Abstract
Objectives
Single-dose nevirapine (sd-NVP) for prevention of mother-to-child HIV-1 transmission is associated with selection of resistant viral variants, particularly the Lysine (K) to Asparagine (N) mutation at codon 103 (K103N) of reverse transcriptase. As this may influence subsequent treatment responses, a better understanding of the dynamics of decay and persistence of this variant is needed.
Design and methods
We measured the frequency of K103N mutants among a cohort of HIV-1-infected pregnant women recruited at an out-patient clinic in Johannesburg, South Africa. Samples taken 6 weeks, 3, 7 and 12 months after delivery from 67 HIV-1-infected women who received sd-NVP during labor to prevent transmission were analyzed. Quantification of K103N mutants in maternal plasma viral RNA and cellular DNA was done using an allele-specific real-time polymerase chain reaction assay capable of detecting codons AAC and AAT if their frequency was > 0.002 of the total viral population.
Results
Using the allele-specific assay, 87.1% (27/31) of RNA samples and 52.3% (23/44) of DNA samples collected 6 weeks after sd-NVP had detectable K103N variants. This declined to 65.4% (17/26), 38.9% (14/36), and 11.3% (6/53) in RNA at 3, 7 and 12 months respectively, and to 4.2% (2/48) in DNA at 12 months.
Conclusions
K103N resistant variants were present in almost all women at 6 weeks post-sd-NVP but declined rapidly over time. Resistant variants were detected less frequently in cellular DNA with persistence in this compartment by 12 months post-sd-NVP among only a minority.
Keywords: drug resistance, nevirapine, mother-to-child HIV transmission (MTCT)
Introduction
The simplest regimen for reduction of mother-to-child HIV-1 transmission utilizes a single dose of nevirapine (sd-NVP) for the mother at labor onset and one for the child soon after birth [1,2]. The simplicity and low cost of sd-NVP allows programs to achieve high population coverage making the reduction in new cases of pediatric HIV-1 infection a reality even in low resource settings.
A worrying consequence of sd-NVP is the rapid selection of viral variants resistant to nevirapine and to other non-nucleoside reverse transcriptase inhibitors (NNRTI). Viral mutations associated with NNRTI resistance can be detected among 20–40% of nevirapine-exposed women using standard sequencing-based methods [3–8]. The Lysine (K) to Asparagine (N) mutation at codon 103 (K103N) is the most common mutation [4,5,7]. Standard sequencing-based methods generally detect minority species only when present at frequencies > 20% of the viral population, so these studies under-estimate the presence of resistant variants [9]. If more sensitive methods are applied, the majority of nevirapine-exposed women have detectable resistance mutations 1–2 months after exposure [10–12].
These observations have raised concern about the possible long-term efficacy of sd-NVP for prevention of mother-to-child transmission of HIV-1, including whether sd-NVP may be as efficacious in subsequent pregnancies, and whether treatment with NNRTI-based regimens will need to be avoided for nevirapine-exposed women and infants [13,14]. Although the percentage of individuals with detectable genotypic resistance measured using population sequencing methods declines with time after exposure [5,15] interpretation is hampered by lack of knowledge about the viral dynamics occurring below the threshold of detection of these assays. There are also no data on the presence of K103N in cellular DNA following sd-NVP. A better understanding of these dynamics is critical to the development of strategies to reduce possible adverse clinical consequences.
To address this problem, we applied a quantitative, allele-specific, real-time polymerase chain reaction (PCR) method to measure the most common nevirapine resistance mutation, K103N, at various time-points post-sd-NVP among HIV-1-infected women. This sensitive assay allowed us to track minority K103N viral RNA populations in plasma over time after sd-NVP and was also used to determine persistence of low-frequency mutants in cellular DNA.
Methods
Study population
Samples were obtained from a cohort of 164 HIV-1-infected women giving birth at Coronation Hospital, Johannesburg, South Africa. The women received antenatal voluntary counseling and testing and were given 200 mg nevirapine at the onset of labor. Their infants were given 0.6 ml of nevirapine after delivery and were scheduled for post-natal follow-up as part of the prevention of mother-to-child transmission (PMTCT) program at the hospital [16]. Women were enrolled at 6 weeks post-delivery and consumption of nevirapine was confirmed by interview and by review of records. None of the women had been exposed to antiretroviral drugs before this pregnancy. CD4+ T cell counts (BD Biosciences, Palo Alto, California, USA) were performed on maternal blood samples at various time-points post-delivery and viral load (Versant HIV-1 RNA 3.0 bDNA Assay) at 6 weeks. Infant blood samples were collected at 6 weeks and 3 months and tested for HIV-1 DNA by PCR (Roche AMPLICOR HIV-1 qualitative assay v1.5; Roche Diagnostics, Mannheim, Germany). Plasma and buffy coat fractions were stored at −70°C.
A subset of 67 women for whom longitudinal samples were available were selected for this study. Plasma samples collected at 6 weeks, 3, 7 and 12 months post-delivery (n = 160) and buffy coat samples collected at 6 weeks and 12 months (n = 101) were tested using real-time PCR. These women had lower CD4 cell counts and higher viral loads than the larger cohort, but their other characteristics, including transmission risks, were similar. For 16 patients, plasma samples were available from all four time-points; these had similar viral loads to the 67 women in the subset. Real-time PCR data was obtained from 146 (91.3%) of the plasma samples and 92 (91.1%) of the buffy coat samples. Buffy coats from 31 drug-naive HIV-1 pregnant women from a neighboring clinic were used as negative controls.
Population sequencing of HIV-1 reverse transcriptase gene fragments
Plasma samples (0.5 ml) collected at 6 weeks post-sd-NVP were used to identify NNRTI resistance mutations using an in-house nested PCR and population sequencing as described (Loubser SA, et al., in preparation). Briefly, complementary DNA (cDNA) was generated by reverse transcription of viral RNA using an antisense primer (LC-OUT-REV 5′-GAGTTCATACCCCATC CA-3′, nucleotide position 3234–3251 of HIV-1HXB2) followed by a first-round PCR reaction generating a 473 base pair (bp) product spanning the 103 codon (primers LC-OUT-FOR 5′-TTCAGGGAACTCAATA AAAG-3′, 2778–2797 of HIV-1HXB2 and LC-OUT-REV). A second-round PCR amplification generating a 419 bp fragment of reverse transcriptase (RT) was performed using primers LC-IN-FOR (5′-TTTTGGG AAGTTCAATTAGG-3′, 2808–2827 of HIV-1HXB2) and LC-IN-REV (5′-TGGGGGTTCTTTCTGATG-3′, 3210–3227 of HIV-1HXB2). Bidirectional DNA sequencing of PCR products was performed using an ABI Prism BigDye 3.0 kit and resolved on an ABI3100 Genetic Analyzer (Applied Biosystems, Foster City, California, USA).
PCR amplification of HIV-1 RT gene fragments for real-time polymerase chain reaction analysis
cDNA was generated by reverse transcription of a 15 μl aliquot of viral RNA using the method described above with the primer pREV-OUT (5′-ATGGGTCATAATA TACTCCATG-3′, 3492–3513 of HIV-1HXB2) in a 20 μl reaction volume. A 1128 bp RT gene fragment was amplified using the primers pFOR-OUT (5′-AAAATG ATAGGAGGAATTGG-3′, 2385–2404 of HIV-1HXB2) and pREV-OUT. After an initial denaturation at 94°C for 2 min, 35 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 60 s were performed, with a final extension at 72°C for 10 min. A second-round PCR reaction with primers pFOR-IN (5′-CACCTGTCAACATAATT GG-3′, 2491–2509 of HIV-1HXB2) and pREV-IN (5′-AATTTCCCTGTTCTCTGC-3′, 3459–3476 of HIV-1HXB2) was performed with cycling parameters of 94°C for 2 min followed by 35 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 60 s, with a final extension at 72°C for 10 min to generate a 985 bp product. The same PCR method was used with 15 μl of DNA to generate RT gene fragments from cellular viral DNA.
Allele-specific real-time polymerase chain reaction detection of K103N
An allele-specific real-time PCR method was developed to distinguish between the wild-type codons for lysine (AAA and AAG) and the mutant codons for asparagine (AAC and AAT) at position 103 of RT. Primers for detection of all four possible alleles of codon 103 were designed by determining the consensus of 153 known subtype C sequences from South Africa. A common forward primer was used in combination with one of four reverse primers designed with a 3′-base complimentary to the third nucleotide position of codon 103. Each primer combination was tested using plasmid clones representative of each of the four codons at position 103 to confirm the discriminatory power of the method and establish background cut-off values. The PCR products were diluted 1 : 1500 in water and real-time PCR reactions performed on an ABI7000 Real-Time PCR instrument in 10 μl volumes using the SYBR GREEN PCR Master Mix Kit (Applied Biosystems) containing 5 μl of diluted PCR product, a common upstream primer pB (5′-GTTAAACAATGGCCATTGACA-3′, 2610–2630 of HIV-1HXB2) and one of the four allele-specific down-stream primers p103A, p103C, p103G or p103T (5′-CC CACATCCAGTACTGTCACTGATTTT/A/G/C-3′, 2858–2884 of HIV-1HXB2 identical except for their last 3′ nucleotide). Cycling parameters were an initial denaturation and activation of DNA polymerase at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min generating a 274 bp product. Threshold cycle (Ct) values were generated automatically and used to calculate relative frequencies of each codon per sample tested.
Confirmation of K103N by single genome real-time allele-specific polymerase chain reaction
To confirm the presence of low frequency K103N variants, the second-round PCR product was used for ligation into plasmid vectors (TOPO TA Cloning Kit for Sequencing; Invitrogen Life Technologies, Carlsbad, California, USA) and transformed into competent bacteria (TOP10 chemically competent; Invitrogen Life Technologies). Individual colonies selected on kanamycin agar plates were picked and re-suspended in 2 ml of water. K103N real-time allele-specific PCR was performed using 5 μl of diluted colony suspension to determine codon 103 usage.
Sensitivity and sample variance of real-time polymerase chain reaction K103N assay
Plasmids were generated from subtype C pol amplicons bearing all four variants of the 103 codon (pCR2. 1TOPO; Invitrogen Life Technologies). Plasmids were sequenced and both the pB and the p103 primer binding sites shown to differ only at the position under test. Mixtures of these plasmids were used to measure sensitivity and establish background cut-off values for each reaction. Synthetic plasmid mixtures showed a cut-off of detection of 0.002 for the minor variant. Analysis of 15 samples repeated in quadruplicate showed a mean standard deviation of 0.011 (range 0–0.036).
Statistical methods
Since the allele-specific assay is quantitative with a low threshold of detection, we examined two parameters: the percentage with detectable K103N (compared across groups using chi-squared tests) and the frequency of K103N defined as the proportion of the viral population that was 103 variant (compared across groups using non-parametric Wilcoxon tests). Spearman rank correlations were calculated to describe associations between frequencies of K103N variants at different time points and with other quantitative parameters, including maternal viral load and CD4 cell count. Generalized estimating equations (GEE) models were used to estimate the rate of decline per month in K103N frequency (slope) by covariates taking into account repeat measures on the same individual. To test if the rate of decline differed by specific covariates, an interaction term on the multiplicative scale between time and the covariate of interest was included in the GEE model. Statistical analyses were undertaken with SAS software (Cary, North Carolina, USA).
Results
Study population
The median CD4 cell counts and viral loads of the 67 study participants indicated that most women were in the chronic phase of infection (Table 1). Using a nested ‘in-house’ population sequencing method, 54.5% (36/66) of plasma samples taken 6 weeks after sd-NVP were found to harbor K103N viral mutations and all, except 1 (subtype A), were infected with subtype C.
Table 1.
Characteristics of 67 HIV-1-infected women in Johannesburg, South Africa exposed to single-dose nevirapine (sd-NVP) for prevention of mother-to-child HIV-1 transmission.
| N | Median (IQR) | |
|---|---|---|
| CD4+ cell count 6 weeks post-delivery | 67 | 419 (248–590) |
| Plasma HIV-1 RNA copies/ml 6 weeks post-delivery | 58 | 8164 (2256–27820) |
| n (%) | ||
| Transmitted HIV-1 to the child | 67 | 8 (11.9) |
| Subtype C | 66a | 65 (98.5) |
| K103N detected on population sequencing 6 weeks post-deliverya | 66a | 36 (54.5) |
Six-week sample from one patient was not available.
IQR, inter-quartile range.
Sensitivity of allele-specific real-time polymerase chain reaction compared to standard genotyping
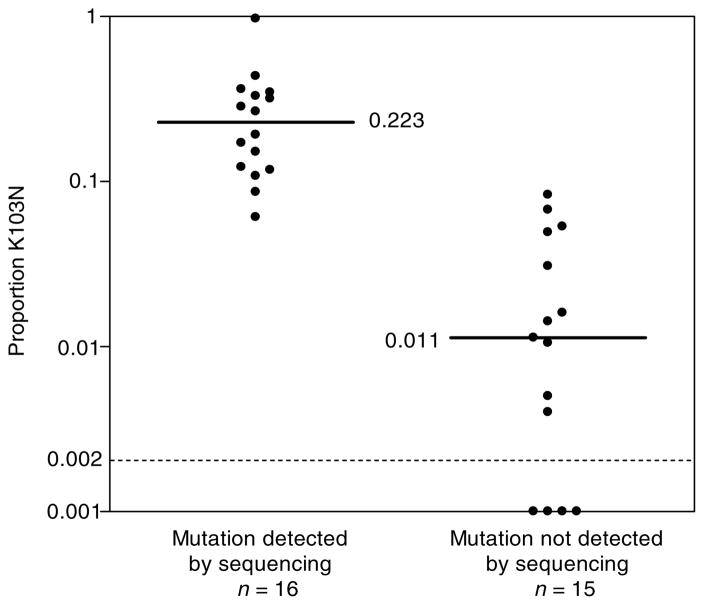
Among 31 plasma samples collected 6 weeks after sd-NVP, K103N variants were detected using the allele-specific assay in all 16 samples in which K103N had originally been detected by sequencing, and in 11 of 15 (73.0%) samples in which K103N had not (Fig. 1). The allele-specific assay also quantified the frequency of these variants which were significantly higher (P < 0.0001) when K103N was also detected by sequencing. The median frequency of K103N variant for genotype positive samples was 0.223 (range, 0.061–0.969) and for genotype negative samples the median frequency of K103N variants if detectable was 0.016 (range, 0.004–0.083). No K103N mutants present at a frequency of < 0.10 of the viral population were detected using the original population sequencing method.
Fig. 1. Relative frequency of K103N variants in maternal plasma viral RNA 6 weeks after single dose nevirapine (sd-NVP) exposure measured by allele-specific real-time polymerase chain reaction stratified by the results of population sequencing-based detection of K103N.
Solid lines indicate the medians in each group calculated to include those samples with frequencies below detection (< 0.002). Medians calculated only in those samples with frequencies above detection are presented in the text. Dashed line indicates the threshold of detection of the assay.
Temporal changes in the relative frequency of K103N variants in plasma RNA
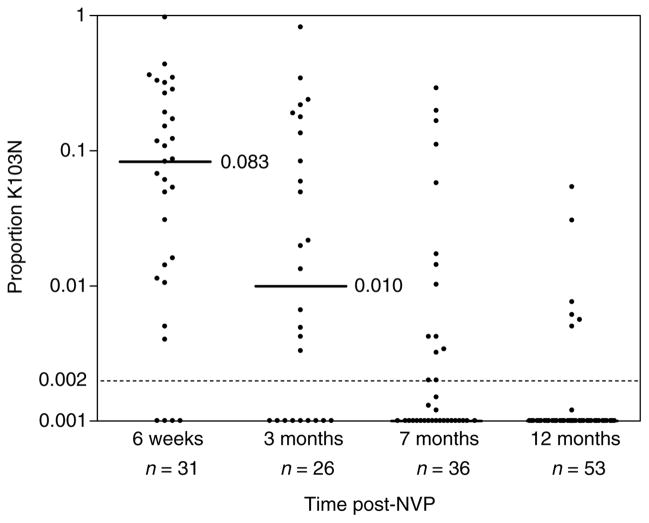
As shown above, K103N variants were detected by real-time PCR in 27 of 31 (87.1%) plasma RNA samples obtained 6 weeks post-sd-NVP. Among the 27 in which K103N variants could be detected (i.e. frequency > 0.002), the median proportion of the viral population that was K103N variant was 0.108 (range, 0.04–0.969). Both the number of patients with detectable K103N variants and the proportion of the viral population that harbored these variants declined rapidly in samples collected at later time points (Fig. 2). At 3 months, 65.4% (17/26) of the samples had detectable K103N variants (median if detected 0.059; range, 0.003–0.819). This declined to 38.9% (14/36) at 7 months (median if detected 0.012; range, 0.002–0.290) and to 11.3% (6/53) at 12 months after sd-NVP (median if detected 0.007; range, 0.005–0.054). All the women who had detectable K103N in plasma at 12 months by real-time PCR also had K103N detected by standard genotyping at 6 weeks.
Fig. 2. Relative frequency of K103N variants in maternal plasma viral RNA at 6 weeks, 3 months, 7 months and 12 months after single dose nevirapine (sd-NVP).
Medians and dotted lines as described in Fig. 1.
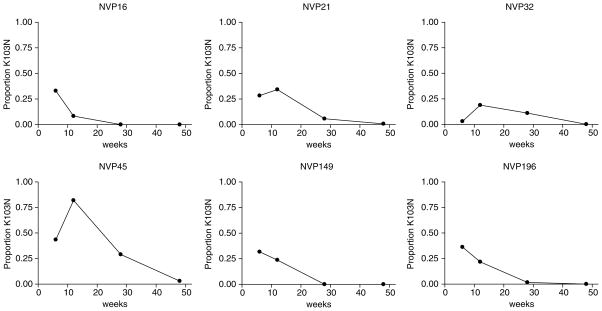
Representative examples of patterns of decline are displayed in Fig. 3. Of 43 women with two or more sequential RNA measurements, 27 (62.8%) had lower frequencies of K103N variants at later time points and 13 (30.2%) had frequencies below detection or very low levels at all time points measured. Three women (7%) had higher frequencies of K103N variants at 3 months than at 6 weeks, thereafter frequencies of their K103N variants declined.
Fig. 3. Longitudinal analysis of the relative frequency of K103N variants in maternal plasma viral RNA at time-points after single-dose nevirapine for individual women to illustrate different patterns of decline.
NVP16, NVP149 and NVP196 showed a decline typical of the whole group. All three women (NVP21, NVP32 and NVP45) with atypical patterns are shown. Women who had no or only very low frequency K103N variants detected at all time points measured are not shown.
Women with high K103N frequencies 6 weeks after sd-NVP were more likely to have K103N mutants detectable later than women with low frequencies. Among women with ≥ 0.1 K103N variant at 6 weeks, frequencies of the mutant was higher at 3 months (P = 0.02), 7 months (P = 0.02) and 12 months (P = 0.01) than among women with < 0.1 K103N variant at 6 weeks. There was significant within-person correlation of K103N frequencies from one visit to the next.
Since 88.7% of women had no detectable K103N variants in RNA by 12 months, the rate of decline between 6 weeks and 12 months post-sd-NVP was significantly faster for those women with higher frequencies of K103N variants at 6 weeks. Assuming the decline in K103N frequencies was linear per month on a log-scale, then the decline was more rapid per month if 6-week levels were high (≥ 0.1; β-coefficient, −0.189) than if they were low (< 0.1; β-coefficient, −0.093) (interaction term P < 0.001).
Higher maternal viral load 6 weeks post-sd-NVP tended to be associated with higher K103N frequencies but this only reached significance for the 7 month time point. Lower CD4 T-cell counts tended to be associated with higher K103N frequencies at 7 and 12 months but not with more proximate time-points.
Relative frequency of K103N variants in cellular DNA
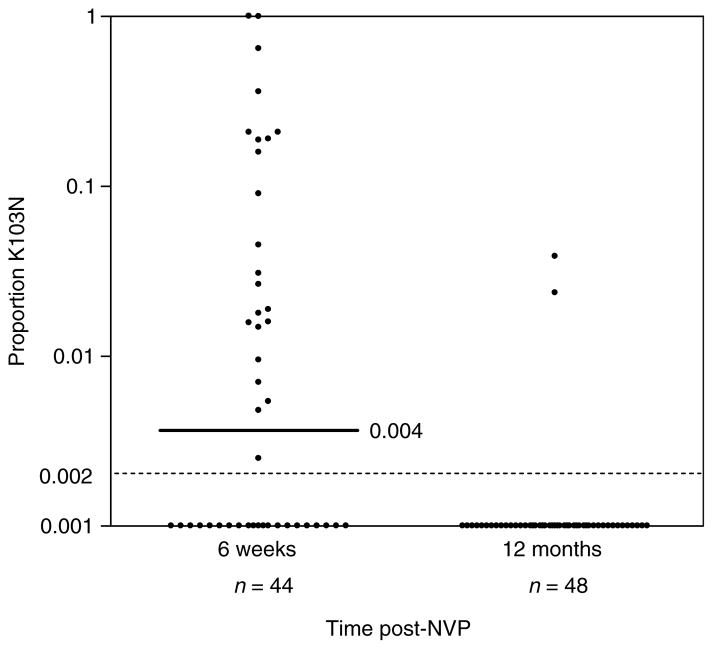
K103N frequencies were determined for DNA samples taken at 6 weeks (n = 44) and 1 year (n = 48) after sd-NVP. At 6 weeks, 52.3% (23/44) of cellular DNA samples had detectable mutant genotypes, with a median frequency of the K103N variant of 0.031 (range, 0.003–1.0) if detectable (Fig. 4). Two of these samples contained only K103N mutant variants. By 12 months, the frequency of K103N variants declined to 4.2% (2/48). The frequency of K103N variants was 0.039 for patient NVP16 and 0.024 for patient NVP196. The presence of K103N was confirmed by real-time allele-specific PCR analysis of clones generated from the NVP196 12 month DNA sample. Of 80 clones tested one (1.3%) was positive for the mutant codon whereas the remainder were wild type. DNA sequencing of this positive clone confirmed the presence of the AAC codon at position 103. None of 31 DNA samples from drug-naive HIV-1-infected pregnant women had any mutant populations detectable by real-time PCR.
Fig. 4. Relative frequency of K103N variants in cellular DNA at 6 weeks and 12 months after single dose nevirapine (sd-NVP).
Medians and dotted lines as described in Fig. 1.
Of those with K103N variants quantified in both RNA and DNA samples collected at 6 weeks (n = 17), all with detectable K103N variants in cellular DNA also had detectable K103N variants in RNA. The proportion of the cellular viral DNA population quantified as K103N variant was significantly correlated with the proportion in RNA (rho = 0.85; P < 0.0001) and with maternal viral load (rho = 0.61, P < 0.0001) at 6 weeks. Correlations between the frequency of K103N variants in cellular DNA at 6 weeks with frequencies of K103N variants in RNA at 3, 7 and 12 months became steadily weaker over time. Neither of the two patients with detectable K103N variants in DNA at 12 months had detectable K103N in RNA at 12 months although both had K103N detected in > 0.1 of the viral RNA population at 6 weeks (Fig. 3).
Discussion
We have shown, as have others [10–12], that viral mutations associated with nevirapine resistance are much more common 6 weeks after sd-NVP than suggested by conventional sequence-based genotyping. Using an allele-specific real-time PCR assay for the most common mutation (K103N), we found almost all women had detectable levels of this mutation at 6 weeks. K103N is important since it is associated with resistance to nevirapine and efavirenz, which are both popular NNRTI for therapeutic regimens and it is also the most persistent [15]. The nested ‘in-house’ population-sequencing method we used on these same samples detected mutations only if present in > 0.1 of the viral population. This is a slightly greater sensitivity than has been observed with other commercial population-sequencing assays possibly because of the nested PCR reaction and shorter target region. However, the lower limit of detection of the allele-specific assay was 0.002 which is at least 50-fold more sensitive than our ‘in-house’ assay and closer to 100-fold more sensitive than commercial genotyping assays.
Over time both detection of K103N-resistant variants and the proportion of the viral RNA population found to harbor these variants declined rapidly in plasma. By 12 months after sd-NVP, our sensitive allele-specific assay could detect K103N variants in only 11% of RNA samples, and these variants were present at low frequency. It remains to be determined whether resistant variants at such low levels have clinical consequences. A study in Thailand showed that women who received nevirapine for prevention of mother-to-child transmission had a poorer virological response to treatment than unexposed women even in the absence of detectable resistance mutations [17]. These results imply a clinical role for these low frequency mutants. However, the Thai study also found that among the nevirapine-exposed, those without detectable resistance mutations were more likely to achieve virologic suppression after treatment than women with detectable resistance [17]. As shown here, women without detectable K103N by conventional genotyping have resistant variants at significantly lower frequency than those detected by conventional genotyping. Thus the frequency of resistant variants may influence treatment response in a dose-dependent manner. Clinical studies should investigate specifically whether sd-NVP has any influence on women’s response to an NNRTI-treatment regimen when started more than 12 months later by which time clinical consequences, if any, may be small.
There were appreciable individual differences in the frequency and persistence of resistant variants. Women who had higher frequencies of mutant viral RNA at 6 weeks (who would have been detected by conventional genotyping) were more likely to have resistance variants detected at later time points. However, the rate of decline was faster among those starting with higher frequencies. These results are consistent with more rapid viral kinetics being associated both with higher peak frequencies of resistant variants and with their more rapid decline. The net result was that for most women resistant variants had declined below detection by one year. Individual variation in drug clearance, viral dynamics or host immune factors may explain the small number of women who displayed an increase in the frequency of K103N variants between 6 weeks and 3 months before declining. Our data also suggest that markers of more advanced disease, including low CD4 cell count and higher viral load, tended to be associated with persistence of resistance mutations. Further clarification of these predictors may have clinical utility. These associations also caution against uncritical comparison across different clinical cohorts since the profile of disease within cohorts may affect the extent of resistant mutations observed.
A limitation of our study is that we examined only the most common resistant variant, K103N. Other mutants may show different dynamics due to differences in viral fitness [18]. A further limitation is that pre-treatment samples were not available. Although resistance mutations may exist at very low frequencies even in the absence of drug pressure [19] other studies among drug-naive, subtype C-infected women have not detected K103N mutations [11,12]. This suggests that pre-existing mutants, if present, are below the detection threshold of even these kinds of assays. Resistance mutations and other polymorphisms may occur in primer binding sites which could affect the sensitivity of the real-time PCR assay. However, we designed subtype C-specific PCR primers in highly conserved regions and single nucleotide changes would not result in complete loss of primer binding. These limitations seem unlikely to explain the dramatic changes in the detection and frequency of K103N variants as time elapsed after sd-NVP.
Our study documented that even once mutants are no longer detectable in plasma viral RNA, persistence in cellular DNA can occur after sd-NVP. However, this could be detected in less than 5% of women. We only examined persistence in the total leukocyte pool and therefore cannot rule out whether there might be mutants present in other cell reservoirs or in lymph nodes. A limitation is that we were unable to quantify cellular HIV DNA copies for input into our PCR. Furthermore it was not possible to determine whether viral DNA was integrated or extra-chromosomal. These factors may have resulted in an underestimation of K103N in DNA. It will be important to test in clinical studies whether the presence of these variants in DNA identifies women most vulnerable to failing NNRTI-based regimens.
Our data suggest that although readily selected by sd-NVP therapy, resistant viral genotypes do not persist for long periods in peripheral blood viral RNA and, although persistence in the cellular DNA compartment occurs, it can be detected in only a minority. If these markers predict subsequent response to NNRTI treatment, when treatment is started more than 12 months post-delivery, previous exposure to sd-NVP may affect treatment responses among only a minority. This is reassuring since the numbers of women needing treatment in the immediate post-partum period will decrease as HIV care programs are implemented more widely and women who meet criteria for drug treatment (low CD4 cell count and symptomatic disease) are started on treatment before or during pregnancy. Furthermore, as subsequent deliveries will generally occur only at some interval of more than 12 months the consequences of previous exposure to sd-NVP for future transmission may be less than anticipated.
Acknowledgments
We thank Stephanie Jones for sample collection, Adrian Puren and Wendy Stevens for diagnostic work, Sarah Cohen for sample and database management and Jefferson Smith for assistance with the real-time PCR assays.
Sponsorship: This work was supported by Bristol-Myers Squibb ‘Secure the Future’ Grants (RES212-01 and RES105-01), Columbia University Center for AIDS Research (AI 42848), and a National Institutes of Child Health and Human Development grant (HD 47177). L.M. is a Wellcome Trust International Senior Research Fellow in Biomedical Sciences.
References
- 1.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIV-NET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JB, Becker-Pergola G, Guay LA, Musoke P, Mracna M, Fowler MG, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 4.Martinson N, Morris L, Gray G, Moodley D, Lupondwana P, Chezzi C. HIV resistance and transmission following single-dose nevirapine in a PMTCT cohort. Eleventh Conference on Retroviruses and Opportunistic Infections; San Francisco. February 2004; [abstract 38] [Google Scholar]
- 5.Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15 :1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Eshleman SH, Becker-Pergola G, Deseyve M, Guay LA, Mracna M, Fleming T, et al. Impact of human immunodeficiency virus type 1 (HIV-1) subtype on women receiving single-dose nevirapine prophylaxis to prevent HIV-1 vertical transmission (HIV Network for Prevention Trials 012 study) J Infect Dis. 2001;184:914–917. doi: 10.1086/323153. [DOI] [PubMed] [Google Scholar]
- 7.Eshleman SH, Guay LA, Mwatha A, Brown ER, Cunningham SP, Musoke P, et al. Characterization of nevirapine resistance mutations in women with subtype A vs. D HIV-1 6–8 weeks after single-dose nevirapine (HIVNET 012) J Acquir Immun Defic Syndr. 2004;35:126–130. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham CK, Chaix ML, Rekacewicz C, Britto P, Rouzioux C, Gelber RD, et al. Development of resistance mutations in women receiving standard antiretroviral therapy who received intrapartum nevirapine to prevent perinatal human immunodeficiency virus type 1 transmission: a substudy of pediatric AIDS clinical trials group protocol 316. J Infect Dis. 2002;186:181–188. doi: 10.1086/341300. [DOI] [PubMed] [Google Scholar]
- 9.Palmer S, Kearney M, Maldarelli F, Halvas EK, Bixby CJ, Bazmi H, et al. Multiple, linked human immunodeficiency virus type 1 drug resistance mutations in treatment-experienced patients are missed by standard genotype analysis. J Clin Microbiol. 2005;43:406–413. doi: 10.1128/JCM.43.1.406-413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flys T, Nissley DV, Claasen CW, Jones D, Shi C, Guay LA, et al. Sensitive drug-resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after the administration of single-dose NVP. J Infect Dis. 2005;192:24–29. doi: 10.1086/430742. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Li JF, Morris L, Martinson N, Gray G, McIntyre J, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 12.Palmer S, Boltz V, Maldarelli F, Martinson N, Gray G, McIntyre J, et al. Persistence of NNRTI-resistant variants after single dose nevirapine in HIV-1 subtype C-infected women. Twelfth Conference on Retroviruses and Opportunistic Infections; Boston. February 2005; [abstract 101] [Google Scholar]
- 13.Nolan M, Fowler MG, Mofenson LM. Antiretroviral prophylaxis of perinatal HIV-1 transmission and the potential impact of antiretroviral resistance. J Acquir Immun Defic Syndr. 2002;30 :216–229. doi: 10.1097/00042560-200206010-00011. [DOI] [PubMed] [Google Scholar]
- 14.Morris L, Pillay C, Gray G, McIntyre J. HIV-1 drug resistance and mother-to-child transmission. S Afr Dental J. 2001;56:614–616. [PubMed] [Google Scholar]
- 15.Morris L, Martinson N, Pillay C, Moodley D, Chezzi C, Lupondwana P, et al. Persistence of nevirapine resistance mutations 6 months following single dose nevirapine. XV International AIDS Conference; Bangkok, Thailand. July 2004; [abstract ThOrB1353] [Google Scholar]
- 16.Sherman GG, Jones SA, Coovadia AH, Urban MF, Bolton KD. PMTCT from research to reality–results from a routine service. S Afr Med J. 2004;94:289–292. [PubMed] [Google Scholar]
- 17.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 18.Collins JA, Thompson MG, Paintsil E, Ricketts M, Gedzior J, Alexander L. Competitive fitness of nevirapine-resistant human immunodeficiency virus type 1 mutants. J Virol. 2004;78 :603–611. doi: 10.1128/JVI.78.2.603-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najera I, Holguin A, Quinones-Mateu ME, Munoz-Fernandez MA, Najera R, Lopez-Galindez C, et al. Pol gene quasispecies of human immunodeficiency virus: mutations associated with drug resistance in virus from patients undergoing no drug therapy. J Virol. 1995;69:23–31. doi: 10.1128/jvi.69.1.23-31.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]