recurrence, or metastases in prostate cancer patients. 111In-capromab pendetide binds to sites of disease in soft tissues, lymph nodes, and bone, and has been found to be particularly useful in the exclusion of lymph node metastases when planning definitive therapy, in the assessment of response to primary therapy, in the determination of patterns of relapse or progression, and in the localization of disease foci in cases of other positive indicators of disease (e.g., rising PSA) without evidence of relapse by computed tomography (CT) or bone scan [14, 15]. Given its proven utility in the imaging realm, the potential of this antibody to target prostate cancer for therapy with a radionuclide is evident.
When 111In-capromab pendetide is used for radioimmunoscintigraphy, the bifunctional chelator glycyl-tyrosyl-(N,-diethylenetriaminepentaacetic acid)-lysine hydrochloride (DTPA) is linked to the antibody to chelate the 111In-radiometal. Toward the goal of optimizing the antibody for radioimmunotherapy using lutetium-177 (177Lu), several linker-chelators for 177Lu conjugation with 7E11/CYT356 PSMA have been tested in an attempt to decrease nonspecific uptake, increase the rate of blood clearance, and minimize toxicity to normal tissues. Investigators have reported recently that the 7E11/CYT356 antibody linked to the tetraazacyclododecanetetraacetic acid (DOTA) chelator demonstrates a highly stable chelation [16, 17]. The 177Lu-DOTA-7E11/CYT500 radioimmunoconjugate therefore is expected to provide little nonspecific radiation dose due to secondary loss of the radionuclide from the bifunctional chelator, and is a promising candidate for radioimmunotherapy in targeting small volume prostate cancer metastases.
In order to further explore its potential as a PSMA targeting agent for prostate cancer radioimmunotherapy, the biodistribution of 177Lu-DOTA-7E11/CYT500 (177Lu-7E11) in the LNCaP xenograft mouse model of prostate cancer was evaluated. In addition, the biodistributions and tumor targeting capabilities of 177Lu-DOTA-7E11/CYT500 (177Lu-7E11) and 111In-DTPA-7E11/CYT356 (111In-7E11) were compared to assess their potential as complementary radioimmunoimaging and radioimmunotherapy of prostate cancer.
Materials and Methods
Cell Culture
LNCaP cells transfected with luciferase were supplied by the Contag laboratory at Stanford University. Cells were grown in RPMI 1640, supplemented with 10% fetal calf serum and 1% penicillin-streptomycin, at 37°C. The cells were then trypsinzed, counted, and suspended in Matrigel (BD Bioscience, San Jose, CA) before implantation.
Xenograft Preparation
Male SCID (severe combined immunodeficiency) mice 7–9 weeks old were inoculated subcutaneously in the right flank with ~106 LNCaP cells in a suspension of 0.2 ml Matrigel. The LNCaP cell line was a stable luciferase-transfected cell line allowing bioluminescent imaging to establish viable tumor presence. Sham control mice were prepared by injecting 0.2 ml Matrigel into the right flank at the time of inoculation as test xenograft mice.
7E11 Radiolabeling Procedure
DOTA-7E11/CYT500 and DTPA-7E11/CYT356 were provided by Cytogen Corporation (Princeton, NJ). The DOTA-7E11/CYT500 was labeled with 177Lu (PerkinElmer, Inc., Waltham, MA) to the specific activity of 123 MBq/mg. The DTPA-7E11/CYT356 was radiolabeled with 111In (PerkinElmer, Inc., Waltham, MA) according to the vendor’s procedure. Following labeling, the radioimmunoconjugates were isolated using a centrifugal filter (Microcon YM-3, Millipore, Bilerica, MA). Thin-layer chromatography (TLC) was performed to determine the labeling efficiency.
Biodistribution Studies
Following 8–10 weeks of xenograft growth, viable tumor volume was established using a combination of calliper measurement and bioluminescent imaging. Bioluminescent imaging using IVIS 50 (Xenogen Corporation, Alameda, CA) was performed under isoflurane anesthesia following the administration of luciferin (150 mg/kg body weight) via intraperitoneal (IP) injection. Mice with tumors ranging from 8 mm to 15 mm in diameter (approximately 1 cm3 in volume) were selected for biodistribution studies. 177Lu (40 ± 0.5 MBq) or 111In (28 ±0.34 MBq) conjugated antibodies in approximately 0.2 ml were injected via tail vein. Animal cohorts (n=3 per cohort) were euthanized at 2, 8, 12, 24, 72 hours, and 7 days following radiolabeled MAb injection in 177Lu conjugated studies, and at 8, 24, and 72 hours for studies with 111In-7E11. Control sham mice were injected with 177Lu-7E11 or 111In-7E11 radioimmunoconjugate and euthanized at 8, 24, and 72 hours (n=3 per cohort). Radionuclide imaging was performed using small animal SPECT/CT (FLEX™ X-SPECT™/X-O™, Gamma Medica-Ideas, Inc., Northridge, CA) at each time point immediately followed by euthanasia. Mice were anesthetized with 1.2% isoflurane. SPECT was performed using a 2-mm pinhole collimator (360° of rotation, 64 projections, 15 seconds per projection, and an 82×82 imaging matrix), and volumetric SPECT images were reconstructed using 10 iterations and 8 subsets of an ordered subsets-expectation maximization (OS-EM) reconstruction algorithm. For anatomical localization of SPECT images, x-ray CT data were acquired at 75 kVp and 315 µA, and volumetric CT images were reconstructed in a 512×512×512 format with voxel dimensions of (170 µm)3 using a generalized cone beam Feldkamp algorithm provided by the manufacturer.
Following euthanasia, major organs including heart, lungs, brain, liver, pancreas, kidneys, small intestine, and large intestine were isolated, the tumor was resected, and samples of bone (femur), skin, and muscle were dissected. These samples were weighed and counted with appropriate standards using a calibrated gamma scintillation counter (Wizard 1480, Perkin Elmer, Wellesley, MA) to determine the localization of the specific radiolabeled antibody in each organ. The results of scintillation counting were expressed as percentage of the injected dose per gram of tissue (%ID/g), and were corrected for physical radionuclide decay. To determine differences in the biodistributions of antibodies labeled with two different isotopes (177Lu and 111In), statistical analysis (Student’s t-test) was performed using SPSS 11 software package (SPSS, Inc., Chicago, IL).
Autoradiography
Tumor tissue samples were immediately frozen in crushed dry ice and embedded in tissue medium (O.C.T. 4583, Sakura Finetec, Torrance, CA). Tissue samples were cryosectioned at 20 µm thickness. Sections were mounted on glass coverslips, placed on cardboard, and exposed on a storage phosphor image plate (35×43 cm) for 1 h. The plate then was scanned with a phosphor imaging system (PhosphorImager 445 SI, Molecular Dynamics, Sunnyvale, CA).
Results
177Lu and 111In Radiolabeling
Both 177Lu- and 111In- 7E11 MAb conjugates were labeled with 98% efficiency as determined by the thin layer chromatography.
Mouse xenograft mass, model survival, and tolerance of radioimmunoconjugates
The average tumor mass of all mice included in the biodistribution study was 1056 ± 422 mg in the 177Lu-7E11 group and 2072±478 mg in 111In-7E11 group. One out of 30 (3.3%) LNCaP-bearing SCID mice died prior to completion of the biodistribution study. This event occurred immediately following the injection of 177Lu radiolabeled conjugates, and was believed to be secondary to air embolization.
Biodistribution Studies
The results of all biodistribution studies in xenograft-containing mice are summarized in Figure 1 and Figure 2 and detailed in Table 1 and Table 2.
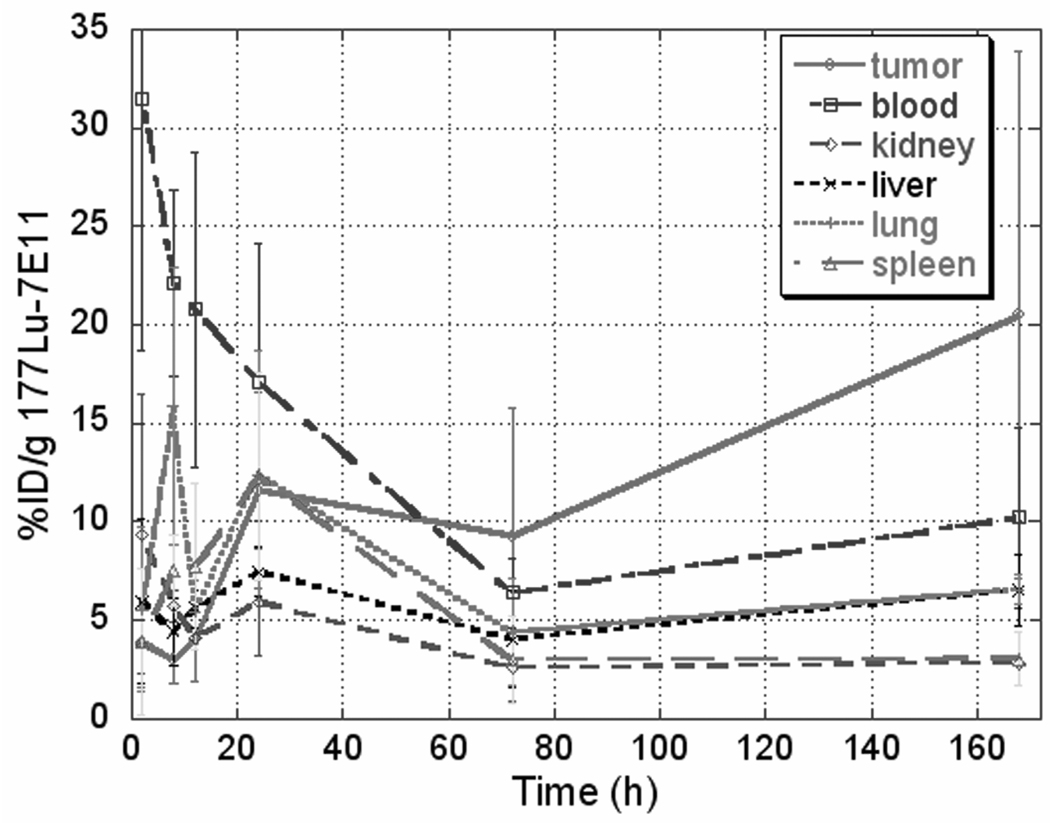
Figure 1.
Summary of pharmacodynamics for 177Lu-7E11 in LNCaP xenograft murine model.
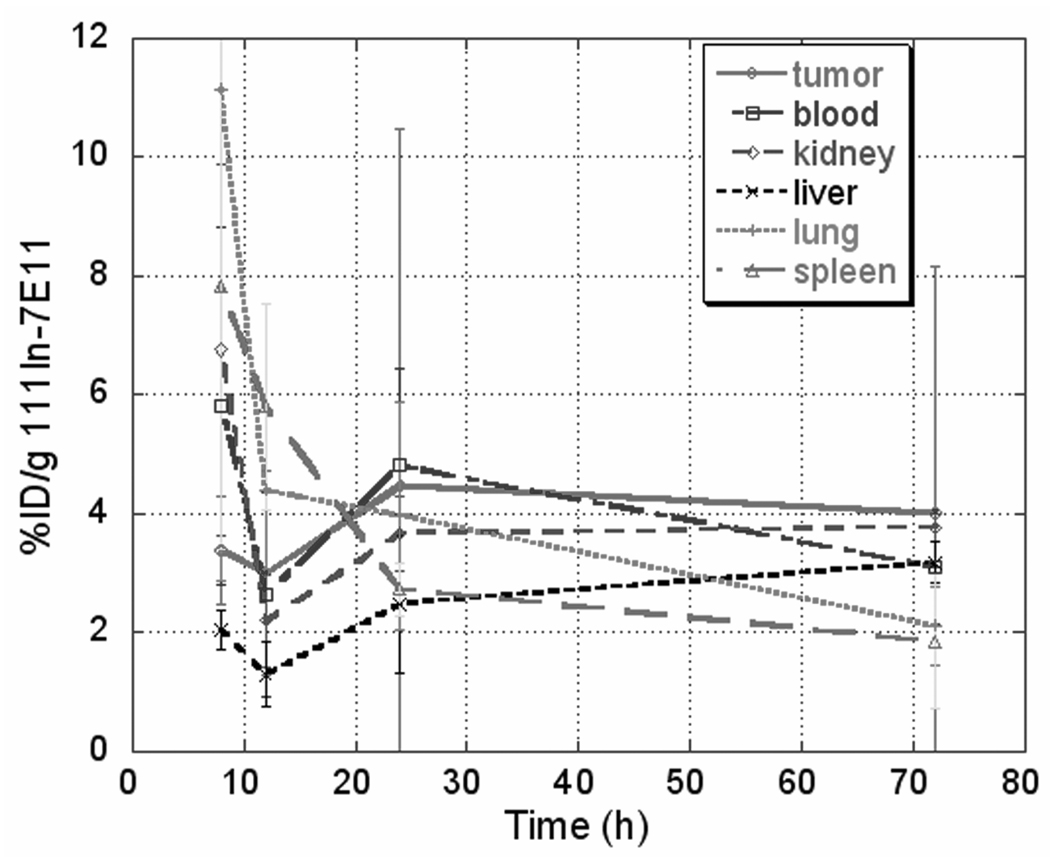
Figure 2.
Summary of pharmacodynamics 111In-7E11 in LNCaP xenograft murine model.
Table 1.
Biodistribution (%ID/g) and tumor uptake of 177Lu-7E11 at 6 time points in LNCaP tumor-bearing SCID mice ( n=3 per group)
| 2 h | 8 h | 12 h | 24 h | 72 h | 7 d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %ID/g | SD | %ID/g | SD | %ID/g | SD | %ID/g | SD | %ID/g | SD | %ID/g | SD | |
| Tumor | 3.86 | 2.26 | 3.01 | 1.26 | 4.01 | 2.14 | 11.6 | 4.98 | 9.29 | 6.49 | 20.5 | 13.4 |
| Blood | 31.5 | 12.8 | 22.1 | 4.70 | 20.8 | 8.01 | 17.1 | 7.06 | 6.38 | 1.76 | 10.2 | 4.59 |
| Kidney | 9.38 | 7.08 | 5.69 | 0.79 | 4.07 | 0.16 | 5.96 | 2.72 | 2.60 | 1.73 | 2.85 | 0.13 |
| Liver | 5.97 | 4.17 | 4.40 | 1.68 | 5.61 | 0.95 | 7.47 | 1.29 | 4.04 | 2.44 | 6.53 | 1.79 |
| Lung | 5.56 | 4.20 | 15.9 | 7.04 | 5.41 | 1.59 | 12.4 | 6.33 | 4.39 | 2.70 | 6.56 | 0.78 |
| Heart | 5.36 | 3.44 | 6.27 | 1.22 | 4.92 | 0.63 | 6.87 | 5.02 | 2.76 | 1.45 | 2.89 | 0.96 |
| Spleen | 3.92 | 3.68 | 7.55 | 1.80 | 7.72 | 4.26 | 12.3 | 5.31 | 3.00 | 2.22 | 3.05 | 1.36 |
| Large intestine | 1.17 | 0.33 | 2.18 | 0.42 | 1.25 | 0.36 | 2.29 | 0.76 | 1.69 | 1.21 | 1.34 | 0.41 |
| Small intestine | 1.76 | 1.45 | 2.25 | 0.73 | 2.07 | 0.99 | 2.95 | 2.13 | 1.29 | 0.22 | 1.20 | 0.34 |
| Bone | 1.36 | 1.47 | 2.07 | 0.36 | 2.13 | 0.42 | 3.55 | 2.36 | 1.25 | 0.05 | 2.33 | 0.95 |
| Muscle | 0.34 | 0.17 | 0.85 | 0.38 | 0.59 | 0.36 | 1.77 | 0.18 | 0.85 | 0.45 | 0.98 | 0.18 |
| Stomach | 0.80 | 0.56 | 2.20 | 0.17 | 1.46 | 0.60 | 1.81 | 0.22 | 0.96 | 0.58 | 1.15 | 0.43 |
| Brain | 0.36 | 0.22 | 0.52 | 0.04 | 0.27 | 0.06 | 0.44 | 0.11 | 0.19 | 0.13 | 0.28 | 0.02 |
| Tumor/blood | 0.09 | 0.05 | 0.13 | 0.03 | 0.20 | 0.12 | 1.17 | 0.82 | 1.76 | 1.34 | 2.36 | 1.66 |
| Tumor/liver | 0.63 | 0.29 | 0.70 | 0.18 | 0.71 | 0.34 | 1.65 | 0.93 | 1.94 | 0.53 | 2.97 | 1.23 |
Table 2.
Biodistribution(%ID/g) and tumor uptake of 111In-7E11 at 4 time points in LNCaP tumor-bearing SCID mice ( n=3 per group)
| 8 h | 12 h | 24 h | 72 h | |||||
|---|---|---|---|---|---|---|---|---|
| %ID/g | SD | %ID/g | SD | %ID/g | SD | %ID/g | SD | |
| Tumor | 3.38 | 0.90 | 3.00 | 1.72 | 4.47 | 6.01 | 4.01 | 4.14 |
| Blood | 5.82 | 3.01 | 2.64 | 1.71 | 4.81 | 1.64 | 3.10 | 0.95 |
| Kidney | 6.76 | 3.12 | 2.20 | 0.77 | 3.67 | 0.64 | 3.77 | 0.22 |
| Liver | 2.05 | 0.32 | 1.30 | 0.54 | 2.48 | 1.15 | 3.18 | 0.35 |
| Lung | 11.14 | 8.26 | 4.39 | 1.32 | 3.97 | 1.92 | 2.11 | 0.65 |
| Heart | 9.60 | 4.45 | 3.85 | 0.49 | 4.28 | 2.82 | 1.21 | 0.17 |
| Spleen | 7.82 | 4.42 | 5.80 | 1.74 | 2.73 | 0.44 | 1.85 | 1.14 |
| Large intestine | 2.56 | 1.49 | 1.48 | 0.35 | 0.98 | 0.28 | 0.62 | 0.18 |
| Small intestine | 2.60 | 1.09 | 2.18 | 0.61 | 1.20 | 0.13 | 0.66 | 0.38 |
| Bone | 0.62 | 0.27 | 1.58 | 0.19 | 1.33 | 0.79 | 0.47 | 0.14 |
| Muscle | 0.51 | 0.25 | 0.94 | 0.14 | 0.74 | 0.43 | 0.43 | 0.01 |
| Stomach | 1.38 | 0.24 | 1.60 | 0.39 | 0.70 | 0.26 | 0.34 | 0.02 |
| Brain | 0.85 | 0.42 | 0.31 | 0.04 | 0.27 | 0.06 | 0.12 | 0.00 |
| Tumor/blood | 0.58 | 1.14 | 0.93 | 1.30 | ||||
| Tumor/liver | 1.65 | 2.32 | 1.80 | 1.26 | ||||
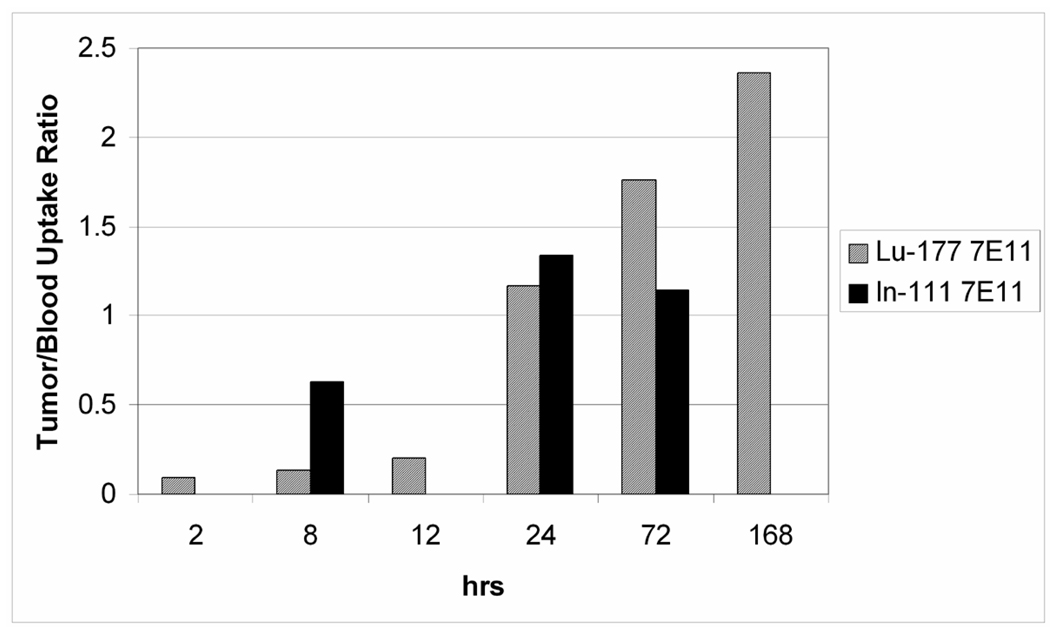
Uptake and retention of the 177Lu-7E11 conjugates within tumor was most prominent after 24 hours, following a blood pool phase in which uptake was dominated by the blood, lung, and liver. Tumor uptake of the radioimmunoconjugates continued for the entire time course studied (7 days), and ratio of uptake within the tumor compared to other organs increased over the 7 day time course following initial administration. Percentage of injected dose per gram (%ID/g) for LNCaP xenograft was 11.6 and 20.5 at 24 h and 7 d, respectively. The tumor to blood ratio (%ID/g / %ID/g) was 0.094 ± 0.051 at 2 h, 1.7± 1.3 at 72 h, and 2.00±1.7 at 7 d following initial injection (Figure 5). The presence of radiolabeled conjugates in the blood pool decreased over time, the uptake in liver remained constant over 7 days.
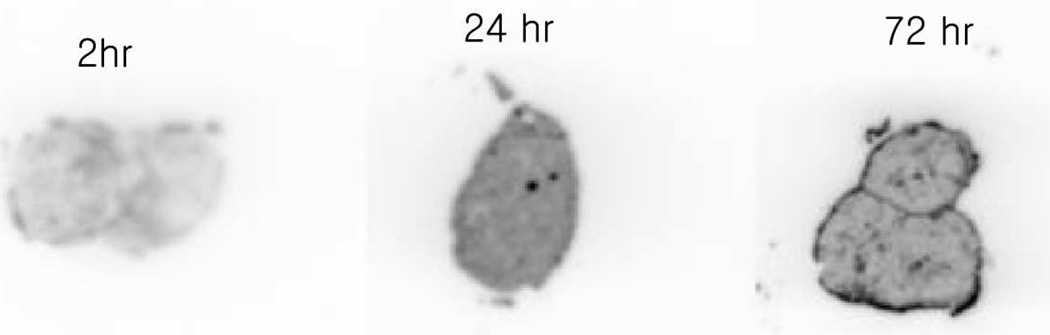
Figure 5.
Representative ex vivo autoradiography demonstrating distribution of 177Lu-7E11 around LNCaP tumor at three time points.
The patterns of 111In-7E11 conjugates in tumor and blood did not differ significantly between mice administered with 177Lu-7E11 versus 111In-7E11; however uptakes (%ID/g) of 111In-7E11 in tumor and blood were less than those of 177Lu-7E11. Total accumulation in tumor using 177Lu-7E11 was notably higher than 111In-7E11 conjugates 24 hours postinjection. 177Lu-7E11 and 111In-7E11 %ID/g were 11.6 and 4.5 at 24 hours, and 9.3 and 4.0 at 72 hours, respectively. The uptake in tumor reached a plateau after 24 hours; whereas the uptake in other organs (lung, blood, kidney and spleen) had initial prominent retention which decreased with time. Hepatic uptake, unlike other organs, continued to rise after 72 hours. Uptake of 111In-7E11 was significantly lower (p<0.05) than that of 177Lu-7E11 studies at all time points. This result was likely due to the DOTA chelator for 177Lu-7E11 which could have resulted in little nonspecific radiation dose without secondary loss of the radionuclide from the bifunctional DTPA chelator used for 111In-7E11. In addition, although the initial tumor sizes for both groups were similar, tumor sizes for the 177Lu-7E11 group were relatively smaller (p<0.15) at the time points since 24 h, which could have resulted in higher %ID/g in tumors for the 177Lu-7E11 group. This tumor size change could be due to the therapeutic radiation dose from 177Lu.
177Lu-7E11 results from sham xenograft control mice did not demonstrate measurable uptake of the radioimmunoconjugates in the region of the sham tumor inoculation over that of background soft tissue (Table 3). Compared to the tumor-bearing groups at matched time points, the %ID/g uptake and retention of radioconjugated MAb were increased in the blood and liver at 8 and 24 hours following administration (p<0.05) with a 2 to 3-fold increase in all major organs except lung and heart at 72 hours after injection (p<0.05) (Table 4). Results for the 111In-7E11 biodistribution studies in sham xenograft control mice were similar.
Table 3.
Biodistribution(%ID/g) and tumor uptake of 177Lu-7E11 at 3 time points in control (sham inoculated) SCID mice ( n=3 per group)
| 8 h | 24 h | 72 h | ||||
|---|---|---|---|---|---|---|
| %ID/g | SD | %ID/g | SD | %ID/g | SD | |
| blood | 8.57 | 2.37 | 8.42 | 0.08 | 3.51 | 0.29 |
| kidney | 1.87 | 0.34 | 1.42 | 0.16 | 1.49 | 1.35 |
| liver | 4.03 | 1.95 | 6.09 | 1.23 | 5.84 | 5.84 |
| lung | 1.26 | 0.59 | 1.53 | 0.26 | 0.94 | 0.93 |
| heart | 0.58 | 0.11 | 0.77 | 0.78 | 0.94 | 1.17 |
| spleen | 0.16 | 0.04 | 0.50 | 0.65 | 0.20 | 0.28 |
| large intestine | 0.41 | 0.22 | 0.84 | 0.99 | 1.62 | 2.15 |
| small intestine | 1.62 | 1.03 | 0.73 | 0.42 | 1.99 | 2.25 |
| bone | 0.10 | 0.08 | 0.10 | 0.08 | 0.03 | 0.03 |
| muscle | 0.11 | 0.07 | 0.23 | 0.12 | 0.20 | 0.15 |
| stomach | 0.41 | 0.04 | 0.70 | 0.66 | 0.17 | 0.10 |
| brain | 0.19 | 0.02 | 0.13 | 0.02 | 0.13 | 0.13 |
Table 4.
Ratio of 177Lu-7E11 uptake of nontumor bearing group (sham xenografts) to tumor bearing group (LNCaP xenografts)
| 8 h | 24 h | 72 h | |
|---|---|---|---|
| blood | 1.66 | 1.73 | 3.86 |
| kidney | 1.00 | 1.06 | 2.11 |
| Liver | 2.07 | 1.65 | 2.83 |
| Lung | 1.04 | 0.34 | 1.92 |
| heart | 1.55 | 1.06 | 1.82 |
| spleen | 0.96 | 0.78 | 3.60 |
| large intestine | 0.98 | 1.55 | 2.32 |
| small intestine | 1.58 | 1.31 | 2.75 |
| brain | 1.13 | 1.25 | 2.33 |
Bioluminescence Imaging
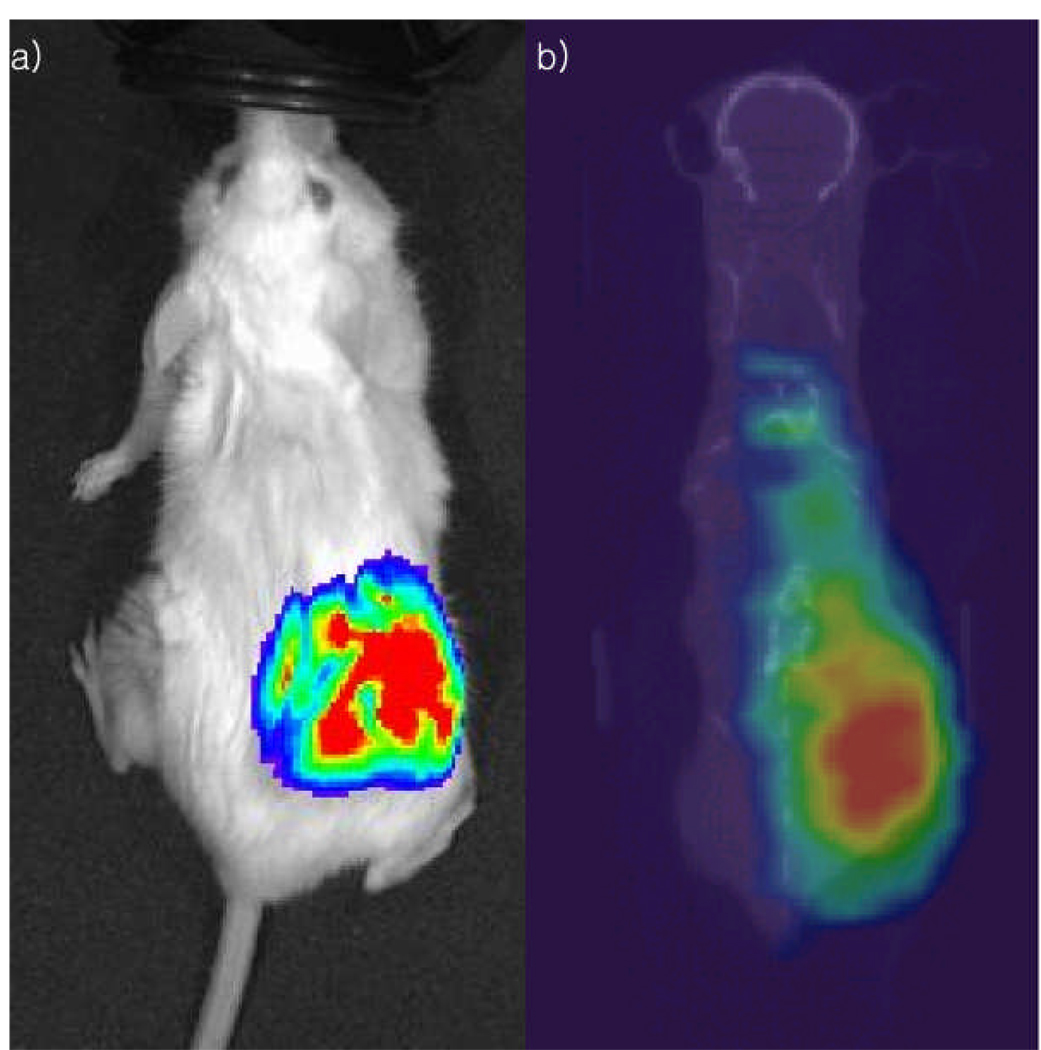
Bioluminescence imaging performed prior to administration of the radioimmunoconjugates demonstrated presence of viable LNCaP tumor in all mice studied. SPECT imaging of 177Lu-7E11 and 111In-7E11 confirmed the ex vivo biodistribution studies, and demonstrated a high tumor-to-background ratio with tumor uptake easily distinguishable from uptake in surrounding soft tissues on SPECT images at the 24 h time point and beyond. Representative bioluminescence and SPECT images of 177Lu-7E11 distribution at 24 h after initial radiopharmaceutical administration from the same animal are shown in Figure 5.
Autoradiography
Representative autoradiography images of 177Lu-7E11 tumor localization at 2, 24, and 72 h are shown in Figure 5. At later time points, signs of necrosis were shown particularly in the autoradiograph at 72 h. Nevertheless, within the tumor boundaries, 7E11 MAb distribution was homogeneous within all tumors.
Discussion
The history of the use of 111In-7E11 as an FDA-approved imaging agent (capromab pendetide), its retained targeting ability during concurrent hormonal therapy, and the recent success and demonstrated stability of the conjugation of 7E11 with DOTA support the potential use of 177Lu-7E11 as a radioimmunotherapeutic for prostate cancer.
The 7E11 PSMA antibody has been constantly compared with other PSMA antibodies, most notably J591. The major difference between 7E11 and J591 is that 7E11 has been shown to target an epitope on the intracellular portion of PSMA, while J591 targets an external membrane PSMA epitope [18]. It has been suggested that the external membrane PSMA epitope should be available for binding on viable prostate cancer cells; whereas the internal epitope, targeted by the 7E11, is only available once cells have undergone necrosis or apoptosis. PSMA is expressed on the apical surface of the prostate cancer epithelium where tight junctions actually impede accessibility of intravenously-injected agents to the apical side of the epithelial cell, so even antibodies to the extracellular surface would probably not be able to reach the intact tissues of viable well-differentiated prostate cancer cells [19]. This suggests that 7E11 is not necessarily at a true disadvantage when compared to antibodies targeting the external epitope of PSMA when used as potential radiotherapeutics for prostate cancer. However, as suggested by other authors, there may be a need for enhanced vascular permeability and intraepithelial transport of any of these agents, an area that will require further research and innovation [19].
In addition, the choice of 177Lu as a radiotherapeutic agent for the 7E11 PSMA antibody is well compared with the current Phase I clinical trial of J591 antibody. The clinical trials of J591 antibodies radiolabeled with either yttrium-90 (90Y) or lutetium-177 (177Lu) have been performed to assess toxicity and initial potential to treat large (28 to 42 mm diameter) and small (1.2 to 3 mm diameter) prostate tumor sites [20, 21]. These trials have demonstrated a high potential for delivering multiple doses of radiation to prostate cancer with acceptable toxicity. Due to the long range of their β− particle, 90Y-labeled agents provide a greater radiation dose to tumors with larger diameters. 177Lu-labeled agents emit a shorter range β− particle enabling high radiation doses to tumors of smaller diameters with minimal radiation to nearby structures. The choice of 177Lu as the therapeutic radionuclide in the present study represents a decision to focus on smaller foci of prostate cancer present within lymph nodes or other small metastases [22]. Our choice of 177Lu as a radioimmunotherapy agent for the 7E11 antibody lies in the same rationale. Besides, because of gamma emission from 177Lu, 177Lu-labeled agents can be imaged in its own right, and produce generally good images comparable to even imaging-only 111In-labeled agents due to less septal penetration of gamma camera collimators shown in our studies presented in this paper.
7E11 labeled with 111In has been used for targeted prostate cancer imaging with SPECT for several years, but this study represents the first published report of investigation into its suitability for radioimmunotherapy when labeled with 177Lu. Using the LNCaP xenograft mouse model, this study investigated the biodistribution of 177Lu-labeled 7E11 over a 7 day period. The presented findings indicate that 177Lu-7E11 localizes within LNCaP PSMA-expressing tumors. The tumor-to-background uptake of 177Lu-7E11 was observed to increase over a 7 day period and its uptake was measured to be 20.5% ±13.3%ID/g at 7 days following administration. This high fractional uptake suggests the potential radioimmunotherapeutic utility of this agent. However, there were also relatively high blood values for all the time points, which may have contributed also high fractional tumor uptake of 177Lu-7E11 at later time points. This prolonged blood uptake values may underestimate the potential efficacy of the 177Lu-7E11 for tumor sites, and may limit the administration dose for radioimmunotherapy because of high bone marrow exposure from the blood uptake. For this reason, a careful dosimetry study of 177Lu-7E11 will be needed.
This study also investigated the similarities and differences in the biodistributions of 111In- and 177Lu-labeled 7E11 PSMA antibody. The extensive experience with 111In-7E11 in Nuclear Medicine community suggests that this diagnostic agent could provide pretherapeutic dosimetry estimates for 177Lu-7E11 therapy. 111In-7E11 is recognized to present some challenges when used alone as imaging agent. These challenges include high nonspecific uptake in background soft tissues, and long tumor uptake times. Nevertheless, 111In-7E11 has been successfully implemented as a prostate cancer imaging tool at many healthcare centers. Furthermore, the use of combined dual-modality SPECT/CT imaging has potential of increased sensitivity of radioimmunoscintigraphy in general, and the addition of corrections for photon attenuation and geometric blurs caused by radionuclide collimator has shown the increase of the tumor target-to-background ratio of 111In-capromab pendetide imaging [23]. As a result, the overall sophistication of PSMA immunoscintigraphy with 111In-capromab pendetide has increased significantly over the past few years, and it would be optimal to capture that experience by utilizing 111In-7E11 for pretherapeutic imaging. Thus, the comparison study of the biodistributions of 111In-7E11 and 177Lu-7E11, presented in this paper, is the first step in determining the potential role of 111In-7E11 as a pretherapeutic imaging agent. The present study demonstrates the feasibility to apply 111In-7E11 as a pretherapeutic agent to predict uptake of the radioimmunotherapeutic in tumor and major organs, except liver. In order to determine whether 111In-7E11 reflects the anticipated uptake of 177Lu-7E11 accurately, further studies are required to determine whether their uptake is proportional within the same animal and tumor focus. One of the future studies needs to focus on 111In-7E11 with the DOTA chelator that is used in 177Lu-7E11 so that there is little variability of chelation of 7E11 antibody with radiometals (111In and 177Lu) to potentially minimize the differences between biodistributions of these two agents.
Conclusions
Biodistribution studies of 177Lu-7E11 in LNCaP xenograft mouse models support its potential application as a radioimmunotherapeutic for prostate cancer by demonstrating its accumulation in prostate cancer xenografts. Companion studies of 111In-7E11 (DTPA) versus 177Lu-7E11 (DOTA) biodistributions support its potential as a pretherapy imaging agent to predict the potential uptake of the 177Lu-7E11 agent within prostate tumor. The gamma emission from the 177Lu-7E11 radiotherapeutic may also be used for posttreatment dosimetry studies and comparison to pretherapy 111In-7E11 imaging. To further validate these points, biodistribution studies with a larger number of animals and the same DOTA chelators for 111In-7E11 and 177Lu-7E11 would be desirable.
Figure 3.
Graphical representation of tumor-to-blood uptake ratio of 177Lu-7E11 and 111In-7E11 over time (%ID/g).
Figure 4.
a) In vivo Bioluminescence image demonstrating location of luciferase-transfected LNCaP tumor and b) representative coronal image from in vivo SPECT imaging 24 hours following 177Lu-7E11 (40 MBq) administration.
Acknowledgements
Research Funding and 7E11 antibody were provided by the Cytogen Corporation for this project. LNCaP cells transfected with luciferase were kindly supplied by the Contag laboratory at Stanford University. The authors also acknowledge support from NIH Grant 5R01EB000348 and 5K25CA114254.
References
- 1.Swanson GP, Thompson IM, Basler J. Current status of lymph node-positive prostate cancer: Incidence and predictors of outcome. Cancer. 2006;107:439–450. doi: 10.1002/cncr.22034. [DOI] [PubMed] [Google Scholar]
- 2.Swanson GP, Thompson IM, Basler J. Treatment options in lymph node-positive prostate cancer. Cancer. 2006;106:2531–2539. doi: 10.1002/cncr.21947. [DOI] [PubMed] [Google Scholar]
- 3.Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993;71:1098–1109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 5.Russell PJ, Hewish D, Carter T, et al. Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivo studies. Cancer Immunol Immunother. 2004;53:411–421. doi: 10.1007/s00262-003-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, Bander NH. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237–5243. [PubMed] [Google Scholar]
- 7.Wright GL, Jr, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 8.Fair WR, Israeli RS, Heston WD. Prostate-specific membrane antigen. Prostate. 1997;32:140–148. doi: 10.1002/(sici)1097-0045(19970701)32:2<140::aid-pros9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troyer JK, Beckett ML, Wright GL., Jr Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62:552–558. doi: 10.1002/ijc.2910620511. [DOI] [PubMed] [Google Scholar]
- 11.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30:232–242. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 13.Barren RJ, 3rd, Holmes EH, Boynton AL, Misrock SL, Murphy GP. Monoclonal antibody 7E11.C5 staining of viable LNCaP cells. Prostate. 1997;30:65–68. doi: 10.1002/(sici)1097-0045(19970101)30:1<65::aid-pros10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 14.Elgamal AA, Troychak MJ, Murphy GP. ProstaScint scan may enhance identification of prostate cancer recurrences after prostatectomy, radiation, or hormone therapy: analysis of 136 scans of 100 patients. Prostate. 1998;37:261–269. doi: 10.1002/(sici)1097-0045(19981201)37:4<261::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Feneley MR, Jan H, Granowska M, et al. Imaging with prostate-specific membrane antigen (PSMA) in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:47–52. doi: 10.1038/sj.pcan.4500390. [DOI] [PubMed] [Google Scholar]
- 16.Tedesco J, Goeckeler W, Becker M, Frank K, Gulyas G, Young S. Development of Op-timal Lu-177 Labeled Monoclonal Antibody (7E11) Construct (CYT-500) for Radioimmunotherapy of Hormone Refractory Prostate Cancer. J Clin Oncol. 2005;23(16S):4765. [Google Scholar]
- 17.Tedesco J, Wolodzco J, Goeckeler W, Becker M, Hasegawa B, Franc B. Development and characterization of a novel 177Lu-MeO-DOTA-7E11 antibody construct (CYT-500) for the treatment and imaging of prostate adenocarcinoma. AACR Annual Meeting. 2006 [Google Scholar]
- 18.Nargund V, Al Hashmi D, Kumar P, et al. Imaging with radiolabelled monoclonal antibody (MUJ591) to prostate-specific membrane antigen in staging of clinically localized prostatic carcinoma: comparison with clinical, surgical and histological staging. BJU Int. 2005;95:1232–1236. doi: 10.1111/j.1464-410X.2005.05511.x. [DOI] [PubMed] [Google Scholar]
- 19.Christiansen JJ, Rajasekaran SA, Inge L, Cheng L, Anilkumar G, Bander NH, Rajasekaran AK. N-glycosylation and microtubule integrity are involved in apical targeting of prostate-specific membrane antigen: implications for immunotherapy. Mol Cancer Ther. 2005;4:704–714. doi: 10.1158/1535-7163.MCT-04-0171. [DOI] [PubMed] [Google Scholar]
- 20.Bander NH, Trabulsi EJ, Kostakoglu L, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170:1717–1721. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 21.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J Clin Oncol. 2004;22:2522–2531. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–1909. [PubMed] [Google Scholar]
- 23.Seo Y, Wong KH, Sun M, Franc BL, Hawkins RA, Hasegawa BH. Correction of photon attenuation and collimator response for a body-contouring SPECT/CT imaging system. J Nucl Med. 2005;46:868–877. [PubMed] [Google Scholar]