Abstract
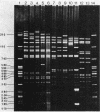
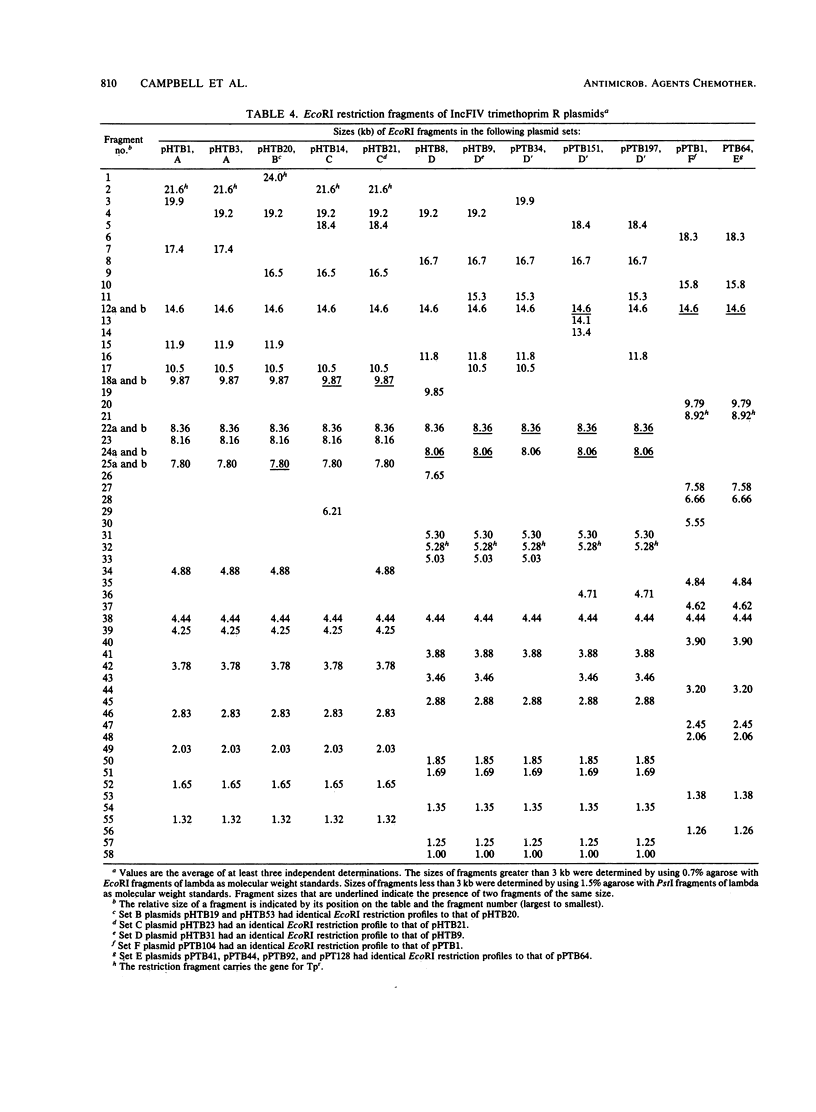
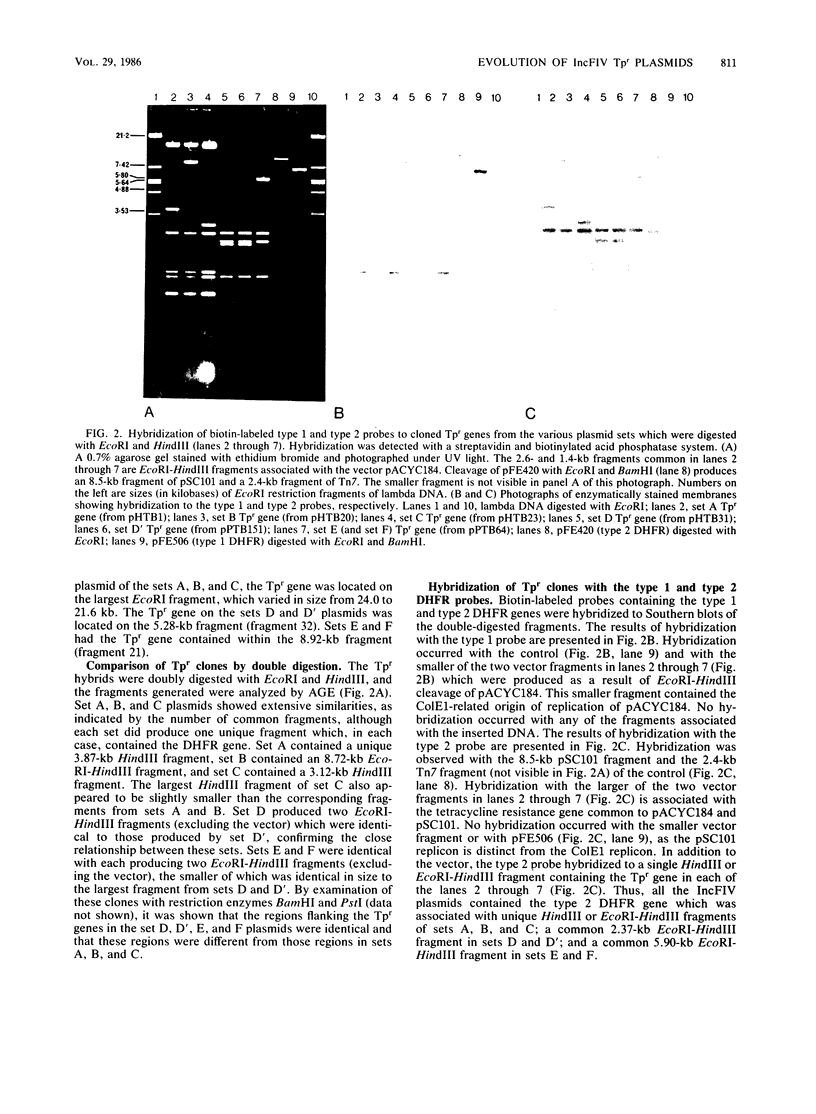
Twenty-one IncFIV-group plasmids conferring trimethoprim resistance in Escherichia coli isolates from humans and pigs were examined. Three evolutionary lines of plasmids were identified on the basis of restriction enzyme analysis. One was found exclusively in human isolates and another was found in pig isolates, while the third line consisted of plasmids from both sources. All R plasmids readily transferred to laboratory strains, and evidence was found for transfer to other biotypes of E. coli in the environment. The Tpr genes from representatives of the plasmid lines were cloned and compared by restriction analysis and by hybridization with two characterized Tpr dihydrofolate reductase genes. The sequences flanking the Tpr genes were different for each line, but all showed homology with the type 2 dihydrofolate reductase gene, irrespective of whether they were of human or animal origin. There was no hybridization to the type 1 gene. The remarkable degree of similarity among plasmids of the third line provided clear evidence of the exchange of plasmid-bearing E. coli between humans and pigs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun D., Cho D. T., Seol S. Y., Suh M. H., Lee Y. C. R plasmids conferring multiple drug resistance from shigella isolated in Korea. J Hyg (Lond) 1984 Apr;92(2):153–160. doi: 10.1017/s0022172400064160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. B., Bird P. I., Nikoletti S. M., Praszkier J., Pittard J. The use of mini-Gal plasmids for rapid incompatibility grouping of conjugative R plasmids. Plasmid. 1984 May;11(3):234–242. doi: 10.1016/0147-619x(84)90029-5. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Elwell L. P. Protein expression in Escherichia coli minicells containing recombinant plasmids specifying trimethoprim-resistant dihydrofolate reductases. J Bacteriol. 1980 Feb;141(2):779–785. doi: 10.1128/jb.141.2.779-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. J., Bird P. I., Pittard J. Naturally occurring plasmids exhibiting incompatibility with members of incompatibility groups I and P. J Bacteriol. 1980 Nov;144(2):758–765. doi: 10.1128/jb.144.2.758-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Shannon K. P. Resistance to apramycin in Escherichia coli isolated from animals: detection of a novel aminoglycoside-modifying enzyme. J Gen Microbiol. 1984 Mar;130(3):473–482. doi: 10.1099/00221287-130-3-473. [DOI] [PubMed] [Google Scholar]
- Jørgensen S. T., Grinsted J., Bennett P., Richmond M. H. Persistence and spread of a chloramphenicol resistance-mediating plasmid in antigenic types of Escherichia coli, pathogenic for piglets. Plasmid. 1980 Sep;4(2):123–129. doi: 10.1016/0147-619x(80)90001-3. [DOI] [PubMed] [Google Scholar]
- Jørgensen S. T. Relatedness of chloramphenicol resistance plasmids in epidemiologically unrelated strains of pathogenic Escherichia coli from man and animals. J Med Microbiol. 1983 May;16(2):165–173. doi: 10.1099/00222615-16-2-165. [DOI] [PubMed] [Google Scholar]
- Konarska-Kozlowska M., Iyer V. N. Sequence homology between Inc N group plasmids. Plasmid. 1983 Nov;10(3):211–223. doi: 10.1016/0147-619x(83)90035-5. [DOI] [PubMed] [Google Scholar]
- Mayer K. H., Fling M. E., Hopkins J. D., O'Brien T. F. Trimethoprim resistance in multiple genera of Enterobacteriaceae at a U.S. hospital: spread of the type II dihydrofolate reductase gene by a single plasmid. J Infect Dis. 1985 May;151(5):783–789. doi: 10.1093/infdis/151.5.783. [DOI] [PubMed] [Google Scholar]
- Mee B. J., Nikoletti S. M. Plasmids encoding trimethoprim resistance in bacterial isolates from man and pigs. J Appl Bacteriol. 1983 Apr;54(2):225–235. doi: 10.1111/j.1365-2672.1983.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tschäpe H., Tietze E. Genetic and molecular characterization of R plasmids incompatible with R387 (IncK). J Gen Microbiol. 1980 Jun;118(2):515–521. doi: 10.1099/00221287-118-2-515. [DOI] [PubMed] [Google Scholar]
- Wise P. J., Towner K. J., Webster C. A., Slack R. C., Jones T. O. Trimethoprim resistance plasmids in Escherichia coli isolated from cases of diarrhoea in cattle, pigs and sheep. J Appl Bacteriol. 1985 Jun;58(6):555–561. doi: 10.1111/j.1365-2672.1985.tb01711.x. [DOI] [PubMed] [Google Scholar]
- de la Cruz F., Zabala J. C., Ortiz J. M. Incompatibility among alpha-hemolytic plasmids studied after inactivation of the alpha-hemolysin gene by transposition of Tn802. Plasmid. 1979 Oct;2(4):507–519. doi: 10.1016/0147-619x(79)90050-7. [DOI] [PubMed] [Google Scholar]