Abstract
Plasmodium sporozoites are the product of a complex developmental process in the mosquito vector and are destined to infect the mammalian liver. Attention has been drawn to the mosquito stages and preerythrocytic stages owing to recognition that these are bottlenecks in the parasite life cycle and that intervention at these stages can block transmission and prevent infection. Parasite progression in the Anopheles mosquito, sporozoite transmission to the mammalian host by mosquito bite, and subsequent infection of the liver are characterized by extensive migration of invasive stages, cell invasion, and developmental changes. Preparation for the liver phase in the mammalian host begins in the mosquito with an extensive reprogramming of the sporozoite to support efficient infection and survival. Here, we discuss what is known about the molecular and cellular basis of the developmental progression of parasites and their interactions with host tissues in the mosquito and during the early phase of mammalian infection.
INTRODUCTION
The malaria parasite life cycle constitutes one of the most complicated and fascinating life cycles of any organism and thus poses intriguing areas of study for cell biology, molecular biology, and immunology alike. Malaria as a disease is devastating developing countries, especially those in sub-Saharan Africa, causing approximately one million deaths each year, which are mainly attributable to a single parasite species, Plasmodium falciparum (46, 47). The intricacy of malaria parasite biology has vexed vaccinologists and immunologists for nearly a century and is a major impediment to the development of a fully protective vaccine. A major part of the complexity associated with the malaria parasite life cycle is due to the parasite’s ability to (a) change its cellular and molecular makeup, which is controlled by a genome with more than 5000 recognized genes, and (b) develop in intracellular and extracellular niches in the mammalian host and the mosquito vector.
The continuous host habitat changes are always associated with severe losses for the malaria parasite, leading to great fluctuations in population densities. This is due mostly to the action of host defense mechanisms initiated upon infection (115). However, the malaria parasite effectively compensates for losses by growth and replication in cellular niches hidden away from the host’s immune responses (36). Parasite stages that suffer such severe losses are the ookinete and the sporozoite, both of which form and migrate within the insect vector (50, 157). The ookinete develops from a zygote in the bloodfed mosquito midgut lumen, a product of fertilization of a female macrogamete by a male microgamete. Notably, the ookinete is the only invasive stage that is not preceded by a replication step, and thus ookinete numbers are a direct product of the number of fertilization events. The ookinete starts its short journey by traversing the midgut epithelial cell layer from the apical side and then egresses from the basal end to reach the basal lamina. This invasion step is accompanied by a severe reduction in ookinete numbers due to the intervention of host protective mechanisms (50). The surviving ookinetes become sessile and transform into oocysts. The oocyst is the only parasite developmental stage that grows extracellularly and results in the formation of sporozoites. Sporozoites are released into the mosquito body cavity and invade the salivary glands, and they suffer severe losses on this journey (51). The ookinete and the sporozoite are thus bottleneck stages in the malaria parasite life cycle. Sporozoites are transmitted during the next mosquito blood meal and initiate liver infection in the mammalian host. Liver infection does not result in overt pathology but leads to a 10,000- fold amplification of parasite numbers, culminating in the release of exoerythrocytic merozoites into the bloodstream, which in turn infect erythrocytes to initiate the pathogenic erythrocytic cycle (152).
The complexity of parasite development in the mosquito is but one reason why the mode of malaria transmission to humans remained unknown up to the end of the nineteenth century. After discovery of mosquito transmission of malaria, further studies on how malaria parasites infect the mammalian host had low priority because of Fritz Schaudinn’s (104) infamously erroneous observation in 1903 of a sporozoite directly infecting an erythrocyte. This blunder stymied further work on the initial infection in the mammalian host. However, Shortt & Garnham (108) discovered in 1948 that sporozoites infect hepatocytes, where they develop into liver stages before infection of erythrocytes. Attention was further redrawn to mosquito stages after the seminal discovery in 1967 that immunization with irradiation attenuated mouse malaria parasite sporozoites conferred long-lasting protection against infectious sporozoite challenge (93). Furthermore, vaccination with irradiated P. falciparum sporozoites in human volunteers conferred long lasting protection against malaria infection (21).
Nevertheless, the pre-erythrocytic stages have been least permissive to study owing to the scarcity and difficulty of isolating mosquito stages and liver stages without contaminating host material. Recent studies have overcome these technical difficulties and have obtained gene expression and proteomic data sets for mosquito stages and pre-erythrocytic stages (49, 61, 70, 79, 84, 131). Furthermore, the advancements of gene manipulation technologies (137) and in vivo imaging approaches (7) have enabled the dissection of the various molecular and cellular mechanisms used by the sporozoite en route to the liver. Altogether, this has led to the identification of numerous genes (Table 1) that have essential roles through- out mosquito stages and pre-erythrocytic stage developmental progression, as well as new features of this progression. The discovery of genes that are essential for liver stage development and that, when deleted by genetic manipulation render sporozoites attenuated, have reinvigorated live-attenuated malaria vaccine efforts and reinforced the notion that studies of basic parasite biology inform the development of potential interventional tools for malaria (82). In this review, we shed light on the biology of developmental progression of mosquito stages and pre-erythrocytic stages within host tissues during their journey from the mosquito midgut to the mammalian liver.
Table 1.
Overview of sporozoite proteins characterized through reverse genetics techniques
| Gene | Locationa | Targeting signals | Domains | Gene knockout phenotype | Likely function |
|---|---|---|---|---|---|
| AMA1 | Micronemes | SP/TM | Hypotheticalf | Refractory to deletion | Erythrocyte invasion and possibly hepatocyte invasion |
| CelTOS/S4 | Micronemes | SP | Hypothetical | Defect in ookinete midgut traversal/sporozoite transmission to the liver | Ookinete/sporozoite cell traversal |
| CSP | Surface | SP/GPI | TSRd | Lack of sporozoite formation | Multiple essential functions throughout preerythrocytic stage development |
| P52/P36p | Micronemes | SP/GPI | 6-Cysteine | Defect in early LS development | Hepatocyte invasion/PVM formation? |
| P36 | Secretory organelles | SP | 6-Cysteine | Defect in LS development | Hepatocyte invasion/PVM formation? |
| PL/UIS10 | Secretory organelles | SP | LCAT | Partial defect in sporozoite transmission to the liver | Sporozoite cell traversal |
| SAP1 | Cytoplasm | None | Hypothetical | Blockage of LS development | Regulation of liver-infectivity-associated gene expression |
| SPECT1 | Micronemes | SP | Hypothetical | Defect in Kupffer cell traversal/skin traversal | Sporozoite cell traversal |
| SPECT2/PLP1 | Micronemes | SP/TM | MACPF | Defect in Kupffer cell traversal/skin traversal | Sporozoite cell traversal |
| TLP | Secretory organelles | SP/TM | TSR and A-domain | Partial defect in sporozoite transmission to the liver | Sporozoite cell traversal |
| TRAP | Micronemes | SP/TM | TSR and A-domaine | Blockage of salivary gland invasion | Sporozoite motility, salivary gland invasion, and hepatocyte invasion |
| TRSP | Secretory organelles | SP/TM | TSR | Partial defect in hepatocyte invasion | Hepatocyte invasion |
| UIS3 | Secretory organelles/PVM | SP/TM | Hypothetical | Arrest of LS development | Unidentified essential function in LS growth/likely fatty acid uptake |
| UIS4 | Secretory organelles/PVM | SP/TM | Hypothetical | Arrest of LS development | Unidentified essential function in LS growth |
| UOS3/S6/TREP | Micronemes | SP/TM | TSR | Blockage of salivary gland invasion | Sporozoite motility/salivary gland invasion |
Putative subcellular localization based on available data
(in the abbreviations list)
(in the abbreviations list)
No known functional domain identified
MOSQUITO STAGE DEVELOPMENT AND GENERATION OF SPOROZOITES
Gametogenesis and Ookinete Formation
The Plasmodium parasite undergoes sexual reproduction once during a life cycle, and this occurs in the mosquito (Figure 1). Its genome is haploid, except briefly after fertilization. The factors that initiate and regulate the formation of gametocytes are not yet well understood (67). Ingestion of gametocytes by a mosquito during a blood meal activates the formation of gametes (gametogenesis) in the mosquito midgut lumen. A mosquito-derived molecule, xanthurenic acid, in addition to temperature shift and pH change, can trigger male gametogenesis in the form of exflagellation (14, 15). P48/45 is a male-gamete-specific surface protein belonging to the 6-cysteine repeat protein family. Using a genetic deletion analysis study, van Dijk et al. (144) showed that P48/45 is essential for the male gamete’s ability to fertilize a female gamete in P. berghei. A paralog of P48/45, named P47, is exclusively expressed on the surface of female gametes. However, in contrast to P48/45, genetic deletion of P47 did not have any impact on female gamete fertilization by a male gamete (146). Another member of the 6-cysteine repeat protein family is P230, which is expressed on the surface of male and female gametes and is recognized as a candidate for malaria transmission blocking strategies (33). Knockout studies of P230 in P. falciparum revealed an important but unidentified function during gamete fertilization in the mosquito midgut (32).
Figure 1.
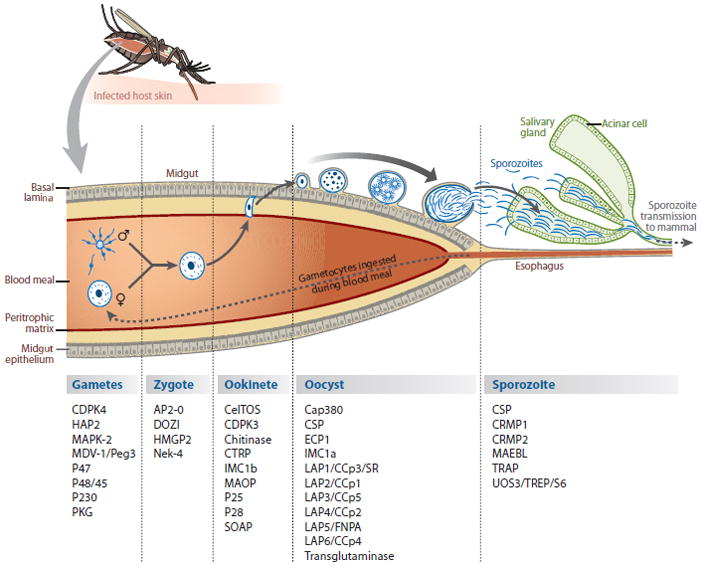
Another gene associated with gamete formation appears to have different functions when comparing P. falciparum with P. berghei. The male development gene-1 (MDV-1) (also called Peg3) is expressed in intracellular vesicles in both male and female gametes but is later localized to the surface of developing ookinetes (121, 40, 69). Deletion of MDV-1 from P. falciparum suggested a role in male gamete activation (40). In P. berghei, however, targeted deletion of MDV-1 revealed a function during female gametocyte activation and macrogamete development (69).
Plasmodium calcium-dependent kinase 4 (CDPK4) and mitogen-activated protein kinase 2 (MAPK-2) have essential functions for mature male gamete development in P. berghei (13, 101, 134). In contrast to the rodent model data, MAPK-2 is not essential for P. falciparum gametogenesis; however, it is essential for asexual blood stage growth (31). Moreover, in P. falciparum a cGMP-dependent protein kinase (PKG) is essential for xanthurenic acid–mediated activation of male gamete exflagellation in the mosquito midgut (80). Collectively, these findings show that malaria parasite protein kinases are essential in multistep functions during the signaling cascade that controls male gametogenesis (30). In addition, a Plasmodium protein with homology to the plant sterility factor HAP2 has an essential function for the gamete membrane fusion in Plasmodium. This mechanism is conserved across phyla because it also occurs in the green alga Chlamydomonas minus (74). Targeted disruption of HAP2 in P. berghei generated parasites that formed mature gametocytes and gametes but failed to fertilize and form zygotes. Moreover, HAP2 localizes to the gamete fusion site and is essential for gamete membrane fusion in C. minus (74). The zygote inherits the maternal gamete plasma membrane, cytoplasm, and intracellular organelles. Translational repression in female gametocytes regulates expression of gene products needed later during zygote formation and development. Genetic deletion of the putative RNA helicase DOZI (development of zygote inhibited) directed about 370 translationally repressed transcripts for degradation, which had a severe negative effect on the production of zygotes (76). Another study pointed to an additional Plasmodium factor, high mobility group protein 2 (HMGP2), as critical for the expression of 35 genes, which in turn are important for completion of the sexual cycle of Plasmodium in the mosquito and for oocyst development (44). Although, the authors of this study did not reveal how, when, and where exactly HMGP2 functions, it is predicted that HMGP2 is involved in activating the transcription of gene products in gametocytes that will be functional later during zygote and ookinete formation.
Because sequencing of the Plasmodium genome revealed an apparent paucity of known transcription factors (9, 42) but the presence of many of the posttranscriptional regulation factors found in other eukaryotes (107), it was suggested that regulation of gene expression in Plasmodium is mainly accomplished at the posttranscriptional and translational levels. However, more recently, a bioinformatics approach led to the identification of Apetala2 (AP2) plantlike transcription factors in Plasmodium and revealed that transcriptional regulation might be utilized significantly by the parasite (10). Indeed, a groundbreaking study demonstrated that Plasmodium AP2-like domains bind DNA (25). Another recent elegant study by Yuda et al. (171) showed that one member of the AP2 family, AP2-O, has an essential role in ookinete development and ookinete invasive function by regulating expression of a specific set of genes. Targeted deletion of AP2-O in P. berghei generated mutant ap2-o-parasites that were capable of fertilization and early ookinete development but failed to produce mature functional ookinetes. The Plasmodium AP2 family binds specific conserved DNA sequences upstream of its target genes (25). Therefore, the mechanism of AP2-O function is predicted to be the transcriptional activation of a specific subset of genes through binding to upstream cis-regulatory elements.
Ookinete Invasion of the Midgut Epithelium
Soon after zygote formation, meiosis is executed and genetic recombination occurs. The spherical zygote transforms into an ookinete (Figure 1). A NIMA (never in mitosis/Aspergillus)-related protein kinase (Nek-4) is crucial for the development of ookinetes from zygotes (102). The ookinete is an elongated motile cell that possesses secretory organelles called micronemes that contain proteins involved in motility, tissue traversal, and invasion. The ookinete uses motility to leave the blood meal bolus and to penetrate the peritrophic matrix that encloses the blood meal. In P. falciparum and P. gallinaceum, but not in P. berghei, the parasite enzyme chitinase seems to be critical for the ookinete to cross the peritrophic matrix layer (27, 154, 155). The micronemal proteins CDPK3 (calcium dependent protein kinase 3) (54, 110) and CTRP (circumsporozoite and TRAP-related protein) (26, 172) are involved in motility and infectivity of the ookinete. Targeted disruption of CTRP drastically reduced the gliding motility of ctrp– ookinetes and abolished the midgut epithelium infectivity, and no oocysts were produced in vivo (26). Similarly, targeted disruption of CDPK3 generated mutant ookinetes that were immotile and failed to invade the midgut epithelium in vivo (54, 110). After breaching the peritrophic matrix, the ookinete then penetrates the apical end of the mosquito midgut epithelium. A candidate for initial host cell membrane disruption and penetration by the ookinete is a micronemal protein with a perforin-like membrane attack complex domain called the membrane attack ookinete protein (MAOP) (56). CTRP is also thought to play an essential role in this process (73). The ookinete traverses a number of epithelial cells before exiting through the basal side of the epithelium. This phenomenon is called cell traversal, and it is also displayed by salivary gland sporozoites in the skin and in the liver sinusoid. The secreted micronemal protein CelTOS (cell traversal protein for ookinetes and sporozoites) plays an important role but is not essential for ookinete traversal through midgut epithelial cells, presumably similar to the traversal of the sporozoite through hepatocytes (63). Transmigration through epithelial cells activates the ookinete to switch from cell traversal mode to sessile mode. The ookinetes’ exit at the basal side of the epithelium marks the end point of migration and initiates its transformation into an oocyst.
Ookinete to Oocyst Transformation
Ookinetes injected into the hemocoel of mosquitoes (161, 164) or even the hemocoel of Drosophila melanogaster (105) can attach to the midgut wall and transform into oocysts that subsequently develop into sporozoites. Ookinetes can also transform in vitro into oocysts and develop sporozoites in the presence of matrigel, which includes a laminin-like substrate (3). The basal lamina, which consists mainly of laminin and collagen, covers the entire coelomic cavity of the mosquito, including the midgut. Mainly laminin, probably in association with other host factors in the basal lamina, induces the transformation from a moving ookinete to a sessile oocyst (2). It is thought that the laminin-mediated transformation is triggered by parasite ligands on the surface of the ookinete and the major glycosylphosphatidylinositol (GPI)-anchored ookinete surface proteins P25 and P28 bind and interact with laminin to participate in ookinete transformation (156). Yet, the action of both proteins is probably redundant (139) because generation of single-gene deletions showed only a mild negative effect on ookinete to oocyst transformation, whereas double-gene deletion mutants showed a severe defect not only in ookinete to oocyst transformation but also in midgut epithelium invasion (139). Other ookinete surface proteins that possibly participate in the process of ookinete to oocyst transformation are two proteins with adhesive domains, SOAP (secreted ookinete adhesive protein) (28) and the aforementioned CTRP (73, 75). P. berghei SOAP interacts with Anopheles laminin in a yeast two-hybrid assay (28). Targeted deletion of SOAP generated mutant ookinetes that were impaired in their ability to invade midgut epithelium and transform into oocysts. In addition to its function during the invasion of midgut epithelium, CTRP binds to Anopheles laminin (75). Interestingly, CTRP and SOAP are not important for ookinete to oocyst transformation in the in vitro cell free system (91).
Oocyst Development and Sporozoite Differentiation
The fate of the malaria parasite in the mosquito and its transmission to the next host are dependent on the few ookinetes that cross the midgut epithelial barrier and develop into oocysts (Figure 1). Defects in gametogenesis, fertilization, ookinete formation, ookinete motility, and cell traversal as well as oocyst development all lead to a reduction in the number of mature oocysts. Oocyst development is relatively long, approximately 10–12 days. The oocyst is the only extracellular developmental stage of the malaria parasite life cycle. Life in an extracellular environment presents distinct challenges, but little is known about how the oocyst interacts with its host.
LAPs (LCCL/lectin adhesive-like proteins), which are expressed only in female gametocytes and ookinetes, play a decisive role in sporozoite formation several days after the oocyst develops and grows in size (17, 20, 71, 97, 99, 141). After the transformation of the ookinete into an oocyst, mitotic divisions take place. A hallmark of Plasmodium replicating stages is the clear temporal separation of karyokinesis from cytokinesis. For the small oocyst syncytium to develop further and grow, it likely acquires nutrients from the host insect. The actual mechanisms that govern the selective influx of nutrients to the oocyst, presumably from the hemolymph through the basal lamina, are not yet known. However, it is assumed that the oocyst wall or capsule is a main organizer of nutrient acquisition. The oocyst capsule is bipartite and consists of a thick outer layer, formed mainly of mosquito laminin (92), and an inner oocyst plasma membrane (118). In addition to the presence of parasite transglutaminase (1), another parasite protein, Cap380 (oocyst capsule protein), is localized specifically to the laminin-rich outer capsule layer (127). The oocyst grows to 50–60 μm in diameter, making it one of the largest life cycle stages. Ultimately, the real success of oocyst development is assessed by the formation of sporozoites.
Prior to sporozoite formation, an important sporozoite protein called circumsporozoite protein (CSP) (Figure 2) plays a major role in oocyst development. CSP has an N-terminal signal peptide and a C-terminal GPI transfer sequence and is thus most likely bound to the parasite membrane by a GPI anchor (85). CSP expression begins a few days after oocyst formation and accumulates on the growing oocyst’s plasma membrane (136). At the onset of sporozoite formation, the oocyst plasma membrane retracts through internal invaginations to form lobes of cytoplasm with nuclei undergoing their final mitotic divisions (117, 119, 121). These syncytial lobes are called the sporoblasts. The sporoblast is marked by the presence of CSP on its membrane and the localization of the daughter nuclei to its periphery (136). CSP is essential for the formation of the sporoblasts, and hence sporozoites (81).
The microtubule organizing centers (MTOCs) are located just underneath the sporoblast membrane (136). MTOCs lead the formation of the apical complex and position the nuclei for the later incorporation into daughter sporozoites. The molecular mechanism that drives the formation and organization of MTOCs under the sporoblast plasma membrane is not yet known. However, it is assumed that CSP plays an important role in this process (136). After assembly of the apical ring, formation and polymerization of the subpellicular microtubules, and attachment to the inner membrane complex (IMC), the sporozoites bud-off from the sporoblast (147). The budding of the sporozoites is asynchronous and appears to happen from the sporoblast membrane in successive waves (121, 133, 147). IMC1, which resides in the IMC, is an important protein that determines the shape of sporozoites and is critical for motility (65). Targeted disruption of IMC1a in P. berghei results in misshaped sporozoites that are defective in motility (65). The mature sporozoites develop their crescent shape after completion of budding from the sporoblasts. The crescent shape is believed to result from the uneven distribution of the microtubules attached to the IMC (119). After completion of sporozoite formation, thousands of sporozoites are waiting in the oocyst to be released into the mosquito hemolymph.
SPOROZOITE MATURATION AND DEVELOPMENT OF INFECTIVITY
Egress of the Sporozoites into the Mosquito Hemocoel
Sporozoites acquire motility before they get released from the oocyst. This form of motility is believed to be incomplete and not as vigorous as the gliding motility displayed by mature salivary gland sporozoites (129, 148), and the development of motility was not thought to directly cause sporozoite egress from the oocyst. Rupture of the oocyst was thought to be a passive result of oocyst expansion and the accumulation of thousands of sporozoites (117). In this model, the oocyst capsule wall is perforated by small holes, due to growth, through which the sporozoites can escape to the hemocoel. However, it is now known that a putative cysteine protease, ECP1 (egress cysteine protease 1), is essential for the egress of sporozoites from oocysts (4). ECP1 deletion in P. berghei created parasites with fully mature oocysts packed with sporozoites, but these sporozoites were incapable of freeing themselves from their oocyst compartment. ecp1– sporozoites showed continuous circular movement inside oocysts (4). The maturation of mutant sporozoites to the extent that they acquire motility without escaping the oocyst strongly suggested that protease activity, not growth or motility, is responsible for the egress of the sporozoites. This intraoocyst circular motility could be compared to the in vivo gliding motility displayed inside the salivary glands (39) and the mammalian skin (8).
Mutation of the positively charged conserved region II plus of the CSP protein led to a phenotype similar to that of ecp1– sporozoites (159). In this study, replacement of the positively charged region II plus of CSP with neutral alanine residues generated transgenic parasites that produced a normal number of oocyst sporozoites that failed to exit the oocyst. This result implied that ECP1 might be involved directly or indirectly in the processing of CSP in the oocyst, which in turn is necessary for sporozoite release. Moreover, deletion of an oocyst hypothetical protein in P. berghei, first identified in a P. falciparum sporozoite proteome study (70), exhibited an egress defect similar to ECP1 disruption and mutation of region II plus of CSP (4, 159). This demonstrates that more than one parasite protein is involved in the process of egress from midgut oocysts. However, it remains to be determined what sequence of molecular events and interactions leads to sporozoite release.
Attachment and Invasion of the Salivary Glands
After their release into the hemocoel, sporozoites are carried by the circulation of hemolymph to all tissues of the mosquito, even to the legs and wing veins (120). The hemocoel sporozoites eventually pass by the basal lamina of the mosquito’s salivary glands (Figure 1). During this brief encounter, parasite ligands recognize specific host receptors that allow the sporozoite to adhere to the basal lamina of the salivary gland but not to any other mosquito tissue (120). The attached sporozoites breach the basal lamina and invade the salivary gland secretory acinar cells through their basal plasma membrane (95). Invasion is thought to occur in a vacuolar membrane of host cell origin and not by plasma membrane disruption. The existence of this vacuole is brief and only accompanies the sporozoite through the cytoplasm of the acinar cells (95). Thus, the transient salivary gland invasion vacuole differs substantially from the persistent parasitophorous vacuole (PV) that surrounds developing liver and blood stages. However, the development and nature of such a transit vacuole are not understood. Finally, the sporozoites exit from the transit vacuole as they emerge from the apical side of the acinar cell plasma membrane into the salivary gland duct (95).
A number of parasite ligands necessary for the initial attachment of sporozoites to the salivary glands have been identified. A recombinant protein of the conserved central region I of CSP binds specifically to the salivary glands’ distal and lateral lobes in a way that resembles the presence of the sporozoites within the respective salivary gland ducts (112). Moreover, competition studies showed that the N terminus of CSP and full-length CSP block the attachment of sporozoites to the salivary glands (90, 112). Furthermore, a number of different studies have shown that antibodies to CSP block salivary gland invasion by sporozoites (11, 29, 162).
The thrombospondin-related anonymous protein (TRAP) is also essential for attachment and invasion of the salivary glands (59, 78, 129, 165). Disruption of TRAP in P. berghei resulted in the accumulation of viable hemocoel sporozoites in the hemolymph that are no longer motile and are unable to invade the salivary glands (129). The functional region of TRAP involved in salivary gland invasion is in the conserved extracellular adhesive A domain and the thrombospondin repeat domain, both of which also have a role in hepatocyte invasion (78). TRAP has a carboxyl terminus cytoplasmic domain containing a penultimate tryptophan. Mutations or deletions in the cytoplasmic tail of TRAP, including the penultimate tryptophan, provided genetic evidence that the carboxyl terminus of TRAP is directly responsible for sporozoite gliding and cell invasion (59).
Interestingly, saglin, a molecule expressed at the surface of the distal lobes of Anopheles salivary glands, interacts with TRAP (43), and antibodies to saglin cause a reduction in sporozoite salivary gland invasion (94). A small peptide (SM1) that binds to salivary gland distal lobes ex vivo and to saglin in vitro can compete for the interaction between TRAP and saglin (43). Moreover, antibodies to the SM1 peptide can detect TRAP in oocyst sporozoites and saglin can bind TRAP in vitro (43). Further molecular dissection of the interaction between TRAP and saglin will reveal the role of saglin in sporozoite attachment, entry into the salivary glands, or both.
Numerous research efforts have determined that gliding motility and host cell invasion by sporozoites and other invasive stages are powered by an actin-myosin motor located between the parasite plasma membrane and the IMC. Furthermore, the IMC and the actin-myosin motor are connected to host cell receptors through TRAP or TRAP-like molecules (60). A TRAP-like molecule called UOS3 (upregulated in oocyst sporozoites 3) has an important role in salivary gland invasion (84). UOS3 has also been named TREP (TRAP-related protein) (22) and S6 (128). UOS3 has a cytoplasmic domain similar to that of TRAP as well as a thrombospondin repeat domain. Targeted deletion of UOS3 in P. yoelii or P. berghei generated viable hemocoel sporozoites that could not invade the salivary glands. Although it is not yet clear how UOS3 functions, initial data suggest that a motility defect due to a lack of UOS3 protein might result in the absence of salivary gland invasion (22, 128). However, unlike TRAP, UOS3 has no function in hepatocyte infection since uos3– oocyst and hemocoel sporozoites are able to infect the liver when injected into mice, leading to a blood stage infection. This observation is thus difficult to reconcile with a general defect in motility (84).
Other proteins that have an important role in salivary gland invasion are cysteine repeat modular proteins (CRMPs), and targeted deletion of CRMP-1 and CRMP-2 in P. berghei revealed a specific role for these sporozoite surface proteins in salivary gland invasion (138). MAEBL also has a specific role in salivary gland invasion, and it is a micronemal protein expressed in oocyst, hemocoel, and salivary gland sporozoites (64). Targeted disruption of MAEBL in P. berghei generated sporozoites that were unable to attach to the basal lamina of the salivary glands (64). In marked contrast to TRAP-deficient parasites, which cannot infect the mammalian host, MAEBL-deficient hemocoel sporozoites were still able to glide and infect the mammalian host. MAEBL undergoes differential alternative splicing and posttranslational modifications in a number of life cycle stages (62, 98, 123), indicating that variants of MAEBL could serve different functions throughout the malaria parasite life cycle.
Differential Gene Expression in Sporozoites and Regulation of Infectivity in Different Host Tissues
The sporozoite maturation within mosquito tissues has remained a puzzle for decades. Initial observations confirmed that whereas the sporozoites in the salivary glands are motile and infectious to the vertebrate host, the sporozoites within the oocysts are not (148, 149). If injected into the hemocoel, salivary-gland derived sporozoites cannot reinvade the salivary glands (140). This finding suggested that salivary gland sporozoites were irreversibly programmed to infect the mammalian host. The first indication that distinct transcriptional repertoires are associated with discrete sporozoite phenotypes came from cDNA subtraction screens of oocyst and salivary gland sporozoites (79). This led to the isolation of a set of genes that were upregulated in infectious sporozoites (UISs), and these genes were thought to be involved in the infection of the mammalian host. Indeed, two transmembrane proteins encoded by UIS genes (UIS3 and UIS4) were subsequently shown to be essential for liver stage development after sporozoite invasion (88, 89). Another UIS-encoded protein, UIS10, a putative phospholipase, has a role in sporozoite tissue traversal before hepatocyte invasion (12). Recently, a more comprehensive microarray analysis of the UIS gene expression profile was conducted in P. yoelii and identified a set of 141 UIS genes (84). The same study found a further set of genes that are upregulated in oocyst sporozoites (UOS) but downregulated in salivary gland sporozoites. This study also demonstrated for the first time that differential gene expression also occurs in P. falciparum sporozoites. UOS genes are predicted to play a role in sporozoite function prior to and during salivary gland invasion. Indeed, the aforementioned UOS3 was identified in this set of genes and is essential only for salivary gland invasion (84). The regulation of infectivity-associated gene expression, however, is still poorly understood.
As discussed above in regard to gametogenesis and ookinete formation, the identification of the AP2-like family of transcription factors (10, 25, 171) revealed that transcriptional control can play an important role in parasite development. However, AP2 participation in the regulation of sporozoite gene expression has not been demonstrated. The low complexity protein SAP1 (sporozoite asparagine-rich protein 1) localizes to the sporozoite cytoplasm and is instrumental in regulating sporozoite infectivity (5). Targeted deletion of SAP1 generated salivary gland sporozoites that showed normal tissue traversal activity and invasion of hepatocytes. However, SAP1-deficient sporozoites were unable to initiate liver stage development (5). UIS3, UIS4, and P52 were not significantly expressed in sap1– sporozoites. This was due to a dramatic reduction in the transcript abundance of UIS genes. However, transcript abundance of other sporozoite-expressed genes that encode proteins involved in tissue traversal activity such as SPECT1, SPECT2, and S4/CelTOS (52, 55, 63), as well as the main constitutive sporozoite proteins CSP and TRAP, were unaffected. Because SAP1 is a putative cytoplasmic protein and lacks any known DNA binding motifs, it was predicted to be involved in posttranscriptional regulation of UIS gene expression because this should occur in the cytoplasm. Another study reported nuclear localization of SAP1 (114), and thus the role of SAP1 could include transcriptional regulation as well. However, data from our laboratory suggest that SAP1 is involved in the posttranscriptional regulation of UIS mRNA stability (A.S.I. Aly & S.H.I. Kappe, unpublished data).
Another recent study showed that the temperature shift that occurs when sporozoites are injected into the host and host cell contact cause an increase in transcript abundance in a number of genes involved in P. falciparum hepatocyte infection (109). The array of genes appears to have two different time courses in infected hepatocytes—transient and sustained—that are proposed to be linked to hepatocyte invasion and liver stage development, respectively. Antibodies raised against two of the transient proteins (SIAP-1 and SIAP-2) and two of the sustained proteins (LSAP-1 and LSAP-2) showed that they were expressed preferentially on the sporozoite surface and the developing liver stage, respectively (109). Antibodies to SIAP-1 and SIAP-2 were able to cause diminished sporozoite traversal and hepatocyte invasion, suggesting that SIAP-1 and SIAP-2 are involved in these processes.
Sporozoite Transmission and Infection of the Mammalian Host
During the mosquito bite, infectious sporozoites leave the salivary gland of the mosquito, enter the host, and eventually make their way to the liver, where they invade hepatocytes and ultimately become liver stages. Although it was shown in 1939 that sporozoites could be found in the avascular tissue surrounding the site of the mosquito bite (16), it was assumed that most of the sporozoites discharged by an infectious mosquito at the moment of biting were expelled directly into the lumen of a capillary. This scenario was questioned when it was shown that mosquitoes that were only allowed to probe the skin, and not actually take a blood meal, were able to infect mice (77). Conversely, if the site of a blood meal was treated with heat immediately after the mosquito imbibed, the mice did not develop a blood stage infection (77). The authors also showed that mice would develop a blood stage infection if sporozoites were injected into the skin, muscle, peritoneum, and tail end. Similarly, it was shown that if the site of the mosquito bite on a mouse ear was removed five minutes after the blood meal had been taken by a P. yoelii–infected mosquito, there was a dramatic drop in the development of a blood stage infection (111). These results suggested that the sporozoites that ultimately led to a malaria infection were not entering the bloodstream during the blood meal; they were leaving the site of injection and finding the bloodstream on their own.
With the development of fluorescently labeled P. berghei parasites (35) and advances in intravital microscopy, it was recently shown that significant numbers of sporozoites are indeed injected into the avascular tissue of mouse ear pinnae as the mosquito probes for its blood meal (151). Real-time imaging allowed the authors to visualize for the first time the efficient gliding motility of the released salivary gland sporozoites and ultimately their invasion of blood vessels and disappearance from the bite site. Many sporozoites remained motile for at least 30 min at the bite site. Amino and coauthors have also captured images of the robust forward-gliding motion of injected fluorescent P. berghei sporozoites in avascular tissue, which occurs at a rate of 1–2 μm s−1 (8) and is 10–20 times faster than their motion in the mosquito salivary duct (39). The authors also noted sporozoites invading blood vessels, and this was typically preceded first by a reduced gliding pattern of movement along the vessel and then by invasion, which took place in about a minute. Additional studies have revealed that about half of the sporozoites remain in the skin for up to seven hours and, surprisingly, that between 15% and 20% of the sporozoites enter the lymphatic system, causing enlargement of the draining lymph node (8, 168). The sporozoites entering the lymphatic system appeared to be slowly drifting away from the bite site in a sideward motion and ultimately interacted with dendritic cells and even partially developed in the endothelial cells lining the proximal lymph node (8).
The findings described above underscore the necessity for (a) sporozoite motility for the progression of the inoculated sporozoite from the avascular tissue of the skin to a blood vessel, and (b) sporozoite entry into the blood vessel endothelium in order to reach the liver. The ability of sporozoites to traverse through host cells by membrane disruption was first documented in 1990 (150). Further studies have shown that sporozoites wound the cells they traverse through but that subsequently the host cell membrane can be repaired (87).
A number of sporozoite proteins play a role in host cell traversal. Two of these are SPECT1 (sporozoite microneme protein essential for cell traversal 1) and SPECT2/PLP1 (perforin-like protein 1). P. berghei SPECT1 is a micronemal sporozoite protein with no known function, yet its targeted disruption completely abrogated the ability of sporozoites to traverse cells (55) without affecting their gliding motility. PLP1, also a micronemal protein, contains a membrane attack complex/perforin-related domain (57), typical of proteins involved in pore formation and plasma membrane lysis. The phenotype resulting from the deletion of PLP1 was similar to that from spect1– sporozoites (52). A more recent study has established the importance of the SPECT proteins in sporozoite migration in the skin after mosquito deposition (6). In the study, in vivo behavior of fluorescent P. berghei spect1– and plp1– sporozoites in the skin of mouse ear pinnae was observed. The authors showed that SPECT1 and PLP1 were important for sporozoite transit in the skin, and although the spect1– and plp1– sporozoites glide in 3D matrices, they were immobilized in the skin presumably because they were unable to traverse host cells. The mutant sporozoites were cleared by dermal phagocytes and arrested in dermal fibroblasts, but despite their apparent lack of cell traversal, a number of spect knockout parasites breached the endothelial barrier to reach the bloodstream and invaded hepatocytes with the formation of a PV. The authors suggest that host cell traversal and extracellular gliding are important attributes the sporozoite requires to reach the liver (6).
As well as the SPECT proteins, P. berghei sporozoites secrete a phospholipase (PbPL) that is important in cell traversal (12). Using a PbPL specific antibody, it was shown that PbPL is expressed at the surface of the sporozoite and when expressed heterologously it had membrane lytic activity (12). Disruption of PbPL did not affect sporozoite gliding or hepatocyte invasion, but it greatly reduced cell traversal activity, leading to a one-day delay in the time from infectious mosquito bite to blood stage parasitemia (12). As for the SPECT proteins, PbPL obviously plays an important role in cell traversal, likely due to its membrane lytic activity.
Finally, the protein CelTOS is involved in cell traversal in the ookinete and the sporozoite (63). CelTOS is localized to micronemes, and its targeted disruption in P. berghei reduced parasite infectivity in the mosquito host and sporozoite infectivity in the liver, as well as significantly reducing its cell traversal ability. These results suggest that conserved cell passage mechanisms are used by both ookinetes and sporozoites to breach host cellular barriers (63).
The ongoing research in sporozoite biology has uncovered the importance of the skin stage of the life cycle. Sporozoites can reside in the skin for hours as well as migrate from the skin to a blood vessel for passage to the liver or to the draining lymph node. Once the sporozoites are in the lymph node, dendritic cells prime a cohort of CD8(+) T cells that then travel to the liver and recognize parasitized hepatocytes (19). Although we are now aware of candidate molecules necessary for cell traversal, the process by which this event actually occurs and the function of the molecules involved are still poorly understood and warrant further research.
CSP: A MULTIFUNCTIONAL PROTEIN
CSP is one of the most studied of all Plasmodium proteins, and hundreds of papers have been published documenting the many biological functions of this ubiquitous cell surface protein as well as its utility as a vaccine (Figure 2). CSP uniformly covers the sporozoite and is attached to the plasma membrane by a GPI anchor. CSP is essential for sporozoite development and its GPI anchor is crucial for its function during sporozoite formation (160). In all malaria species, CSP has a similar structural organization consisting of a variable central region of repeat units and two highly conserved motifs, regions I and II plus, located at the amino- and carboxyl-terminal of the molecule, respectively. Studies utilizing P. berghei parasites expressing P. falciparum CSP have shown that region II plus is essential for sporozoite motility and necessary for robust salivary gland invasion and hepatocyte infection (135). In 1992 it was shown that region II plus of CSP was involved in the binding of CSP to the basolateral domain of hepatocyte membranes, and the authors concluded that this binding provided a rational explanation of why sporozoites target the liver (18). It was then discovered that CSP recognized and bound to heparan sulfate proteoglycans (HSPGs) that are expressed on the surface of both hepatocytes and hepatoma cells (38), and the recognition was shown to occur via the glycosaminoglycan chains of the HSPGs. Further work demonstrated that region II plus did indeed bind directly to HSPGs and that certain basic amino acids as well as the interspersed hydrophobic residues of region II plus are required for binding of CSP to hepatocyte HSPGs (124). On the basis of this information, the authors constructed a multiple antigen peptide that mimicked the hepatocyte binding ligand and showed that the peptide inhibits binding of CSP to HepG2 cells in vitro. A closer examination of region II plus demonstrated a specific requirement for the carboxyl positively charged residues that bind to the negatively charged glycosaminoglycan chains of the heparan sulfate expressed on the surface of cells (41). The degree of sulfation of the glycosaminoglycan chains at both the N- and O-positions of the HSPGs is important for the binding of CSP to cells (96), and highly sulfated heparins (naturally occurring HSPGs) had an enhanced ability to competitively inhibit the attachment of sporozoites to HepG2 cells (169). These and other studies have demonstrated that CSP has high-affinity binding for the highly sulfated heparins found specifically in the liver and that these HSPGs provide a signal for sporozoites to arrest in the liver and proceed with a productive invasion of hepatocytes.
The overall extent of sulfation of heparan sulfate is highest in hepatoma cells when compared with other cell types, and the subsequent change in sporozoite behavior, from host cell traversal to invasion, when encountering hepatoma cells is directly related to CSP cleavage (24). CSP cleavage takes place in region I and is mediated by a cysteine protease of sporozoite origin (23). CSP cleavage is unnecessary for cell traversal, but treatment of sporozoites with the cysteine protease inhibitor E-64, prior to an in vitro infection of hepatoma cells, completely inhibits sporozoite invasion of cells. A similar result was demonstrated in vivo when mice were treated with E-64 prior to a sporozoite inoculation (23). This work demonstrated the requirement of CSP processing for active sporozoite invasion. The incubation of sporozoites with soluble heparin also triggered the invasion response, allowing the sporozoites to invade typically nonpermissive cells such as dermal fibroblasts and endothelial cells as well as Hepa 1–6 cells that had been treated with the sulfation inhibitor chlorate (24). The triggering of CSP cleavage is associated with signaling events that enable the onset of sporozoite invasion. Such signaling cascades are typically mediated by protein kinases, and the protein kinase inhibitor staurosporine inhibits sporozoite invasion (86).
The Plasmodium genome possesses a large number of predicted kinases including a family of CDPKs (163), and the triggering of calcium-mediated signaling pathways within the sporozoite are thought to be necessary to induce exocytosis of molecules required for sporozoite invasion (86). To date, no specific Plasmodium CDPK inhibitors have been discovered, although the antagonist W-7 inhibits plant CDPKs and KN-93 inhibits structurally similar animal CDPKs. These inhibitors also decrease sporozoite invasion and CSP processing (24), suggesting a role for CDPKs in the signaling cascade involved in sporozoite invasion. The transcription level of a newly described member of the Plasmodium CDPK-family, CDPK-6, was high in P. falciparum sporozoites (72), and CDPK-6 was subsequently deleted from P. berghei (24). The cdpk-6– sporozoites showed a marked decrease in invasive capabilities and a severe decrease in CSP cleavage when compared with wild-type sporozoites. Furthermore, incubation of the cdpk-6– sporozoites with heparin did not increase their ability to invade Hepa 1–6 cells (24). Thus, the sporozoite-specific kinase CDPK-6 is involved in the signaling cascade that occurs when CSP binds to hepatocyte HSPGs, resulting in region I cleavage, and this cleavage is then followed by sporozoite invasion of hepatocytes.
The HSPGs in the liver sinusoid are produced by stellate cells within the space of Disse, and the sinusoidal cell layer also contains Kupffer cells, which are resident liver macrophages. An elegant study using intravital imaging has shown the movement of fluorescent sporozoites in the liver (37). Once sporozoites have reached the liver, they glide freely for a number of minutes along the sinusoidal epithelium, a process that can occur both with and against the flow of blood. It appears that sporozoites then invade Kupffer cells, traverse them, and cross into the space of Disse (37). Although SPECT mutants may have a deficiency in Kupffer cell traversal (52, 55), recent evidence indicates that they can traverse endothelia from the avascular space into the lumen of skin capillaries (6). Therefore, it is possible that sporozoites also exit liver sinusoids by routes other than via Kupffer cell traversal, perhaps by passing between sinusoidal epithelial cells. Once inside the liver parenchyma, sporozoites continue to traverse through several hepatocytes before invasion and formation of a PV.
Because the Kupffer cell is the liver’s main line of defense against foreign bodies, it is exceedingly surprising that this resident macrophage is unable to eliminate the sporozoite. CSP is playing a role here too, by preventing the respiratory burst necessary for Kupffer cells to destroy the hepatocyte-seeking sporozoite. Sporozoites and CSP alone induce the generation of cyclic AMP (cAMP) by stimulating Kupffer cell adenylyl cyclase, the enzyme necessary for cAMP production (142). Adenylyl cyclase thus causes an upregulation of cAMP activity, which then inhibits the assembly of the NADPH oxidase necessary for the generation of reactive oxygen species—a potent macrophage defense mechanism. Sporozoites induce cAMP via HSPGs and the low-density lipoprotein receptor-related protein LRP-1, both of which are expressed by Kupffer cells (142).
Finally, CSP even plays a role in promoting liver stage development. CSP contains PEXEL (Plasmodium export element)/VTS (vacuolar transport signal) motifs, which are important for the export of parasite proteins to the host cell. This was elegantly demonstrated by Singh and colleagues for CSP (122). They showed that there are two functional PEXEL/VTS motifs in the amino terminus of CSP. This was achieved by fusing the amino terminus of P. berghei CSP to GFP and demonstrating that the fusion protein could be expressed in the cytoplasm of infected erythrocytes and that mutation of the critical arginine and lysine residues of both PEXEL/VTS motifs to alanine prevented GFP expression in the cytoplasm of the erythrocyte. The deletion of the CSP PEXEL/VTS motif from P. berghei CSP had no effect on sporozoite maturation, but it did prevent the transport of the mutated CSP into the hepatocyte cytoplasm, causing a subsequent decrease in liver stage development. What role was CSP playing in the hepatocyte cytoplasm? On further examination, the authors found that CSP was also present in the hepatocyte nucleus and that targeting of CSP to the nucleus was due to a functional bipartite nuclear localization signal (NLS). Deletion of the NLS prevented CSP from entering the nucleus, and furthermore, the NLS domain of CSP competes with the proinflammatory transcription factor NF-κB for binding to importin-α3, a protein involved in the import of molecules into the nucleus. Thus, CSP plays a role in inhibiting the host inflammatory responses necessary to prevent liver stage development by reducing the translocation of NF-κB from the cytoplasm to the nucleus (122). CSP truly is a multifunctional protein that plays a dominant role in sporozoite development, salivary gland infection, sporozoite targeting to the liver, silencing of Kupffer cells, hepatocyte invasion, and even liver stage growth.
ESSENTIAL PROTEINS FOR PLASMODIUM HEPATOCYTE INVASION AND LIVER STAGE DEVELOPMENT
After traversing through a number of hepatocytes, the sporozoite actively invades a final hepatocyte using its membrane-associated actin-myosin motor. Invasion requires the formation of a parasite–host cell junction termed the moving junction, and it was originally thought that the host cell was essentially passive during this process. However, a recent elegant study shows that host cell F-actin accumulates underneath the stable moving junction and contributes to sporozoite internalization of the host cell (45). Staining of host cell F-actin during sporozoite invasion showed the presence of a host actin sheath around the moving junction. Actin dynamics was shown to be important for sporozoite invasion as jasplakinolide, which prevented actin filament disassembly and markedly decreased the ability of P. berghei sporozoites to enter cells (45). A similar result was seen with mycalolide B, a toxin that irreversibly severs actin filaments and sequesters actin monomers. The authors went on to show that actin polymerization at the site of the moving junction was an active process involving the recruitment of the actin-related protein 2/3 complex (Arp 2/3) to the site of the moving junction and that sequestration of the Arp 2/3 prevented P. berghei sporozoite invasion of HepG2 cells (45). However, it is currently not known how the parasite utilizes host cell actin for invasion and the parasite signals that promote this change in the host remain unidentified.
Once the invasive salivary gland sporozoite enters its final hepatocyte with the resulting formation of a parasitophorous vacuole membrane (PVM), which provides a barrier between the parasite and its host cell, a number of parasite proteins essential for liver stage development are expressed. Deletion of the genes coding for these proteins causes arrest of liver stage growth in knockout parasites, and in vivo studies have shown that these parasites are unable to initiate a blood stage infection. The control of gene transcription and translation in malaria parasites has only recently been studied in depth, and transcriptional comparisons made between infectious salivary gland sporozoites with either oocyst sporozoites or blood stage merozoites allowed for the isolation of the aforementioned UIS genes (58, 79). A number of these UIS genes are vital only for liver stage development, but not for any other stage of the life cycle. Gene deletion of P. berghei UIS3 gave rise to parasites that, although able to transform into early trophozoites, arrested early on in liver stage development—even at 24 h post infection, uis3– liver stages were far smaller than their wild-type counterparts (89).
Utilizing yeast two-hybrid screening, Mikolajczak et al. (83) showed that UIS3 interacts with the host cell’s liver fatty acid binding protein (L-FABP). UIS3 is a member of a family of early-transcribed membrane proteins (ETRAMPs) that traffic to the PVM, and these proteins have a carboxyl terminus that faces the host cell cytoplasm (125, 126). An antibody raised against the carboxyl terminus of UIS3 was used to show that UIS3 was expressed at the liver stage PVM, and the carboxyl terminus was also used as bait in the yeast two-hybrid screen that isolated the L-FABP binding partner (83). L-FABP traffics lipids within hepatocytes and delivers them to membrane compartments. Decreasing L-FABP expression in hepatoma cells using siRNA methodologies decreased the growth of liver stage parasites, whereas increasing its expression increased the growth of liver stage parasites (83). This study was the first to suggest a host binding partner for a parasite PVM protein, and the soluble portion of the carboxy terminus of UIS3 has recently been crystallized and is bound to the phospholipid, phosphatidyl ethanolamine (106). It is tempting to hypothesize that L-FABP delivers lipid to UIS3, which can then unload this cargo to the developing liver stage, providing it with the lipids it needs for growth, but this has yet to be shown experimentally.
Two additional host cell factors involved in lipid organization have links to parasite growth. The first is the tetraspanin cluster of differentiation 81 (CD81), the expression of which is required on the hepatocyte plasma membrane for P. yoelii sporozoite invasion and subsequent PVM formation (116). Antibodies raised against mouse and human CD81 inhibit the in vitro hepatic development of P. yoelii and P. falciparum, respectively (116). Using a similar tetraspanin, CD9, to produce CD9/CD81 chimeras, Yalaoui et al. (167) have shown that a 21-amino-acid stretch of CD81 located in a domain structurally conserved in the large extracellular loop of all tetraspanins is sufficient in an otherwise CD9 background to confer susceptibility to in vitro P. yoelii infection. Cholesterol is involved in the assembly of CD81 microdomains on the cell surface of hepatocytes and is necessary for sporozoite infection (113). Cholesterol is likely supplied via the host hepatocyte scavenger receptor BI (SR-BI), which mediates the selective uptake of cholesterol esters from both high- and low-density lipoproteins. Two bodies of work have recently shown that this second host cell factor, SR-BI, plays a critical role in Plasmodium hepatocyte infection (103, 166). Primary hepatocytes isolated from SR-BI transgenic mice had an enhanced permissiveness to both P. yoelii and P. berghei sporozoite infection, whereas hepatocytes isolated from SR-BI−/− mice and hypomorphic- SR-BI mice (expressing approximately 10% of normal SR-BI levels) were less permissive to infection (103, 166). In addition, there appears to be a strong connection between SR-BI and CD81 (166). SR-BI deficiency caused a decreased expression of CD81 on the cell surface, and Yalaoui et al. concluded that SR-BI functions to provide the necessary cholesterol for tetraspanin microdomain assembly (166). Thus, SR-BI appears to have a dual role in liver stage development: It is required to provide (a) the cholesterol necessary for the correct cell surface expression of CD81, a necessity of sporozoite invasion, and (b) the lipids needed for parasite growth.
Continuing with the subject of lipid requirements of developing liver stages, two groups have demonstrated that a parasite liver stage- specific metabolic pathway involved in de novo lipid synthesis is essential only for late liver stage development (153, 170). Plasmodium parasites possess an apicoplast, a relict plastid organelle (66). Plant and algal plastids harbor several key biosynthetic pathways, and a detailed analysis of the proteins of known function that were targeted to the apicoplast (34) allowed the construction of an apicoplast specific metabolic map (100). The apicoplast is cyanobacterial in origin, and bacteria-like type II fatty acid synthesis (FAS II) is present in the plastid (158). FAS II is a de novo pathway by which Plasmodium can synthesize fatty acids from derivatives of acetate and malonate. The fatty acid chain extension step of FAS II is catalyzed by four key enzymes, FabB/F, FabG, FabI, and FabZ. An earlier study identified FabI from P. falciparum and showed that the FabI inhibitor triclosan kills blood stage parasites (130), suggesting that FAS II is active in blood stages. A recent study showed that the transcription of FAS II genes was increased in P. yoelii liver stages compared with blood stages and that FAS II enzymes were present in the liver stage proteome (131). These results suggested that FAS II might be important for parasite liver infection. Strikingly, gene knockouts of P. yoelii FabB/F and FabZ, two of the enzymes involved in fatty acid synthesis, demonstrated that FAS II is critical only for late liver stage development, not for blood stage or mosquito stage development (153). Analysis of mouse liver sections infected wit h fabb/f – sporozoites showed normal liver stage development for the first 24 h post infection, after which the fabb/f – liver stages failed to undergo complete nuclear replication and merozoite formation, as determined by the lack of cytomere formation and merozoite surface protein 1 (MSP1) expression (153). Both P. yoelii fabb/f – and fabz– sporozoites failed to produce a blood stage infection when injected intravenously into mice, suggesting that FAS II is essential for late liver stage development. The study of P. falciparum FabI showed that its deletion had no effect on blood stage replication and did not alter the parasite’s sensitivity to triclosan (170), thereby demonstrating that although triclosan kills parasites, it does not kill parasites by inhibiting FabI. Gene deletion of P. berghei FabI gave a rise to a phenotype in late liver stage development similar to that seen for P. yoelii fabb/f – and fabz–, but P. berghei fabi– sporozoites ultimately gave rise to a blood stage infection (170). This difference might be explained by the use of P. berghei and C57BL/6 mice, instead of P. yoelii and BALB/c mice, for analysis of knockout parasites, but it is also possible that the parasite has some means to compensate for the loss of FabI but not for FabB/F or FabZ. It will be interesting to see if a similar liver stage phenotype is seen for P. falciparum fabi– parasites. As well as demonstrating the first gene deletions that arrest liver stages late in development, these studies draw attention to the fact that parasite metabolic pathways are not necessarily expressed at every life cycle stage but are expressed when the needs of the parasite cannot be met by host nutrient acquisition.
In a manner similar to UIS3, gene deletion of a second PVM protein, UIS4, from P. berghei gave rise to parasites that were unable to progress through liver stage development (88). An antibody to UIS4 was utilized to demonstrate that, like UIS3, it is localized to the PVM and also to vesicular protrusions that extend from the PVM into the hepatocyte cytoplasm (88). This liver stage tubovesicular network was similar to that seen in the erythrocytic stages (48). Thus, UIS4 could be involved in the maintenance of the tubovesicular network or, alternatively, in trafficking molecules to or from the PVM to promote liver stage development. Further study awaits.
What about sporozoite invasion of hepatocytes with the formation of a PVM? Two parasite genes, P36 and P52 (53, 143), that are arranged in tandem within the Plasmodium genome are involved in this process. P36 and P52 code for proteins that are members of the 6-cysteine protein family (132), and P52 has a putative GPI-anchoring domain that allows the attachment of the protein to the sporozoite plasma membrane. Gene disruptions of either P52 or P36 in P. berghei gave rise to sporozoites that traversed cells but were defective in the final invasion of hepatocytes (53, 143). In P. yoelii, the simultaneous disruption of both P36 and P52 gave rise to sporozoites that were unable to form a PVM (68). The result of this dual gene deletion was that the liver stage of the parasite did not develop and a blood stage infection did not occur, indicating that P52 and P36 might have cooperative functions. Together, they have a critical role in a process that leads to PVM formation, but the identification of host cell receptors that bind P36 and P52 has yet to be accomplished. Most research conducted on liver stage proteins has been carried out in rodent malaria models, but recent work on P. falciparum has shown that p52– sporozoites show normal gliding motility, cell traversal, and in vitro invasion of human hepatocytes compared with wild-type sporozoites (145). However, inside the hepatocyte, liver stage development was arrested soon after invasion. The simultaneous disruption of both P36 and P52 resulted in an even more severe defect of liver stage development in vitro (K.M. VanBuskirk, M. O’Neill, P. De La Vega, A.G. Maier, U. Krzych, et al., unpublished data). This work shows that disruption of the P52 and P36 genes in P. falciparum leads to a phenotype similar to that seen in rodent Plasmodium species and thus paves the way for the generation of P. falciparum genetically attenuated parasite vaccines.
Supplementary Material
Acknowledgments
Stefan H. I. Kappe is funded by grants from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative and the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Adini A, Krugliak M, Ginsburg H, Li L, Lavie L, Warburg A. Transglutaminase in Plasmodium parasites: activity and putative role in oocysts and blood stages. Mol Biochem Parasitol. 2001;117:161–68. doi: 10.1016/s0166-6851(01)00345-0. [DOI] [PubMed] [Google Scholar]
- 2.Adini A, Warburg A. Interaction of Plasmodium gallinaceum ookinetes and oocysts with extracellular matrix proteins. Parasitology. 1999;119(Pt 4):331–36. doi: 10.1017/s0031182099004874. [DOI] [PubMed] [Google Scholar]
- 3.Al-Olayan EM, Beetsma AL, Butcher GA, Sinden RE, Hurd H. Complete development of mosquito phases of the malaria parasite in vitro. Science. 2002;295:677–79. doi: 10.1126/science.1067159. [DOI] [PubMed] [Google Scholar]
- 4.Aly AS, Matuschewski K. A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. J Exp Med. 2005;202:225–30. doi: 10.1084/jem.20050545. Demonstrates that sporozoite egress from oocysts is a protease dependent process, implying that proteases also play a role in egress of other life cycle stages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aly AS, Mikolajczak SA, Rivera HS, Camargo N, Jacobs-Lorena V, et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol Microbiol. 2008;69:152–63. doi: 10.1111/j.1365-2958.2008.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, et al. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3:88–96. doi: 10.1016/j.chom.2007.12.007. Shows that sporozoites require host cell traversal activity during their brief presence in the host dermis but breach the endothelium without cell traversal. [DOI] [PubMed] [Google Scholar]
- 7.Amino R, Menard R, Frischknecht F. In vivo imaging of malaria parasites—recent advances and future directions. Curr Opin Microbiol. 2005;8:407–14. doi: 10.1016/j.mib.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Amino R, Thiberge S, Martin B, Celli S, Shorte S, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–24. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 9.Aravind L, Iyer LM, Wellems TE, Miller LH. Plasmodium biology: genomic gleanings. Cell. 2003;115:771–85. doi: 10.1016/s0092-8674(03)01023-7. [DOI] [PubMed] [Google Scholar]
- 10.Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005;33:3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: Sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;81:332–43. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- 12.Bhanot P, Schauer K, Coppens I, Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J Biol Chem. 2005;280:6752–60. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- 13.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–14. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 14.Billker O, Lindo V, Panico M, Etienne AE, Paxton T, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–92. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 15.Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115(Pt 1):1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- 16.Boyd MF, Kitchen SF. The demonstration of sporozoites in human tissues. Am J Trop Med. 1939;19:27–31. [Google Scholar]
- 17.Carter V, Shimizu S, Arai M, Dessens JT. PbSR is synthesized in macrogametocytes and involved in formation of the malaria crystalloids. Mol Microbiol. 2008;68:1560–69. doi: 10.1111/j.1365-2958.2008.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, et al. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–33. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–41. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 20.Claudianos C, Dessens JT, Trueman HE, Arai M, Mendoza J, et al. A malaria scavenger receptor like protein essential for parasite development. Mol Microbiol. 2002;45:1473–84. doi: 10.1046/j.1365-2958.2002.03118.x. [DOI] [PubMed] [Google Scholar]
- 21.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite induced falciparum malaria. Am J Med Sci. 1973;266:169–77. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Combe A, Moreira C, Ackerman S, Thiberge S, Templeton TJ, Ménard R. TREP, a novel protein necessary for gliding motility of the malaria sporozoite. Int J Parasitol. 2009;39:489–96. doi: 10.1016/j.ijpara.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppi A, Tewari R, Bishop JR, Bennett BL, Lawrence R, et al. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2007;2:316–27. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Silva EK, Gehrke AR, Olszewski K, Leon I, Chahal JS, et al. Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci USA. 2008;105:8393–98. doi: 10.1073/pnas.0801993105. Demonstrates that AP2 plant-like transcription factors of Plasmodium bind specific DNA sequences of Plasmodium, and opens the field for studying transcriptional regulation in the parasite. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dessens JT, Beetsma AL, Dimopoulos G, Wengelnik K, Crisanti A, et al. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–27. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dessens JT, Mendoza J, Claudianos C, Vinetz JM, Khater E, et al. Knockout of the rodent malaria parasite chitinase pbCHT1 reduces infectivity to mosquitoes. Infect Immun. 2001;69:4041–47. doi: 10.1128/IAI.69.6.4041-4047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dessens JT, Siden-Kiamos I, Mendoza J, Mahairaki V, Khater E, et al. SOAP, a novel malaria ookinete protein involved in mosquito midgut invasion and oocyst development. Mol Microbiol. 2003;49:319–29. doi: 10.1046/j.1365-2958.2003.03566.x. [DOI] [PubMed] [Google Scholar]
- 29.do Rosario VE, Appiah A, Vaughan JA, Hollingdale MR. Plasmodium falciparum: Administration of anti-sporozoite antibodies during sporogony results in production of sporozoites which are not neutralized by human anti-circumsporozoite protein vaccine sera. Trans R Soc Trop Med Hyg. 1989;83:305–7. doi: 10.1016/0035-9203(89)90481-1. [DOI] [PubMed] [Google Scholar]
- 30.Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC. Protein kinases of malaria parasites: an update. Trends Parasitol. 2008;24:570–77. doi: 10.1016/j.pt.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Dorin-Semblat D, Quashie N, Halbert J, Sicard A, Doerig C, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol Microbiol. 2007;65:1170–80. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 32.Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. Malaria transmission blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol. 2006;61:991–98. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 33.Eksi S, Stump A, Fanning SL, Shenouda MI, Fujioka H, Williamson KC. Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Mol Microbiol. 2002;44:1507–16. doi: 10.1046/j.1365-2958.2002.02986.x. [DOI] [PubMed] [Google Scholar]
- 34.Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz M, et al. Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science. 2003;299:705–8. doi: 10.1126/science.1078599. [DOI] [PubMed] [Google Scholar]
- 35.Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, Van Der Keur M, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Frevert U. Sneaking in through the back entrance: the biology of malaria liver stages. Trends Parasitol. 2004;20:417–24. doi: 10.1016/j.pt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Frevert U, Engelmann S, Zougbede S, Stange J, Ng B, et al. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol. 2005;3:e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B, Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993;177:1287–98. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S, et al. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol. 2004;6:687–94. doi: 10.1111/j.1462-5822.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 40.Furuya T, Mu J, Hayton K, Liu A, Duan J, et al. Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proc Natl Acad Sci USA. 2005;102:16813–18. doi: 10.1073/pnas.0501858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gantt SM, Clavijo P, Bai X, Esko JD, Sinnis P. Cell adhesion to a motif shared by the malaria circumsporozoite protein and thrombospondin is mediated by its glycosaminoglycan-binding region and not by CSVTCG. J Biol Chem. 1997;272:19205–13. doi: 10.1074/jbc.272.31.19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner MJ, Hall N, Fung E, White O, Berriman M, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gissot M, Ting LM, Daly TM, Bergman LW, Sinnis P, Kim K. High mobility group protein HMGB2 is a critical regulator of Plasmodium oocyst development. J Biol Chem. 2008;283:17030–38. doi: 10.1074/jbc.M801637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, et al. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe. 2009;5:259–72. doi: 10.1016/j.chom.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet. 2005;365:1487–98. doi: 10.1016/S0140-6736(05)66420-3. [DOI] [PubMed] [Google Scholar]
- 47.Guinovart C, Navia MM, Tanner M, Alonso PL. Malaria: burden of disease. Curr Mol Med. 2006;6:137–40. doi: 10.2174/156652406776055131. [DOI] [PubMed] [Google Scholar]
- 48.Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL. Transport mechanisms in Plasmodium infected erythrocytes: lipid rafts and a tubovesicular network. Int J Parasitol. 2001;31:1393–401. doi: 10.1016/s0020-7519(01)00251-x. [DOI] [PubMed] [Google Scholar]
- 49.Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 50.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–40. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hillyer JF, Barreau C, Vernick KD. Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. Int J Parasitol. 2007;37:673–81. doi: 10.1016/j.ijpara.2006.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 53.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–75. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 54.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol Microbiol. 2006;59:1175–84. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 55.Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M. Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci USA. 2004;101:16310–15. doi: 10.1073/pnas.0406187101. Defines the function of a Plasmodium protein with a MACPF lytic domain and its role in breaching the lasma membrane of host cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol. 2004;133:15–26. doi: 10.1016/j.molbiopara.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SH. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–32. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- 59.Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Menard R. Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J Cell Biol. 1999;147:937–44. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kappe SH, Buscaglia CA, Bergman LW, Coppens I, Nussenzweig V. Apicomplexan gliding motility and host cell invasion: overhauling the motor model. Trends Parasitol. 2004;20:13–16. doi: 10.1016/j.pt.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Kappe SH, Gardner MJ, Brown SM, Ross J, Matuschewski K, et al. Exploring the transcriptome of the malaria sporozoite stage. Proc Natl Acad Sci USA. 2001;98:9895–900. doi: 10.1073/pnas.171185198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kappe SH, Noe AR, Fraser TS, Blair PL, Adams JH. A family of chimeric erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1998;95:1230–35. doi: 10.1073/pnas.95.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59:1369–79. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 64.Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–23. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khater EI, Sinden RE, Dessens JT. A malaria membrane skeletal protein is essential for normal morphogenesis, motility, and infectivity of sporozoites. J Cell Biol. 2004;167:425–32. doi: 10.1083/jcb.200406068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, et al. A plastid of probable green algal origin in apicomplexan parasites. Science. 1997;275:1485–89. doi: 10.1126/science.275.5305.1485. [DOI] [PubMed] [Google Scholar]
- 67.Kooij TW, Matuschewski K. Triggers and tricks of Plasmodium sexual development. Curr Opin Microbiol. 2007;10:547–53. doi: 10.1016/j.mib.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun. 2007;75:3758–68. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lal K, Delves MJ, Bromley E, Wastling JM, Tomley FM, Sinden RE. Plasmodium male development gene-1 (mdv-1) is important for female sexual development and identifies a polarised plasma membrane during zygote development. Int J Parasitol. 2009;39:755–61. doi: 10.1016/j.ijpara.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog. 2008;4:e1000195. doi: 10.1371/journal.ppat.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavazec C, Moreira CK, Mair GR, Waters AP, Janse CJ, Templeton TJ. Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol Biochem Parasitol. 2009;163:1–7. doi: 10.1016/j.molbiopara.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 73.Limviroj W, Yano K, Yuda M, Ando K, Chinzei Y. Immuno-electron microscopic observation of Plasmodium berghei CTRP localization in the midgut of the vector mosquito Anopheles stephensi. J Parasitol. 2002;88:664–72. doi: 10.1645/0022-3395(2002)088[0664:IEMOOP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–68. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahairaki V, Voyatzi T, Siden-Kiamos I, Louis C. The Anopheles gambiae gamma1 laminin directly binds the Plasmodium berghei circumsporozoite- and TRAP-related protein (CTRP) Mol Biochem Parasitol. 2005;140:119–21. doi: 10.1016/j.molbiopara.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–69. doi: 10.1126/science.1125129. Identifies a specific translational repression factor in Plasmodium and demonstrates that Plasmodium transcription and translation are not necessarily tightly coupled. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsuoka H, Yoshida S, Hirai M, Ishii A. A rodent malaria, Plasmodium berghei, is experimentally transmitted to mice by merely probing of infective mosquito, Anopheles stephensi. Parasitol Int. 2002;51:17–23. doi: 10.1016/s1383-5769(01)00095-2. [DOI] [PubMed] [Google Scholar]
- 78.Matuschewski K, Nunes AC, Nussenzweig V, Menard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002;21:1597–606. doi: 10.1093/emboj/21.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J Biol Chem. 2002;277:41948–53. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- 80.McRobert L, Taylor CJ, Deng W, Fivelman QL, Cummings RM, et al. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS Biol. 2008;6:e139. doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–40. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 82.Mikolajczak SA, Aly AS, Kappe SH. Preerythrocytic malaria vaccine development. Curr Opin Infect Dis. 2007;20:461–66. doi: 10.1097/QCO.0b013e3282ef6172. [DOI] [PubMed] [Google Scholar]
- 83.Mikolajczak SA, Jacobs-Lorena V, MacKellar DC, Camargo N, Kappe SH. L-FABP is a critical host factor for successful malaria liver stage development. Int J Parasitol. 2007;37:483–89. doi: 10.1016/j.ijpara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Mikolajczak SA, Silva-Rivera H, Peng X, Tarun AS, Camargo N, et al. Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol Cell Biol. 2008;28:6196–207. doi: 10.1128/MCB.00553-08. A comprehensive differential transcript abundance analysis to identify differentially expressed genes in sporozoites; it also shows the functional significance of differentially expressed genes for salivary gland invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moran P, Caras IW. Requirements for glycosylphosphatidylinositol attachment are similar but not identical in mammalian cells and parasitic protozoa. J Cell Biol. 1994;125:333–43. doi: 10.1083/jcb.125.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002;8:1318–22. doi: 10.1038/nm785. [DOI] [PubMed] [Google Scholar]
- 87.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, et al. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–44. doi: 10.1126/science.291.5501.141. Functionally analyzes host cell traversal of sporozoites through hepatocytes and shows that it occurred by membrane wounding. [DOI] [PubMed] [Google Scholar]
- 88.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–27. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–67. doi: 10.1038/nature03188. Identifies the first gene that is essential for early liver stage development, and enables development of genetically attenuated parasites as live malaria vaccine strains. [DOI] [PubMed] [Google Scholar]
- 90.Myung JM, Marshall P, Sinnis P. The Plasmodium circumsporozoite protein is involved in mosquito salivary gland invasion by sporozoites. Mol Biochem Parasitol. 2004;133:53–59. doi: 10.1016/j.molbiopara.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Nacer A, Underhill A, Hurd H. The microneme proteins CTRP and SOAP are not essential for Plasmodium berghei ookinete to oocyst transformation in vitro in a cell free system. Malar J. 2008;7:82–89. doi: 10.1186/1475-2875-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nacer A, Walker K, Hurd H. Localisation of laminin within Plasmodium berghei oocysts and the midgut epithelial cells of Anopheles stephensi. Parasites Vectors. 2008;1:33. doi: 10.1186/1756-3305-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–62. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 94.Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, et al. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. 2007;16:711–22. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 95.Pimenta PF, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol. 1994;41:608–24. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 96.Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. The binding of the circumsporozoite protein to cell surface heparan sulfate proteoglycans is required for Plasmodium sporozoite attachment to target cells. J Biol Chem. 2001;276:26784–91. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pradel G, Hayton K, Aravind L, Iyer LM, Abrahamsen MS, et al. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J Exp Med. 2004;199:1533–44. doi: 10.1084/jem.20031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Preiser P, Renia L, Singh N, Balu B, Jarra W, et al. Antibodies against MAEBL ligand domains M1 and M2 inhibit sporozoite development in vitro. Infect Immun. 2004;72:3604–8. doi: 10.1128/IAI.72.6.3604-3608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raine JD, Ecker A, Mendoza J, Tewari R, Stanway RR, Sinden RE. Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog. 2007;3:e30. doi: 10.1371/journal.ppat.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, et al. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol. 2004;2:203–16. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 101.Rangarajan R, Bei AK, Jethwaney D, Maldonado P, Dorin D, et al. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Rep. 2005;6:464–69. doi: 10.1038/sj.embor.7400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reininger L, Billker O, Tewari R, Mukhopadhyay A, Fennell C, et al. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J Biol Chem. 2005;280:31957–64. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- 103.Rodrigues CD, Hannus M, Prudencio M, Martin C, Goncalves LA, et al. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe. 2008;4:271–82. doi: 10.1016/j.chom.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 104.Schaudinn F. Studien ueber krankheitserregende Protozoen II. Plasmodium vivax (Grassi und Feletti), der Erreger des Tertianfiebers beim Menschen. Arb Kaiserl Gesundheits. 1903;19:169–250. [Google Scholar]
- 105.Schneider D, Shahabuddin M. Malaria parasite development in a Drosophila model. Science. 2000;288:2376–79. doi: 10.1126/science.288.5475.2376. [DOI] [PubMed] [Google Scholar]
- 106.Sharma A, Yogavel M, Akhouri RR, Gill J, Sharma A. Crystal structure of soluble domain of malaria sporozoite protein UIS3 in complex with lipid. J Biol Chem. 2008;283:24077–88. doi: 10.1074/jbc.M801946200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shock JL, Fischer KF, DeRisi JL. Whole-genome analysis of mRNA decay in Plasmodium falciparum reveals a global lengthening of mRNA half-life during the intra-erythrocytic development cycle. Genome Biol. 2007;8:R134. doi: 10.1186/gb-2007-8-7-r134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shortt HE, Garnham PCC. Pre-erythrocytic stage in mammalian malaria parasites. Nature. 1948;161:126. doi: 10.1038/161126a0. [DOI] [PubMed] [Google Scholar]
- 109.Siau A, Silvie O, Franetich JF, Yalaoui S, Marinach C, et al. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog. 2008;4:e1000121. doi: 10.1371/journal.ppat.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei alcium dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Mol Microbiol. 2006;60:1355–63. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sidjanski S, Vanderberg JP. Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. Am J Trop Med Hyg. 1997;57:426–29. doi: 10.4269/ajtmh.1997.57.426. [DOI] [PubMed] [Google Scholar]
- 112.Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, et al. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;19:1992–2002. doi: 10.1242/jcs.02911. [DOI] [PubMed] [Google Scholar]
- 114.Silvie O, Goetz K, Matuschewski K. A sporozoite asparagine-rich protein controls initiation of Plasmodium liver stage development. PLoS Pathog. 2008;4:e1000086. doi: 10.1371/journal.ppat.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silvie O, Mota MM, Matuschewski K, Prudencio M. Interactions of the malaria parasite and its mammalian host. Curr Opin Microbiol. 2008;11:352–59. doi: 10.1016/j.mib.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, et al. Hepatocyte CD81 is required for plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 117.Sinden RE. Excystment by sporozoites of malaria parasites. Nature. 1974;252:314. doi: 10.1038/252314a0. [DOI] [PubMed] [Google Scholar]
- 118.Sinden RE, Alavi Y, Raine JD. Mosquito–malaria interactions: a reappraisal of the concepts of susceptibility and refractoriness. Insect Biochem Mol Biol. 2004;34:625–29. doi: 10.1016/j.ibmb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 119.Sinden RE, Garnham PC. A comparative study on the ultrastructure of Plasmodium sporozoites within the oocyst and salivary glands, with particular reference to the incidence of the micropore. Trans R Soc Trop Med Hyg. 1973;67:631–37. doi: 10.1016/0035-9203(73)90031-x. [DOI] [PubMed] [Google Scholar]
- 120.Sinden RE, Matuschewski K. The sporozoite. In: Sherman IW, editor. Molecular Approaches to Malaria. Washington, DC: ASM Press; 2005. pp. 169–90. [Google Scholar]
- 121.Sinden RE, Strong K. An ultrastructural study of the sporogonic development of Plasmodium falciparum in Anopheles gambiae. Trans R Soc Trop Med Hyg. 1978;72:477–91. doi: 10.1016/0035-9203(78)90167-0. [DOI] [PubMed] [Google Scholar]
- 122.Singh AP, Buscaglia CA, Wang Q, Levay A, Nussenzweig DR, et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131:492–504. doi: 10.1016/j.cell.2007.09.013. Confirms that CSP is exported into the host cell and has important functions during liver stage development. [DOI] [PubMed] [Google Scholar]
- 123.Singh N, Preiser P, Renia L, Balu B, Barnwell J, et al. Conservation and developmental control of alternative splicing in maebl among malaria parasites. J Mol Biol. 2004;343:589–99. doi: 10.1016/j.jmb.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 124.Sinnis P, Clavijo P, Fenyo D, Chait BT, Cerami C, Nussenzweig V. Structural and functional properties of region II-plus of the malaria circumsporozoite protein. J Exp Med. 1994;180:297–306. doi: 10.1084/jem.180.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spielmann T, Fergusen DJ, Beck HP. etramps, a new Plasmodium falciparum gene family coding for developmentally regulated and highly charged membrane proteins located at the parasite-host cell interface. Mol Biol Cell. 2003;14:1529–44. doi: 10.1091/mbc.E02-04-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spielmann T, Gardiner DL, Beck HP, Trenholme KR, Kemp DJ. Organization of ETRAMPs and EXP-1 at the parasite-host cell interface of malaria parasites. Mol Microbiol. 2006;59:779–94. doi: 10.1111/j.1365-2958.2005.04983.x. [DOI] [PubMed] [Google Scholar]
- 127.Srinivasan P, Fujioka H, Jacobs-Lorena M. PbCap380, a novel oocyst capsule protein, is essential for malaria parasite survival in the mosquito. Cell Microbiol. 2008;10:1304–12. doi: 10.1111/j.1462-5822.2008.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steinbuechel M, Matuschewski K. Role for the Plasmodium sporozoite-specific transmembrane protein S6 in parasite motility and efficient malaria transmission. Cell Microbiol. 2009;11:279–88. doi: 10.1111/j.1462-5822.2008.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–22. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 130.Surolia N, Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat Med. 2001;7:167–73. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 131.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105:305–10. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Templeton TJ, Kaslow DC. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol. 1999;101:223–27. doi: 10.1016/s0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 133.Terzakis JA, Sprinz H, Ward RA. The transformation of the Plasmodium gallinaceum oocyst in Aedes aegypti mosquitoes. J Cell Biol. 1967;34:311–26. doi: 10.1083/jcb.34.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005;58:1253–63. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 135.Tewari R, Spaccapelo R, Bistoni F, Holder AA, Crisanti A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J Biol Chem. 2002;277:47613–18. doi: 10.1074/jbc.M208453200. [DOI] [PubMed] [Google Scholar]
- 136.Thathy V, Fujioka H, Gantt S, Nussenzweig R, Nussenzweig V, Menard R. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2002;21:1586–96. doi: 10.1093/emboj/21.7.1586. Dissects the essential role of CSP in the regulation of sporozoite morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thathy V, Menard R. Gene targeting in Plasmodium berghei. Methods Mol Med. 2002;72:317–31. doi: 10.1385/1-59259-271-6:317. [DOI] [PubMed] [Google Scholar]
- 138.Thompson J, Fernandez-Reyes D, Sharling L, Moore SG, Eling WM, et al. Plasmodium cysteine repeat modular proteins 1–4: complex proteins with roles throughout the malaria parasite life cycle. Cell Microbiol. 2007;9:1466–80. doi: 10.1111/j.1462-5822.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 139.Tomas AM, Margos G, Dimopoulos G, van Lin LH, de Koning-Ward TF, et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 2001;20:3975–83. doi: 10.1093/emboj/20.15.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Touray MG, Warburg A, Laughinghouse A, Krettli AU, Miller LH. Developmentally regulated infectivity of malaria sporozoites for mosquito salivary glands and the vertebrate host. J Exp Med. 1992;175:1607–12. doi: 10.1084/jem.175.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trueman HE, Raine JD, Florens L, Dessens JT, Mendoza J, et al. Functional characterization of an LCCL-lectin domain containing protein family in Plasmodium berghei. J Parasitol. 2004;90:1062–71. doi: 10.1645/GE-3368. [DOI] [PubMed] [Google Scholar]
- 142.Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007;9:2610–28. doi: 10.1111/j.1462-5822.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 143.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci USA. 2005;102:12194–99. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–64. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 145.van Schaijk BC, Janse CJ, van Gemert GJ, van Dijk MR, Gego A, et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149:216–22. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 147.Vanderberg J, Rhodin J. Differentiation of nuclear and cytoplasmic fine structure during sporogonic development of Plasmodium berghei. J Cell Biol. 1967;32:C7–10. doi: 10.1083/jcb.32.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vanderberg JP. Studies on the motility of Plasmodium sporozoites. J Protozool. 1974;21:527–37. doi: 10.1111/j.1550-7408.1974.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 149.Vanderberg JP. Development of infectivity by the Plasmodium berghei sporozoite. J Parasitol. 1975;61:43–50. [PubMed] [Google Scholar]
- 150.Vanderberg JP, Chew S, Stewart MJ. Plasmodium sporozoite interactions with macrophages in vitro: a videomicroscopic analysis. J Protozool. 1990;37:528–36. doi: 10.1111/j.1550-7408.1990.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 151.Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–96. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 152.Vaughan AM, Aly AS, Kappe SH. Malaria parasite pre-erythrocytic stage infection: gliding and hiding. Cell Host Microbe. 2008;4:209–18. doi: 10.1016/j.chom.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaughan AM, O’Neill MT, Tarun AS, Camargo N, Phuong TM, et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–20. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vinetz JM, Dave SK, Specht CA, Brameld KA, Xu B, et al. The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc Natl Acad Sci USA. 1999;96:14061–66. doi: 10.1073/pnas.96.24.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vinetz JM, Valenzuela JG, Specht CA, Aravind L, Langer RC, et al. Chitinases of the avian malaria parasite Plasmodium gallinaceum, a class of enzymes necessary for parasite invasion of the mosquito midgut. J Biol Chem. 2000;275:10331–41. doi: 10.1074/jbc.275.14.10331. [DOI] [PubMed] [Google Scholar]
- 156.Vlachou D, Lycett G, Siden-Kiamos I, Blass C, Sinden RE, Louis C. Anopheles gambiae laminin interacts with the P25 surface protein of Plasmodium berghei ookinetes. Mol Biochem Parasitol. 2001;112:229–37. doi: 10.1016/s0166-6851(00)00371-6. [DOI] [PubMed] [Google Scholar]
- 157.Vlachou D, Schlegelmilch T, Runn E, Mendes A, Kafatos FC. The developmental migration of Plasmodium in mosquitoes. Curr Opin Genet Dev. 2006;16:384–91. doi: 10.1016/j.gde.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 158.Waller RF, Keeling PJ, Donald RG, Striepen B, Handman E, et al. Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci USA. 1998;95:12352–57. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Q, Fujioka H, Nussenzweig V. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 2005;1:e9. doi: 10.1371/journal.ppat.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Q, Fujioka H, Nussenzweig V. Mutational analysis of the GPI-anchor addition sequence from the circumsporozoite protein of Plasmodium. Cell Microbiol. 2005;7:1616–26. doi: 10.1111/j.1462-5822.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 161.Warburg A, Schneider I. In vitro culture of the mosquito stages of Plasmodium falciparum. Exp Parasitol. 1993;76:121–26. doi: 10.1006/expr.1993.1014. [DOI] [PubMed] [Google Scholar]
- 162.Warburg A, Touray M, Krettli AU, Miller LH. Plasmodium gallinaceum: antibodies to circumsporozoite protein prevent sporozoites from invading the salivary glands of Aedes aegypti. Exp Parasitol. 1992;75:303–7. doi: 10.1016/0014-4894(92)90215-v. [DOI] [PubMed] [Google Scholar]
- 163.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weathersby AB. The role of the stomach wall in the exogenous development of Plasmodium gallinaceum as studies by means of haemocoel injections of susceptible and refractory mosquitoes. J Infect Dis. 1952;91:198–205. doi: 10.1093/infdis/91.2.198. [DOI] [PubMed] [Google Scholar]
- 165.Wengelnik K, Spaccapelo R, Naitza S, Robson KJ, Janse CJ, et al. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yalaoui S, Huby T, Franetich JF, Gego A, Rametti A, et al. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe. 2008;4:283–92. doi: 10.1016/j.chom.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 167.Yalaoui S, Zougbede S, Charrin S, Silvie O, Arduise C, et al. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog. 2008;4:e1000010. doi: 10.1371/journal.ppat.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–22. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ying P, Shakibaei M, Patankar MS, Clavijo P, Beavis RC, et al. The malaria circumsporozoite protein: interaction of the conserved regions I and II-plus with heparin-like oligosaccharides in heparan sulfate. Exp Parasitol. 1997;85:168–82. doi: 10.1006/expr.1996.4134. [DOI] [PubMed] [Google Scholar]
- 170.Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe. 2008;4:567–78. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol. 2009;71:1402–14. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- 172.Yuda M, Sakaida H, Chinzei Y. Targeted disruption of the Plasmodium berghei CTRP gene reveals its essential role in malaria infection of the vector mosquito. J Exp Med. 1999;190:1711–16. doi: 10.1084/jem.190.11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


